Drug-induced liver injury (DILI) is a term used to describe an unexpected liver reaction to drugs, but also to herbal products and dietary supplements (HDS). In the current therapeutic armamentarium, only a small number of available drugs are still capable of causing liver damage in overdose. These include, in addition to paracetamol—the prototypical example of this group—niacin (now rarely used to treat hypercholesterolaemia), intravenous methotrexate and probably ketamine (an intravenous hypnotic used in critically ill patients for rapid sequence induction) (1). This type of liver injury contrasts with the majority of toxic liver injury events that occur in clinical practice, which are unpredictable, not related to the dose or pharmacological action of the drug, and therefore mostly dependent on the “idiosyncrasy” of the host. The variety of drugs that can, occasionally, provoke DILI is enormous, with more than 600 different compounds identified as potentially hepatotoxic. As a result, not only general practitioners, or specialists, but almost any physician who prescribes drugs (surgeons, gynaecologists, urologists, ophthalmologists, etc...) is likely to encounter DILI cases at least once in their practice. In fact, DILI is one of the most challenging situations that clinicians must deal with in their practice. This is because there is no validated diagnostic test that can confidently distinguish DILI from other causes of acute liver injury, so the diagnosis is based on the acumen of clinical information, particularly regarding the time of exposure to the drug or HDS in relation to the clinical or biochemical presentation, and the careful exclusion of competing liver diseases (viral hepatitis, alcoholic liver disease, autoimmune liver diseases, etc.) (Figure 1). The relative rarity of idiosyncratic DILI and its uncertain diagnosis in many cases has led to a lack of well-powered studies to identify risk factors, test biomarkers, or investigate efficacy and safety. Therefore, unlike other liver diseases, the level of evidence in idiosyncratic DILI is generally low for most aspects of its characterisation and management. Nevertheless, European, Asian Pacific and American clinical practice guidelines (CPG) endorsed by the European Association for the Study of the liver (EASL), the Asian-Pacific Society for Liver Diseases (APSL) and American College Gastroenterology (ACG) respectively, have been published in recent years (1-3) using the levels of evidence recommended by the Oxford Centre for Evidence-based Medicine (1) or the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system (2,3). Taking a more conservative approach, the Practice Guidelines Committee of the American Association for the Study of Liver Diseases appointed a group of experts to produce a ‘guidance’ rather than a guideline, recognising the paucity of randomised controlled trials in this area. Therefore, the authors provide recommendations and expert advice on the management of DILI based on observational data (4). In line with this DILI guidance, in this article we review and discuss the key issues to be consider when approaching a potential DILI case to assist clinicians and surgeons in their decision making.
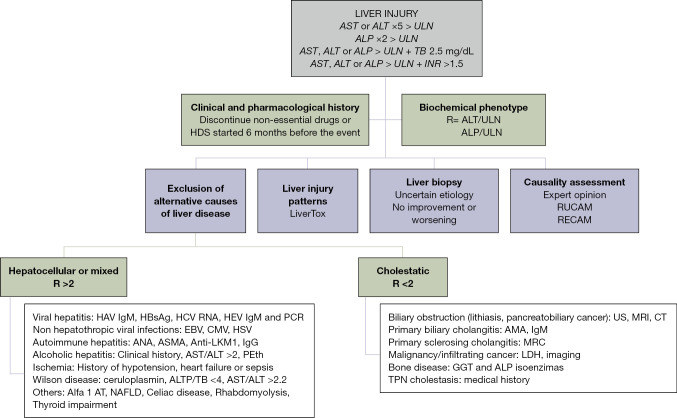
Figure 1.
DILI diagnostic algorithm. AST, aspartate aminotransferase; ALT, alanine aminotransferase; ULN, upper limit of normal; ALP, alkaline phosphatase; TB, total bilirubin; INR, international normalized ratio; HDS, herbal and dietary supplement; RUCAM, Roussel-Uclaf Causality Assessment Method; RECAM, Revised Electronic Causality Assessment Method; HAV, hepatitis A virus; IgM, immunoglobulin M; HCV, hepatitis C virus; HEV, hepatitis E virus; PCR, polymerase chain reaction; EBV, Epstein-Barr virus; CMV, cytomegalovirus; HSV, herpes simplex virus; ANA, antinuclear antibody; ASMA, anti-smooth muscle antibody; Anti-LKM1, anti-liver/kidney microsomal antibody type 1; IgG, immunoglobulin G; PEth, phosphatidylethanol; AT, antitrypsin; NAFLD, non-alcoholic fatty liver disease; US, ultrasound; MRI, magnetic resonance imaging; CT, computerized tomography; AMA, anti-mitochondrial antibody; MRC, magnetic resonance cholangiography; GGT, LDH, lactate dehydrogenase; TPN, total parenteral nutrition.
Idiosyncratic DILI usually occurs with a variable latency, although there are some hepatotoxic reactions that can appear after the withdrawal of the drug (i.e., amoxicillin-clavulanate) (5). Clinically significant acute DILI is defined by elevated aminotransferases, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) greater than 5 times the upper limit of normal (ULN), alkaline phosphatase (ALP) greater than 2 times the ULN, or elevated AST, ALT or ALP in combination with total bilirubin greater than 2.5 mg/dL. Unlike the European DILI CPG, the AASLD CPG also includes an altered international normalized ratio (INR) greater than 1.5 with elevated aminotransferases or ALP as a DILI detection criterion (4). Meanwhile, laboratory markers of severity, such as INR and albumin, will support the prognostic assessment of liver injury. The pattern of liver damage can be calculated by the ratio (R) between ALT/ULN or AST/ULN divided by ALP/ULN, where R >5 identifies hepatocellular pattern, R value <2 reflects cholestatic liver injury, and R value between 2 and 5 classifies liver injury in a mixed pattern (6,7).
The usual presentation of DILI is acute hepatocellular damage sometimes detected as asymptomatic elevation in transaminases. However, it can also present with a cholestatic pattern and can cause jaundice, general malaise, abdominal discomfort, and fever (5), and in this case, a differential diagnosis with acute biliary disease is mandatory. Upper abdominal pain is a typical symptom in biliary events, but may also be present in acute hepatitis. On the other hand, acute cholangitis/cholecystitis can occasionally be associated with marked elevation of transaminases, in range of acute hepatitis, typically lasting for a few hours (usually less than 24–36 hours), followed by an abrupt drop. A persistently elevated liver profile, would suggest parenchymal damage. Likewise, gallbladder wall thickening in ultrasound examination does not necessarily indicate gallbladder inflammation as it is often observed in patients with acute hepatitis. When all of the above is combined with the concurrent use of any potentially hepatotoxic xenobiotics, the possible diagnosis of DILI should be kept in mind by clinicians and surgeons.
Although the diagnosis of DILI is based on the exhaustive exclusion of other causes of liver damage, it is important for the surgeon to suspect acute DILI, as the most important therapeutic measure is the early withdrawal of the offending drug (1). Some agents commonly prescribed in gastrointestinal surgery have significant hepatotoxic potential and are associated with a cholestatic pattern of damage. Examples of such drugs include amoxicillin-clavulanic acid, cephalosporins and non-steroidal anti-inflammatory drugs (NSAIDs). Livertox® PubMed is a very useful tool when hepatotoxicity is suspected, as it allows clinicians to quickly and easily review the published evidence on any suspected agent (Table 1), including specific clinical phenotypes or signatures of more than 600 drugs and HDS (8).
Table 1. Main pharmacological agents used by hepatobiliary surgeons, hepatotoxic potential (according to Livertox®) and specific damage patterns and phenotypes associated to these drugs.
| Agents | Hepatotoxic potential | Pattern of damage | Specific phenotype |
|---|---|---|---|
| Anticoagulants | |||
| Low molecular weight heparins | E | Hepatocellular | Asymptomatic hypertransaminasemia |
| Fondaparinux | E | Hepatocellular | Asymptomatic hypertransaminasemia |
| NSAIDs | |||
| Ibuprofen | A | Hepatocellular/Cholestatic | Immunoallergic-like syndrome |
| Acute liver failure | |||
| Vanishing bile duct syndrome | |||
| Flurbiprofen | C | Cholestatic/Hepatocellular | Asymptomatic hypertransaminasemia |
| Acute liver failure | |||
| Naproxen | B | Hepatocellular/Cholestatic | Asymptomatic hypertransaminasemia |
| Acute liver failure | |||
| Diclofenac | A | Hepatocellular/Cholestatic | Asymptomatic hypertransaminasemia |
| Acute liver failure | |||
| Immunoallergic-like syndrome | |||
| Autoimmune-like hepatitis | |||
| Chronic hepatitis | |||
| Piroxicam | B | Cholestatic | Asymptomatic hypertransaminasemia |
| Acute liver failure | |||
| Vanishing bile duct syndrome | |||
| Pyrazolone derivatives | |||
| Metamizole | NA | Hepatocellular | Acute hepatitis |
| Autoimmune-like hepatitis | |||
| Antibiotics | |||
| Amoxicillin-clavulanate | A | Cholestatic (older people)/Hepatocellular (young people) | Acute hepatitis |
| Autoimmune-like hepatitis | |||
| Immunoallergic-like syndrome | |||
| Vanishing bile duct syndrome | |||
| Cephalosporins | B | Cholestatic/mixed | Acute hepatitis |
| Immunoallergic-like syndrome | |||
| Penicillins (2nd generation) | B/C | Cholestatic/Hepatocellular | Asymptomatic hypertransaminasemia |
| Immunoallergic-like syndrome | |||
| Piperacillin-Tazobactam | B | Cholestatic/mixed | Prolonged cholestasis |
| Immunoallergic-like syndrome | |||
| Ciprofloxacin | B | Hepatocellular/Cholestatic | Acute hepatitis |
| Chronic cholestasis | |||
| Vanishing bile duct syndrome | |||
| Meropenem | D | Cholestatic | Asymptomatic hypertransaminasemia |
| Acute hepatitis | |||
| Vanishing bile duct syndrome | |||
| Metronidazole | C | Hepatocellular | Asymptomatic hypertransaminasemia |
| Acute hepatitis | |||
| Vancomycin | B | Hepatocellular | Asymptomatic hypertransaminasemia |
| Immunoallergic-like syndrome | |||
| Acute liver failure | |||
| Tigecycline | E | NA | Asymptomatic hypertransaminasemia |
| Antifungal | |||
| Amphotericin B | C | Hepatocellular/mixed | Asymptomatic hypertransaminasemia |
| Acute cholestasis | |||
| Azoles | B | Hepatocellular | Asymptomatic hypertransaminasemia |
| Immunoallergic-like syndrome | |||
| Echinocandins | D | NA | Asymptomatic hypertransaminasemia |
| Acute hepatitis | |||
Likelihood score: A (well known cause of clinically apparent liver injury), B (highly likely cause of clinically apparent liver injury), C (probable rare cause clinically apparent liver injury), D (possible rare cause of clinically apparent liver injury), E (unproven but suspected cause of clinically apparent liver injury, largely due to bleeding episodes). NSAIDs, nonsteroidal anti-inflammatory drug; NA, no available.
It is imperative that clinicians are able to recognise the early signs of severity, such as the presence of jaundice +/− coagulopathy and/or hepatic encephalopathy, so that, in addition to withdrawing the suspected agent, they must urgently refer the patient to a hepatology unit and, consequently, to a transplant centre if the patient meets transplant criteria. On the other hand, elderly and frail patients (Charlson index >2) and those with underlying liver disease who suffer DILI may have a worse prognosis with increased mortality during the first 6 months after DILI onset (9).
The exclusion of alternative causes of liver injury is the most important step in the diagnosis of DILI. As mentioned above, the biochemical profile of the liver can assist in this assessment, guiding the exclusion of different etiologies of liver injury depending on the pattern of liver injury (Figure 1). Histological evaluation in hepatotoxicity is highly variable and liver biopsy is only required in cases of uncertain diagnosis or poor clinical outcome. The histological findings can guide the diagnosis of DILI or an alternative etiological diagnosis and provide prognostic information. In this regard, the presence of eosinophils or granulomas is considered a good prognostic factor, whereas the presence of necrosis or ductal reaction excludes a worse outcome in DILI (10).
In recent decades, several causality assessment methods have been developed with the intention of standardizing DILI assessment and providing a probability category for a drug or HDS as the cause of the DILI event. Today, the most accepted and commonly used tools are the Roussel-Uclaf Causality Assessment Method (RUCAM), the Revised Electronic Causality Assessment Method (RECAM) and the Structured Expert Opinion Scale developed by DILIN (11,12).
The RUCAM was developed by a panel of DILI experts in 1993 and has been the main method for assessing causality for many years. However, certain limitations, such as the inclusion of unclear risk factors and some complex steps in the assessment, have led to the development of alternative tools (13).
The DILIN Expert Opinion Scale is a causality assessment method that requires at least three hepatologists to independently assess the case. The assessment is based on a retrospective review of the case history, laboratory data and prospective follow-up visits. Each reviewer assigns a causality score corresponding to a percentage of the likelihood of DILI, where 1= definite (>95% likelihood), 2= very likely (75–95%), 3= probable (50–74%), 4= possible (25–49%) and 5= unlikely (<25%). Consensus scores are reached through discussion by e-mail or telephone conference. The main strengths of the DILIN Expert Opinion Scale are its ability to assess atypical hepatotoxicity events, e.g., cases with discontinuous toxic exposure or subtle biopsy findings, albeit with the obvious disadvantage of requiring expert judgement (14).
The RECAM is a new online digital tool that is easier to use for non-DILI experts. It is an automated electronic platform that may provide a faster and more reliable causality assessment than the RUCAM by using standardized, quantitative and categorical data fields, although this tool still needs to be validated in different DILI populations around the world (15).
In conclusion, DILI remains an uncommon but serious threat to successful pharmacological therapy in many clinical contexts, including the pre- and post-operative patient. The current AASLD guidelines are a helpful tool to assist clinicians in the evaluation and management of patients with suspected DILI.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: The study was supported by grants from Instituto de Salud Carlos III cofounded by Fondo Europeo de Desarrollo Regional—FEDER [contract numbers: FIS 21_01248; PI18/00901, UMA18-FEDERJA-193]. CIBERehd is funded by Instituto de Salud Carlos III (ISCIII). All authors are members of the COST ACTION “CA-17112”-Prospective European Drug-Induced Liver Injury Network, supported by COST (European Cooperation in Science and Technology). www.cost.eu. JMPB holds a Rio Hortega contract from ISCIII.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-319/coif). The authors have no conflicts of interest to declare.
References
- 1.European Association for the Study of the Liver . Electronic address: easloffice@easloffice.eu; Clinical Practice Guideline Panel: Chair:; Panel members; EASL Governing Board representative:. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol 2019;70:1222-61. 10.1016/j.jhep.2019.02.014 [DOI] [PubMed] [Google Scholar]
- 2.Devarbhavi H, Aithal G, Treeprasertsuk S, et al. Drug-induced liver injury: Asia Pacific Association of Study of Liver consensus guidelines. Hepatol Int 2021;15:258-82. 10.1007/s12072-021-10144-3 [DOI] [PubMed] [Google Scholar]
- 3.Chalasani NP, Maddur H, Russo MW, et al. ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am J Gastroenterol 2021;116:878-98. 10.14309/ajg.0000000000001259 [DOI] [PubMed] [Google Scholar]
- 4.Fontana RJ, Liou I, Reuben A, et al. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology 2023;77:1036-65. 10.1002/hep.32689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrade RJ, Chalasani N, Björnsson ES, et al. Drug-induced liver injury. Nat Rev Dis Primers 2019;5:58. 10.1038/s41572-019-0105-0 [DOI] [PubMed] [Google Scholar]
- 6.Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 1993;46:1323-30. 10.1016/0895-4356(93)90101-6 [DOI] [PubMed] [Google Scholar]
- 7.Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol 1990;11:272-6. 10.1016/0168-8278(90)90124-A [DOI] [PubMed] [Google Scholar]
- 8.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [Google Scholar]
- 9.Ghabril M, Gu J, Yoder L, et al. Development and Validation of a Model Consisting of Comorbidity Burden to Calculate Risk of Death Within 6 Months for Patients With Suspected Drug-Induced Liver Injury. Gastroenterology 2019;157:1245-52.e3. 10.1053/j.gastro.2019.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleiner DE. Liver histology in the diagnosis and prognosis of drug-induced liver injury. Clin Liver Dis (Hoboken) 2014;4:12-6. 10.1002/cld.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs--II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol 1993;46:1331-6. 10.1016/0895-4356(93)90102-7 [DOI] [PubMed] [Google Scholar]
- 12.Hayashi PH, Lucena MI, Fontana RJ, et al. A revised electronic version of RUCAM for the diagnosis of DILI. Hepatology 2022;76:18-31. 10.1002/hep.32327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García-Cortés M, Stephens C, Lucena MI, et al. Causality assessment methods in drug induced liver injury: strengths and weaknesses. J Hepatol 2011;55:683-91. 10.1016/j.jhep.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 14.Hayashi PH, Barnhart HX, Fontana RJ, et al. Reliability of causality assessment for drug, herbal and dietary supplement hepatotoxicity in the Drug-Induced Liver Injury Network (DILIN). Liver Int 2015;35:1623-32. 10.1111/liv.12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology 1997;26:664-9. 10.1002/hep.510260319 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as