Abstract
Objectives: The emergence of COVID‐19 has revealed its association with croup. The objective of this study was to compare outcomes of COVID‐19 related croup to non‐COVID‐19 related croup during the COVID‐19 pandemic.
Methods: This retrospective propensity matched study used data from 2020–2023 in the United States Cohort of the TriNetX database that includes 56 major health care organizations. The analysis compared the outcomes of 2 cohorts of patients between 2 months and 7 years of age: Cohort A had croup and a positive test for COVID‐19 and Cohort B had croup without a positive COVID‐19 test, both within 1 week before or after presentation with croup. Outcomes were death, admission to the hospital, intensive care unit (ICU) admission, respiratory rate >60, and oxygen saturation <90 within 7 days after the diagnosis of croup.
Results: There were 2590 patients with COVID‐19 related croup and 103,439 patients with non‐COVID‐19 croup. The final propensity matched cohort included 5180 patients evenly divided between groups. When both groups were compared based on outcomes after matching, there was twice the risk of the patient being admitted to the hospital with COVID‐19 croup (risk ratio [RR] = 2.12; 95% confidence interval [CI] 1.59–2.84; P < 0.001). Those with COVID‐19 related croup had significantly increased risk of being admitted to the ICU (RR = 4.90; 95% CI 3.11–7.73; P < 0.001). The patients with COVID‐19 related croup were more likely to have a respiratory rate ≥60 (RR = 2.00; 95% CI 1.18–3.37; P = 0.008) and oxygen saturation ≤90% (RR = 2.12; 95% CI 1.21–3.70; P = 0.007). There were no deaths in the final cohorts. There were no worse outcomes in the Omicron COVID‐19 related croup group.
Conclusions: The patients with COVID‐19 related croup exhibited more severe disease manifestations. These children were more likely to be admitted to the hospital/ICU and had more significant respiratory distress.
1. INTRODUCTION
1.1. Background
Croup (laryngotracheitis) is usually caused by viral infections, most commonly parainfluenza, in the fall and winter months. Generally, croup occurs in those >2 months to <7 years of age. 1 , 2 Most patients do not have severe croup. Before the COVID‐19 pandemic, hospital admission for croup occurred in 5%–10% of children, ICU admissions in 1% and rare deaths, occurring in <1%. 3 , 4 Compared to adults, children with COVID‐19 infections have lower mortality and less need for hospitalization. 5 , 6 , 7 , 8 With the advent of COVID‐19, the association of COVID‐19 with croup has been reported in the literature. One of the first publications on croup and COVID‐19 was a case series of 3 children who had a more severe and longer duration of symptoms requiring admission and respiratory support. 9 , 10 , 11 There have been other case studies and 1 other case series showing COVID‐19 is associated with more hospitalization 10 additional treatments of dexamethasone, racemic epinephrine (RE), 12 , 13 and intubation. 14 .
In general, the COVID‐19 Omicron variant is more transmissible but less severe in humans. 15 , 16 Omicron‐related COVID‐19 croup in children has been previously shown to be worse than the non‐Omicron related COVID‐19 croup. 17 , 18 In a study where COVID‐19 presented as croup in children during the Omicron predominance, of 75 children in a single children's hospital in Boston, Massachusetts, 9 patients (12% of total) required admission. Four patients (9% of total) of those admitted needed intensive care. Their conclusion was that croup is more severe with the COVID‐19 Omicron variant. 18 In the United States, hospital admission for croup before the COVID‐19 pandemic was relatively infrequent, suggesting potentially more severe pathophysiology with COVID‐19 croup than with non‐COVID‐19 related croup. 3 , 4
1.2. Importance
The value of this study includes providing additional information about the incidence and severity of COVID‐19 related croup, identifying options for improved treatment, and emphasizing the usefulness of determining if croup is COVID‐19 related. Although the American Academy of Pediatrics has not mandated COVID‐19 testing for croup patients, they did recommend testing to enhance the appropriate implementation of contact precautions. 19 Knowing if a croup infection is COVID‐19 related or not on presentation to the emergency department or hospital will help guide physicians to know if the patient needs to be observed for a longer period, admitted, or receive more treatments with RE or dexamethasone. Because polymerase chain reaction (PCR) for COVID‐19 is available and quick, taking a few minutes to a few hours for a result, it is advantageous for those physicians and health care workers initially evaluating children with croup. Genotyping for variant analysis is a much longer process, and although not beneficial to those health care workers on the frontlines, it might assist epidemiologists and scientists in developing life‐saving vaccines.
1.3. Goals of this investigation
The purpose of this study was to review and compare outcomes of COVID‐19 related croup to non‐COVID‐19 related croup during the COVID‐19 pandemic. The secondary aim was to compare outcomes of Omicron COVID‐19 related croup to non‐Omicron COVID‐19 related croup.
2. METHODS
2.1. Study design
This was a retrospective, propensity matched study from the US Collaborative Network, a network of 56 health care organizations in the United States. This study was considered to be not‐human‐subjects research by our institutional review board.
THE BOTTOM LINE
This retrospective analysis compared children with emergency department presentations for croup with and without confirmed COVID‐19 using propensity matching. They found that children with COVID‐19 had more severe cases evidenced by increased risk of admission to the hospital and ICU.
2.2. Participant selection
Patients were included if they were diagnosed with croup, >2 months of age, <7 years of age, and had a COVID‐19 PCR test during the period from January 1, 2020–January 1, 2023. Both patients who were discharged home or hospitalized were included in the study. Patients were excluded if they presented outside the study time period, were <2 months of age, or were >7 years of age. For the subgroup analysis, we compared patients with croup who were COVID‐19 positive during the Omicron predominance period (December 1, 2021–February 28, 2022) with patients who were COVID‐19 positive outside the Omicron predominance period (January 01, 2020–November 30, 2021, or March 01, 2022–January 01, 2023). All diagnoses were identified using the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD‐10‐CM) codes, and laboratory tests were identified using the TriNetX Curated Codes (TNX) (Figure 1)
FIGURE 1.
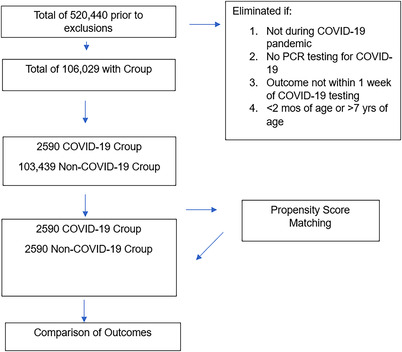
Process of patient selection. Abbreviation: PCR, polymerase chain reaction.
2.3. Outcomes
The outcomes, as defined by TNX, VISIT, Logical Observation identifiers Names and Codes (LOINC), and Current Procedural Terminology (CPT) codes, were death, admission to the ICU (CPT: 1019130 or LNC: 95420–6) or the hospital (VISIT: INPATIENT), respiratory rate ≥60 (TNX: 9073), and oxygen saturation ≤90% on room air (TNX: 9075). VISIT refers to a term within the context of the HL7 Encounter type. The same cohorts and outcomes were used for the subgroup analysis during the Omicron and non‐Omicron time periods.
2.4. Analysis
A 1:1 propensity score matching was done with linear and logistic regression on the demographics of both groups of COVID‐19 related croup and non‐COVID‐19 croup, and the subgroup analysis of COVID‐19 Omicron related croup and COVID‐19 non‐Omicron related croup, for the variables of age, race or ethnicity, and gender. Greedy nearest‐neighbor matching was used with a tolerance of 0.1 and a difference between propensity scores less than or equal to 0.1. TriNetX randomizes the order of data rows to mitigate bias introduced by the nearest‐neighbor algorithm. This study methodology has been previously validated. Comparisons were made between cohorts before and after propensity matching.
The analysis compared the outcomes of 2 cohorts: Cohort A, denoted Croup COVID‐19 (ICD‐10‐CM: J050.0), >2 months and l<7 years of age and had a positive COVID‐19 PCR (TNX: 9088) test 1 week before or 1 week after presentation. Cohort B, denoted Croup no COVID‐19, >2 months and <7 years of age who did not have a positive COVID‐19 PCR result 1 week before or after presentation. The same cohorts and outcomes were used for the subgroup analysis during the Omicron and non‐Omicron time periods.
After propensity matching, all of the demographics were no longer statistically different. Measure of association tool in TriNetX was used to perform univariate analysis where risk ratios (RRs), 95% confidence intervals (CIs), and probability values (p) were calculated to compare outcomes. The univariate analysis uses both the chi‐square test and the t test to examine the data sets. Statistical significance was set at a 2‐sided alpha <0.05.
3. RESULTS
There was a total of 106,029 patients in the cohorts with 2590 patients with COVID‐19 related croup and 103,439 patients with non‐COVID‐19 related croup. There was no difference in the percentage of females and males in each group (Table 1). When comparing age, the COVID‐19 related group was significantly younger compared to the non‐COVID‐19 group. Concerning race, White patients (P < 0.001) and Asian patients (P < 0.001) had a lower risk of COVID‐19 croup versus non‐COVID‐19 croup. Hispanic patients (P < 0.001) and Black patients (P = 0.004) had a higher risk of COVID‐19 croup versus non‐COVID‐19 croup (Table 1). After propensity matching, there was no significant differences in age, race, or ethnicity between groups.
TABLE 1.
Cohort demographics before and after propensity matching.
| Before propensity matching | After propensity matching | |||||||
|---|---|---|---|---|---|---|---|---|
| COVID‐19 | Non‐COVID‐19 | P value | Std diff. | COVID‐19 | Non‐COVID‐19 | P value | Std diff. | |
| Age (years) | 1.9 | 2.4 | <0.001 | 0.245 | 1.9 | 1.9 | 1.0 | <0.001 |
| Gender | ||||||||
| Males | 63% | 63% | 0.740 | 0.007 | 63% | 63% | 1.0 | <0.001 |
| Females | 37% | 37% | 0.730 | 0.007 | 37% | 37% | 1.0 | <0.001 |
| Race or ethnicity | ||||||||
| White patients | 61% | 65% | <0.001 | 0.094 | 61% | 61% | 1.0 | <0.001 |
| Black patients | 13% | 11% | 0.004 | 0.055 | 13% | 13% | 1.0 | <0.001 |
| Hispanic patients | 22% | 16% | <0.001 | 0.168 | 22% | 22% | 1.0 | <0.001 |
| Asian patients | 4% | 2% | <0.001 | 0.076 | 4% | 4% | 1.0 | <0.001 |
Abbreviation: Std diff, standard difference.
After propensity score matching there was a total of 5180 patients, evenly matched with 2590 in each group. With propensity matching, we found there was a 2‐fold increased risk of hospital admission for the COVID‐19 related croup patient (RR = 2.12; 95% CI 1.58–2.84; P < 0.001). If a patient had COVID‐19 related croup, they had a significant risk of ICU admission (RR = 4.9; 95% CI 3.11–7.73; P < 0.001). When the groups were compared based on respiratory rate ≥60 (RR = 1.00; 95% CI 1.18–3.37; P = 0.008), or oxygen saturation ≤90 (RR = 2.12; 95% CI 1.21–3.70; P = 0.007), there was a significant number of patients in the COVID‐19 related croup group who had these 2 signs of respiratory distress (Table 2). There were no children with croup, in either group, who died. For patients in our study with COVID‐19 related croup, the incidence of admission to the ICU was 1%. The number of patients requiring intubation was small, so analysis of intubation rates was not feasible.
TABLE 2.
Comparisons of outcomes 1 week after croup diagnosis (after propensity matching).
| Outcomes | COVID‐19 | Non‐COVID‐19 | RR (95% CI) | P value | Omicron | Non‐omicron | RR (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|
| Admission | 5.7% | 2.7% | 2.1 (1.58–2.95) | <0.001 | 5.7% | 5.6% | 1.02 (0.74–1.42) | 0.897 |
| ICU admission | 4.3% | 0.9% | 4.9 (3.11–7.13) | <0.001 | 3.5% | 5.1% | 0.69 (0.47–0.99) | 0.044 |
| RR ≥ 60 | 1.7% | 0.8% | 2.0 (1.19–3.37) | 0.008 | 1.0% | 2.4% | 0.42 (0.22–0.80) | 0.006 |
| Oxygen sat ≤ 90% | 1.5% | 0.7% | 2.1 (1.21–3.70) | 0.007 | 1.1% | 1.6% | 0.72 (0.37–1.38) | 0.315 |
Abbreviations: CI, confidence interval; RR, risk ratio
For the subgroup analysis comparing outcomes of Omicron COVID‐19 related croup to the non‐Omicron COVID‐19 related croup, there was a total of 2941 patients before propensity score matching and 2684 after propensity score matching. There was no difference in the percentage of males, females, and age in each group. When comparing race among both groups, White patients had a significantly lower risk of having the COVID‐19 Omicron virus, and Hispanic patients had a significantly higher risk of having COVID‐19 Omicron virus. There were no significant differences in hospital admission and oxygen saturation ≤ 90. Respiratory rate ≥ 60 (RR = 0.42; 95% CI 0.22–0.80; P = 0.006) and ICU Admission (RR = 0.69; 95% CI 0.47–0.99; P = 0.04) were significantly higher in those with non‐Omicron COVID‐19.
4. LIMITATIONS
Our study has several limitations. The retrospective nature of the study may have missed important data and introduced bias. Because comprehensive viral testing was not available on every patient, we cannot entirely exclude the possibility of viral coinfection. Although we propensity matched for demographics such as age, sex, and race or ethnicity, there may be other preexisting conditions not considered that could affect outcomes. In the subgroup analysis, there is a possibility that a small number of patients may have had overlapping records and could have been included in both cohorts on separate visits.
5. DISCUSSION
This study, among the most extensive in the United States, underscores the severity of COVID‐19‐associated croup in children. We observed a heightened risk of both hospital and ICU admissions for these children. Furthermore, the COVID‐related croup group exhibited more instances of respiratory distress, elevated respiratory rates, and hypoxia. Interestingly, children with the Omicron variant of COVID‐19‐related croup showed decreased respiratory rates and fewer ICU admissions compared to those with non‐Omicron related croup. The demographic findings of our study concur with the prevailing understanding of COVID‐19. Specifically, Hispanic and Black patients exhibited a higher incidence of COVID‐19 croup. Prior research similarly indicates that Black, Hispanic, and Southern Asian populations account for a disproportionately large number of COVID‐19 infections in both the United States and the United Kingdom. This discrepancy is potentially tied to genetic factors and disparities in the social determinants of health. 20 , 21
Typically, croup has low morbidity and mortality rates. 15 , 16 However, COVID‐19 worsens the severity, 9 , 12 , 13 , 14 , 20 , 22 , 23 mandating higher rates of hospitalization and ICU admission compared to the prepandemic period. In the largest study of 43 US children's hospitals with a sample size similar to ours, when comparing Omicron COVID‐19 croup to a pre‐COVID‐19 croup group, there was a higher admission rate to the hospital, ICU, and hypoxia 20 in the Omicron COVID‐19 croup group. Unlike the studies on Omicron COVID‐19 related croup, our study did not show worse outcomes in the Omicron COVID‐19 related croup group. This study adds to the current body of literature because the results include a longer time frame of 3 years, as compared with shorter periods in the other studies currently in the literature. Other studies included a historical group, whereas this study included those with non‐COVID‐19 related croup during the same time period (during the pandemic), which generates a more accurate comparison.
Dexamethasone, usually administered to mild croup to mitigate inflammation, and RE for moderate to severe cases 10 , 11 , 24 may need to be administered more frequently in light of the greater severity of COVID‐19 related croup. 9 , 12 , 13 , 14 , 25 In 1 series, patients with COVID croup needed more than 2 RE treatments and more than 1 dose of dexamethasone. 9 Other studies have shown that multiple doses of RE are often required in these croup patients. 12 , 13 , 14 These studies included all COVID‐19 variants.
In conclusion, COVID‐19 related croup was more severe, requiring higher admission rates to the hospital and ICU due to respiratory distress. In terms of demographics, Hispanic and Black patients were significantly more at risk for COVID‐19 related croup, aligning with current knowledge of COVID‐19. Interestingly, Omicron‐related croup did not significantly worsen outcomes compared to the non‐Omicron COVID‐19 croup. This study underlines the necessity of refining treatment strategies with more aggressive management of COVID‐19 related croup in children.
AUTHOR CONTRIBUTIONS
Donna R. Mendez, Gregory Rumph, and Dietrich Jehle conceived the concept and design of the study. Donna R. Mendez, Krishna K. Paul, and Dietrich Jehle supervised the data collection. Donna R. Mendez, Gregory Rumph, Joan Richardson, Krishna K. Paul, and Dietrich Jehle undertook data acquisition, analysis, or interpretation of data. Donna R. Mendez, Gregory Rumph, Joan Richardson, Krishna K. Paul, and Dietrich Jehle provided statistical advice on study design and analyzed the data. Donna R. Mendez drafted the manuscript, and all authors contributed substantially to its revision. All authors take responsibility for the paper as a whole.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch – Galveston, supported in part by a Clinical and Translational Science Award (UL1 TR001439) from the National Center for Advancing Translational Sciences, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biography
Donna R. Mendez, MD, EdD, is the Director of Pediatric Emergency Medicine Education for the Emergency Medicine Department. She is a faculty member at The University of Texas Medical Branch at Galveston.

Mendez DR, Rumph G, Richardson J, Paul KK, Jehle D. Outcomes of croup in children: COVID‐19 versus non‐COVID‐19 cases. JACEP Open. 2023;4:e13053. 10.1002/emp2.13053
Funding and support By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors declare no conflicts of interest.
Supervising Editor: Matthew Hansen, MD, MCR
DATA AVAILABILITY STATEMENT
Deidentified data were collected from the TriNetX database (https://trinetx.com/) and are made available as of May 11, 2023 by contacting Dietrich Jehle, MD, at email: dijehle@utmb.edu.
REFERENCES
- 1. Tovar PLJ, Cherry JD. Croup (laryngitis, laryngotracheitis, spasmodic croup, laryngotracheobronchitis, bacterial tracheitis and laryngotracheobronchopneumonitis) and epiglottitis (supraglottis). In: Cherry JD, Harrison GJ, Kaplan SL. Steinbach WJ, Jotez PJ, eds. Feigin and Cherry's Textbook of Pediatric Infectious Diseases. 8th ed. Elsevier Saunders; 2019:175‐190. [Google Scholar]
- 2. Cherry JD. Clinical practice. Croup. N Engl J Med. 2008;358(4):384‐391. doi: 10.1056/NEJMcp072022 [DOI] [PubMed] [Google Scholar]
- 3. Tyler A, McLeod L, Beaty B, et al. Variation in inpatient croup management and outcomes. Pediatrics. 2017;139:e20163582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McEniery J, Gillis J, Kilham H, et al. Croup in children. Pediatrics. 1991;87(6):847. [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . COVID‐19 weekly cases and deaths per 100,000 population by age, race/ethnicity, and sex. 2022. Accessed July 26, 2022. https://covid.cdc.gov/covid‐data‐tracker/#demographicsovertime
- 6. Centers for Disease Control and Prevention . Demographic trends of COVID‐19 cases and deaths in the US reported to CDC. 2022. Accessed April 1, 2023. https://covid.cdc.gov/covid‐data‐tracker/#demographics
- 7. Centers for Disease Control and Prevention . COVID‐NET laboratory‐confirmed COVID‐19 hospitalizations. 2022. Accessed April 1, 2023. https://covid.cdc.gov/covid‐data‐tracker/#covidnet‐hospitalization‐network
- 8. Centers for Disease Control and Prevention . Provisional COVID‐19 deaths: focus on ages 0–18 years. 2022. Accessed April 1, 2023. https://data.cdc.gov/NCHS/Provisional‐COVID‐19‐Deaths‐Focus‐on‐Ages‐0‐18‐Yea/nr4s‐juj3
- 9. Venn AMR, Schmidt JM, Mullan P. Pediatric croup with COVID‐19. Am J EM. 2021;287:e1–287.e3. doi: 10.1016/j.ajem.2020.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gates A, Gates M, Vandermeer B, et al. Glucocorticoids for croup in children. Cochrane Database of Syst Rev. 2018;8(8):CD001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dobrovoljac M, Geelhoed GC. How fast does oral dexamethasone work in mild to moderately severe croup? A randomized double‐blinded clinical trial: oral dexamethasone for croup. Emerg Med Australas. 2012;24(1):79‐85. doi: 10.1111/j.1742-6723.2011.01475.x [DOI] [PubMed] [Google Scholar]
- 12. Peterson K, Parel J, Collier C, et al. SARS‐CoV‐2 and croup, not a rare coincidence. Am J Emerg Med. 2022;57:175. doi: 10.1016/j.ajem.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matthew D, Calderon JC. Mild‐moderate croup presentations in patients with COVID‐19. J Surg Case Rep Cureus. 2022;14(8):e27893. doi: 10.7759/cureus.27893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim CC, j Saniasiaya, Kulasegarah J. Croup and COVID‐19 in a child: a case report and literature review. BMJ. 2021;14(9):e244769. doi: 10.1136/bcr-2021-244769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yao C, Song W, Wang L, et al. Enhanced transmissibility of XBB.1.5 is contributed by both strong ACE2 binding and antibody evasion. Lancet Infect Dis. 2023;23(3):278‐280. doi: 10.1016/S1473-3099(23)00010-510.1016/S1473-3099(23)00010-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bouzid D, Visseaux B, Kassasseya C, et al. Comparison of patients infected with delta versus omicron COVID‐19 variants presenting to Paris emergency departments: a retrospective cohort study. Ann Intern Med. 2022;175(6):831‐837. doi: 10.7326/M22-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Acker KP, Levine DA, Varghese M, et al. Indications for hospitalization in children with SARS‐CoV‐2 infection during the omicron wave in New York City. Children. 2022;9(7):1043. doi: 10.3390/children907104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brewster RC, Parsons C, Laird‐Gion J, et al. COVID‐19‐associated croup in children. Pediatrics. 2022;149(6):e2022056492. doi: 10.1542/peds.2022-056492 [DOI] [PubMed] [Google Scholar]
- 19. COVID‐19 testing guidance. 2022. Accessed: June 23, 2002. https://www.aap.org/en/pages/2019‐novelcoronavirus‐covid‐19‐infections/clinical‐guidance/covid‐19‐testing‐guidance/
- 20. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and white patients with Covid‐19. N Eng J Med. 2020;38:2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pitstick CE, Rodriguez KM, Smith AC, et al. Curious case of croup: laryngotracheitis caused by COVID‐19. Pediatr Int. 2022;64:e14952. doi: 10.1542/peds.2020-012179 [DOI] [PubMed] [Google Scholar]
- 23. Scribner C, Patel KI, Tunic M. Pediatric croup due to omicron infection is more severe than non‐COVID croup. Pediatr Emerg Care. 2023;39(9):651‐653. [DOI] [PubMed] [Google Scholar]
- 24. Sharma S, Agha B, Delgado C, et al. Croup associated with SARS‐CoV‐2: pediatric laryngotracheitis during the omicron surge. JPIDS. 2022;11:371‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aregbesola A, Tam CM, Kothari A, et al. Cochrane Database Syst Rev. 2023;1(1):CD001955. doi: 10.1002/14651858.CD001955.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data were collected from the TriNetX database (https://trinetx.com/) and are made available as of May 11, 2023 by contacting Dietrich Jehle, MD, at email: dijehle@utmb.edu.


