Abstract
Introduction
This study aimed to address therapeutic inertia in the management of type 2 diabetes (T2D) by investigating the potential of early treatment with oral semaglutide.
Methods
A cross-sectional survey was conducted between October 2021 and April 2022 among specialists treating individuals with T2D. A scientific committee designed a data collection form covering demographics, cardiovascular risk, glucose control metrics, ongoing therapies, and physician judgments on treatment appropriateness. Participants completed anonymous patient questionnaires reflecting routine clinical encounters. The preferred therapeutic regimen for each patient was also identified.
Results
The analysis was conducted on 4449 patients initiating oral semaglutide. The population had a relatively short disease duration (42% < 5 years), and a minority (15.6%) had a history of cardiovascular events. Importantly, oral semaglutide was started in subjects with various disease durations and background therapies. Notably, its initiation was accompanied by de-prescription of sulfonylureas, pioglitazone, DPP-4 inhibitors, and insulin. Choice of oral semaglutide was influenced by patient profiles and ongoing glucose-lowering regimens. Factors such as younger age, higher HbA1c, and ongoing SGLT-2 inhibitor therapy drove the choice of oral semaglutide with the aim of improving glycemic control. Projected glycemic effectiveness analysis revealed that oral semaglutide could potentially lead HbA1c to target in > 60% of patients, and more often than sitagliptin or empagliflozin.
Conclusion
The study supports the potential of early implementation of oral semaglutide as a strategy to overcome therapeutic inertia and enhance T2D management.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-023-01490-6.
Keywords: Therapeutic inertia, Survey, GLP-1, Cardiovascular risk, Type 2 diabetes, Management
Key Summary Points
| Why carry out this study? |
| Although guidelines and recommendations on the management of type 2 diabetes prioritize the use of GLP-1 receptor agonists, this class of drugs is still used in a minority of patients who could benefit from them |
| Implementation of an oral therapy in place of an injectable one may help overcome such inertia |
| We undertook a survey among diabetes care specialists in Italy to describe the characteristics and trajectories of patients with type 2 diabetes candidate for initiating oral semaglutide |
| What was learned from the study? |
| We analyzed 4449 patients initiating oral semaglutide who had a relatively short diabetes duration; a minority had a history of cardiovascular disease |
| Initiation of oral semaglutide allowed to de-prescribe sulfonylureas, pioglitazone, DPP-4 inhibitors, and insulin |
| Projected glycemic effectiveness analysis showed that oral semaglutide could potentially lead HbA1c to target in the majority of patients |
Introduction
Oral semaglutide is the first orally delivered peptidic hormone-based therapy for the management of type 2 diabetes (T2D). It utilizes an advanced pharmaceutical technology to ensure its absorption and effectiveness when taken orally: semaglutide is combined with SNAC (sodium N-[8-(2-hydroxybenzoyl) amino] caprylate) to protect the peptide from enzymatic degradation in the stomach and increasing permeability and absorption [1]. Compared to injectable GLP-1 receptor agonists (GLP-1RA), it has the advantage of greater patient acceptability [2].
Based on results of the PIONEER trial program, oral semaglutide is the most effective drug for controlling glycemia and body weight among available oral medications for T2D [3]. In addition, oral semaglutide has shown ability to improve cardiovascular risk biomarkers, including blood pressure, lipids, abdominal adiposity, and inflammation [4, 5]. These characteristics make oral semaglutide an ideal option for the early treatment of patients with T2D after failure of metformin monotherapy or in the presence of contraindications or intolerance to metformin.
Treatment of patients with T2D with oral semaglutide results in an improvement of cardiovascular risk factors [6]. Although there is still no evidence that oral semaglutide improves cardiovascular outcomes of T2D, the same active compound, when administered subcutaneously in the SUSTAIN-6 trial, reduced the rate of 3-point major adverse cardiovascular events (3P-MACE) compared to placebo [7]. In the pre-marketing PIONEER-6 trial, a similarly low HR for 3P-MACE was observed for oral semaglutide versus placebo, which was not statistically significant because of fewer events than in SUSTAIN-6 [8]. Nominally significant reductions in death from any cause and for cardiovascular causes were nonetheless observed with oral semaglutide versus placebo [8]. The rates of cardiovascular events among individuals with T2D receiving oral semaglutide or placebo are being further compared in the ongoing SOUL trial [9].
Prior studies with injectable GLP-1RA suggest that, in clinical practice, these drugs have been used in the later stages of T2D in patients with increasing prevalence of cardiovascular disease and concomitant insulin use [10]. Whether oral semaglutide will be positioned earlier in the management of T2D is still unclear. This opportunity would provide a new way to overcome therapeutic inertia and optimize the probability of reaching and maintaining therapeutic goals in T2D.
Oral semaglutide became part of the therapeutic armamentarium for the management of T2D in Italy in July 2021. In this study, we aimed to describe the clinical features of patients with T2D who were candidate for oral semaglutide treatment under specialist care. We aimed to evaluate whether oral semaglutide was positioned early according to patient characteristics (e.g., age, diabetes duration, presence of complications) and therapeutic regimen (e.g., oral monotherapy, dual therapy, or injectable therapy) and in relation to the cardiovascular risk profile.
Methods
Study Design
This project was developed to improve awareness of therapeutic inertia and relevance of early treatment in patients with T2D. Between October 2021 and April 2022, a cross-sectional survey was proposed to endocrinologist and diabetologist specialists who treated people with T2D. During this period, only diabetes specialists, but not general practitioners, could prescribe DPP-4 inhibitors, SGLT-2 inhibitors, and GLP-1RA. Preliminarily, a scientific committee analyzed the open issues of this specific scenario and designed a dedicated form for data collection. Participants in the survey (n = 218 diabetes care specialists) were asked to complete at least 25 questionnaires relative to anonymous records of patients seen in their routine clinical practice. Data were entered in an online form that included demographics, cardiovascular risk profile, glucose control metrics, ongoing therapies, HbA1c targets, and the physician’s judgment on whether diabetes treatment was appropriate or needed intensification. Finally, participants were asked to identify the suggested most appropriate therapeutic regimen that they chose or would choose for each patient. None of the items on the form were mandatory to complete the record, and there was no direct control over the collected data. We are here presenting observations leading to the choice of oral semaglutide (Fig. S1) as a strategy to overcome clinical inertia and address unmet needs in the management of T2D.
According to the Italian Medicines Agency det. 20/03/2008 on retrospective observational studies on anonymous data, preemptive approval by an ethics committee was not mandatory and the need for informed consent was waived. Given that the survey collected anonymous data, not referable to specific individuals, and was not performed in hospitals or other healthcare settings, approval by one or more ethical committee(s) was not requested. Informed consent could also not be obtained because the identity of patients was unknown. The protocol, in the form of an educational project, was approved by the National Agency for Regional Health Services (AGENAS, id ECM: 5310-329114) and conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. All survey participants provided informed consent and agreed to be mentioned in the supplementary material.
Editorial support was provided by IGMED COMM, and the project was unconditionally supported by Novo Nordisk.
Variables
The following variables were recorded and analyzed: age; sex; duration of diabetes; height and weight for the calculation of BMI; presence of concomitant risk factors (including smoking habit, obesity, hypertension, dyslipidemia); HbA1c; systolic and diastolic blood pressure; estimated glomerular filtration rate (eGFR) calculated from serum creatinine using the CKD-EPI equation; presence of micro-/macro-albuminuria; KDOQI class for chronic kidney disease (CKD); lipid profile; comorbidities (heart failure, chronic liver disease, chronic obstructive pulmonary disease); prevalent cardiovascular disease (history of stroke, myocardial infarction or angina, or peripheral arterial disease); physical activity; type of ongoing glucose-lowering medications. Cardiovascular risk (very high, high, moderate) was estimated based on the ESC/EASD position statement [11]. Organ damage was defined by the presence of at least of one of the following: macroalbuminuria, left ventricular hypertrophy, eGFR < 30 ml/min/1.73 m2, severe retinopathy, and peripheral artery disease (PAD). Information on pre-treatment and recommended glucose-lowering medications was collected. We also recorded information on the reasons for eventual changes in the glucose-lowering medications, potential barriers to therapeutic intensification, and ideal timeline for next outpatient evaluation. The individualized HbA1c target was calculated as previously suggested by Cahn et al. [12].
Statistical Analysis
Continuous variables are reported as mean (standard deviation) or as median and interquartile range. Categorical variables are reported as number of subjects and proportions. McNemar's test was used to compare the proportion of patients being treated with specific medication (or class of medication) prior to and after modification of treatments or between alternative treatments. The differences in clinical characteristics of patients in the different groups (e.g., according to previous glucose-lowering regimen) were tested with unadjusted logistic or linear regression. To account for the multiple hypotheses being tested, we considered 0.0005 as a significant threshold (i.e., accounting for approximately 100 comparisons, corresponding to 6 different background treatment groups tested with 18 independent traits each—the number of independent traits has been estimated with the simpleM methods [13]).
The HbA1c reduction expected from initiation of oral semaglutide was estimated according to the effect reported in the randomized controlled trial (RCT) program (PIONEER 1–8). As described in Table S1, the expected HbA1c reduction ranged from – 0.7% to – 1.1% and was chosen for each patient, according to background therapy aiming at maximizing the overlap of clinical characteristics of patients in this study with those enrolled in the respective trial. As an alternative estimate, we used the reported effect of oral semaglutide in the IGNITE real-world evidence (RWE) study [14], using the estimated HbA1c reduction stratified by baseline HbA1c levels (e.g., – 0.1% among those with HbA1c ≤ 7% vs – 1.1% among those with HbA1 > 7%). The comparison between the estimated effect of adding oral semaglutide compared to adding sitagliptin 100 mg or empagliflozin 25 mg was estimated from data reported in PIONEER 2, 3, or 7 [4, 15, 16] among patients who were not already on a DPP-4 or SGLT-2 inhibitor.
Results
Patient Demographics and Clinical Characteristics
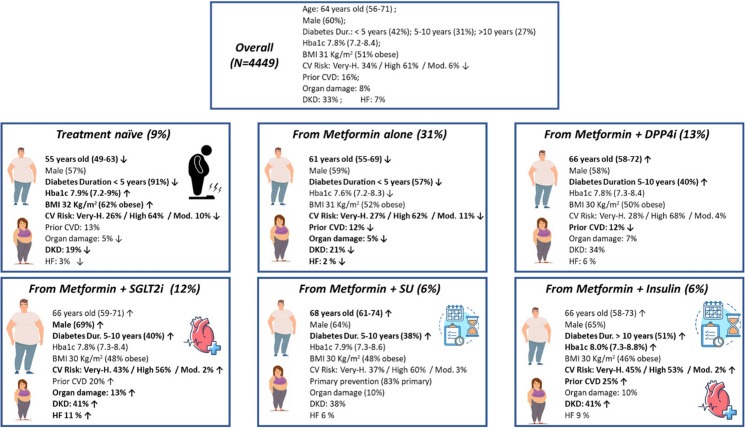
This study collected information on 4449 patients with T2D candidate to oral semaglutide therapy (Fig. S1). As described in Table 1, these patients were 60% males, with a mean age of 63.4 (10.1) years, a relatively short duration of diabetes (< 5 years in 42% of cases), and a mean HbA1c of 7.9 (1.1)%, which was, on average, 1.2% above the recommended target. Most patients had obesity (51%), hypertension (76%), or dyslipidemia (66%), with 19% having three or more cardiovascular risk factors in addition to T2D. Only 14% performed regular physical activity. According to ESC-EASD 2019 definition, the estimated cardiovascular risk was moderated in 6% of the population, high in 61%, and very high in 34%. A total of 15.6% had a prior history of MACE.
Table 1.
Characteristics of the population
| Characteristics | Avail (%) | Total | Naïve | 1 oral | 2+ oral | Injectable |
|---|---|---|---|---|---|---|
| No. of subjects | 4449 | 425 | 1583 | 1935 | 498 | |
| Female | 99 | 1736 (39.6%) | 179 (42.5%) | 644 (41.4%) | 718 (37.7%) | 192 (38.9%) |
| Age, years | 96 | 63.4 ± 10.1 | 56.9 ± 10.4 | 62.1 ± 10.1 | 65.6 ± 9.2 | 64.8 ± 10.2 |
| Age < 50 years | 96 | 482 (11.2%) | 129 (30.6%) | 203 (13.6%) | 109 (5.8%) | 39 (7.8%) |
| Age 50–70 years | 96 | 2691 (62.8%) | 243 (57.7%) | 965 (64.7%) | 1176 (62.9%) | 305 (61.4%) |
| Age > 70 years | 96 | 1112 (26.0%) | 49 (11.6%) | 323 (21.7%) | 585 (31.3%) | 153 (30.8%) |
| Duration < 5 years | 100 | 1853 (41.8%) | 385 (90.8%) | 853 (54.1%) | 505 (26.1%) | 106 (21.3%) |
| Duration 5–10 yrs | 100 | 1381 (31.1%) | 22 (5.2%) | 463 (29.4%) | 739 (38.3%) | 156 (31.4%) |
| Duration > 10 years | 100 | 1204 (27.1%) | 17 (4.0%) | 261 (16.6%) | 688 (35.6%) | 235 (47.3%) |
| Active smoker | 99 | 1194 (27.1%) | 141 (33.3%) | 394 (25.4%) | 549 (28.6%) | 107 (21.6%) |
| Compliance to diet | ||||||
| Good-optimal | 93 | 1069 (25.8%) | 141 (35.6%) | 339 (23.0%) | 470 (26.3%) | 117 (24.5%) |
| Average | 93 | 1172 (28.3%) | 95 (24.0%) | 424 (28.8%) | 489 (27.3%) | 162 (34.0%) |
| Sufficient | 93 | 1086 (26.2%) | 64 (16.2%) | 426 (28.9%) | 485 (27.1%) | 110 (23.1%) |
| Insufficient | 93 | 814 (19.7%) | 96 (24.2%) | 283 (19.2%) | 345 (19.3%) | 88 (18.4%) |
| Regular physical activity | 98 | 608 (13.9%) | 79 (18.9%) | 246 (15.8%) | 215 (11.3%) | 67 (13.7%) |
| Latest HbA1c (%) | 96 | 7.9 ± 1.1 | 8.2 ± 1.5 | 7.9 ± 1.2 | 7.9 ± 0.9 | 8.0 ± 1.2 |
| HbA1c < 7% | 96 | 616 (14.4%) | 54 (13.0%) | 256 (17.2%) | 229 (12.2%) | 77 (15.6%) |
| HbA1c 7–8% | 96 | 1870 (43.8%) | 159 (38.4%) | 661 (44.4%) | 868 (46.4%) | 178 (36.1%) |
| HbA1c 8–10% | 96 | 1592 (37.2%) | 156 (37.7%) | 489 (32.8%) | 737 (39.4%) | 208 (42.2%) |
| HbA1c > 10% | 96 | 196 (4.6%) | 45 (10.9%) | 83 (5.6%) | 38 (2.0%) | 30 (6.1%) |
| HbA1c target, % | 82 | 6.8 ± 0.3 | 6.6 ± 0.4 | 6.7 ± 0.3 | 6.8 ± 0.3 | 6.9 ± 0.4 |
| Distance to target, % | 82 | 1.2 ± 1.1 | 1.6 ± 1.5 | 1.2 ± 1.2 | 1.0 ± 0.9 | 1.1 ± 1.2 |
| Body mass index, kg/m2 | 92 | 30.6 ± 5.2 | 31.8 ± 5.5 | 30.8 ± 5.2 | 30.3 ± 5.1 | 30.3 ± 5.5 |
| Obesity | 92 | 2078 (50.5%) | 257 (62.1%) | 725 (50.9%) | 865 (48.5%) | 228 (47.0%) |
| Hypertension | 100 | 3387 (76.1%) | 267 (62.8%) | 1122 (70.9%) | 1586 (82.0%) | 406 (81.5%) |
| SBP, mmHg | 87 | 135.6 ± 18.3 | 133.4 ± 13.9 | 135.7 ± 15.3 | 136.1 ± 21.7 | 135.8 ± 15.9 |
| DBP, mmHg | 87 | 80.2 ± 8.7 | 80.8 ± 8.9 | 80.2 ± 8.8 | 80.0 ± 8.5 | 80.2 ± 8.6 |
| Dyslipidemia | 100 | 2915 (65.5%) | 223 (52.5%) | 1023 (64.6%) | 1341 (69.3%) | 323 (64.9%) |
| Tot-cholesterol, mg/dl | 82 | 176.4 ± 41.2 | 188.7 ± 47.7 | 180.0 ± 41.1 | 173.1 ± 39.7 | 168.8 ± 38.9 |
| HDL-cholesterol, mg/dl | 81 | 47.7 ± 11.6 | 46.0 ± 11.0 | 47.7 ± 11.9 | 47.8 ± 11.3 | 48.4 ± 11.9 |
| LDL-cholesterol, mg/dl | 80 | 97.5 ± 37.0 | 107.4 ± 38.7 | 100.9 ± 36.4 | 94.7 ± 36.0 | 90.9 ± 38.7 |
| Triglycerides, mg/dl | 82 | 156.0 ± 87.0 | 172.1 ± 97.2 | 157.2 ± 79.4 | 154.3 ± 78.4 | 147.7 ± 121.7 |
| Non-HDL, mg/dl | 81 | 128.6 ± 40.8 | 142.4 ± 47.9 | 132.1 ± 40.4 | 125.4 ± 39.4 | 120.6 ± 37.9 |
| Kidney damage | 100 | 1466 (33.0%) | 81 (19.1%) | 394 (24.9%) | 757 (39.1%) | 232 (46.6%) |
| eGFR, ml/min/1.73 m2 | 85 | 79.2 ± 19.9 | 84.1 ± 20.7 | 81.8 ± 19.0 | 76.9 ± 19.2 | 76.2 ± 22.3 |
| CKD stage 3 + | 85 | 683 (18.1%) | 36 (10.2%) | 171 (13.2%) | 353 (21.4%) | 121 (26.2%) |
| Micro-albuminuria | 92 | 989 (24.2%) | 54 (13.8%) | 258 (18.1%) | 514 (28.5%) | 161 (34.5%) |
| Macro-albuminuria | 92 | 92 (2.2%) | 8 (2.1%) | 20 (1.4%) | 44 (2.4%) | 20 (4.3%) |
| CV risk assessments | ||||||
| Very high risk | 100 | 1494 (33.6%) | 112 (26.4%) | 443 (28.0%) | 703 (36.3%) | 235 (47.2%) |
| High risk | 100 | 2703 (60.8%) | 270 (63.5%) | 990 (62.5%) | 1183 (61.1%) | 254 (51.0%) |
| Moderate risk | 100 | 252 (5.7%) | 43 (10.1%) | 150 (9.5%) | 49 (2.5%) | 9 (1.8%) |
| No. of CVRF ≥ 3 | 100 | 822 (18.5%) | 67 (15.8%) | 275 (17.4%) | 383 (19.8%) | 96 (19.3%) |
| Complications | ||||||
| Prior CV events | 100 | 695 (15.6%) | 56 (13.2%) | 191 (12.1%) | 314 (16.2%) | 133 (26.7%) |
| Stroke | 100 | 147 (3.3%) | 12 (2.8%) | 30 (1.9%) | 63 (3.3%) | 42 (8.4%) |
| Myocardial infarction | 100 | 466 (10.5%) | 41 (9.6%) | 129 (8.1%) | 213 (11.0%) | 82 (16.5%) |
| Angina | 100 | 68 (1.5%) | 4 (0.9%) | 29 (1.8%) | 27 (1.4%) | 8 (1.6%) |
| PAD | 100 | 82 (1.8%) | 4 (0.9%) | 22 (1.4%) | 37 (1.9%) | 19 (3.8%) |
| Organ damage | 100 | 356 (8.0%) | 20 (4.7%) | 86 (5.4%) | 194 (10.0%) | 55 (11.0%) |
| Heart failure | 100 | 302 (6.8%) | 13 (3.1%) | 61 (3.9%) | 161 (8.3%) | 65 (13.1%) |
Data are shown for the entire population (total) and in groups of patients divided by the ongoing treatment regimen prior to the suggested introduction of oral semaglutide
CV cardiovascular, CVRF CV risk factors, SBP systolic blood pressure, DBP diastolic blood pressure, PAD peripheral artery disease
According to ongoing treatment complexity, prior to the recommended change, 9.6% of patients were drug-naïve, 35.6% on oral monotherapy, 35.8% on two oral drugs, 7.7% on three or more oral drugs, and 11.2% on injectable medications, including 1.9% on injectable GLP1-RA and 9.3% on insulin treatment (Table 2). Patients’ characteristics stratified by ongoing treatment complexity are described in Table 1. Further details by patients grouping based on background therapy are available in the supplementary material.
Table 2.
Ongoing regimen before oral semaglutide
| Ongoing | Proposed | p | |
|---|---|---|---|
| Number of drug classes used (incl. 0) | 1.6 ± 0.9 | 2.2 ± 0.6 | < 0.001 |
| Net change in the number of drugs | 1 (0—1) | n.a. | |
| Increased number of drugs (add-on) | 2414 (54.3%) | n.a. | |
| Add-on from naïve treatment | 425 (9.6%) | n.a. | |
| Unchanged number of drugs (switch) | 1714 (38.5%) | n.a. | |
| Reduced number of drug (switch 1 vs > 1) | 321 (7.2%) | n.a. | |
| Specific medications | |||
| Metformin | 3553 (79.9%) | 3668 (82.4%) | < 0.001 |
| Metformin alone | 1380 (31.0%) | 0 (0.0%) | n.a. |
| Sulfonylurea | 649 (14.6%) | 112 (2.5%) | < 0.001 |
| Pioglitazone | 219 (4.9%) | 92 (2.1%) | < 0.001 |
| DPP-4 inhibitors | 1108 (24.9%) | 13 (0.3%) | < 0.001 |
| GLP1-RA | 114 (2.6%) | 4449 (100.0%) | n.a. |
| SGLT-2 inhibitors | 929 (20.9%) | 939 (21.1%) | 0.65 |
| Insulin | 412 (9.3%) | 299 (6.7%) | < 0.001 |
| Basal insulin | 403 (9.1%) | 298 (6.7%) | < 0.001 |
| Bolus insulin | 79 (1.8%) | 10 (0.2%) | < 0.001 |
| Regimen before oral semaglutide | |||
| No treatment | 425 (9.6%) | 0 (0.0%) | n.a. |
| Oral monotherapy | 1583 (35.6%) | 521 (11.7%) | < 0.001 |
| Dual oral therapy | 1592 (35.8%) | 2749 (61.8%) | < 0.001 |
| Triple oral therapy | 343 (7.7%) | 880 (19.8%) | < 0.001 |
| Injectable GLP1-RA | 86 (1.9%) | n.a. | |
| Basal insulin | 333 (7.5%) | 289 (6.5%) | < 0.001 |
| Bolus insulin | 79 (1.8%) | 10 (0.2%) | < 0.001 |
Changes in Medications According to the Proposed Regimen
As described in Table 2, 54.3% of patients added oral semaglutide to the ongoing regimen, leading to an increase in net number of medications (including 1976 subjects adding only oral semaglutide and 438 adding oral semaglutide and other medications at the same time). A switch from other drug classes to oral semaglutide was recommended in 1714 subjects (38.5%), leading to no change in the net number of drug classes. In 321 subjects (7.2%), the addition of oral semaglutide was a substitute for two or more drug classes, leading to a reduction in the number of medications. In the proposed regimen, there was also a minor increase in concomitant use of metformin (from 79.9 to 82.4%) and a significant drop in the use of sulfonylurea (from 14.6 to 2.5%) and insulin (from 9.3% to 6.7%; all p < 0.001), with no change in the concomitant use of SGLT-2i. As expected the concomitant use of DPP4i dropped to almost zero.
Reasons for Choosing Oral Semaglutide
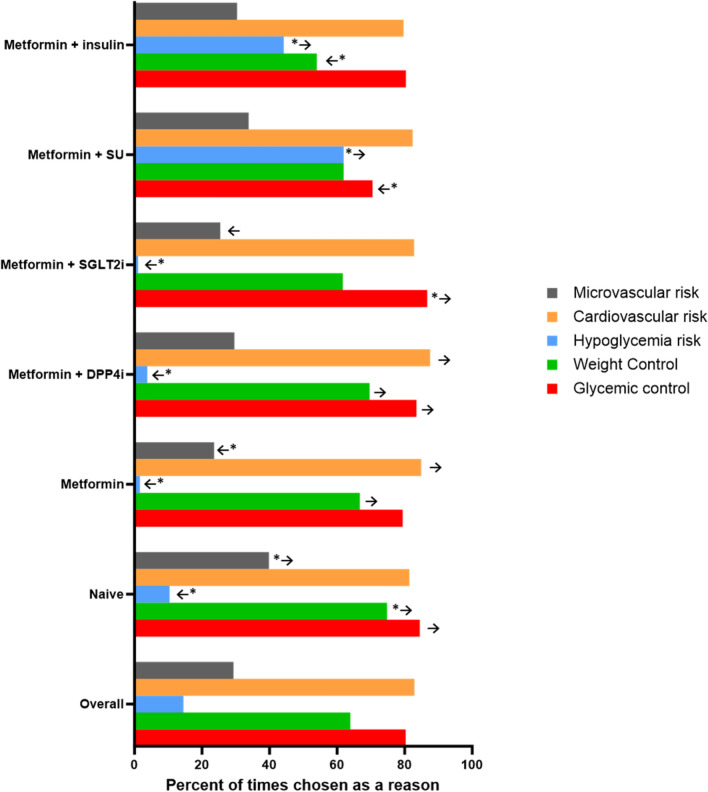
Physicians were asked to report the reason for choosing the initiation of oral semaglutide in each patient. Improvement of metabolic control (80.3%) and reduction of cardiovascular risk (82.9%) were the most common reasons, followed by weight control (63.9%), reduction of microvascular risk (29.3%), and lowering hypoglycemia risk (14.5%).
Patient Phenotypes and Trajectories
We then evaluated patient phenotypes in relation to the indicated reasons for choosing oral semaglutide. First, as described in Figs. 1 and 2 (and detailed in Table S2), we stratified the population according to the ongoing regimen. While the improvement of metabolic control and reduction of cardiovascular risk were the most reported reasons to choose the drug in all groups, we found some differences. Among patients without concomitant medications (characterized by higher prevalence of obesity and younger age with shorter duration of diabetes), physicians reported a higher impact of weight control (74.8%) and control of microvascular risk (39.8%) compared to patients in other groups (p < 0.0005 for both). Among patients on concomitant therapy with metformin and SGLT-2i, the addition of oral semaglutide was even more driven by the need for improving glycemic control (86.7%). As expected, reduction of hypoglycemia risk was frequently a reported reason among those on metformin plus sulfonylureas (61.9%) or plus insulin (44.2%).
Fig. 1.
Patient phenotypes and trajectories by ongoing glucose-lowering regimen. Note: Arrows are reported when the difference between the reported variable in the specific group is significant compared to that in the remaining population. Bold arrows: significant vs overall with study-wide significance p < 0.0005; regular arrows: significant vs overall with nominal p < 0.05
Fig. 2.
Reasons for choosing oral semaglutide by ongoing glucose-lowering regimen. Note: Arrows are reported when the difference between the reported variable in the specific group is significant compared to that in the remaining population. Arrows with asterisk: significant vs overall with study-wide significance p < 0.0005; regular arrows: significant vs overall with nominal p < 0.05
When we combined different clinical characteristics in multivariable logistic regression conducted in the entire population (heatmap in Figure S2), we found that younger age, being on SGLT-2i, and higher HbA1c were all independently associated with higher relevance of improving glycemic control as a reason for choosing oral semaglutide. The independent clinical characteristics associated with other reasons to choose oral semaglutide were as follows: younger age and obesity were linked to weight control; lowering hypoglycemia risk was independently associated with prior cardiovascular events and insulin treatment while inversely associated with ongoing use of SGLT-2i (i.e., hypoglycemia was less of a concern when adding oral semaglutide to patients who were already on SGLT-2i); reduction of cardiovascular risk was associated with prior cardiovascular events and younger age and inversely related to ongoing use of injectable therapies (i.e., less of a concern); presence of kidney disease and younger age were associated with reducing microvascular risk as a reason for choosing oral semaglutide.
Estimated Benefits of Adding Oral Semaglutide
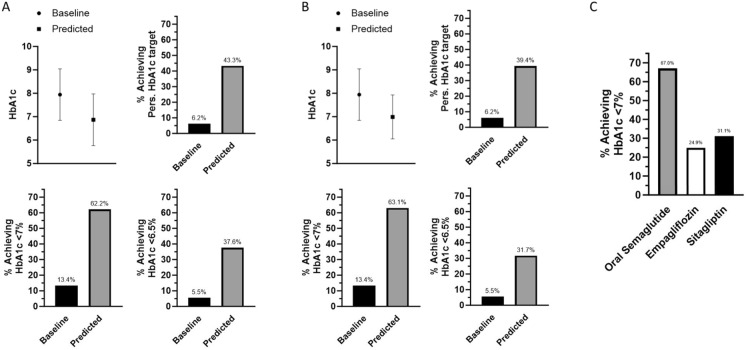
We evaluated the expected glycemic improvement in patients who were candidate for oral semaglutide without the concomitant introduction of other diabetes drugs (n = 1869). As shown in Fig. 3A, using the expected HbA1c reduction derived from RCTs, we estimated a reduction of HbA1c from 7.9% to 6.9% (p < 0.0001), with 43% of patients achieving personalized targets, 62% reaching HbA1c < 7%, and 37.6% reaching HbA1c < 6.5%. Similar results were found using estimates from RWE (Fig. 3B).
Fig. 3.
Predicted improvements in HbA1c. Prediction of HbA1c reduction and achievement of different glycemic targets were calculated among 1869 patients using oral semaglutide only as add-on therapy. A Estimates done using data from RCTs (PIONEER 2,3,7). B Estimates done using data from the IGNITE real-world evidence (RWE) study. C Comparison of expected benefit (proportion of subjects achieving HbA1c < 7%) from the addition of oral semaglutide versus empagliflozin or sitagliptin, among n = 1259 subjects without SGLT-2 or DPP-4 inhibitors as background regimen
Finally, we compared the proportion of patients who were expected to achieve a HbA1c value < 7% among those with clinical characteristics similar to the respective RCT evaluating oral semaglutide versus sitagliptin or versus empagliflozin. Based on RCT results applied to our population (Fig. 3C), a larger proportion of patients was expected to reach HbA1c < 7% with oral semaglutide than with sitagliptin or empagliflozin (p < 0.0001 for both comparisons).
Discussion
In this study, we examined the clinical phenotypes and trajectories of patients who were deemed to be candidate for initiating therapy with oral semaglutide by specialists usually taking care of them in the Italian clinical practice. Patients had a relatively short disease duration (42% had had diabetes for < 5 years); despite a high-to-very high cardiovascular risk, a minority (15.6%) had a history of cardiovascular events. These data may suggest an earlier positioning of oral semaglutide in the management of T2D in Italy compared to what was observed for injectable GLP-1RA [10]. Indeed, over 9 years of observation, we previously found that injectable GLP-1RA had been prescribed to patients with progressively more advanced disease. The possibility to administer semaglutide orally may increase patient acceptability and help positioning this drug earlier in the management of T2D. Nonetheless, our report shows that oral semaglutide was chosen in patients with a wide range of disease duration and background therapy.
Notably, the reported choice of oral semaglutide led to a significant reduction in the use of sulfonylureas, pioglitazone, DPP-4 inhibitors (expected), and insulin, without a significant change in the use of SGLT-2 inhibitors. These findings suggest that initiation of oral semaglutide could lead to an improvement in the overall adherence of the patient’s glucose-lowering regimen to the recommended standards that deprioritize DPP-4 inhibitors, sulfonylurea, and insulin [17]. The striking reduction in the use of sulfonylureas likely reflects the application of the Italian guidelines for the treatment of T2D (which have both clinical and medico-legal implications) recommending not to start and to de-prescribe this class of drugs [18, 19].
Reasons for choosing oral semaglutide as the next step in the management of these patients differed significantly according to the patient profile and ongoing glucose-lowering regimen. Specifically, younger age, higher HbA1c, and ongoing therapy with a SGLT-2 inhibitor were drivers of the need to improve glycemic control with the addition of oral semaglutide. While it is reasonable that higher HbA1c and younger age should prompt an optimization of glycemic control, the association with the ongoing use of SGLT-2 inhibitors may reflect a perceived lower glycemic efficacy of this class of drugs. Indeed, in the PIONEER-2 trial, oral semaglutide was superior to empagliflozin in reducing HbA1c over 52 weeks [4]. In patients with higher cardiovascular risk or a prior cardiovascular event, oral semaglutide was mainly proposed to reduce cardiovascular risk. While there is still no significant evidence that oral semaglutide can reduce the rates of cardiovascular events in people with T2D, this finding may rely on a supposed “class effect” of GLP-1RA in protecting from cardiovascular disease [20]. In addition, oral semaglutide can improve cardiovascular risk factors [6], and a pooled analysis of the SUSTAIN-6 and PIONEER-6 trials identified a strong consistency in the effects on cardiovascular outcomes of injectable and oral semaglutide [21]. The effects of oral semaglutide on cardiovascular outcomes in individuals with T2D and established atherosclerotic cardiovascular disease and/or chronic kidney disease are being tested in the SOUL trial [9], the results of which are expected to be presented in 2025. Meanwhile, in view of the shortage that has afflicted injectable GLP-1RA availability in many countries [22], oral semaglutide may be perceived as a reasonable alternative to improve glycemic and extraglycemic targets that allow reducing cardiovascular risk [22, 23].
In addition to describing the baseline features and therapeutic trajectories of patients who are candidates for oral semaglutide, we analyzed the projected glycemic effectiveness that could be expected based on data from clinical trials or RWE. We estimated that the HbA1c at 6–12 months would be reduced to < 7% in 62% of patients and that 43% of patients would achieve their optimal individualized HbA1c target [12]. Furthermore, in this specific population, oral semaglutide was expected to allow reaching an HbA1c level < 7% significantly more often than empagliflozin or sitagliptin. While these results are projections, it should be highlighted that there is still a paucity of real-world studies examining the effectiveness of oral semaglutide in clinical practice. The IGNITE study reported data on 782 patients prescribed oral semaglutide: from a baseline HbA1c of 8.4%, the mean reduction was 0.9%, but a substantial proportion of patients (37%) received only the 3 mg dose, thereby identifying a potential treatment gap [14]. Indeed, titration to the 7 mg and 14 mg dose in all patients is expected to optimize the glycemic and weight-reducing effect of oral semaglutide. In smaller studies conducted in Japan, patients receiving oral semaglutide experienced a reduction in HbA1c of 1.2% and in body weight of 1.4 kg, with a mean dose slightly above 7 mg [24] and improvements in cardiovascular risk factors [6].
Therefore, our study offers a new perspective on the uptake of oral semaglutide by diabetes care specialists and describes the patient’s journey as well as the expected outcomes.
We acknowledge several limitations of the present analysis. First, we only collected baseline information, and no follow-up data were available to analyze the true effectiveness of oral semaglutide under routine care. Second, data were entered manually by participants, such that there was no possible control over data quality. In addition, the study covered the initial period of oral semaglutide use after commercialization when the learning curve for the best positioning of this new medication was still ongoing. Moreover, estimation of the glycemic effects relies on several assumptions, and we had no information on dose escalation, gastrointestinal tolerability, and other side effects that may affect persistence on treatment. For these reasons, the estimated effect based on trials or RWE needs to be considered cautiously. Further studies are therefore needed, e.g., longitudinal RWE or pooled analyses of RCTs, that might identify subgroups of patients with different benefits from oral semaglutide. Finally, this survey involved Italian specialists expert in diabetes care; therefore, generalizability of results to other countries or to other healthcare settings (e.g., primary care) requires caution.
Conclusion
We argue that more real-world studies are needed to complement findings from clinical trials and to better describe the benefits of using oral semaglutide under routine care. Specifically, studies also need to focus on physicians’ and patients’ perspectives (e.g., investigating reason for specific choice of treatments) to improve our understanding of clinical inertia and hence improve implementation of national and international diabetes guidelines.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Editorial Assistance
Editorial support was provided by IGMED COMM. The authors acknowledge the members of the PIONEERING EXPERIENCE Study Group (see supplementary material).
Author Contributions
Mario Luca Morieri. Riccardo Candido, Simona Frontoni, Olga Disoteo, Anna Solini, and Gian Paolo Fadini made a substantial contribution to the conception or design of the work, data acquisition, data analysis, or interpretation. All authors contributed to drafting the work or revising it critically for important intellectual content. All authors approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of the work are appropriately investigated and resolved.
Funding
The project was supported by an educational grant from Novo Nordisk, including the journal’s Rapid Service Fee. The sponsor had no role in the conduction of the study, analysis and interpretation of data, and preparation of the manuscript.
Data Availability
The dataset generated during the current study is available from the corresponding author on reasonable request, but restrictions may apply.
Declarations
Conflict of Interest
Mario Luca Morieri received lecture, consultancy, or advisory board fees from Amarin, Amgen, Eli Lilly, Merck Sharp & Dohme, Mylan, Novo Nordisk, Novartis, Servier, and SlaPharma. Riccardo Candido received grants, consultancy or lecture fees from Abbott, AstraZeneca, Bayer, Boehringer, Lilly, MSD, Menarini Diagnostics, Mundipharma, Novo Nordisk, Roche Diabetes Care, Sanofi. Simona Frontoni received lecture fees from Eli-Lilly and Novo Nordisk. Olga Disoteo received lecture, consultancy, grant or advisory board fees from Eli Lilly, Novo Nordisk, Astra Zeneca, Daiichi Sankyo, Boehringer Ingelheim, Sanofi, MSD, Novartis. Anna Solini received grants, consultancy or lecture fees from Astra Zeneca, Bayer, Boehringer, Lilly, Novo Nordisk, Sankyo, Sanofi. Gian Paolo Fadini received grants, consultancy or lecture fees from Abbott, AstraZeneca, Boehringer, Lilly, MSD, Mundipharma, Novartis, Novo Nordisk, Sanofi, Servier and Takeda.
Ethical Approval
According to the Italian Medicines Agency det. 20/03/2008 on retrospective observational studies on anonymous data, preemptive approval by an ethical committee is not mandatory and the need for informed consent is waived. Given that the survey collected anonymous data, not referable to specific individuals, and was not performed in hospitals or other healthcare settings, approval by one or more ethical committee(s) was not requested. Informed consent could not be obtained also because the identity of patients was unknown. The protocol, in the form of an educational project, was approved by the National Agency for Regional Health Services (AGENAS, id ECM:5310-329114) and conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. All study participants provided informed consent and agreed to be mentioned in the supplementary material.
Footnotes
Simona Frontoni: Died during the publication process.
Contributor Information
Gian Paolo Fadini, Email: gianpaolo.fadini@unipd.it.
for the PIONEERING EXPERIENCE study group:
Francesco Bellanti, Massimiliano Caprio, Michele Cutolo, Gloria Formoso, Elisa Forte, Vera Frison, Giovanna Gregori, Cristina Lencioni, Gaetano Leto, Salvatore Mandica, Alberto Marangoni, Pasqualina Memoli, Giuseppe Memoli, Carlo Negri, Laura Nollino, Andrea Perrelli, Sebastio Perrini, Flavia Prodam, Alberto Rebora, Daniela Sansone, Marcello Sciaraffia, Silvio Settembrini, Gaetano Sodo, Francesco Tassone, Valentina Todisco, Antonio Vetrano, Giacomo Accardo, Valeria Albanese, Irene Alemanno, Stefano Allasia, Rosario Alosa, Anna Altomari, Anna Maria Letizia Amato, Eleonora Ambrosetti, Angela Angarano, Stefania Angotti, Roberto Anichini, Fabio Baccetti, Marcella Balbo, Elisabetta Balestra, Sara Balzano, Maria Barone, Walter Baronti, Veronica Basso, Guglielmo Beccuti, Iaele Maria Bellone, Alessandra Bertolotto, Michela Bettio, Cristina Bittante, Nadia Bonelli, Marzia Bongiovanni, Benedetta Maria Bonora, Barbara Bonsembiante, Laura Borgognoni, Daniela Bracaglia, Antonia Francesca Braione, Clementina Brancario, Sabrina Braucci, Lucia Briatore, Elisabetta Brun, Valeria Cambria, Elena Cantino, Paolo Capitanata, Sergio Cappello, Marina Caputo, Barbara Carabba, Alberto Carpenito, Marco Castellana, Anna Castrovilli, Donato Cataldo, Giuliana Cazzetta, Francesca Cecoli, Nino Cristiano Chilelli, Marco Cianciullo, Federica Coccia, Sara Colarusso, Caterina Colella, Isabella Colletti, Sara Coluzzi, Marisa Conte, Marco Corigliano, Alessandra Cosma, Silvana Costa, Pantaleo Daniele, Maria D’aurizio, Alessandra De Bellis, Lorenzo De Candia, Giovanni De Gennaro, Ezechiele De Luca, Claudia De Natale, Giuseppina De Simone, Raffaele De Simone, Andrea Del Buono, Vincenza Delmonte, Eleonora Devangelio, Nicolina Biase, Giuseppe Di Giovanni, Mariarosaria Di Palo, Caterina Divella, Mara Dolcino, Oreste Egione, Anna Farese, Saverio Fatone, Alessio Filippi, Daniela Fiore, Paolo Fiorentini, Rossana Fiori, Maria Rosa Fittipaldi, Giuseppina Floriddia, Luca Franco, Alessandra Fusco, Sergio Galdieri, Alessandra Gallo, Maria Alessandra Gardini, Francesca Garino, Adriano Gatti, Valentina Gatto, Carlotta Gauna, Luigi Gesuè, Anna Giacchini, Raffeale Giannettino, Debora Giannini, Filomena Gioia, Domenica Giuffrida, Umberto Goglia, Francesco Golia, Lucia Gottardo, Elena Gramaglia, Marco Grasso, Massimo Graziuso, Roberto Gualdiero, Rita Graziella Guarnieri, Nicolangelo Iazzetta, Marco Infante, Francesca Innelli, Angelantonio Iovino, Giovanni Izzo, Antonio Lampitella, Antonio Lanzilli, Emanuela Lapice, Anna Pia Lassandro, Adele Latina, Mario Laudato, Angelo Lauria Pantano, Paola Leporati, Filomena Lo Conte, Barbara Giovanna Lucatello, Tiziano Lucianer, Barbara Macerola, Valeria Maggi, Chiara Maggioli, Emilia Maglione, Francesco Manetti, Mariangela Manicone, Andrea Marcocci, Valentina Mariano, Elisa Marinazzo, Anna Maria Mariniello, Giuseppe Marrazzo, Emilia Martedì, Paolo Martini, Michela Masin, Elisa Me, Marika Menduni, Chiara Alberta Mesturino, Sabato Mignano, Nicola Milano, Monica Modugno, Eleonora Monti, Mary Mori, Elena Nazzari, Giuseppe Pietro Nunziata, Domenica Oliva, Marcello Orio, Antonio Pio Palena, Pio Paraggio, Lisangela Pascale, Maria Divina Pascuzzo, Angela Peluso, Dorotea Peragine, Ettore Petraroli, Giuliana Petraroli, Giovanni Paolo Piccolo, Marco Piscopo, Roberta Poli, Stella Potenziani, Maria Chiara Quinto, Andrea Renzullo, Gaetano Emanuele Rizzo, Rossella Romano, Ernesto Rossi, Ilaria Rubbo, Gilda Ruga, Angela Sabbatini, Francesca Santilli, Giovanna Saraceno, Patrizia Savino, Francesco Scalabrì, Carla Scarano, Maria Pia Scioti, Rachele Scotton, Antonello Selleri, Antonella Senesi, Marilena Sidoti, Maria Rosaria Sorrentino, Marco Strazzabosco, Daniela Strippoli, Miryam Talco, Anna Tedeschi, Annamaria Terracciano, Gilda Tirelli, Domenico Tricò, Salvatore Turco, Anna Amelia Turco, Livio Valente, Valeria Vallone, Carmela Vinci, and Danuta Teresa Wolosinska
References
- 1.Buckley ST, Baekdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10:467. doi: 10.1126/scitranslmed.aar7047. [DOI] [PubMed] [Google Scholar]
- 2.Gallwitz B, Giorgino F. Clinical perspectives on the use of subcutaneous and oral formulations of semaglutide. Front Endocrinol (Lausanne) 2021;12:645507. doi: 10.3389/fendo.2021.645507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodbard HW, Dougherty T, Taddei-Allen P. Efficacy of oral semaglutide: overview of the PIONEER clinical trial program and implications for managed care. Am J Manag Care. 2020;26:S335–S343. doi: 10.37765/ajmc.2020.88554. [DOI] [PubMed] [Google Scholar]
- 4.Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42:2272–2281. doi: 10.2337/dc19-0883. [DOI] [PubMed] [Google Scholar]
- 5.Mosenzon O, Capehorn MS, De Remigis A, Rasmussen S, Weimers P, Rosenstock J. Impact of semaglutide on high-sensitivity C-reactive protein: exploratory patient-level analyses of SUSTAIN and PIONEER randomized clinical trials. Cardiovasc Diabetol. 2022;21:172. doi: 10.1186/s12933-022-01585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanai H, Hakoshima M, Adachi H, Katsuyama H. A significant effect of oral semaglutide on cardiovascular risk factors in patients with type 2 diabetes. Cardiol Res. 2022;13:303–308. doi: 10.14740/cr1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 8.Husain M, Birkenfeld AL, Donsmark M, et al. Oral Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 9.McGuire DK, Busui RP, Deanfield J, et al. Effects of oral semaglutide on cardiovascular outcomes in individuals with type 2 diabetes and established atherosclerotic cardiovascular disease and/or chronic kidney disease: Design and baseline characteristics of SOUL, a randomized trial. Diabetes Obes Metab. 2023;25:1932–1941. doi: 10.1111/dom.15058. [DOI] [PubMed] [Google Scholar]
- 10.Fadini GP, Frison V, Rigato M, et al. Trend 2010–2018 in the clinical use of GLP-1 receptor agonists for the treatment of type 2 diabetes in routine clinical practice: an observational study from Northeast Italy. Acta Diabetol. 2020;57:367–375. doi: 10.1007/s00592-019-01445-z. [DOI] [PubMed] [Google Scholar]
- 11.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 12.Cahn A, Raz I, Kleinman Y, et al. Clinical assessment of individualized glycemic goals in patients with type 2 diabetes: formulation of an algorithm based on a survey among leading worldwide diabetologists. Diabetes Care. 2015;38:2293–2300. doi: 10.2337/dc15-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao X, Starmer J, Martin ER. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol. 2008;32:361–369. doi: 10.1002/gepi.20310. [DOI] [PubMed] [Google Scholar]
- 14.Aroda VR, Faurby M, Lophaven S, Noone J, Wolden ML, Lingvay I. Insights into the early use of oral semaglutide in routine clinical practice: the IGNITE study. Diabetes Obes Metab. 2021;23:2177–2182. doi: 10.1111/dom.14453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321:1466–1480. doi: 10.1001/jama.2019.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:528–539. doi: 10.1016/S2213-8587(19)30194-9. [DOI] [PubMed] [Google Scholar]
- 17.Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2022;65:1925–1966. doi: 10.1007/s00125-022-05787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannucci E, Candido R, Monache LD, et al. 2023 update on Italian guidelines for the treatment of type 2 diabetes. Acta Diabetol. 2023;60:1119–1151. doi: 10.1007/s00592-023-02107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mannucci E, Candido R, Monache LD, et al. Italian guidelines for the treatment of type 2 diabetes. Acta Diabetol. 2022;59:579–622. doi: 10.1007/s00592-022-01857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheen AJ. GLP-1 receptor agonists and cardiovascular protection: a class effect or not? Diabetes Metab. 2018;44:193–196. doi: 10.1016/j.diabet.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Husain M, Bain SC, Jeppesen OK, et al. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes Metab. 2020;22:442–451. doi: 10.1111/dom.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teague M, Martinez A, Walker E, El-Rifai M, Carris NW. Use and interchange of incretin mimetics in the treatment of metabolic diseases: a narrative review. Clin Ther. 2023;45:248–261. doi: 10.1016/j.clinthera.2023.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Whitley HP, Trujillo JM, Neumiller JJ. Special report: potential strategies for addressing GLP-1 and dual GLP-1/GIP receptor agonist shortages. Clin Diabetes. 2023;41:467–473. doi: 10.2337/cd23-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada H, Yoshida M, Funazaki S, et al. Retrospective analysis of the effectiveness of oral semaglutide in type 2 diabetes mellitus and its effect on cardiometabolic parameters in Japanese Clinical Settings. J Cardiovasc Dev Dis. 2023;10:176. doi: 10.3390/jcdd10040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated during the current study is available from the corresponding author on reasonable request, but restrictions may apply.