Abstract
Background:
Although Black/African American older adults bear significant inequities in prevalence, incidence, and outcomes of Alzheimer’s disease and related dementias, they are profoundly under-included in Alzheimer’s Disease research. Community-Engaged Research (e.g., equitable community/science partnerships) is an evidence-based approach for improving engagement of underrepresented populations into Alzheimer’s Disease research, but has lacked scalability to the national level. As internet use among older adults from underrepresented populations continues to grow, internet-based research shows promise as a feasible, valid approach to engagement and longitudinal assessment. The Community Engaged Digital Alzheimer’s Research (CEDAR) study utilizes a community-engaged research approach to increase the engagement and research participation of Black/African American adults in the Brain Health Registry (BHR) and Alzheimer Disease clinical research.
Objectives:
To describe the methods and evaluate the feasibility of the CEDAR culturally-informed digital platform within BHR.
Methods:
Design:
All Black/African American participants in BHR were invited to enroll in CEDAR and to consider serving on a newly convened Community-Scientific Partnership Board to guide the study. The community board guided the development a culturally-informed cadre of engagement materials and strategies to increase research participation. Engagement strategies included incentives for study task completion, culturally-informed communications (e.g., landing page, emails and social media), resources about brain health, and video and written testimonials by CEDAR participants.
Setting:
BHR, an Internet-based registry and cohort.
Participants:
BHR participants self-identifying as Black/African American were invited to enroll. All participants who signed an online informed consent document were enrolled.
Measurements:
We report the number of participants invited, enrolled, completed tasks, and volunteered to join the community board. We compared the demographics, cognitive profile, and baseline BHR task completion rates between CEDAR participants and all those invited to join the study.
Results:
Of 3738 invited, 349 (9.34%) enrolled in CEDAR. 134 (37% of CEDAR participants) volunteered to join the community board, of which 19 were selected for the community board. Compared to those invited, the CEDAR cohort had a higher percentage of female participants (84.5%) and a lower percentage of participants who identify as belonging to more than one ethnocultural group (21.8%). Compared to those did not enroll in CEDAR, those enrolled in CEDAR had a higher percentage of participants completing all BHR tasks (22%) and a higher percentage of participants completing at least one cognitive test (76%). Those enrolled in CEDAR also had a higher percentage of participants having an enrolled study partner (18%).
Conclusions:
A culturally-informed Community-Engaged Research approach, including a remotely-convened community board, to engagement of Black/African American participants in an online research registry is feasible. This approach can be adapted for use in various clinical studies and other settings. Future studies will evaluate the effectiveness of the engagement strategies.
Keywords: Brain Health Registry, Engagement, Community-Engaged Research, Alzheimer’s Disease, Black/African American
1. INTRODUCTION
1.1. Health Inequities in Alzheimer’s disease and related dementias
Underrepresented populations (i.e., Black/African American and Latinx/a/o adults) experience significant inequities in the prevalence, incidence, and outcomes of Alzheimer’s disease and related dementias [1-11]. Underrepresented older adults are 1.5 - 2x as likely to develop dementia as non-Latinx white adults.[2, 12]. Although ethnocultural status (e.g., race, ethnicity) is a sociocultural construct rather than a biological variable, it serves as a proxy for numerous exposures that may result from a complex combination of sociocultural (e.g., racial discrimination,[13-15] socioeconomic status, low years/quality of education), environmental, and structural factors that influence important biological (e.g., diabetes, vascular) [16-18] vulnerabilities. Yet, underrepresented populations are profoundly under-included in Alzheimer’s disease and related dementias research.[19-22]. This has the potential to affect the generalizability and external validity of research studies and clinical trials, and amplify health disparities [23].
Inclusion and engagement (e.g., retention, task completion) strategies in the vast majority of observational and interventional studies have generally failed to engage underrepresented populations.[24-36] With the exception of a few studies focused on underrepresented population samples,[1, 11, 32, 37-45] most “samples of convenience” largely enroll non-Latinx white adults with high education and socioeconomic status.[25, 46] Further, participants from underrepresented populations are less likely to complete study tasks, return for longitudinal follow-up, and participate in genetic and biomarker research.[47-52] Although some investigators, often from underrepresented populations themselves, have effectively engaged and included participants from these communities, their methods and results have not successfully been taken up by most clinical investigators. This is a particular problem in large multisite observational and interventional trials, wherein funders, corporate partners, principal investigators, co-investigators, and local study staff may lack the expertise, dedicated resources, and/or motivation to make the substantial investments needed to genuinely increase enrollment of underrepresented populations and engagement in research of Alzheimer’s disease and related dementias.
1.2. A Community-Engaged Research Approach
A recent systematic review by Gilmore-Bykovsky[21] demonstrates that a community-engaged research approach, founded on Community-Based Participatory Research principles, has repeatedly yielded promising results for improving the representation of Black and Latinx American adults in Alzheimer’s disease and related dementias research. Community-engaged research is founded upon active, sustained community collaboration and engagement at all stages of the research process, from study inception to dissemination.[21, 53-55] Inclusion and engagement strategies shaped by community-engaged research methods include: authentic, equitable community-science partnerships rather than often marginalized community advisory boards; listening/responding to unique community concerns; and sustained and active engagement with communities. This work takes long-term investment and is more intensive than traditional, colonized approaches to research. Despite the promising preliminary results of community-engaged research approaches to Alzheimer’s disease research, the work to date has mostly been hyper local and on a smaller scale as these approaches have historically lacked scalability for broad implementation in largescale, multi-site studies.
1.3. Digital Engagement Approaches to Increasing Research Participation of Underrepresented Populations
Growing evidence supports the feasibility and validity of digital engagement (e.g., digital communications, outreach) for large multi-site studies, and establishment of Internet-based platforms (e.g., registries) to enroll participants and refer them to research studies.[56-64] . Major advantages of Internet-based data collection include scalability, efficiency, reduced cost, frequent data collection, and ability to engage those who cannot participate in in-clinic studies due to geography and travel or financial burden. The recent COVID pandemic, which greatly limited in-person research, further highlights the need for remote, Internet-based approaches. Increasing numbers of older adults,[65] including older adults from underrepresented populations[66, 67] use the internet for a wide variety of activities,[68] and internet-based telemedicine is increasing. However, like in-clinic studies, Internet-based registries under-include underrepresented group participants.[61, 63, 69] [66-68]
Research is needed to adapt well-established community-engaged research methods to an Internet-based approach to scale up previously small-scale, localized, community-engaged inclusion and engagement efforts. The Brain Health Registry (BHR)[63] offers a unique opportunity to do just that. The BHR (N>90,000) is one of the world’s largest Internet-based registries dedicated to Alzheimer’s disease and aging research. However, similar to most cohort studies and registries, the BHR also has had a poor record of underrepresented population inclusion. BHR includes N=3738 Black participants, comprising only 4% of all BHR participants. This is a major limitation of the current BHR approach.
The overall aim of this project was to evaluate the effectiveness of a culturally-informed, community-engaged, digital research approach to increase research participation (e.g., retention, task completion) of Black/African American (hereafter referred to as Black) participants within the BHR. The purpose of this manuscript is 1) to describe the Community-engaged Digital Alzheimer’s Research (CEDAR) study, including how we developed and implemented the culturally-informed digital engagement efforts; and 2) to evaluate feasibility by reporting results from an interim analysis of enrolled participants.
2. METHODS
2.1. The Brain Health Registry (BHR)
The BHR is a public online registry to recruit, screen, and longitudinally monitor participants for aging and cognitive-related research, as well as to refer enrolled participants to other studies.[63, 70] Anyone over the age of 18 is eligible to participate. BHR includes online consent, self-administered cognitive tests, self-report questionnaires, and study partner enrollment and questionnaires[71]. The questionnaires collect demographic, health, cognitive, and lifestyle data. Participants are asked to complete questionnaires and cognitive tests every six months. Participants do not receive feedback about their questionnaire replies or cognitive test results.
2.2. CEDAR Study enrollment
Eligible participants were current BHR participants who self-identify as Black and agreed to be contacted about future research opportunities. BHR participants who did not identify as Black were not included in this study. Study participants were recruited by a series of four culturally-tailored, automated email invitations describing the study. Interested participants could click a link in the email invitation, prompting them to log in to their BHR account and view a page describing the study in detail. Then, after signing an electronic consent form, they could proceed to their study tasks. All CEDAR activities were completed using the BHR online infrastructure.
2.3. CEDAR study activities
2.3.1. Barriers and Facilitators Survey.
All enrolled participants were directed to a brief (5-15 minutes to complete), voluntary, uncompensated, cross-sectional online survey about motivators (e.g., reasons for joining and continuing to participate) and barriers (e.g., reasons that make it difficult to participate) to BHR participation, as well as preferences for engagement strategies and communication channels. Survey respondents could provide their answers using rating scales and free text responses. The final question in the survey allowed participants to volunteer to serve on the Community-Science Partnership Board. Interested participants are asked to enter their name, email address, brief details about their background (where they live, education, occupation, experience with or interest in research and/or interest in helping the Black community), and whether compensation with electronic gift cards is satisfactory.
2.3.2. Community-Science Partnership Board (community board).
Based on our prior community-engaged research experience and expertise, we aimed to have the composition of the community board include no more than 15 – 20 community members and 4 – 6 study scientists. For the selection of our community members, we examined the demographic characteristics (e.g., age, gender, education, geographic location, occupation), self-reported reasons for offering to participate, and relevant experiences of the 134 community members who expressed interest in serving on the community board. We prioritized diversity in all demographic characteristics and interest in or experience with Alzheimer’s disease and related dementias. For the selection of study scientists, all 7 study scientists were invited to join the community board. In addition, study staff members participated in an ex officio capacity to facilitate certain aspects of the meetings (e.g., logistics, presentation of engagement materials). Two-hour board meetings were convened on a quarterly basis via videoconference as the community board was convened during the pandemic and members resided all over the country and in-person meetings. Thus, remote meetings facilitated the feasibility of community board implementation.
The initial community board meeting oriented the entire board to the study aims, community-engaged research principles, and then the group development of jointly decided processes and goals for the community board. Over the course of the following meetings, the board guided the development of a culturally-informed cadre of engagement materials and strategies to increase research participation of Black adults in the study. Through a process iterative feedback and discussion, the community board informed engagement strategies, including included incentives for study task completion, culturally-informed communications (e.g., landing page, emails and social media), resources about brain health, and video and written testimonials by CEDAR participants. Also of note, all meetings included dedicated “listening sessions” for unstructured time for board members to share their views and sentiments with each other to further promote a sense of teamwork and trust. Towards the end of the project, all members also voted to continue community board meetings, regardless of whether dedicated grant funding would be available.
2.3.3. Financial compensation for completing tasks.
Participants received electronic gift cards for completing each study visit. Partway through the study, the researchers received IRB approval to increase the gift card amount from $25 to $50. The community board members were compensated with $100 gift cards for each board meeting attended.
2.4. Engagement materials/strategies.
The research team employs several strategies for engaging CEDAR participants and improving study retention (see Table 1), including: compensation for task completion, a private Facebook group to share knowledge and facilitate dialogue among prospective and existing CEDAR participants, blog posts and an email campaign to provide resources and educational materials about Alzheimer’s disease and the Black community, participant videos and written testimonials, and a culturally-tailored landing page.
Table 1:
List of Engagement strategies
| Engagement Strategy | Details |
|---|---|
| CEDAR Facebook group | A private Facebook group was developed to disseminate knowledge and facilitate dialogue among prospective and existing CEDAR participants Posts include resources and educational materials pertaining to Black brain health |
| Participant testimonials | 3 Community-Science Partnership Board members shared their motivations for joining CEDAR and personal experiences with Alzheimer’s disease Include both written statements posted to the Brain Health Registry website and brief videos shared with CEDAR participants via email and social media |
| Investigator videos | Two CEDAR investigators recorded videos explaining the study and the importance of including Black adults in research Videos distributed via social media and email |
| 13-week email engagement campaign | Series of 13 emails sent to CEDAR participants to provide educational resources pertaining to Black brain health Emails also share recent news in the Alzheimer’s field |
| Blog posts | Educational blog posts about brain health are distributed to prospective and existing CEDAR participants via social media channels |
| Compensation | Compensation for completing study tasks was increased from $25 to $50 partway through the study community board members are compensated $100 per quarterly meeting attended |
| Landing page | Participants see a culturally tailored landing page when they enroll in CEDAR Landing page contains links to learn more about the study and links to the CEDAR Facebook page |
| Referrals to other studies | All Black Brain Health Registry participants received an email featuring BHR and collaborator studies Objective was to increase Black representation in studies |
2.5. Participant metrics
After enrollment in BHR, participants complete a questionnaire, which asks them to self-report sociodemographic information. This analysis focused on the following variables: age (continuous), gender (Male, Female, Other, Prefer not to say), race (Asian, Black or African American, Native American or Alaska Native, Pacific Islander, White, Other, Prefer not to say), ethnicity (Latino, non-Latino, Prefer not to say), education attainment (categorical), endorsement of subjective memory concern (“Are you concerned that you have a memory problem?”), family history of Alzheimer disease/dementia, and the participants’ self-report Everyday Cognition Scale score. It (continuous, numeric scores range from 1-4) is a 39-item instrument which assesses functional change by asking about the participant’s self- or study partner-reported capabilities to perform everyday tasks in the present versus 10 years prior. These tasks include activities that map to cognitive abilities across six domains[72]. In BHR, participants are asked to complete an Everyday Cognition Scale that has been adapted for an online setting[73]. Analyses also included self-report diagnosis of Alzheimer’s disease, mild cognitive impairment (MCI), and/or dementia. The categorical variable education attainment was converted into a continuous variable called “years of education,” ranging from 6-20 years. Based on the original race variable, we created a dichotomous variable of Black only (self identifies as Black/African American and no other race categories) or More than One Race (self identifies as Black/African American and at least one additional race category).
2.6. Task completion metrics
We measured task completion of invited and enrolled CEDAR participants during participants’ last BHR visit prior to the CEDAR invitation. Metrics of task completion included whether they completed at least the BHR core questionnaire (this is the first questionnaire participants complete which asks about demographic information, family history of Alzheimer’s disease/dementia, mood, health, medications, and memory) (yes, no), completed all BHR tasks (yes, no), completed at least one cognitive test (yes, no), began at least one cognitive test but had technical difficulties (yes, no), and whether they have an enrolled study partner through the BHR Caregiver and Study Partner Portal.[73] Participants were considered to have an enrolled study partner if their potential study partner completed online informed consent (yes/no).
2.7. Statistical Methods
We compared characteristics of participants who enrolled in CEDAR to characteristics of Black BHR participants who did not enroll. For continuous variables, independent sample t tests were conducted to compare the group means. Cohen’s d was reported as effect size. For categorical variables, Chi-square tests were used if ≤20% of expected cell counts were less than 5 and Cramer’s V was reported as effect size. Otherwise, Fisher’s exact tests were used if > 20% of expected cell counts were less than 5.
3. RESULTS
3.1. Recruitment and enrollment
A total of 3738 Black BHR participants were invited to join CEDAR. 364 (9.74%) participants indicated interest in CEDAR by clicking on the email study link, and 349 (9.34%) enrolled in CEDAR (Figure 1).
Figure 1.
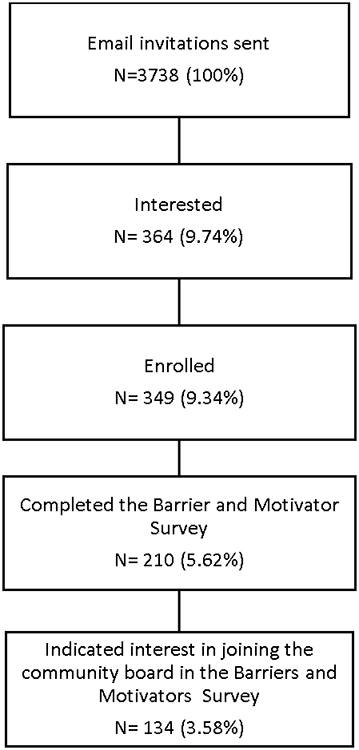
Participant flow from invitation to enrollment
3.2. Participant characteristics
Compared to those who did not enroll in CEDAR, those enrolled (Table 2) were older (t 3387 = 6.43, p < .001, Cohen’s d = .33), had higher education levels (t 3375 = 6.30, p < .001, Cohen’s d = .34), and lower self-report everyday cognition scale score, indicating less self-report cognitive and functional decline (t 1246 = −3.61, p < .001, Cohen’s d = .21). Those who enrolled had a higher percentage of family history of Alzheimer’s disease/dementia (χ2 = 24.27, p < .001, Cramer’s V = .09). Among those who completed or partially completed the survey questions, those who volunteered to join the community board (Table 3) were older (t 156 = 2.00, p = .047, Cohen’s d = .29) and had higher education levels (t 191 = 2.63, p = .009, Cohen’s d = .36) than those who did not volunteer. The cohort of community board volunteers had a higher percentage of those who self-identify as African American/Black only (χ2 = 9.12, p = .003, Cramer’s V = .21).
Table 2.
Participant characteristics of invited, enrolled, and not enrolled participants
| Participants invited to join CEDAR N= 3738 |
Black BHR participants enrolled in CEDAR N=349 |
Black BHR participants who did not enroll in CEDAR N=3389 |
p value from significant test between enrolled vs. not enrolled (Effect size) |
|
|---|---|---|---|---|
| Age in years, M(SD) | 54.48(12.94) | 58.29(11.49) | 54.08(13.02) | <.001 (.33) 1 |
| Min, Max | 18, 90 | 23, 84 | 18, 90 | |
| Years education, M(SD) | 15.48(2.52) | 16.25(2.4) | 15.40(2.52) | <.001 (.34) 1 |
| Min, Max | 6, 20 | 12, 20 | 6, 20 | |
| Gender, n(%) | ||||
| Male | 620(16.6%) | 54(15.5%) | 566(16.7%) | .609(.01) 2 |
| Female | 3118(83.4%) | 295(84.5%) | 2823(83.3%) | |
| Ethnicity, n(%) | ||||
| Latino | 303(3.0%) | 25(7.2%) | 278(8.2%) | .495(.01) 2 |
| Non-Latino | 3322(88.9%) | 320(91.7%) | 3002(88.6%) | |
| Declined to state | 113(3.0%) | 4(1.2%) | 109(3.2%) | |
| Race, n(%) | ||||
| African American/Black only | 2953(79.0%) | 273(78.2%) | 2680(79.1%) | .761(.01) 2 |
| African American/Black mixed | 785(21.0%) | 76(21.8%) | 709(20.9%) | |
| Self-report memory concern, n (%) | 2509(67.1%) | 250(71.6%) | 2259(66.7%) | .284(.02) 2 |
| Report family history of Alzheimer’s disease , n (%) | 1098(29.4%) | 146(41.8%) | 952(28.1%) | <.001 (.09) 2 |
| Self-report diagnosis of MCI, n (%) | 135(3.6%) | 28(8.0%) | 107(3.2%) | .307 (.03) 2 |
| Self-report diagnosis of dementia, n (%) | 38(1.0%) | 7(2.0%) | 31(0.9%) | 1.000 (.00) 2 |
| Self-report diagnosis of Alzheimer’s disease, n (%) | 20(0.6%) | 4(1.2%) | 16(0.5%) | .764 3 |
| Everyday Cognition Scale score, M(SD) | 1.47(0.51) | 1.39(0.44) | 1.49(0.52) | < .001 (.21) 1 |
| Min, Max | 1, 4 | 1, 4 | 1, 3.97 |
Note.
based on independent t test with Cohen’s d as effect size.
based on Chi-Square test with Cramer’s V as effect size.
based on Fisher’s Exact test.
Table 3.
Participant characteristics of participants who volunteered to join and not
| Participants who completed/completed partially the survey |
Participants who volunteered |
Participants who did not volunteer |
p value from significant test between volunteered vs. not |
|
|---|---|---|---|---|
| Age in years, M(SD) | 57.38(11.36) | 58.74(9.76) | 55.47(13.10) | .047 (.29) 1 |
| Min, Max | 24, 81 | 31, 81 | 24, 79 | |
| Years education, M(SD) | 16.25(2.33) | 16.60(2.29) | 15.77(2.31) | .009 (.36) 1 |
| Min, Max | 12, 20 | 12, 20 | 12, 20 | |
| Gender, n(%) | ||||
| Male | 36(16.7%) | 20(15.9%) | 16(17.8%) | .853(.01) 2 |
| Female | 180(83.3%) | 106(84.1%) | 74(82.2%) | |
| Ethnicity, n(%) | ||||
| Latino | 15(6.9%) | 5(4.0%) | 10(11.1%) | .074(.12) 2 |
| Non-Latino | 200(92.6%) | 121(96.0%) | 79(87.8%) | |
| Declined to state | 1(0.5%) | 0(0.0%) | 1(1.1%) | |
| Race, n(%) | ||||
| African American/Black only | 163(75.5%) | 105(83.3%) | 58(64.6%) | .003(.21) 2 |
| African American/Black mixed | 53(24.5%) | 21(16.7%) | 32(35.6%) | |
| Self-report memory concern, n (%) | 157(73.0%) | 90(71.4%) | 6775.3%) | .637(.03) 2 |
| Report family history of Alzheimer’s disease, n (%) | 94(43.7%) | 55(43.7%) | 39(43.8%) | 1.000 (.00) 2 |
| Self-report diagnosis of MCI, n (%) | 17(8.3%) | 13(10.8%) | 4(4.7%) | .190 (.09) 2 |
| Self-report diagnosis of dementia, n (%) | 6(2.9%) | 5(4.2%) | 1(1.2%) | .404 3 |
| Self-report diagnosis of Alzheimer’s disease, n (%) | 3(1.5%) | 2(1.7%) | 1(1.2%) | 1.000 3 |
| Everyday Cognition Scale score, M(SD) | 1.37(0.44) | 1.36(0.46) | 1.37(0.42) | .899 (.02) 1 |
| Min, Max | 1, 4 | 1, 4 | 1, 3.03 |
Note.
based on independent t test with Cohen’s d as effect size.
based on Chi-Square test with Cramer’s V as effect size.
based on Fisher’s Exact test.
3.3. Prior BHR task completion
Among all Black BHR participants invited to join CEDAR, 100% completed at least the BHR questionnaire, 4.1% completed all tasks, 53.2% completed at least one cognitive test, 11.1% attempted a cognitive test but had technical difficulties, and 5.0% have an enrolled study partner (see Table 4). Compared to those who did not enroll in CEDAR, CEDAR participants had a higher percentage of completing all BHR tasks (χ2 = 439.57, p < .001, Cramer’s V = .36) and a higher percentage of participants completing at least one cognitive test (χ2 = 179.41, p < .001, Cramer’s V = .23). CEDAR also had a higher percentage of those with cognitive test technical difficulties (χ2 = 32.55, p < .001, Cramer’s V = .10) and a higher percentage of participants having an enrolled study partner (χ2 = 190.34, p < .001, Cramer’s V = .23).
Table 4.
Prior task completion of all invited, enrolled and not enrolled in CEDAR
| Total Black participants enrolled in BHR (invited to join (CEDAR) N= 3738 |
Black BHR participants who did enroll in CEDAR N=349 |
Black BHR participants who did not enroll in CEDAR N= 3389 |
p values from Chi-Square tests between enrolled vs. not enrolled (Effect size) |
|
|---|---|---|---|---|
| Completed at least BHR core questionnaire, n (%) | 3738(100.0%) | 349(100.0%) | 3389(100.0%) | NA |
| Completed all BHR tasks, n (%) | 154(4.1%) | 89(22.4%) | 65(1.9%) | <.001(.36) |
| Completed at least one Cognitive Test, n (%) | 1988(53.2%) | 305(76.8%) | 1683(49.7%) | <.001(.23) |
| Had difficult completing Cognitive Test, n (%) | 414(11.1%) | 71(17.9%) | 343(10.1%) | <.001(.10) |
| Have an enrolled study partner, n (%) | 185(5.0%) | 71(17.9%) | 114(3.4%) | <.001(.24) |
3.4. Community-Scientific Partnership Board
A total of 134 (3.58% of all those invited to CEDAR; 37% of those enrolled in CEDAR) community members volunteered to join the community board, of which 19 were selected for the community board. Currently the CEDAR community board has a total of 27 members consisting of 19 Black BHR participants, seven study scientists, and a Latinx marketing/inclusion expert. Community board meetings to date have introduced community members to community-engaged research methods, the study team, and the project goals; included multiple community listening sessions; and offered community board members the opportunity to provide feedback on proposed digital outreach strategies and materials (e.g., website, social media strategy, digital advertising themes and messaging, images and text used in participant communications, dissemination plans).
4. DISCUSSION
The major finding of this study was that a culturally-informed, community-engaged research approach, is a feasible and scalable strategy to enroll Black participants into a research study to increase engagement and research participation. The approach included a novel, remotely-convened community board comprised of individuals residing across the US. Demographic selection biases reflect some of the overall biases of the BHR cohort, including overrepresentation of older adults and those with high educational attainment. Compared to those who did not enroll, those enrolled in CEDAR had higher rates of self-reported family history of Alzheimer’s disease/dementia, suggesting that this may be a motivator for enrolling. Remaining challenges are improving overall enrollment rates and increasing sample diversity (e.g., gender, education, cognitive). Future analyses will evaluate the effectiveness of engagement strategies to increase BHR participation by comparing participation levels before and after CEDAR enrollment. This approach can be adapted for use in multiple studies and settings to facilitate inclusion and engagement of Black older adults, and potentially other historically under-included populations in Alzheimer’s disease and related dementias research.
We enrolled 349 Black BHR participants into the CEDAR study, representing 9.74% of those invited to join. This enrollment rate is comparable to the average enrollment rate when BHR participants are asked to enroll in additional studies (10%). However, in terms of BHR task completion, participants enrolled in CEDAR showed a higher level of engagement compared to participants not enrolled in CEDAR, suggesting that the participants enrolled CEDAR were already more engaged. Comparing task completion rates (e.g., having completed at least one cognitive test) with other BHR communities, participants invited to CEDAR (53.2%) had a slightly lower rate compared the overall BHR community (56%), but higher than a large Latinx cohort recently enrolled in BHR (47.8%)[74]. The CEDAR results build on our previous work, in which a combination of community-engaged research methods and digital methods were used to recruit underrepresented populations into Alzheimer’s disease research studies. For example, the California Latino BHR (CAL-BHR)[74] demonstrated the feasibility and effectiveness of a similar approach for inclusion and engagement of Latino individuals. Recently, the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Diversity Taskforce used culturally informed digital advertising to improve underrepresented population representation in ADNI[54, 75]. To our knowledge, CEDAR is the first study to demonstrate the feasibility of a digital, culturally-informed engagement approach, informed by a community board, focused on improving engagement, retention, and participation of Black adults in Alzheimer’s disease and related dementias research. Our findings support the use of digital communications as a tool for engagement in studies[76-79] and emphasize the importance of integrating culturally-informed engagement materials with guidance from community members into the study design[28, 30-32, 51, 80-84]. Since the digital material can be tailored to other ethnocultural populations, this strategy has high potential for scalability and improving the reach of engagement and recruitment.
Researchers interested in this digital community-engaged research approach should consider strategies for fostering dialogue and long-term, bidirectional relationships with community members. This includes strategies for sustaining community boards and financially compensating community board members. Additionally, previous research[82] has demonstrated the effectiveness of direct partnerships with community-based leaders and organizations such as (1) churches and other faith-based institutions, (2) Black fraternities/sororities, and (3) community health clinics for ameliorating distrust and increasing retention of Black participants in clinical Alzheimer’s research.
Limitations of the study include multiple selection biases that limit generalizability. First, enrollment in BHR requires participants to have regular access to a device and the Internet, and the cognitive capacity to navigate an unsupervised, remote assessment platform. Our analyses highlight further selection biases for those who chose to join CEDAR from BHR, including a bias for those with high baseline engagement levels in BHR. This may obscure the results of subsequent analyses that evaluate effectiveness of CEDAR engagement strategies by comparing engagement levels before and after the culturally-informed materials were deployed. Additional retention efforts and strategies must be developed to engage participants with lower baseline levels of BHR engagement. Additional analyses to explore the role of social media in engagement, and related issues around privacy concerns, are crucial. The CEDAR study under-samples Black male individuals and Black individuals with lower levels of educational attainment. To improve generalizability, future efforts will include engagement strategies targeted to men and individuals with lower education levels. To better optimize future efforts, we will evaluate the effectiveness of individual engagement strategies in a later study.
This manuscript focused on feasibility and baseline engagement levels of study participants. Future analyses will evaluate the effectiveness of methods by comparing specific BHR registry behaviors (task completion, longitudinal retention) before and after deployment of the engagement strategies. BHR also refers participants to additional research studies[63]. The CEDAR study will also evaluate whether a culturally informed approach improves further research participation of CEDAR participants.
Conclusion
Combining community-engaged research and digital participant communication methods has the potential to efficiently engage Black adults in online Alzheimer’s disease research. Future analyses evaluating the effectiveness of specific engagement strategies for improving research participation can inform the development of evidence-based best practices for including and retaining Black individuals in Alzheimer’s disease and related dementias research.
ACKNOWLEDGEMENTS
We would like to acknowledge all the hard work and insightful comments from all CEDAR Community-Scientific Partnership Board members, including Anna Middleton, Bernadette Waddell, Regene P. Ross. We are also grateful to all BHR participants who enrolled through this effort. This work was supported by the Genentech Health Equity Innovations 2020 Fund (Grant #: G-89294; MPIs: M. Rivera Mindt, R. Nosheny)
FUNDING
Funding for this work was provided by the Genentech Health Equity Innovations 2020 Fund (#G-89294). The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
AUTHOR DISCLOSURES
Danqi Zhu, Dr. Heining Cham, Anna Aaronson, Catherine Conti, Xinyue Deng, Jennefer Sorce, Carole Cypress, Philip Griffin, Derek Flenniken, Monica Camacho, Juliet Fockler, Diana Truran, Dr. R. Scott Mackin, Dr. Carl V. Hill and Dr. Robert Turner have nothing to disclose.
Dr. Rivera Mindt reports grants from NIH/NIA Pending # 1R01AG079285-01, during the conduct of the study; grants from U19AG078109-01; R01AG066471; R56AG075744; R13AG071313-01; & R01AG065110 - 01A1; SC3GM141996; Genentech Health Equity Innovations 2020 Fund G-89294, outside the submitted work.
Dr. Ashford reports grants from NIA during the conduct of the study (F32 AG072730-02).
Roxanne Alaniz reports personal fees from eVAL, personal fees from ADNI4, personal fees from SALUD, personal fees from US Pointer, personal fees from Active6, outside the submitted work.
Roxanne Alaniz reports personal fees from eVAL, personal fees from ADNI4, personal fees from SALUD, personal fees from US Pointer, personal fees from Active6, outside the submitted work.
Dr. Byrd reports grants from Genetech during the conduct of the study.
Dr. Weiner reports grants from National Institutes of Health (NIH), grants from Department of Defense (DOD), grants from Patient-Centered Outcomes Research Institute (PCORI), grants from California Department of Public Health (CDPH), grants from University of Michigan, grants from Siemens, grants from Biogen, grants from Hillblom Foundation, grants from Alzheimer’s Association, grants from The State of California, grants from Johnson & Johnson, grants from Kevin and Connie Shanahan, grants from GE, grants from VUmc, grants from Australian Catholic University (HBI- BHR), grants from The Stroke Foundation, grants from Veterans Administration, personal fees from Acumen Pharmaceutical, personal fees from Cerecin, personal fees from Dolby Family Ventures, personal fees from Eli Lilly, personal fees from Merck Sharp & Dohme Corp., personal fees from National Institute on Aging (NIA), personal fees from Nestle/Nestec, personal fees from PCORI/PPRN, personal fees from Roche, personal fees from University of Southern California (USC), personal fees from NervGen, personal fees from Baird Equity Capital, personal fees from BioClinica, personal fees from Cytox, personal fees from Duke University, personal fees from Eisai, personal fees from FUJIFILM-Toyama Chemical (Japan), personal fees from Garfield Weston, personal fees from Genentech, personal fees from Guidepoint Global, personal fees from Indiana University, personal fees from Japanese Organization for Medical Device Development, Inc. (JOMDD), personal fees from Medscape, personal fees from Peerview Internal Medicine, personal fees from Roche, personal fees from T3D Therapeutics, personal fees from WebMD, personal fees from Vida Ventures, personal fees from The Buck Institute for Research on Aging, personal fees from China Association for Alzheimer’s Disease (CAAD), personal fees from Japan Society for Dementia Research, personal fees from Korean Dementia Society, outside the submitted work; and I hold stocks or options with Alzheon Inc., Alzeca, and Anven.
Dr. Nosheny reports grants from NIH, grants from Genentech, Inc., grants from California Department of Public Health during the conduct of the study.
REFERENCES
- 1.Barnes LL, et al. , Change in cognitive function in Alzheimer's disease in African-American and white persons. Neuroepidemiology, 2006. 26(1): p. 16–22. [DOI] [PubMed] [Google Scholar]
- 2.Tang MX, et al. , The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA, 1998. 279(10): p. 751–5. [DOI] [PubMed] [Google Scholar]
- 3.Green RC, et al. , Risk of dementia among white and African American relatives of patients with Alzheimer disease. Jama, 2002. 287(3): p. 329–36. [DOI] [PubMed] [Google Scholar]
- 4.Hendrie HC, et al. , Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. Jama, 2001. 285(6): p. 739–47. [DOI] [PubMed] [Google Scholar]
- 5.Shadlen MF, et al. , Education, cognitive test scores, and black-white differences in dementia risk. J Am Geriatr Soc, 2006. 54(6): p. 898–905. [DOI] [PubMed] [Google Scholar]
- 6.Turner AD, et al. , Perceived Stress and Cognitive Decline in Different Cognitive Domains in a Cohort of Older African Americans. Am J Geriatr Psychiatry, 2017. 25(1): p. 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson RS, et al. , Factors related to racial differences in late-life level of cognitive function. Neuropsychology, 2016. 30(5): p. 517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arentoft A., et al. , Socioeconomic Status and Neuropsychological Functioning: Associations in an Ethnically Diverse HIV+ Cohort. Clin Neuropsychol, 2015. 29(2): p. 232–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arentoft A., et al. , Multidimensional effects of acculturation on English-language neuropsychological test performance among HIV+ Caribbean Latinas/os. J Clin Exp Neuropsychol, 2012. 34(8): p. 814–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivera Mindt M., et al. , The Neuropsychological Norms for the U.S.-Mexico Border Region in Spanish (NP-NUMBRS) Project: Overview and considerations for life span research and evidence-based practice. Clin Neuropsychol, 2021. 35(2): p. 466–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamar M., et al. , Cardiovascular disease risk factor burden and cognition: Implications of ethnic diversity within the Hispanic Community Health Study/Study of Latinos. PLoS One, 2019. 14(4): p. e0215378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta P., et al. , CDC Grand Rounds: National Amyotrophic Lateral Sclerosis (ALS) Registry Impact, Challenges, and Future Directions. MMWR Morb Mortal Wkly Rep, 2017. 66(50): p. 1379–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thames AD, et al. , Effects of stereotype threat, perceived discrimination, and examiner race on neuropsychological performance: simple as black and white? J Int Neuropsychol Soc, 2013. 19(5): p. 583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell WR, et al. , Association of Neighborhood-Level Disadvantage With Alzheimer Disease Neuropathology. JAMA Netw Open, 2020. 3(6): p. e207559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes LL, et al. , Perceived discrimination and cognition in older African Americans. J Int Neuropsychol Soc, 2012. 18(5): p. 856–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneiderman N., et al. , Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care, 2014. 37(8): p. 2233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haan MN, et al. , Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc, 2003. 51(2): p. 169–77. [DOI] [PubMed] [Google Scholar]
- 18.Vega IE, et al. , Alzheimer's Disease in the Latino Community: Intersection of Genetics and Social Determinants of Health. J Alzheimers Dis, 2017. 58(4): p. 979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fargo KN, et al. , The crisis in recruitment for clinical trials in Alzheimer's and dementia: An action plan for solutions. Alzheimer's & dementia : the journal of the Alzheimer's Association, 2016. 12(11): p. 1113–1115. [DOI] [PubMed] [Google Scholar]
- 20.Shin J and Doraiswamy PM, Underrepresentation of African-Americans in Alzheimer's Trials: A Call for Affirmative Action. Front Aging Neurosci, 2016. 8: p. 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilmore-Bykovskyi AL, et al. , Recruitment and retention of underrepresented populations in Alzheimer's disease research: A systematic review. Alzheimers Dement (N Y), 2019. 5: p. 751–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birkenbihl C., et al. , Evaluating the Alzheimer's disease data landscape. Alzheimers Dement (N Y), 2020. 6(1): p. e12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franzen S., et al. , Diversity in Alzheimer's disease drug trials: The importance of eligibility criteria. Alzheimer's & Dementia, 2022. 18(4): p. 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong R., et al. , Strategies for the Recruitment and Retention of Racial/Ethnic Minorities in Alzheimer Disease and Dementia Clinical Research. Curr Alzheimer Res, 2019. 16(5): p. 458–471. [DOI] [PubMed] [Google Scholar]
- 25.Canevelli M., et al. , Race reporting and disparities in clinical trials on Alzheimer's disease: A systematic review. Neurosci Biobehav Rev, 2019. 101: p. 122–128. [DOI] [PubMed] [Google Scholar]
- 26.Areán PA, et al. , Recruitment and retention of older minorities in mental health services research. Gerontologist, 2003. 43(1): p. 36–44. [DOI] [PubMed] [Google Scholar]
- 27.Bergeron CD, et al. , Clinical trial recruitment in rural South Carolina: a comparison of investigators' perceptions and potential participant eligibility. Rural Remote Health, 2013. 13(4): p. 2567. [PubMed] [Google Scholar]
- 28.Chadiha LA, et al. , Building a Registry of Research Volunteers Among Older Urban African Americans: Recruitment Processes and Outcomes From a Community-Based Partnership. The Gerontologist, 2011. 51(suppl_1): p. S106–S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grill JD, et al. , Attitudes toward Potential Participant Registries. J Alzheimers Dis, 2017. 56(3): p. 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McHenry JC, et al. , Recruitment of older adults: success may be in the details. The Gerontologist, 2015. 55(5): p. 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glover CM, et al. , Facilitators of research registry enrollment and potential variation by race and gender. Journal of clinical and translational science, 2018. 2(4): p. 234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epps FR, Skemp L, and Specht J, Using culturally informed strategies to enhance recruitment of African Americans in dementia research: A nurse researcher's experience. Journal of Research Practice, 2015. 11(1): p. M2–M2. [Google Scholar]
- 33.Jefferson AL, et al. , Clinical research participation among aging adults enrolled in an Alzheimer's Disease Center research registry. J Alzheimers Dis, 2011. 23(3): p. 443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JY, et al. , “If it helps someone, then I want to do it”: Perspectives of persons living with dementia on research registry participation. Dementia, 2020. 19(8): p. 2525–2541. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y., et al. , African Americans are less likely to enroll in preclinical Alzheimer's disease clinical trials. Alzheimers Dement (N Y), 2017. 3(1): p. 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auster J and Janda M, Recruiting older adults to health research studies: a systematic review. Australasian journal on ageing, 2009. 28(3): p. 149–151. [DOI] [PubMed] [Google Scholar]
- 37.Hendrie H., et al. , Apolipoprotein E genotypes and Alzheimer's disease in a community study of elderly African Americans. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 1995. 37(1): p. 118–120. [DOI] [PubMed] [Google Scholar]
- 38.Marquez DX, et al. , Representation of Older Latinxs in Cohort Studies at the Rush Alzheimer’s Disease Center. Neuroepidemiology, 2020. 54(5): p. 404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeki Al Hazzouri A., et al. , Life-course socioeconomic position and incidence of dementia and cognitive impairment without dementia in older Mexican Americans: results from the Sacramento area Latino study on aging. Am J Epidemiol, 2011. 173(10): p. 1148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Miller EE, et al. , Acculturation, Cognitive Performance and Decline, and Incident Dementia/CIND: The Sacramento Area Latino Study on Aging. Am J Epidemiol, 2020. 10(12): p. 84. [Google Scholar]
- 41.González HM, et al. , Neurocognitive function among middle-aged and older Hispanic/Latinos: results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol, 2015. 30(1): p. 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mindt MR, et al. , Aging and HIV/AIDS: neurocognitive implications for older HIV-positive Latina/o adults. Behav Med, 2014. 40(3): p. 116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes LL, et al. , Social resources and cognitive decline in a population of older African Americans and whites. Neurology, 2004. 63(12): p. 2322–6. [DOI] [PubMed] [Google Scholar]
- 44.Avila JF, et al. , Education differentially contributes to cognitive reserve across racial/ethnic groups. Alzheimers Dement, 2021. 17(1): p. 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roosa MW, et al. , Sampling and recruitment in studies of cultural influences on adjustment: a case study with Mexican Americans. J Fam Psychol, 2008. 22(2): p. 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson JL, et al. , Obstacles and opportunities in Alzheimer's clinical trial recruitment. Health Aff (Millwood), 2014. 33(4): p. 574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashford MT, et al. , Effects of sex, race, ethnicity, and education on online aging research participation. Alzheimers Dement (N Y), 2020. 6(1): p. e12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grill JD, et al. , Retention of Alzheimer Disease Research Participants. Alzheimer Dis Assoc Disord, 2019. 33(4): p. 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kennedy RE, et al. , Challenging Assumptions About African American Participation in Alzheimer Disease Trials. Am J Geriatr Psychiatry, 2017. 25(10): p. 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bardach SH, et al. , Genetic Sample Provision Among National Alzheimer's Coordinating Center Participants. J Alzheimers Dis, 2019. 69(1): p. 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bilbrey AC, et al. , The Impact of Latino Values and Cultural Beliefs on Brain Donation: Results of a Pilot Study to Develop Culturally Appropriate Materials and Methods to Increase Rates of Brain Donation in this Under-Studied Patient Group. Clin Gerontol, 2018. 41(3): p. 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moulder KL, et al. , P4-038: Factors influencing lumbar puncture participation in Alzheimer's research. Alzheimer's & Dementia, 2015. 11(7S_Part_17): p. P780–P780. [Google Scholar]
- 53.Gilmore-Bykovskyi A., et al. , Traversing the aging research and health equity divide: Toward intersectional frameworks of research justice and participation. The Gerontologist, 2022. 62(5): p. 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rivera Mindt MO,O; Weiner MW; Veitch DP; Aisen P; Ashford MT; Coker G; Langa KM; Miller G; Petersen R; Raman R; Nosheny RL, Improving generalizability and study design of Alzheimer’s disease clinical trials by including underrepresented populations in Alzheimer’s cohort studies. Alzheimers Dement, 2022. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Israel BA, et al. , Introduction to methods in community-based participatory research for health. Methods in community-based participatory research for health, 2005. 3: p. 26. [Google Scholar]
- 56.Leach V., et al. , Research participation registers can increase opportunities for patients and the public to participate in health services research. J Health Serv Res Policy, 2016. 21(3): p. 183–7. [DOI] [PubMed] [Google Scholar]
- 57.Aisen P., et al. , Registries and Cohorts to Accelerate Early Phase Alzheimer's Trials. A Report from the E.U./U.S. Clinical Trials in Alzheimer's Disease Task Force. J Prev Alzheimers Dis, 2016. 3(2): p. 68–74. [DOI] [PubMed] [Google Scholar]
- 58.Saunders KT, et al. , Arizona Alzheimer's Registry: Strategy and Outcomes of a Statewide Research Recruitment Registry. J Prev Alzheimers Dis, 2014. 1(2): p. 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langbaum JB, et al. , GeneMatch: A novel recruitment registry using at-home APOE genotyping to enhance referrals to Alzheimer's prevention studies. Alzheimers Dement, 2019. 15(4): p. 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong K and Cummings J, Healthybrains.org: From Registry to Randomization. J Prev Alzheimers Dis, 2016. 3(3): p. 123–126. [DOI] [PubMed] [Google Scholar]
- 61.Grill JD, et al. , Constructing a Local Potential Participant Registry to Improve Alzheimer's Disease Clinical Research Recruitment. J Alzheimers Dis, 2018. 63(3): p. 1055–1063. [DOI] [PubMed] [Google Scholar]
- 62.Johnson SC, et al. , The Wisconsin Registry for Alzheimer's Prevention: A review of findings and current directions. Alzheimers Dement (Amst), 2018. 10: p. 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiner MW, et al. , The Brain Health Registry: An internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement, 2018. 14(8): p. 1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langbaum JB, et al. , The Alzheimer's Prevention Registry: A Large Internet-Based Participant Recruitment Registry to Accelerate Referrals to Alzheimer's-Focused Studies. J Prev Alzheimers Dis, 2020. 7(4): p. 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perrin A and Duggan M, Americans' internet access: 2000-2015. 2015. [Google Scholar]
- 66.Smith A., African Americans and technology use: A demographic portrait. Washington, DC: Pew Research Center. 2014. [Google Scholar]
- 67.Brown A, López G, and Lopez MH, Digital divide narrows for Latinos as more Spanish speakers and immigrants go online. 2016. [Google Scholar]
- 68.Center, P.R., Mobile fact sheet. Pew Research Center: Internet. Science & Tech, 2017. [Google Scholar]
- 69.Langbaum JB, et al. , P3-024: THE ALZHEIMER'S PREVENTION REGISTRY'S GENEMATCH PROGRAM: UPDATE ON PROGRESS AND LESSONS LEARNED IN HELPING TO ACCELERATE ENROLLMENT INTO ALZHEIMER'S PREVENTION STUDIES. Alzheimer's & Dementia, 2018. 14(7S_Part_20): p. P1073–P1073. [Google Scholar]
- 70.Mackin RS, et al. , Unsupervised online neuropsychological test performance for individuals with mild cognitive impairment and dementia: Results from the Brain Health Registry. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring, 2018. 10: p. 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yesavage JA, et al. , Development and validation of a geriatric depression screening scale: a preliminary report. Journal of psychiatric research, 1982. 17(1): p. 37–49. [DOI] [PubMed] [Google Scholar]
- 72.Farias ST, et al. , The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology, 2008. 22(4): p. 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nosheny RL, et al. , Online study partner-reported cognitive decline in the Brain Health Registry. Alzheimer's & Dementia: Translational Research & Clinical Interventions, 2018. 4: p. 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ashford MT, et al. , Culturally tailored internet advertising recruitment websites for recruiting Latino participants in a web-based registry: Baseline metrics from the Brain Health Registry. Alzheimer's & Dementia. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ashford MT, et al. , Screening and enrollment of underrepresented ethnocultural and educational populations in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Alzheimer's & Dementia, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watson B., et al. , The inclusion of African-American study participants in web-based research studies. Journal of medical Internet research, 2016. 18(6): p. e5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cowie JM and Gurney ME, The use of Facebook advertising to recruit healthy elderly people for a clinical trial: baseline metrics. JMIR research protocols, 2018. 7(1): p. e7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wozney L., et al. , Facebook ads to the rescue? Recruiting a hard to reach population into an Internet-based behavioral health intervention trial. Internet interventions, 2019. 17: p. 100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bour C., et al. , The Use of Social Media for Health Research Purposes: Scoping Review. Journal of Medical Internet Research, 2021. 23(5): p. e25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brewer LC, et al. , Back to the future: achieving health equity through health informatics and digital health. JMIR mHealth and uHealth, 2020. 8(1): p. e14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.García AA, Zuñiga JA, and Lagon C, A personal touch: The most important strategy for recruiting Latino research participants. Journal of Transcultural Nursing, 2017. 28(4): p. 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hall LN, et al. , Promoting retention: African American older adults in a research volunteer registry. Gerontology and Geriatric Medicine, 2016. 2: p. 2333721416677469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gelman CR, Learning from recruitment challenges: Barriers to diagnosis, treatment, and research participation for Latinos with symptoms of Alzheimer's disease. Journal of gerontological social work, 2010. 53(1): p. 94–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Massett HA, et al. , Facilitators, Challenges, and Messaging Strategies for Hispanic/Latino Populations Participating in Alzheimer’s Disease and Related Dementias Clinical Research: A Literature Review. Journal of Alzheimer's Disease, 2021(Preprint): p. 1–21. [DOI] [PubMed] [Google Scholar]


