Highlights
-
•
A reduced dose strategy to subclinical nodal regions may be feasible in HPV-associated OPC.
-
•
We simulated the effects of this de-escalation strategy.
-
•
This de-escalation plans generally met the dose constraints for the targets and all OAR.
-
•
This de-escalation strategy prevents a QOL decline, particularly xerostomia, dysphagia, and hypothyroidism.
Keywords: Intensity-modulated radiotherapy, Human papillomavirus, Oropharyngeal cancer, Adverse effects, NTCP
Abstract
Introduction
In this simulation study, we examined the effects of a de-escalation strategy with a reduced dose to subclinical nodal regions in patients with human papillomavirus (HPV)-associated oropharyngeal carcinoma (OPC).
Methods
We created two patterns of intensity-modulated radiotherapy for 16 patients with HPV-associated OPC. In the standard and de-escalation plans, the initial field including elective nodal regions received 46 and 30 Gy, followed by 20 and 36 Gy to the cutdown field, respectively. Comparison metrics were set for each organ at risk (OAR). We compared these metric values and the probability of adverse effects based on the normal tissue complication probability (NTCP) model between the two plans.
Results
Both plans generally met the dose constraints for the targets and all OAR. Among the comparison metrics, the mean doses to the brain, pharyngeal constrictor muscle, thyroid, and skin and the dose to a 1 % volume of the skin were higher in the standard plan than in the de-escalation plan (P = 0.031, 0.007, < 0.001, < 0.001, and 0.006, respectively). NTCP analyses revealed that the probability of adverse effects in the ipsilateral parotid gland and thyroid was higher in the standard plan than in the de-escalation plan (standard vs. de-escalation plans: ipsilateral parotid gland, 6.4 % vs. 5.0 %, P = 0.016; thyroid, 3.3 % vs. 0.5 %, P < 0.001).
Conclusions
A de-escalation strategy with elective nodal regions is a promising treatment to prevent a decline in the quality of life in patients with HPV-associated OPC, particularly xerostomia, dysphagia, and hypothyroidism.
Introduction
Patients with head and neck cancer (HNC) may develop various adverse effects (AE) during and after radiotherapy (RT) [1], [2], [3], [4]. RT-related AE are closely associated with the irradiated doses delivered to each organ at risk (OAR) [5]. One of the major AE compromising quality of life (QOL) is xerostomia, which depends on irradiated doses to the parotid glands [6]. Xerostomia may trigger mucositis, gum disease, dental caries, taste disorders, and dysphagia [7]. The doses irradiated to the pharyngeal constrictor muscle have also been associated with the development of dysphagia [8], [9]. Intensity-modulated radiation therapy (IMRT) may reduce doses to OAR to lower than those with 3-dimensional conformal RT (3DCRT) due to its highly conformal dose distribution. However, many HNC patients still develop AE in the era of IMRT [10], [11].
A dose of 66–70 Gy is generally prescribed to tumors, while a prophylactic dose of 46–50 Gy is delivered to elective nodal regions in HNC patients [12], [13]. RT to elective nodal regions has a major impact on the doses delivered to many critical OAR, such as the parotid glands, pharyngeal constrictor muscle, larynx, and thyroid, all of which contribute to post-treatment QOL. Prospective trials showed that the risk of regional failure was not increased by the delivery of 40 Gy to elective nodal regions in HNC patients [14], [15]. Human papillomavirus (HPV)-associated oropharyngeal cancer (OPC) is more likely to be diagnosed in younger patients and has a favorable prognosis [16]. Therefore, a reduction in late AE and the maintenance of QOL are important in HPV-associated OPC patients. A retrospective study recently demonstrated that a systematic de-escalation to 30 Gy to elective nodal regions was associated with minimal toxicity and good locoregional control in HPV-associated OPC [17]. However, this study had a short observation period of approximately 2 years. In this simulation study, we examined the effects of this de-escalation strategy in HPV-associated OPC.
Materials & methods
Patient selection
We selected 16 patients with HPV-associated OPC who were treated with IMRT in our institution so that there were approximately equal numbers of patients with N0, N1, and N2-3. All patients received concurrent chemoradiotherapy (CCRT) with cisplatin between 2019 and 2021. Table 1 shows the primary sites and stages for 16 patients according to the eighth edition of the American Joint Committee on Cancer (AJCC) staging manual in the section on HPV-positive OPC [18]. All patients were non-smokers and had p16-positive, T1-4 N0-3 M0 cancer. All primary sites were the tonsils. The median age of patients was 63 years (range, 42–78). Eleven patients were male and five were female. None of the simulation plans were used for actual clinical RT plans. The present study was performed after approval by the Institutional Review Board.
Table 1.
Patient characteristics.
| Case | Primary site | T | N | Stage |
|---|---|---|---|---|
| 1 | Lt tonsil | 2 | 0 | I |
| 2 | Rt tonsil | 1 | 0 | I |
| 3 | Lt tonsil | 1 | 0 | I |
| 4 | Lt tonsil | 2 | 0 | I |
| 5 | Rt tonsil | 2 | 0 | I |
| 6 | Lt tonsil | 2 | 1 | I |
| 7 | Rt tonsil | 2 | 1 | I |
| 8 | Lt tonsil | 2 | 1 | I |
| 9 | Rt tonsil | 2 | 1 | I |
| 10 | Lt tonsil | 3 | 1 | II |
| 11 | Rt tonsil | 2 | 2 | II |
| 12 | Lt tonsil | 2 | 2 | II |
| 13 | Lt tonsil | 3 | 2 | II |
| 14 | Rt tonsil | 3 | 2 | II |
| 15 | Rt tonsil | 4 | 2 | III |
| 16 | Lt tonsil | 2 | 3 | III |
Lt, left; Rt, right.
Treatment volumes and normal structures
All patients were immobilized with a shell in a supine position and simulated by computed tomography (CT) with a slice thickness of 2.5 mm. The targets and OAR were contoured using the RayStation treatment planning system (RaySearch Medical Laboratories AB, Stockholm, Sweden).
An adaptive two-step IMRT method was used. So, simulation CT scans and IMRT planning were performed twice. The targets were named as target-1 in the initial plan and as target-2 in the cutdown field plan. Gross tumor volume-1 (GTV-1) and GTV-2 were defined as all known gross primary tumor and nodes according to clinical information, CT, magnetic resonance imaging, and 18F-fluorodeoxyglucose positron emission tomography/CT. Clinical target-1 (CTV-1) included GTV-1 with an additional margin of 0.5 cm and elective nodal regions of bilateral levels II, III, IVa, V, and VIIa [19]. Planning target-1 (PTV-1) was defined as CTV-1 plus a 0.5-cm margin, excluding the skin, which was defined as the surface of the body plus 0.2-cm margins in an inward direction. CTV-2 included GTV-2 with an additional margin of 0.5 cm. PTV-2 was defined as CTV-2 plus a 0.5-cm margin excluding the skin. OAR included the spinal cord, brain, brainstem, inner ear, parotid glands, oral cavity, mandible, pharyngeal constrictor muscle, larynx, thyroid, and skin and were delineated according to consensus guidelines [20]. The planning organs at risk volume (PRV) was used for the spinal cord, brain, and brainstem and was defined as the corresponding structure plus 0.3-cm margins.
IMRT planning
Plans were optimized to achieve a target coverage with 95 % of PTV-1 and PTV-2 receiving 100 % of the prescription dose, and to provide a dose distribution that was as homogenous as possible within the target. Plans were optimized using dose-volume histogram (DVH) dose constraints to reduce the dose to OAR to as low as achievable while maintaining target coverage. The prescribed doses to PTV-1 and PTV-2 were simulated for a total of 66 Gy over 33 fractions in 7 weeks. Dose constraints are shown in Appendix Table 1. IMRT plans were generated using 6-MV X-ray beams of Radixact X9 (Accuray, Sunnyvale, USA). The Precision Treatment Planning System (Accuray, Sunnyvale, USA) was used with the superposition algorithm for plan calculations. The calculation grid was 1.0 × 1.0 mm. A 2.5-cm field width, pitch of 0.287, and modulation factor of 2.0 were used.
The prescribed doses of 66 Gy to PTV-1 and PTV-2 were extracted and two patterns of IMRT plans were created as follows: standard plans; 46 Gy in 23 fractions to PTV-1, followed by 20 Gy in 10 fractions to PTV-2: de-escalation plans; 30 Gy in 15 fractions to PTV-1, followed by 36 Gy in 18 fractions to PTV-2.
NTCP model
The Lyman-Kutcher-Burman (LKB) model was used in analyses of a normal tissue complication probability (NTCP) [21], [22]. The probabilities of AE at the parotid glands (xerostomia), mandible (joint dysfunction), larynx (laryngeal edema), thyroid (hypothyroidism), and skin (ulceration) were calculated using RayBiology (RaySearch Medical Laboratories AB, Stockholm, Sweden). AE noted in brackets were the endpoint for each OAR. The NTCP based on LKB model can be estimated by the equation as follows.
| (1) |
| (2) |
| (3) |
Where, D is total dose, TD50 represents the dose for a homogenous dose distribution to an organ at which 50 % of patients are likely to exhibit defined toxicity within 5 years. n indicates the volume effect of the organ being assessed. m is related to the standard deviation of TD50 and describes the steepness of the dose–response curve. M indicates the total number of voxels. In this study, the parameters to predict the development of the above AE were used as follows [23]: (1) the parotid gland: n = 0.70, m = 0.18, TD50 = 46 Gy; (2) mandible: n = 0.07, m = 0.10, TD50 = 72 Gy; (3) larynx: n = 0.08, m = 0.17, TD50 = 70 Gy; (4) thyroid: n = 0.22, m = 0.26, TD50 = 80 Gy; (5) skin: n = 0.10, m = 0.12, TD50 = 70 Gy. The endpoint for each structure follows the report of Emami et al. and the most clinically important (i.e., severe) endpoint were shown: parotid gland, xerostomia; mandible, joint dysfunction; larynx, laryngeal edema; thyroid, hypothyroidism; skin, ulceration [24]. An α/β ratio of 3.0 Gy was used in NTCP analyses.
Plan comparison criteria
Comparison metrics between the standard and de-escalation plans were as follows: (1) maximal doses for the spinal cord PRV, brain PRV, and brainstem PRV; (2) mean doses (Dmean) for the brain PRV, brainstem PRV, inner ears, parotid glands, oral cavity, mandible, pharyngeal constrictor muscle, larynx, thyroid, and skin; (3) doses to 1 % volumes (D1%) of the spinal cord PRV and skin; (4) doses to a 2 % volume (D2%) of the mandible. The Student’s t-test was employed to compare comparison metrics and AE probabilities with NTCP analyses between the standard and de-escalation plans. All statistical analyses were performed using EZR [25], a graphical user interface for R (version 3.4.1; R Foundation for Statistical Computing, Vienna, Austria). A P-value of < 0.05 was significant.
Results
DVH analysis
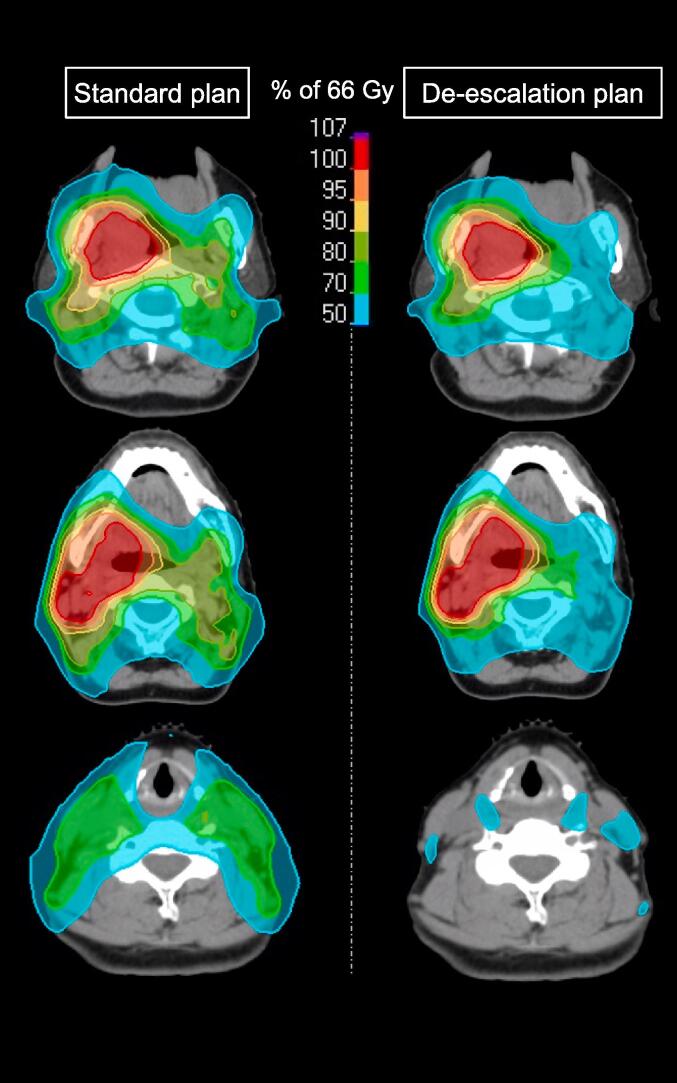
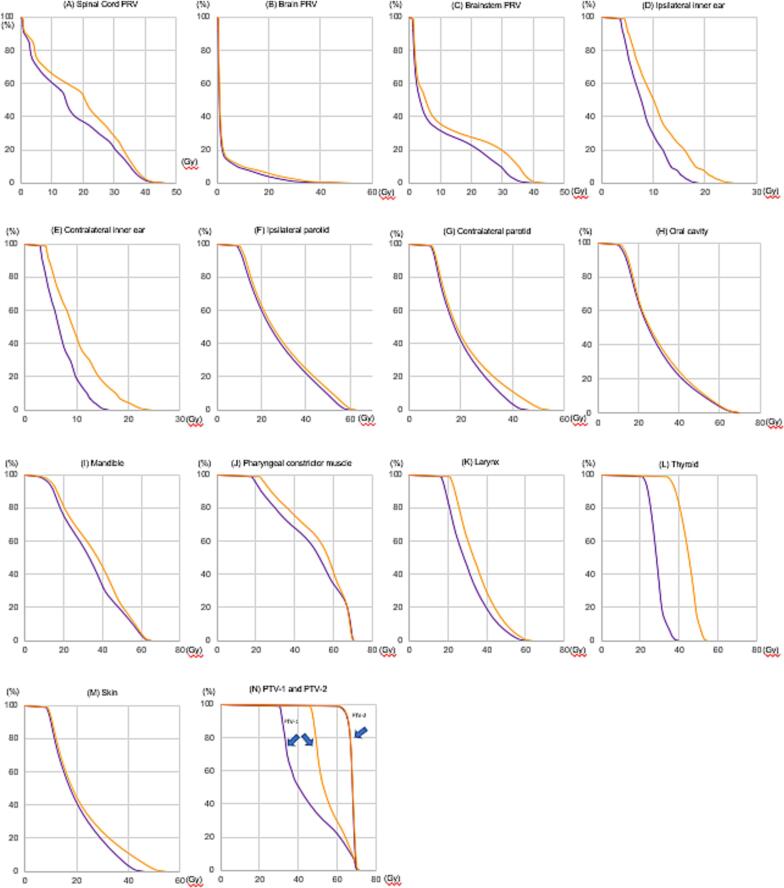
A representative example of dose distributions in the standard and de-escalation plans is shown in Fig. 1. These plans were created in case 7 of Table 1 (primary cancer, T2 tumor at the right tonsil; nodal metastasis, N1 at right level II). In Fig. 1, the spread of 70–80 % doses to the contralateral and lower nodal regions was less in the de-escalation plan than in the standard plan. Fig. 2 shows DVH comparisons of OAR and PTV between the two plans. DVH are presented as an average of 16 patients. When DVH were comprehensively evaluated, those of the spinal cord PRV, brainstem PRV, bilateral inner ears, contralateral parotid glands, mandible, pharyngeal constrictor muscle, larynx, thyroid, and skin were slightly lower in the de-escalation plan than in the standard plan, while DVH of the brain, ipsilateral parotid gland, and oral cavity appeared to be similar between the two plans.
Fig. 1.
Examples of dose distributions in standard and de-escalation plans. The stage of this case was T2N1M0. The primary cancer was at the right tonsil and nodal metastasis at right level II.
Fig. 2.
Comparison of the dose-volume histogram (DVH) of organs at risk (OAR) between standard and de-escalation plans. (A) Planning organ at risk volume (PRV) of the spinal cord; (B) brain PRV; (C) brainstem PRV; (D) ipsilateral inner ear; (E) contralateral inner ear; (F) ipsilateral parotid; (G) contralateral parotid; (H) oral cavity; (I) mandible; (J) pharyngeal constrictor muscle; (K) larynx; (L) thyroid; (M) skin; and (N) planning target volume-1 (PTV-1) and PTV-2.
Quantitative analysis
The results of a quantitative analysis of PTV are shown in Appendix Table 2. In view of the dose constraints shown in Appendix Table 1, PTV-2 coverage and homogeneity met the constraint goals at least within acceptable ranges in both plans and were similar between the two plans. Since the prescribed doses to PTV-1 were different between the two plans, PTV-1 coverage naturally differed between the two plans.
Table 2 shows the comparison metric values of OAR. Dmean of the brain PRV, pharyngeal constrictor muscle, thyroid, and skin were significantly higher in the standard plan than in the de-escalation plan (P = 0.031, 0.007, < 0.001, and < 0.001). Dmean of the larynx tended to be higher in the standard plan than in the de-escalation plan (P = 0.085). Regarding other parameters, D1% of the skin was significantly higher in the standard plan than in the de-escalation plan (P = 0.006).
Table 2.
Doses to organs at risk.
| Structure | Parameter | Standard plan | De-escalation plan | P-value |
|---|---|---|---|---|
| Spinal cord PRV | Dmax | 47.0 ± 2.1 | 46.5 ± 3.0 | 0.61 |
| D1% | 42.0 ± 1.8 | 41.4 ± 2.4 | 0.60 | |
| Brain PRV | Dmax | 53.7 ± 7.4 | 49.6 ± 11.6 | 0.27 |
| Dmean | 3.6 ± 0.8 | 2.9 ± 0.9 | 0.031 | |
| Brainstem PRV | Dmax | 44.2 ± 4.9 | 40.6 ± 8.1 | 0.15 |
| Dmean | 11.8 ± 3.4 | 9.9 ± 3.5 | 0.14 | |
| Ipsilateral inner ear | Dmean | 10.9 ± 5.8 | 8.3 ± 4.7 | 0.20 |
| Contralateral inner ear | Dmean | 10.2 ± 6.1 | 7.6 ± 4.7 | 0.19 |
| Ipsilateral parotid gland | Dmean | 28.8 ± 9.9 | 27.5 ± 9.5 | 0.70 |
| Contralateral parotid gland | Dmean | 22.7 ± 5.7 | 19.8 ± 5.2 | 0.16 |
| Oral cavity | Dmean | 29.9 ± 3.9 | 28.9 ± 4.4 | 0.49 |
| Mandible | D2% | 61.9 ± 6.8 | 61.1 ± 8.2 | 0.80 |
| Pharyngeal constrictor muscle | Dmean | 52.8 ± 2.7 | 48.9 ± 4.2 | 0.007 |
| Larynx | Dmean | 35.3 ± 6.1 | 30.5 ± 8.3 | 0.085 |
| Thyroid | Dmean | 44.9 ± 5.3 | 30.3 ± 4.5 | < 0.001 |
| Skin | Dmean | 8.0 ± 1.1 | 6.2 ± 1.1 | < 0.001 |
| D1% | 48.7 ± 5.4 | 40.4 ± 9.3 | 0.006 | |
| Body | Dmax | 71.7 ± 1.2 | 71.1 ± 1.0 | 0.12 |
Values are the mean ± standard deviation.
Abbreviations: Dmax, maximum dose; Dmean, mean dose; PRV, planning risk volume; D1% or D2%, dose to a 1% or 2% volume of organs at risk; Lt, left; Rt, right.
Fig. 3 shows the rates of reductions in comparison metric values between the two plans, which were shown separately due to the status of nodal metastasis. When the rates of reductions in comparison metric values were comprehensively evaluated, the largest decrease was observed in Dmean of the thyroid with 33 %, followed by the contralateral and ipsilateral inner ears with 24 and 25 %, the skin with 23 %, and then D1% of the skin with 17 % and Dmean of the larynx with 14 %. In addition, the rates of reductions in comparison metric values were slightly larger in N0 patients than in N1 and N2-3 patients.
Fig. 3.
Differences in percent reductions in comparison metrics of each OAR between standard and de-escalation plans according to the stage of nodal metastases. * means D1%.
NTCP analysis
Table 3 shows the probabilities of AE in the parotid glands, mandible, larynx, thyroid, and skin. The probability of xerostomia due to irradiation to the ipsilateral parotid gland was significantly higher in the standard plan than in the de-escalation plan (6.4 % vs. 5.0 %, P = 0.016). The probability of xerostomia of the contralateral parotid gland was similar between the two plans (0.1 % vs. 0.1 %, P = 1.0). The probability of hypothyroidism was significantly higher in the standard plan than in the de-escalation plan (3.3 % vs. 0.5 %, P < 0.001). The probabilities of AE in the mandible, larynx, and skin did not significantly differ between the two plans.
Table 3.
Probabilities of adverse events using the normal tissue complication probability (NTCP) model.
| Structure | Endpoint | Standard plan (%) | De-escalation plan (%) | P-value |
|---|---|---|---|---|
| Ipsilateral parotid gland | Xerostomia | 6.4 ± 12.0 | 5.0 ± 10.5 | 0.016 |
| Contralateral parotid gland | Xerostomia | 0.1 ± 0.3 | 0.1 ± 0.5 | 1.0 |
| Mandible | Joint dysfunction | 0.7 ± 0.7 | 0.6 ± 0.6 | 0.16 |
| Larynx | Laryngeal edema | 6.4 ± 8.2 | 5.8 ± 7.0 | 0.17 |
| Thyroid | Hypothyroidism | 3.3 ± 1.6 | 0.5 ± 0.9 | < 0.001 |
| Skin | Ulceration | 0 | 0 | NA |
Discussion
This simulation study investigated the effects of a reduced dose to elective nodal regions in HPV-associated OPC. RT is an integral component in the treatment of HPV-associated OPC patients. However, various types of RT-related AE and decreased QOL are serious issues [26]. Xerostomia may trigger taste disorders and dysphagia during RT. Mucositis due to irradiation remains a critical issue during RT, even in the era of IMRT [27]. Furthermore, the management of radiation dermatitis is frequently challenging. Regarding late AE, xerostomia and dysphagia are the two of the main AE, and others, such as mandibular osteomyelitis, hypothyroidism, and skin ulcers, may occur after RT [28]. Younger patients with HPV-associated OPC may develop very late AE, such as carotid artery stenosis, in the near future [29].
Radiosensitivity-adjusted doses may improve QOL without compromising oncologic outcomes. Clinical trials showed that the total dose delivered to HPV-associated OPC patients may be safely reduced to approximately to 60 Gy or lower [30], [31], [32], [33], [34], [35]. These trials did not systematically evaluate radiation doses in elective nodal regions. Among total doses of 66–70 Gy, a prophylactic dose of 46–50 Gy is typically delivered to elective nodal regions in HNC patients. These prophylactic doses have been used and validated since the era of 2-dimensional RT or 3DCRT [1], [2], [3], [4]. The findings of 2 recent prospective trials using 40-Gy elective neck volumes revealed uncompromised locoregional control regardless of the anatomical site of disease and the HPV status [14], [15]. We consider radiation dose reductions in line with RT sensitivity to be fully applicable to elective nodal regions. In a recent retrospective study with a median follow-up of 26 months, a systematic de-escalation to 30 Gy to elective nodal regions was associated with minimal toxicity and excellent locoregional control in HPV-associated OPC [17]. This study group reported their dose prescriptions and briefly explained the rationale behind these choices [36]. However, to the best of our knowledge, a quantitative analysis of OAR or AE predictions for the de-escalation strategy have not yet been conducted. Therefore, we performed this simulation study to estimate the effects of reduced doses to elective nodal regions in HPV-associated OPC.
The present results demonstrated that Dmean of the brain, pharyngeal constrictor muscle, thyroid, and skin were significantly lower in the de-escalation plan than in the standard plan. However, since Dmean of the brain was sufficiently low in both groups (standard plan vs. de-escalation plan: 3.6 Gy vs. 2.9 Gy), this difference was not considered to contribute to late AE or post-treatment QOL. Therefore, NTCP analyses of the brain were omitted. NTCP analyses of other OAR, such as the spinal cord, brainstem, and bilateral inner ears, were also omitted for similar reasons. The three parameters of TD50, n, and m in the LKB model were unclear for the oral cavity and pharyngeal constrictor muscle; therefore, NTCP analyses were not performed. However, Dmean of the pharyngeal constrictor muscle were significantly different and high in both groups (standard plan vs. de-escalation plan: 52.8 Gy vs. 48.9 Gy, P < 0.007), which may contribute to the development of late dysphagia. In Fig. 3, the largest decrease was Dmean of the thyroid with 33 % (standard plan vs. de-escalation plan: 44.9 Gy vs.30.3 Gy, P < 0.001), followed by the contralateral and ipsilateral inner ears with 24 and 25 %. Nevertheless, since Dmean of the bilateral inner ears was sufficiently low in both groups, these differences did not contribute to late AE. Although the merit of the de-escalation strategy for subclinical nodal regions was generally more apparent in N0 patients than in N1 and N2-3 patients, as shown in Fig. 3, this treatment strategy is expected to reduce OAR doses and potential AE, even in N1 and N2-3 patients.
NTCP analyses showed that the probabilities of xerostomia at the ipsilateral parotid gland and hypothyroidism were significantly higher in the standard plan than in the de-escalation plan (xerostomia, 6.4 % vs. 5.0 %, P = 0.016; hypothyroidism, 3.3 %vs. 0.5 %, P < 0.001). Although the DVH of the contralateral parotid gland and mandible differed between the two plans, as shown in Fig. 2G and 2I, the probabilities of AE at these OAR did not significantly differ in NTCP analyses. These results were attributed to the low irradiated doses delivered to these OAR in both plans. The probabilities of laryngeal edema did not significantly differ between the two plans in NTCP analyses. However, Dmean of the larynx and the probability of laryngeal edema were lower in the de-escalation plan than in the standard plan (standard plan vs. de-escalation plan: 35.3 Gy vs. 30.5 Gy, P = 0.085; 6.4 % vs. 5.8 %, P = 0.17). Therefore, the de-escalation strategy for subclinical nodal regions may reduce potential AE at the larynx. Since Dmean and D1% of the skin were low in both groups, the probabilities of skin ulceration were 0 % in both plans in NTCP analyses. However, if the skin endpoint was changed, such as to subcutaneous induration, a difference may have been observed in the incidence of AE between the two groups because of the marked difference in the skin dose between the two groups.
The present study had some limitations. First, since this was a simulation study, a prospective study is needed in the future to confirm the efficacy of this strategy. Second, if IMRT plans is created in a different way than ours, e.g., for total dose or target margin, the results may be slightly different. Third, when the NTCP model is analyzed by methods other than LKB model, the results may be slightly different.
In conclusion, we estimated the effects of a de-escalation dose plan for elective nodal regions in HPV-associated OPC. We compared the dosimetric values and the AE probability based on the NTCP model between the standard and de-escalation plans. A de-escalation strategy with a reduced dose to elective nodal regions is a promising treatment to prevent a decline in QOL in patients with HPV-associated OPC, particularly xerostomia, dysphagia, and hypothyroidism.
Waiver of patient consent
This is a retrospective case study. Patient consent has been waived by Ethic committee.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Funding
This study is supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 19K08183.
Appendix Table 1. Dose constraints for targets and organs at risk
| Structure | Parameter | Constraint goal | Acceptable range |
|---|---|---|---|
| PTV1, PTV2 | D98% | >61.4 Gy (93 %) | >59.4 Gy (90 %) |
| D95% | =66 Gy (100 %) | >63.4 Gy (96 %) | |
| D50% | <69.3 Gy (105 %) | <70.6 Gy (107 %) | |
| D10% | <72.6 Gy (110 %) | <75.9 Gy (115 %) | |
| D2% | <79.2 Gy (120 %) | <82.5 Gy (125 %) | |
| Body | Dmax dose | <82.5 Gy (125 %) | <85.8 Gy (130 %) |
| Spinal cord PRV | Dmax | <50 Gy | <54 Gy |
| D1cc | <46 Gy | <50 Gy | |
| Brain PRV | Dmax | <66 Gy | <70 Gy |
| Brainstem PRV | Dmax | <54 Gy | <60 Gy |
| Lt or Rt parotid gland | Dmean | <26 Gy | <30 Gy |
| Oral cavity | Dmean | <30 Gy | <40 Gy |
| Mandible | D2% | <66 Gy | <70 Gy |
| Pharyngeal constrictor muscle | Dmean | <54 Gy | <60 Gy |
| Larynx | Dmean | <45 Gy | <50 Gy |
| Lt or Rt inner ear | Dmean | <45 Gy | <50 Gy |
| Thyroid | Dmean | – | – |
Abbreviations: PTV, planning target volume; PRV, planning risk volume; Lt, left; Rt, right;
Dmax, maximum dose; Dmean, mean dose; D2, 10, 50, 95, 98 %, dose to a 2, 10, 50, 95, 98 %.
volume of the target or organ at risk; D1cc, dose to a 1 cc volume of the target or organ at risk.
Appendix Table 2. Target coverage and homogeneity
| Parameter | Standard plan (Gy) | De-escalation plan (Gy) | P-value |
|---|---|---|---|
| PTV1 | |||
| D98% | 46.2 ± 0.7 | 30.5 ± 0.6 | < 0.001 |
| D95% | 47.0 ± 0.8 | 31.0 ± 0.7 | < 0.001 |
| D50% | 52.8 ± 4.5 | 40.1 ± 8.7 | < 0.001 |
| D10% | 67.6 ± 2.2 | 66.9 ± 3.7 | 0.52 |
| D2% | 69.8 ± 0.7 | 69.5 ± 0.7 | 0.27 |
| PTV2 | |||
| D98% | 62.8 ± 1.4 | 62.2 ± 1.7 | 0.42 |
| D95% | 64.6 ± 0.7 | 64.4 ± 0.9 | 0.47 |
| D50% | 68.0 ± 0.4 | 67.9 ± 0.4 | 0.61 |
| D10% | 69.6 ± 0.4 | 69.3 ± 0.5 | 0.13 |
| D2% | 70.3 ± 0.4 | 69.9 ± 0.6 | 0.14 |
Values are the mean ± standard deviation.
Abbreviations D2, 10, 50, 95, and 98, dose to 2, 95, and 98, volume of the target; PTV, planning target volume.
References
- 1.Goto Y., Kodaira T., Fuwa N., Mizoguchi N., Nakahara R., Nomura M., et al. Alternating chemoradiotherapy in patients with nasopharyngeal cancer: prognostic factors and proposal for individualization of therapy. J Radiat Res. 2013;54(1):98–107. doi: 10.1093/jrr/rrs071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakahara R., Kodaira T., Furutani K., Tachibana H., Tomita N., Inokuchi H., et al. Treatment outcomes of definitive chemoradiotherapy for patients with hypopharyngeal cancer. J Radiat Res. 2012;53(6):906–915. doi: 10.1093/jrr/rrs052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ichimiya Y., Fuwa N., Kamata M., Kodaira T., Furutani K., Tachibana H., et al. Treatment results of stage I oral tongue cancer with definitive radiotherapy. Oral Oncol. 2005;41(5):520–525. doi: 10.1016/j.oraloncology.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Tomita N., Kodaira T., Furutani K., Tachibana H., Hasegawa Y., Terada A., et al. Long-term follow-up and a detailed prognostic analysis of patients with oropharyngeal cancer treated with radiotherapy. J Cancer Res Clin Oncol. 2010;136(4):617–623. doi: 10.1007/s00432-009-0700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dirix P., Nuyts S. Evidence-based organ-sparing radiotherapy in head and neck cancer. Lancet Oncol. 2010;11(1):85–91. doi: 10.1016/S1470-2045(09)70231-1. [DOI] [PubMed] [Google Scholar]
- 6.Deasy J.O., Moiseenko V., Marks L., Chao K.S., Nam J., Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S58–S63. doi: 10.1016/j.ijrobp.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore C., McLister C., Cardwell C., O'Neill C., Donnelly M., McKenna G. Dental caries following radiotherapy for head and neck cancer: A systematic review. Oral Oncol. 2020;100 doi: 10.1016/j.oraloncology.2019.104484. [DOI] [PubMed] [Google Scholar]
- 8.Grepl J, Sirak I, Vosmik M, Tichy A. The Changes in Pharyngeal Constrictor Muscles Related to Head and Neck Radiotherapy: A Systematic Review Technol Cancer Res Treat. 2020;19, 1533033820945805. 10.1177/1533033820945805. [DOI] [PMC free article] [PubMed]
- 9.Dirix P., Abbeel S., Vanstraelen B., Hermans R., Nuyts S. Dysphagia after chemoradiotherapy for head-and-neck squamous cell carcinoma: dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2009;75(2):385–392. doi: 10.1016/j.ijrobp.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 10.Daly M.E., Le Q.T., Maxim P.G., Loo B.W., Jr., Kaplan M.J., Fischbein N.J., et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys. 2010;76(5):1339–1346. doi: 10.1016/j.ijrobp.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Kodaira T., Tomita N., Tachibana H., Nakamura T., Nakahara R., Inokuchi H., et al. Aichi cancer center initial experience of intensity modulated radiation therapy for nasopharyngeal cancer using helical tomotherapy. Int J Radiat Oncol Biol Phys. 2009;73(4):1129–1134. doi: 10.1016/j.ijrobp.2008.06.1936. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher G.H. Elective irradiation of subclinical disease in cancers of the head and neck. Cancer. 1972;29(6):1450–1454. doi: 10.1002/1097-0142(197206)29:6<1450::aid-cncr2820290605>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 13.Kunieda F., Kiyota N., Tahara M., Kodaira T., Hayashi R., Ishikura S., et al. Randomized phaseII/III trial of post-operative chemoradiotherapy comparing 3-weekly cisplatin with weekly cisplatin in high-risk patients with squamous cell carcinoma of head and neck: Japan Clinical Oncology Group Study (JCOG1008) Jpn J Clin Oncol. 2014;44(8):770–774. doi: 10.1093/jjco/hyu067. [DOI] [PubMed] [Google Scholar]
- 14.Deschuymer S., Nevens D., Duprez F., Daisne J.F., Dok R., Laenen A., et al. Randomized clinical trial on reduction of radiotherapy dose to the elective neck in head and neck squamous cell carcinoma; update of the long-term tumor outcome. Radiother Oncol. 2020;143:24–29. doi: 10.1016/j.radonc.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Sher D.J., Pham N.L., Shah J.L., Sen N., Williams K.A., Subramaniam R.M., et al. Prospective Phase 2 Study of Radiation Therapy Dose and Volume De-escalation for Elective Neck Treatment of Oropharyngeal and Laryngeal Cancer. Int J Radiat Oncol Biol Phys. 2021;109(4):932–940. doi: 10.1016/j.ijrobp.2020.09.063. [DOI] [PubMed] [Google Scholar]
- 16.Ang K.K., Harris J., Wheeler R., Weber R., Rosenthal D.I., Nguyen-Tân P.F., et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai C.J., McBride S.M., Riaz N., Kang J.J., Spielsinger D.J., Waldenberg T., et al. Evaluation of substantial reduction in elective radiotherapy dose and field in patients with human papillomavirus–associated oropharyngeal carcinoma treated with definitive chemoradiotherapy. JAMA Oncol. 2022;8(3):364–372. doi: 10.1001/jamaoncol.2021.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and neck cancers—major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122–137. http://doi.org/10.3322/caac.21389. [DOI] [PubMed]
- 19.Grégoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, et al. Delineation of the neck node levels for head and neck tumors: A 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110(1):172-181. http://doi.org/10.1016/j.radonc.2013.10.010. [DOI] [PubMed]
- 20.Brouwer C.L., Steenbakkers R.J., Bourhis J., Budach W., Grau C., Grégoire V., et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother Oncol. 2015;117(1):83–90. doi: 10.1016/j.radonc.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 21.Lyman JT. Complication probability as assessed from dose-volume histograms Radiat Res Suppl. 1985;8:S13–S19, PMID: 3867079. [PubMed]
- 22.Friedrich T., Grun R., Scholz U., Elsasser T., Durante M., Scholz M. Sensitivity analysis of the relative biological effectiveness predicted by the local effect model. Phys Med Biol. 2013;58(19):6827–6849. doi: 10.1088/0031-9155/58/19/6827. [DOI] [PubMed] [Google Scholar]
- 23.Burman C., Kutcher G.J., Emami B., Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991;21(1):123–135. doi: 10.1016/0360-3016(91)90172-z. [DOI] [PubMed] [Google Scholar]
- 24.Emami B., Lyman J., Brown A., Coia L., Goitein M., Munzenrider J.E., et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(1):109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 25.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura K., Kodaira T., Tomita N., Tachibana H., Makita C., Yoshida M., et al. Clinical results of definitive intensity-modulated radiation therapy for oropharyngeal cancer: retrospective analysis of treatment efficacy and safety. Jpn J Clin Oncol. 2016;46(1):78–85. doi: 10.1093/jjco/hyv157. [DOI] [PubMed] [Google Scholar]
- 27.Kamal M., Peeler C.R., Yepes P., Mohamed A.S.R., Blanchard P., Frank S., et al. Radiation-induced hypothyroidism after radical intensity modulated radiation therapy for oropharyngeal carcinoma. Adv Radiat Oncol. 2019;7;5(1):111–119. doi: 10.1016/j.adro.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried D.V., Das S.K., Shen C., Marks L.B., Chera B.S. Impact of oral cavity dosimetry on patient reported xerostomia and dysgeusia in the setting of deintensified chemoradiotherapy. Adv Radiat Oncol. 2022;25;7(4):100952. doi: 10.1016/j.adro.2022.100952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leboucher A., Sotton S., Gambin Flandrin I., Magné N. Head and neck radiotherapy-induced carotid toxicity: Pathophysiological concepts and clinical syndromes. Oral Oncol. 2022;129 doi: 10.1016/j.oraloncology.2022.105868. [DOI] [PubMed] [Google Scholar]
- 30.Yom S.S., Torres-Saavedra P., Caudell J.J., Waldron J.N., Gillison M.L., Xia P., et al. Reduced-dose radiation therapy for HPV-associated oropharyngeal carcinoma (NRG Oncology HN002) J Clin Oncol. 2021;39(9):956–965. doi: 10.1200/jco.20.03128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seiwert T.Y., Foster C.C., Blair E.A., Karrison T.G., Agrawal N., Melotek J.M., et al. OPTIMA: a phase II dose and volume de-escalation trial for human papillomavirus-positive oropharyngeal cancer. Ann Oncol. 2019;30(2):297–302. doi: 10.1093/annonc/mdy522. [DOI] [PubMed] [Google Scholar]
- 32.Marur S., Li S., Cmelak A.J., Gillison M.L., Zhao W.J., Ferris R.L., et al. E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx— ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2017;35(5):490–497. doi: 10.1200/jco.2016.68.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma D.J., Price K.A., Moore E.J., Patel S.H., Hinni M.L., Garcia J.J., et al. Phase II evaluation of aggressive dose de-escalation for adjuvant chemoradiotherapy in human papillomavirus–associated oropharynx squamous cell carcinoma. J Clin Oncol. 2019;37(22):1909–1918. doi: 10.1200/jco.19.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chera B.S., Amdur R.J., Green R., Shen C., Gupta G., Tan X., et al. Phase II trial of de-intensified chemoradiotherapy for human papillomavirus–associated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2019;37(29):2661–2669. doi: 10.1200/jco.19.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen A.M., Felix C., Wang P.-C., Hsu S., Basehart V., Garst J., et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol. 2017;18(6):803–811. doi: 10.1016/S1470-2045(17)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai C.J., McBride S.M., Riaz N., Lee N.Y. Reducing the radiation therapy dose prescription for elective treatment areas in human papillomavirus–associated oropharyngeal carcinoma being treated with primary chemoradiotherapy at Memorial Sloan Kettering Cancer Center. Pract Radiat Oncol. 2019;9(2):98–101. doi: 10.1016/j.prro.2018.10.015. [DOI] [PubMed] [Google Scholar]