Abstract
Ceftriaxone and ciprofloxacin were effective in the treatment of Yersinia enterocolitica O9 intestinal infection in mice. Amikacin was less effective. The impact of these drugs on indigenous bacteria from the intestinal microbiota was studied.
Pathogenic serotypes of Yersinia enterocolitica are the cause of food-borne enteritis in humans (4). Usually, human infections with low-virulence serotypes, such as O3 and O9, are self-limiting and do not require antibiotic therapy (15). However, enteric infection with low-virulence yersiniae may last for periods of up to 14 weeks without symptoms (12). Since Y. enterocolitica possesses immunomodulatory activity (13, 14), the prolonged intestinal carriage of yersiniae may exert nonspecific effects on the immune system of the host and may be involved in the induction of secondary immunologically mediated disorders that have been described as nonseptic sequelae of human yersiniosis (reviewed in reference 4). Thus, the use of antibiotics to shorten the carriage of yersiniae should be considered. Moreover, antibiotic therapy is necessary for treating systemic infections caused by Y. enterocolitica in immunocompromised hosts and patients with iron overload (4, 6, 15). Ceftriaxone, fluoroquinolones, and antipseudomonal aminoglycosides have been recommended for treatment against Y. enterocolitica infection (16). The purpose of this study was to test the efficacy of ceftriaxone, ciprofloxacin, and amikacin in enteric yersiniosis and to compare the impacts of these agents on the intestinal flora of mice.
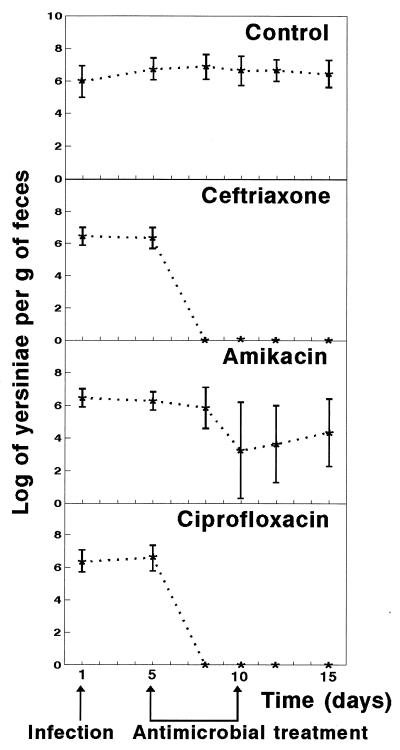
The experiments were performed with specific-pathogen-free BALB/c female mice weighing 20 to 24 g (Animal Research Unit, University of Granada, Granada, Spain). Strain IP383 of Y. enterocolitica belongs to serotype O9, biotype 2, and carries the virulence plasmid that is required for pathogenicity in this species (11). For experimental infection of mice by the oral route, yersiniae were grown on tryptic soy agar (Difco Laboratories, Detroit, Mich.) at 25°C for 24 h. Bacterial cells were harvested in sterile phosphate-buffered saline, washed twice, and resuspended in sterile water to obtain about 109 bacteria per ml. After an 18-h period of water deprivation, the mice were allowed to drink ad libitum for 24 h from the bacterial suspension (7). The viability of yersiniae in water suspensions did not decrease for a 24-h period at room temperature. Since the water intake was about 4 ml per mouse per day, the average challenge dose was about 4 × 109 yersiniae per mouse. To quantify fecal excretion of yersiniae, samples of feces from infected mice were obtained directly on sterile plastic petri dishes and processed in less than 15 min after collection. The feces were weighed and homogenized in 10 ml of sterile saline solution (0.9% [wt/vol] NaCl), and suitable dilutions of the homogenates were plated onto Yersinia CIN (cefsulodin, irgasan, and novobiocin) agar plates for viable bacterial counts. Results were expressed as the number of CFU per gram of feces. As shown in Fig. 1, fecal excretion of yersiniae was detected on day 1 postchallenge in all mice challenged with strain IP383. All animals were apparently asymptomatic, except for 30% of mice that had intermittent diarrhea. To verify the ileum mucosal colonization by IP383, pieces of the terminal ileum (1 cm) that did not contain Peyer’s patches were excised, rinsed, and homogenized in sterile phosphate-buffered saline, and appropriate dilutions were plated onto Yersinia CIN agar plates. Viable yersiniae were present in all samples from infected mice (data not shown). When mice were sacrificed between days 3 and 15 following infection, significant splenomegaly was observed. Bakour et al. (2) reported the recovery of viable yersiniae from spleens of mice after infection with the serotype O9 strain by the oral route. Thus, the mouse model reproduces a self-limiting infection that resembles human infection with low-virulence serotypes of Y. enterocolitica.
FIG. 1.
Effects of antimicrobial agents on Y. enterocolitica intestinal infection in mice. Infected mice were treated for 5 days with ceftriaxone, amikacin, or ciprofloxacin. Data represent the mean log10 ± standard deviation (indicated by error bars) of viable yersiniae per gram of feces for 10 mice. Similar results were obtained in two experiments.
Ceftriaxone, amikacin, and ciprofloxacin were obtained from Sigma Chemical Co. (St. Louis, Mo.). MICs for IP383 were determined by the macrobroth dilution method as described by Woods and Washington (18). MICs of ceftriaxone, amikacin, and ciprofloxacin were, respectively, 0.287, 8, and 0.0718 μg/ml (data are geometric means of five replicates). These results are similar to MICs previously reported for Y. enterocolitica strains (10). To assess the in vivo efficacy of the antimicrobial agents against Y. enterocolitica, therapy was begun 5 days after infection. Mice (10 animals per group) were injected twice a day at 12-h intervals for 5 consecutive days. The dosages were similar to those used in human therapy by the parenteral route: ceftriaxone, 28 mg/kg/day; amikacin, 15 mg/kg/day; and ciprofloxacin, 5 mg/kg/day. The results of antimicrobial therapy are presented in Fig. 1. Fecal excretion of yersiniae persisted for more than 15 days in animals in the untreated groups. Ceftriaxone and ciprofloxacin suppressed yersinia excretion 3 days after the antimicrobial treatment was started, and yersiniae did not reappear when the antibiotics were discontinued. Amikacin was unable to eliminate Y. enterocolitica from the intestinal tract of all animals, but MICs for recovered Y. enterocolitica clones did not significantly differ from that for the original strain.
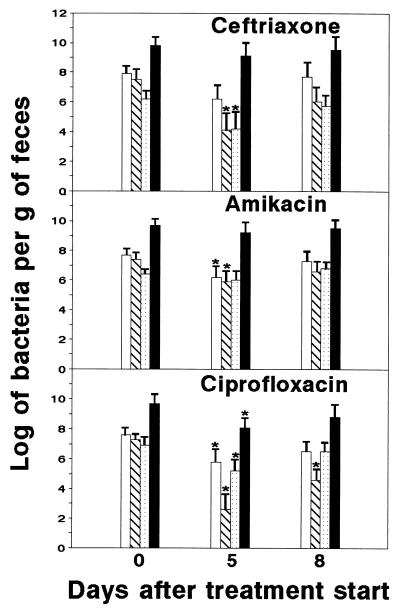
The ecological balance of intestinal microbiota may be disturbed by the administration of antimicrobial agents. To evaluate the impact of ceftriaxone, amikacin, and ciprofloxacin treatments on intestinal microbiota of mice, we quantified in feces the total aerobic bacteria, anaerobic bacteria, enterobacteria, and enterococci as representative indicators of the condition of the intestinal microbiota. Total aerobic bacteria were quantified by growing in tryptic soy agar or in CLED (cystine-lactose-electrolyte-deficient) agar. Enterobacteria were counted in Tergitol 7 agar supplemented with triphenyl-tetrazolium chloride. Enterococci were quantified by growing in KF Streptococcus agar supplemented with triphenyl-tetrazolium chloride. Clostridia and other anaerobic and microaerophilic bacteria were grown on Brewer Anaerobic agar plates under an anoxic atmosphere (GasPak, BBL Microbiology Systems, Cockeysville, Md.). All culture media were purchased from Difco Laboratories. Bacterial quantifications were performed at the start of treatment (5 days after infection with IP383), at the end of treatment (10 days after infection), and 2 days after the treatment was completed (12 days after infection). The results illustrated in Fig. 2 show that ciprofloxacin caused the largest alterations in the examined populations of indigenous bacteria. The number of aerobic bacteria was significantly reduced by amikacin and ciprofloxacin. The number of enterobacteria was significantly decreased by ceftriaxone, amikacin, and ciprofloxacin. The number of enterococci was significantly reduced by ceftriaxone and ciprofloxacin. Finally, anaerobic bacteria were significantly reduced in feces from ciprofloxacin-treated mice; the affected populations were composed mainly of spore-forming bacilli and Fusobacterium strains. Antibiotic-associated diarrhea was not observed.
FIG. 2.
Changes of bacterial populations in feces following treatment with antimicrobial agents. Total aerobic bacteria (open bars), enterobacteria (hatched bars), enterococci (stippled bars), and anaerobic bacteria (solid bars) were quantified. Data represent the mean log10 ± standard deviation (indicated by error bars) of viable bacteria per gram of feces for 10 mice. ∗, significantly different (P < 0.05 by the Student t test) from value for untreated controls. Similar results were obtained in two experiments.
Our results demonstrated that ceftriaxone and ciprofloxacin were efficacious in the treatment of experimental infection with Y. enterocolitica O9. Both drugs exerted a significant impact on the intestinal microbiota. In the case of ceftriaxone, a comparable picture has been observed in humans: the number of enterobacteria decreased after ceftriaxone administration, whereas anaerobes were not affected (1). Ciprofloxacin caused marked reductions in aerobic and anaerobic indigenous bacteria. In humans, the oral administration of ciprofloxacin also resulted in decreases in the aerobic portion of fecal microbiota, whereas the changes in the anaerobic part were not so pronounced (3, 5). In agreement with our data, Gismondo et al. reported that clostridia were significantly reduced by ciprofloxacin in a mouse model (8). In our experimental model, antibiotics were given by the parenteral route but ciprofloxacin can reach the intestinal lumen by biliary excretion (5). It is interesting to note that ciprofloxacin-mediated killing of indigenous bacteria has been proposed as a cause of release of bacterial fractions with immunomodulatory properties (9).
An important criticism of many animal models is the use of unnatural routes of inoculation (17). On the basis of the data cited above, it appears that experimental infection of mice with moderately virulent serotypes of Y. enterocolitica, such as serotype O9, by the oral route, may be a suitable model for experimental therapeutic studies.
Acknowledgments
This work was supported by the Junta de Andalucía (Research Group CVI 201).
REFERENCES
- 1.Ambrose N S, Johnson M, Burdon B W, Keighley M R. The influence of single dose intravenous antibiotics on faecal flora and emergence of Clostridium difficile. J Antimicrob Chemother. 1985;15:319–326. doi: 10.1093/jac/15.3.319. [DOI] [PubMed] [Google Scholar]
- 2.Bakour R, Balligand G, Laroche Y, Cornelis G, Wauters G. A simple adult-mouse test for tissue invasiveness in Yersinia enterocolitica strains of low experimental virulence. J Med Microbiol. 1985;19:237–246. doi: 10.1099/00222615-19-2-237. [DOI] [PubMed] [Google Scholar]
- 3.Bergan T, Delin C, Johansen S, Kolstad I M, Nord C E, Thorsteinsson S B. Pharmacokinetics of ciprofloxacin and effect of repeated dosage on salivary and fecal microflora. Antimicrob Agents Chemother. 1986;29:298–302. doi: 10.1128/aac.29.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bottone E J. Yersinia enterocolitica: the charisma continues. Clin Microbiol Rev. 1997;10:257–276. doi: 10.1128/cmr.10.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brumfitt W, Franklin I, Grady D, Hamilton-Miller J M T, Iliffe A. Changes in the pharmacokinetics of ciprofloxacin and fecal flora during administration of a 7-day course to human volunteers. Antimicrob Agents Chemother. 1984;26:757–761. doi: 10.1128/aac.26.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cover T L, Aber R C. Yersinia enterocolitica. N Engl J Med. 1989;321:16–24.9. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- 7.Gemski P, Lazere J R, Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980;27:682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gismondo M R, Drago L, Lombardi A, Fassina C, Cesana M. Impact of rufloxacin and ciprofloxacin on the intestinal microflora in a germ-free mice model. Chemotherapy. 1995;41:281–288. doi: 10.1159/000239357. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez-Valera M, Sampedro A, Moreno E, Ruiz-Bravo A. Modification of immune response in mice by ciprofloxacin. Antimicrob Agents Chemother. 1995;39:150–154. doi: 10.1128/aac.39.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwaga J, Iversen J O. In vitro antimicrobial susceptibilities of Yersinia enterocolitica and related species isolated from slaughtered pigs and pork products. Antimicrob Agents Chemother. 1990;34:2423–2425. doi: 10.1128/aac.34.12.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazigh D, Alonso J M, Mollaret H H. Simple method for demonstration of differential colony morphology of plasmid-associated virulent clones of Yersinia enterocolitica. J Clin Microbiol. 1983;17:555–557. doi: 10.1128/jcm.17.3.555-557.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris J G, Prado V, Ferreccio C, Robins-Browne R M, Bordun A M, Cayazzo M, Kay B A, Levine M M. Yersinia enterocolitica isolated from two cohorts of young children in Santiago, Chile: incidence of and lack of correlation between illness and proposed virulence factors. J Clin Microbiol. 1991;29:2784–2788. doi: 10.1128/jcm.29.12.2784-2788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz-Bravo A, Jimenez-Valera M, Alvarez de Cienfuegos G, Ruiz C, Kouwatli K, Ramos-Cormenzana A. Immunomodulation in mice by experimental infection with Yersinia enterocolitica. Microbiol Immunol. 1985;29:1089–1097. doi: 10.1111/j.1348-0421.1985.tb00899.x. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Bravo A, Jimenez-Valera M, Roman S M. Nonspecific modification of cellular immunity by Yersinia enterocolitica. Immunol Lett. 1996;49:57–61. doi: 10.1016/0165-2478(95)02481-6. [DOI] [PubMed] [Google Scholar]
- 15.Salyers A A, Whitt D D. Bacterial pathogenesis: a molecular approach. Washington, D.C: American Society for Microbiology; 1994. [Google Scholar]
- 16.Sanford J P. Guide to antimicrobial therapy. Dallas, Tex: Antimicrobial Therapy, Inc.; 1992. [Google Scholar]
- 17.Smith H. The state and future of studies on bacterial pathogenicity. In: Roth J A, editor. Virulence mechanisms of bacterial pathogens. Washington, D.C: American Society for Microbiology; 1988. pp. 365–382. [Google Scholar]
- 18.Woods G L, Washington J A. Antibacterial susceptibility tests: dilution and disk diffusion methods. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 1327–1341. [Google Scholar]