Abstract
We investigated the antifungal activities of itraconazole and voriconazole on Aspergillus species by time kill studies, and the results were compared with those obtained for Candida species. Exposure of Aspergillus fumigatus conidia to varying concentrations (1.25 to 10 μg/ml) of itraconazole and voriconazole resulted in cellular death; the cytocidal effect was time and concentration dependent. In contrast, no killing of Candida albicans occurred in the presence of itraconazole and voriconazole at concentrations as high as 10 μg/ml, although candidal growth was inhibited compared to the drug-free control. Amphotericin B (1.25 to 10 μg/ml), on the other hand, killed both A. fumigatus and C. albicans. Similar results were obtained for non-A. fumigatus aspergilli and non-C. albicans Candida species. These observations indicate that both itraconazole and voriconazole are cytocidal agents for Aspergillus species but not for Candida species, suggesting that azoles possess organism-dependent fungicidal activities.
Amphotericin B and certain members of the azole family of antifungals are the most commonly used antibiotics for the treatment of systemic fungal infections (3–6, 14). Amphotericin B acts on the cytoplasmic membrane, in particular the ergosterol component (1, 13), leading to irreversible damage of the membrane and consequent leakage of essential nutrients. The lack of a permeability barrier to essential nutrients and ions is believed to be responsible for the fungicidal activity of amphotericin B (2, 11, 12). In contrast, the azoles are known to be fungistatic agents. These heterocyclic compounds inhibit the synthesis of sterol in fungi by inhibiting cytochrome P-450-dependent 14α-lanosterol demethylase (P-45014DM), which specifically removes the methyl group on C-14 of lanosterol (5, 16–18). This demethylation is an essential intermediate step in the synthesis of ergosterol, the major sterol found in fungi. The action of azoles on P-45014DM of yeasts appears to be reversible; once the drug is removed, the organism recovers rapidly and functions normally. As a result of the static effect of azoles on yeasts, it is difficult to obtain clearly defined endpoints during susceptibility studies, and the so-called “trailing phenomenon” is very common (9, 15). On the other hand, the trailing phenomenon is uncommon during susceptibility tests with fungicidal agents such as amphotericin B and nystatin. During our recent studies of the susceptibility of Aspergillus species to voriconazole, we observed clearly defined endpoints, and prolonged incubation of the MIC tubes rarely resulted in higher MICs (8). Moreover, the minimum fungicidal concentrations were only two- to fourfold higher than the MICs. These findings suggested that voriconazole acts possibly as a fungicidal agent for Aspergillus fumigatus and prompted us to examine the activity of voriconazole against pathogenic yeasts and Aspergillus species by kill curve experiments and to compare the results with those obtained for itraconazole and amphotericin B.
Clinical isolates of A. fumigatus and non-A. fumigatus species were obtained from the Microbiology Laboratory, Detroit Medical Center, Wayne State University. Working cultures were maintained on peptone-yeast extract-glucose (PYG) (peptone, 1 g; yeast extract, 1 g; glucose, 3 g [per liter of distilled water]) agar at room temperature. The primary cultures obtained from the Detroit Medical Center were subcultured on PYG agar to assure the purity of the cultures. Long-term storage of the cultures was done as conidial suspensions in 25% glycerol at −70°C.
Working cultures of Candida species used in this study were grown for 48 h at 30°C on Sabouraud dextrose agar from stock cultures stored at −70°C in litmus milk (Becton Dickinson Microbiology Systems, Cockeysville, Md.). Single colonies from the 2-day-old cultures were used as the source of the inoculum for all subsequent experiments.
Conidial suspensions of various Aspergillus species were prepared, and the MICs of various antifungals were determined as described previously (7, 10). Briefly, fresh conidia were resuspended in PYG medium at a density of 2 × 104 conidia/ml. Twice the required concentrations of the drugs were prepared in PYG medium (0.5 ml) by serial dilution in sterile 6-ml polystyrene tubes (Falcon 2054) and inoculated with an equal volume (0.5 ml) of the conidial suspension. The tubes were incubated at 35°C for 48 h and scored for visible growth after gentle vortexing of the tubes or scraping of the walls of the tubes followed by vortexing. The MIC was defined as the lowest concentration of drug at which no visible growth occurred. The MIC determination for each isolate was repeated at least once, and the data were within ±1 dilution.
The MICs of amphotericin B, itraconazole, and voriconazole for various Candida species used in this study were determined by the broth microdilution method, as recommended by the National Committee for Clinical Laboratory Standards (9). The MIC was defined as the lowest concentration of drug that inhibited growth by 80% compared to the drug-free control after 48 h of incubation at 35°C.
Aspergillus spp.
Five milliliters of conidial suspension prepared in PYG broth (106 conidia/ml) was incubated at 35°C in the presence of various concentrations of amphotericin B, itraconazole, and voriconazole (0 to 10 μg/ml). At various time intervals, 0.1-ml aliquots of the conidial suspension were removed and diluted appropriately to obtain 10- to 104-fold dilutions, and 0.1-ml aliquots were spread in duplicate on PYG agar plates. The plates were incubated at 35°C for 48 h, and the numbers of CFU per milliliter of conidial suspension were determined.
Candida spp.
Fresh 24-h-old cultures of Candida species prepared in PYG broth were diluted approximately 1,000-fold to obtain a cell density of 106 CFU per ml. One-milliliter aliquots of the diluted cultures were incubated with 0 to 10 μg of the antifungals per ml at 35°C. At various time intervals, aliquots (0.05 ml) of the drug-treated cell suspensions were removed and serially diluted (10- to 106-fold), and 0.1-ml amounts were spread on Sabouraud dextrose agar plates in duplicate. The plates were incubated at 35°C for 24 h, and the numbers of CFU/ml of culture were determined. A similar treatment without the drug was used as a growth control where applicable.
Itraconazole (R 51,211, batch no. STAN-9304-005-1), voriconazole, and amphotericin B (batch no. 20-914-29670) were obtained from Janssen Pharmaceutica, Beerse, Belgium; Pfizer Pharmaceuticals, New York, N.Y.; and Squibb Institute for Medical Research, Princeton, N.J.; respectively. All antifungals were dissolved in dimethyl sulfoxide at concentrations of 1 mg/ml and stored as 0.25-ml aliquots at −20°C. The frozen stocks were thawed at room temperature and gently vortexed several times to ensure that any remaining crystals were completely dissolved before use. Drug concentrations ranging from 0 to 16 μg/ml were used for MIC determinations. Comparable concentrations of dimethyl sulfoxide were tested to examine its effect on the growth of A. fumigatus. No detectable inhibition of growth occurred at the concentrations used.
The MICs of amphotericin B, itraconazole, and voriconazole obtained for various Aspergillus and Candida species are shown in Table 1. As shown, all of the isolates used in this study were susceptible to low concentrations of amphotericin B (MIC range, 0.02 to 4 μg/ml), itraconazole (MIC range, 0.031 to 4 μg/ml), and voriconazole (MIC range, 0.015 to 2 μg/ml).
TABLE 1.
Susceptibilities of microorganisms testeda
| Microorganism | Sourceb | MIC (μg/ml)c
|
||
|---|---|---|---|---|
| AMB | ITZ | VCZ | ||
| A. fumigatusW73355 | DMC | 0.5 | 0.5 | 0.5 |
| A. fumigatusF55064 | DMC | 0.5 | 0.25 | 1 |
| A. fumigatusH52950 | DMC | 1 | 0.25 | 0.5 |
| A. fumigatusT52654 | DMC | 0.5 | 0.25 | 0.5 |
| A. fumigatusZ88896 | DMC | 1 | 0.5 | 1 |
| Aspergillus nigerS11338 | DMC | 4 | 4 | 1 |
| A. nigerF51729 | DMC | 1 | 4 | 1 |
| A. nigerI71775 | DMC | 1 | 4 | 1 |
| A. niger W7884 | DMC | 2 | 0.25 | 0.25 |
| A. nigerT57275 | DMC | 2 | 0.5 | 2 |
| Aspergillus flavusI65850 | DMC | 0.5 | 0.25 | 1 |
| A. flavusI65680 | DMC | 8 | 0.25 | 0.5 |
| A. flavusW69597 | DMC | 4 | 0.25 | 0.5 |
| A. flavusS47511 | DMC | 4 | 0.5 | 0.25 |
| A. flavusW72335 | DMC | 4 | 0.25 | 0.25 |
| Aspergillus sp. M65388 | DMC | 0.5 | 0.5 | 0.5 |
| Aspergillus sp. I35077 | DMC | 1 | 0.5 | 0.25 |
| Aspergillus sp. R26451 | DMC | 2 | 4 | 0.5 |
| C. albicans 90028 | ATCC | 0.02 | 0.031 | 0.015 |
| Candida guilliermondii 9390 | ATCC | 0.25 | 0.5 | 0.25 |
| Candida lusitaniae 40438 | DMC | 0.5 | 0.25 | 0.5 |
| Candida parapsilosis CM205.95 | DMC | 0.5 | 0.125 | 0.125 |
| Candida kefyr LK061.90 | DMC | 0.5 | 0.125 | 0.125 |
| Candida stellatoidea GW575.90 | DMC | 0.125 | 0.125 | 0.125 |
| Candida tropicalis 44508 | ATCC | 0.5 | 0.25 | 0.25 |
| Candida glabrata 33554 | ATCC | 0.5 | 0.5 | 0.5 |
Results shown are from a typical experiment. Each value represents the mean of two independent determinations. MIC determinations were repeated at least once, and the results were within ±1 dilution.
DMC, Detroit Medical Center; ATCC, American Type Culture Collection.
AMB, amphotericin B; ITZ, itraconazole; VCZ, voriconazole.
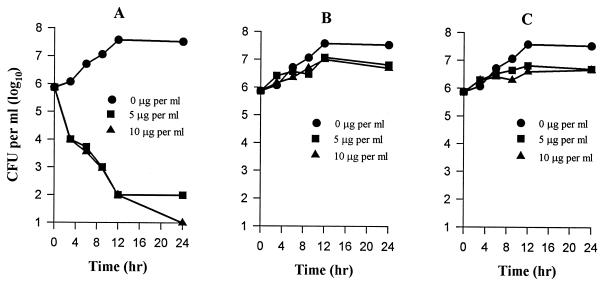
The effects of amphotericin B, itraconazole, and voriconazole over a 24-h period on the ability of A. fumigatus W73355 conidia to produce colonies are shown in Fig. 1. The concentrations (1.25 to 10 μg/ml) of the drugs used for the kill curve studies were 2.5- to 20-fold higher than the MICs of the drugs for efficient killing. All three compounds at the concentrations used reduced the number of CFU with time in a dose-dependent manner, compared to the initial inoculum. For example, approximately 99% of the conidia were killed by amphotericin B at 5 μg/ml (Fig. 1A) within 24 h. Under the same conditions, itraconazole at 5 μg/ml (Fig. 1B) provided 85% killing. Approximately 95% killing was obtained with voriconazole at 5 μg/ml (Fig. 1C), suggesting that it has slightly better fungicidal activity than itraconazole but is not as efficient as amphotericin B.
FIG. 1.
Comparison of the fungicidal activities of amphotericin B (A), itraconazole (B), and voriconazole (C) against A. fumigatus W73355. Each point represents the mean of two independent determinations. Experiments were repeated three times with similar results; the data shown here are from a typical experiment.
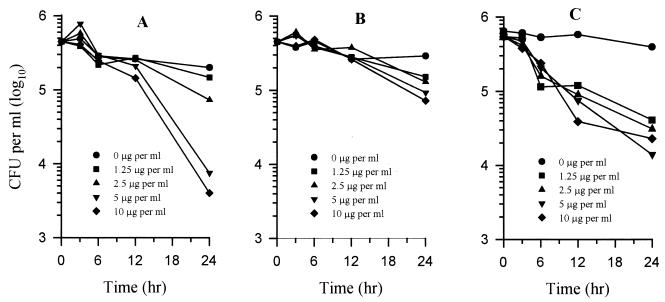
Figure 2 shows the effects of exposure of Candida albicans to amphotericin B, itraconazole, and voriconazole over a 24-h period. In contrast to the fungicidal activities of the three drugs against Aspergillus, only amphotericin B showed a reduction in CFU per milliliter with time against Candida species. Amphotericin B at 5 and 10 μg/ml provided ≥99.99 and 100% killing, respectively (Fig. 2A) within 24 h, whereas no killing was obtained at either 5 or 10 μg of itraconazole (Fig. 2B) or voriconazole (Fig. 2C) per ml. With both azoles, the growth of the organism after 24 h was inhibited approximately 80 to 85% compared to the drug-free control. However, there was an increase in CFU per milliliter compared to the initial inoculum.
FIG. 2.
Comparison of the fungicidal or fungistatic activities of amphotericin B (A), itraconazole (B), and voriconazole (C) against C. albicans 90028. Each point represents the mean of two independent determinations. Experiments were repeated twice with similar results; the data shown here are from a typical experiment.
In addition to A. fumigatus and C. albicans, we investigated the fungicidal activities of amphotericin B, itraconazole, and voriconazole on other clinically important species of Aspergillus and Candida. As shown in Table 2, non-A. fumigatus aspergilli examined showed ≥87% reduction in CFU per milliliter within 24 h of drug exposure. Hence, itraconazole and voriconazole were fungicidal against A. fumigatus and other Aspergillus species tested. All seven non-C. albicans Candida species examined were killed (≥95%) within 24 h by amphotericin B, whereas no killing was obtained with voriconazole and itraconazole. This finding demonstrated that the azoles tested have only fungistatic activity against C. albicans and the other yeasts examined.
TABLE 2.
Fungicidal or fungistatic activities of amphotericin B, itraconazole, and voriconazole against Aspergillus and Candida speciesa
| Microorganism | 105 CFU/ml at T0 | 105 CFU/ml at T24 (% change)b
|
||
|---|---|---|---|---|
| AMB | ITZ | VCZ | ||
| A. fumigatus(n = 5) | 8.52 ± 2.67 | 0.071 ± 0.091 (−99.2) | 0.195 ± 0.297 (−97.7) | 0.096 ± 0.055 (−98.9) |
| A. niger(n = 5) | 4.19 ± 1.85 | 0.074 ± 0.097 (−98.2) | 0.541 ± 0.349 (−87.1) | 0.224 ± 0.140 (−94.7) |
| A. flavus(n = 5) | 9.06 ± 4.78 | 0.173 ± 0.288 (−98.1) | 0.317 ± 0.223 (−96.5) | 0.376 ± 0.243 (−95.9) |
| Aspergillus sp. (n = 3) | 5.57 ± 5.08 | 0.081 ± 0.123 (−98.5) | 0.207 ± 0.284 (−96.3) | 0.052 ± 0.023 (−99.1) |
| C. albicans | 7.35 ± 0.68 | 0.00005 (−100) | 65.3 ± 5.2 (+788.4) | 50 ± 7.2 (+580.2) |
| C. guilliermondii | 12.3 ± 4.2 | 0.00005 (−100) | 16.3 ± 7.7 (+32.5) | 19.2 ± 15.6 (+56.1) |
| C. lusitaniae | 8.5 ± 2.12 | 0.028 ± 0.012 (−99.7) | 20.1 ± 5.9 (+136.5) | 131 ± 39 (+1,441.2) |
| C. parapsilosis | 4.0 ± 1.4 | 0.129 ± 0.026 (−96.8) | 12.8 ± 0.28 (+220.0) | 13.2 ± 2.9 (+230) |
| C. kefyr | 25.0 ± 2.4 | 0.092 ± 0.025 (−99.6) | 60.5 ± 20.5 (+142) | 66 ± 19 (+164) |
| C. stellatoidea | 12.5 ± 4.2 | 0.00005 (−100) | 84.0 ± 21.4 (+572) | 108 ± 43 (+764) |
| C. tropicalis | 11.7 ± 2.5 | 0.0067 (−99.9) | 137 ± 65 (+1,070.9) | 150 ± 37 (+1,182.1) |
| C. glabrata | 7.0 ± 0 | 0.00005 (−100) | 152 ± 30 (+2,071.4) | 46.5 ± 23.3 (+564.3) |
Results shown are from a typical experiment. Each value represents the mean of two independent determinations. T0 and T24 denote the times immediately prior to and 24 h after addition of the antifungal agent, respectively.
Percent change is the decrease or increase from the original inoculum (T0). AMB, amphotericin B; ITZ, itraconazole; VCZ, voriconazole. All drugs were used at 5 μg/ml.
Our present observations show that itraconazole and voriconazole, like amphotericin B (albeit to a lesser degree), have fungicidal activity against A. fumigatus and the other Aspergillus species examined. Shorter incubation times of conidia in the presence of higher concentrations of drugs were not very effective. This finding is not surprising considering that any killing of cells via inhibition of the sterol synthetic pathway would take longer than production of a leaky cytoplasmic membrane by direct action on ergosterol (e.g., amphotericin B). Furthermore, our studies support previous findings that the azoles have fungistatic activity against Candida species. Varying concentrations (including levels achievable in humans) of itraconazole and voriconazole showed time-dependent cytocidal activities against Aspergillus species but only cytostatic effects against Candida species. The exact reason(s) for such differential activities of azoles against fungi is not clear. It is possible that the sterol synthetic pathway in Aspergillus is essential and that inhibition of ergosterol synthesis may lead to cell death in Aspergillus but not in Candida. As fungal infections due to filamentous fungi are seen increasingly in patients with immunocompromised states, the cytocidal activities of azoles against Aspergillus species may be of clinical significance and hold promise in the treatment of these frequently fatal infections.
Acknowledgments
We thank William Brown and Joe Vazquez (Detroit Medical Center, Wayne State University) for kindly providing the various isolates of Aspergillus and Candida species used in this study.
REFERENCES
- 1.Bolard J. How do the polyene macrolide antibiotics affect cellular membrane properties? Biochim Biophys Acta. 1986;864:257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 2.Cohn B E. Concentration and time-dependence of amphotericin B induced permeability changes across ergosterol-containing liposomes. Biochim Biophys Acta. 1985;857:117–122. doi: 10.1016/0005-2736(86)90104-5. [DOI] [PubMed] [Google Scholar]
- 3.Elewski B E. Mechanisms of action of systemic antifungal agents. J Am Acad Dermatol. 1993;28:S28–S34. doi: 10.1016/s0190-9622(09)80305-8. [DOI] [PubMed] [Google Scholar]
- 4.Georgopapadakou N H, Walsh T J. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279–291. doi: 10.1128/aac.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heeres J, Backx L J J, Mostmans J H. Antimycotic imidazoles. Part 4. Synthesis and antifungal activity of ketoconazole. A new potent orally active broad-spectrum antifungal agent. J Med Chem. 1979;22:1003–1005. doi: 10.1021/jm00194a023. [DOI] [PubMed] [Google Scholar]
- 6.Lyman C A, Walsh T J. Systemically administered antifungal agents: a review of their clinical pharmacology and therapeutic applications. Drugs. 1992;44:9–35. doi: 10.2165/00003495-199244010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Manavathu E K, Alangaden G J, Lerner S A. A comparative study of the broth micro- and macro-dilution techniques for the determination of the in vitro susceptibility of Aspergillus fumigatus. Can J Microbiol. 1996;42:960–964. doi: 10.1139/m96-123. [DOI] [PubMed] [Google Scholar]
- 8.Manavathu, E. K., J. L. Cutright, and P. H. Chandrasekar. 1997. In vitro susceptibility of itraconazole-resistant isolates of Aspergillus fumigatus to voriconazole. Clin. Microbiol. Infect. 3(Suppl. 2):81. [DOI] [PubMed]
- 9.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 10.Oakley K L, Moore C B, Denning D W. In vitro activity of SCH-56592 and comparison with activities of amphotericin B and itraconazole against Aspergillus spp. Antimicrob Agents Chemother. 1997;41:1124–1126. doi: 10.1128/aac.41.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palacios J, Serano R. Proton permeability induced by polyene antibiotics: a plausible mechanism for their inhibition of maltose fermentation in yeast. FEBS Lett. 1978;91:198–201. doi: 10.1016/0014-5793(78)81171-5. [DOI] [PubMed] [Google Scholar]
- 12.Sokol-Anderson M L, Brajtburg J, Medoff G. Amphotericin B-induced oxidation damage and killing of Candida albicans. J Infect Dis. 1986;154:76–83. doi: 10.1093/infdis/154.1.76. [DOI] [PubMed] [Google Scholar]
- 13.Thomas A H. Suggested mechanisms for the antimycotic activity of the polyene antibiotics and the N-substituted imidazoles. J Antimicrob Chemother. 1986;17:269–279. doi: 10.1093/jac/17.3.269. [DOI] [PubMed] [Google Scholar]
- 14.Vanden Bossche H, Warnock D W, Dupont B, Kerridge D. Mechanisms and clinical impact of antifungal drug resistance. J Med Vet Mycol. 1994;32:189–202. doi: 10.1080/02681219480000821. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez J A, Lynch M, Sobel J D. In vitro activity of a new pneumocandin antifungal agent, L-733,560 against azole-susceptible and -resistant Candida and Torulopsis species. Antimicrob Agents Chemother. 1995;39:2689–2691. doi: 10.1128/aac.39.12.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright G D, Parent T, Honek J F. Nonsterol structural probes of the lanosterol 14α-demethylase from Saccharomyces cerevisiae. Biochim Biophys Acta. 1990;1040:95–101. doi: 10.1016/0167-4838(90)90151-5. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida Y, Aoyama Y. Yeast cytochrome P-450 catalyzing lanosterol 14α-demethylation. J Biol Chem. 1984;259:1655–1660. [PubMed] [Google Scholar]
- 18.Yoshida Y, Aoyama Y. Interaction of azole antifungal agents with cytochrome P-45014DM purified from Saccharomyces cerevisiae microsomes. Biochem Pharmacol. 1987;36:229–235. doi: 10.1016/0006-2952(87)90694-0. [DOI] [PubMed] [Google Scholar]