Abstract
Understanding the genetic basis of novel adaptations in new species is a fundamental question in biology. Here we demonstrate a new role for galr2 in vertebrate craniofacial development using an adaptive radiation of trophic specialist pupfishes endemic to San Salvador Island, Bahamas. We confirmed the loss of a putative Sry transcription factor binding site upstream of galr2 in scale-eating pupfish and found significant spatial differences in galr2 expression among pupfish species in Meckel's cartilage using in situ hybridization chain reaction (HCR). We then experimentally demonstrated a novel role for Galr2 in craniofacial development by exposing embryos to Garl2-inhibiting drugs. Galr2-inhibition reduced Meckel's cartilage length and increased chondrocyte density in both trophic specialists but not in the generalist genetic background. We propose a mechanism for jaw elongation in scale-eaters based on the reduced expression of galr2 due to the loss of a putative Sry binding site. Fewer Galr2 receptors in the scale-eater Meckel's cartilage may result in their enlarged jaw lengths as adults by limiting opportunities for a circulating Galr2 agonist to bind to these receptors during development. Our findings illustrate the growing utility of linking candidate adaptive SNPs in non-model systems with highly divergent phenotypes to novel vertebrate gene functions.
Keywords: gene expression, galanin, trophic morphology, craniofacial divergence, evo-devo, adaptive radiation
1. Introduction
Craniofacial developmental anomalies are the most common source of birth defects in humans, present in 1 out of 700 births [1–3]. While Mendelian craniofacial defects are well characterized (e.g. Treacher Collins syndrome [4], Apert syndrome [5] and Crouzon syndrome [6,7]), the developmental genetics of complex craniofacial defects, such as micrognathia, are poorly understood [8–14]. With the continued lowering costs of genomic sequencing and functional genetic tools, it is increasingly feasible to develop new genetic models for understanding human development and disease.
Understanding the genetic bases of naturally occurring, highly divergent adaptive phenotypes in novel systems that parallel human clinical variation, such as ‘evolutionary mutant’ models [15–18], provides a powerful approach combining the tractable functional investigations possible in vertebrate model systems with genome-wide association scans of small-effect regulatory loci underlying natural craniofacial diversity. In particular, the most remarkable diversity of vertebrate craniofacial morphology is represented in teleost fishes, often associated with their diverse and sometimes highly specialized modes of feeding (e.g. [19–24]).
Emerging fish model systems include the rapidly evolving East African and Cameroon cichlid radiations, in which a small number of genetic changes underlie immense morphological disparity [25–32] and the repeated parallel speciation of stickleback ecomorphs in glacial lakes [33–36]. These systems provide excellent examples of leveraging naturally occurring and highly divergent craniofacial phenotypes as ‘evolutionary mutants' or ‘evolutionary forward genetics’ models to gain novel insights into the genetics of natural human craniofacial variation [17,37–42].
Here we demonstrate the utility of an evolutionary radiation of Cyprinodon pupfishes for discovering and validating the craniofacial function of a new gene associated with jaw evolution via QTL and GWAS analyses. Pupfishes offer some advantages over other evolutionary fish systems because they (1) rapidly evolved highly divergent and unique craniofacial phenotypes (figure 1) with minimal genetic differentiation among species [43–50], (2) speciated in the face of ongoing gene flow resulting in very few highly differentiated genomic regions associated with species-specific craniofacial traits [51–57], and (3) are highly amenable to laboratory rearing and imaging due to their high fecundity, daily egg production, and egg transparency comparable to zebrafish [58,59]. This radiation contains the widespread algae-eating generalist pupfish, Cyprinodon variegatus (figure 1a), which is broadly distributed across the Caribbean and North American Atlantic coast and occurs in sympatry with two microendemic trophic specialist species found only in the hypersaline lakes of San Salvador Island (SSI), Bahamas. Each trophic specialist displays highly divergent behaviour, pigmentation and craniofacial morphology: the molluscivore C. brontotheroides has a novel nasal protrusion that is a skeletal extension of the maxilla and foreshortened robust oral jaws (figure 1b); and the scale-eater, C. desquamator, exhibits two-fold larger oral jaws and overall brachycephalic features (figure 1c) [46,47,60,61]. There is also a fourth intermediate scale-eating ecotype in some lakes [62].
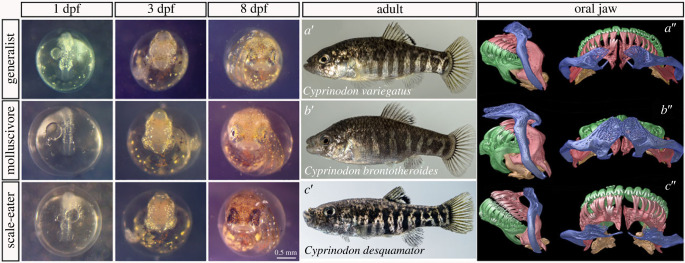
Figure 1.
Divergent craniofacial morphology and development of the San Salvador Island Cyprinodon pupfish radiation. C. variegatus (first row) is a trophic generalist distributed across the western Atlantic and Caribbean; C. brontotheroides (second row) is a molluscivore and C. desquamator (third row) is a scale-eater, both endemic to the hypersaline lakes of San Salvador Island (SSI), Bahamas. Left panel: development at 1-, 3-, and 8-days post-fertilization (dpf). Middle panel: laboratory-reared adult female pupfishes of each species. Right panel: Lateral and dorsal views of µCT scans of craniofacial morphology (modified from [43]). The maxilla is coloured in blue, premaxilla in red, dentary in green, articular in orange.
Previous genomic and transcriptomic work on the SSI pupfishes identified dozens of new candidate craniofacial genes never previously characterized as craniofacial or directly investigated in other systems [54,56,57,62–67]. One of the most promising candidates was galanin receptor 2a, the second receptor type for the galanin peptide. Using a genome-wide association (GWA) test across 202 individuals from the SSI radiation and outgroup populations, we previously found an association of the regulatory region of galr2a with lower jaw length, confirming an earlier pilot study that found the galr2a region to be among the top five strongest associations with lower jaw length, containing highly differentiated SNPs between trophic specialist species [56,63]. An analysis of hard selective sweeps using both site frequency spectrum (SweeD) and linkage disequilibrium (Omegle) based summary statistics additionally found evidence of a putative adaptive allele in the 20 kb regulatory region upstream of galr2 that swept to fixation in the scale-eater C. desquamator population on SSI 696–1008 (95% credible interval) years ago, potentially providing a pivotal stage in adaptation to scale-eating [56]. Furthermore, an independent quantitative trait loci (QTL) mapping study in F2 intercross hybrids between scale-eater and molluscivore parents found a significant QTL on linkage group 15 containing galr2a that accounted for 15.3% of the phenotypic variance in premaxilla length (n = 178) [67], with a positive effect on jaw length in the scale-eater genotype. Similarly, a second independent study of an F2 hybrid intercross from a second lake found evidence of a QTL in this region explaining 8% of the phenotypic variance in the length of the coronoid process on the articular bone of the lower jaw (jaw closing in-lever; n = 227; [57]). The combined strength of evidence for a role of galr2a in craniofacial development across independent analyses of GWA, QTL, selective sweeps and genetic differentiation between species indicated that this was one of our highest priority candidates for functional studies.
In humans, galr2 is abundantly expressed within the hypothalamus and hippocampus of the central nervous system and in the heart, kidney, liver, colon and small intestine, and it has genetic associations with epilepsy and Alzheimer's [68,69]. Classified as an orexigenic (appetite stimulant) gene, galr2 is expressed in the human hypothalamus and in the ventral telencephalon of larval and adult zebrafish [68,70,71]. Although a role of galr2 in craniofacial development has not previously been reported in the literature, its ligand, the neuropeptide galanin (GAL), is highly expressed in bones from early to post-embryonic development [72,73], with demonstrated effects on bone mass [74], muscle contraction [75] and periodontal regeneration [76].
Here we used Sanger sequencing to confirm two highly differentiated SNPs between SSI specialist species detected in our previous genomic studies affecting two predicted transcription factor binding sites in the galr2 regulatory region, characterized the divergent craniofacial expression of galr2 across all three SSI pupfish species using in situ hybridization chain reaction (HCR) [77,78] at two key developmental timepoints, and demonstrated that treatment with two different Galr2 receptor inhibitors reduced Meckel's cartilage length and increased chondrocyte density dependent on the species' genetic backgrounds.
2. Results
(a) . Two highly differentiated SNPs are associated with galr2a transcription factor binding sites
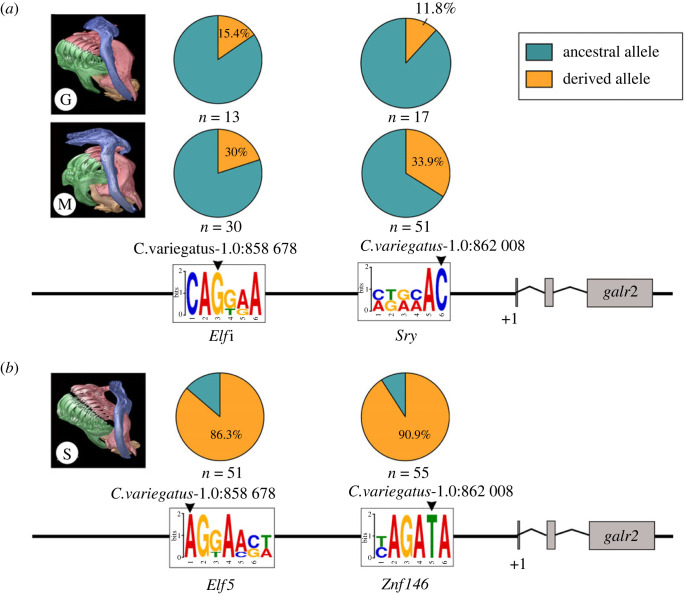
Previous whole genome resequencing of over one hundred San Salvador Island (SSI) pupfishes identified only two highly differentiated single nucleotide polymorphisms (SNP) within the 20 kb regulatory region of galr2a between trophic specialists across different lake populations on SSI [56]. We designed primers (electronic supplementary material, table S5) and used Sanger sequencing to further genotype these SNPs in a large panel of wild-caught specialists from six lake populations. We confirmed the presence of a transversion from G to A approximately 11 kb upstream of the galr2a transcription starting site (TSS; figure 2a; electronic supplementary material, figure S1 and table S1), which changes a predicted CAGCAA Elf1/Erg transcription factor binding site (TFBS) to a predicted AGGGASW Elf5 TFBS at this locus (using the Multiple Expectation maximizations for Motif Elicitation (MEME) server and the motif database scanning algorithm TOMTOM [79]). This transversion was observed in 86.3% of scale-eaters across four lake populations (n = 51) versus 20% of molluscivores across six lake populations on SSI (n = 30) (figure 2a, S1).
Figure 2.
The two most differentiated single-nucleotide polymorphisms (SNP) between trophic specialists lie within the 20 kb regulatory region of galr2a. (a) Putative ancestral TFBS for Elf1/Erg and Sry, respectively, in the generalist (G), Cyprinodon variegatus, and the molluscivore (M) Cyprinodon brontotheroides. (b) Derived changes in predicted TFBS to Elf5 and Znf146 in the scale-eater (S) Cyprinodon desquamator. Pie charts indicate frequencies of ancestral (teal) and derived (orange) alleles at each locus. +1 represents the transcription starting site of galr2a (Galanin receptor 2a). µCT scans of craniofacial morphology are modified from [43]. Sequence logos of the position weight matrices (PWM) identified using MEME and TOMTOM.
Using Sanger sequencing, we genotyped a second transversion from C to T approximately 8 kb upstream of galr2a TSS, which changes the predicted AGACAA Sry TFBS to a predicted YAGATA Znf146 TFBS (figure 2b; electronic supplementary material, figure S2 and table S1). This transversion was observed in 90.9% of scale-eaters across six lake populations (n = 55) versus 33.9% of molluscivores across six lake populations on SSI (n = 53) (electronic supplementary material, figure S2). Notably, all scale-eaters sampled from Crescent Pond (CRP) contained the T transversion (n = 30) (electronic supplementary material, figure S2). The predicted TFBS changes in the galr2a cis-regulatory region across pupfishes, combined with a previous genetic mapping study that found a significant QTL in this region explaining 15% of phenotypic variance in oral jaw size [51], suggests that different spatial or temporal galr2a expression may underlie some of the craniofacial divergence in SSI pupfishes. Previous studies of allele-specific expression in SSI pupfishes were inconclusive due to lack of heterozygous sites in the galr2a transcripts [53].
(b) . Different spatio-temporal patterns of galr2a expression in craniofacial tissues
To determine if spatial or temporal changes in galr2a expression underlie SSI craniofacial divergence, we assayed galr2a expression in 2 dpf embryos and 8 dpf larvae from two independent lake populations for each of the three SSI species using fluorescent in-situ hybridization chain reaction (HCR) for galr2a and tropomyosin 3b (tpm3b), a component of thin filaments of myofibrils expressed in fish skeletal muscles [80], to visualize jaw and other cranial muscles. We also tested galr2a expression using orthogonal amplifiers labelled with three distinct fluorophores (see Methods for details) to ensure reliable detection of galr2a transcripts in situ across species and developmental stages (electronic supplementary material, video S1).
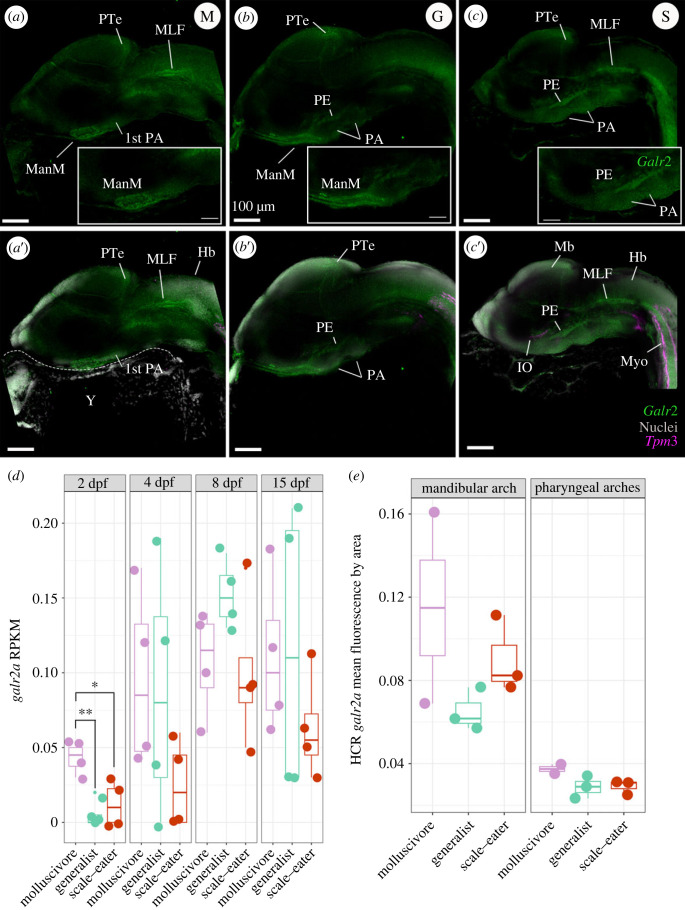
At 2 dpf, galr2a expression was detected in broad regions of the central nervous system (e.g. the posterior tectum and medial longitudinal fasciculus) of specialists (figure 3a,c) and generalists, with apparent higher expression in both specialist species than in generalists. We also observed an apparent increase of galr2a expression anterior to the first pharyngeal arches in the molluscivore and generalist pupfishes relative to the scale-eaters.
Figure 3.
Spatio-temporal patterns of galr2a expression differ among SSI species at 2 dpf. Representative images of Galr2a (green) and Tpm3b (magenta) expression revealed by hybridization chain reaction (HCR) and DAPI nuclear staining (grey) in the: (a) molluscivore, C. brontotheroides; (b) generalist, C. variegatus; and (c) scale-eater, C. desquamator. (d) Galr2a reads per kilobase of transcript per million reads (RPKM) from an existing mRNAseq study sampling the entire head of each species at four developmental stages [81]. Galr2a showed significantly higher expression in the molluscivore relative to generalists (p = 0.003, Tukey's HSD test) and scale-eaters (p = 0.014, Tukey's HSD test) at 2 days post fertilization (dpf). (e) Mean fluorescence per area for galr2a quantified at 2 dpf in all three species showed similarly elevated levels of expression in the molluscivore for the mandibular and pharyngeal arches. Images shown are representative Z-stack maximum projection images of 30 optical sections taken every 3 µm from whole-mounted embryos imaged using a Zeiss LSM880 laser confocal microscope. PA: pharyngeal arches, PE: pharyngeal endoderm, ManM: mandibular mesenchyme, MLF: medial longitudinal fasciculus, PTe: posterior tectum, Di: diencephalon; Mb: midbrain, Hb: hindbrain, Y: yolk, Myo: somitic myofibers, IO: inferior oblique muscle.
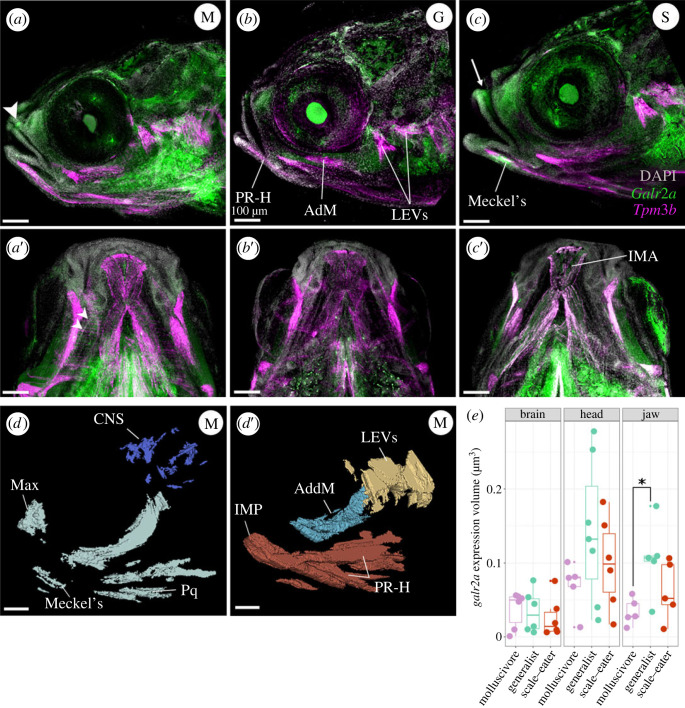
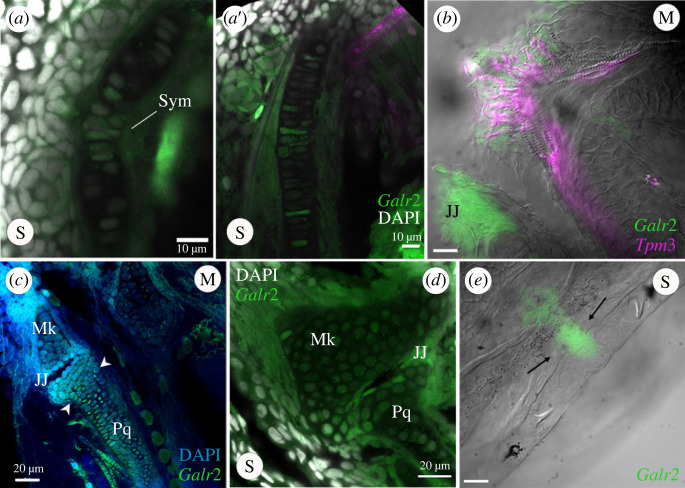
Using three-dimensional reconstruction and volume rendering analysis of HCR data for whole-mounted pupfishes at hatching time (8 dpf), we found that the galr2a expression domain was expanded in the jaws of the molluscivores relative to the generalists (figure 4; p = 0.03, Tukey's HSD), consistent with either greater tissue volume or an increased gene expression domain. By contrast, galr2a showed no differences in expression volume among species in the brain and head (figure 4; electronic supplementary material, table S3). Galr2a expression was detected in the Meckel's and palatoquadrate cartilages in all SSI pupfishes, in the premaxilla of the generalist and the scale-eater SSI specialist (figure 4b–b′,c–c′), the maxilla of the molluscivore SSI specialist (figure 4a–a′), and in the intermandibular muscles of the scale-eaters (figure 4c′).
Figure 4.
Spatio-temporal patterns of galr2a expression differ in molluscivores at 8 dpf. Representative images of Galr2a (green) and Tpm3b (magenta) expression revealed by hybridization chain reaction (HCR) and DAPI nuclear staining (grey). (a) Lateral view of an 8 dpf molluscivore (M in white circle), (a′) ventral view. (b) Lateral view of an 8 dpf generalist (G in white circle), (b′) ventral view. (c) Lateral view of an 8 dpf scale-eater (S in white circle), (c′) ventral view. The arrowhead in (b) and the arrow in (c) point to high expression of galr2a in the maxilla and premaxilla of the molluscivore and scale-eater specialists, respectively. White arrowheads in (a′) point to high expression of galr2a in the palatoquadrate of the molluscivore. Galr2a expression in the IMA was found only in the scale-eaters (c′). Images shown here are representative Z-stack maximum projection images of 30 optical sections taken every 15 µm from whole-mounted larvae imaged using a Zeiss LSM880 laser confocal microscope. (d–d′) 3D-Slicer view of the expression volume of galr2a (d) and tpm3b (d′) in an 8 dpf molluscivore pupfish larvae (C. brontotheroides). (e) Volume of galr2a expression in the brain (left panel), whole head (middle panel), and jaw (right panel) across SSI pupfishes. CNS: central nervous system, Max: maxilla. LEVs: levator arcus palatini and operculi, AdM (or AddM): adductor mandibular. IMA: intermandibularis anterior, PR-H: protractor hyoideus, Pq: palatoquadrate cartilage, Meckel's: Meckel's cartilage. Total volume rendered: 450 µm. All scale bars: 100 µm.
At the subcellular level, galr2a mRNA was detected in the cytoplasm of chondrocytes at the Meckel's symphysis (figure 5a). By contrast, on the distal edge of the Meckel's cartilage expression was detected only in the cytoplasm of the elongated chondrocytes (figure 5a′). Towards the most posterior region of the Meckel's cartilage closest to the palatoquadrate cartilage, we observed galr2a expression in the cells surrounding the jaw joint (figure 5b–d; electronic supplementary material, video S1). We conclude that galr2a was significantly differentially expressed in specific and distinct craniofacial tissues in the specialists at hatching time, suggesting an important role for craniofacial divergence in the SSI radiation.
Figure 5.
Galr2a is expressed in the chondrocytes of the Meckel's and palatoquadrate cartilages at hatching (8 dpf) in SSI pupfishes. HCRs showing tissue expression of Galr2a in green, and the muscle marker Tropomyosin 3b, (Tpm3b), in magenta. Nuclei are stained with DAPI (grey). (a–a′) Scale-eater galr2a expression in the chondrocytes of the Meckel's symphysis (Sym) and lateral side. (b) Ventral view of a molluscivore jaw at 8 dpf showing the expression of Tpm3b in the intermandibular muscles of the lower jaw. Galr2a is expressed in the jaw joint and the most anterior region of the lower jaw. (c,d) Galr2a is expressed in the chondrocytes of the Meckel's and palatoquadrate cartilages and around the jaw joint. (e) Galr2a expression in the premaxilla of a scale-eater. Unlabelled scale-bars: 10 µm.
We further tested for quantitative differences in galr2a expression at 2 dpf and 8 dpf by quantifying transcript counts from the only existing RNAseq data set for the heads of SSI pupfishes during development [81]. Only galr2a, but not galr1a, galr1b, galr2a, galr2b or galanin, showed significantly higher mRNA expression in the molluscivores relative to generalists (p = 0.003, Tukey's HSD test) and scale-eaters (p = 0.014, Tukey's HSD test) at 2 dpf (electronic supplementary material, table S2), whereas galr2a showed overall similar levels of expression in the head from 4 to 15 dpf in all three species (electronic supplementary material, table S2).
By using Tpm3b expression to label muscle cells, we observed at 2 dpf that Tpm3b expression was detected in somitic myofibers in all SSI pupfishes (figure 3a″–c″). However, only in the scale-eaters, Tpm3b expression was detected in the eye's inferior oblique muscle primordial cells (figure 3c″). At hatching time, Tpm3b expression was detected in all larval head muscles (figure 4a-a′, b-b′, c-c′; figure 5b). The scale-eater and molluscivore Tpm3b expression volume was significantly larger than in the generalists (electronic supplementary material, table S3; p < 0.05; 1-way ANOVA, Tukey's HSD test).
(c) . Chemical inhibition of Galr2 receptors affects Meckel's cartilage length and chondrocyte density
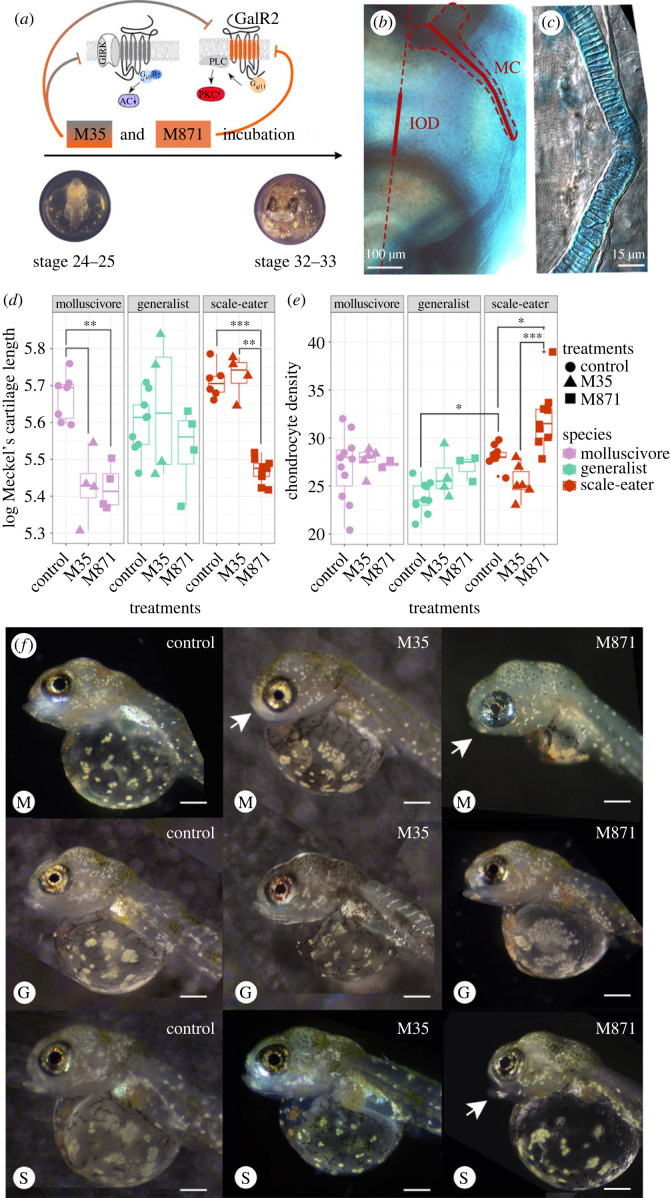
To study the effect of Galr2 in craniofacial development, we inhibited the endogenous activation of all four known galanin receptors in teleost fishes (Galr1a and b, Galr2a and b [68,82]) using M35 (Innopep, Inc.), a synthetic peptide antagonist of Galr1+2 galanin receptors [83], and M871 (Abcam), a Galr2-specific synthetic peptide antagonist [84]. Embryos of all three species from two different lake populations were exposed from stages 24–25 (2 dpf, with the appearance of the first pharyngeal arches) until hatching at stages 32–33 (8 dpf; figure 6a).
Figure 6.
Galr2 receptor inhibition reduced Meckel's cartilage length in both specialists and increased chondrocyte density in scale-eaters. (a) Galr2 and Galr1 + 2 inhibition protocol during pupfish development. C. desquamator embryos are depicted. (b) Alcian blue cartilage staining at Stage 32–33. IOD: interocular distance, MC: Meckel's cartilage. The MC length and IOD are shown in red. (c) Chondrocytes at the symphysis. (d) Changes in the log-transformed Meckel's length and the log-transformed interocular distance at stage 32–33 across species and treatments. (e) Chondrocyte numbers at the symphysis across species and treatments. *p < 0.05, **p < 0.01, ***p < 0.001 Tukey's HSD test. f. Representative control and treated larvae of each species at stage 32–33. Arrows point to abnormal lower jaws. M: molluscivores, G: generalist, S: scale-eaters. Scale-bars = 0.4 mm. Galr1 and Galr2 cartoons were modified from [85].
At approximate hatching time (8 dpf), both trophic specialist species raised under laboratory common garden conditions exhibited increased Meckel's cartilage length (figure 6d), consistent with the longer jaws of adult scale-eaters and more robust jaws of the molluscivore relative to the more gracile jaws of the generalist [47,59]. We found that exposure to M871, the Galr2-specific antagonist, significantly reduced the length of the Meckel's cartilage in both specialists relative to the generalists (figure 6d; p = 7.89 × 10−5 for scale-eaters; p = 0.001 for molluscivores; 2-way ANOVA, Tukey's HSD test) while the interocular distance remained unchanged between control and treated larvae (electronic supplementary material, figure S4). By contrast, M35 (Galr1 and Galr2 antagonist) only significantly reduced Meckel's length in the molluscivore (figure 6c). Meckel's length of generalists was unaffected by exposure to M35 or M871 (figure 6d).
To understand the cellular effect of Galr1 and 2 antagonists on Meckel's length across species, we quantified the number of chondrocytes 100 µm from the symphysis and the mean width of ten chondrocytes nearest to the symphysis. We found no significant differences in the mean chondrocyte width among pupfish species but observed increased chondrocyte density in untreated scale-eaters relative to generalists (figure 6e; electronic supplementary material, table S4; p = 0.009, ANOVA, Tukey's HSD test). Moreover, only scale-eaters responded to M871, but not M35, by further increasing chondrocyte density relative to the control larvae (figure 6e; electronic supplementary material, table S4; p = 0.03, ANOVA, Tukey's HSD test; mean ± 1 s.e.; control = 28.22 ± 0.38; M871 = 32.06 ± 1.06). Despite having shorter jaws after treatment with M871, chondrocyte density was significantly increased (figure 6e; mean ± 1 s.e.; control = 28.22 ± 0.38; M871 = 32.06 ± 1.06).
3. Discussion
We used an evolutionary radiation of trophic specialist pupfishes, endemic to San Salvador Island in the Bahamas, to discover a novel function for galr2a in craniofacial divergence. Specifically, we confirmed that two transcription factor binding sites upstream of galr2a display highly divergent allele frequencies between trophic specialist species, visualized galr2a expression in craniofacial tissues in all three SSI pupfish species at two developmental stages, and demonstrated a phenotypic effect on Meckel's cartilage length and chondrocyte density using synthetic peptides to inhibit the activity of Galr2 and Galr1 + 2. Our findings demonstrate a crucial role for Galr2 in craniofacial divergence within this pupfish radiation. Our study also provides a roadmap in a non-model vertebrate system for rapidly identifying previously uncharacterized candidate genes important for adaptation to novel ecological niches (e.g. trophic specialization) that can be quickly validated through classic and state-of-the-art developmental biology tools. Overall, our research contributes to understanding the genetic basis of phenotypic evolution and adaptation in non-model organisms.
(a) . Putative loss of a transcription factor binding site for galr2a in scale-eating pupfish
One of the most common evolutionary changes associated with phenotypic changes among closely related species is the gain or loss of cis-regulatory elements [86,87]. More than 85% of scale-eaters carry two transversions in the regulatory region of galr2a (figure 2). Combined with our observations of reduced galr2a expression in the mandibular mesenchyme during early jaw development in this species (figure 3), we conclude that the putative loss of a predicted Sry transcription factor binding site in scale-eaters is the most likely explanation for changes in gene expression, rather than a gain of a new predicted TFBS for Znf416 at this locus (figure 2). This is further supported by the critical role of the Sry-related HMG box (Sox) family of transcription factors (especially the SoxE group including Sox8, Sox9 and Sox10) in craniofacial development as Sry transcription factors specify the behaviour, multipotency and survival of neural crest cells during vertebrate development [88–92]. Moreover, the maintenance of the Elf (E74-like ETS transcription factor) family TFBS upstream of the loss of the Sry TFB in scale-eaters suggests that Elf TF recruitment to the regulatory region of galr2a may play a broader role in head development than species-specific craniofacial differences. Interestingly, morphant zebrafish larvae for elf3 show craniofacial cartilage defects [93], suggesting still unexplored possible roles for elf genes in craniofacial development and evolution.
Along with the identification of an Sry TFBS site using MEME, the Sry-related HMG box sox21 and sox7 were the second and third top-hits for predicted TFBS for the galr2a downstream SNP in molluscivores and generalists (TFBS predicted to be lost in scale-eaters). We checked both sox21 and sox7 expression through time and across species and found that they are mostly expressed at 2 and 4 dpf, with sox21b expressed more than sox7 (greater than 25 RPKM for sox21b, greater than 3 RPKM for sox7). We did not find species differences across time for sox21b; however, sox7 expression was significantly higher in scale-eaters than in molluscivores and generalists at 2 dpf (electronic supplementary material, table S2).
Alternatively, we cannot rule out a gain of a TFBS upstream of galr2a in scale-eaters, additive or epistatic effects of both upstream transversions, or more complex regulatory architectures, such as many interacting functional cis- and trans-acting regulatory variants or combinations of variants segregating at lower frequencies in trophic specialists that we have not prioritized [53,94,95]. However, our mapping cross of a single outbred pair of trophic specialists indicates that a single moderate-effect QTL containing galr2a explains 15% of phenotypic variation in oral jaw length between these species, consistent with causative variants affecting jaw size originating from this region [51].
(b) . Differential Galr2a expression during development is associated with craniofacial divergence in SSI pupfishes
We found galr2a expression in our in situ hybridization experiments to be consistent with previously published RNAseq data for craniofacial tissues in this radiation (figure 3) [53,64,96–98], with galr2a being differentially expressed only at 2 dpf. We observed distinctive spatial galr2a expression among SSI species at 2 and 8 dpf suggesting important time and tissue-specific regulation of galr2a expression during pupfish development. At 2 dpf, we found a strong association between decreased expression of galr2a in the mandibular mesenchyme anterior to the first pharyngeal arch with the future Meckel's cartilage and oral jaw lengths in the adults of each species; with increasing galr2 abundance in the molluscivores associated with shorter, but more robust jaws in adults [47,59]. By contrast, the reduced galr2a expression in the mandibular mesenchyme is associated with the development of longer oral jaws in scale-eaters, which is apparent as early as hatching time (figure 6d) [44].
We noted expression of galr2a in the maxilla of only molluscivores at hatching (figure 4a–c), absent in the scale-eaters and generalists, consistent with the uniquely enlarged and anteriorly protruding head of the maxilla in this species [43,47,49,50,59,98]. Furthermore, galr2a expression in the intermandibular muscles of the scale-eaters at hatching time (figure 4c) suggests that galr2a expression can also modulate the development of the observed hypertrophic musculature of the adductor mandibulae in the adult scale-eating pupfish [43]. Altogether, these interspecific differences in spatial expression support a novel role for galr2a in musculoskeletal development and may contribute to the divergent craniofacial morphology observed in SSI pupfishes.
(c) . Receptor inhibition supports a novel function for GALR2 in craniofacial divergence of SSI pupfishes
Interestingly, the response of Meckel's cartilage length and chondrocyte density to the inhibition of Galr-receptor pathways was highly dependent on the species' genetic background. Scale-eaters responded only to the Galr2-specific antagonist M871 with substantially reduced Meckel's cartilage length and increased chondrocyte density but were unaffected by the Galr1 + 2 antagonist M35 (figure 6c). Importantly, M871 exhibits a higher binding constant for Galr2 receptors than the Galr1 + 2 antagonist M35 [84,99]. Previous transcriptomic data from craniofacial tissues during early development [96] also indicates that galanin and Galr1 mRNA transcript abundance does not vary among the three SSI species (electronic supplementary material, table S3).
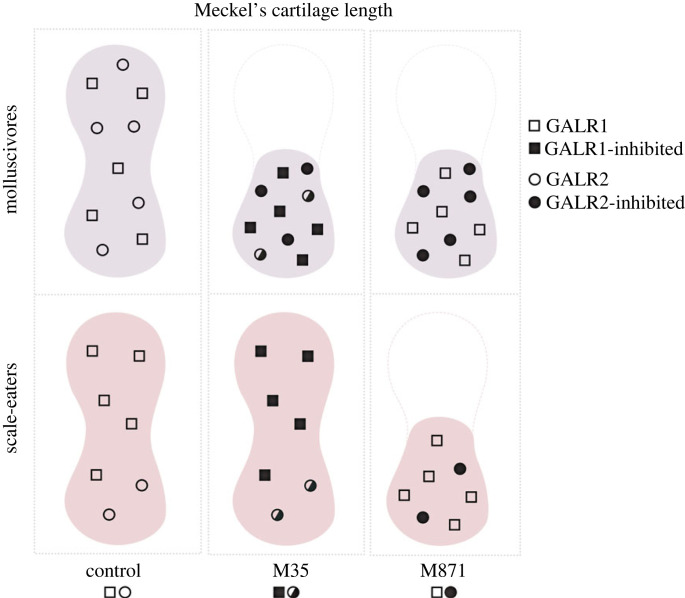
Therefore, we conclude that even though scale-eaters expressed the lowest amount of galr2a transcripts and presumably contain overall fewer Galr2 receptors in their craniofacial tissue, M871's higher binding affinity [85] was sufficient to inhibit these fewer receptors, resulting in reduced Meckel's cartilage length (figure 7). By contrast, the presumably greater concentration of Galr2 receptors in molluscivore craniofacial tissue due to increased Galr2 expression during development resulted in the inhibition of these receptors by both M871 and M35, despite its lower binding affinity for Galr2 [85]. We also conclude that inhibition of Galr1 receptors by M35 does not affect Meckel's cartilage length or chondrocyte density and that the specific inhibition of Galr2 by M871 was sufficient to drive equal Meckel's cartilage length shortening. Finally, the generalists' lack of significant response to M35 is consistent with their decreased levels of galr2a at 2 dpf, suggesting that, as in scale-eaters treated with M35, fewer Galr2 receptors may be present for inhibition in these species’ background. However, the response to M871 appears to be specialist-specific, with little effect on the generalist Meckel's cartilage length, and thus, we cannot rule out more complex species-specific factors interacting with Galr regulatory pathways.
Figure 7.
Proposed mechanism for how galanin receptor abundance affects Meckel's cartilage length in the two pupfish trophic specialists after exposure to M35 (weak Galr1 + Galr2 antagonist) or M871 (strong Galr2 antagonist). Molluscivores (top row) express more galr2a at 2 and 8 dpf than scale-eaters (bottom row) in their Meckel's cartilage (see figures 3 and 4), resulting in more Galr2 receptors (open circles). Increased Galr2 receptor putative abundance in molluscivores results in their reduced Meckel's cartilage length (figure 6) due to inhibition by both the weak antagonist (M35: middle column; half-filled circles) and strong antagonist (M871: right column; filled circles) of Galr2. By contrast, reduced Galr2 receptor putative abundance in scale-eaters (bottom row) results in their reduced Meckel's cartilage length only after exposure to the strong Galr2-specific antagonist M871.
Our work is consistent with previous work showing that Galr2 activation inhibits cell proliferation in neuronal cell lines [85,100], suggesting that the TFBS allele frequency shifts observed in scale-eaters are the cause for low galr2a abundance and, consequently, have low Galr2 activity during craniofacial development resulting in increased chondrocyte density and Meckel's cartilage length. Thus, the increased chondrocyte density observed in the M871-treated scale-eater larvae likely results from the strong and continued inhibition of the fewer Galr2 receptors available during development due to their genetic background. Lastly, due to the variable expression of galr2a between species at stage 24 but similar expression in chondrocytes of the Meckel's and palatoquadrate cartilages of different species at stage 33, we speculate that galr2a may have a species-specific morphogenetic role during pharyngeal arch differentiation and early jaw development and an osteogenic role at later stages.
In conclusion, we propose that the inhibition of Galr2 and not Galr1 induces the reduction of Meckel's cartilage length. Thus, a greater number of Galr2 receptors in the molluscivore specialist due to increased expression of galr2a during early development may result in their reduced oral jaw lengths as adults through increased opportunities for endogenous agonistic binding interactions (figure 7). Fewer Galr2 receptors on the scale-eater jaw may result in their enlarged jaw lengths as adults by limiting opportunities for Galr2 endogenous agonists to bind to these receptors during development. In the generalists, a greater number of Galr2 receptors but smaller overall Meckel's cartilage length may limit the sensitivity of this species to Galr2 and Galr1 + 2 endogenous agonists, however, resulting in their intermediate length and least robust oral jaws among the three species under control conditions [43,46].
4. Conclusion
Our results support a novel role of the second receptor for galanin, Galr2, as a craniofacial modulator gene important in controlling craniofacial development and interspecific divergence through modifying its transcript abundance and receptor activity. Galr2a transcript abundance changes among species are associated with genetic changes in the regulatory region of galr2a, consistent with the loss of a transcription factor binding site in the scale-eating pupfish. We propose a model in which reduced Galr2 receptor abundance in the oral jaws of scale-eaters results in fewer endogenous agonistic interactions, increasing Meckel's cartilage length presumably by decreased inhibition of chondrocyte proliferation (as a downstream effect of endogenous Galr2 activation). We also acknowledge the polygenic nature of jaw development and note that our previous genetic mapping experiments place an upper bound of 15% of oral jaw variation that may be due to differences in galr2a regulation among the myriad craniofacial genes driving the evolution of divergent craniofacial traits in this system.
5. Material and methods
Full details on pupfish husbandry, HCR FISH staining, microscopy, Galr2-inhibition, sequencing, TFBS prediction, gene expression and statistical analyses are available in the supplemental material.
Acknowledgements
We thank Reviewer no. 1 and J. Todd Streelman for their valuable comments on the manuscript. We also thank Tyler Square, Michelle St. John, David Tian, Mara Van Tassell, Chloe Clair, Dylan Chau and Heidi Buratti for valuable comments and discussion of the results, and Lydia Smith for her help in the Evolutionary Genetics Lab at the University of California, Berkeley. We are particularly thankful to Austin Patton, who extracted the C. brontotheroides mRNA reads to create the HCR probes tested here, and for his insightful ideas and comments on the early stages of this project. We thank the Gerace Research Centre and Troy Day for logistical support and the government of the Bahamas for permission to collect and export samples.
Ethics
All protocols and procedures employed were reviewed and approved by the University of California, Berkeley Animal Care and Use Committee (AUP-2015-01-7053) and the Institutional Biosafety Committee for Biological Use Authorization #522 animals were collected and exported from the Bahamas with research and export permits from the Bahamas Environmental, Science, and Technology commission through the Gerace Research Centre from 2016 to 2018.
Data accessibility
All data and R scripts used for this study are included as electronic supplementary material [101].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
M.F.P.: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing—original draft, writing—review and editing; V.M.: data curation, investigation, methodology; E.J.R.: software, writing—review and editing; C.T.M.: conceptualization, writing—review and editing; C.H.M.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This research was funded by the National Science Foundation DEB CAREER grant 1749764, National Institutes of Health grant 5R01DE027052-02, the University of North Carolina at Chapel Hill and the University of California, Berkeley to C.H.M.
References
- 1.Hall D, Tegstrom C, Ingvarsson PK. 2010. Using association mapping to dissect the genetic basis of complex traits in plants. Brief. Funct. Genom. 9, 157-165. ( 10.1093/bfgp/elp048) [DOI] [PubMed] [Google Scholar]
- 2.Meng L, Bian Z, Torensma R, Von den Hoff JW. 2009. Biological mechanisms in palatogenesis and cleft palate. J. Dent. Res. 88, 22-23. ( 10.1177/0022034508327868) [DOI] [PubMed] [Google Scholar]
- 3.Palmer K, et al. 2016. Discovery and characterization of spontaneous mouse models of craniofacial dysmorphology. Dev. Biol. 415, 216-227. ( 10.1016/j.ydbio.2015.07.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadakia S, Helman SN, Badhey AK, Saman M, Ducic Y. 2014. Treacher Collins syndrome: the genetics of a craniofacial disease. Int. J. Pediatr. Otorhinolaryngol. 78, 893-898. ( 10.1016/j.ijporl.2014.03.006) [DOI] [PubMed] [Google Scholar]
- 5.Wilkie AO, et al. 1995. Apert syndrome results from localized mutations of FGFR2 and is allelic with Crouzon syndrome. Nat. Genet. 9, 165-172. ( 10.1038/ng0295-165) [DOI] [PubMed] [Google Scholar]
- 6.Helman SN, Badhey A, Kadakia S, Myers E. 2014. Revisiting Crouzon syndrome: reviewing the background and management of a multifaceted disease. Oral Maxillofac. Surg. 18, 373-379. ( 10.1007/s10006-014-0467-0) [DOI] [PubMed] [Google Scholar]
- 7.Reardon W, Winter RM, Rutland P, Pulleyn LJ, Jones BM, Malcolm S. 1994. Mutations in the fibroblast growth factor receptor 2 gene cause Crouzon syndrome. Nat. Genet. 8, 98-103. ( 10.1038/ng0994-98) [DOI] [PubMed] [Google Scholar]
- 8.Carlborg O, Haley CS. 2004. Epistasis: too often neglected in complex trait studies? Nat. Rev. Genet. 5, 618-625. ( 10.1038/nrg1407) [DOI] [PubMed] [Google Scholar]
- 9.Glazier AM. 2002. Finding genes that underlie complex traits. Science (New York, N.Y.) 298, 2345-2349. ( 10.1126/science.1076641) [DOI] [PubMed] [Google Scholar]
- 10.Hirschhorn JN, Daly MJ. 2005. Genome-wide association studies for common diseases and complex traits. Nat. Rev. 6, 95-108. ( 10.1038/nrg1521) [DOI] [PubMed] [Google Scholar]
- 11.Hochheiser H, et al. 2011. The FaceBase Consortium: a comprehensive program to facilitate craniofacial research. Dev. Biol. 355, 175-182. ( 10.1016/j.ydbio.2011.02.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manolio TA, et al. 2009. Finding the missing heritability of complex diseases. Nature 461, 747-753. ( 10.1038/nature08494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monteiro A, Podlaha O. 2009. Wings, horns, and butterfly eyespots: how do complex traits evolve? PLoS Biol. 7, e37. ( 10.1371/journal.pbio.1000037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberg SM, Cornell R, Leslie EJ. 2018. Craniofacial genetics: where have we been and where are we going? PLoS Genet. 14, e1007438. ( 10.1371/journal.pgen.1007438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albertson RC, Cresko W, Detrich HW, Postlethwait JH. 2009. Evolutionary mutant models for human disease. Trends Genet. 25, 74-81. ( 10.1016/j.tig.2008.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Concannon MR, Albertson RC. 2015. The genetic and developmental basis of an exaggerated craniofacial trait in East African cichlids. J. Exp. Zool. B Mol. Dev. Evol. 324, 662-670. ( 10.1002/jez.b.22641) [DOI] [PubMed] [Google Scholar]
- 17.Powder KE, Albertson RC. 2016. Cichlid fishes as a model to understand normal and clinical craniofacial variation. Dev. Biol. 415, 338-346. ( 10.1016/j.ydbio.2015.12.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohner N. 2018. Cavefish as an evolutionary mutant model system for human disease. Dev. Biol. 441, 355-357. ( 10.1016/j.ydbio.2018.04.013) [DOI] [PubMed] [Google Scholar]
- 19.Liem KF. 1991. A functional approach to the development of the head of teleosts: implications on constructional morphology and constraints. In Constructional morphology and evolution (eds Schmidt-Kittler N, Vogel K). Berlin, Germany: Springer. [Google Scholar]
- 20.Otten E. 1983. Vision and jaw mechanism during growth of the cichlid fish Haplochromis elegans. Doctoral dissertation, University of Leiden, Netherlands. [Google Scholar]
- 21.Albertson RC, Streelman JT, Kocher TD. 2003. Directional selection has shaped the oral jaws of Lake Malawi cichlid fishes. PNAS 100, 5252-5257. ( 10.1073/pnas.0930235100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin CH, Richards EJ. 2019. The paradox behind the pattern of rapid adaptive radiation: how can the speciation process sustain itself through an early burst?. Ann. Rev. of Ecol. Evol. Systematics 50, 569-593. ( 10.1146/ecolsys.2019.50.issue-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konings AF, Wisor JM, Stauffer JR. 2021. Microcomputed tomography used to link head morphology and observed feeding behavior in cichlids of Lake Malaŵi. Ecol. Evol. 11, 4605-4615. ( 10.1002/ece3.v11.9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans KM, Larouche O, Watson S-J, Farina S, Habegger ML, Friedman M. 2021. Integration drives rapid phenotypic evolution in flatfishes. PNAS 118. ( 10.1073/pnas.2101330118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brawand D, et al. 2014. The genomic substrate for adaptive radiation in African cichlid fish. Nature 513, 375-381. ( 10.1038/nature13726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conith AJ, Lam DT, Albertson RC. 2019. Muscle-induced loading as an important source of variation in craniofacial skeletal shape. Genesis 57, e23263. ( 10.1002/dvg.23263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kocher TD, Albertson RC, Carleton KL, Streelman JT. 2003. The genetic basis of biodiversity, genomic studies of cichlid fishes. In Aquatic genomics: steps toward a great future, pp. 34-44. [Google Scholar]
- 28.Martin CH. 2012. Weak disruptive selection and incomplete phenotypic divergence in two classic examples of sympatric speciation: Cameroon crater lake cichlids. Am. Nat. 180, E90-E109. ( 10.1086/667586) [DOI] [PubMed] [Google Scholar]
- 29.Martin CH. 2013. Strong assortative mating by diet, color, size, and morphology but limited progress toward sympatric speciation in a classic example: Cameroon crater lake cichlids. Evolution 67, 2114-2123. ( 10.1111/evo.12090) [DOI] [PubMed] [Google Scholar]
- 30.Martin CH, Genner MJ. 2009. High niche overlap between two successfully coexisting pairs of Lake Malawi cichlid fishes. Can. J. Fish. Aquat. Sci. 66, 579-588. ( 10.1139/F09-023) [DOI] [Google Scholar]
- 31.Martin CH, Cutler JS, Friel JP, Dening T, Coop G, Wainwright PC. 2015. Complex histories of repeated colonization and hybridization cast doubt on the clearest examples of sympatric speciation in the wild. Evolution 69, 1406-1422. ( 10.1111/evo.12674) [DOI] [PubMed] [Google Scholar]
- 32.Navon D, Male I, Tetrault ER, Aaronson B, Karlstrom RO, Albertson RC. 2020. Hedgehog signaling is necessary and sufficient to mediate craniofacial plasticity in teleosts. Proc. Natl Acad. Sci. USA 117, 19 321-19 327. ( 10.1073/pnas.1921856117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan YF, et al. (2010). Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science (New York, N.Y.) 327, 302-305. ( 10.1126/science.1182213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson PA, Ellis NA, Miller CT. 2016. Microinjection for transgenesis and genome editing in threespine sticklebacks. J. Vis. Exp. 13, e54055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones FC, et al. 2012. The genomic basis of adaptive evolution in threespine sticklebacks. Nature 484, 55-61. ( 10.1038/nature10944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller CT, Beleza S, Pollen AA, Schluter D, Kittles RA, Shriver MD, Kingsley DM. 2007. cis-Regulatory changes in Kit ligand expression and parallel evolution of pigmentation in sticklebacks and humans. Cell 131, 1179-1189. ( 10.1016/j.cell.2007.10.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erickson PA, Glazer AM, Cleves PA, Smith AS, Miller CT. 2014. Two developmentally temporal quantitative trait loci underlie convergent evolution of increased branchial bone length in sticklebacks. Proc. R. Soc. B 281, 20140822. ( 10.1098/rspb.2014.0822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glazer AM, Killingbeck EE, Mitros T, Rokhsar DS, Miller CT. 2015. Genome assembly improvement and mapping convergently evolved skeletal traits in sticklebacks with genotyping-by-sequencing. Genes,Genomes,Genetics 5, 1463-1472. ( 10.1534/g3.115.017905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsons J, McWhinnie K, Armstrong T. 2021. An evo-devo view of post-genomic African cichlid biology: enhanced models for evolution and biomedicine. In The behavior, ecology and evolution of cichlid fishes (eds Abate ME, Noakes DLG), pp. 779-802. Dordrecht: Springer Netherlands. [Google Scholar]
- 40.Kocher TD. 2004. Adaptive evolution and explosive speciation: the cichlid fish model. Nat. Rev. Genet. 5, 288-298. ( 10.1038/nrg1316) [DOI] [PubMed] [Google Scholar]
- 41.Roberts RB, Ser JR, Kocher TD. 2009. Sexual conflict resolved by invasion of a novel sex determiner in Lake Malawi cichlid fishes. Science (New York, N.Y.) 326, 998-1001. ( 10.1126/science.1174705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Streelman JT, Peichel CL, Parichy DM. 2007. Developmental genetics of adaptation in fishes: the case for novelty. Annu. Rev. Ecol. Evol. Syst. 38, 655-681. ( 10.1146/annurev.ecolsys.38.091206.095537) [DOI] [Google Scholar]
- 43.Hernandez LP, Adriaens D, Martin CH, Wainwright PC, Masschaele B, Dierick M. 2018. Building trophic specializations that result in substantial niche partitioning within a young adaptive radiation. J. Anat. 232, 173-185. ( 10.1111/joa.12742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lencer ES, Riccio ML, McCune AR. 2016. Changes in growth rates of oral jaw elements produce evolutionary novelty in bahamian pupfish. J. Morphol. 277, 935-947. ( 10.1002/jmor.20547) [DOI] [PubMed] [Google Scholar]
- 45.Lencer ES, Warren WC, Harrison R, McCune AR. 2017. The Cyprinodon variegatus genome reveals gene expression changes underlying differences in skull morphology among closely related species. BMC Genomics 18, 424. ( 10.1186/s12864-017-3810-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin CH, Wainwright PC. 2011. Trophic novelty is linked to exceptional rates of morphological diversification in two adaptive radiations of Cyprinodon pupfishes. Evolution 65, 2197-2212. ( 10.1111/j.1558-5646.2011.01294.x) [DOI] [PubMed] [Google Scholar]
- 47.Martin CH, Wainwright PC. 2013. A remarkable species flock of Cyprinodon pupfishes endemic to San Salvador Island, Bahamas. Bull. Peabody Mus. Nat. Hist. 54, 231-240. ( 10.3374/014.054.0201) [DOI] [Google Scholar]
- 48.Martin CH, McGirr JA, Richards EJ, St John ME. 2019. How to investigate the origins of novelty: insights gained from genetic. Behav. Fitness Perspect. Integr. Org. Biol. 1, obz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.St John ME, Holzman R, Martin CH. 2020. Rapid adaptive evolution of scale-eating kinematics to a novel ecological niche. J. Exp. Biol. 223, jeb217570. ( 10.1242/jeb.217570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.St John ME, Dixon KE, Martin CH. 2020. Oral shelling within an adaptive radiation of pupfishes: testing the adaptive function of a novel nasal protrusion and behavioural preference. J. Fish Biol. 97, 163-171. ( 10.1111/jfb.14344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin CH, Erickson PA, Miller CT. 2017. The genetic architecture of novel trophic specialists: higher effect sizes are associated with exceptional oral jaw diversification in a pupfish adaptive radiation. Mol. Ecol. 26, 624-638. ( 10.1111/mec.13935) [DOI] [PubMed] [Google Scholar]
- 52.McGirr JA, Martin CH. 2016. Novel candidate genes underlying extreme trophic specialization in Caribbean pupfishes. Mol. Biol. Evol. 34, 873-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGirr JA, Martin CH. 2021. Few fixed variants between trophic specialist pupfish species reveal candidate cis-regulatory alleles underlying rapid craniofacial divergence. Mol. Biol. Evol. 38, 405-423. ( 10.1093/molbev/msaa218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richards EJ, Martin CH. 2017. Adaptive introgression from distant Caribbean islands contributed to the diversification of a microendemic adaptive radiation of trophic specialist pupfishes. PLoS Genet. 13, 1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richards EJ, Martin CH. 2022. We get by with a little help from our friends: shared adaptive variation provides a bridge to novel ecological specialists during adaptive radiation. Proc. Biol. Sci. 289, 20220613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richards EJ, McGirr JA, Wang JR, St John ME, Poelstra JW, Solano MJ, O'Connell DC, Turner BJ, Martin CH. 2021. A vertebrate adaptive radiation is assembled from an ancient and disjunct spatiotemporal landscape. Proc. Natl Acad. Sci. USA 118, e2011811118. ( 10.1073/pnas.2011811118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.St. John ME, Dunker JC, Richards EJ, Romero S, Martin CH. 2021. Parallel genetic changes underlie integrated craniofacial traits in an adaptive radiation of trophic specialist pupfishes. bioRxiv 2021.07.01.450661.
- 58.Martin CH, Gould KJ. 2020. Surprising spatiotemporal stability of a multi-peak fitness landscape revealed by independent field experiments measuring hybrid fitness. Evol Lett 4, 530-544. ( 10.1002/evl3.195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin CH, Wainwright PC. 2013. Multiple fitness peaks on the adaptive landscape drive adaptive radiation in the wild. Science (New York, N.Y.) 339, 208-211. ( 10.1126/science.1227710) [DOI] [PubMed] [Google Scholar]
- 60.Holtmeier CL. 2001. Heterochrony, maternal effects, and phenotypic variation among sympatric pupfishes. Evolution 55, 330-338. [DOI] [PubMed] [Google Scholar]
- 61.Martin CH. 2016. The cryptic origins of evolutionary novelty: 1000-fold faster trophic diversification rates without increased ecological opportunity or hybrid swarm. Evolution 70, 2504-2519. ( 10.1111/evo.13046) [DOI] [PubMed] [Google Scholar]
- 62.Richards EJ, Martin CH. 2022. We get by with a little help from our friends: shared adaptive variation provides a bridge to novel ecological specialists during adaptive radiation. Proc. R. Soc. B 289, 20220613. ( 10.1098/rspb.2022.0613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGirr JA, Martin CH. 2016. Novel candidate genes underlying extreme trophic specialization in Caribbean pupfishes. Mol. Biol. Evol., msw286. ( 10.1093/molbev/msw286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGirr JA, Martin CH, Etges WJ. 2019. Hybrid gene misregulation in multiple developing tissues within a recent adaptive radiation of Cyprinodon pupfishes. PLOS ONE 14, e0218899. ( 10.1371/journal.pone.0218899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGirr JA, Martin CH. 2020. Ecological divergence in sympatry causes gene misexpression in hybrids. Mol. Ecol. 29, 2707-2721. ( 10.1111/mec.v29.14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patton AH, Richards EJ, Gould KJ, Buie LK, Martin CH. 2022. Hybridization alters the shape of the genotypic fitness landscape, increasing access to novel fitness peaks during adaptive radiation. eLife 11, 229. ( 10.7554/eLife.72905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin CH, Erickson PA, Miller CT. 2017. The genetic architecture of novel trophic specialists: larger effect sizes are associated with exceptional oral jaw diversification in a pupfish adaptive radiation. Molecular Ecology 26, 624-638. ( 10.1111/mec.2017.26.issue-2) [DOI] [PubMed] [Google Scholar]
- 68.Li L, Wei S, Huang Q, Feng D, Zhang S, Liu Z. 2013. A novel galanin receptor 1a gene in zebrafish: tissue distribution, developmental expression roles in nutrition regulation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 164, 159-167. ( 10.1016/j.cbpb.2012.12.004) [DOI] [PubMed] [Google Scholar]
- 69.Ma A, Bai J, He M, Wong AOL. 2018. Spexin as a neuroendocrine signal with emerging functions. Gen. Comp. Endocrinol. 265, 90-96. ( 10.1016/j.ygcen.2018.01.015) [DOI] [PubMed] [Google Scholar]
- 70.Ahi EP, Brunel M, Tsakoumis E, Schmitz M. 2019. Transcriptional study of appetite regulating genes in the brain of zebrafish (Danio rerio) with impaired leptin signalling. Scientific Reports 9, 213. ( 10.1038/s41598-019-56779-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim E, et al. 2016. Distribution of galanin receptor 2b neurons and interaction with galanin in the zebrafish central nervous system. Neurosci. Lett. 628, 153-160. ( 10.1016/j.neulet.2016.06.025) [DOI] [PubMed] [Google Scholar]
- 72.Xu Z-Q, Shi T-J, Hökfelt T. 1996. Expression of galanin and a galanin receptor in several sensory systems and bone anlage of rat embryos. PNAS 93, 14901-14905. ( 10.1073/pnas.93.25.14901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones M, Perumal P, Vrontakis M. 2009. Presence of galanin‐like immunoreactivity in mesenchymal and neural crest origin tissues during embryonic development in the mouse. Anat. Record 292, 481-487. ( 10.1002/ar.v292:4) [DOI] [PubMed] [Google Scholar]
- 74.Idelevich A, Sato K, Nagano K, Rowe G, Gori F, Baron R. 2018. Neuronal hypothalamic regulation of body metabolism and bone density is galanin dependent. J. Clin. Invest. 128, 2626-2641. ( 10.1172/JCI99350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kakuyama H, Kuwahara A, Mochizuki T, Hoshino M, Yanaihara N. 1997. Role of N-terminal active sites of galanin in neurally evoked circular muscle contractions in the guinea-pig ileum. European J. Pharmacol. 329, 85-91. ( 10.1016/S0014-2999(97)10109-1) [DOI] [PubMed] [Google Scholar]
- 76.Ma W, Lyu H, Pandya M, Gopinathan G, Luan X, Diekwisch TGH. 2021. Successful application of a galanin-coated scaffold for periodontal regeneration. J. Dental Res. 100, 1144-1152. ( 10.1177/00220345211028852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi HMT, Schwarzkopf M, Fornace ME, Acharya A, Artavanis G, Stegmaier J, Cunha A, Pierce NA. 2018. Third-generation in situ hybridization chain reaction: multiplexed, quantitative, sensitive, versatile, robust. Development 145, 169. ( 10.1242/dev.165753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ibarra-García-Padilla R, Howard AGA, Singleton EW, Uribe RA. 2021. A protocol for whole-mount immuno-coupled hybridization chain reaction (WICHCR) in zebrafish embryos and larvae. STAR Protocols 2, 100709. ( 10.1016/j.xpro.2021.100709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Research 37, W202-W208. ( 10.1093/nar/gkp335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dube DK, et al. 2017. Identification, characterization, and expression of sarcomeric tropomyosin isoforms in zebrafish. Cytoskeleton 74, 125-142. ( 10.1002/cm.v74.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lencer Ezra, McCune Amy R, Mullen Sean. 2020. Differences in cell proliferation and craniofacial phenotype of closely related species in the pupfish genus Cyprinodon. J. Heredity 111, 237-247. ( 10.1093/jhered/esz074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Butler JM, Herath EM, Rimal A, Whitlow SM, Maruska KP. 2020. Galanin neuron activation in feeding, parental care, and infanticide in a mouthbrooding African cichlid fish. Hormones Behav. 126, 104870. ( 10.1016/j.yhbeh.2020.104870) [DOI] [PubMed] [Google Scholar]
- 83.Wiesenfeld-Hallin Z, Xu X-J. 1993. The differential roles of substance P and neurokinin A in spinal cord hyperexcitability and neurogenic inflammation. Regulatory Peptides 46, 165-173. ( 10.1016/0167-0115(93)90027-6) [DOI] [PubMed] [Google Scholar]
- 84.Sollenberg UE, Lundström L, Bartfai T, Langel Ü. 2006. M871—a novel peptide antagonist selectively recognizing the galanin receptor Type 2. Int. J. Pept. Res. Ther. 12, 115-119. ( 10.1007/s10989-005-9008-x) [DOI] [Google Scholar]
- 85.Lang R, Gundlach A, Kofler B. 2007. The galanin peptide family: Receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmac. Therapeut. 115, 177-207. ( 10.1016/j.pharmthera.2007.05.009) [DOI] [PubMed] [Google Scholar]
- 86.Mack KL, Nachman MW. 2017. Gene regulation and speciation. Trends Genet. 33, 68-80. ( 10.1016/j.tig.2016.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Signor SA, Nuzhdin SV. 2018. The evolution of gene expression in cis and trans. Trends Genet. 34, 532-544. ( 10.1016/j.tig.2018.03.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leathers TA, Rogers CD. 2022. Time to go: neural crest cell epithelial-to-mesenchymal transition. Development 149, 2048. ( 10.1242/dev.200712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simões-Costa M, Bronner ME. 2015. Establishing neural crest identity: a gene regulatory recipe. Development 142, 242-257. ( 10.1242/dev.105445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haldin CE, LaBonne C. 2010. SoxE factors as multifunctional neural crest regulatory factors. Int. J. Biochem. Cell Biol. 42, 441-444. ( 10.1016/j.biocel.2009.11.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Betancur P, Bronner-Fraser M, Sauka-Spengler T. 2010. Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. PNAS 107, 3570-3575. ( 10.1073/pnas.0906596107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kamachi Y, Kondoh H. 2013. Sox proteins: regulators of cell fate specification and differentiation. Development 140, 4129-4144. ( 10.1242/dev.091793) [DOI] [PubMed] [Google Scholar]
- 93.Sarmah S, Hawkins MR, Manikandan P, Farrell M, Marrs JA. 2022. Elf3 deficiency during zebrafish development alters extracellular matrix organization and disrupts tissue morphogenesis. PLoS ONE 17, e0276255. ( 10.1371/journal.pone.0276255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boyle EA, Li YI, Pritchard JK. 2017. An expanded view of complex traits: from polygenic to omnigenic. Cell 169, 1177-1186. ( 10.1016/j.cell.2017.05.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Price AL, Spencer CCA, Donnelly P. 2015. Progress and promise in understanding the genetic basis of common diseases. Proc. R. Soc. B 282, 20151684. ( 10.1098/rspb.2015.1684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lencer ES, Warren WC, Harrison R, McCune AR. 2017. The Cyprinodon variegatus genome reveals gene expression changes underlying differences in skull morphology among closely related species. BMC Genomics 18, 682. ( 10.1186/s12864-017-3810-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McGirr JA, Martin CH. 2018. Parallel evolution of gene expression between trophic specialists despite divergent genotypes and morphologies. Evol. Lett. 2, 62-75. ( 10.1002/evl3.41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martin CH, Feinstein LC.. 2014. Novel trophic niches drive variable progress towards ecological speciation within an adaptive radiation of pupfishes. Molec. Ecol. 23, 1846-1862. ( 10.1111/mec.2014.23.issue-7) [DOI] [PubMed] [Google Scholar]
- 99.Sollenberg UE, Runesson J, Sillard R, Langel Ü. 2010. Binding of chimeric peptides M617 and M871 to galanin receptor Type 3 reveals characteristics of galanin receptor–ligand interaction. Int. J. Pept. Res. Ther. 16, 17-22. ( 10.1007/s10989-009-9197-9) [DOI] [Google Scholar]
- 100.Berger A, Lang R, Moritz K, Santic R, Hermann A, Sperl W, Kofler B. 2004. Galanin receptor subtype GalR2 mediates apoptosis in SH-SY5Y neuroblastoma cells. Endocrinology 145, 500-507. ( 10.1210/en.2003-0649) [DOI] [PubMed] [Google Scholar]
- 101.Palominos MF, Muhl V, Richards EJ, Miller CT, Martin CH. 2023. Jaw size variation is associated with a novel craniofacial function for galanin receptor 2 in an adaptive radiation of pupfishes. Figshare. ( 10.6084/m9.figshare.c.6879612) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Palominos MF, Muhl V, Richards EJ, Miller CT, Martin CH. 2023. Jaw size variation is associated with a novel craniofacial function for galanin receptor 2 in an adaptive radiation of pupfishes. Figshare. ( 10.6084/m9.figshare.c.6879612) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data and R scripts used for this study are included as electronic supplementary material [101].