Abstract
Mitochondria are a main source of cellular energy. Oxidative phosphorylation (OXPHOS) is the major process of aerobic respiration. Enzyme complexes of the electron transport chain (ETC) pump protons to generate a protonmotive force (Δp) that drives OXPHOS. Complex I is an electron entry point into the ETC. Complex I oxidizes nicotinamide adenine dinucleotide (NADH) and transfers electrons to ubiquinone in a reaction coupled with proton pumping. Complex I also produces reactive oxygen species (ROS) under various conditions. The enzymatic activities of complex I can be regulated by metabolic conditions and serves as a regulatory node of the ETC. Complex I ROS plays diverse roles in cell metabolism ranging from physiologic to pathologic conditions. Progress in our understanding indicates that ROS release from complex I serves important signaling functions. Increasing evidence suggests that complex I ROS is important in signaling a mismatch in energy production and demand. In this article, we review the role of ROS from complex I in sensing acute hypoxia.
Keywords: Mitochondrial complex I, Oxygen sensing, ROS signaling, Acute hypoxia
Graphical abstract
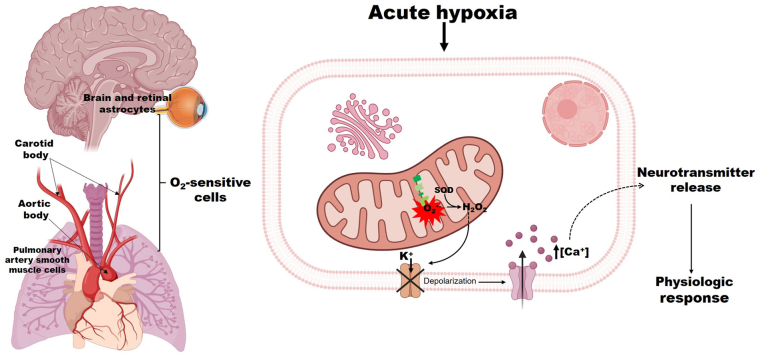
Oxygen sensing in acute hypoxia. The astrocytes in the central nervous system and retina, the glomus cells in the carotid and aortic bodies, and the pulmonary artery smooth muscle cells respond to acute hypoxia-induced alteration in hydrogen peroxide (H2O2) production from complex I. The subsequent increase in intracellular calcium ion concentration [Ca2+] elicits the release of neurotransmitters and other mediators that produce a physiologic response. Created with BioRender.com.
Highlights
-
•
Mitochondria complex I is structurally and functionally dynamic and regulates OXPHOS.
-
•
Complex I ROS is critical in redox and stress signaling.
-
•
Complex I ROS release is key in peripheral and central O2 sensing.
-
•
Reversible modification of conserved cysteine residues in complex I mediates acute hypoxia-induced responses.
1. Introduction
Cellular energy generation is a complex multistep process involving the catabolism of metabolic fuels (e.g., carbohydrates, lipids, and proteins) to produce adenosine 5′ trisphosphate (ATP) [1]. ATP serves as the global energy currency of cells in addition to other functions including signaling. ATP is produced on demand by substrate-level phosphorylation and OXPHOS [2,3]. Mitochondrial complex I NADH:ubiquinone oxidoreductase or NADH dehydrogenase) is an important electron entry point for OXPHOS. Complex I is the first enzyme complex of the ETC. It oxidizes NADH generated by the tricarboxylic acid (TCA) cycle, mitochondrial fatty acid β-oxidation (FAO), and amino acid catabolism in the mitochondrial matrix [[4], [5], [6]]. Following oxidation of NADH, paired electron transfer reduces ubiquinone (UQ) to ubiquinol (UQH2). UQH2 shuttles electrons from complex I to reduce oxygen (O2) to water (H2O). Electron transfer through complex I is coupled with transmembrane proton (H+) pumping, which generates Δp necessary for ATP synthesis (Fig. 1). In addition to regenerating NAD+ following oxidation of NADH, complex I ROS. ROS is a generic term used to define a range of oxidant molecules with a broad spectrum of biological functions including signaling [7]. Hereinafter, ROS refers to superoxide anion (O2•‾), hydrogen peroxide (H2O2) or their derivatives and, when possible, we will refer to the specific type of ROS meditating the response. NAD+ and ROS are important complex I metabolites that play key roles in regulation of cellular metabolism. Complex I adopts structural and conformational changes to regulate ROS production, and modulate the activity of the ETC.
Fig. 1.
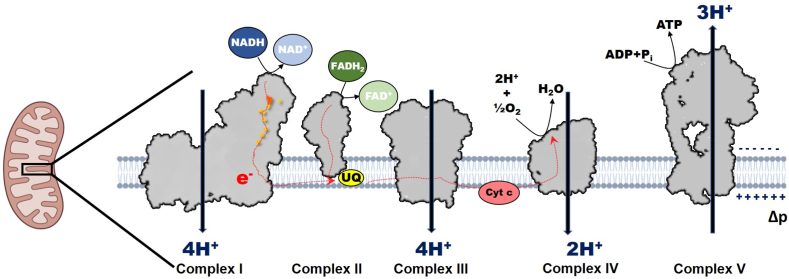
Mitochondrial electron transport chain. Electrons (e−) following oxidation of reducing equivalent (NADH) are transferred through series of iron-sulfur clusters (orange spots) in complex I to reduce ubiquinone (UQ) and pump protons (H+). Complex II oxidizes FADH2 to reduce UQ. Electrons are then transferred by UQ2 to complex III, then to cytochrome c and finally to oxygen (O2) at complex IV. During this process protons pumped by complexes I, III and IV generative protonmotive force (Δp) utilized by complex V for the synthesis of ATP.
Complex I is important in maintaining metabolic homeostasis by sensing and initiating responses to mitochondrial and extramitochondrial stress, such as hypoxia [[8], [9], [10]]. Hypoxia signaling has been shown to involve mitochondrial ROS. Studies now highlight the importance of complex I ROS in acute hypoxia signaling. The role and contribution of complex I to hypoxia signaling is an emerging area of research with potential for therapeutics and management of many disease conditions.
1.1. Oxidative phosphorylation
Mitochondrial ATP production proceeds via coupling series of reduction-oxidation (redox) reactions in the ETC with the phosphorylation machinery through a process known as OXPHOS. Together, OXPHOS consists of the ETC and complex V (ATP synthase, F1F0-ATPase). The ETC consists of four enzyme complexes (complex I – IV) and mobile electron carriers (the hydrophobic UQ and the hydrophilic cytochrome c), which are located in the inner mitochondrial membrane (IMM) [[1], [3], [6]]. These enzyme complexes include complex I, complex II (succinate:ubiquinone oxidoreductase or succinate dehydrogenase), complex III (ubiquinol:cytochrome c oxidoreductase or cytochrome bc1 complex), and complex IV (cytochrome c oxidase) [[1], [3], [6]]. These enzyme complexes catalyze redox reactions, and are involved in generation of the Δp that drives synthesis of ATP by complex V (Fig. 1) [[1], [6]]. This Δp is the driving force for several mitochondrial functions beyond ATP synthesis, such as ROS production, uptake and homeostasis of ions (including calcium Ca2+, sodium Na+, iron Fe2+, magnesium Mg2+, manganese Mn2+) [[11], [12], [13], [14]], import and export of proteins [[15], [16], [17]], biomolecule synthesis [18], and mitochondrial dynamics and modeling [19]. The Δp is critical for many facets of health by influencing cell homeostasis directly or indirectly through the regulating of various processes including but not limited to redox and pH microenvironments, cell proliferation, thermogenesis, immune and inflammatory responses, and apoptosis signaling [[20], [21], [22], [23]].
1.2. ETC redox reactions
Complex I oxidizes NADH to reduce UQ to UQH2 in a proton coupled electron transfer reaction [[24], [25], [26], [27], [28]]. UQ can also be reduced by complex II upon oxidation of succinate, linking the ETC and the TCA cycle. UQ can be reduced by other non-canonical ETC oxidoreductases including electron transfer flavoprotein:ubiquinone oxidoreductase, glycerol 3-phosphate dehydrogenase, proline dehydrogenase, choline dehydrogenase, dihydroorotate dehydrogenase, sulfide:quinone oxidoreductase [[6], [29], [30], [31], [32]]. The redox status of the UQ pool, which is the ratio of UQH2 to UQ serves as a sensor of ETC efficiency. Alterations in the UQ pool redox status is important in complex I ROS production since changes in the UQH2/UQ ratio will affect the direction of electron flow and ROS production [30,33]. UQH2 is oxidized by complex III which, facilitated by the UQ cycle transfers electrons singly to cytochrome c. Complex IV oxidizes cytochrome c, ultimately reducing cellular O2, the terminal electron acceptor to H2O. The difference in the redox potentials of NADH (Eo′=−340 mV) and O2 (Eo′=+810 mV) energetically favors flux of electrons through the ETC from complex I down the electrochemical gradient to complex IV [29,34].
1.3. Generation of protonmotive force (Δp)
Electron flux through complex I, III and IV is coupled with H+ translocation from the matrix across the IMM to the intermembrane space (IMS) [1,3,23]. Complex I and III pump H+ at a stoichiometry of 4H+/2e−, while complex IV pumps at lower stoichiometry of 2H+/2e− [1,3]. The Δp consists of an electrochemical potential (Δψm, of about −120 to −200 mV) and a pH gradient (ΔpH, of 0.3–0.8 pH units) under physiologic conditions [1,[35], [36], [37]]; [23,38]. The Δp acts as a transducer, serving to store energy that drives protons into the proton-conducting channel of F0 domain of complex V. This produces a rotatory motion that is transmitted to the catalytic head of the F1 domain through the central stalk of complex V. Rotation of the catalytic head of F1 domain produces a conformation change that catalyze the phosphorylation of adenosine 5′ diphosphate (ADP) in the presence of orthophosphate to produce ATP [23,39,40]. OXPHOS generates about 90 % of ATP used in cellular metabolism, of which complex I contributes about 40 % of the total Δp [1,2,[1], [2], [6], [41], [42], [43]].
In tissues and individual cells, O2 gradients exist that affect oxidative metabolisms and health [[44], [45], [46]]. Changes in O2 gradient affects ETC function [[47], [48], [49]]. However, OXPHOS can operate under limited O2 as low as 2–10 % O2 (physiologic hypoxia), but is inhibited at near anoxic level, ≤1 % O2 (pathologic hypoxia) [43,47,50,51]. Low O2 tension (<2 %) impedes ETC function and may limit OXPHOS [43,51]. Important here is the “apparent” kM of complex III. While the kM measured for complex IV is in the low micromolar to high nanomolar range, various cellular factors such as prevailing concentrations of nitric oxide can modulate this, such that the apparent kM can be as high as 1–10 μM [52,53]. Dependency on O2 for ATP synthesis necessitates a system of O2 sensing to inform adoption of alternative mode of energy generation such as anaerobic respiration. Failure to quickly meet the energy demand may necessitate induction of physiologic changes such as cardiopulmonary responses including angiogenesis, conservation of energy through metabolic suppression, or behavioral response to avoid anoxic/hypoxic environments [[54], [55], [56], [57]]. Earlier studies have established that complex III is critical in chronic hypoxia signaling [54,58,59]. However, recent studies indicate that acute hypoxia sensing operates through a disparate mechanism that may involve signals upstream of complex III [[60], [61], [62], [63], [64], [65]]. This review highlights recent progress in unveiling the role of complex I in acute hypoxia signaling. Understanding the role of complex I in acute hypoxia signaling is important in unveiling the mechanisms of many pathologies and the discovery of targeted medical intervention for such conditions.
2. Complex I: functional complexity
2.1. Complex I structure
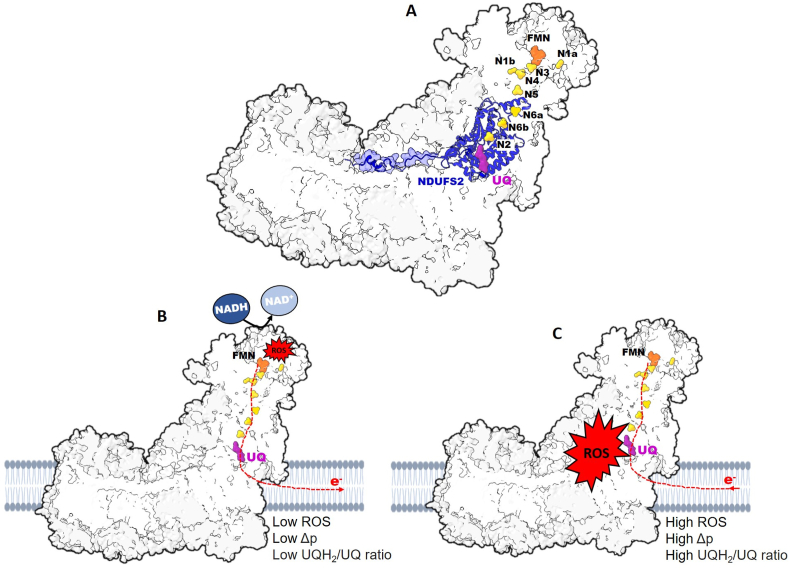
Complex I is the largest (∼980 kDa) of the ETC protein complexes having 45 protein subunits encoded by 44 genes, 37 by the nuclear deoxyribonucleic acid (DNA, nDNA) and 7 by the mitochondrial DNA (mtDNA) [27,28,66]. The 7 mtDNA-encoded subunits (ND1, ND2, ND3, ND4, ND5, ND6 and ND4L), and the 7 nDNA-encoded “core” subunits (NDUFV1, NDUFV2, NDUFS1, NDUFS2, NDUFS3, NDUFS7 and NDUFS8) form the conserved central subunits responsible for the core bioenergetic function of complex I. The remaining proteins (about 30 in mammalian mitochondria), referred to as accessory or supernumerary subunits, are required for assembly and structural stability [27,[66], [67], [68]]. Complex I is structurally asymmetric and has a modular L-shaped architecture consisting of a hydrophilic peripheral matrix arm and a hydrophobic membrane arm. The peripheral matrix arm comprises the N module and Q module, and consists of the 7 nDNA-encoded core subunits [27,67]. The N module contains the NADH docking cavity (site IF), and contains a tightly but noncovalently bound flavin mononucleotide (FMN) cofactor as the electron acceptor. The N module is connected to the Q module, which contains the UQ-binding pocket (site IQ) by a series of iron-sulfur (Fe–S) clusters (N3, N1b, N4, N5, N6a, N6b, N2) that is ∼100 Å long [27,67,69]. The peripheral arm interfaces at an angle of ∼100° with the membrane arm (P module) at the UQ-binding pocket [67]. The membrane arm contains 3 highly hydrophobic proton pumping subunits, ND2, ND4 and ND5; a fourth proton pump is suggested to exist but is yet to be characterized [70].
2.2. NADH:ubiquinone oxidoreduction
Complex I can adopt two structurally and catalytically different states, the active form (A-form), which can reversibly transition to the deactive, dormant form (D-form) [28,[71], [72], [73], [74]]. In the absence of NADH and UQ, complex I slowly but spontaneously transitions to the D-form, whereas with the addition of NADH it rapidly transitions to the A-form catalyzing redox reactions at high rates [28,72]. Oxidation of NADH regenerates NAD+, which rapidly dissociates from the docking site while FMNH2 transfers two electrons to N3 of the Fe–S clusters [69]. The electrons are transferred through the Fe–S clusters of the Q module to reduce UQ via proton-coupled electron transfer reaction. Reduction of UQ occurs at the UQ-binding pocket (site IQ) located at the interface of the peripheral and membrane arms (Fig. 2A). This pocket is ∼20 Å above the membrane interface and within 12 Å of N2. It is accessed by a long narrow channel (Q-channel) long enough to contain most of the approximately 50 Å-long isoprenoid tail of UQ-10 [[75], [76], [77], [78]]. The mechanism of UQ reduction at this site is still under investigation. However, reduction of UQ was recently proposed to occur through a “concerted two-electron two-proton transfer” reaction. Here, one electron comes from N2, and another electron together with a proton comes from the conserved Tyr87 of NDUFS2, while NDUFS2 His38 supply the second proton [77,79]. This overcomes the thermodynamically unfavorable energetics of one-electron sequential reduction of UQ to semiubiquinone radical (UQH•−), and finally to UQH2. A transient tyrosyl radical formed in the reaction is eventually reduced following arrival of an electron from the Fe–S cluster [79]. The flux of electrons upon oxidation of NADH2 to UQH2 describes the catalytic activity of complex I in the forward electron transfer (FET) mode. Operation of complex I in FET is important in maintaining a high NADH/NAD+ ratio and a reduce UQ pool as well as generating Δp (Fig. 2B). Thus, complex I is important in intracellular redox-sensing through NADH/NAD+ ratio.
Fig. 2.
Directional flow of electrons through complex I produce ROS. A Complex I consists of the peripheral and membrane arms. The peripheral arm contains the NADH oxidation site at docking to flavin mononucleotide (FMN, orange) from which, electrons are transferred through a series of iron-sulfur cluster subunits (N1a, N3, N1b, N4, N5, N6a, N6b and N2, yellow) to the ubiquinone (UQ, purple) at the UQ-binding site, neighboring the NDUFS2 subunit. B Electron flow (dashed red lines) from NADH to reduce UQ to UQH2 through forward electron transfer (FET). Low protonmotive force (Δp) and low UQH2/UQ during FET promotes the generation of relatively low level of ROS from FMN (site IF). C Favored by high Δp and high UQH2/UQ, electrons are transferred from reduced UQH2 during reverse electron transfer (RET) to generate bursts of ROS from the UQ-binding site (site IQ).
2.3. UQ binding and proton translocation
Conformational changes in complex I is affected by interaction with other metabolites. For example, the entry and docking of UQ in the binding pocket results in a local structural and conformational change in this region. Electron flux through the Fe–S cluster, and the subsequent UQ reduction chemistry, induces an electrostatic interaction that produces a global conformational change of the peripheral arm [24,70,[80], [81], [82]]. The induced conformational change in the peripheral arm, hydration and protonation of residues at the UQ-binding pocket is propagated to the proton pumps ∼200 Å away in the peripheral arm to drive proton translocation from the matrix across the IMM [24,70,[80], [81], [82], [83], [84]]. UQ reduction at this site can be specifically inhibited using a number of structurally different compounds including piericidin, rotenone, and capsaicin [85]. Inhibition of this site using rotenone stops proton translocation and dissipates Δp [83,84,86]. Additionally, inhibition of UQ reduction results in buildup of electrons, reduction of the Fe–S cluster and leakage of electrons to O2 leading to the formation of ROS [29,87].
Seminal studies by Chance and his group reported that electron flux through complex I in isolated mitochondria can operate conditionally in reverse [[88], [89], [90]]. Here, electrons may backup from the UQ pool in the presence of highly reduced UQ and at the expense of Δp in the reverse electron transfer (RET) mode [72,74,[91], [92], [93]]. Thus, increase in cellular NADH/NAD+ ratio favors RET by complex I (Fig. 2C) [94]. Mitochondria maintain a pool of NAD that is distinct from the rest of the cell, since NAD cannot diffuse across the IMM thus, allowing the NADH/NAD+ ratio to regulate mitochondrial redox homeostasis and OXPHOS [95,96]. A high mitochondrial NADH/NAD+ ratio exerts a negative feedback regulation of glycolytic enzymes, such as glyceraldehyde 3-phosphate dehydrogenase and pyruvate dehydrogenase complex (PDC) [97]. The high mitochondrial NADH/NAD+ ratio also alters the α-ketoglutarate/citrate ratio and limits entry of acetyl-CoA into the TCA cycle. This results in diminution in the generation of NADH, and flavin adenine dinucleotide (FADH2) from TCA cycle [96,97]. Modulation in the generation of reducing equivalents, entry of electrons following oxidation of NADH into the ETC, and maintenance of the NADH/NAD+ ratio makes complex I a “gatekeeper” of OXPHOS. Changes in mitochondrial NADH/NAD+ ratio has been reported during ischemia-reperfusion, dysfunctional cellular metabolism; and it is also a hallmark of diabetes, cancer, neurodegeneration, stroke, and heart failure [[95], [98], [99]]. Many of these pathologies display dysregulated complex I activity, and mitochondrial ROS homeostasis.
3. Complex I ROS
3.1. Production of ROS by complex I
ROS are produced by various enzymatic and non-enzymatic processes in the cell [100,101]. O2 exists as a paramagnetic biradical with two electrons occupying separate π* orbitals with parallel spins. This feature imposes spin restriction, which makes single-electron reduction of O2 thermodynamically favored compared with paired-electron reduction [29,102,103]. Premature single-electron reduction of O2 leads to the production of O2•‾, which undergoes rapid dismutation spontaneously or catalyzed by superoxide dismutase (SOD) into H2O2 [[102], [103], [104], [105], [106]]. Single-electron reduction of H2O2 in the presence of transition metals, such as Fe2+ and Cu2+ yields hydroxyl radical (•OH) via the Fenton reaction [104,[106], [107], [108], [109]]. H2O2 is not a radical, and crosses membranes through aquaporin-mediated diffusion compared with O2•‾ or •OH, which have limited membrane permeability due to a negative charge or reactivity, respectively [29,91,102,105,110]. The pKa of O2•‾ is ∼5, such that at physiologic pH around 1 % is present as perhydroxyl radical (HO2•) which can freely cross membranes, although the importance of this in the overall context of mitochondrial ROS signaling is unclear.
3.2. Sites and modes of ROS production
Among the different (≥41) ROS production sites within the cell, the mitochondrion is a major source of ROS, having up to 16 different sites of ROS production associated with substrate catabolism and OXPHOS [111,112]. Complex I has high ROS production capacity. The FMN-containing NADH docking pocket (site IF) and site IQ are the canonical sites of ROS production [29,91,104,113,114]. Complex I predominantly produce O2•‾, but also can directly produce H2O2 [115]. The ratio of O2•‾ to H2O2 that is produced is influenced by various factors including substrate type and concentration, NADH2/NAD+ ratio, direction of electron flux, supercomplex formation, polarity of the IMM, redox state of the UQ pool, and O2 concentration [115,116]. Low (≤3 μM) NADH results primarily in O2•‾ production, while high (≥50 μM) NADH levels results in ≥60 % of the ROS being produced as H2O2 [115]. Complex I can produce measurable rates of ROS in FET or RET modes. In FET, site IF produces ROS due to leakage of electrons from the reduced flavin (FMNH or FMNH2) to O2. The rate of ROS production depends on high NADH/NAD ratio and the steady-state concentration of FMNH or FMNH2 [29,113,117] (Fig. 2B). However, complex I ROS production capacity by RET is greater [29,91,104,113,114,118]. Conditions that favor ROS production by RET include high UQ2/UQ ratio, and presence of Δp. During ischemia (hypoxia), the ETC is hyperpolarized, succinate accumulates due to high UQ2/UQ ratio and large Δp (Fig. 2C). Upon reperfusion (reoxygenation) there is rapid oxidation of the accumulated succinate leading to rapid ROS burst from complex I [91,[119], [120], [121], [122], [123]]. In RET, both O2•‾ (∼65 %) and H2O2 (∼35 %) are produced [115]. Recent studies show that site IQ is the main contributor to ROS production in RET mode (RET ROS) in different tissues including heart [91,119,121,122,124], brain [125]; [null]; [null], kidney [124], skeletal muscles [113,126], and liver [127]. However, site IF has been reported to also contribute significantly to RET ROS [91,128]. Importantly, in mitochondria oxidizing complex I substrates (glutamate and pyruvate with malate) rotenone-inhibition of electron flux through site IQ stimulates ROS production from site IF, and other proximal sites in the TCA cycle [29,113].
ROS production by RET is modulated by the degree and duration of O2 deprivation [[129], [130], [131], [132], [133]]. The Siesjö group first reported damaging effect of incomplete ischemia (with cerebral blood flow less than 10 %) compared to complete ischemia on rat brain, which they hypothesized to be due to the formation of ROS upon reperfusion [134,135]. Kang et al. [130] have shown that ischemia-reperfusion is more consequential than ischemia alone, and majority of the damage from ischemia-reperfusion injury occurs in the first few minutes of reperfusion. RET ROS production occurs during reoxygenation following ischemic/hypoxic exposure when Δψm is not dissipated, but not in anoxic exposure that dissipates the Δψm [131]. Additionally, RET ROS production is highly sensitive to O2 concentration [91,136,137]. Hoffman and Brookes [136] reported an apparent Km of 0.9 μM O2 for complex I RET ROS production compared with 2.0 μM O2 for complex III outer ubiquinone-binding site (site IIIQo). This indicates that complex I RET ROS production is more sensitive to O2 concentration compared with complex III, which also has a high native rate of ROS production capacity and is important in sustained hypoxia signaling.
Following exposure to hypoxia complex I spontaneously transitions to the D-form [73,137] (Fig. 3). Structural rearrangement of site IQ subunits upon transition to the D-form disrupts electron flux between the N2 terminal and site IQ similar to the inhibitory effect of rotenone (site IQ inhibitor) on electron flux [82,83,138]. Transitioning to the D-form is associated with increased rate of NADH-dependent ROS production that is time- and pH-dependent [73,139]. In the D-form, alkaline pH (9.4) unlike physiological pH (7.4) caused significant increase in O2•‾ production in rat heart submitochondrial particles. Acute hypoxia increased O2•‾ production in the first 10 min of exposure in bovine aortic endothelial cells [140], and after 30 min in mouse hippocampal slices [73]. In the D-form, reoxygenation with addition of succinate showed slower rate of ROS production with an initial lag phase compared with the A-form in rat brain mitochondrial [137,141]. Thus, transitioning to the D-form may serve to protect against oxidative damage due to greater magnitude of ROS release (as bursts) following ischemia-reperfusion in the A-form [142,137]. Furthermore, studies have demonstrated a redox-dependent reversible dissociation of the tightly but noncovalently bound FMN from the N module of the peripheral arm of brain complex I [143,144,133]. This has been reported to be independent of O2 concentration or ROS release and may serve to maintain the native protein conformation [144,133].
Fig. 3.
Active and deactive forms of complex I. Complex I reversibly transitions from active to deactive states during hypoxia. Hypoxic conditions and limited NADH induce complex I to transition from active to deactive forms, changing protein conformation and altering enzymatic function.
The position of the sites of ROS production in complex I (site IF at the tip of the N module and site IQ close to the matrix side of the IMM) results in release of ROS into to the matrix. Studies are now elucidating the importance of complex I site-specific ROS emission in physiology and disease, such that we are beginning to understand what the physiologic consequence would be for ROS made at site IF compared with IQ. The impact of ROS from complex I also will be influenced by the duration of release. Key among the protein targets of complex I RET ROS are inner membrane proteins such as cytochrome bcI complex, complex II flavoprotein, and the electron transferring flavoprotein-ubiquinone oxidoreductase. Others are matrix aconitase, and the 2-oxoacid dehydrogenase complexes including the ROS generating flavin sites of PDC and oxoglutarate dehydrogenase complex (OGDC) [29,[145], [146], [147], [148]]. PDC and OGDC play crucial role in mitochondrial redox sensing through a negative feedback response to complex I [145,[148], [149], [150], [151]]. PDC and OGDC are entry points for pyruvate and amino acids into the TCA cycle, respectively. Ischemia-reperfusion has been shown to inhibit the activity of PDC in canine [152], and rat brain mitochondria [153]. Similarly, the activity of OGDC was reported to be inhibited by ischemia-reperfusion in rat cardiac mitochondria [154]. Inhibition of the activity of these enzymes especially OGDC, will result in reduced supply of NADH [155]. It can thus, be suggested from the reports of Bleier et al. [145], and Tretter and Adam-Vizi [148] that complex I RET ROS-induced inhibition of aconitase, PDC, and OGDC may be protective, serving to limit the supply of reducing equivalents from the TCA cycle to the ETC. Limiting the supply of reducing equivalents to the ETC will in turn reduce ROS production.
Complex I is also a target of ROS-induced inhibition and oxidative thiol-modifications [130,142,156,157]. RET ROS causes oxidative thiol-modification of several of the cysteine residues of complex I resulting in diminution of the catalytic activity of the enzyme complex [158,130,156,159]. Glutathionylation of complex I subunits including NDUFS1 and NDUFV1 leads to inhibition of complex I activity and ROS production [160]. Moreover, the D-form is more susceptible to oxidative thiol-modification [142]. Exposure of the ND3 Cys39 is generally accepted as a marker of transition of complex I into the catalytically inactive D-form. However, a recent report [161] demonstrated that in the catalytically active A-form, about 65 % of Cys39 is exposed compared with about 93 % in the catalytic D-form. ND3 Cys39 is a critical residue, which when exposed in the D-form is readily modified by ROS resulting in inhibition of complex I activity [138,142,162].
Complex I may be distributed individually as single free (uncomplexed) units or may form higher order respiratory supercomplexes in association with complex III referred to as supercomplex, or with complex III and IV referred to as respirosomes [163,164]. Formation of higher order respiratory supercomplexes has been reported to improve the enzyme stability, modulate the catalytic function and reduce formation of ROS [[163], [164], [165]]. In addition, formation of higher order respiratory supercomplexes has been shown to reduce exposure of ND3 Cys39 [161]. Adoption of different conformations, dissociation of FMN and transitioning to the D-form may serve to regulate OXPHOS, maintain redox status, and protect against oxidative damage [null]; [null]; [28]. Thus, complex I shows a high degree of autoregulation thereby modulating the activities of the ETC and ROS production.
4. Complex I ROS signaling
Complex I ROS signaling involves the oxidation of key cysteine residues to trigger downstream signaling [166]. Cysteine is one of the least abundant amino acid residues, but is the most highly conserved and is often seen in functionally important sites of proteins [167,168]. Cysteine accounts for about 2 % of cellular amino acid content, and constitutes the highest concentration of thiols in mitochondria [34,169]. Bak et al. [170] reported ∼1500 reactive cysteine residues on ∼450 mitochondrial proteins; while Danielson et al. [171] reported 130 cysteine residues within murine complex I. Complex I cysteine residues play critical role in diverse functions such as protein assembly and structure, metal binding, catalytic activity and regulation, redox sensing, and signaling [34,91,111,171,172]. Among these are the redox-sensitive cysteines with thiol-containing side chains, which are critical in sensing changes in redox status [173,174].
The thiol groups in redox-sensitive cysteines are deprotonated, and present as highly reactive thiolate anions (RS−) at physiologic conditions compared to the protonated (RSH) and largely nonreactive cytoplasmic cysteines [173]. Nominally, the higher pH in mitochondrial matrix would drive a less protonated state, which may render intra-mitochondrial cysteines particularly susceptible to redox reactions [175,176]. Oxidative thiol modifications are generally reversible, making these thiol cysteines act as redox switches. Reversible oxidative modifications of cysteines include but not limited to S-nitrosylation, S-glutathionylation, and sulfenic acid formation [177,178]. The thiolate anions are readily oxidized by ROS to the sulfenic acid intermediate which modulates the effector protein structure and function [34,111,173,174]. However, inordinate and sustained ROS release can progressively oxidize the sulfenic acid intermediates to sulfinic and to the sulfonic acids which, irreversibly deactivates the protein resulting in loss of function [34,174,179]. It is important to note that redox signaling involves reversible oxidative modification of cysteine thiols by H2O2 to sulfenic acid, whereas O2•‾ causes formation of thiyl radical at a rate constant as high as 103 M−1 s−1 without specificity. However, higher rate constants (>109 M−1 s−1) for the dismutation of O2•‾ by SOD to H2O2 and O2 compared with cysteine reactions limits the signaling function of O2•‾ [180]. Overall, ROS signaling depends on location, duration and quantity of production wherein transient and low-level ROS production may be beneficial or physiologic (oxidative eustress), whereas inordinate and sustained release may be detrimental or pathologic (oxidative distress) [[181], [182], [183]]. Localization of ROS release restricts the effect within cellular microdomains. Additionally, antioxidant systems scavenge ROS thereby restricting and fine-tuning the signaling function. Dysregulation of ROS release in favor of prooxidants may cause biomolecular damage associated with the ischemia-reoxygenation injury associated with heart attack and stroke [120,184], neurodegeneration, senescence [[185], [186], [187]], and dysfunction signaling.
4.1. Complex I ROS in adaptive O2 signaling
Complex I ROS is important in adaptive signaling and have been shown to promote sensory behavior in response to oxidative stress by activating different transcription cascades [188,189]. Complex I RET ROS is critical in maintaining homeostasis, and response to different stressors. Complex I RET ROS initiates metabolic reprogramming by redirecting glycolytic intermediates to the pentose phosphate pathway for the maintenance of NADPH levels and elicits transcriptional changes in response to thermal stress in Drosophila melanogaster [190,191]. Activation of RET ROS signaling has also been demonstrated to diminish with age, and loss of this signaling is detrimental for survival [190,191]. Induction of transient neuronal complex I H2O2 release has been reported to enhanced stress resistance and fitness resulting ultimately in the extension of lifespan in Caenorhabditis elegans by activation of PMK-1/p38 MAP kinase and SKN-1/NRF-2 [192].
4.2. Complex I ROS in acute O2 sensing
Organisms are exposed to local and global O2 transients, and dependence on O2 for OXPHOS necessitates mechanisms for detecting (within seconds to minutes) acute changes in O2 gradients. The ability to sense and respond to changes in O2 levels is described as O2 sensing [193]. Acute changes in O2 gradients elicits physiologic responses such as hypoxic ventilatory response and hypoxic pulmonary vasoconstriction in mammals (HPV) [61,65,[194], [195], [196], [197], [198], [199]], and avoidance locomotory response in C. elegans [57,200]. In mammals, acute O2 sensing mechanisms is found in strategically located tissues including the O2-sensitive neuron-like type I cells, also called glomus or chief cells of the carotid body (CB), and aortic body. These are the major systemic chemoreceptor organs that detect hypoxia and hypercapnia [63,64,[201], [202], [203]]. The adrenal medulla (AM) chromaffin cells and pulmonary artery smooth muscle cells (PASMCs) are components of the peripheral chemoreceptor organs forming the homeostatic O2-sensing system [[61], [62], [63], [64]].
The CB and AM working in concert constitute the CB-AM axis, and function to maintain cardiorespiratory homeostasis [63]. The glomus and chromaffin cells are neural crest-derived electrically excitable cells, that can generate action potentials repetitively [63,64,204]. These cells express a variety of O2-sensitive ion channels, particularly the potassium ion (K+) channels including the voltage-gated K+ channels (KV: KV 1.5, KV 1.2, KV 2.1 and KV 3.1), maxi-K channels, and background K+ channels [63,64,[204], [205], [206], [207]]. These K+ channels are reversibly inhibited by acute hypoxia-induced H2O2 release resulting in membrane depolarization [63,64,204,[206], [207], [208]]. The hypoxia-induced membrane depolarization activates voltage-dependent Ca2+ channels, initiating influx of extracellular Ca2+ which, in turn triggers exocytotic release of neurotransmitters [63,64,202,204,208]. The release of neurotransmitters (including ATP and acetylcholine) by the glomus and chromaffin cells stimulates the central respiratory and autonomic centers through the afferent sensory fibers to initiate HPV [63,64,202,204,208,209]. This cascade of events is the basis of the “membrane model” of acute O2 sensing by peripheral chemoreceptor organs. However, reports indicate that acute hypoxia signaling proximal to the acute O2 sensitive-K+ channels is multimodal, involving processes other than those of the membrane model and have mitochondrial metabolism as a unifying factor [63,64]. Key among these processes is the alteration in mitochondrial metabolism resulting in accumulation of succinate, NADH and lactate, and increased ROS production [63,65,210].
These metabolites may act as upstream signals that modulate the activities of the O2 sensitive-K+ channels. NADH but not NAD+, ADP, ATP, Na+, or Mg2+ have been reported to inhibit the activity of rabbit PASMCs large conductance Ca2+-activated K (KC) channels [211]. The inhibition of PASMCs KC by NADH was hypothesized to be due to change in the redox state of the channel rather than direct binding of NADH on the channel, suggesting that the KC may be acting as a redox sensor [211]. In support, Archer et al. [212] showed evidence of a redox-based O2 sensor in the PASMCs K+ channels that depends on mitochondrial ROS release. Use of rotenone or antimycin A (specific inhibitors of mitochondrial complex I and III, respectively) mimicked acute hypoxia by causing a change in the redox status which, in turn reversibly inhibited PASMCs K+ channels [212]. Reports show that in response to moderate hypoxia, lactate production is increased as a protective signal to modulate vasodilation [213]. Lactate signaling activates the glomus cell and potentiates hypoxia-induced activation [210]. Lactate activation of mouse and rat glomus cells in response to acute hypoxia was shown to be Ca2+ dependent and results in a dose-dependent reversible increase in matrix and IMS ROS release [210]. However, responsiveness to hypoxia requires complex I since ablation of Ndufs2, which encodes the core nuclear-encoded 49 kDa protein necessary for complex I assembly and activity, results in loss of responsiveness to acute hypoxia [27,[60], [61], [62],214,215]. These studies suggest that complex I ROS is key in O2-sensing.
4.3. Complex I ROS signaling in peripheral acute O2-sensing
Complex I activity is important in the O2-sensing function of CB and AM. The CB and AM have unique metabolic and redox status that facilitates their O2-sensing function. Studies have shown that CB unlike other neural cells have elevated succinate levels [62,216]. Mitochondria of the CB are atypical, expressing both high and low O2 affinity cytochrome a3, which are required for O2-sensing [217,218]. The O2-sensing of glomus cell is due in part to hypoxia-inducible factor subunit 2 alpha (Hif2α)-dependent expression of atypical nuclear-encoded mitochondrial subunits including NDUFA4l2, COX4i2, and COX8b, which are responsible for the accelerated oxidative metabolism and strict O2-dependent complex IV activity [65,219]. Hypoxia induces a decrease in complex IV activity and causes a buildup of electrons along the ETC. The resultant high UQH2/UQ ratio leads to RET ROS and altered complex I activity [63,65]. Thus, hypoxia produces a reversible increase in ROS in the cytosol and IMS, but a decrease in matrix ROS [60,[62], [63], [64], [65],214]. Similar to the reported decrease in matrix ROS, previous studies show that acute hypoxia produced a rapid and reversible reduction in the levels of matrix H2O2 in neonatal but not juvenile rat chromaffin cells [220] PASMCs [61,221]. However, using microfluorimetry with genetically-encoded probes, Jiménez-Gómez et al. [214] showed that acute hypoxia produced a reversible increase in IMS and matrix ROS levels in complex I-deficient glomus cells. These studies suggest possible tissue-specific mitochondrial microdomains and compartmentalization of ROS production in response to acute hypoxia.
ROS signaling will depend on the magnitude and duration of ROS release, and the redox poise of the environment [183,222,223]. However, the diffusion of H2O2 is limited by antioxidant systems including catalase, and the peroxiredoxin thioredoxin couple [222,[224], [225], [226]]. To overcome this limitation, H2O2 may use redox relays mediated by peroxiredoxins and or peroxidases to transmit signals across cellular microdomains or compartments. Here, peroxiredoxins are oxidized to sulfenates and disulfide forms, which in turn oxidize the redox targets [[227], [228], [229], [230], [231]]. Peroxiredoxin, especially mammalian mitochondrial peroxiredoxin 3 plays a key role in mitochondrial ROS redox relays. Peroxiredoxin 3 has been reported to react with about 90 % of mitochondrial H2O2 due to relative higher abundance and reactivity [232,233] hence, may be important in the redox relay of complex I H2O2 that is release into the matrix. Other peroxiredoxins are also important in functioning as a redox relay. The cytosolic peroxiredoxin 2 acts as a H2O2 receptor, which transmits the oxidative equivalents to the signal transducer and activator of transcription 3 (STAT3) [228]. The resultant disulfide-linked dimers and tetramers of STAT3 then regulates the transcription machinery in response to stress [231]. Peroxiredoxins are also important in the “floodgate model” of redox signaling. The peroxiredoxin are proposed in the floodgate model to be inactivated (oxidized to the sulfinic and sulfonic moieties) locally by H2O2. This leaves only a remote peroxiredoxin in the redox-active sulfenic form to transmit the redox signal to target proteins [234]. H2O2-induced signal transduction is therefore, predicated on the presence of robust antioxidant systems and redox-sensitive proteins particularly the thiol cysteine redox switches in the domain of release. These serve to maintain the localization, rapidity and reversibility of the H2O2-induced redox events. Thus, the proximity of mitochondrial ROS release to the redox switches is important in signaling.
Complex I has been demonstrated as the primary source of H2O2 signaling in response to acute hypoxia in the peripheral O2-sensitive chemoreceptor organs. Studies have shown that rotenone treatment in the glomus cells [60,[62], [63], [64], [65]], PASMCs [61], and pulmonary artery myocytes [235,236] abolished the H2O2 signal and responsiveness to acute hypoxia. This strongly suggests that ROS release from complex I is important in acute hypoxia signaling. In confirmation of complex I as the source of the H2O2 signal, Ndufs2 has been shown to have critical regulatory role necessary for acute hypoxia response in peripheral O2-sensitive chemoreceptor organs [61,62,64]. NDUFS2 is redox-sensitive being reduced during acute hypoxia. It is required for acute hypoxia-induced intracellular Ca2+ concentration ([Ca2+]i) release, and induction of HPV [60,61,64]. Ablation, inactivation or deficiency of Ndufs2 results in loss of acute hypoxia response in peripheral O2-sensitive chemoreceptor organs and absence of HPV [[60], [61], [62],64,214]. Reversible oxidation of NDUFS2 cysteine residues by complex I H2O2 was shown to be responsible for acute hypoxia response [61].
4.4. Putative astrocyte complex I ROS in central acute O2-sensing
The brain is sensitive to hypoxia due to the high energy demand and minimal energy reserves [[237], [238], [239]]. Additionally, the central nervous system (CNS) is subject to varying levels of oxygenation due to variable degree of blood supply, which cannot be adequately monitored by the peripheral O2-sensing chemoreceptor organs [239]. Furthermore, the brain shows selective neuronal vulnerability where specific neurons within different regions in the brain such as the cerebellar granule, hippocampus, and amygdala are highly sensitive to oxidative stress [240,241]. These regions coincidentally, have a relatively high astrocyte density [242]. Astrocytes exhibit heterogeneity within and between regions of the CNS indicating functional dynamism in response to the needs imposed on them [243,244]. Astrocytes are a specialized subset of glial cells, which are closely integrated into neural networks having remarkable adaptive plasticity enabling them to support neuronal viability, metabolism, and function in addition to regulating CNS homeostasis [244,245]. Astrocytes in the different brain regions show varying sensitivity to O2 deprivation, and H2O2 exposure. Among different brain regions, hippocampal astrocytes show more sensitivity to O2 deprivation compared with cortical or striatal astrocytes in rats [246,247]. Contrastingly, cortical astrocytes show more sensitivity to H2O2 compared with hippocampal or striatal astrocytes [246].
Astrocytes have a metabolic signature distinct from neurons. Astrocytes have a functional ETC, but depend more on glycolysis compared with neurons [[248], [249], [250], [251], [252]]. Furthermore, astrocytes unlike neurons have the capacity for FAO, and oxidize fatty acids as an alternative fuel to augment energy supply during metabolic stress [[253], [254], [255]]. FAO has been shown to increased ROS release in mouse astrocytes [255]. Astrocytes reportedly have an abundance of complex I in the free form that are not assembled as supercomplex in contrast to neurons where complex I are assembled into supercomplex [251]. The free complex I contributes to the higher ROS production capacity of astrocytes compared with neurons [251]. Moreover, neurons have lower antioxidant levels and are more sensitive to oxidative stress than astrocytes. Additionally, neurons that produce more mitochondria-derived ROS relative to other neurons are more vulnerable to oxidative stress [[256], [257], [258]]. The distribution and function of astrocytes within specific chemoreceptor sites in the brain have been suggested to mediate the response to ROS release and redox stress [[259], [260], [261], [262]]. Astrocytic mitochondrial ROS has been reported to modulate neuronal metabolism and function, and also alter organismal behavior [248,263,264].
To maintain neuronal homeostasis, astrocytes function as key chemosensors for CO2 and pH, which are linked to Ca2+ signaling [[265], [266], [267]]. Astrocytes are positioned closer to and are part of the blood-brain barrier, and thus are subject to greater fluctuations in pO2 in circulation compared to neurons, which are buffered from this by glial cells [268,269]. Studies are now highlighting the role of astrocytes as central O2-sensing chemoreceptors in addition to other functions. O2-sensing has been demonstrated in vivo and in cultured cortical, hippocampal, midbrain and brainstem astrocytes [270]. In their study, Angelova et al. [270] showed that hypoxia resulted in elevated [Ca2+]i and increased exocytosis of ATP-containing vesicles. Their study showed that astrocytic O2-sensing is dependent on mitochondrial ROS and Δψm. Using MitoSOX, a mitochondria-targeted fluorescent probe, they Angelova et al. [270] showed an immediate increase in mitochondrial ROS in astrocytes in response to hypoxia. The observed increase in ROS was reduced by pretreatment with 0.5 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP, a mitochondrial uncoupler) or 100 nM MitoQ (a mitochondrial-targeted antioxidant). Treatment with 0.5 μM FCCP or 100 nM MitoQ in vivo and in cultured astrocytes abolished the hypoxia-induced increase in [Ca2+]i. Simultaneous measurement of Δψm and [Ca2+]i in cultured rat astrocytes using Rh123 and fura-2, respectively, showed hypoxia induces a decrease in Δψm that precedes increase in [Ca2+]i [270]. Supporting the central O2-sensing role of astrocytes [271], showed that nucleus of the solitary tract in neonatal rat brain responded to acute hypoxia by altering Ca2+ activities. Astrocytes of the ventral surface of the medulla oblongata have also been shown to act as central O2-sensing chemoreceptors. In these astrocytes, acute hypoxia inhibits the O2-induced suppression of sensor cation channel transient receptor potential (TRP) ankyrin 1 (TRPA1) activity. Complementing the report of Angelova et al. [270], rat cortical astrocytes in vivo have been shown to respond to decreases in brain perfusion with increased frequency and duration of [Ca2+]i signals, which are required for compensatory sympathetic and cardiovascular response to cerebral ischemia [272]. Acute hypoxia-induced mitochondrial ROS release was demonstrated to contribute to activation of plasma membrane-inserted TRPA1 resulting in Ca2+ influx, which leads to ATP release from these astrocytes [239,273]. It is therefore, possible that complex I may contribute to mitochondrial ROS release necessary for increased [Ca2+]i, subsequent activation of inositol triphosphate (IP3) receptors, and ultimately to vesicular ATP release in astrocytes [270,274,275].
Angelova et al. [270] demonstrated that astroglia have a lower pO2 threshold for activating [Ca2+]i signaling of about 17 mmHg compared with about 37 mmHg for glomus cells. This suggests that astrocytes may detect O2 levels below the threshold for glomus cells. Supporting the findings of Angelova et al. [270], Barioni et al. [276] showed that intermediolateral nucleus astrocytes contribute to spinal O2 sensing in rats. Their study showed that acute hypoxia activated [Ca2+]i signal through ROS-dependent TRP channels in addition to other mechanisms. The spinal O2 sensing capacity was reported to be similar to that of the carotid bodies [276].
The central respiratory O2 sensing function of astrocytes has been suggested to be in part due to the mitochondrial complex I-derived ROS [251]. Specifically [251], showed that higher rate of H2O2 release by astrocytes compared with neurons is not due to contributions from other major nonmitochondrial ROS sources such as xanthine oxidase, NADPH oxidases, and nitric oxide synthase. Interestingly, astrocytes that utilize glycolysis for energy metabolism showed similar activities for different ETC enzymes including complex I compared with neurons. However, the higher rate of ROS release in glycolytic astrocytes may be partly due to the lower abundance of NDUFS1 subunit in astrocytes [251]. NDUFS1 expression was demonstrated to be necessary for assembly of supercomplexes, higher electron transfer efficiency, and lower rates of ROS release. Corroborating this [277], showed reduced Ndufs1 expression in myocardium of human heart failure patients and in mice with myocardial infarction. However, overexpression of Ndufs1 prevented the hypoxia-induced high ROS rates in mice [277]. Furthermore, a biallelic mutation in nuclear Ndufs1 in a human patient has been reported to cause reduced complex I N-module stability resulting in impaired supercomplex formation, reduced electron transfer rate between N4 and N5 subunits of the Fe–S cluster, upregulation of glycolysis, and increased ROS production [278]. Altogether, it appears that the lower abundance of NDUFS1 may be a default redox poise of astrocyte complex I, which predisposes it to higher rates of ROS release. However, whether astrocyte complex I ROS release is the proximal signal in central O2-sensing remains to be conclusively demonstrated.
Similar to the brain, the retina has high metabolism with low functional O2 reserve and is considered one of the most O2-sensitive tissues [279,280]. Retinal mitochondrial-dense photoreceptor cells canonically depend on oxidation of glucose via glycolysis for energy metabolism and the products, lactate and aspartate are then oxidized by Müller glia cells [281]. In addition, the free fatty acid receptor 1 senses availability of fatty acids and activates FAO energy metabolism [282]. Interestingly the glycolytic retinal photoreceptor cells are vulnerable to O2 deprivation, and ischemia-reoxygenation damage that is typical of complex I RET ROS [280,283,284]. Recent studies demonstrate that mitochondrial ROS following ischemia-reperfusion is responsible for the death of retinal ganglion cells, which can be inhibited by metformin or mito-TEMPO [284]. Response to acute hypoxia in the retina involves promoting blood flow by mechanisms yet to elucidated [280]. In the optic nerve, astrocytes have also been proposed to function as hypoxia sensors in the unmyelinated nerves [285].
Complex I has been shown to be important in astrocyte function. Mutation in different subunits of complex I leads to various neurodegenerative conditions. Mutations in various complex I subunits including NDUFS1 [251], NDUFS2 [[286], [287], [288]], NDUFS4 [22,289,290], ND1 [291], ND3 [292,293] results in defective complex I assembly, altered mitochondrial activity and dysregulation of ROS production. Further, loss of Ndufs4 results in significant diminution of astrocyte complex I activity [294]. Similarly, exposure to pharmacologic inhibitors of complex I diphenyleneiodonium or rotenone inhibited hypoxia sensing and HPV in rat pulmonary myocytes implicating RET ROS in O2-sensing [235,236]. Furthermore, using suppressors of site IQ electron leak (S1QEL), which do not cause ROS production or impair mitochondrial function, Brand et al. [295] showed that site IQ is a major source of RET ROS in astrocytes exposed to ambient and low O2 tension conditions. In support of the role of complex I ROS in signaling acute changes in O2 tension, Read et al. [296] demonstrated that S1QEL inhibited the rapid increase in ROS production and the subsequent elevation of [Ca2+]i following normoxic exposure in human ductus arteriosus smooth muscle cells (DASMC). The DASMCs contain O2 sensors that respond to elevation of pO2 leading to constriction of the ductus arteriosus following the first breath, and inhibition of ROS production from complex I with S1QEL prevented closure of rabbit ductus arteriosus in vivo [296]. They [296] demonstrated that inhibition of complex I ROS release specifically, and not complex III ROS is responsible for O2 signaling. While astrocytes function to protect neurons and retinal photoreceptor cells from ROS, excessive ROS release due to mutations in complex I subunits may overwhelm the Ca2+ or ROS-buffering capacity of glial mitochondria, thereby activate these astrocytes. The activated astrocytes then release chemokines and inordinate levels of ROS ultimately leading to neuronal and retinal degeneration [297,298]. Thus, elucidating the involvement of complex I in astrocyte O2-sensing will be valuable in understanding the pathophysiology of oxidative neuronal and retinal degeneration.
5. NDUFS2 residues mediate acute hypoxia behavioral response
Altogether, mitochondrial complex I activity is key in peripheral and central respiratory O2 sensing tissues. Critical for the assembly and function of complex I is the ubiquitously expressed NDUFS2, which is located at the interface of the membrane and matrix arm and constitutes part of the ubiquinone binding pocket together with ND1 and NDUFS7 [62,75,78,215,287,299]. Loss of Ndufs2 has been shown to induce the degradation of complex I and disappearance of other subunits including NDUFS1, NDUFV2, NDUFS4 and NDUFB8 [62,299,300]. Demonstrating the importance of NDUFS2 in mitochondrial complex I physiology, Cabello-Rivera et al. [301] reported that loss of NDUFS2 resulted in perinatal death, defective proliferation of neural progenitor cells, and dysfunctional differentiation of neurons and oligodendrocytes in NDUFS2-knockout mice. In support, the disruption of NDUFS2 was also reported to induce a metabolic shift that downregulated complex I driven respiration and promoted glycolysis in neuronal mitochondria [302]. Disruption of NDUFS2 in human embryonic kidney cell line 293 (HEK293) resulted in reduction in growth, complex I-supported respiration, glycolytic capacity, cell membrane integrity but increased complex II-supported respiration and ROS production [300]. Disruption of Ndufs2 resulted in altered ROS homeostasis and loss of acute hypoxia behavioral response [[60], [61], [62]] whereas inactivation of Ndufs4 resulted only in about 50 % decrease in complex I activity without loss of hypoxia response [60,62]. This suggests that ROS from complex I may be acting locally, producing redox events within complex I putatively on NDUFS2 to elicit acute hypoxia responses.
NDUFS2 contains a number of cysteine residues that undergo oxidative modification resulting in altered complex I activity. The location and interaction of NDUFS2 cysteine residues with neighboring redox active amino acids influences the extent to which NDUFS2 can modulate redox events. The privileged location of NDUFS2 brings it in contact with other subunits and exposes it directly to ROS from complex I especially from site IQ. NDUFS2 contacts and interacts with a number of other core and accessory subunits including but not limited to ND1, ND3, NDUFS7, NDUFS3, NDUFS6, NDUFA5 and NDUFA10-13 [60,303] (Fig. 2A). These interactions create a local electrostatic environment produced by the charge differentials of different residues in these subunits. This electrostatic environment permits fine-tuning of redox active residues, which in turn induces modification of other neighboring redox active residues [166,304,305]. Also, charged modifications of residues produce conformational transitions of the individual subunits that is propagated to other subunit leading to change in catalytic activity of the complex I [70,77,306]. For example, the loop between the first and second β-strands in NDUFS2 (β1- β2 loop) which contains highly conserved histidine residues critical for UQ binding and catalysis shows great conformational dynamism [5,307]. Changes in the conformation of this loop may induce alteration in complex I activity [308] that may lead to altered ROS homeostasis.
NDUFS2 cysteine residues including Cys146, Cys326, and Cys347 are reported to be susceptible to direct oxidative modification resulting in conformational change of the subunit and altered complex I activity [158,130]). The Cys146 residue is buried inside NDUFS2 in contrast to Cys326 and Cys347 residues, which are surface-exposed and located at the same α-helix of NDUFS2 [130]. The surface exposed Cys326 and Cys347 undergo oxidative modification following ischemia-reperfusion in rats, which may indirectly lead to oxidation of Cys158 and Cys188 at the N2 cluster of NDUFS7 [130]. Redox proteomics assay for S-glutathionylation of mouse gastrocnemius muscles after fatigue show that NDUFS2 Cys347 was among the cysteines of complex I subunits that was oxidatively modified [309]. Similarly, using quantitative redox proteomics analysis with GELSILOX of hypoxic mitochondrial protein of COX10 knockout mouse skin fibroblast cells, Guarás et al. [33] show that the Cys347 detected is mainly in the reduced form and undergoes oxidation following reoxygenation (Fig. 4A).
Fig. 4.
An evolutionarily conserved redox-sensing cysteine in NDUFS2. A NDUFS2 interacts with the final Fe–S cluster N2, critically stabilizing its assembly and stability to support electron flow through Complex I. The Cys347 (Cys366 in C. elegans) residue is susceptible to redox modification and important in oxygen-sensing. The position of Cys347 residue is highly conserved across NDUFS2 structure. B Cys347 and surrounding residues are highly conserved in listed species (indicated by color, paired with A).
Recent studies using both genetic and pharmacologic interventions demonstrate the involvement of NDUFS2 subunit in complex I ROS-mediated behavioral response to acute hypoxia in C. elegans [57]. Acute hypoxia in C. elegans produces avoidance behavior characterized by rapid and transient increase in locomotory speed and turning angle [310]. In C. elegans mutants, GAS-1 and ISP-1 were identified by Zhao et al. [200] as mitochondrial components required for acute hypoxia response. Mutation in gas-1, the C. elegans ortholog of Ndufs2, increased ROS levels and entirely abolished the locomotory response to acute hypoxia. To confirm ROS is mediating the behavioral response, sod-2 (encoding primarily mitochondrial manganese-dependent SOD) mutant C. elegans showed partially inhibited locomotory response to acute hypoxia. However, mutation of all SOD genes produced a complete inhibition of acute hypoxia response [200]. C. elegans has five sod genes: sod-1, sod-2 and sod-4, which are the primary cytoplasmic, mitochondrial and extracellular sod genes, respectively; while sod-3 and sod-5 are inducible mitochondrial and cytoplasmic genes, respectively. Loss of any individual SOD may be compensated by presence or upregulation other SOD genes [311,312]. Therefore, the partially inhibited locomotory response to acute hypoxia with sod-2 mutants reported by Zhao et al. [200] may be due compensation by other SOD genes.
Comparable to the loss of acute hypoxia response with sod-2 mutants reported by Zhao et al. [200], Onukwufor et al. [57] also reported that loss of sod-2 and sod-2/-3 abolished activation of acute hypoxia locomotory response. The two mitochondrial isoforms of SOD, sod-2 and sod-3 have been reported to associate with supercomplexes in C. elegans and in mouse mitochondria [311]. Loss of sod-2 resulted in damage to proteins specific to the ETC, dissociation of supercomplexes, decrease in NDUFS2, and reduction in complex I function [311]. Similarly, previous reports show ROS-induced reduction of complex I and mitochondrial aconitase activity in sod-2 mutant mouse heart and brain mitochondria [313,314]. This is supported by reports that loss of SOD results in absence of the acute hypoxia behavioral response since SOD dismutates the precursor O2•‾ to H2O2 and O2. This supports the finding by Onukwufor et al. [57] that the acute hypoxia avoidance behavior in C. elegans is mediated by specifically by H2O2. These reports show a conserved protective location of SOD at sites of high ROS emission that serves to maintain a redox environment conducive for ROS signaling. Therefore, O2•‾ may be primarily a precursor for H2O2 and not necessarily involved directly in signaling. Together these studies strongly suggest that H2O2 produced locally at complex I may be the proximal signal in acute hypoxia sensing.
Treatment with known complex I redox cyclers such as paraquat, and juglone generates ROS increased release. Treatment with 200 mM paraquat, 1 mM juglone, and 10 μM rotenone for 10, 2 and 60 min, respectively in the bacterial food abolished the locomotory response to acute hypoxia [200]. Interestingly, Onukwufor et al. [57] reported that treatment with lower doses of paraquat (1 mM) or rotenone (1 μM) 24 h prior to assay resulted in increased C. elegans locomotion. The observed differences in locomotory behavior may be as a result of dose- and time-dependent effect of the pharmacological agents. In C. elegans, the increase in ROS emission following mutation of isp-1, the ortholog of complex III Rieske Fe–S protein, was abolished by rotenone [200]. This recapitulates the hypothesis that complex I ROS may be the proximal signal mediating acute hypoxia response or that electrons from complex I are required for complex III ROS since rotenone, which is a specific inhibitor of site IQ abolishes the locomotory behavioral response to acute hypoxia.
UQ and inhibitors of site IQ including rotenone, piericidin A1, aureothin and pyridaben are believed to bind to His59 and Tyr108, key residues of NDUFS2 that form part of the amphipathic redox active site of Q-channel [75,78]. Using simulations of mouse complex I, rotenone binding has been shown to reorder the NDUFS2 β1-β2 loop of the Q-channel resulting in structural changes similar to that observed during transition from the open to closed states of complex I [78]. While the use of pharmacological inhibitors highlights the importance of NDUFS2; use of these toxins may produce off-target effects and does not provide spatial or temporal control. The use of optogenetics confers better spatiotemporal control in the investigation of complex I ROS signaling compared with the use of genetics or pharmacological inhibitors. With SuperNova, an optogenetic ROS-generating monomeric variant of KillerRed fused to NUO-1 (NADH:ubiquinone oxidoreductase-1; mammalian ortholog, NDUFV1, encoding complex I site IF) Onukwufor et al. [57] showed that ROS emanating from complex I mediated a rapid reversible acute hypoxia-induced behavioral response in C. elegans. They [57] show that O2•‾ specifically, from the matrix arm of complex I is converted to H2O2 to signal change in the membrane arm to ultimately evoke the organismal behavioral response. Additionally, with SuperNova tagged to complex II or complex III, the magnitude of locomotory response to acute hypoxia was not recapitulated compared with SuperNova tagged to complex I [57]. This indicated the specificity and compartmentalization of local complex I ROS in signaling.
The acute hypoxia avoidance behavior was shown by Onukwufor et al. [57] to be due to reversible oxidative modification of a single cysteine, Cys366 (Cys347 in mammals) residue in complex I (Fig. 4b). They [57] reported that mimicking redox modification of a key cysteine residue (Cys366) of NDUFS2 to serine (Cys366Ser, non-oxidizable) or aspartate (Cys366Asp, oxidized) altered organismal behavior in response to ROS. The ROS generated following photoactivation of SuperNova produced avoidance behavior in C. elegans similar to that induced by paraquat [57]. This shows that reversible oxidative modification of a single NDUFS2 cysteine residue modulates complex I activity and mediates rapid organismal behavioral response to acute hypoxia.
The identification of NDUFS2 Cys366 in mediating acute hypoxia behavioral response opens opportunity for interventions in pathophysiologies involving complex I ROS metabolism. The conserved nature of NDUFS2 Cys366 (Cys347 in mouse, rat, bovine and human) (Fig. 4B), the importance of this residue in complex I stability and function, and proximity of Cys366 to the site of ROS production in complex I indicate a sentinel-like function as an “early warning sign” for hypoxia signaling. However, how the reversible modification of this single cysteine residue transmits signal out of the mitochondrial to the whole organism remains an important question for further investigation.
6. Concluding remarks
Recent advances into understanding O2 sensing has made tremendous progress, particularly highlighting the role of mitochondrial complex I during acute hypoxia. This has been made possible by understanding the complex functional morphology, redox biology, and ROS production mechanisms in mitochondrial complex I. Here, we highlighted modalities of ROS production from complex I and the involvement in hypoxia signaling. Importantly, we present recent progress in the understanding of complex I H2O2 hypoxic signaling in peripheral and central O2-sensing chemoreceptor tissues. Use of novel techniques such as live in vivo optogenetics to target ROS production and measurement to select sites with spatiotemporal precision. Understanding how complex I H2O2 signaling mediates behavioral response to acute hypoxia is of importance in future studies and in the development of new therapeutic interventions in conditions associated with hypoxia, ischemia-reoxygenation injuries in stroke, and other pathologies associated with altered ROS homeostasis including neurodegeneration and aging.
Author contributions
C.N.O. wrote the manuscript. C.N.O. and S.A.K. made figures. S.A.K., and A.P.W. edited the manuscript and figures. All authors read and agreed to the published version of the manuscript.
Funding
A.P.W. is supported by grants from National Institutes of Health (R01 NS092558, R01 NS115906).
Declaration of competing interest
The authors declare that they have no conflicts of interest that could influence the contents of this article.
Acknowledgments
We are grateful to the Mitochondrial Research & Innovation Group (MRIG) at University of Rochester Medical Center and the Western New York Worm Group (WNYMG) for insightful comments and suggestions.
Abbreviations
- (OXPHOS)
Oxidative phosphorylation
- (ETC)
electron transport chain
- (Δp)
protonmotive force
- (NADH)
reduced nicotinamide adenine dinucleotide
- (ROS)
reactive oxygen species
- (ATP)
adenosine 5′ trisphosphate
- (NADH)
reduced nicotinamide adenine dinucleotide
- (TCA) cycle
tricarboxylic acid
- (FAO)
fatty acid β-oxidation
- (UQ)
ubiquinone
- (UQH2)
ubiquinol
- (O2)
oxygen
- (H2O)
water
- (H+)
proton
- (NAD+)
oxidized nicotinamide adenine dinucleotide
- (O2•‾)
superoxide anion
- (H2O2)
hydrogen peroxide
- (redox)
reduction-oxidation
- (Ca2+)
calcium
- (Na+)
sodium
- (Fe2+)
iron
- (Mg2+)
magnesium
- (Mn2+)
manganese
- (IMM)
inner mitochondrial membrane
- (IMS)
intermembrane space
- (Δψm)
electrochemical potential
- (ΔpH)
pH gradient
- (ADP)
adenosine 5′ diphosphate
- (kDa)
kilodalton
- (DNA, nDNA)
nuclear deoxyribonucleic acid
- (mtDNA)
mitochondrial DNA
- (FMN)
flavin mononucleotide
- (Fe–S)
iron-sulfur
- (A-form)
active form
- (D-form)
deactive, dormant form
- (UQH•−)
semiubiquinone radical
- (FET)
forward electron transfer
- (RET)
reverse electron transfer
- (PDC)
pyruvate dehydrogenase complex
- (acetyl-CoA)
acetyl-coenzyme A
- (FADH2)
reduced flavin adenine dinucleotide
- (SOD)
superoxide dismutase
- (•OH)
hydroxyl radical
- (HO2•)
perhydroxyl radical
- (RET ROS)
ROS production in RET
- (OGDC)
oxoglutarate dehydrogenase complex
- (RS−)
reactive thiolate anions
- (RSH)
protonated thiol
- (HPV)
hypoxic pulmonary vasoconstriction
- (CB)
carotid body
- (AM)
adrenal medulla
- (PASMCs)
pulmonary artery smooth muscle cells
- (K+)
potassium ion
- (Hif2α)
hypoxia-inducible factor subunit 2 alpha
- (STAT3)
signal transducer and activator of transcription 3
- ([Ca2+]i)
intracellular Ca2+ concentration
- (CNS)
central nervous system
- (FCCP)
carbonyl cyanide p-trifluoromethoxyphenylhydrazone
- (MitoQ)
mitochondria-targeted antioxidant
- (TRPA1)
transient receptor potential (TRP) ankyrin 1
- (IP3)
inositol triphosphate
- (mito-TEMPO)
mitochondria-targeted SOD mimetic
- (S1QEL)
suppressors of site IQ electron leak
- (DASMC)
ductus arteriosus smooth muscle cells
- (HEK293)
human embryonic kidney cell line 293
Data availability
No data was used for the research described in the article.
References
- 1.Bonora M., Patergnani S., Rimessi A., De Marchi E., Suski J.M., Bononi A., Giorgi C., Marchi S., Missiroli S., Poletti F., Wieckowski M.R., Pinton P. ATP synthesis and storage. Purinergic Signal. 2012;8(8):343–357. doi: 10.1007/s11302-012-9305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 3.Senior A.E. ATP synthesis by oxidative phosphorylation. Physiol. Rev. 1988;68(1):177–231. doi: 10.1152/physrev.1988.68.1.177. [DOI] [PubMed] [Google Scholar]
- 4.Hirst J. Mitochondrial complex I. Annu. Rev. Biochem. 2013;82:551–575. doi: 10.1146/annurev-biochem-070511-103700. [DOI] [PubMed] [Google Scholar]
- 5.Laube E., Meier-Credo J., Langer J.D., Kühlbrandt W. Conformational changes in mitochondrial complex I of the thermophilic eukaryote Chaetomium thermophilum. Sci. Adv. 2022;8(47) doi: 10.1126/sciadv.adc9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vercellino I., Sazanov L.A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 2022 doi: 10.1038/s41580-021-00415-0. [DOI] [PubMed] [Google Scholar]
- 7.Sies H., Belousov V.V., Chandel N.S., Davies M.J., Jones D.P., Mann G.E., Murphy M.P., Yamamoto M., Winterbourn C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022;23(7):499–515. doi: 10.1038/s41580-022-00456-z. [DOI] [PubMed] [Google Scholar]
- 8.Emmerzaal T.L., Preston G., Geenen B., Verweij V., Wiesmann M., Vasileiou E., Grüter F., de Groot C., Schoorl J., de Veer R., Roelofs M., Arts M., Hendriksen Y., Klimars E., Donti T.R., Graham B.H., Morava E., Rodenburg R.J., Kozicz T. Impaired mitochondrial complex I function as a candidate driver in the biological stress response and a concomitant stress-induced brain metabolic reprogramming in male mice. Transl. Psychiatry. 2020;10(1):176. doi: 10.1038/s41398-020-0858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han S., Lee M., Shin Y., Giovanni R., Chakrabarty R.P., Herrerias M.M., Dada L.A., Flozak A.S., Reyfman P.A., Khuder B., Reczek C.R., Gao L., Lopéz-Barneo J., Gottardi C.J., Budinger G.R.S., Chandel N.S. Mitochondrial integrated stress response controls lung epithelial cell fate. Nature. 2023;620(7975):890–897. doi: 10.1038/s41586-023-06423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trushina E., Trushin S., Hasan M.F. Mitochondrial complex I as a therapeutic target for Alzheimer's disease. Acta Pharm. Sin. B. 2022;12(2):483–495. doi: 10.1016/j.apsb.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aw T.Y., Andersson B.S., Jones D.P. Suppression of mitochondrial respiratory function after short-term anoxia. Am. J. Physiol. 1987;252(4 Pt 1):C362–C368. doi: 10.1152/ajpcell.1987.252.4.C362. [DOI] [PubMed] [Google Scholar]
- 12.Fiedler J., Daniels A.J. Uptake of magnesium by chromaffin granules in vitro: role of the proton electrochemical gradient. J. Neurochem. 1984;42(5):1291–1297. doi: 10.1111/j.1471-4159.1984.tb02786.x. [DOI] [PubMed] [Google Scholar]
- 13.Lange H., Kispal G., Lill R. Mechanism of iron transport to the site of heme synthesis inside yeast mitochondria. J. Biol. Chem. 1999;274(27):18989–18996. doi: 10.1074/jbc.274.27.18989. [DOI] [PubMed] [Google Scholar]
- 14.Ohsumi Y., Anraku Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Biol. Chem. 1983;258(9):5614–5617. https://www.sciencedirect.com/science/article/pii/S0021925820819358?via%3Dihub [PubMed] [Google Scholar]
- 15.Martin J., Mahlke K., Pfanner N. Role of an energized inner membrane in mitochondrial protein import. Delta psi drives the movement of presequences. J. Biol. Chem. 1991;266(27):18051–18057. https://www.sciencedirect.com/science/article/pii/S0021925818552352?via%3Dihub [PubMed] [Google Scholar]
- 16.Mavridou V., King M.S., Tavoulari S., Ruprecht J.J., Palmer S.M., Kunji E.R.S. Substrate binding in the mitochondrial ADP/ATP carrier is a step-wise process guiding the structural changes in the transport cycle. Nat. Commun. 2022;13(1):3585. doi: 10.1038/s41467-022-31366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfanner N., Neupert W. Transport of proteins into mitochondria: a potassium diffusion potential is able to drive the import of ADP/ATP carrier. Embo j. 1985;4(11):2819–2825. doi: 10.1002/j.1460-2075.1985.tb04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw G.C., Cope J.J., Li L., Corson K., Hersey C., Ackermann G.E., Gwynn B., Lambert A.J., Wingert R.A., Traver D., Trede N.S., Barut B.A., Zhou Y., Minet E., Donovan A., Brownlie A., Balzan R., Weiss M.J., Peters L.L., Kaplan J., Zon L.I., Paw B.H. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440(7080):96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 19.Monzel A.S., Enríquez J.A., Picard M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat. Metab. 2023;5(4):546–562. doi: 10.1038/s42255-023-00783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlieb E., Armour S.M., Harris M.H., Thompson C.B. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003;10(6):709–717. doi: 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]
- 21.Rajagopal M.C., Brown J.W., Gelda D., Valavala K.V., Wang H., Llano D.A., Gillette R., Sinha S. Transient heat release during induced mitochondrial proton uncoupling. Commun. Biol. 2019;2:279. doi: 10.1038/s42003-019-0535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serrano-Lorenzo P., Gobelli D., Garrido-Moraga R., Esteban-Amo M.J., López-López J.R., Orduña A., de la Fuente M.A., Martín M.A., Simarro M. Development of a novel in vitro model to study the modulatory role of the respiratory complex I in macrophage effector functions. PLoS One. 2023;18(9) doi: 10.1371/journal.pone.0291442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zorova L.D., Popkov V.A., Plotnikov E.Y., Silachev D.N., Pevzner I.B., Jankauskas S.S., Babenko V.A., Zorov S.D., Balakireva A.V., Juhaszova M., Sollott S.J., Zorov D.B. Mitochondrial membrane potential. Anal. Biochem. 2018;552:50–59. doi: 10.1016/j.ab.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutiérrez-Fernández J., Kaszuba K., Minhas G.S., Baradaran R., Tambalo M., Gallagher D.T., Sazanov L.A. Key role of quinone in the mechanism of respiratory complex I. Nat. Commun. 2020;11(1):4135. doi: 10.1038/s41467-020-17957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi T., Stuchebrukhov A.A. Electron tunneling in respiratory complex I. Proc. Natl. Acad. Sci. U. S. A. 2010;107(45):19157–19162. doi: 10.1073/pnas.1009181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi T., Stuchebrukhov A.A. Quantum electron tunneling in respiratory complex I. J. Phys. Chem. B. 2011;115(18):5354–5364. doi: 10.1021/jp109410j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wirth C., Brandt U., Hunte C., Zickermann V. Structure and function of mitochondrial complex I. Biochim. Biophys. Acta Bioenerg. 2016;1857(7):902–914. doi: 10.1016/j.bbabio.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Yin Z., Burger N., Kula-Alwar D., Aksentijević D., Bridges H.R., Prag H.A., Grba D.N., Viscomi C., James A.M., Mottahedin A., Krieg T., Murphy M.P., Hirst J. Structural basis for a complex I mutation that blocks pathological ROS production. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-021-20942-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brand M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Burger N., Logan A., Prime T.A., Mottahedin A., Caldwell S.T., Krieg T., Hartley R.C., James A.M., Murphy M.P. A sensitive mass spectrometric assay for mitochondrial CoQ pool redox state in vivo. Free Radic. Biol. Med. 2020;147:37–47. doi: 10.1016/j.freeradbiomed.2019.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orr A.L., Quinlan C.L., Perevoshchikova I.V., Brand M.D. A refined analysis of superoxide production by mitochondrial sn-glycerol 3-phosphate dehydrogenase. J. Biol. Chem. 2012;287(51):42921–42935. doi: 10.1074/jbc.M112.397828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perevoshchikova I.V., Quinlan C.L., Orr A.L., Gerencser A.A., Brand M.D. Sites of superoxide and hydrogen peroxide production during fatty acid oxidation in rat skeletal muscle mitochondria. Free Radic. Biol. Med. 2013;61:298–309. doi: 10.1016/j.freeradbiomed.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guarás A., Perales-Clemente E., Calvo E., Acín-Pérez R., Loureiro-Lopez M., Pujol C., Martínez-Carrascoso I., Nuñez E., García-Marqués F., Rodríguez-Hernández M.A., Cortés A., Diaz F., Pérez-Martos A., Moraes C.T., Fernández-Silva P., Trifunovic A., Navas P., Vazquez J., Enríquez J.A. The CoQH2/CoQ ratio serves as a sensor of respiratory chain efficiency. Cell Rep. 2016;15(1):197–209. doi: 10.1016/j.celrep.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Mailloux R.J., Jin X., Willmore W.G. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol. 2013;2:123–139. doi: 10.1016/j.redox.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komlódi T., Geibl F.F., Sassani M., Ambrus A., Tretter L. Membrane potential and delta pH dependency of reverse electron transport-associated hydrogen peroxide production in brain and heart mitochondria. J. Bioenerg. Biomembr. 2018;50(5):355–365. doi: 10.1007/s10863-018-9766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maldonado E.N., Lemasters J.J. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion. 2014;19 Pt A:78–84. doi: 10.1016/j.mito.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieger B., Arroum T., Borowski M.T., Villalta J., Busch K.B. Mitochondrial F1 FO ATP synthase determines the local proton motive force at cristae rims. EMBO Rep. 2021;22(12) doi: 10.15252/embr.202152727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vergun O., Votyakova T.V., Reynolds I.J. Spontaneous changes in mitochondrial membrane potential in single isolated brain mitochondria. Biophys. J. 2003;85(5):3358–3366. doi: 10.1016/S0006-3495(03)74755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nesci S., Trombetti F., Algieri C., Pagliarani A.A. Therapeutic role for the F1FO-ATP synthase. SLAS Discov. 2019;24(9):893–903. doi: 10.1177/2472555219860448. [DOI] [PubMed] [Google Scholar]
- 40.Song J., Pfanner N., Becker T. Assembling the mitochondrial ATP synthase. Proc. Natl. Acad. Sci. U. S. A. 2018;115(12):2850–2852. doi: 10.1073/pnas.1801697115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rafikov R., Sun X., Rafikova O., Louise Meadows M., Desai A.A., Khalpey Z., Yuan J.X., Fineman J.R., Black S.M. Complex I dysfunction underlies the glycolytic switch in pulmonary hypertensive smooth muscle cells. Redox Biol. 2015;6:278–286. doi: 10.1016/j.redox.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salin K., Villasevil E.M., Auer S.K., Anderson G.J., Selman C., Metcalfe N.B., Chinopoulos C. Simultaneous measurement of mitochondrial respiration and ATP production in tissue homogenates and calculation of effective P/O ratios. Physiological reports. 2016;4(20) doi: 10.14814/phy2.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solaini G., Baracca A., Lenaz G., Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochim. Biophys. Acta. 2010;1797(6–7):1171–1177. doi: 10.1016/j.bbabio.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Sedlack A.J.H., Penjweini R., Link K.A., Brown A., Kim J., Park S.J., Chung J.H., Morgan N.Y., Knutson J.R. Computational modeling and imaging of the intracellular oxygen gradient. Int. J. Mol. Sci. 2022;23(20) doi: 10.3390/ijms232012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi E., Doi K. Impact of diffusional oxygen transport on oxidative metabolism in the heart. Jpn. J. Physiol. 1998;48(4):243–252. doi: 10.2170/jjphysiol.48.243. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi E., Sato M. Anaerobic respiration sustains mitochondrial membrane potential in a prolyl hydroxylase pathway-activated cancer cell line in a hypoxic microenvironment. Am. J. Physiol. Cell Physiol. 2014;306(4):C334–C342. doi: 10.1152/ajpcell.00255.2013. [DOI] [PubMed] [Google Scholar]
- 47.Maltepe E., Saugstad O.D. Oxygen in health and disease: regulation of oxygen homeostasis--clinical implications. Pediatr. Res. 2009;65(3):261–268. doi: 10.1203/PDR.0b013e31818fc83f. [DOI] [PubMed] [Google Scholar]
- 48.Mas-Bargues C., Sanz-Ros J., Román-Domínguez A., Inglés M., Gimeno-Mallench L., El Alami M., Viña-Almunia J., Gambini J., Viña J., Borrás C. Relevance of oxygen concentration in stem cell culture for regenerative medicine. Int. J. Mol. Sci. 2019;20(5):1195. doi: 10.3390/ijms20051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tretter V., Zach M.L., Böhme S., Ullrich R., Markstaller K., Klein K.U. Investigating disturbances of oxygen homeostasis: from cellular mechanisms to the clinical practice. Front. Physiol. 2020;11:947. doi: 10.3389/fphys.2020.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Figueiredo S.M., Sousa F.O.C., Lopes M.A.G., Quinta-Ferreira R.M., Quinta-Ferreira M.E. Effect of oxygen levels in cellular activity. Energy Rep. 2020;6(8):286–291. doi: 10.1016/j.egyr.2020.11.145. [DOI] [Google Scholar]
- 51.Wheaton W.W., Chandel N.S. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am. J. Physiol. Cell Physiol. 2011;300(3):C385–C393. doi: 10.1152/ajpcell.00485.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brookes P.S., Kraus D.W., Shiva S., Doeller J.E., Barone M.C., Patel R.P., Lancaster J.R., Jr., Darley-Usmar V. Control of mitochondrial respiration by NO*, effects of low oxygen and respiratory state. J. Biol. Chem. 2003;278(34):31603–31609. doi: 10.1074/jbc.M211784200. [DOI] [PubMed] [Google Scholar]
- 53.Mason M.G., Nicholls P., Wilson M.T., Cooper C.E. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc. Natl. Acad. Sci. U. S. A. 2006;103(3):708–713. doi: 10.1073/pnas.0506562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guzy R.D., Schumacker P.T. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 2006;91(5):807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 55.Kierans S.J., Taylor C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J. Physiol. 2021;599(1):23–37. doi: 10.1113/JP280572. [DOI] [PubMed] [Google Scholar]
- 56.Michiels C. Physiological and pathological responses to hypoxia. Am. J. Pathol. 2004;164(6):1875–1882. doi: 10.1016/s0002-9440(10)63747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Onukwufor J.O., Farooqi M.A., Vodičková A., Koren S.A., Baldzizhar A., Berry B.J., Beutner G., Porter G.A., Jr., Belousov V., Grossfield A., Wojtovich A.P. A reversible mitochondrial complex I thiol switch mediates hypoxic avoidance behavior in C. elegans. Nat. Commun. 2022;13(1):2403. doi: 10.1038/s41467-022-30169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chandel N.S., McClintock D.S., Feliciano C.E., Wood T.M., Melendez J.A., Rodriguez A.M., Schumacker P.T. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J. Biol. Chem. 2000;275(33):25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 59.Guzy R.D., Hoyos B., Robin E., Chen H., Liu L., Mansfield K.D., Simon M.C., Hammerling U., Schumacker P.T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metabol. 2005;1(6):401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Arias-Mayenco I., González-Rodríguez P., Torres-Torrelo H., Gao L., Fernández-Agüera M.C., Bonilla-Henao V., Ortega-Sáenz P., López-Barneo J. Acute O(2) sensing: role of coenzyme QH(2)/Q ratio and mitochondrial ROS compartmentalization. Cell Metabol. 2018;28(1):145–158.e144. doi: 10.1016/j.cmet.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Dunham-Snary K.J., Wu D., Potus F., Sykes E.A., Mewburn J.D., Charles R.L., Eaton P., Sultanian R.A., Archer S.L. Ndufs2, a core subunit of mitochondrial complex I, is essential for acute oxygen-sensing and hypoxic pulmonary vasoconstriction. Circ. Res. 2019;124(12):1727–1746. doi: 10.1161/circresaha.118.314284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernández-Agüera M.C., Gao L., González-Rodríguez P., Pintado C.O., Arias-Mayenco I., García-Flores P., García-Pergañeda A., Pascual A., Ortega-Sáenz P., López-Barneo J. Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metabol. 2015;22(5):825–837. doi: 10.1016/j.cmet.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Gao L., Ortega-Sáenz P., López-Barneo J. Acute oxygen sensing-Role of metabolic specifications in peripheral chemoreceptor cells. Respir. Physiol. Neurobiol. 2019;265:100–111. doi: 10.1016/j.resp.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Gao L., Ortega-Sáenz P., Moreno-Domínguez A., López-Barneo J. Mitochondrial redox signaling in O(2)-sensing chemoreceptor cells. Antioxidants Redox Signal. 2022;37(4–6):274–289. doi: 10.1089/ars.2021.0255. [DOI] [PubMed] [Google Scholar]
- 65.Ortega-Sáenz P., Moreno-Domínguez A., Gao L., López-Barneo J. Molecular mechanisms of acute oxygen sensing by arterial chemoreceptor cells. Role of Hif2α. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.614893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Formosa L.E., Muellner-Wong L., Reljic B., Sharpe A.J., Jackson T.D., Beilharz T.H., Stojanovski D., Lazarou M., Stroud D.A., Ryan M.T. Dissecting the roles of mitochondrial complex I intermediate assembly complex factors in the biogenesis of complex I. Cell Rep. 2020;31(3) doi: 10.1016/j.celrep.2020.107541. [DOI] [PubMed] [Google Scholar]
- 67.Mimaki M., Wang X., McKenzie M., Thorburn D.R., Ryan M.T. Understanding mitochondrial complex I assembly in health and disease. Biochim. Biophys. Acta. 2012;1817(6):851–862. doi: 10.1016/j.bbabio.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 68.Stroud D.A., Surgenor E.E., Formosa L.E., Reljic B., Frazier A.E., Dibley M.G., Osellame L.D., Stait T., Beilharz T.H., Thorburn D.R., Salim A., Ryan M.T. Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature. 2016;538(7623):123–126. doi: 10.1038/nature19754. [DOI] [PubMed] [Google Scholar]
- 69.Saura P., Kaila V.R.I. Energetics and dynamics of proton-coupled electron transfer in the NADH/FMN site of respiratory complex I. J. Am. Chem. Soc. 2019;141(14):5710–5719. doi: 10.1021/jacs.8b11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parey K., Lasham J., Mills D.J., Djurabekova A., Haapanen O., Yoga E.G., Xie H., Kühlbrandt W., Sharma V., Vonck J., Zickermann V. High-resolution structure and dynamics of mitochondrial complex I-Insights into the proton pumping mechanism. Sci. Adv. 2021;7(46) doi: 10.1126/sciadv.abj3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Babot M., Birch A., Labarbuta P., Galkin A. Characterisation of the active/de-active transition of mitochondrial complex I. Biochim. Biophys. Acta. 2014;1837(7):1083–1092. doi: 10.1016/j.bbabio.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galkin A., Moncada S. Modulation of the conformational state of mitochondrial complex I as a target for therapeutic intervention. Interface Focus. 2017;7(2) doi: 10.1098/rsfs.2016.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hernansanz-Agustín P., Ramos E., Navarro E., Parada E., Sánchez-López N., Peláez-Aguado L., Cabrera-García J.D., Tello D., Buendia I., Marina A., Egea J., López M.G., Bogdanova A., Martínez-Ruiz A. Mitochondrial complex I deactivation is related to superoxide production in acute hypoxia. Redox Biol. 2017;12:1040–1051. doi: 10.1016/j.redox.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kotlyar A.B., Vinogradov A.D. Slow active/inactive transition of the mitochondrial NADH-ubiquinone reductase. Biochim. Biophys. Acta. 1990;1019(2):151–158. doi: 10.1016/0005-2728(90)90137-s. [DOI] [PubMed] [Google Scholar]
- 75.Chung I., Serreli R., Cross J.B., Di Francesco M.E., Marszalek J.R., Hirst J. Cork-in-bottle mechanism of inhibitor binding to mammalian complex I. Sci. Adv. 2021;7(20) doi: 10.1126/sciadv.abg4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fedor J.G., Jones A.J.Y., Di Luca A., Kaila V.R.I., Hirst J. Correlating kinetic and structural data on ubiquinone binding and reduction by respiratory complex I. Proc. Natl. Acad. Sci. U. S. A. 2017;114(48):12737–12742. doi: 10.1073/pnas.1714074114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Galemou Yoga E., Schiller J., Zickermann V. Ubiquinone binding and reduction by complex I-open questions and mechanistic implications. Front. Chem. 2021;9 doi: 10.3389/fchem.2021.672851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pereira C.S., Teixeira M.H., Russell D.A., Hirst J., Arantes G.M. Mechanism of rotenone binding to respiratory complex I depends on ligand flexibility. Sci. Rep. 2023;13(1):6738. doi: 10.1038/s41598-023-33333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hagras M.A., Stuchebrukhov A.A. Concerted two-electron reduction of ubiquinone in respiratory complex I. J. Phys. Chem. B. 2019;123(25):5265–5273. doi: 10.1021/acs.jpcb.9b04082. [DOI] [PubMed] [Google Scholar]
- 80.Baradaran R., Berrisford J.M., Minhas G.S., Sazanov L.A. Crystal structure of the entire respiratory complex I. Nature. 2013;494(7438):443–448. doi: 10.1038/nature11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verkhovskaya M., Bloch D.A. Energy-converting respiratory Complex I: on the way to the molecular mechanism of the proton pump. Int. J. Biochem. Cell Biol. 2013;45(2):491–511. doi: 10.1016/j.biocel.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 82.Zickermann V., Wirth C., Nasiri H., Siegmund K., Schwalbe H., Hunte C., Brandt U. Structural biology. Mechanistic insight from the crystal structure of mitochondrial complex I. Science. 2015;347(6217):44–49. doi: 10.1126/science.1259859. [DOI] [PubMed] [Google Scholar]
- 83.Kampjut D., Sazanov L.A. The coupling mechanism of mammalian respiratory complex I. Science. 2020;370(6516) doi: 10.1126/science.abc4209. [DOI] [PubMed] [Google Scholar]
- 84.Kravchuk V., Petrova O., Kampjut D., Wojciechowska-Bason A., Breese Z., Sazanov L. A universal coupling mechanism of respiratory complex I. Nature. 2022;609(7928):808–814. doi: 10.1038/s41586-022-05199-7. [DOI] [PubMed] [Google Scholar]
- 85.Okun J.G., Lümmen P., Brandt U. Three classes of inhibitors share a common binding domain in mitochondrial complex I (NADH:ubiquinone oxidoreductase) J. Biol. Chem. 1999;274(5):2625–2630. doi: 10.1074/jbc.274.5.2625. [DOI] [PubMed] [Google Scholar]
- 86.Roberts P.G., Hirst J. The deactive form of respiratory complex I from mammalian mitochondria is a Na+/H+ antiporter. J. Biol. Chem. 2012;287(41):34743–34751. doi: 10.1074/jbc.M112.384560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bridges H.R., Fedor J.G., Blaza J.N., Di Luca A., Jussupow A., Jarman O.D., Wright J.J., Agip A.A., Gamiz-Hernandez A.P., Roessler M.M., Kaila V.R.I., Hirst J. Structure of inhibitor-bound mammalian complex I. Nat. Commun. 2020;11(1):5261. doi: 10.1038/s41467-020-18950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem. J. 1972;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chance B., Hollunger G. The interaction of energy and electron transfer reactions in mitochondria. I. General properties and nature of the products of succinate-linked reduction of pyridine nucleotide. J. Biol. Chem. 1961;236:1534–1543. https://www.sciencedirect.com/science/article/pii/S0021925818642103?via%3Dihub [PubMed] [Google Scholar]
- 90.Chance B., Hollunger G. The interaction of energy and electron transfer reactions in mitochondria. IV. The pathway of electron transfer. J. Biol. Chem. 1961;236:1562–1568. [PubMed] [Google Scholar]
- 91.Robb E.L., Hall A.R., Prime T.A., Eaton S., Szibor M., Viscomi C., James A.M., Murphy M.P. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018;293(25):9869–9879. doi: 10.1074/jbc.RA118.003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wright J.J., Biner O., Chung I., Burger N., Bridges H.R., Hirst J. Reverse electron transfer by respiratory complex I catalyzed in a modular proteoliposome system. J. Am. Chem. Soc. 2022;144(15):6791–6801. doi: 10.1021/jacs.2c00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zu Y., Shannon R.J., Hirst J. Reversible, electrochemical interconversion of NADH and NAD+ by the catalytic (Ilambda) subcomplex of mitochondrial NADH:ubiquinone oxidoreductase (complex I) J. Am. Chem. Soc. 2003;125(20):6020–6021. doi: 10.1021/ja0343961. [DOI] [PubMed] [Google Scholar]
- 94.Xiao W., Wang R.S., Handy D.E., Loscalzo J. NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxidants Redox Signal. 2018;28(3):251–272. doi: 10.1089/ars.2017.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu Q., Wu D., Walker M., Wang P., Tian R., Wang W. Genetically encoded biosensors for evaluating NAD(+)/NADH ratio in cytosolic and mitochondrial compartments. Cell Rep Methods. 2021;1(7) doi: 10.1016/j.crmeth.2021.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin S.J., Guarente L. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr. Opin. Cell Biol. 2003;15(2):241–246. doi: 10.1016/s0955-0674(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 97.Yang Y., Sauve A.A. NAD(+) metabolism: bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta. 2016;1864(12):1787–1800. doi: 10.1016/j.bbapap.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amjad S., Nisar S., Bhat A.A., Shah A.R., Frenneaux M.P., Fakhro K., Haris M., Reddy R., Patay Z., Baur J., Bagga P. Role of NAD(+) in regulating cellular and metabolic signaling pathways. Mol. Metabol. 2021;49 doi: 10.1016/j.molmet.2021.101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen C., Mossman E., Malko P., McDonald D., Blain A.P., Bone L., Erskine D., Filby A., Vincent A.E., Hudson G., Reeve A.K. Astrocytic changes in mitochondrial oxidative phosphorylation protein levels in Parkinson's disease. Mov. Disord. 2022;37(2):302–314. doi: 10.1002/mds.28849. [DOI] [PubMed] [Google Scholar]
- 100.Auten R.L., Davis J.M. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr. Res. 2009;66(2):121–127. doi: 10.1203/PDR.0b013e3181a9eafb. [DOI] [PubMed] [Google Scholar]
- 101.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122(6):877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Andreyev A.Y., Kushnareva Y.E., Murphy A.N., Starkov A.A. Mitochondrial ROS metabolism: 10 Years later. Biochemistry (Mosc.) 2015;80(5):517–531. doi: 10.1134/s0006297915050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fridovich I. Oxygen: how do we stand it? Med. Princ. Pract. 2013;22(2):131–137. doi: 10.1159/000339212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hernansanz-Agustín P., Enríquez J.A. Generation of reactive oxygen species by mitochondria. Antioxidants. 2021;10(3) doi: 10.3390/antiox10030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417(1):1–13. doi: 10.1042/bj20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94(3):909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fransen M., Nordgren M., Wang B., Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim. Biophys. Acta. 2012;1822(9):1363–1373. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 108.Kremer M.L. Promotion of the fenton reaction by Cu2+ ions: evidence for intermediates. Int. J. Chem. Kinet. 2006;38(12):725–736. doi: 10.1002/kin.20205. [DOI] [Google Scholar]
- 109.Liochev S.I., Fridovich I. The Haber-Weiss cycle -- 70 years later: an alternative view. Redox Rep. 2002;7(1):55–57. doi: 10.1179/135100002125000190. author reply 59-60. [DOI] [PubMed] [Google Scholar]
- 110.Wang H., Schoebel S., Schmitz F., Dong H., Hedfalk K. Characterization of aquaporin-driven hydrogen peroxide transport. Biochim. Biophys. Acta Biomembr. 2020;1862(2) doi: 10.1016/j.bbamem.2019.183065. [DOI] [PubMed] [Google Scholar]
- 111.Mailloux R.J. Protein S-glutathionylation reactions as a global inhibitor of cell metabolism for the desensitization of hydrogen peroxide signals. Redox Biol. 2020;32 doi: 10.1016/j.redox.2020.101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21(7):363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 113.Quinlan C.L., Perevoschikova I.V., Goncalves R.L., Hey-Mogensen M., Brand M.D. The determination and analysis of site-specific rates of mitochondrial reactive oxygen species production. Methods Enzymol. 2013;526:189–217. doi: 10.1016/b978-0-12-405883-5.00012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Treberg J.R., Quinlan C.L., Brand M.D. Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I) J. Biol. Chem. 2011;286(31):27103–27110. doi: 10.1074/jbc.M111.252502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grivennikova V.G., Vinogradov A.D. Partitioning of superoxide and hydrogen peroxide production by mitochondrial respiratory complex I. Biochim. Biophys. Acta. 2013;1827(3):446–454. doi: 10.1016/j.bbabio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 116.Duong Q.V., Levitsky Y., Dessinger M.J., Strubbe-Rivera J.O., Bazil J.N. Identifying site-specific superoxide and hydrogen peroxide production rates from the mitochondrial electron transport system using a computational strategy. Function. 2021;2(6) doi: 10.1093/function/zqab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gibbs E.T., Lerner C.A., Watson M.A., Wong H.S., Gerencser A.A., Brand M.D. Site IQ in mitochondrial complex I generates S1QEL-sensitive superoxide/hydrogen peroxide in both the reverse and forward reactions. Biochem. J. 2023;480(5):363–384. doi: 10.1042/bcj20220611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kussmaul L., Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2006;103(20):7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chouchani E.T., Pell V.R., Gaude E., Aksentijević D., Sundier S.Y., Robb E.L., Logan A., Nadtochiy S.M., Ord E.N.J., Smith A.C., Eyassu F., Shirley R., Hu C.H., Dare A.J., James A.M., Rogatti S., Hartley R.C., Eaton S., Costa A.S.H., Brookes P.S., Davidson S.M., Duchen M.R., Saeb-Parsy K., Shattock M.J., Robinson A.J., Work L.M., Frezza C., Krieg T., Murphy M.P. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chouchani E.T., Pell V.R., James A.M., Work L.M., Saeb-Parsy K., Frezza C., Krieg T., Murphy M.P. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metabol. 2016;23(2):254–263. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 121.Milliken A.S., Kulkarni C.A., Brookes P.S. Acid enhancement of ROS generation by complex-I reverse electron transport is balanced by acid inhibition of complex-II: relevance for tissue reperfusion injury. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang J., Wang Y.T., Miller J.H., Day M.M., Munger J.C., Brookes P.S. Accumulation of succinate in cardiac ischemia primarily occurs via canonical krebs cycle activity. Cell Rep. 2018;23(9):2617–2628. doi: 10.1016/j.celrep.2018.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Y., Zhang M., Zhu W., Yu J., Wang Q., Zhang J., Cui Y., Pan X., Gao X., Sun H. Succinate accumulation induces mitochondrial reactive oxygen species generation and promotes status epilepticus in the kainic acid rat model. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tomar N., Zhang X., Kandel S.M., Sadri S., Yang C., Liang M., Audi S.H., Cowley A.W., Jr., Dash R.K. Substrate-dependent differential regulation of mitochondrial bioenergetics in the heart and kidney cortex and outer medulla. Biochim. Biophys. Acta Bioenerg. 2022;1863(2) doi: 10.1016/j.bbabio.2021.148518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Niatsetskaya Z.V., Sosunov S.A., Matsiukevich D., Utkina-Sosunova I.V., Ratner V.I., Starkov A.A., Ten V.S. The oxygen free radicals originating from mitochondrial complex I contribute to oxidative brain injury following hypoxia-ischemia in neonatal mice. J. Neurosci. 2012;32(9):3235–3244. doi: 10.1523/jneurosci.6303-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goncalves R.L.S., Watson M.A., Wong H.S., Orr A.L., Brand M.D. The use of site-specific suppressors to measure the relative contributions of different mitochondrial sites to skeletal muscle superoxide and hydrogen peroxide production. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Okoye C.N., Chinnappareddy N., Stevens D., Kamunde C. Factors affecting liver mitochondrial hydrogen peroxide emission. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022;259 doi: 10.1016/j.cbpb.2022.110713. [DOI] [PubMed] [Google Scholar]
- 128.Lambert A.J., Buckingham J.A., Brand M.D. Dissociation of superoxide production by mitochondrial complex I from NAD(P)H redox state. FEBS Lett. 2008;582(12):1711–1714. doi: 10.1016/j.febslet.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 129.Abramov A.Y., Scorziello A., Duchen M.R. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J. Neurosci. 2007;27(5):1129–1138. doi: 10.1523/jneurosci.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kang P.T., Chen C.L., Lin P., Zhang L., Zweier J.L., Chen Y.R. Mitochondrial complex I in the post-ischemic heart: reperfusion-mediated oxidative injury and protein cysteine sulfonation. J. Mol. Cell. Cardiol. 2018;121:190–204. doi: 10.1016/j.yjmcc.2018.07.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Korge P., Ping P., Weiss J.N. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation. Circ. Res. 2008;103(8):873–880. doi: 10.1161/CIRCRESAHA.108.180869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Okoye C.N., Stevens D., Kamunde C. Modulation of mitochondrial site-specific hydrogen peroxide efflux by exogenous stressors. Free Radic. Biol. Med. 2021;164:439–456. doi: 10.1016/j.freeradbiomed.2020.12.234. [DOI] [PubMed] [Google Scholar]
- 133.Yoval-Sánchez B., Ansari F., James J., Niatsetskaya Z., Sosunov S., Filipenko P., Tikhonova I.G., Ten V., Wittig I., Rafikov R., Galkin A. Redox-dependent loss of flavin by mitochondria complex I is different in brain and heart. Redox Biol. 2022;51 doi: 10.1016/j.redox.2022.102258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chapman A.G., Nordström C.H., Siesjö B.K. Influence of phenobarbital anesthesia on carbohydrate and amino acid metabolism in rat brain. Anesthesiology. 1978;48(3):175–182. doi: 10.1097/00000542-197803000-00003. [DOI] [PubMed] [Google Scholar]
- 135.Nordström C.H., Rehncrona S., Siesjö B.K. Effects of phenobarbital in cerebral ischemia. Part II: restitution of cerebral energy state, as well as of glycolytic metabolites, citric acid cycle intermediates and associated amino acids after pronounced incomplete ischemia. Stroke. 1978;9(4):335–343. doi: 10.1161/01.str.9.4.335. [DOI] [PubMed] [Google Scholar]
- 136.Hoffman D.L., Brookes P.S. Oxygen sensitivity of mitochondrial reactive oxygen species generation depends on metabolic conditions. J. Biol. Chem. 2009;284(24):16236–16245. doi: 10.1074/jbc.M809512200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stepanova A., Konrad C., Guerrero-Castillo S., Manfredi G., Vannucci S., Arnold S., Galkin A. Deactivation of mitochondrial complex I after hypoxia-ischemia in the immature brain. J. Cerebr. Blood Flow Metabol. 2019;39(9):1790–1802. doi: 10.1177/0271678x18770331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gorenkova N., Robinson E., Grieve D.J., Galkin A. Conformational change of mitochondrial complex I increases ROS sensitivity during ischemia. Antioxidants Redox Signal. 2013;19(13):1459–1468. doi: 10.1089/ars.2012.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Matsuzaki S., Humphries K.M. Selective inhibition of deactivated mitochondrial complex I by biguanides. Biochemistry. 2015;54(11):2011–2021. doi: 10.1021/bi501473h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hernansanz-Agustín P., Izquierdo-Álvarez A., Sánchez-Gómez F.J., Ramos E., Villa-Piña T., Lamas S., Bogdanova A., Martínez-Ruiz A. Acute hypoxia produces a superoxide burst in cells. Free Radic. Biol. Med. 2014;71:146–156. doi: 10.1016/j.freeradbiomed.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 141.Vinogradov A.D., Grivennikova V.G. Generation of superoxide-radical by the NADH:ubiquinone oxidoreductase of heart mitochondria. Biochemistry (Mosc.) 2005;70(2):120–127. doi: 10.1007/s10541-005-0090-7. [DOI] [PubMed] [Google Scholar]
- 142.Dröse S., Stepanova A., Galkin A. Ischemic A/D transition of mitochondrial complex I and its role in ROS generation. Biochim. Biophys. Acta. 2016;1857(7):946–957. doi: 10.1016/j.bbabio.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gostimskaya I.S., Grivennikova V.G., Cecchini G., Vinogradov A.D. Reversible dissociation of flavin mononucleotide from the mammalian membrane-bound NADH: ubiquinone oxidoreductase (complex I) FEBS Lett. 2007;581(30):5803–5806. doi: 10.1016/j.febslet.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stepanova A., Sosunov S., Niatsetskaya Z., Konrad C., Starkov A.A., Manfredi G., Wittig I., Ten V., Galkin A. Redox-dependent loss of flavin by mitochondrial complex I in brain ischemia/reperfusion injury. Antioxidants Redox Signal. 2019;31(9):608–622. doi: 10.1089/ars.2018.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bleier L., Wittig I., Heide H., Steger M., Brandt U., Dröse S. Generator-specific targets of mitochondrial reactive oxygen species. Free Radic. Biol. Med. 2015;78:1–10. doi: 10.1016/j.freeradbiomed.2014.10.511. [DOI] [PubMed] [Google Scholar]
- 146.Kumar V., Kleffmann T., Hampton M.B., Cannell M.B., Winterbourn C.C. Redox proteomics of thiol proteins in mouse heart during ischemia/reperfusion using ICAT reagents and mass spectrometry. Free Radic. Biol. Med. 2013;58:109–117. doi: 10.1016/j.freeradbiomed.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 147.McLain A.L., Cormier P.J., Kinter M., Szweda L.I. Glutathionylation of α-ketoglutarate dehydrogenase: the chemical nature and relative susceptibility of the cofactor lipoic acid to modification. Free Radic. Biol. Med. 2013;61:161–169. doi: 10.1016/j.freeradbiomed.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tretter L., Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: a key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J. Neurosci. 2000;20(24):8972–8979. doi: 10.1523/jneurosci.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fisher-Wellman K.H., Gilliam L.A.A., Lin C.T., Cathey B.L., Lark D.S., Darrell Neufer P. Mitochondrial glutathione depletion reveals a novel role for the pyruvate dehydrogenase complex as a key H2O2-emitting source under conditions of nutrient overload. Free Radic. Biol. Med. 2013;65:1201–1208. doi: 10.1016/j.freeradbiomed.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mailloux R.J., Craig Ayre D., Christian S.L. Induction of mitochondrial reactive oxygen species production by GSH mediated S-glutathionylation of 2-oxoglutarate dehydrogenase. Redox Biol. 2016;8:285–297. doi: 10.1016/j.redox.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tretter L., Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J. Neurosci. 2004;24(36):7771–7778. doi: 10.1523/jneurosci.1842-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bogaert Y.E., Rosenthal R.E., Fiskum G. Postischemic inhibition of cerebral cortex pyruvate dehydrogenase. Free Radic. Biol. Med. 1994;16(6):811–820. doi: 10.1016/0891-5849(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 153.Zaidan E., Sheu K.F., Sims N.R. The pyruvate dehydrogenase complex is partially inactivated during early recirculation following short-term forebrain ischemia in rats. J. Neurochem. 1998;70(1):233–241. doi: 10.1046/j.1471-4159.1998.70010233.x. [DOI] [PubMed] [Google Scholar]
- 154.Lucas D.T., Szweda L.I. Declines in mitochondrial respiration during cardiac reperfusion: age-dependent inactivation of α-ketoglutarate dehydrogenase. Proc. Natl. Acad. Sci. USA. 1999;96(12):6689–6693. doi: 10.1073/pnas.96.12.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tretter L., Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360(1464):2335–2345. doi: 10.1098/rstb.2005.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dröse S., Brandt U., Wittig I. Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim. Biophys. Acta. 2014;1844(8):1344–1354. doi: 10.1016/j.bbapap.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 157.Paradies G., Petrosillo G., Pistolese M., Venosa N.D., Federici A., Ruggiero F.M. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart. Circ. Res. 2004;94(1):53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- 158.Chen C.L., Zhang L., Jin Z., Kasumov T., Chen Y.R. Mitochondrial redox regulation and myocardial ischemia-reperfusion injury. Am. J. Physiol. Cell Physiol. 2022;322(1):C12–c23. doi: 10.1152/ajpcell.00131.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tompkins A.J., Burwell L.S., Digerness S.B., Zaragoza C., Holman W.L., Brookes P.S. Mitochondrial dysfunction in cardiac ischemia-reperfusion injury: ROS from complex I, without inhibition. Biochim. Biophys. Acta. 2006;1762(2):223–231. doi: 10.1016/j.bbadis.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 160.Mailloux R.J., Grayson C., Koufos O. Regulation of mitochondrial hydrogen peroxide availability by protein S-glutathionylation. Cells. 2022;12(1) doi: 10.3390/cells12010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Burger N., James A.M., Mulvey J.F., Hoogewijs K., Ding S., Fearnley I.M., Loureiro-López M., Norman A.A.I., Arndt S., Mottahedin A., Sauchanka O., Hartley R.C., Krieg T., Murphy M.P. ND3 Cys39 in complex I is exposed during mitochondrial respiration. Cell Chem. Biol. 2022;29(4):636–649.e614. doi: 10.1016/j.chembiol.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chouchani E.T., Methner C., Nadtochiy S.M., Logan A., Pell V.R., Ding S., James A.M., Cochemé H.M., Reinhold J., Lilley K.S., Partridge L., Fearnley I.M., Robinson A.J., Hartley R.C., Smith R.A., Krieg T., Brookes P.S., Murphy M.P. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013;19(6):753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maranzana E., Barbero G., Falasca A.I., Lenaz G., Genova M.L. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxidants Redox Signal. 2013;19(13):1469–1480. doi: 10.1089/ars.2012.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reyes-Galindo M., Suarez R., Esparza-Perusquía M., de Lira-Sánchez J., Pardo J.P., Martínez F., Flores-Herrera O. Mitochondrial respirasome works as a single unit and the cross-talk between complexes I, III(2) and IV stimulates NADH dehydrogenase activity. Biochim. Biophys. Acta Bioenerg. 2019;1860(8):618–627. doi: 10.1016/j.bbabio.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 165.Lapuente-Brun E., Moreno-Loshuertos R., Acín-Pérez R., Latorre-Pellicer A., Colás C., Balsa E., Perales-Clemente E., Quirós P.M., Calvo E., Rodríguez-Hernández M.A., Navas P., Cruz R., Carracedo Á., López-Otín C., Pérez-Martos A., Fernández-Silva P., Fernández-Vizarra E., Enríquez J.A. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science. 2013;340(6140):1567–1570. doi: 10.1126/science.1230381. [DOI] [PubMed] [Google Scholar]
- 166.Xiao H., Jedrychowski M.P., Schweppe D.K., Huttlin E.L., Yu Q., Heppner D.E., Li J., Long J., Mills E.L., Szpyt J., He Z., Du G., Garrity R., Reddy A., Vaites L.P., Paulo J.A., Zhang T., Gray N.S., Gygi S.P., Chouchani E.T. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell. 2020;180(5):968–983.e924. doi: 10.1016/j.cell.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bak D.W., Bechtel T.J., Falco J.A., Weerapana E. Cysteine reactivity across the subcellular universe. Curr. Opin. Chem. Biol. 2019;48:96–105. doi: 10.1016/j.cbpa.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marino S.M., Gladyshev V.N. Cysteine function governs its conservation and degeneration and restricts its utilization on protein surfaces. J. Mol. Biol. 2010;404(5):902–916. doi: 10.1016/j.jmb.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Habich M., Salscheider S.L., Riemer J. Cysteine residues in mitochondrial intermembrane space proteins: more than just import. Br. J. Pharmacol. 2019;176(4):514–531. doi: 10.1111/bph.14480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bak D.W., Pizzagalli M.D., Weerapana E. Identifying functional cysteine residues in the mitochondria. ACS Chem. Biol. 2017;12(4):947–957. doi: 10.1021/acschembio.6b01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Danielson S.R., Held J.M., Oo M., Riley R., Gibson B.W., Andersen J.K. Quantitative mapping of reversible mitochondrial Complex I cysteine oxidation in a Parkinson disease mouse model. J. Biol. Chem. 2011;286(9):7601–7608. doi: 10.1074/jbc.M110.190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.van der Reest J., Lilla S., Zheng L., Zanivan S., Gottlieb E. Proteome-wide analysis of cysteine oxidation reveals metabolic sensitivity to redox stress. Nat. Commun. 2018;9(1):1581. doi: 10.1038/s41467-018-04003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brandes N., Schmitt S., Jakob U. Thiol-based redox switches in eukaryotic proteins. Antioxidants Redox Signal. 2009;11(5):997–1014. doi: 10.1089/ars.2008.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Groitl B., Jakob U. Thiol-based redox switches. Biochim. Biophys. Acta. 2014;1844(8):1335–1343. doi: 10.1016/j.bbapap.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Booty L.M., Gawel J.M., Cvetko F., Caldwell S.T., Hall A.R., Mulvey J.F., James A.M., Hinchy E.C., Prime T.A., Arndt S., Beninca C., Bright T.P., Clatworthy M.R., Ferdinand J.R., Prag H.A., Logan A., Prudent J., Krieg T., Hartley R.C., Murphy M.P. Selective disruption of mitochondrial thiol redox state in cells and in vivo. Cell Chem. Biol. 2019;26(3):449–461.e448. doi: 10.1016/j.chembiol.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lin T.K., Hughes G., Muratovska A., Blaikie F.H., Brookes P.S., Darley-Usmar V., Smith R.A., Murphy M.P. Specific modification of mitochondrial protein thiols in response to oxidative stress: a proteomics approach. J. Biol. Chem. 2002;277(19):17048–17056. doi: 10.1074/jbc.M110797200. [DOI] [PubMed] [Google Scholar]
- 177.Chung H.S., Wang S.-B., Venkatraman V., Murray C.I., Eyk J.E.V. Cysteine oxidative posttranslational modifications. Circ. Res. 2013;112(2):382–392. doi: 10.1161/CIRCRESAHA.112.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Murray C.I., Van Eyk J.E. Chasing cysteine oxidative modifications: proteomic tools for characterizing cysteine redox status. Circ Cardiovasc Genet. 2012;5(5):591. doi: 10.1161/circgenetics.111.961425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lo Conte M., Carroll K.S. The redox biochemistry of protein sulfenylation and sulfinylation. J. Biol. Chem. 2013;288(37):26480–26488. doi: 10.1074/jbc.R113.467738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Forman H.J., Ursini F., Maiorino M. An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 2014;73:2–9. doi: 10.1016/j.yjmcc.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sies H. Oxidative eustress: on constant alert for redox homeostasis. Redox Biol. 2021;41 doi: 10.1016/j.redox.2021.101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 183.Wojtovich A.P., Berry B.J., Galkin A. Redox signaling through compartmentalization of reactive oxygen species: implications for health and disease. Antioxidants Redox Signal. 2019;31(9):591–593. doi: 10.1089/ars.2019.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Granger D.N., Kvietys P.R. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 186.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., Abete P. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/cia.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stefanatos R., Sanz A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018;592(5):743–758. doi: 10.1002/1873-3468.12902. [DOI] [PubMed] [Google Scholar]
- 188.Jia Q., Sieburth D. Mitochondrial hydrogen peroxide positively regulates neuropeptide secretion during diet-induced activation of the oxidative stress response. Nat. Commun. 2021;12(1):2304. doi: 10.1038/s41467-021-22561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li G., Gong J., Lei H., Liu J., Xu X.Z. Promotion of behavior and neuronal function by reactive oxygen species in C. elegans. Nat. Commun. 2016;7 doi: 10.1038/ncomms13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Graham C., Stefanatos R., Yek A.E.H., Spriggs R.V., Loh S.H.Y., Uribe A.H., Zhang T., Martins L.M., Maddocks O.D.K., Scialo F., Sanz A. Mitochondrial ROS signalling requires uninterrupted electron flow and is lost during ageing in flies. Geroscience. 2022;44(4):1961–1974. doi: 10.1007/s11357-022-00555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Scialò F., Sriram A., Stefanatos R., Spriggs R.V., Loh S.H.Y., Martins L.M., Sanz A. Mitochondrial complex I derived ROS regulate stress adaptation in Drosophila melanogaster. Redox Biol. 2020;32 doi: 10.1016/j.redox.2020.101450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schmeisser S., Priebe S., Groth M., Monajembashi S., Hemmerich P., Guthke R., Platzer M., Ristow M. Neuronal ROS signaling rather than AMPK/sirtuin-mediated energy sensing links dietary restriction to lifespan extension. Mol. Metabol. 2013;2(2):92–102. doi: 10.1016/j.molmet.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang Q., Yan Q., Yang H., Wei W. Oxygen sensing and adaptability won the 2019 Nobel Prize in Physiology or medicine. Genes Dis. 2019;6(4):328–332. doi: 10.1016/j.gendis.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Burtscher M., Millet G.P., Burtscher J. Hypoxia conditioning for high-altitude pre-acclimatization. Journal of Science in Sport and Exercise. 2022;4(4):331–345. doi: 10.1007/s42978-021-00150-0. [DOI] [Google Scholar]
- 195.Dunham-Snary K.J., Wu D., Sykes E.A., Thakrar A., Parlow L.R.G., Mewburn J.D., Parlow J.L., Archer S.L. Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest. 2017;151(1):181–192. doi: 10.1016/j.chest.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pamenter M.E., Powell F.L. Time domains of the hypoxic ventilatory response and their molecular basis. Compr. Physiol. 2016;6(3):1345–1385. doi: 10.1002/cphy.c150026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Swenson E.R. Early hours in the development of high-altitude pulmonary edema: time course and mechanisms. J. Appl. Physiol. 2020;128(6):1539–1546. doi: 10.1152/japplphysiol.00824.2019. 1985. [DOI] [PubMed] [Google Scholar]
- 198.Wu D., Dasgupta A., Read A.D., Bentley R.E.T., Motamed M., Chen K.H., Al-Qazazi R., Mewburn J.D., Dunham-Snary K.J., Alizadeh E., Tian L., Archer S.L. Oxygen sensing, mitochondrial biology and experimental therapeutics for pulmonary hypertension and cancer. Free Radic. Biol. Med. 2021;170:150–178. doi: 10.1016/j.freeradbiomed.2020.12.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yu J.J., Non A.L., Heinrich E.C., Gu W., Alcock J., Moya E.A., Lawrence E.S., Tift M.S., O'Brien K.A., Storz J.F., Signore A.V., Khudyakov J.I., Milsom W.K., Wilson S.M., Beall C.M., Villafuerte F.C., Stobdan T., Julian C.G., Moore L.G., Fuster M.M., Stokes J.A., Milner R., West J.B., Zhang J., Shyy J.Y., Childebayeva A., Vázquez-Medina J.P., Pham L.V., Mesarwi O.A., Hall J.E., Cheviron Z.A., Sieker J., Blood A.B., Yuan J.X., Scott G.R., Rana B.K., Ponganis P.J., Malhotra A., Powell F.L., Simonson T.S. Time domains of hypoxia responses and -omics insights. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.885295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhao L., Fenk L.A., Nilsson L., Amin-Wetzel N.P., Ramirez-Suarez N.J., de Bono M., Chen C. ROS and cGMP signaling modulate persistent escape from hypoxia in Caenorhabditis elegans. PLoS Biol. 2022;20(6) doi: 10.1371/journal.pbio.3001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cummins E.P., Strowitzki M.J., Taylor C.T. Mechanisms and consequences of oxygen and carbon dioxide sensing in mammals. Physiol. Rev. 2020;100(1):463–488. doi: 10.1152/physrev.00003.2019. [DOI] [PubMed] [Google Scholar]
- 202.Gao L., González-Rodríguez P., Ortega-Sáenz P., López-Barneo J. Redox signaling in acute oxygen sensing. Redox Biol. 2017;12:908–915. doi: 10.1016/j.redox.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Prabhakar N. Academic Press; 2016. O2 and CO2 Detection by the Carotid and Aortic Bodies. [DOI] [Google Scholar]
- 204.Sobrino V., Platero-Luengo A., Annese V., Navarro-Guerrero E., González-Rodríguez P., López-Barneo J., Pardal R. Neurotransmitter modulation of carotid body germinal niche. Int. J. Mol. Sci. 2020;21(21) doi: 10.3390/ijms21218231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dunham-Snary K.J., Hong Z.G., Xiong P.Y., Del Paggio J.C., Herr J.E., Johri A.M., Archer S.L. A mitochondrial redox oxygen sensor in the pulmonary vasculature and ductus arteriosus. Pflügers Archiv. 2016;468(1):43–58. doi: 10.1007/s00424-015-1736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Moudgil R., Michelakis E.D., Archer S.L. The role of k+ channels in determining pulmonary vascular tone, oxygen sensing, cell proliferation, and apoptosis: implications in hypoxic pulmonary vasoconstriction and pulmonary arterial hypertension. Microcirculation. 2006;13(8):615–632. doi: 10.1080/10739680600930222. [DOI] [PubMed] [Google Scholar]
- 207.Veit F., Pak O., Brandes R.P., Weissmann N. Hypoxia-dependent reactive oxygen species signaling in the pulmonary circulation: focus on ion channels. Antioxidants Redox Signal. 2015;22(6):537–552. doi: 10.1089/ars.2014.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chang A.J. Acute oxygen sensing by the carotid body: from mitochondria to plasma membrane. J. Appl. Physiol. 2017;123(5):1335–1343. doi: 10.1152/japplphysiol.00398.2017. 1985. [DOI] [PubMed] [Google Scholar]
- 209.López-Barneo J., González-Rodríguez P., Gao L., Fernández-Agüera M.C., Pardal R., Ortega-Sáenz P. Oxygen sensing by the carotid body: mechanisms and role in adaptation to hypoxia. Am. J. Physiol. Cell Physiol. 2016;310(8):C629–C642. doi: 10.1152/ajpcell.00265.2015. [DOI] [PubMed] [Google Scholar]
- 210.Torres-Torrelo H., Ortega-Sáenz P., Gao L., López-Barneo J. Lactate sensing mechanisms in arterial chemoreceptor cells. Nat. Commun. 2021;12(1):4166. doi: 10.1038/s41467-021-24444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lee S., Park M., So I., Earm Y.E. NADH and NAD modulates Ca(2+)-activated K+ channels in small pulmonary arterial smooth muscle cells of the rabbit. Pflügers Archiv. 1994;427(3–4):378–380. doi: 10.1007/bf00374548. [DOI] [PubMed] [Google Scholar]
- 212.Archer S.L., Huang J., Henry T., Peterson D., Weir E.K. A redox-based O2 sensor in rat pulmonary vasculature. Circ. Res. 1993;73(6):1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 213.Vestergaard M.B., Ghanizada H., Lindberg U., Arngrim N., Paulson O.B., Gjedde A., Ashina M., Larsson H.B.W. Human cerebral perfusion, oxygen consumption, and lactate production in response to hypoxic exposure. Cerebr. Cortex. 2022;32(6):1295–1306. doi: 10.1093/cercor/bhab294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jiménez-Gómez B., Ortega-Sáenz P., Gao L., González-Rodríguez P., García-Flores P., Chandel N., López-Barneo J. Transgenic NADH dehydrogenase restores oxygen regulation of breathing in mitochondrial complex I-deficient mice. Nat. Commun. 2023;14(1):1172. doi: 10.1038/s41467-023-36894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.McElroy G.S., Chakrabarty R.P., D'Alessandro K.B., Hu Y.S., Vasan K., Tan J., Stoolman J.S., Weinberg S.E., Steinert E.M., Reyfman P.A., Singer B.D., Ladiges W.C., Gao L., Lopéz-Barneo J., Ridge K., Budinger G.R.S., Chandel N.S. Reduced expression of mitochondrial complex I subunit Ndufs2 does not impact healthspan in mice. Sci. Rep. 2022;12(1):5196. doi: 10.1038/s41598-022-09074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Swiderska A., Coney A.M., Alzahrani A.A., Aldossary H.S., Batis N., Ray C.J., Kumar P., Holmes A.P. Mitochondrial succinate metabolism and reactive oxygen species are important but not essential for eliciting carotid body and ventilatory responses to hypoxia in the rat. Antioxidants. 2021;10(6) doi: 10.3390/antiox10060840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Buckler K.J., Turner P.J. Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J. Physiol. 2013;591(14):3549–3563. doi: 10.1113/jphysiol.2013.257741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Holmes A.P., Ray C.J., Coney A.M., Kumar P. Is carotid body physiological O(2) sensitivity determined by a unique mitochondrial phenotype? Front. Physiol. 2018;9:562. doi: 10.3389/fphys.2018.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Moreno-Domínguez A., Ortega-Sáenz P., Gao L., Colinas O., García-Flores P., Bonilla-Henao V., Aragonés J., Hüttemann M., Grossman L.I., Weissmann N., Sommer N., López-Barneo J. Acute O(2) sensing through HIF2α-dependent expression of atypical cytochrome oxidase subunits in arterial chemoreceptors. Sci. Signal. 2020;13(615) doi: 10.1126/scisignal.aay9452. [DOI] [PubMed] [Google Scholar]
- 220.Thompson R.J., Buttigieg J., Zhang M., Nurse C.A. A rotenone-sensitive site and H2O2 are key components of hypoxia-sensing in neonatal rat adrenomedullary chromaffin cells. Neuroscience. 2007;145(1):130–141. doi: 10.1016/j.neuroscience.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 221.Read A.D., Bentley R.E., Archer S.L., Dunham-Snary K.J. Mitochondrial iron-sulfur clusters: structure, function, and an emerging role in vascular biology. Redox Biol. 2021;47 doi: 10.1016/j.redox.2021.102164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Koren S.A., Ahmed Selim N., De la Rosa L., Horn J., Farooqi M.A., Wei A.Y., Müller-Eigner A., Emerson J., Johnson G.V.W., Wojtovich A.P. All-optical spatiotemporal mapping of ROS dynamics across mitochondrial microdomains in situ. Nat. Commun. 2023;14(1):6036. doi: 10.1038/s41467-023-41682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Veal E.A., Day A.M., Morgan B.A. Hydrogen peroxide sensing and signaling. Mol. Cell. 2007;26(1):1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 224.Laporte A., Lortz S., Schaal C., Lenzen S., Elsner M. Hydrogen peroxide permeability of cellular membranes in insulin-producing cells. Biochim. Biophys. Acta Biomembr. 2020;1862(2) doi: 10.1016/j.bbamem.2019.183096. [DOI] [PubMed] [Google Scholar]
- 225.Ledo A., Fernandes E., Salvador A., Laranjinha J., Barbosa R.M. In vivo hydrogen peroxide diffusivity in brain tissue supports volume signaling activity. Redox Biol. 2022;50 doi: 10.1016/j.redox.2022.102250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Seaver L.C., Imlay J.A. Hydrogen peroxide fluxes and compartmentalization inside growing <i>Escherichia coli</i>. J. Bacteriol. 2001;183(24):7182–7189. doi: 10.1128/jb.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Calabrese G., Peker E., Amponsah P.S., Hoehne M.N., Riemer T., Mai M., Bienert G.P., Deponte M., Morgan B., Riemer J. Hyperoxidation of mitochondrial peroxiredoxin limits H2O2-induced cell death in yeast. EMBO J. 2019;38(18) doi: 10.15252/embj.2019101552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sobotta M.C., Liou W., Stöcker S., Talwar D., Oehler M., Ruppert T., Scharf A.N.D., Dick T.P. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 2015;11(1):64–70. doi: 10.1038/nchembio.1695. [DOI] [PubMed] [Google Scholar]
- 229.Travasso R.D.M., Sampaio Dos Aidos F., Bayani A., Abranches P., Salvador A. Localized redox relays as a privileged mode of cytoplasmic hydrogen peroxide signaling. Redox Biol. 2017;12:233–245. doi: 10.1016/j.redox.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.van Dam L., Pagès-Gallego M., Polderman P.E., van Es R.M., Burgering B.M.T., Vos H.R., Dansen T.B. The human 2-cys peroxiredoxins form widespread, cysteine-dependent- and isoform-specific protein-protein interactions. Antioxidants. 2021;10(4) doi: 10.3390/antiox10040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Xia Q., Casas-Martinez J.C., Zarzuela E., Muñoz J., Miranda-Vizuete A., Goljanek-Whysall K., McDonagh B. Peroxiredoxin 2 is required for the redox mediated adaptation to exercise. Redox Biol. 2023;60 doi: 10.1016/j.redox.2023.102631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cox Andrew G., Winterbourn Christine C., Hampton Mark B. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem. J. 2009;425(2):313–325. doi: 10.1042/bj20091541. [DOI] [PubMed] [Google Scholar]
- 233.Forred B.J., Daugaard D.R., Titus B.K., Wood R.R., Floen M.J., Booze M.L., Vitiello P.F. Detoxification of mitochondrial oxidants and apoptotic signaling are facilitated by thioredoxin-2 and peroxiredoxin-3 during hyperoxic injury. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0168777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Reczek C.R., Chandel N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Waypa G.B., Chandel N.S., Schumacker P.T. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ. Res. 2001;88(12):1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 236.Waypa G.B., Marks J.D., Mack M.M., Boriboun C., Mungai P.T., Schumacker P.T. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ. Res. 2002;91(8):719–726. doi: 10.1161/01.RES.0000036751.04896.F1. [DOI] [PubMed] [Google Scholar]
- 237.Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metabol. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 238.Casas A.I., Geuss E., Kleikers P.W.M., Mencl S., Herrmann A.M., Buendia I., Egea J., Meuth S.G., Lopez M.G., Kleinschnitz C., Schmidt H. NOX4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Proc. Natl. Acad. Sci. U. S. A. 2017;114(46):12315–12320. doi: 10.1073/pnas.1705034114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Uchiyama M., Nakao A., Kurita Y., Fukushi I., Takeda K., Numata T., Tran H.N., Sawamura S., Ebert M., Kurokawa T., Sakaguchi R., Stokes A.J., Takahashi N., Okada Y., Mori Y. O(2)-Dependent protein internalization underlies astrocytic sensing of acute hypoxia by restricting multimodal TRPA1 channel responses. Curr. Biol. 2020;30(17):3378–3396. doi: 10.1016/j.cub.2020.06.047. e3377. [DOI] [PubMed] [Google Scholar]
- 240.Wang X., Michaelis E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang X., Zaidi A., Pal R., Garrett A.S., Braceras R., Chen X.W., Michaelis M.L., Michaelis E.K. Genomic and biochemical approaches in the discovery of mechanisms for selective neuronal vulnerability to oxidative stress. BMC Neurosci. 2009;10:12. doi: 10.1186/1471-2202-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Keller D., Erö C., Markram H. Cell densities in the mouse brain: a systematic review. Front. Neuroanat. 2018;12:83. doi: 10.3389/fnana.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Clarke B.E., Taha D.M., Tyzack G.E., Patani R. Regionally encoded functional heterogeneity of astrocytes in health and disease: a perspective. Glia. 2021;69(1):20–27. doi: 10.1002/glia.23877. [DOI] [PubMed] [Google Scholar]
- 244.Verkhratsky A., Nedergaard M. Physiology of astroglia. Physiol. Rev. 2018;98(1):239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gourine A.V., Kasymov V., Marina N., Tang F., Figueiredo M.F., Lane S., Teschemacher A.G., Spyer K.M., Deisseroth K., Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329(5991):571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Xu L., Sapolsky R.M., Giffard R.G. Differential sensitivity of murine astrocytes and neurons from different brain regions to injury. Exp. Neurol. 2001;169(2):416–424. doi: 10.1006/exnr.2001.7678. [DOI] [PubMed] [Google Scholar]
- 247.Zhao G., Flavin M.P. Differential sensitivity of rat hippocampal and cortical astrocytes to oxygen-glucose deprivation injury. Neurosci. Lett. 2000;285(3):177–180. doi: 10.1016/s0304-3940(00)01056-9. [DOI] [PubMed] [Google Scholar]
- 248.Almeida A., Jimenez-Blasco D., Bolaños J.P. Cross-talk between energy and redox metabolism in astrocyte-neuron functional cooperation. Essays Biochem. 2023;67(1):17–26. doi: 10.1042/ebc20220075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Almeida A., Moncada S., Bolaños J.P. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 2004;6(1):45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- 250.Bonvento G., Bolaños J.P. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metabol. 2021;33(8):1546–1564. doi: 10.1016/j.cmet.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 251.Lopez-Fabuel I., Le Douce J., Logan A., James A.M., Bonvento G., Murphy M.P., Almeida A., Bolaños J.P. Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc. Natl. Acad. Sci. U. S. A. 2016;113(46):13063–13068. doi: 10.1073/pnas.1613701113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Silva E.A., Dalla Costa A.P., Ruas J.S., Siqueira-Santos E.S., Francisco A., Castilho R.F. Proliferating astrocytes in primary culture do not depend upon mitochondrial respiratory complex I activity or oxidative phosphorylation. Cells. 2023;12(5) doi: 10.3390/cells12050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Eraso-Pichot A., Brasó-Vives M., Golbano A., Menacho C., Claro E., Galea E., Masgrau R. GSEA of mouse and human mitochondriomes reveals fatty acid oxidation in astrocytes. Glia. 2018;66(8):1724–1735. doi: 10.1002/glia.23330. [DOI] [PubMed] [Google Scholar]
- 254.Natarajaseenivasan K., Cotto B., Shanmughapriya S., Lombardi A.A., Datta P.K., Madesh M., Elrod J.W., Khalili K., Langford D. Astrocytic metabolic switch is a novel etiology for Cocaine and HIV-1 Tat-mediated neurotoxicity. Cell Death Dis. 2018;9(4):415. doi: 10.1038/s41419-018-0422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Polyzos A.A., Lee D.Y., Datta R., Hauser M., Budworth H., Holt A., Mihalik S., Goldschmidt P., Frankel K., Trego K., Bennett M.J., Vockley J., Xu K., Gratton E., McMurray C.T. Metabolic reprogramming in astrocytes distinguishes region-specific neuronal susceptibility in huntington mice. Cell Metabol. 2019;29(6):1258–1273. doi: 10.1016/j.cmet.2019.03.004. e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Herrero-Mendez A., Almeida A., Fernández E., Maestre C., Moncada S., Bolaños J.P. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat. Cell Biol. 2009;11(6):747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 257.Jimenez-Blasco D., Santofimia-Castaño P., Gonzalez A., Almeida A., Bolaños J.P. Astrocyte NMDA receptors' activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ. 2015;22(11):1877–1889. doi: 10.1038/cdd.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wang X., Pal R., Chen X.W., Limpeanchob N., Kumar K.N., Michaelis E.K. High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res Mol Brain Res. 2005;140(1–2):120–126. doi: 10.1016/j.molbrainres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 259.Park S.J., Lee J.H., Kim H.Y., Choi Y.H., Park J.S., Suh Y.H., Park S.M., Joe E.-h., Jou I. Astrocytes, but not microglia, rapidly sense H2O2 via STAT6 phosphorylation, resulting in cyclooxygenase-2 expression and prostaglandin release. J. Immunol. 2012;188(10):5132–5141. doi: 10.4049/jimmunol.1101600. [DOI] [PubMed] [Google Scholar]
- 260.Patabendige A., Singh A., Jenkins S., Sen J., Chen R. Astrocyte activation in neurovascular damage and repair following ischaemic stroke. Int. J. Mol. Sci. 2021;22(8) doi: 10.3390/ijms22084280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Peng L., Zhao Y., Li Y., Zhou Y., Li L., Lei S., Yu S., Zhao Y. Effect of DJ-1 on the neuroprotection of astrocytes subjected to cerebral ischemia/reperfusion injury. J. Mol. Med. (Berl.) 2019;97(2):189–199. doi: 10.1007/s00109-018-1719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sheikhbahaei S., Turovsky E.A., Hosford P.S., Hadjihambi A., Theparambil S.M., Liu B., Marina N., Teschemacher A.G., Kasparov S., Smith J.C., Gourine A.V. Astrocytes modulate brainstem respiratory rhythm-generating circuits and determine exercise capacity. Nat. Commun. 2018;9(1):370. doi: 10.1038/s41467-017-02723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jimenez-Blasco D., Busquets-Garcia A., Hebert-Chatelain E., Serrat R., Vicente-Gutierrez C., Ioannidou C., Gómez-Sotres P., Lopez-Fabuel I., Resch-Beusher M., Resel E., Arnouil D., Saraswat D., Varilh M., Cannich A., Julio-Kalajzic F., Bonilla-Del Río I., Almeida A., Puente N., Achicallende S., Lopez-Rodriguez M.L., Jollé C., Déglon N., Pellerin L., Josephine C., Bonvento G., Panatier A., Lutz B., Piazza P.V., Guzmán M., Bellocchio L., Bouzier-Sore A.K., Grandes P., Bolaños J.P., Marsicano G. Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature. 2020;583(7817):603–608. doi: 10.1038/s41586-020-2470-y. [DOI] [PubMed] [Google Scholar]
- 264.Vicente-Gutierrez C., Bonora N., Bobo-Jimenez V., Jimenez-Blasco D., Lopez-Fabuel I., Fernandez E., Josephine C., Bonvento G., Enriquez J.A., Almeida A., Bolaños J.P. Astrocytic mitochondrial ROS modulate brain metabolism and mouse behaviour. Nat. Metab. 2019;1(2):201–211. doi: 10.1038/s42255-018-0031-6. [DOI] [PubMed] [Google Scholar]
- 265.Howarth C., Sutherland B., Choi H.B., Martin C., Lind B.L., Khennouf L., LeDue J.M., Pakan J.M., Ko R.W., Ellis-Davies G., Lauritzen M., Sibson N.R., Buchan A.M., MacVicar B.A. A critical role for astrocytes in hypercapnic vasodilation in brain. J. Neurosci. 2017;37(9):2403–2414. doi: 10.1523/jneurosci.0005-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Turovsky E., Theparambil S.M., Kasymov V., Deitmer J.W., Del Arroyo A.G., Ackland G.L., Corneveaux J.J., Allen A.N., Huentelman M.J., Kasparov S., Marina N., Gourine A.V. Mechanisms of CO2/H+ sensitivity of astrocytes. J. Neurosci. 2016;36(42):10750–10758. doi: 10.1523/jneurosci.1281-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.van de Wiel J., Meigh L., Bhandare A., Cook J., Nijjar S., Huckstepp R., Dale N. Connexin26 mediates CO(2)-dependent regulation of breathing via glial cells of the medulla oblongata. Commun. Biol. 2020;3(1):521. doi: 10.1038/s42003-020-01248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hösli L., Zuend M., Bredell G., Zanker H.S., Porto de Oliveira C.E., Saab A.S., Weber B. Direct vascular contact is a hallmark of cerebral astrocytes. Cell Rep. 2022;39(1) doi: 10.1016/j.celrep.2022.110599. [DOI] [PubMed] [Google Scholar]
- 269.Toth A.E., Klepe A., Lipka D.V., Goldeman C., Brodin B., Nielsen M.S. SorLA in astrocytes regulates blood-brain barrier integrity [Brief Research Report] Frontiers in Drug Delivery. 2023;2 doi: 10.3389/fddev.2022.1082689. [DOI] [Google Scholar]
- 270.Angelova P.R., Kasymov V., Christie I., Sheikhbahaei S., Turovsky E., Marina N., Korsak A., Zwicker J., Teschemacher A.G., Ackland G.L., Funk G.D., Kasparov S., Abramov A.Y., Gourine A.V. Functional oxygen sensitivity of astrocytes. J. Neurosci. 2015;35(29):10460–10473. doi: 10.1523/jneurosci.0045-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Onimaru H., Yazawa I., Takeda K., Fukushi I., Okada Y. Calcium imaging analysis of cellular responses to hypercapnia and hypoxia in the NTS of newborn rat brainstem preparation. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.645904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Marina N., Christie I.N., Korsak A., Doronin M., Brazhe A., Hosford P.S., Wells J.A., Sheikhbahaei S., Humoud I., Paton J.F.R., Lythgoe M.F., Semyanov A., Kasparov S., Gourine A.V. Astrocytes monitor cerebral perfusion and control systemic circulation to maintain brain blood flow. Nat. Commun. 2020;11(1):131. doi: 10.1038/s41467-019-13956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Pires P.W., Earley S. Neuroprotective effects of TRPA1 channels in the cerebral endothelium following ischemic stroke. Elife. 2018;7 doi: 10.7554/eLife.35316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Rajani V., Zhang Y., Jalubula V., Rancic V., SheikhBahaei S., Zwicker J.D., Pagliardini S., Dickson C.T., Ballanyi K., Kasparov S., Gourine A.V., Funk G.D. Release of ATP by pre-Bötzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca(2+) -dependent P2Y(1) receptor mechanism. J. Physiol. 2018;596(15):3245–3269. doi: 10.1113/jp274727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Thakore P., Alvarado M.G., Ali S., Mughal A., Pires P.W., Yamasaki E., Pritchard H.A., Isakson B.E., Tran C.H.T., Earley S. Brain endothelial cell TRPA1 channels initiate neurovascular coupling. Elife. 2021;10 doi: 10.7554/eLife.63040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Barioni N.O., Derakhshan F., Tenorio Lopes L., Onimaru H., Roy A., McDonald F., Scheibli E., Baghdadwala M.I., Heidari N., Bharadia M., Ikeda K., Yazawa I., Okada Y., Harris M.B., Dutschmann M., Wilson R.J.A. Novel oxygen sensing mechanism in the spinal cord involved in cardiorespiratory responses to hypoxia. Sci. Adv. 2022;8(12):eabm1444. doi: 10.1126/sciadv.abm1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Qi B., Song L., Hu L., Guo D., Ren G., Peng T., Liu M., Fang Y., Li C., Zhang M., Li Y. Cardiac-specific overexpression of Ndufs1 ameliorates cardiac dysfunction after myocardial infarction by alleviating mitochondrial dysfunction and apoptosis. Exp. Mol. Med. 2022;54(7):946–960. doi: 10.1038/s12276-022-00800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ni Y., Hagras M.A., Konstantopoulou V., Mayr J.A., Stuchebrukhov A.A., Meierhofer D. Mutations in NDUFS1 cause metabolic reprogramming and disruption of the electron transfer. Cells. 2019;8(10) doi: 10.3390/cells8101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Arduini A., Escobar J., Vento M., Escrig R., Quintás G., Sastre J., Saugstad O.D., Solberg R. Metabolic adaptation and neuroprotection differ in the retina and choroid in a piglet model of acute postnatal hypoxia. Pediatr. Res. 2014;76(2):127–134. doi: 10.1038/pr.2014.70. [DOI] [PubMed] [Google Scholar]
- 280.Lange C.A., Bainbridge J.W. Oxygen sensing in retinal health and disease. Ophthalmologica. 2012;227(3):115–131. doi: 10.1159/000331418. [DOI] [PubMed] [Google Scholar]
- 281.Lindsay K.J., Du J., Sloat S.R., Contreras L., Linton J.D., Turner S.J., Sadilek M., Satrústegui J., Hurley J.B. Pyruvate kinase and aspartate-glutamate carrier distributions reveal key metabolic links between neurons and glia in retina. Proc. Natl. Acad. Sci. U. S. A. 2014;111(43):15579–15584. doi: 10.1073/pnas.1412441111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Joyal J.-S., Sun Y., Gantner M.L., Shao Z., Evans L.P., Saba N., Fredrick T., Burnim S., Kim J.S., Patel G., Juan A.M., Hurst C.G., Hatton C.J., Cui Z., Pierce K.A., Bherer P., Aguilar E., Powner M.B., Vevis K., Boisvert M., Fu Z., Levy E., Fruttiger M., Packard A., Rezende F.A., Maranda B., Sapieha P., Chen J., Friedlander M., Clish C.B., Smith L.E.H. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat. Med. 2016;22(4):439–445. doi: 10.1038/nm.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Country M.W. Retinal metabolism: a comparative look at energetics in the retina. Brain Res. 2017;1672:50–57. doi: 10.1016/j.brainres.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 284.Zhang K., Wang T., Sun G.F., Xiao J.X., Jiang L.P., Tou F.F., Qu X.H., Han X.J. Metformin protects against retinal ischemia/reperfusion injury through AMPK-mediated mitochondrial fusion. Free Radic. Biol. Med. 2023;205:47–61. doi: 10.1016/j.freeradbiomed.2023.05.019. [DOI] [PubMed] [Google Scholar]
- 285.Mesentier-Louro L.A., Shariati M.A., Dalal R., Camargo A., Kumar V., Shamskhou E.A., de Jesus Perez V., Liao Y.J. Systemic hypoxia led to little retinal neuronal loss and dramatic optic nerve glial response. Exp. Eye Res. 2020;193 doi: 10.1016/j.exer.2020.107957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Gerber S., Ding M.G., Gérard X., Zwicker K., Zanlonghi X., Rio M., Serre V., Hanein S., Munnich A., Rotig A., Bianchi L., Amati-Bonneau P., Elpeleg O., Kaplan J., Brandt U., Rozet J.M. Compound heterozygosity for severe and hypomorphic NDUFS2 mutations cause non-syndromic LHON-like optic neuropathy. J. Med. Genet. 2017;54(5):346–356. doi: 10.1136/jmedgenet-2016-104212. [DOI] [PubMed] [Google Scholar]
- 287.Ngu L.H., Nijtmans L.G., Distelmaier F., Venselaar H., van Emst-de Vries S.E., van den Brand M.A., Stoltenborg B.J., Wintjes L.T., Willems P.H., van den Heuvel L.P., Smeitink J.A., Rodenburg R.J. A catalytic defect in mitochondrial respiratory chain complex I due to a mutation in NDUFS2 in a patient with Leigh syndrome. Biochim. Biophys. Acta. 2012;1822(2):168–175. doi: 10.1016/j.bbadis.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 288.Tuppen H.A.L., Hogan V.E., He L., Blakely E.L., Worgan L., Al-Dosary M., Saretzki G., Alston C.L., Morris A.A., Clarke M., Jones S., Devlin A.M., Mansour S., Chrzanowska-Lightowlers Z.M.A., Thorburn D.R., McFarland R., Taylor R.W. The p.M292T NDUFS2 mutation causes complex I-deficient Leigh syndrome in multiple families. Brain. 2010;133(10):2952–2963. doi: 10.1093/brain/awq232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.de Haas R., Das D., Garanto A., Renkema H.G., Greupink R., van den Broek P., Pertijs J., Collin R.W.J., Willems P., Beyrath J., Heerschap A., Russel F.G., Smeitink J.A. Therapeutic effects of the mitochondrial ROS-redox modulator KH176 in a mammalian model of Leigh Disease. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-09417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Yu A.K., Song L., Murray K.D., van der List D., Sun C., Shen Y., Xia Z., Cortopassi G.A. Mitochondrial complex I deficiency leads to inflammation and retinal ganglion cell death in the Ndufs4 mouse. Hum. Mol. Genet. 2015;24(10):2848–2860. doi: 10.1093/hmg/ddv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ji Y., Zhang J., Lu Y., Yi Q., Chen M., Xie S., Mao X., Xiao Y., Meng F., Zhang M., Yang R., Guan M.-X. Complex I mutations synergize to worsen the phenotypic expression of Leber's hereditary optic neuropathy. J. Biol. Chem. 2020;295(38):13224–13238. doi: 10.1074/jbc.RA120.014603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Bruhn H., Samuelsson K., Schober F.A., Engvall M., Lesko N., Wibom R., Nennesmo I., Calvo-Garrido J., Press R., Stranneheim H., Freyer C., Wedell A., Wredenberg A. Novel mutation m.10372A>G in <em>MT-ND3</em> causing sensorimotor axonal polyneuropathy. Neurology Genetics. 2021;7(2):e566. doi: 10.1212/nxg.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sarzi E., Brown M.D., Lebon S., Chretien D., Munnich A., Rotig A., Procaccio V. A novel recurrent mitochondrial DNA mutation in ND3 gene is associated with isolated complex I deficiency causing Leigh syndrome and dystonia. Am. J. Med. Genet. 2007;143A(1):33–41. doi: 10.1002/ajmg.a.31565. [DOI] [PubMed] [Google Scholar]
- 294.Bird Matthew J., Wijeyeratne Xiaonan W., Komen Jasper C., Laskowski A., Ryan Michael T., Thorburn David R., Frazier Ann E. Neuronal and astrocyte dysfunction diverges from embryonic fibroblasts in the Ndufs4fky/fky mouse. Biosci. Rep. 2014;34(6) doi: 10.1042/bsr20140151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Brand M.D., Goncalves R.L., Orr A.L., Vargas L., Gerencser A.A., Borch Jensen M., Wang Y.T., Melov S., Turk C.N., Matzen J.T., Dardov V.J., Petrassi H.M., Meeusen S.L., Perevoshchikova I.V., Jasper H., Brookes P.S., Ainscow E.K. Suppressors of superoxide-H(2)O(2) production at site I(Q) of mitochondrial complex I protect against stem cell hyperplasia and ischemia-reperfusion injury. Cell Metabol. 2016;24(4):582–592. doi: 10.1016/j.cmet.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Read A.D., Bentley R.E.T., Martin A.Y., Mewburn J.D., Alizadeh E., Wu D., Lima P.D.A., Dunham-Snary K.J., Thébaud B., Sharp W., Archer S.L. Electron leak from the mitochondrial electron transport chain complex I at site I(Q) is crucial for oxygen sensing in rabbit and human ductus arteriosus. J. Am. Heart Assoc. 2023;12(13) doi: 10.1161/jaha.122.029131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Chen Y., Qin C., Huang J., Tang X., Liu C., Huang K., Xu J., Guo G., Tong A., Zhou L. The role of astrocytes in oxidative stress of central nervous system: a mixed blessing. Cell Prolif. 2020;53(3) doi: 10.1111/cpr.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Quintana A., Kruse S.E., Kapur R.P., Sanz E., Palmiter R.D. Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc. Natl. Acad. Sci. U. S. A. 2010;107(24):10996–11001. doi: 10.1073/pnas.1006214107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yoon J.Y., Daneshgar N., Chu Y., Chen B., Hefti M., Vikram A., Irani K., Song L.S., Brenner C., Abel E.D., London B., Dai D.F. Metabolic rescue ameliorates mitochondrial encephalo-cardiomyopathy in murine and human iPSC models of Leigh syndrome. Clin. Transl. Med. 2022;12(7):e954. doi: 10.1002/ctm2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Bandara A.B., Drake J.C., James C.C., Smyth J.W., Brown D.A. Complex I protein NDUFS2 is vital for growth, ROS generation, membrane integrity, apoptosis, and mitochondrial energetics. Mitochondrion. 2021;58:160–168. doi: 10.1016/j.mito.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Cabello-Rivera D., Sarmiento-Soto H., López-Barneo J., Muñoz-Cabello A.M. Mitochondrial complex I function is essential for neural stem/progenitor cells proliferation and differentiation. Front. Neurosci. 2019;13:664. doi: 10.3389/fnins.2019.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.González-Rodríguez P., Zampese E., Stout K.A., Guzman J.N., Ilijic E., Yang B., Tkatch T., Stavarache M.A., Wokosin D.L., Gao L., Kaplitt M.G., López-Barneo J., Schumacker P.T., Surmeier D.J. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature. 2021;599(7886):650–656. doi: 10.1038/s41586-021-04059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Padavannil A., Ayala-Hernandez M.G., Castellanos-Silva E.A., Letts J.A. The mysterious multitude: structural perspective on the accessory subunits of respiratory complex I. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.798353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Chithra Y., Dey G., Ghose V., Chandramohan V., Gowthami N., Vasudev V., Srinivas Bharath M.M. Mitochondrial complex I inhibition in dopaminergic neurons causes altered protein profile and protein oxidation: implications for Parkinson's disease. Neurochem. Res. 2023 doi: 10.1007/s11064-023-03907-x. [DOI] [PubMed] [Google Scholar]
- 305.Hameedi M.A., Grba D.N., Richardson K.H., Jones A.J.Y., Song W., Roessler M.M., Wright J.J., Hirst J. A conserved arginine residue is critical for stabilizing the N2 FeS cluster in mitochondrial complex I. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Grba D.N., Blaza J.N., Bridges H.R., Agip A.A., Yin Z., Murai M., Miyoshi H., Hirst J. Cryo-electron microscopy reveals how acetogenins inhibit mitochondrial respiratory complex I. J. Biol. Chem. 2022;298(3) doi: 10.1016/j.jbc.2022.101602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lasham J., Haapanen O., Zickermann V., Sharma V. Tunnel dynamics of quinone derivatives and its coupling to protein conformational rearrangements in respiratory complex I. Biochim. Biophys. Acta Bioenerg. 2023;1864(2) doi: 10.1016/j.bbabio.2022.148951. [DOI] [PubMed] [Google Scholar]
- 308.Kim H., Saura P., Pöverlein M.C., Gamiz-Hernandez A.P., Kaila V.R.I. Quinone catalysis modulates proton transfer reactions in the membrane domain of respiratory complex I. J. Am. Chem. Soc. 2023;145(31):17075–17086. doi: 10.1021/jacs.3c03086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kramer P.A., Duan J., Gaffrey M.J., Shukla A.K., Wang L., Bammler T.K., Qian W.J., Marcinek D.J. Fatiguing contractions increase protein S-glutathionylation occupancy in mouse skeletal muscle. Redox Biol. 2018;17:367–376. doi: 10.1016/j.redox.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Ma D.K., Vozdek R., Bhatla N., Horvitz H.R. CYSL-1 interacts with the O2-sensing hydroxylase EGL-9 to promote H2S-modulated hypoxia-induced behavioral plasticity in C. elegans. Neuron. 2012;73(5):925–940. doi: 10.1016/j.neuron.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Suthammarak W., Somerlot B.H., Opheim E., Sedensky M., Morgan P.G. Novel interactions between mitochondrial superoxide dismutases and the electron transport chain. Aging Cell. 2013;12(6):1132–1140. doi: 10.1111/acel.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Van Raamsdonk J.M., Hekimi S. Superoxide dismutase is dispensable for normal animal lifespan. Proc. Natl. Acad. Sci. U. S. A. 2012;109(15):5785–5790. doi: 10.1073/pnas.1116158109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Melov S., Coskun P., Patel M., Tuinstra R., Cottrell B., Jun A.S., Zastawny T.H., Dizdaroglu M., Goodman S.I., Huang T.T., Miziorko H., Epstein C.J., Wallace D.C. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96(3):846–851. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Van Remmen H., Williams M.D., Guo Z., Estlack L., Yang H., Carlson E.J., Epstein C.J., Huang T.T., Richardson A. Knockout mice heterozygous for Sod2 show alterations in cardiac mitochondrial function and apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2001;281(3):H1422–H1432. doi: 10.1152/ajpheart.2001.281.3.H1422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.