Abstract
BACKGROUND AND OBJECTIVES
Multisystem inflammatory syndrome in children (MIS-C) is a novel, severe condition following severe acute respiratory syndrome coronavirus 2 infection. Large epidemiologic studies comparing MIS-C to Kawasaki disease (KD) and evaluating the evolving epidemiology of MIS-C over time are lacking. We sought to understand the illness severity of MIS-C compared with KD and evaluate changes in MIS-C illness severity over time during the coronavirus disease 2019 pandemic compared with KD.
METHODS
We included hospitalizations of children with MIS-C and KD from April 2020 to May 2022 from the Pediatric Health Information System administrative database. Our primary outcome measure was the presence of shock, defined as the use of vasoactive/inotropic cardiac support or extracorporeal membrane oxygenation. We examined the volume of MIS-C and KD hospitalizations and the proportion of hospitalizations with shock over time using 2-week intervals. We compared the proportion of hospitalizations with shock in MIS-C and KD patients over time using generalized estimating equations adjusting for hospital clustering and age, with time as a fixed effect.
RESULTS
We identified 4868 hospitalizations for MIS-C and 2387 hospitalizations for KD. There was a higher proportion of hospitalizations with shock in MIS-C compared with KD (38.7% vs 5.1%). In our models with time as a fixed effect, we observed a significant decrease in the odds of shock over time in MIS-C patients (odds ratio 0.98, P < .001) but not in KD patients (odds ratio 1.00, P = .062).
CONCLUSIONS
We provide further evidence that MIS-C is a distinct condition from KD. MIS-C was a source of lower morbidity as the pandemic progressed.
What’s Known on This Subject:
MIS-C is a severe condition following severe acute respiratory syndrome coronavirus 2 infection. Large studies comparing MIS-C with KD and evaluating the epidemiology and illness severity of MIS-C and KD over the course of the COVID-19 pandemic are lacking.
What This Study Adds:
We report the epidemiology of MIS-C and KD during COVID-19 in a large, geographically diverse cohort. There was a significant decrease in the odds of shock over time in MIS-C patients. KD hospitalization volume returned to pre-pandemic levels.
A subset of children with coronavirus disease 2019 (COVID-19) will develop multisystem inflammatory syndrome in children (MIS-C), a severe condition that often requires intensive care. The Centers for Disease Control and Prevention defines MIS-C as a patient with fever, evidence of inflammation, and clinically severe illness with involvement of multiple organ systems requiring hospitalization and no likely alternative diagnosis, along with recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or exposure to a confirmed or suspected COVID-19 case.1 MIS-C was originally described in April 2020 as a Kawasaki disease (KD)-like illness with similarities including fever, evidence of inflammation, and symptomatology.2–7 However, in contrast to KD, which rarely necessitates intensive care, case series and observational studies have revealed substantial rates of critical illness in MIS-C.6–11 Large-scale epidemiologic studies comparing patient demographics and illness severity in patients with MIS-C and KD over time during the COVID-19 pandemic are lacking. Understanding the similarities and differences between these 2 entities may help inform diagnostic and treatment recommendations.
The epidemiology of MIS-C and KD has evolved over the course of the COVID-19 pandemic, with observed differences in rates of MIS-C during different waves of COVID-1910,12–15 and a decreased incidence of KD early in the pandemic.16–20 Yet, there is a dearth of literature both evaluating the severity of illness in MIS-C over time and describing the epidemiology of KD after the first year of the pandemic. The recent identification of validated diagnostic algorithms to identify patients with MIS-C in administrative databases21 now allows for epidemiologic studies of MIS-C in these multisite data sources. A better understanding of epidemiologic trends may guide the identification of risk factors for severe disease, inform proper patient disposition and monitoring, and aid prioritization of future research.
We sought to compare MIS-C to KD and to investigate the temporal trends in MIS-C epidemiology using a large cohort of children from geographically diverse children’s hospitals in the United States identified from administrative data dating back to the beginning of the COVID-19 pandemic. Specifically, our aims were to understand the severity of illness in MIS-C compared with KD and to evaluate changes in the severity of illness in MIS-C over time compared with KD. We hypothesized that children hospitalized with MIS-C would develop shock more often than children hospitalized with KD before and during the COVID-19 pandemic.
Methods
Study Design and Data Source
We conducted a serial cross-sectional study of hospitalizations using the Pediatric Health Information System (PHIS), an administrative database maintained by the Children’s Hospital Association (Lenexa, KS) that contains demographics, daily billing data, and diagnosis and procedure codes (using International Classification of Diseases, Tenth Edition [ICD-10] codes and Current Procedural Terminology codes, respectively) from all discharges from 49 children’s hospitals in 34 US states. The Children’s Hospital Association and participating hospitals jointly ensure data quality. We included data from 39 hospitals, excluding 10 hospitals that did not have data for every month during the study period. This study was deemed not human subjects research by the Cincinnati Children’s Hospital Institutional Review Board.
Study Population
We included hospitalizations for children aged ≥30 days to <21 years with hospitalization for MIS-C or KD at a PHIS-participating hospital from April 1, 2020 to May 31, 2022 (hereafter the COVID-19 period). We used the ICD-10 code for MIS-C (M35.81) to identify hospitalizations for MIS-C from January 2021 to May 2022. We excluded children with diagnoses of oncologic disease and transplant because this improves the accuracy of the MIS-C code.21 Before 2021, there was no dedicated ICD-10 code for MIS-C. To identify patients with MIS-C from April 2020 to December 2020 and minimize the risk of misclassification bias, we used a previously developed algorithm21 (Supplemental Table 5) with high sensitivity and positive predictive value for identifying MIS-C hospitalizations. We used the ICD-10 code for KD (M30.3) to identify hospitalizations for KD. For historical comparisons, we also included children aged ≥30 days to <21 years with hospitalization for KD that occurred before the COVID-19 pandemic from January 1, 2016 to March 31, 2020. To minimize the risk of misclassification bias and not include hospitalizations for which there was diagnostic uncertainty, we excluded hospitalizations with discharge diagnoses for both KD and MIS-C from our primary analysis and examined them separately.
Patient Demographics and Clinical Characteristics
We examined patient demographics, including age, sex, race/ethnicity, primary payor, and Child Opportunity Index (COI).22 We categorized age to match COVID-19 vaccine age categories (<5 years, 5–11, 12–15, 16–20) and with children <1 year as a standalone category recognizing the differences in clinical presentation and outcomes in this group for KD.23 Race and ethnicity were examined as social constructs and included in our analysis because of previously reported disparities in outcomes related to MIS-C that may reflect disparities in timely access to care, structural racism, and implicit bias.24 COI is an aggregate measure of resource availability at the neighborhood level based on the patient’s residential ZIP code and has been previously reported to be associated with a diagnosis of MIS-C.25
We examined patient clinical characteristics, including the number of complex chronic conditions (CCC),26 except that we excluded the diagnosis code for KD as a CCC because all children in our KD group had a cardiac CCC. Because clinical data, such as vital signs and laboratory results, are not included in PHIS, we assessed the severity of illness using Hospitalization Resource Intensity Scores for Kids (H-RISK), a pediatric-specific measure of illness severity and resource utilization.27 H-RISK assigns relative weights to each All Patient Refined Diagnosis Group (3M Healthcare) and severity of illness level to facilitate comparison across All Patient Refined Diagnosis Groups. We also captured the use of echocardiography in all groups by evaluating billing data.
Outcome Measures
Our primary outcome measure was the presence of shock, defined as the use of vasoactive/inotropic cardiac support with epinephrine, norepinephrine, phenylephrine, dopamine, dobutamine, vasopressin, or milrinone, or the use of extracorporeal membrane oxygenation (ECMO). Death was evaluated as a secondary outcome. We examined the number of KD and MIS-C hospitalizations and the proportion of hospitalizations with shock over time at 2-week intervals.
Statistical Analysis
We examined demographics, clinical characteristics, and outcomes using χ2 tests. We performed unadjusted comparisons of demographics, clinical characteristics, and outcomes in 3 groups of patients: (1) patients with MIS-C compared with patients with KD during the COVID-19 pandemic (April 2020 through May 2022), (2) patients with MIS-C, KD, or a diagnosis of both MIS-C and KD during the COVID-19 pandemic, and (3) patients with KD during the COVID-19 pandemic compared with the historical KD patients (January 2016 through March 2020).
We examined the proportion of hospitalizations with shock during each COVID-19 wave time period captured in our study: March 1, 2020 to June 20, 2020 (wave 1), June 21, 2020 to September 19, 2020 (wave 2), September 20, 2020 to March 6, 2021 (wave 3), March 7, 2021 to July 3, 2021 (wave 4), July 4, 2021 to November 6, 2021 (wave 5), and November 7, 2021 to the end of the study period (wave 6). The endpoint of each wave was identified by using the nadirs of COVID-19 hospitalizations on Centers for Disease Control and Prevention charts.28 We compared the proportion of hospitalizations with shock across age categories during each COVID-19 wave using χ2 tests. We also compared the proportion of hospitalizations with shock across the COVID-19 waves for each age category using the Cochran-Armitage Trend Test. The proportion of hospitalizations with shock for patients with MIS-C and KD was evaluated over time by using generalized estimating equations with time as a fixed effect and adjusting for age and hospital clustering. All statistical analyses were performed by using SAS v.9.4 (SAS Institute, Cary, NC) and GraphPad Prism v.9.3 (GraphPad Software, San Diego, CA), with P < .05 considered statistically significant.
Results
MIS-C and Kawasaki Disease During the COVID-19 Pandemic
Demographic and Clinical Characteristics
We identified 4868 hospitalizations for MIS-C and 2387 hospitalizations for KD during the COVID-19 period (Table 1). We also identified 914 hospitalizations with diagnoses of both MIS-C and KD during the COVID-19 period (Supplemental Table 6). In comparison with KD patients, there were increased proportions of MIS-C patients who were older (79.1% ≥5 years vs 22.3% ≥5 years), non-Hispanic Black (28.2% vs 20.7%), and had government insurance (52.9% vs 49.3%). There were no differences in the COI between the 2 groups. We observed an increased proportion of children with medical complexity in the MIS-C group and higher illness severity in the MIS-C group (H-RISK 5.5 vs 2.1).
TABLE 1.
Patient Demographics and Clinical Characteristics of MIS-C and KD Patients During the COVID-19 Pandemic
| Total | MIS-C | KD | P | |
|---|---|---|---|---|
| # Encounters | 7255 | 4868 (67.1) | 2387 (32.9) | |
| Age | <.001 | |||
| <1 y | 586 (8.1) | 107 (2.2) | 479 (20.1) | |
| 1–4 y | 2287 (31.5) | 911 (18.7) | 1376 (57.6) | |
| 5–11 y | 2815 (38.8) | 2336 (48) | 479 (20.1) | |
| 12–15 y | 1072 (14.8) | 1033 (21.2) | 39 (1.6) | |
| 16–20 y | 495 (6.8) | 481 (9.9) | 14 (0.6) | |
| Sex | .275 | |||
| Female | 2895 (39.9) | 1921 (39.5) | 974 (40.8) | |
| Race/ethnicity | <.001 | |||
| Non-Hispanic White | 2749 (37.9) | 1824 (37.5) | 925 (38.8) | |
| Non-Hispanic Black | 1865 (25.7) | 1372 (28.2) | 493 (20.7) | |
| Hispanic | 1814 (25) | 1226 (25.2) | 588 (24.6) | |
| Asian | 341 (4.7) | 129 (2.6) | 212 (8.9) | |
| Other | 486 (6.7) | 317 (6.5) | 169 (7.1) | |
| Payor | .005 | |||
| Government | 3753 (51.7) | 2577 (52.9) | 1176 (49.3) | |
| Private | 3037 (41.9) | 1973 (40.5) | 1064 (44.6) | |
| Other | 465 (6.4) | 318 (6.5) | 147 (6.2) | |
| COI | .834 | |||
| Very low | 682 (22.4) | 451 (22.1) | 231 (23) | |
| Low | 713 (23.4) | 480 (23.5) | 233 (23.2) | |
| Moderate | 617 (20.3) | 423 (20.7) | 194 (19.3) | |
| High | 679 (22.3) | 457 (22.4) | 222 (22.1) | |
| Very high | 354 (11.6) | 231 (11.3) | 123 (12.3) | |
| No. of CCC | <.001 | |||
| 0 | 4042 (55.7) | 2386 (49) | 1656 (69.4) | |
| 1–2 | 2958 (40.8) | 2255 (46.3) | 703 (29.5) | |
| ≥3 | 255 (3.5) | 227 (4.7) | 28 (1.2) | |
| Complex chronic condition category | ||||
| Neurologic | 167 (2.3) | 145 (3) | 22 (0.9) | <.001 |
| Cardiovascular | 1440 (19.8) | 1274 (26.2) | 166 (7) | <.001 |
| Respiratory | 52 (0.7) | 46 (0.9) | 6 (0.3) | .001 |
| Renal/urologic | 139 (1.9) | 120 (2.5) | 19 (0.8) | <.001 |
| Gastrointestinal | 282 (3.9) | 252 (5.2) | 30 (1.3) | <.001 |
| Hematologic/immunologic | 494 (6.8) | 384 (7.9) | 110 (4.6) | <.001 |
| Metabolic | 1807 (24.9) | 1340 (27.5) | 467 (19.6) | <.001 |
| Congenital/genetic | 98 (1.4) | 80 (1.6) | 18 (0.8) | .002 |
| Neonatal | 16 (0.2) | 11 (0.2) | 5 (0.2) | .888 |
| Technology | 211 (2.9) | 183 (3.8) | 28 (1.2) | <.001 |
| H-RISK | 4.4 (5.7) | 5.5 (6.4) | 2.1 (2.6) | <.001 |
| Echocardiogram | 6937 (95.6) | 4620 (94.9) | 2317 (97.1) | <.001 |
| Shock | 2005 (27.6) | 1883 (38.7) | 122 (5.1) | <.001 |
| ECMO | 71 (1) | 69 (1.4) | 2 (0.1) | <.001 |
| Epinephrine | 1598 (22) | 1526 (31.3) | 72 (3) | <.001 |
| Norepinephrine | 832 (11.5) | 802 (16.5) | 30 (1.3) | <.001 |
| Phenylephrine | 118 (1.6) | 91 (1.9) | 27 (1.1) | .020 |
| Dopamine | 103 (1.4) | 85 (1.7) | 18 (0.8) | .001 |
| Dobutamine | 21 (0.3) | 21 (0.4) | 0 (0) | .001 |
| Vasopressin | 140 (1.9) | 134 (2.8) | 6 (0.3) | <.001 |
| Milrinone | 598 (8.2) | 586 (12) | 12 (0.5) | <.001 |
| Death | 43 (0.6) | 43 (0.9) | 0 (0) | <.001 |
Data are presented as n (%) with the exception of H-RISK, which is presented as mean (standard deviation).
Outcome Measures
We observed substantial differences in the proportion of hospitalizations with shock between the MIS-C and KD groups. During the COVID-19 period, 38.7% of patients with a diagnosis of MIS-C met our definition of shock compared with 5.1% of patients with a diagnosis of KD. Epinephrine (31.3% of MIS-C patients, 3% of KD patients) and norepinephrine (16.5% of MIS-C patients, 1.3% of KD patients) were the most used inotropic medications in both groups. There were 69 (1.4%) MIS-C patients who received ECMO compared with 2 (0.2%) KD patients. All but 1 patient who received ECMO also received a vasoactive/inotropic medication. There were 43 (0.9%) deaths in the MIS-C group compared with 0 deaths in the KD group during the COVID-19 pandemic.
Patients With a Diagnosis of MIS-C and KD
Patients with both diagnoses had demographic and clinical characteristics that generally resembled those of the MIS-C group (Supplemental Table 6). We observed higher illness severity in the dual diagnosis group compared with the KD group (H-RISK 4.3 vs 2.1) and a proportion of hospitalizations with shock that were higher than the KD group but lower than the MIS-C group (21.9% dual diagnosis vs 38.7% MIS-C vs 5.1% KD). The volume of patients with both diagnoses remained relatively stable over time (Supplemental Fig 2).
Kawasaki Disease Before and During COVID-19 Pandemic
We identified 6355 hospitalizations for KD from January 2016 to March 2020 to compare with the 2387 hospitalizations for KD during the COVID-19 pandemic (Table 2). We observed similar demographic characteristics between the historical and COVID-19-era KD groups. The groups had similar levels of medical complexity and illness severity (H-RISK 1.9 vs 2.1). We also observed similar proportions of hospitalizations with shock in the historic and COVID-19 KD groups (6.9% vs 5.1%).
TABLE 2.
Patient Demographics and Clinical Characteristics of KD Patients Before and During COVID-19 Pandemic
| Total | KD Before COVID-19 | KD During COVID-19 | P | |
|---|---|---|---|---|
| # Encounters | 8742 | 6355 (72.7) | 2387 (27.3) | |
| Age | <.001 | |||
| <1 y | 1514 (17.3) | 1035 (16.3) | 479 (20.1) | |
| 1–4 y | 5116 (58.5) | 3740 (58.9) | 1376 (57.6) | |
| 5–11 y | 1938 (22.2) | 1459 (23) | 479 (20.1) | |
| 12–15 y | 137 (1.6) | 98 (1.5) | 39 (1.6) | |
| 16–20 y | 37 (0.4) | 23 (0.4) | 14 (0.6) | |
| Sex | .701 | |||
| Female | 3538 (40.5) | 2564 (40.4) | 974 (40.8) | |
| Race/ethnicity | .002 | |||
| Non-Hispanic White | 3325 (38) | 2400 (37.8) | 925 (38.8) | |
| Non-Hispanic Black | 1732 (19.8) | 1239 (19.5) | 493 (20.7) | |
| Hispanic | 2046 (23.4) | 1458 (22.9) | 588 (24.6) | |
| Asian | 915 (10.5) | 703 (11.1) | 212 (8.9) | |
| Other | 724 (8.3) | 555 (8.7) | 169 (7.1) | |
| Payor | .006 | |||
| Government | 4182 (47.8) | 3006 (47.3) | 1176 (49.3) | |
| Private | 4101 (46.9) | 3037 (47.8) | 1064 (44.6) | |
| Other | 459 (5.3) | 312 (4.9) | 147 (6.2) | |
| COI | .182 | |||
| Very low | 831 (22.4) | 600 (22.2) | 231 (23) | |
| Low | 831 (22.4) | 598 (22.1) | 233 (23.2) | |
| Moderate | 821 (22.1) | 627 (23.2) | 194 (19.3) | |
| High | 792 (21.3) | 570 (21.1) | 222 (22.1) | |
| Very high | 435 (11.7) | 312 (11.5) | 123 (12.3) | |
| No. of CCC | <.001 | |||
| 0 | 6362 (72.8) | 4706 (74.1) | 1656 (69.4) | |
| 1–2 | 2288 (26.2) | 1585 (24.9) | 703 (29.5) | |
| ≥3 | 92 (1.1) | 64 (1) | 28 (1.2) | |
| Complex chronic condition category | ||||
| Neurologic | 61 (0.7) | 39 (0.6) | 22 (0.9) | .123 |
| Cardiovascular | 637 (7.3) | 471 (7.4) | 166 (7) | .464 |
| Respiratory | 27 (0.3) | 21 (0.3) | 6 (0.3) | .553 |
| Renal/urologic | 56 (0.6) | 37 (0.6) | 19 (0.8) | .264 |
| Gastrointestinal | 97 (1.1) | 67 (1.1) | 30 (1.3) | .421 |
| Hematologic/immunologic | 320 (3.7) | 210 (3.3) | 110 (4.6) | .004 |
| Metabolic | 1394 (15.9) | 927 (14.6) | 467 (19.6) | <.001 |
| Congenital/genetic | 58 (0.7) | 40 (0.6) | 18 (0.8) | .522 |
| Neonatal | 13 (0.1) | 8 (0.1) | 5 (0.2) | .366 |
| Technology | 85 (1) | 57 (0.9) | 28 (1.2) | .241 |
| H-RISK | 2 (2.7) | 1.9 (2.7) | 2.1 (2.6) | <.001 |
| Echocardiogram | 8407 (96.2) | 6090 (95.8) | 2317 (97.1) | .007 |
| Shock | 559 (6.4) | 437 (6.9) | 122 (5.1) | .003 |
| ECMO | 8 (0.1) | 6 (0.1) | 2 (0.1) | .884 |
| Epinephrine | 387 (4.4) | 315 (5) | 72 (3) | <.001 |
| Norepinephrine | 112 (1.3) | 82 (1.3) | 30 (1.3) | .901 |
| Phenylephrine | 91 (1) | 64 (1) | 27 (1.1) | .611 |
| Dopamine | 74 (0.8) | 56 (0.9) | 18 (0.8) | .563 |
| Dobutamine | 5 (0.1) | 5 (0.1) | 0 (0) | .170 |
| Vasopressin | 21 (0.2) | 15 (0.2) | 6 (0.3) | .896 |
| Milrinone | 68 (0.8) | 56 (0.9) | 12 (0.5) | .073 |
| Deaths | 3 (0) | 3 (0) | 0 (0) | .288 |
Data are presented as n (%) with the exception of H-RISK, which is presented as mean (SD).
Volume of Hospitalizations and Proportion of Hospitalizations With Shock Over Time
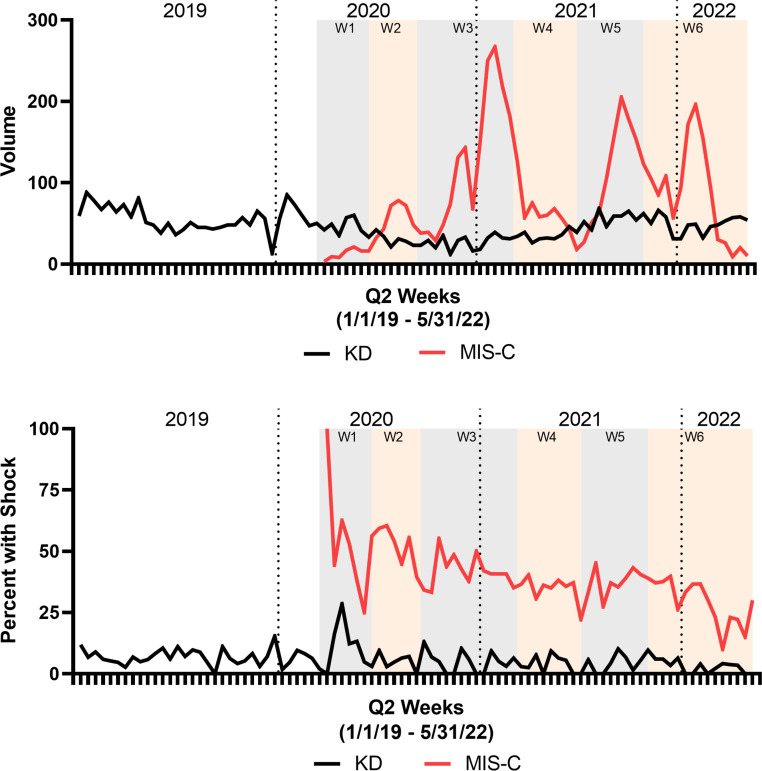
The volume of hospitalizations and proportion of hospitalizations with shock for KD remained relatively constant over time from before the COVID-19 pandemic until the end of our study period, aside from a decrease in volume and increased proportion with shock in late 2020 (Fig 1). The volume of hospitalizations for MIS-C varied over time. The proportion with shock for MIS-C revealed a gradual decline over time from 46.3% in the first wave to 32.6% in the most recent included wave.
FIGURE 1.
Volume of hospitalization and proportion of hospitalizations with shock for MIS-C and KD. (A) Volume of hospitalization for MIS-C and KD. (B) Proportion of hospitalizations with shock for MIS-C and KD. Data shown every 2 weeks from January 2019 to May 2022 with COVID-19 waves indicated with shading.
Throughout all waves of the COVID-19 pandemic, the proportion of MIS-C hospitalizations with shock was statistically different across age groups, with older patients experiencing higher rates of shock (Table 3). We also observed a decreasing proportion of MIS-C hospitalizations with shock over time for each age group. In models with time as a fixed effect and adjusting for hospital clustering and patient age, we observed a significant decrease in the odds of shock over time in MIS-C patients (adjusted odds ratio 0.98, 95% confidence interval [CI] 0.98–0.99, P < .001), representing a 2% decreased odds of shock every 2 weeks (Table 4). We did not observe a decrease in the odds of shock over time in KD patients in our adjusted analysis (adjusted odds ratio 1.00, 95% CI 0.99–1.00, P = .062).
TABLE 3.
Proportion of Hospitalizations with Shock in MIS-C Patients Over Time and Across Age Groups
| Age (y) | Overall | Wave 1 | Wave 2 | Wave 3 | Wave 4 | Wave 5 | Wave 6 | P (Trend) |
|---|---|---|---|---|---|---|---|---|
| Overall | 38.7 (37.3–40) | 46.3 (35.3–57.2) | 51.5 (46.4–56.7) | 40.9 (38.5–43.3) | 36.1 (32–40.1) | 38.7 (35.7–41.7) | 32.6 (30–35.3) | <.001 |
| 0–4 | 23.4 (20.8–26) | 33.3 (2.5–64.1) | 28.1 (17.1–39.1) | 27.9 (23.2–32.7) | 24.8 (16.8–32.7) | 28.3 (21.6–35) | 14.1 (10.3–17.9) | <.001 |
| 5–11 | 38.6 (36.6–40.5) | 32 (13.7–50.3) | 54.2 (46.4–62.1) | 39.7 (36.2–43.2) | 35.7 (29.6–41.8) | 38.1 (34–42.1) | 35.1 (31.4–38.9) | <.001 |
| 12–15 | 51 (48–54.1) | 56.5 (36.3–76.8) | 64.3 (54–74.5) | 51.8 (46.7–56.9) | 42.9 (34.4–51.3) | 48.8 (42.1–55.6) | 51.2 (44.5–57.8) | .0053 |
| 16–20 | 45.1 (40.7–49.6) | 56.5 (36.3–76.8) | 51.9 (38.5–65.2) | 48.6 (41.2–56) | 42.6 (30.9–54.4) | 38.9 (28.8–49) | 38 (26.7–49.3) | .0323 |
| P | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
Wave 1: March 01, 2020–June 20, 2020; Wave 2: June 21, 2020–September 19, 2020; Wave 3: September 20, 2020–March 06, 2021; Wave 4: March 07, 2021–July 03, 2021; Wave 5: July 04, 2021–November 06, 2021; Wave 6: November 07, 2021–May 31, 2022.
TABLE 4.
Unadjusted and Adjusted Proportion of Hospitalizations With Shock Over Time
| Fixed Effect | Unadjusted* OR (95% CI) | P | Adjusted** OR (95% CI) | P | |
|---|---|---|---|---|---|
| MIS-C | Time | 0.97 (0.97–0.98) | <.001 | 0.98 (0.98–0.99) | <.001 |
| KD | Time | 1.00 (0.99–1.00) | .043 | 1.00 (0.99–1.00) | .062 |
OR, odds ratio.
Accounting for hospital clustering only.
Adjusted for age and hospital clustering.
Discussion
In this large multicenter study including patients, over 2 years we observed differences between the clinical course of patients with MIS-C and Kawasaki disease during the COVID-19 pandemic. We observed a higher proportion of hospitalizations with shock in children hospitalized with MIS-C than in those hospitalized with KD, a finding that narrowed but persisted over time during the COVID-19 pandemic. We observed a significant decrease in the odds of shock in MIS-C hospitalizations after controlling for age and hospital, with the proportion of hospitalizations with shock in the most recent wave reduced by 30% as compared with hospitalizations during the first wave. This decrease in illness severity parallels the observed decreased incidence of MIS-C in later COVID-19 waves.10,12–14 The authors of a recent publication using data from the International Kawasaki Disease Registry also report decreasing illness severity with later COVID waves.15 Our findings provide stronger evidence for this association with one of the largest MIS-C cohorts to date and our models that control for important covariates. Potential explanations for the observed decrease in illness severity include improved identification of MIS-C patients, improved treatment, the impact of vaccination and infection-induced immunity, and possible differences in the likelihood of developing MIS-C and severe illness from MIS-C with different SARS-CoV-2 variants. Although uptake has been low, vaccination against SARS-CoV-2 may have contributed to the changing epidemiology of MIS-C. Vaccination decreases the risk of MIS-C by 91%, and most critically ill children with MIS-C have been unvaccinated.29 Vaccines were available to children aged ≥16 in December 2020, 12 to 15 in May 2021, and 5 to 11 in November 2021.
This large cohort of patients with MIS-C also highlights age as a possible risk factor for increased illness severity in MIS-C. In all the COVID-19 waves included in the study, a higher proportion of older children (aged ≥12) with MIS-C experienced shock. The highest proportion of hospitalizations with shock was in the 12 to 15 years group (51%), and the 16 to 20 years group was high but somewhat lower (45.1%). The 16 to 20 years group was also the smallest in our study. Although we observed a decrease in the proportion of hospitalizations with shock in all age groups over time, ∼50% of MIS-C hospitalizations in children 12 to 15 years of age had shock in the most recent waves. The increasing proportion of shock with age may have mechanisms similar to the increased risk of severe COVID-19 with increasing age, with age being the largest risk factor for severe disease.30
Consistent with other observational studies of MIS-C,4,8,9,31 children with MIS-C were older than those with KD. We observed a lower proportion of Asian children and a higher proportion of non-Hispanic Black children in the MIS-C group than in the KD group, differences that were not explained by the COI and did not differ between the groups. Racial and ethnic disparities in the incidence, severity of illness, and outcomes in children with MIS-C have been noted in other studies.8,24,31 These observed differences may reflect disparities in timely access to care and structural racism, potentially contributing to higher SARS-CoV2 infection rates and warrant further investigation to close this equity gap.
The volume of hospitalizations for KD decreased at the beginning of the pandemic, consistent with previous observational studies from 202016–20 but has since rebounded to pre-pandemic levels. The decreased volume associated with maximal social distancing early in the pandemic and rebound to historic volumes after relaxing these measures lend support to a potential infectious trigger of KD. The proportion of KD hospitalizations with shock was stable across the COVID-19 pandemic after controlling for age and hospital. There was an increase in the proportion of KD hospitalizations with shock in the first few months of the pandemic, which has been noted in another observational study from 2020.19 Given the novel nature of MIS-C as a diagnosis and the volume of patients with a diagnosis of both KD and MIS-C in our cohort, this increased proportion with shock may reflect misclassification of patients presenting early in the pandemic when a diagnosis of MIS-C was less likely to be made. The higher proportion of hospitalizations with shock in both groups in the first 1 to 2 months of the pandemic may also partially reflect delayed presentation to care and, therefore, increased illness severity at presentation.
Our findings should be interpreted within the context of certain limitations. The use of an administrative dataset does not allow us to incorporate clinical data, such as vital signs, laboratory results, and vaccination status; thus, there is some misclassification risk in severity of illness. In addition, there is a risk of classification bias given the similarity between the diagnoses. This potential for bias was likely highest in the first few months of the pandemic before an ICD-10 code was available for MIS-C and when MIS-C was a novel diagnosis that clinicians felt uncertain about. We attempted to mitigate this risk by using an algorithm with high sensitivity to identify MIS-C hospitalizations in 2020 and by excluding hospitalizations in which both diagnoses were present. Our data are limited to hospitalizations from 39 children’s hospitals in the United States, which may limit generalizability, although nearly all patients with MIS-C and most patients with KD are cared for at larger pediatric centers. We did not include patients with a diagnosis of both MIS-C and KD in our primary analyses, which represented ∼10% of total patients and may have affected our findings. However, given the stable volume of patients with both diagnoses over time, this likely did not impact our temporal analyses of the individual diagnoses.
The difference in mortality between MIS-C and KD (0.9% vs 0%) is notable in a pediatric population in which mortality is rare. Further investigation is needed to determine the specific causes of MIS-C mortality and modifiable risk factors that may contribute. Future research should examine whether vaccination-induced or infection-induced COVID-19 immunity is associated with a decreased risk of shock or death in patients with MIS-C. This may provide further evidence of the benefits of COVID-19 vaccination in the pediatric population. Lastly, ongoing surveillance of the changing epidemiology of MIS-C will help pediatricians understand what groups remain at risk for developing this severe condition. Although there was a notable decrease in MIS-C in the year after our study period, there were still >500 hospitalizations for MIS-C in the PHIS database from June 2022 to May 2023. Pediatricians will likely continue to encounter MIS-C over the coming years.
Conclusions
This large cohort of patients across >2 years of the COVID-19 pandemic provides further evidence that MIS-C is a distinct, if phenotypically overlapping, disease from KD. We demonstrate that MIS-C was a source of lower morbidity as the pandemic progressed, although it is not clear whether the mechanism is related to public health advances (eg, vaccination), treatment advances, changing virus variants, or increasing herd immunity in our study population. Ongoing and future prospective studies with patient-level data and biomarkers may lead to a better understanding of the mechanism behind these observations, which would have public health implications if new strains of SARS-CoV-2 lead to increases in MIS-C incidence and/or illness severity.
Supplementary Material
Glossary
- CCC
complex chronic conditions
- CI
confidence interval
- COI
Child Opportunity Index
- COVID-19
coronavirus disease 2019
- ECMO
extracorporeal membrane oxygenation
- H-RISK
Hospitalization Resource Intensity Scores for Kids
- ICD-10
International Classification of Diseases, Tenth Edition
- KD
Kawasaki disease
- MIS-C
multisystem inflammatory syndrome in children
- PHIS
Pediatric Health Information System
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Footnotes
Drs Molloy, Auger, and Brady conceptualized and designed the study, drafted the initial manuscript, and assisted with data interpretation; Dr Hall performed the analyses and assisted with data interpretation; Drs Shah, Schondelmeyer, Parikh, Kazmier, Katragadda, Jacob, Jerardi, Ivancie, Hartley, Bryan, Bhumbra, and Arnold conceptualized and designed the study and assisted with data interpretation; and all authors critically reviewed and revised the manuscript, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
FUNDING: Funded by the National Institutes of Health (NIH). Drs Auger, Hall, Hartley, and Brady were supported by the Agency for Healthcare Research and Quality (HS028102-01) and the NIH (HD105619-02). Dr Schondelmeyer’s time in contributing to the manuscript was in part supported by AHRQ (K08 HS026763). The contents are solely the responsibility of the authors and do not necessarily represent official views of AHRQ and NIH.
CONFLICT OF INTEREST DISCLOSURES: The authors have indicated they have no potential conflicts of interest relevant to this article to disclose.
References
- 1. Melgar M, Lee EH, Miller AD, et al. Council of State and Territorial Epidemiologists/CDC surveillance case definition for multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection - United States. MMWR Recomm Rep. 2022;71(4 No. RR-4):1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whittaker E, Bamford A, Kenny J, et al. ; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abrams JY, Godfred-Cato SE, Oster ME, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr. 2020;226:45–54.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20(11):e276–e288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 3. Arthritis Rheumatol. 2022;74(4):e1–e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feldstein LR, Tenforde MW, Friedman KG, et al. ; Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York state. New Engl J Med. 2020;383(4):347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belay ED, Abrams J, Oster ME, et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr. 2021;175(8):837–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Merckx J, Cooke S, El Tal T, et al. ; Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC). Predictors of severe illness in children with multisystem inflammatory syndrome after SARS-CoV-2 infection: a multicentre cohort study. CMAJ. 2022;194(14):E513–E523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin B, DeWitt PE, Russell S, et al. Characteristics, outcomes, and severity risk factors associated with SARS-CoV-2 infection among children in the US National COVID Cohort Collaborative. JAMA Netw Open. 2022;5(2):e2143151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buonsenso D, Perramon A, Català M, et al. ; COPP-consortium; COPEDI-CAT Research Group. Multisystem inflammatory syndrome in children in western countries? Decreasing incidence as the pandemic progresses?: an observational multicenter international cross-sectional study. Pediatr Infect Dis J. 2022;41(12):989–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lopez L, Burgner D, Glover C, et al. ; Australian Vasculitis Working Group and Paediatric Active Enhanced Disease Surveillance (PAEDS) network. Lower risk of multi-system inflammatory syndrome in children (MIS-C) with the omicron variant. Lancet Reg Health West Pac. 2022;27:100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCrindle BW, Harahsheh AS, Handoko R, et al. ; International Kawasaki Disease Registry. SARS-CoV-2 variants and multisystem inflammatory syndrome in children. N Engl J Med. 2023;388(17):1624–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ae R, Makino N, Kuwabara M, et al. Incidence of Kawasaki disease before and after the COVID-19 pandemic in Japan: results of the 26th Nationwide Survey, 2019 to 2020. JAMA Pediatr. 2022;176(12):1217–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iio K, Matsubara K, Miyakoshi C, et al. Incidence of Kawasaki disease before and during the COVID-19 pandemic: a retrospective cohort study in Japan. BMJ Paediatr Open. 2021;5(1):e001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katsumata N, Harama D, Toda T, et al. Prevention measures for COVID-19 and changes in Kawasaki disease incidence. J Epidemiol. 2021;31(11):573–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Phamduy TT, Smith S, Herbst KW, et al. Kawasaki disease hospitalizations in the United States 2016-2020: a comparison of before and during the coronavirus disease 2019 era. Pediatr Infect Dis J. 2021;40(11):e407–e412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burney JA, Roberts SC, DeHaan LL, et al. ; KIDCARE Study Investigators. Epidemiological and clinical features of Kawasaki disease during the COVID-19 pandemic in the United States. JAMA Netw Open. 2022;5(6):e2217436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Auger KA, Hall M, Arnold SD, et al. Identifying and validating pediatric hospitalizations for MIS-C through administrative data. Pediatrics. 2023;151(5):e2022059872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krager MK, Puls HT, Bettenhausen JL, et al. The Child Opportunity Index 2.0 and hospitalizations for ambulatory care sensitive conditions. Pediatrics. 2021;148(2):e2020032755. [DOI] [PubMed] [Google Scholar]
- 23. Gorelik M, Chung SA, Ardalan K, et al. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of Kawasaki disease. Arthritis Care Res (Hoboken). 2022;74(4):538–548 [DOI] [PubMed] [Google Scholar]
- 24. Encinosa W, Moon K, Figueroa J, Elias Y. Complications, adverse drug events, high costs, and disparities in multisystem inflammatory syndrome in children vs COVID-19. JAMA Netw Open. 2023;6(1):e2244975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tyris J, Boggs K, Bost J, et al. Examining the association between MIS-C and the Child Opportunity Index at a single center. Hosp Pediatr. 2022;12(10):e342–e348 [DOI] [PubMed] [Google Scholar]
- 26. Feudtner C, Feinstein JA, Zhong W, et al. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richardson T, Rodean J, Harris M, et al. Development of hospitalization resource intensity scores for kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9):602–608 [DOI] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention. COVID data tracker. Available at: https://covid.cdc.gov/covid-data-tracker/#new-hospital-admissions. Accessed March 13, 2023
- 29. Zambrano LD, Newhams MM, Olson SM, et al. ; Overcoming COVID-19 Investigators. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12–18 years - United States, July-December 2021. MMWR Morb Mortal Wkly Rep. 2022;71(2):52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zimmermann P, Curtis N. Why does the severity of COVID-19 differ with age?: understanding the mechanisms underlying the age gradient in outcome following SARS-CoV-2 infection. Pediatr Infect Dis J. 2022;41(2):e36–e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Payne AB, Gilani Z, Godfred-Cato S, et al. ; MIS-C Incidence Authorship Group. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4(6):e2116420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.