Abstract
Background
Hydrofluoric acid (HF) is associated with systemic toxicity, particularly with high‐concentration formulations. However, most existing data describe dermal exposures; there is a paucity of data related to outcomes after ingestions.
Objective
To determine the morbidity and mortality associated with HF ingestions as reported to the National Poison Data System (NPDS). A secondary objective is to assess for clinical criteria that are associated with serious outcomes after HF ingestion.
Methods
We performed a retrospective review of HF ingestions reported to the NPDS from 2007 to 2017. Data including patient demographics, exposure and caller sites, electrolyte abnormalities, treatments, and serious (moderate or major effect or death as documented in NPDS) and non‐serious outcomes were abstracted from case narratives. Cases meeting the criteria for a qualifiable HF ingestion were included in the study.
Results
During the study period, there were 653 HF ingestions reported to NPDS, of which 142 were included in the final data analysis. Most HF exposures occurred in men (68.3%), and the most common exposure site was at the exposed individual's own residence (78.2%). Nearly half of all exposures (46.5%) were due to transfer into a non‐labeled secondary storage container. Total of 45.8% of the cases resulted in a serious outcome. Electrolyte disturbances were associated with an increased risk of a serious outcome. Hypocalcemia was the most frequently reported electrolyte abnormality, occurring in 24.6% of cases. Nine (6.3%) individuals died.
Conclusions
Mortality after HF ingestion is low. However, a large cohort of exposures occurred after the transfer of HF to secondary containers. Targeted interventions to reduce this practice are necessary to decrease hazardous chemical exposures.
1. INTRODUCTION
1.1. Background
Hydrofluoric acid (HF) is a weak acid with household and industrial applications, including rust removal, glass etching, aluminum brightening, and semiconductor and chemical manufacturing. 1 Primarily available in dilute concentrations (1%–10%) in household products and concentrated forms (up to 70%) for industrial use, 2 HF may cause severe toxicity, even after minor cutaneous exposure, particularly with highly concentrated formulations. 3 , 4
HF exposures are potentially devastating, with small areas of exposure to high‐concentration acid potentially leading to death. 3 HF ingestions may carry a higher risk of serious outcomes due to the potential for increased absorption in the upper gastrointestinal tract, the increased surface area of exposure, and increased blood flow in the intestinal tract over dermal blood flow.
HF exerts its toxicity through a multimodal mechanism. As a weak acid, it stays in a protonated and non‐ionic form for an extended period of time, allowing it to penetrate deeply into tissues where, when deprotonation occurs, the highly reactive fluoride ion readily binds to free calcium and magnesium, leading to whole body depletion of these electrolytes, 5 which may cause severe localized pain, cardiac dysrhythmias, and death. Severe exposures often result in cardiac dysrhythmias despite treatment with large doses of calcium and magnesium. 6 Hyperkalemia is another potentially fatal consequence of HF exposure and occurs as a result of cellular death from fluoride contact with tissues and the effect of fluoride on calcium‐dependent potassium channels. 5 , 7
1.2. Importance
Despite the risk of severe morbidity and mortality, there is a paucity of high‐quality data exploring outcomes after HF ingestions.
1.3. Goals of this investigation
We sought to evaluate outcomes after HF ingestions as reported to the National Poison Data System (NPDS) over 10 years from 2007 to 2017. We also sought to determine criteria that might predict serious versus non‐serious outcomes after HF ingestion.
2. METHODS
2.1. Study design
We performed a retrospective review of HF ingestions reported to the NPDS from 2007 to 2017 and requested all available case narratives to conduct a chart review of HF ingestions. NPDS maintains near real‐time information on poisonings reported to all 55 US poison centers. Although coded data are available centrally in the NPDS repository, narrative documentation is stored locally with each poison center. Case narratives were requested from each of the poison centers; however, only those poison centers that shared their information were included in the analysis. NPDS was queried for the American Association of Poison Control Centers generic category code 118000 for all cases involving any number of coingestants for the study dates. All currently active poison centers were contacted by email to the medical director. Up to 5 emails were sent for outstanding case reports. Emails were sent by the primary investigator and the investigating poison center's assistant director. Among those that did not send cases, 1 poison center said no, and 21 did not reply. Institutional review board approval was received before the initiation of this study.
THE BOTTOM LINE
Hydrofluoric (HF) acid ingestions can cause major morbidity and death through rapid electrolyte disturbances. Using US National Poison Center data for 2007–2017, there were 653 ingestions of HF reported with almost 50% having serious outcomes, most commonly hypocalcemia and death, in 9 cases.
2.2. Selection of participants
Inclusion criteria consisted of qualifiable ingestion of HF, categorized as minor ingestion (sip or spray), moderate ingestion (mouthful or swallow), or severe ingestion (more than a mouthful). Exclusion criteria consisted of cases that could not be followed or confirmed as non‐exposures, unavailable case narratives (case closed by the poison center, non‐oral exposure route, or inability to identify or qualify an ingestion based on the available documentation from NPDS). Table S1 highlights the characteristics of cases that were obtained versus those that could not be obtained. Table S2 highlights the characteristics of the cases that met inclusion criteria and were included in the data analysis versus those that could not be obtained. Of note, there were no significant differences between either group when adjusting the P values using the method developed by Benjamini and Yekutieli (false discovery rate [FDR]‐adj P). 8 Trained, non‐blinded medical toxicology fellows abstracted data from case narratives using standardized templates and abstraction guides. At least 2 team members abstracted data from each case, with disagreements adjudicated by a third team member. The overall interrater reliability between the reviewers was excellent (Cohen's kappa = 0.99).
2.3. Measures
The authors abstracted the following data from each case narrative: age, gender, exposure and caller sites, the severity of exposure, agent ingested, the scenario of ingestion, ECG, serum calcium concentration, serum potassium concentration, serum magnesium concentration, treatments provided, and outcome. Hypocalcemia was determined to be present if the statement “hypocalcemia” was made in the narrative or was coded as a clinical effect (regardless of the actual concentration), or the value provided was <8.5 mg/dL, or ionized calcium was <4.4 mg/dL. Hypomagnesemia was determined to be present if the statement “hypomagnesemia” was made in the narrative or was coded as a clinical effect (regardless of the actual concentration) or the value provided was <1.8 mg/dL. Hyperkalemia was determined to be present if the statement “hyperkalemia” was made in the narrative or was coded as a clinical effect (regardless of the actual concentration) or the value provided was >5.0 mEq/L. Additionally, the study team evaluated whether esophagogastroduodenoscopy (EGD) was performed and, if so, its findings.
2.4. Outcomes
Cases were grouped into serious and non‐serious categories based on outcomes. Documentation of a moderate effect, major effect, or death was categorized as a serious outcome. Documentation of a minor or no effect was categorized as a non‐serious outcome. The definitions used for no effect, minor effect, moderate effect, and major effect are those provided in the NPDS Coding Users' Manual: no effect—the patient developed no symptoms as a result of the exposure; minor effect—the patient exhibited some symptoms as a result of the exposure, but they were minimally bothersome to the patient; moderate effect—the patient exhibited symptoms as a result of the exposure which is more pronounced, more prolonged, or more of a systemic nature than minor symptoms; and major effect—the patient has exhibited symptoms as a result of the exposure that were life threatening or resulted in significant residual disability or disfigurement. 9 Outcome categorization is further defined by the NPDS coding manual with the selection of certain symptoms dictating a more serious outcome. For example, documentation of “cardiac arrest” or “intubation and mechanical ventilation” dictates the minimal outcome of “major effect.”
2.5. Statistical analysis
Categorical variables were described using frequencies and percentages, and continuous variables were described using medians and interquartile ranges. Comparisons were conducted using the Mann–Whitney U test, χ2 test, or exact test, depending on the variable. Due to the number of tests and a need to mitigate the increased risk of false positives, the FDR was controlled by adjusting the P values using the method developed by Benjamini and Yekutieli (FDR‐adj P). 8 Interrater reliability for the chart review procedure was evaluated using Cohen's κ. Statistical analyses were conducted using R (v. 4.2; R Core Team, 2022). 10
3. RESULTS
NPDS returned 9357 coded cases of HF exposure from 2007 to 2017. Of these, 653 (6.9%) were coded as ingestions.
Three hundred ninety‐six (60.6%) case files were obtained from 33 (60.0%) of the poison centers queried. An additional 254 cases were excluded from further analysis as, upon manual review, it was unclear whether an ingestion occurred or there was insufficient information for further analysis. A total of 142 cases were included in the final data analysis (Figure 1), of which 77 (54.2%) had a non‐serious outcome, and 65 (45.8%) had a serious outcome. Of those individuals with a serious outcome, nine (13.8%) died. Characteristics of those who died after HF ingestion are shown in Table 1.
FIGURE 1.
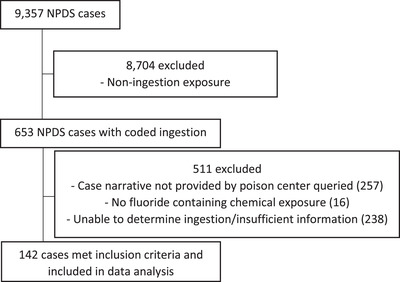
Excluded cases and reasons for exclusion. Abbreviation: NPDS, National Poison Data System.
TABLE 1.
Characteristics of individuals who died after HF ingestion.
| Characteristic |
Death (N = 9) |
|---|---|
| Age, y | 53 (25–64) |
| Gender | |
| Female | 3 (33.3) |
| Male | 6 (66.6) |
| Agent | |
| HF | 5 (55.6) |
| Ammonium bifluoride | 2 (22.2) |
| Other/unknown | 2 (22.2) |
| Exposure site | |
| Own residence | 8 (88.9) |
| Workplace | 1 (11.1) |
| Other | 0 (0) |
| Scenario | |
| In drinking container | 4 (44.4) |
| Suicide/intentional | 3 (33.3) |
| Other/unknown | 2 (22.2) |
| Electrolyte disturbance | |
| Hypocalcemia | 8 (88.9) |
| Hypomagnesemia | 4 (44.4) |
| Hyperkalemia | 0 (0) |
| Ingestion amount, N/% | |
| Minor—Sip or spray to the face | 1 (11.1) |
| Moderate ‐ 1 mouthful or swallow | 2 (22.2) |
| Severe—More than a mouthful | 3 (33.3) |
| Unknown | 3 (33.3) |
Abbreviation: HF, hydrofluoric acid.
The average age of the exposed individuals was 35 years, with no significant differences between outcome categories. The majority of exposures occurred in men (68.3%), and the most common exposure site was at the exposed individual's residence (78.2%), followed by the workplace (16.2%) in both the non‐serious and serious outcome groups. Most calls originated from a health care facility (59.9%), followed by the exposed individual's residence (26.1%). Nearly half of all exposures (46.5%) resulted from HF being transferred from its original container to a drinking container for storage and inadvertently being ingested. Only 8.5% of the reported exposures resulted from an intentional ingestion or suicide attempt. Additional demographic and scenario‐based characteristics are shown in Table 2.
TABLE 2.
Demographic and scenario‐based characteristics of individuals who ingested HF.
| Characteristic | Total (N = 142) | Non‐severe outcome (N = 77) | Severe outcome (N = 65) | P | FDR‐Adj P | κ |
|---|---|---|---|---|---|---|
| Age, y | 35 (15–52) | 35 (10.5–48) | 32 (16.5–53.5) | 0.74 | 1 | – |
| Gender | 0.07 | 0.28 | – | |||
| Female | 45 (31.7) | 29 (37.7) | 16 (24.6) | |||
| Male | 97 (68.3) | 48 (62.3) | 49 (75.4) | |||
| Exposure site | 0.10 | 0.38 | – | |||
| Other | 4 (2.8) | 4 (5.2) | 0 (0) | |||
| Other residence | 1 (0.7) | 0 (0) | 1 (1.5) | |||
| Own residence | 111 (78.2) | 61 (79.2) | 50 (76.9) | |||
| Public area | 1 (0.7) | 0 (0) | 1 (1.5) | |||
| Unknown | 2 (1.4) | 0 (0) | 2 (3.1) | |||
| Workplace | 23 (16.2) | 12 (15.6) | 11 (16.9) | |||
| Caller site | 0.02 | 0.08 | – | |||
| Health care facility | 85 (59.9) | 43 (55.8) | 42 (64.6) | |||
| Other | 14 (9.9) | 5 (6.5) | 9 (13.8) | |||
| Other residence | 3 (2.1) | 3 (3.9) | 0 (0) | |||
| Own residence | 37 (26.1) | 26 (33.8) | 11 (16.9) | |||
| Public area | 1 (0.7) | 0 (0) | 1 (1.5) | |||
| Workplace | 2 (1.4) | 0 (0) | 2 (3.1) | |||
| Ingestion amount, N/% | <0.001 | 0.0003 | 0.99 | |||
| Minor—Sip or spray to the face | 50 (35.2) | 38 (49.4) | 12 (18.5) | |||
| Moderate—1 mouthful or swallow | 50 (35.2) | 28 (36.4) | 22 (33.8) | |||
| Severe—More than a mouthful | 21 (14.8) | 5 (6.5) | 16 (24.6) | |||
| Unknown | 21 (14.8) | 6 (7.8) | 15 (23.1) | |||
| Agent, N/% | <0.001 | 0.002 | 1 | |||
| HF | 87 (61.3) | 45 (58.4) | 42 (64.6) | |||
| Ammonium bifluoride | 37 (26.1) | 28 (36.4) | 9 (13.8) | |||
| Other | 1 (0.7) | 1 (1.3) | 0 (0) | |||
| Unknown | 17 (12) | 3 (3.9) | 14 (21.5) | |||
| Scenario, N/% | 0.06 | 0.28 | 1 | |||
| Child exploration | 17 (12) | 11 (14.3) | 6 (9.2) | |||
| In drinking container | 66 (46.5) | 31 (40.3) | 35 (53.8) | |||
| Industrial/work mishap | 10 (7) | 7 (9.1) | 3 (4.6) | |||
| Other | 19 (13.4) | 14 (18.2) | 5 (7.7) | |||
| Suicide/intentional | 12 (8.5) | 3 (3.9) | 9 (13.8) | |||
| Unknown | 18 (12.7) | 11 (14.3) | 7 (10.8) | |||
| Outcome, N/% | – | – | 1 | |||
| Death | 9 (6.3) | – | 9 (13.8) | |||
| Major effect | 12 (8.5) | – | 12 (18.5) | |||
| Moderate effect | 44 (31) | – | 44 (67.7) | |||
| Minor effect | 50 (35.2) | 50 (64.9) | – | |||
| No effect | 27 (19) | 27 (35.1) | – |
Note: P—P‐values from the Mann–Whitney U, chi‐square, or exact tests. FDR‐adj P—P‐values following the false discovery rate correction. κ—Cohen's kappa. Abbreviations: FDR, false discovery rate; HF, hydrofluoric acid.
Electrolyte disturbances, including hypocalcemia, hypomagnesemia, and hyperkalemia, were associated with an increased risk of a serious outcome. Hypocalcemia was the most frequently reported electrolyte abnormality, occurring in 24.6% of all cases and 47.7% of cases with a major outcome. Not enough cases included serum electrolyte concentrations to allow for further analysis beyond whether these electrolyte derangements existed. Ten individuals (7%) developed dysrhythmia, with ventricular fibrillation (4 cases) and ventricular tachycardia (4 cases), including torsades de pointes, being the most common. Less than half of the individuals (41.5%) were treated with oral calcium or magnesium therapy after HF ingestion, and 35.4% of individuals with a serious outcome received oral calcium or magnesium therapy (Table 3).
TABLE 3.
Electrolyte abnormalities, treatments, and dysrhythmias after HF ingestion.
| Characteristic | Total (N = 142) | Non‐severe outcome (N = 77) | Severe outcome (N = 65) | P | FDR‐Adj P | κ |
|---|---|---|---|---|---|---|
| Hypocalcemia, N/% | <0.001 | <0.001 | 1 | |||
| No | 82 (57.7) | 53 (68.8) | 29 (44.6) | |||
| Yes | 35 (24.6) | 4 (5.2) | 31 (47.7) | |||
| Unknown | 25 (17.6) | 20 (26) | 5 (7.7) | |||
| Hypomagnesemia, N/% | <0.001 | <0.001 | 0.99 | |||
| No | 79 (55.6) | 50 (64.9) | 29 (44.6) | |||
| Yes | 23 (16.2) | 3 (3.9) | 20 (30.8) | |||
| Unknown | 40 (28.2) | 24 (31.2) | 16 (24.6) | |||
| Hyperkalemia, N/% | 0.008 | 0.04 | 0.99 | |||
| No | 96 (67.6) | 51 (66.2) | 45 (69.2) | |||
| Yes | 6 (4.2) | 0 (0) | 6 (9.2) | |||
| Unknown | 40 (28.2) | 26 (33.8) | 14 (21.5) | |||
| ECG dysrhythmia, N/% | 0.005 | 0.03 | 0.99 | |||
| None | 72 (50.7) | 39 (50.6) | 33 (50.8) | |||
| Torsades | 1 (0.7) | 0 (0) | 1 (1.5) | |||
| Ventricular fibrillation | 4 (2.8) | 0 (0) | 4 (6.2) | |||
| Ventricular tachycardia | 3 (2.1) | 0 (0) | 3 (4.6) | |||
| Other | 2 (1.4) | 0 (0) | 2 (3.1) | |||
| Unknown | 60 (42.3) | 38 (49.4) | 22 (33.8) | |||
| Oral calcium/magnesium, N/% | 0.37 | 1 | 0.99 | |||
| No | 49 (34.5) | 25 (32.5) | 24 (36.9) | |||
| Yes | 59 (41.5) | 36 (46.8) | 23 (35.4) | |||
| Unknown | 34 (23.9) | 16 (20.8) | 18 (27.7) |
Note: P—P‐values from the Mann–Whitney U, chi‐square, or exact tests. FDR‐adj P—P‐values following the false discovery rate correction. κ—Cohen's kappa. Abbreviations: FDR, false discovery rate; HF, hydrofluoric acid.
Cases in which an EGD was performed, and the results, are shown in Table 4. Eight (10.4%) of the individuals in the non‐serious outcome group had an EGD performed, of whom 6 (7.8%) had abnormal findings, whereas 33 (50.8%) individuals in the serious outcome group had an EGD performed with 32 (49.2%) having abnormal results.
TABLE 4.
Endoscopy performed and results.
| Characteristic | Total (N = 142) | Non‐severe outcome (N = 77) | Severe outcome (N = 65) | P | FDR‐Adj P | κ |
|---|---|---|---|---|---|---|
| Endoscopy, N/% | <0.001 | <0.001 | ||||
| No | 97 (68.3) | 67 (87) | 30 (46.2) | |||
| Yes | 41 (28.9) | 8 (10.4) | 33 (50.8) | |||
| Unknown | 4 (2.8) | 2 (2.6) | 2 (3.1) | |||
| Endoscopy results, N/% | <0.001 | <0.001 | 1 | |||
| Abnormal | 38 (26.8) | 6 (7.8) | 32 (49.2) | |||
| Normal | 4 (2.8) | 2 (2.6) | 2 (3.1) | |||
| Unknown | 9 (6.3) | 5 (6.5) | 4 (6.2) | |||
| N/A | 91 (64.1) | 64 (83.1) | 27 (41.5) |
Note: P—P‐values from the Mann–Whitney U, chi‐square, or exact tests. FDR‐adj P—P‐values following the false discovery rate correction. κ—Cohen's kappa. Abbreviation: FDR, false discovery rate.
4. LIMITATIONS
This study has several limitations. First, this study reanalyzed prospectively collected data from regional poison centers; thus, the available data may be incomplete or inaccurate. We could not perform laboratory verification of the reportedly ingested chemicals. Second, we were limited to the information documented in the NPDS case files, and long‐term follow‐up could not be performed to assess outcomes. Third, some cases may have been miscoded, including the route of exposure or the chemical exposure. In several instances, we identified cases coded as an ingestion that did not meet our criteria for ingestion. Fourth, NPDS data tend to overestimate severity as it represents only exposures severe enough to warrant consultation with a poison center. Finally, our data may underestimate treatment with calcium and magnesium after HF ingestion as we report only treatment with oral calcium and magnesium. Our data do not capture treatment with intravenous or other non‐oral routes of administration of calcium and magnesium.
5. DISCUSSION
Overall, mortality in our study was low. Nine (6.3%) individuals with an HF ingestion died. The low mortality rate observed may be related to several factors, including exposure to predominantly low‐concentration solutions and small‐volume ingestions. Unfortunately, our source of data, coupled with a lack of details surrounding many cases, precludes further analysis. Mortality summaries were also requested from NPDS, but they did not provide additional insight into the scenarios surrounding the deaths in this study.
We found no statistically significant age difference between outcome groups; working‐aged individuals were primarily affected. Additionally, exposures were almost twice as common in men as women, with the most common exposure sites being an individual's residence and workplace. These findings may reflect that individuals who use and have access to HF are more commonly men with occupational use of HF or home hobbies or chores that involve HF. Interestingly, there was no change in the severity outcome between residential and workplace exposures.
Although an attempt was made to characterize the concentrations of the chemicals ingested, too few cases provided data on concentrations to allow for further analysis. Nonetheless, the majority of the exposures in which the HF concentration was known were low‐concentration (<10%) exposures. There does, however, appear to be a dose‐response effect, as there was a trend toward worse outcomes with increasing ingestion volumes.
Although HF was the predominant species ingested, ammonium bifluoride accounted for almost one quarter of ingestions. However, a statistically significant increase in serious outcomes with HF was noted. This is an interesting finding because ammonium bifluoride contains twice the number of fluoride ions per mole relative to HF. The reason ammonium bifluoride is less frequently associated with a serious outcome in our study is unclear but could be related to the concentration or volume of exposure.
Our study revealed that nearly half of the exposures resulted from a product being transferred from its original container to a drinking container. Additionally, over half of the cases with a serious outcome were associated with transfer to a drinking container. This is a hazardous practice in which severe poisoning may inadvertently result. Prior research has highlighted that this practice is increasing and has led to unintended morbidity and mortality. 11
Of those who ingested HF, suicidal intent was infrequently observed (8.5%). However, among those who attempted suicide with HF, 75% had a serious outcome. Serious outcomes in this group are likely a result of larger volume ingestions due to the intentional nature of the ingestion. Despite the perceived risks of industrial or occupational HF exposures, there were very few industrial HF exposures. Most industrial HF exposure scenarios involved equipment failure, resulting in HF spraying onto skin with no or minimal oral exposure.
A previous study has shown that patients may develop hypocalcemia, ECG abnormalities, and death, even after a low‐concentration HF ingestion. 12 Additionally, there are multiple case reports in which ingestion of HF resulted in refractory ventricular dysrhythmias and death. 2 , 13 , 14 Of those who have survived a significant HF ingestion, the development of critical illness, including cardiac arrest requiring cardiopulmonary resuscitation and defibrillation, was reported. 15 , 16 , 17 In our study, hypocalcemia was strongly associated with serious outcomes, which may be the primary driver of toxicity after HF exposures. Additionally, ECG abnormalities were observed, but rare, with any dysrhythmia occurring in only 10 individuals. However, it was unknown if there was an ECG abnormality in >40% of the cases, limiting the importance of this finding.
Treatment with oral calcium was performed in ≈40% of the cases; however, it was not associated with an outcome severity. Theoretically, oral calcium may help neutralize the HF that has become ionized, improving pain, decreasing the risk of hypocalcemia, and decreasing local tissue destruction. Although the utility of oral calcium has not been thoroughly evaluated in humans, it has been studied using various animal models. Heard et al. used a mouse model that failed to find a mortality benefit, 18 and Coffey et al. found that oral calcium and magnesium delayed, but did not prevent, death in a porcine model. 19 Although the benefit of oral calcium administration may not be clear, the harm is likely minimal. However, which individuals will benefit from oral calcium therapy may not be evident during the initial clinical evaluation.
Endoscopy was associated with more serious outcomes, likely due to selection bias. Patients with severe or persistent symptoms are more likely to be evaluated with endoscopy and are more likely to have been coded as having a serious outcome. The impact endoscopy has on care beyond long‐term prognosis is unclear, and this study was not designed to determine the long‐term consequences of HF ingestions.
In this study of HF ingestions reported to US poison centers, overall mortality was low. However, given its ability to cause rapid and severe toxicity, physicians and advanced practice providers should be aware of the adverse effects and management after HF exposure. Additionally, nearly half of all HF exposures were due to transfer into a non‐labeled secondary storage container. Targeted interventions, including educational public health initiatives and improved product packaging controls, should be considered to reduce hazardous chemical exposures through this practice.
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the United States Air Force, Defense Health Agency, Department of Defense, or US Government.
AUTHOR CONTRIBUTIONS
Brian Patrick Murray, Joseph Carpenter, and Emily Kiernan conceived and designed the study. Brian Patrick Murray, Joseph Carpenter, Zachary Illg, and Emily Kiernan supervised the conduct of the study and data collection. Brian Patrick Murray, Joseph Carpenter, Zachary Illg, and Emily Kiernan managed the data, including quality control. Tim P. Moran provided statistical advice on study design and analyzed the data. Zachary Illg drafted the manuscript, and all authors contributed substantially to its revision. Brian Patrick Murray takes responsibility for the paper as a whole.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to report.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENT
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Illg Z, Carpenter J, Moran TP, Kiernan E, Murray BP. Hydrofluoric acid ingestions: Retrospective evaluations from cases reported to the National Poison Data System 2007–2017. JACEP Open. 2023;4:e13059. 10.1002/emp2.13059
Supervising Editor: Christian Tomaszewski, MD, MS
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Meetings: Preliminary findings from this study were presented at the 2019 American College of Medical Toxicology Annual Scientific Meeting.
REFERENCES
- 1. Kirkpatrick JJ, Enion DS, Burd DA. Hydrofluoric acid burns: a review. Burns. 1995;21(7):483‐493. [DOI] [PubMed] [Google Scholar]
- 2. Kavakli AS, Kavrut Ozturk N. Recurrent ventricular fibrillation associated with acute ingestion of hydrofluoric acid. J Clin Anesth. 2018;46:8‐9. [DOI] [PubMed] [Google Scholar]
- 3. Tepperman PB. Fatality due to acute systemic fluoride poisoning following a hydrofluoric acid skin burn. J Occup Med. 1980;22(10):691‐692. [DOI] [PubMed] [Google Scholar]
- 4. Wu ML, Deng JF, Fan JS. Survival after hypocalcemia, hypomagnesemia, hypokalemia and cardiac arrest following mild hydrofluoric acid burn. Clin Toxicol (Phila). 2010;48(9):953‐955. [DOI] [PubMed] [Google Scholar]
- 5. Hoffman RS, Burns MM, Gosselin S. Ingestion of caustic substances. N Engl J Med. 2020;382(18):1739‐1748. [DOI] [PubMed] [Google Scholar]
- 6. Vohra R, Velez LI, Rivera W, Benitez FL, Delaney KA. Recurrent life‐threatening ventricular dysrhythmias associated with acute hydrofluoric acid ingestion: observations in one case and implications for mechanism of toxicity. Clin Toxicol. 2008;46(1):79‐84. [DOI] [PubMed] [Google Scholar]
- 7. Cummings CC, McIvor ME. Fluoride‐induced hyperkalemia: the role of Ca2+‐dependent K+ channels. Am J Emerg Med. 1988;6(1):1‐3. [DOI] [PubMed] [Google Scholar]
- 8. Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29(4):1165‐1188. [Google Scholar]
- 9. American Association of Poison Control Centers . NPDS Coding Users' Manual Version 3.1. 2014.
- 10. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2022. https://www.R‐project.org/ [Google Scholar]
- 11. Carpenter JE, Murray BP, Moran TP, Dunkley CA, Layer MR, Geller RJ. Poisonings due to storage in a secondary container reported to the National Poison Data System, 2007–2017. Clin Toxicol (Phila). 2021;59(6):521‐527. [DOI] [PubMed] [Google Scholar]
- 12. Kao WF, Dart RC, Kuffner E, Bogdan G. Ingestion of low‐concentration hydrofluoric acid: an insidious and potentially fatal poisoning. Ann Emerg Med. 1999;34(1):35‐41. doi: 10.1016/s0196-0644(99)70269-6 [DOI] [PubMed] [Google Scholar]
- 13. Cordero SC, Goodhue WW, Splichal EM, Kalasinsky VF. A fatality due to ingestion of hydrofluoric acid. J Anal Toxicol. 2004;28(3):211‐213. [DOI] [PubMed] [Google Scholar]
- 14. Ozsoy G, Kendirli T, Ates U, et al. Fatal refractory ventricular fibrillation due to ingestion of hydrofluoric acid. Pediatr Emerg Care. 2019;35(11):e201‐e2. [DOI] [PubMed] [Google Scholar]
- 15. Chan BS, Duggin GG. Survival after a massive hydrofluoric acid ingestion. J Toxicol Clin Toxicol. 1997;35(3):307‐309. [DOI] [PubMed] [Google Scholar]
- 16. Stremski ES, Grande GA, Ling LJ. Survival following hydrofluoric acid ingestion. Ann Emerg Med. 1992;21(11):1396‐1399. [DOI] [PubMed] [Google Scholar]
- 17. Whiteley PM, Aks SE. Case files of the Toxikon Consortium in Chicago: survival after intentional ingestion of hydrofluoric acid. J Med Toxicol. 2010;6(3):349‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heard K, Delgado J. Oral decontamination with calcium or magnesium salts does not improve survival following hydrofluoric acid ingestion. J Toxicol Clin Toxicol. 2003;41(6):789‐792. [DOI] [PubMed] [Google Scholar]
- 19. Coffey JA, Brewer KL, Carroll R, Bradfield J, Meggs WJ. Limited efficacy of calcium and magnesium in a porcine model of hydrofluoric acid ingestion. J Med Toxicol. 2007;3(2):45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


