Abstract
We investigated the postantibiotic effects (PAEs) of four agents against Mycobacterium avium in a human macrophage model under two different experimental conditions. For postantibiotic leukocyte enhancement (PALE), bacteria were exposed to antibiotics prior to their phagocytosis, whereas for pulsed exposure (PE), antibiotics were added after phagocytosis. In both cases, the drugs were used at their peak concentrations in serum (Cmax) for 2 h. The results showed two different patterns: one for the drug for which results under PE and PALE test conditions did not significantly differ (amikacin) and one for drugs for which PAE values were significantly higher under PE test conditions (clarithromycin, clofazimine, and rifampin). These data suggest that even a brief exposure of M. avium to peak concentrations of certain drugs in serum may result in prolonged and persistent suppression of bacterial growth inside human macrophages.
The Mycobacterium avium complex is a major source of opportunistic infection in AIDS patients and mostly results in disseminating infections (6) that remain difficult to treat because of the natural resistance of these organisms to most of the antituberculosis drugs (17). Although the M. avium treatment strategies employed currently are often favorable (2), the fact remains that a more rational basis for the determination of the dosing regimens and the dosing intervals would be particularly useful to guide the scheduling of drug administration for M. avium-infected AIDS patients. Alternative strategies have recently been investigated by using animal models for diseases other than those caused by mycobacteria (4). In this context, the evaluation of postantibiotic effects (PAEs) of drugs against M. avium is an interesting approach that was recently investigated in vitro for amikacin, clarithromycin, clofazimine, and rifampin, which have PAEs within a range of 2.6 ± 1 to 71 ± 3.2 h against M. avium (9). In the present investigation we have concentrated on the evaluation of the activities of these antibiotics in human macrophages under two different test conditions. In the first case, the bacteria were exposed to drugs for 2 h prior to phagocytosis (a test condition that has been termed postantibiotic leukocyte enhancement [PALE]) (13, 19), and in the second case, phagocytosis of the bacteria was followed by a 2-h pulsed exposure of infected macrophages to drugs (a test condition previously termed pulsed exposure [PE]) (1, 14–16). This study was meant to investigate if a prolonged and persistent suppression of bacterial growth inside human macrophages could be observed after a short exposure to clarithromycin, rifampin, amikacin, and clofazimine.
Two M. avium strains isolated from AIDS patients (MAC1 and MAC3) were used. Bacteria were scraped from Löwenstein-Jensen slants and recultured in complete 7H9 broth supplemented with the Middlebrook-ADC (albumin, dextrose, and catalase) enrichment medium (Difco Laboratories, Detroit, Mich.) and Tween 80 (0.05% [vol/vol] to avoid clumping) to their mid-logarithmic phase to an optical density at 650 nm of 0.1. Stock solutions of amikacin (Bristol, Paris, France), rifampin (Sigma Chemical Co, St. Louis, Mo.), clofazimine (Ciba, Basel, Switzerland), and clarithromycin (Laboratories Abbott, Rungis, France) were prepared, sterilized, and stored as described previously (9). Antibiotics were used at their reported peak concentrations in serum (Cmax) in humans, i.e., 20 μg/ml for amikacin, 4.0 μg/ml for clarithromycin, 1.25 μg/ml for clofazimine, and 15 μg/ml for rifampin (20). Human blood-derived macrophages were prepared from heparinized peripheral blood from healthy donors as reported previously (21). Under our experimental conditions, the viability of an isolated cell was greater than 97% as judged by the trypan blue dye exclusion test. The cells were seeded to a concentration of 2 × 106 cells/well in 12-well tissue culture clusters and incubated for 4 h at 37°C in the presence of 5% CO2 to permit the adherence of monocytes, followed by the removal of nonadherent cells. Macrophage monolayers were infected with exponentially growing bacteria as reported previously for 4 h at 37°C in the presence of 5% CO2 (21). After phagocytosis, all the extracellular bacteria were thoroughly washed away with Hanks’ balanced solution. Two successive washings followed by replacement with fresh medium removed about 99.9% of the extracellular bacilli as evidenced by the plating of the final wash for the assessment of CFU, and also by the Ziehl-Neelsen staining of similarly treated parallel controls on coverslips. Furthermore, there was no bacterial multiplication in the extracellular medium used (unpublished data; 14). The number of bacteria effectively phagocytized (around 5 × 104 to 105) was determined by lysing the macrophages by using 0.25% (wt/vol) sodium dodecyl sulfate (SDS), doing immediate serial dilutions, and plating the lysate on 7H11 agar medium for viable-count determinations. The addition of 0.25% SDS to parallel cultures of M. avium, which were immediately serially diluted for viability assessment in parallel control experiments, showed that SDS addition did not lower the bacterial viability counts.
For the drug experiments, the bacteria were exposed to antibiotics prior to phagocytosis (PALE experiments) or the drugs were added to infected macrophages by supplementing the macrophage-containing wells with the desired antibiotics (PE experiments). In agreement with previous PAE determinations with M. avium, the selected contact time was 2 h (9, 14–16). For PALE experiments, the drug-treated bacteria (37°C, 2 h) were washed free of antibiotics as described previously (9), resuspended in complete RPMI 1640 medium, and used for macrophage infection experiments. For PE experiments, the drug-containing medium was removed from macrophage-containing wells and the cells were thoroughly washed to remove all extracellular antibiotics, as described previously, to avoid any drug carryover (21). In both cases, the intracellular growth of the bacteria was monitored for 5 days by lysing the macrophages at various times (at 0, 72, and 120 h) and plating for the determination of CFU per milliliter. The results were expressed as mean values of CFU per milliliter ± standard errors. The persistent suppression of bacterial growth despite the removal of drugs after a 2-h contact period was calculated from the equation PAE = T − C where T is the time required for the drug-exposed organisms to grow by 1 log unit and C is the time required for the untreated bacterial control to grow by 1 log unit (9). PAEs obtained under PE or PALE test conditions were expressed in hours as means ± standard errors. The Student t test was used to compare the experimental results to underline the significance of delayed bacterial growth under PE versus PALE test conditions.
The results obtained are summarized in Table 1 and Fig. 1. Under our experimental conditions, the control untreated M. avium grew by about 2 log units inside human macrophages within the 5 days of the incubation (the division time varied from 18 to 24 h in various experiments). This intracellular bacterial multiplication did not significantly affect cell viability, i.e., that of infected versus noninfected macrophages and that of control versus drug-treated cells. When various drugs were added to infected macrophages at Cmax levels, a significant suppression of bacterial growth was observed in many cases. For example, the suppression of M. avium growth after a single pulsed exposure to 15 μg of rifampin per ml resulted in complete inhibition of bacterial growth for nearly 72 h, followed by a slight increase in bacterial viable counts between 72 and 120 h. In these experiments, untreated control bacteria grew by 1 log unit between 80 and 90 h compared to bacteria in the rifampin-treated sample, which grew by 1 log unit between 118 and 126 h. Thus, the bacterial growth was delayed by an additional 34 to 46 h by a 2-h pulsed exposure to rifampin. However, variability of results between the two isolates was observed; this was probably linked to the differences among the respective MICs of the drugs for the strains studied (Table 1).
TABLE 1.
PAEs of the study drugs against M. avium under PE and PALE experimental conditions
| Druga | Strain MAC1
|
Strain MAC3
|
||||
|---|---|---|---|---|---|---|
| MICb (μg/ml) | Mean PAE (h) ± SEc for:
|
MIC (μg/ml) | Mean PAE (h) ± SEc for:
|
|||
| PE | PALE | PE | PALE | |||
| Amikacin | 1.00 | 15.2 ± 2.8 | 7.6 ± 2.3 | 2.00 | 14.4 ± 2.4 | 10.5 ± 3.1 |
| Clarithromycin | 0.50 | 77.4 ± 2.9d | 58.6 ± 2.3*e | 1.00 | 57.4 ± 2.0 | 10.1 ± 3.3* |
| Clofazimine | 0.25 | 72.9 ± 5.4d | 5.2 ± 1.4* | 0.12 | 112.4 ± 4.9d | 34.3 ± 5.2*d |
| Rifampin | 0.50 | 39.1 ± 1.5 | 9.9 ± 1.5* | 1.00 | 44.6 ± 1.8 | 6.4 ± 2.6* |
All the drugs were used at their reported Cmax values in humans, i.e., 20 μg/ml for amikacin, 4.0 μg/ml for clarithromycin, 1.25 μg/ml for clofazimine, and 15 μg/ml for rifampin.
MICs were determined by the radiometric methodology as reported earlier for M. avium (16).
Drugs were added to already-pathocytized bacteria by supplementing the macrophage-containing wells with the desired antibiotics in PE experiments, whereas bacteria were treated with antibiotics prior to phagocytosis in PALE experiments. In both cases, the contact time with drugs was 2 h at 37°C. Results are based on five to nine observations per condition.
The drug-treated bacteria did not grow by 1 log unit in this case. Therefore, the equation PAE = T − C was applifed by extrapolating the bacterial growth after day 5. For this purpose, the bacterial growth curve between days 3 and 5 was extended to attain the equivalent of 1 log unit of growth compared to the viability counts at time zero.
*, P < 0.001 by Student’s t test. PAEs for these drugs were significantly different between PE and PALE experimental conditions.
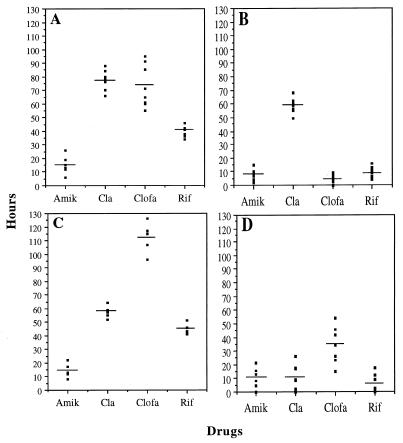
FIG. 1.
Summary of PAE values obtained under PE (A and C) and PALE (B and D) experimental conditions for M. avium isolates MAC1 (A and B) and MAC3 (C and D). Each dot represents an individual PAE result, whereas each bar represents the mean value for five to nine observations. Amik, amikacin; Cla, clarithromycin; Clofa, clofazimine; Rif, rifampin.
The delay in bacterial growth under the PALE experimental condition for various drugs (Table 1 and Fig. 1) showed that organisms in the PALE phase may be more susceptible to the antimycobacterial activity of human macrophages than the untreated controls (3). In this respect, PE experiments were similar to PALE experiments except for the fact that the availability of drugs to intracellular bacteria in PE test conditions also depended on the drugs’ pharmacokinetic properties, such as intracellular penetration and accumulation, as well as on interactions with the macrophage environment that may alter the effect of drugs on intraphagosomal bacteria (20). In conclusion, the results obtained (Table 1 and Fig. 1) underline two different patterns; one for the drug for which results under PE and PALE test conditions did not significantly differ (amikacin) and one for drugs for which significantly higher values were observed under the PE test conditions (clarithromycin, clofazimine, and rifampin; P < 0.001).
In both PE and PALE experiments, a significant part of the antimicrobial activity was related to the PAE and another part was related to the microbicidal action of macrophages (13, 19). Concerning the PAE, factors contributing to the suppression of bacterial growth included the concentration of the drug, the time of contact, and the mode of action of the drug (8). A possible mechanism of PAE includes nonlethal damage induced by an antimicrobial agent followed by drug persistence at the binding site, which interferes with the metabolism of the bacteria (3). Suppression of bacterial growth under nonlethal conditions inside macrophages may further result from delayed recovery of proteins with or without enzyme activities, prolonged but reversible changes in bacterial morphology and metabolism (11), modifications of generation times, altered susceptibility to phagocytosis (13, 19), and finally the persistence of an intracellular drug beyond the 2-h exposure time, which occurs particularly under PE test conditions (14, 16). It is important to underline that, with the exception of amikacin (18), all drugs used in this investigation have been reported to actively concentrate inside macrophages (14–16, 20, 22, 23). Rifampin, which is the “gold standard” in antibiotic activity against intracellular bacteria (20), produced PAEs of 39.1 ± 1.5 and 44.6 ± 1.8 h for the two isolates under PE test conditions, which were four- to sevenfold higher than the PAEs observed in the PALE experiments. Similarly, values for clofazimine under the PE test conditions were 4- to 14-fold higher, whereas those for clarithromycin were about 1.5- to 5-fold higher under the PE conditions (Table 1, Fig. 1). In contrast, the PAEs of 15.2 ± 2.8 and 14.4 ± 2.4 h found for amikacin under the PE test conditions were not significantly different from those observed under PALE test conditions (7.6 ± 2.3 and 10.5 ± 3.1 h; Table 1, Fig. 1). Indeed, unlike rifampin, clofazimine, and clarithromycin, which concentrate manyfold inside macrophages after even a short 2-h exposure, aminoglycosides require more than 24 h of drug contact to produce a moderate accumulation inside macrophages (18, 22). In fact, the intracellular-to-extracellular-concentration ratio for amikacin does not exceed 1.0 in less than a 24-h incubation period (23). Furthermore, the weaker activity of amikacin under PE conditions may also be linked to its low level of intracellular activity inside macrophages due to the acidification of phagolysosomes (5). Although both macrolide and aminoglycoside drugs bind to ribosomal units and interfere with bacterial protein synthesis, the mechanisms by which they produce PAEs are significantly different (12); e.g., unlike the short PAEs for aminoglycosides, which easily dissociate from ribosomes and rediffuse out of the cell (10), the longer PAEs for macrolides result from a resynthesis of relevant proteins rather than the dissociation of drug-protein complexes (7).
Thus, it can be concluded that the prolonged growth-suppressive effect of a single pulsed exposure to clarithromycin, clofazimine, and rifampin in this investigation argues in favor of intermittent administration of selected drugs to M. avium-infected AIDS patients who are overburdened with too many drugs given for various opportunistic infections. However, our findings are best explained by, and are consistent with, the ability of the tested drugs to penetrate intracellularly into macrophages. Whether this observation can be “exported” to the clinical infection setting will depend on numerous factors, including the predominant location of M. avium complex organisms in the patient and the ability of the drugs to reach the organisms. Human clinical trials will, therefore, be needed to confirm our ex vivo observations, since other variables such as the pharmacokinetics of drugs may significantly change the clinical outcome of PAE.
Acknowledgments
We are grateful to the Délégation Générale au Réseau International des Instituts Pasteur et Instituts Associés, Institut Pasteur, Paris, France, and Fondation Française Raoul Follereau, Paris, France, for financial support.
REFERENCES
- 1.Beggs W H, Jenne J W. Isoniazid uptake and growth inhibition of Mycobacterium tuberculosis in relation to time and concentration of pulsed drug exposure. Tubercle. 1969;50:377–385. doi: 10.1016/0041-3879(69)90038-5. [DOI] [PubMed] [Google Scholar]
- 2.Benson C A. Treatment of disseminated Mycobacterium avium complex disease: a clinician’s perspective. Res Microbiol. 1996;147:16–24. doi: 10.1016/0923-2508(96)80198-7. [DOI] [PubMed] [Google Scholar]
- 3.Craig W A. The postantibiotic effect. Clin Microbiol Newsl. 1991;13:121–124. [Google Scholar]
- 4.Craig, W. A. 1993. Relevance of animal models for clinical treatment. Eur. J. Clin. Microbiol. Infect. Dis. 12(Suppl. A):S55–S57. [DOI] [PubMed]
- 5.Düzgünes N, Perumal V K, Kesavalu L, Goldstein J A, Debs R J, Gangadharam P R J. Enhanced effect of liposome-encapsulated amikacin on Mycobacterium avium-M. intracellulare complex infection in beige mice. Antimicrob Agents Chemother. 1988;32:1404–1411. doi: 10.1128/aac.32.9.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falkinham J O., III Epidemiology of Mycobacterium avium infections in the pre- and post-HIV era. Res Microbiol. 1994;145:169–172. doi: 10.1016/0923-2508(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 7.Gerber, A. U., and W. A. Craig. 1981. Growth kinetic of respiratory pathogens after short exposures to ampicillin and erythromycin. J. Antimicrob. Chemother. 8(Suppl.):S81–S91. [DOI] [PubMed]
- 8.Gudmundsson A, Gottfredsson M, Erlendstottir H, Gudmundsson S. Impact of pH and cationic supplementation on in vitro postantibiotic effect. Antimicrob Agents Chemother. 1991;35:2617–2624. doi: 10.1128/aac.35.12.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horgen L, Legrand E, Rastogi N. Postantibiotic effect of amikacin, rifampin, sparfloxacin, clofazimine and clarithromycin against Mycobacterium avium. Res Microbiol. 1997;148:673–681. doi: 10.1016/S0923-2508(99)80066-7. [DOI] [PubMed] [Google Scholar]
- 10.Isaaksson B, Nilsson L, Maller R, Sören L. Postantibiotic effect of aminoglycosides on Gram-negative bacteria evaluated by a new method. J Antimicrob Chemother. 1988;22:23–33. doi: 10.1093/jac/22.1.23. [DOI] [PubMed] [Google Scholar]
- 11.Lorian V, Amaral L, Ernst J. The postantibiotic effect defined by bacterial morphology. J Antimicrob Chemother. 1989;23:485–491. doi: 10.1093/jac/23.4.485. [DOI] [PubMed] [Google Scholar]
- 12.Mackenzie F M, Gould I M. The postantibiotic effect. J Antimicrob Chemother. 1993;32:519–537. doi: 10.1093/jac/32.4.519. [DOI] [PubMed] [Google Scholar]
- 13.McDonald P J, Pruul H, Weterhall B L. Postantibiotic leukocyte enhancement: increased susceptibility of bacteria pretreated with antibiotics to activity of leukocytes. Rev Infect Dis. 1981;3:38–44. doi: 10.1093/clinids/3.1.38. [DOI] [PubMed] [Google Scholar]
- 14.Mor N, Heifets L. Inhibition of intracellular growth of Mycobacterium avium by one pulsed exposure of infected macrophages to clarithromycin. Antimicrob Agents Chemother. 1993;37:1380–1382. doi: 10.1128/aac.37.6.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mor N, Simon B, Mezo N, Heifets L. Comparison of activities of rifapentine and rifampin against Mycobacterium tuberculosis residing in human macrophages. Antimicrob Agents Chemother. 1995;39:2073–2077. doi: 10.1128/aac.39.9.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mor N, Vanderkol J, Mezo N, Heifets L. Effects of clarithromycin and rifabutin alone and in combination on intracellular and extracellular replication of Mycobacterium avium. Antimicrob Agents Chemother. 1994;38:2738–2742. doi: 10.1128/aac.38.12.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris S L, Rouse D A. The genetics of multiple drug resistance in Mycobacterium tuberculosis and the Mycobacterium avium complex. Res Microbiol. 1996;147:68–73. doi: 10.1016/0923-2508(96)80206-3. [DOI] [PubMed] [Google Scholar]
- 18.Murdoch M B, Peterson L R. Antimicrobial penetration into polymophonuclear leukocytes and alveolar macrophages. Semin Respir Infect. 1991;6:112–121. [PubMed] [Google Scholar]
- 19.Pruul H, Lewis G, McDonald P J. Enhanced susceptibility of gram-negative bacteria to phagocytic killing by human polymorphonuclear leukocytes after brief exposure to aztreonam. J Antimicrob Chemother. 1988;22:675–686. doi: 10.1093/jac/22.5.675. [DOI] [PubMed] [Google Scholar]
- 20.Rastogi N. Mycobacteria as intracellular pathogens: current notions of pathogenicity, virulence, and drug resistance and their relation to effective therapy. In: Raoult D, editor. Antimicrobial agents and intracellular pathogens. Boca Raton, Fla: CRC Press; 1993. pp. 245–300. [Google Scholar]
- 21.Rastogi N, Labrousse V, Goh K S, Carvalho de Sousa J P. Antimycobacterial spectrum of sparfloxacin and its activities alone and in association with other drugs against Mycobacterium avium complex growing extracellulary and intracellularly in murine and human macrophages. Antimicrob Agents Chemother. 1991;35:2473–2480. doi: 10.1128/aac.35.12.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tulkens P M. Intracellular distribution and activity of antibiotics. Eur J Clin Microbiol Infect Dis. 1991;10:100–106. doi: 10.1007/BF01964420. [DOI] [PubMed] [Google Scholar]
- 23.VandenBroek P J. Antimicrobial drugs, microorganisms, and phagocytes. Rev Infect Dis. 1989;11:213–245. doi: 10.1093/clinids/11.2.213. [DOI] [PubMed] [Google Scholar]