Abstract
We report a case of blow-out-type left ventricular free wall rupture (LVFWR) after acute myocardial infarction, who presented with unstable hemodynamic condition in New York Heart Association (NYHA) functional class IV. Immediately, we performed a successful LVFWR repair with sutureless technique using a glue and expanded polytetrafluoroethylene patch on cardio-pulmonary bypass support. Postoperative period and recovery was uneventful. Over a period of 2-year follow-up, the patient is in NYHA class I and cardiac magnetic resonance imaging showed adequate left ventricular (LV) function and no evidence of LV aneurysm.
Keywords: Left ventricular free wall rupture, Acute myocardial infarction, Sutureless repair, Cardiopulmonary bypass
Introduction
Left ventricular free wall rupture (LVFWR) is a lethal mechanical complication following acute myocardial infarction (AMI). Although its incidence has decreased due to the introduction of cardiac reperfusion techniques, it is associated with high mortality [1–3]. Many surgical techniques are available for managing this problem and an early intervention can be lifesaving. Here we report a sutureless repair technique in the management of a LVFWR in AMI patient with a 2-year follow-up.
Case report
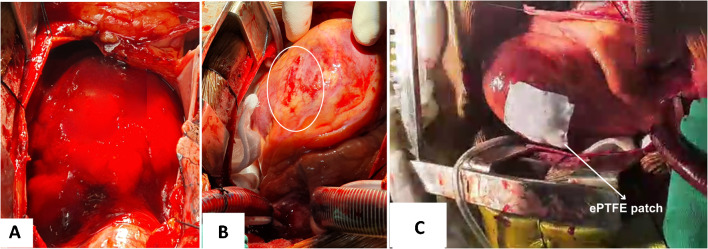
A 42-year-old male, known hypertensive, presented with breathlessness 24 h after the onset of acute chest pain, in New York Heart Association (NYHA) functional class IV. On examination, the patient was drowsy, peripheries were cold and only a feeble carotid pulse was palpable. He was orthopneic and hemodynamically unstable. Electrocardiography showed low-voltage QRS complexes and gross ST elevations in all the leads suggesting AMI. The bedside transthoracic echocardiography (TTE) revealed a large pericardial effusion and cardiac tamponade. A quick left femoral arterial access was obtained, showing a blood pressure of 60/28 mmHg and a heart rate of 140 bpm. The patient was intubated and put on mechanical ventilation in the emergency room. His hemodynamic condition marginally improved with an intra-aortic balloon pump (IABP) and inotropic supports. Repeat bedside TTE revealed a small defect in the distal left anterolateral ventricular wall (Fig. 1). The coronary catheterisation revealed near-total occlusion (99% stenosis) of first diagonal artery. He was immediately taken to operation room for emergency cardiac surgery. The heart–lung machine was assembled and priming was performed with blood with pre-bypass hemofiltration. A quick peripheral femoro-femoral cardiopulmonary bypass (CPB) was established after systemic heparinisation. Sternotomy was performed, pericardium was opened and a large hemopericardium was drained (Fig. 2A). A large infarct of around 5 cm × 4 cm in size was noted in the anterolateral region of the diagonal territory. A linear LVFWR, measuring 20 mm in length and 4 to 5 mm in width, was noted within the infarcted area (Fig. 2B). The active bleeding was stopped by compression. Right superior pulmonary vein (RSPV) vent insertion was done. The entire procedure was performed under normothermia. A central CPB was established for full support and the surgery was performed on an empty beating heart. The rupture site was stabilised by placing posterior pericardial stay suture and wet sponges around the heart. CPB support with RSPV vent and cessation of lung ventilation helped in achieving the blood less field at the infarct area. The infarct area was large and very friable; hence, a sutureless technique was planned. The area at the rupture site was dried up by the oxygen blower mister (without saline). A topical tissue adhesive n-butyl cyanoacrylate (Truseal Healthium MedTech Ltd., India) was applied to a rectangular expanded polytetrafluoroethylene (ePTFE) patch measuring 6 cm by 5 cm in size. After 5 to 10 s, the glue and ePTFE patch were placed on the dried infarcted myocardial surface (Fig. 2C). By using a dry sponge, the patch covering the entire LVFWR and the infarct area was compressed for a period of 2 min. This gentle compression facilitated the adherence of ePTFE patch to the myocardial surface. Adherence was confirmed by gentle traction of the patch. Supportive CPB was extended for brief time and patient was gradually weaned from heart lung machine with minimal inotropic supports and IABP support. The total CPB duration was 90 min. Central and peripheral CPB cannulae were removed. Heparin was reversed with protamine sulphate and adequate haemostasis was achieved. A right ventricle and a right atrial pacing wires were placed. Patient was observed for 45 min to look for any extravasation of blood from the repaired site. Since there was no extravasation, the sternum and chest wounds were closed with a pericardial and a retrosternal drain in situ. Patient was shifted to cardiothoracic surgical intensive care unit. Postoperative period was uneventful and patient was extubated on day one. Inotropic and IABP supports were gradually weaned. Serial echocardiography in postoperative period did not show any pericardial collection. Patient was discharged on postoperative day 10 and was followed up regularly for every 10 days in the first month. Subsequent follow-ups were scheduled at every 3 months for a period of 2 years. On every follow-up, clinical examination and screening echo cardiography was performed. Echocardiography showed adequate cardiac function with left ventricular ejection fraction (LVEF) 45% and no evidence of any left ventricular (LV) aneurysm. Cardiac magnetic resonance imaging was performed 1 year after the surgery and it showed good cardiac function (LVEF 45%) with no evidence of leak or LV aneurysm at the repair site. Presently, the patient is in NYHA class I and he is doing well on pharmacological management with beta-blockers, antiplatelets, angiotensin converting enzyme inhibitors and statins.
Fig. 1.
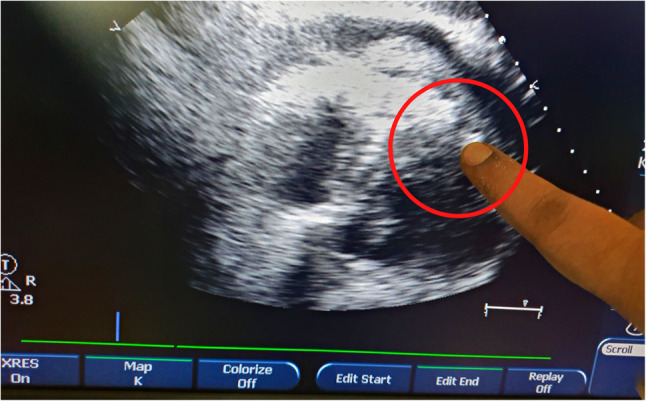
Echocardiographic image showing pericardial collection and finger-pointing the LVFWR defect
Fig. 2.
Intraoperative images showing hemopericardium (A), rupture site (B) and sutureless repair with ePTFE patch (C). (ePTFE—expanded polytetrafluoroethylene)
Discussion
Prior to coronary reperfusion era, the incidence of cardiac rupture was high (6%) [1, 2]. It gradually declined to 1.7% with the progressive use of reperfusion therapy and pharmacological therapy [1, 2]. The LVFWR occurs in up to 0.2 to 7.6% of all acute myocardial infarction cases, with a high mortality of up to 60% in hospitalised patients [3]. Despite the advances in cardiology, some patients present with dreadful complications. Early diagnosis and management of AMI are definitely useful in preventing the incidence of LVFWR.
Based on operative findings, the LVFWRs are categorised into two types, namely blow-out-type LVFWR and oozing-type LVFWR [4]. The blow-out-type LVFWR is a transmural tear causing free communication between the left ventricle (LV) and pericardium. Blow-out type of LVWFR often present with acute unstable conditions including cardiac arrest. The oozing type LVFWR is not associated with macroscopic tear but blood will be oozing from the site. Oozing type usually present as sub-acute conditions with hemopericardium, with or without cardiac tamponade [4].
High index of suspicion for LVWFR should be considered in patients of AMI presenting with unexplained continuous chest pain with or without hemopericardium and persisting ST segment elevations. The risk factors for LVFWR are old age, female sex, hypertension, and first episode of AMI [2, 3]. It is strongly associated with single-vessel disease [2, 3].
Echocardiography is useful in diagnosing LVFWR. Hemopericardium and cardiac tamponade points towards the presence of LVFWR. Sometimes it may show the actual site of the tear. If patient is hemodynamically stable, a coronary artery angiography should be performed, to know the status of coronary artery disease.
Surgical strategies in managing LVFWR include sutured techniques of repair (linear closure of the tear, infarctectomy and patch repair) and sutureless techniques of repair [4, 5]. The sutureless repair (glue and patch technique) has been used successful in managing both oozing-type LVFWR and blow-out-type LVFWR [4, 6, 7]. The sutured and sutureless repairs have shown comparable hospital mortality rates around 13.8% and 14% respectively [8]. Our patient had blow-out-type LVFWR with large and friable infarct area. In such situations, sutured technique of repair can be challenging due to cut through of sutures and also if the myocardial infarct area is advancing. Hence, we chose glue and ePTFE patch technique of sutureless repair in our patient with a patch larger than the infarct area. However, one should be highly alert for any possible chances of re-rupture in blow-out-type LVFWR and in advancing margin of myocardial infarct zone. In a systematic review by Matteucci et al., a 5% higher in-hospital re-rupture was documented in sutureless technique of repairs than the sutured technique of repairs [8]. Few case reports have also shown development of left ventricular pseudoaneurysm in suturless repair techniques [9, 10]. We believe the technique of LVFWR surgery should be individualised based on clinical stability, area of myocardial infarction, presence of myocardial hematoma and anatomical factors. The cardiopulmonary bypass helps in such unstable and emergency condition and also in achieving the complete bloodless field at the repair site. Suturless repair technique is easy and reproducible, but one needs to be cautious in post operative period. We recommend serial follow-up of such patient with echocardiography and cardiac magnetic resonance imaging.
Conclusion
Sutureless repair for LVFWR is a simple and quick technique which helps in reducing the operating time. It is a beneficial technique especially in friable tissue with unsuitable anatomical factors. Use of CPB provides bloodless surgical field for ease and effective application of this technique. We believe that the use of ePTFE patch larger than the infarct size will help in preventing the chances of re-rupture especially in advancing margin of myocardial infarct area.
Author contribution
• Conception: Shivaprasad Babu Mukkannavar.
• Clinical and Surgical management: Shivaprasad Babu Mukkannavar, Jayarama Pai, N. Raja Ramesh and T.K. Radhika.
• Writing the final draft: Shivaprasad Babu Mukkannavar; N. Raja Ramesh; Krishna Shriram Dhanasekaran.
• Literature search and evidence synthesis: Shivaprasad Babu Mukkannavar.
• Critical review and revision: Shivaprasad Babu Mukkannavar; T. Jayarama Pai, N. Raja Ramesh; Krishna Shriram Dhanasekaran.
• Supervision: Shivaprasad Babu Mukkannavar; T. Jayarama Pai.
• Final approval: All the authors read and approved the manuscript, the requirements for authorship contribution have been met and each author believes that the manuscript represents honest work.
Funding
None.
Declarations
Ethical approval
The approval of the institutional review board is not required for the case report.
Informed consent
Informed consent was obtained.
Statement of human and animal rights
This case was not an experiment but actual surgical management. The privacy and confidentiality of the patient’s identity are maintained as per the Declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shivaprasad Babu Mukkannavar, Email: shivaprasad_2000@yahoo.com.
Tonse Jayarama Pai, Email: drjayaramapai@yahoo.co.in.
Nukavarapu Raja Ramesh, Email: nukavarapuraja@gmail.com.
Tirumala Kanduri Radhika, Email: radhikatk73@gmail.com.
Krishna Shriram Dhanasekaran, Email: krishnadhanasekaran98@outlook.com.
References
- 1.Figueras J, Alcalde O, Barrabés JA, Serra V, Alguersuari J, Cortadellas J, et al. Changes in hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation. 2008;118:2783–2789. doi: 10.1161/CIRCULATIONAHA.108.776690. [DOI] [PubMed] [Google Scholar]
- 2.Honda S, Asaumi Y, Yamane T, Nagai T, Miyagi T, Noguchi T, et al. Trends in the clinical and pathological characteristics of cardiac rupture in patients with acute myocardial infarction over 35 years. J Am Heart Assoc. 2014;3:e000984. doi: 10.1161/JAHA.114.000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Formica F, Mariani S, Singh G, D'Alessandro S, Messina LA, Jones N, et al. Postinfarction left ventricular free wall rupture: a 17-year single-centre experience. Eur J Cardiothorac Surg. 2018;53:150–156. doi: 10.1093/ejcts/ezx271. [DOI] [PubMed] [Google Scholar]
- 4.Okamura H. Sutureless repair techniques for post-infarction left ventricular free wall rupture. Ann Cardiothorac Surg. 2022;11:268–272. doi: 10.21037/acs-2021-ami-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matteucci M, Fina D, Jiritano F, Meani P, Blankesteijn WM, Raffa GM, et al. Treatment strategies for post-infarction left ventricular free-wall rupture. Eur Heart J Acute Cardiovasc Care. 2019;8:379–387. doi: 10.1177/2048872619840876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makhoul M, Medalion B, Lorusso R, Bolotin G. Sutureless repair of subacute left ventricular free wall rupture. Ann Cardiothorac Surg. 2022;11:299–303. doi: 10.21037/acs-2022-ami-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimatsu T, Oosawa H, Takai F, Aruga M, Ogiwara F, Mawatari E, et al. Patch-and-glue sutureless repair for blowout rupture after myocardial infarction: report of two cases. Ann Thorac Cardiovasc Surg. 2008;14:48–51. [PubMed] [Google Scholar]
- 8.Matteucci M, Fina D, Jiritano F, Blankesteijn WM, Raffa GM, Kowalewski M, et al. Sutured and sutureless repair of postinfarction left ventricular free-wall rupture: a systematic review. Eur J Cardiothorac Surg. 2019;56:840–848. doi: 10.1093/ejcts/ezz101. [DOI] [PubMed] [Google Scholar]
- 9.Iha K, Ikemura R, Higa N, Akasaki M, Kuniyoshi Y, Koja K. Left ventricular pseudoaneurysm after sutureless repair of subacute left ventricular free wall rupture: a case report. Ann Thorac Cardiovasc Surg. 2001;7:311–314. [PubMed] [Google Scholar]
- 10.Hsieh YK, Lee CH, Chen YS, Wu IH. Pseudoaneurysm after sutureless repair of left ventricular free wall rupture: sequential magnetic resonance imaging demonstration. Asian J Surg. 2015;38:174–176. doi: 10.1016/j.asjsur.2013.07.010. [DOI] [PubMed] [Google Scholar]