Abstract
Objective
The aim of this study was to investigate the clinical features, and treatment outcome of women with preeclampsia and eclampsia at Gondar University Comprehensive Specialized Hospital in Amhara, Northern Ethiopia, in 2021.
Methods
An institutional-based retrospective chart review was conducted at Gondar University Specialized Hospital from March to June 2021. The study participants were chosen using a simple, systematic random sampling method. A pretested check list was used to collect data from medical records. The collected data was coded, entered into Epi-data version 4.6, and exported to SPSS version 26 for descriptive and inferential analysis. A Fisher’s exact test was used to determine statistically significant factors at a p-value of < 0.05.
Results
Of the 311 study participants, more than half (53 %) of mothers have illiterate, nearly half (49.8 %) had preeclampsia with severe features. Eclampsia accounted for 18.6 % of females in the study setting. For various reasons, more than half of the mothers required immediate intervention to terminate the pregnancy via cesarean section. Unfavorable maternal outcomes were present in more than 25 % of cases; the observed unfavorable maternal outcomes were aspiration pneumonia (10.6 %), hemolytic elevated liver function test and low platelet count syndrome (8.7 %), and maternal death (0.6 %). The severity of the disease, mode of delivery, aspartate transaminase, gravidity, gestational age, and antenatal care were all statistically significant predictors of pregnancy outcome.
Conclusion
The prevalence of unfavorable maternal and perinatal outcomes of preeclampsia and eclampsia is considerable in the study area. To prevent these perinatal and postnatal effects, maternal outcomes of pregnancy, antenatal care services, emergency obstetrics, and new born care should be expanded and strengthened.
Keywords: Preeclampsia, Eclampsia, Maternal outcome, Ethiopia
Introduction
A pregnant woman is considered hypertensive if her blood pressure is greater than or equal to 140/90 mmHg on two consecutive measurements, each four hours apart [1]. Hypertensive disorders of pregnancy (HDP) account for nearly 18 % of all maternal deaths globally, with an expected 62,000–77,000 deaths per year [2].
Preeclampsia is a multisystem disorder characterized by hypertension and new-onset proteinuria > 300 mg/24 h) which develops after the 20th week of pregnancy in previously normotensive women [1], [2]. Preeclampsia may be mild or severe based on its presentation. A systolic blood pressure (SBP) of 160 mmHg or a diastolic blood pressure (DBP) of 110 mmHg with proteinuria of 5 g/24 hr or more is considered severe pre-eclampsia [1], [3]. While mild pre-eclampsia is described by an elevated blood pressure less than 160 mmHg (systolic) or 110 mmHg (diastolic) with proteinuria greater than 300 mg, but less than 5 g per day [1], [4].
Eclampsia is one of the serious obstetric emergencies, and it is defined as the new onset of grand mal seizure activity and/or coma unexplained by other mental causes or disorders during pregnancy or postpartum in a woman with signs or symptoms of preeclampsia and/or gestational hypertension [4], [5]. About 10 % of women in Africa develop any form of HDP at some point during pregnancy or puerperium [4], [6]. Among the various clinical types of HDP, preeclampsia accounts for 5.3 % regardless of the degree of severity and was found to be the most common form of HDP in Africa [4], [7].
Pre-eclampsia and eclampsia are one of the leading causes of severe morbidity, long-term disability, and death among both mothers and their babies worldwide [5], [8]. In Africa and Asia, nearly 10 % of all maternal deaths are associated with hypertensive disorders of pregnancy, while one-quarter of maternal deaths in Latin America have been associated with preeclampsia/eclampsia complications [9]. In 2017, approximately 295,000 women died during and after pregnancy and childbirth, according to a World Health Organization report on maternal mortality trends [8], [9]. Likewise, as the 2019 WHO maternal mortality fact sheet reported, around 810 women die every day from pregnancy-related complications. By far most of these deaths (94 %) occurred in low-income countries, and most could have been prevented [10].
Globally, the incidence of preeclampsia and eclampsia varies from country to country, even within the same country, and it is estimated that it affects between 2 % and 10 % of pregnancies every year [11]. According to the WHO assessments, the incidence of preeclampsia in developing countries (2.8 % of live births) is seven times higher than in developed countries (0.4 % of live births) [11], [12]. Eclampsia also increases the risk of maternal death, both in developed (0.5–1.8 %) and developing countries (15 %) [13].
Studies show that in sub-Saharan Africa (SSA) countries, preeclampsia and eclampsia are among the top five leading causes of morbidity and mortality among women and babies [14]. Preeclampsia and eclampsia were also reported to account for 19 % of maternal and 25 % of perinatal mortality in Ethiopia [6], [2]. Due to this and other issues, SSA countries, including Ethiopia, experience the highest maternal and newborn mortality rates [13], [14].
Identification of the common poor maternal and perinatal outcomes of preeclampsia and their determinants is the first step to improving the service given to preeclampsia mother [14], [15]. Studies that specifically addressed this issue are rare and not well studied in Ethiopia, and to the knowledge of the investigator, no study has been conducted at Gondar. As a result, the purpose of this study is to determine the clinical features, maternal and perinatal outcomes, and outcome determinants of patients with preeclampsia or eclampsia treated at Gondar Hospital.
Implications of the study (key massages)
What is already known about this topic?
The cause, clinical presentation and outcomes of preeclampsia and eclamsia is not well studied in Ethiopia.
Why did this study need to be done?
The previous study had a general limitation in that the data collection process was only quantitative, thus, it could not provide in-depth details on the problems.
What does this study add?
This study could be updated and could generate significant evidence for collaborative practice regarding the risk, clinical presentation, and outcomes of preeclampsia and eclampsia.
What are the implications of these findings for clinical practice and/or further research??
The rationale for this study is to help policymakers and programmers, even health care providers working in clinical areas, have a clear picture of the effect of preeclampsia and eclampsia on adverse maternal and perinatal outcomes to make an evidence-based decision for prevention. It aids in the prioritization of interventions and the management of adverse outcomes, as well as the mobilization of resources for the management of preeclampsia and eclampsia and their associated perinatal and maternal complications in the area and country.
Aims
The study investigated the clinical features and treatment outcome of women with preeclampsia and eclampsia at Gondar University Comprehensive Specialized Hospital in Amhara, Northern Ethiopia, in 2021.
Methods
Design, periods and area
A retrospective cross-sectional study was conducted from March to June 2021 in Gondar city. Gondar is found 750 km northwest of Addis Ababa (the capital city of Ethiopia) [15]. The Gondar comprehensive specialized hospital is a teaching and referral hospital that has more than a 500-bed capacity and is used as the referral center for more than 7 million people in its catchment area [14], [15]. It provides both specialty and subspecialty services, including obstetrics and gynecology units in its inpatient and outpatient clinics. According to the hospital's data registry, more than 10,000 mothers receive delivery service annually.
Research questions
What are the main clinical features of maternal preeclampsia and eclampsia?
What are the possible outcomes of those hypertensive disorders, preeclampsia and eclampsia?
What are the potential determinants of favorable and unfavorable preeclampsia and eclampsia outcomes in the study area?
Sample
The actual sample size for the study was determined using a single population proportion formula:{n = [(zα/2)2p (1-p)]/d2}, n = sample size, zα/2 = 95% confidence level, and P = the proportion (46.5 %) [15]. Since the number of preeclampsia and eclampsia patients is less than 10,000, correction formula was applied: nf = {(n * N)/N + n}. After the addition of 10 % of the sample for missing and incomplete charts, the final corrected sample size is 311. Systematic random sampling was used to select the respected patient charts at four intervals. The first chart was selected using simple random sampling.
Criteria for Inclusion and Exclusion
Inclusion criteria
All women who were received treatment for preeclampsia and eclampsia in Gondar Specialized Hospital from March to June 2021 were included.
Exclusion criteria
The study excluded women with preeclampsia and eclampsia who did not have a complete record, lost cards, or were transferred to other hospitals.
Data collection tools
Data collection was done by using a pretested checklist format that was developed from reviewing different kinds of literatures [9], [11], [13]. The checklist contains socio-demographic, obstetric, clinical presentation, management profiles, laboratory results, and treatment outcome questions for preeclampsia and eclampsia.
Data quality control
Before the actual data collection date, data collectors (two BSC nurses) and a supervisor (one MSc, nurse) were trained for two days concerning the overall issue of data collection format. A pretest was done on 5 % of the total sample to ensure the agreement of the data abstraction format with the aim of the study. Any error found in the data abstraction format (checklist) was corrected and modified. Every day, during data collection, close supervision was carried out by the supervisor and principal investigator. In this study, data were collected using checklists.
Patient and public involvement
No patients were involved in this study.
Data processing and analysis
After manual checking of the data for completeness and clarity, the data was entered into Epi-data version 4.6 statistical software and exported to the statistical package for social sciences (SPSS) version 26.0 for further analysis. A descriptive component such as central tendency measures (mean and median), standard deviation, and frequency distribution were used to present the demographic, obstetric, and clinical characteristics of the patients. Due to violations of the variables in other tests and model assumptions, Fisher’s exact test was used for the data analysis and testing of the relationship between predictor and outcome variables. The data were presented in the form of tables, graphs, and paragraphs. Finally, using the Fisher exact test, independent variables with a P-value of less than 0.05 were considered statistically significant with respect to the dependent variable.
Results
Socio-demographic characteristics of respondents
A total of 311 randomly selected patients with a response rate of 98.7 % were included in this study. The maternal age of the study population ranged from 17 to 45 years, with a mean of 27.5 ± 6 years. Most cases (77.2 %) were referred from health centers, primary hospitals, and private clinics. Of the total study subjects, 174 (56 %) were residents of Gondar (Table 1).
Table 1.
Socio-demographic characteristics of mothers with preeclampsia or eclampsia, Ethiopia, 2021.
| Variables | Category | Frequency (n = 311) | Percentage (%) | |
|---|---|---|---|---|
| Referral | Yes | Health center Primary hospital Private clinic |
117 103 20 |
37.6 33.2 6.4 |
| No | 71 | 22.8 | ||
| Age | ≤ ≥35 |
41 211 59 |
13.2 67.8 19 |
|
| Residence | Gondar city Out of Gondar city |
174 137 |
56 44 |
|
| Religion | Orthodox Muslim Protestant |
228 56 27 |
73 18 9 |
|
| Educational level | No formal education Primary school Secondary school College and above |
100 10 13 66 |
53 5 7 35 |
|
| Marital status | Married Unmarried |
226 14 |
94 6 |
|
Clinical presentations of mothers with preeclampsia and eclampsia
In this study, regarding their BP measurement, 180 mmHg (57.9 %) and 150 mmHg (48.2 %) of patients had SBP and DBP, respectively, in the severity feature at admission, whereas after a 4-hour measurement, SBP was 116 (37.3 %) and DBP was 120 mmHg (38.6 %). About the laboratory results of the participants, 92 % of them had normal creatinine, with a mean of 0.76 mg/dl. The majority, 54.4 %, had normal platelets, and 17.3 % of mothers had moderate thrombocytopenia (Table 2).
Table 2.
The clinical signs and symptoms, laboratory results, and management profiles for Ethiopia in 2021.
| Variables | Category | Frequency (n = 311) | Percentage (%) |
|---|---|---|---|
| SBP at admission | <140 140–159 ≥160 Mean + SD |
1 130 180 159 ± 13 |
0.3 41.8 57.9 |
| DBP at admission | <90 90–109 ≥110 Mean + SD |
20 141 150 105 ± 12 |
6.4 45.3 48.2 |
| Platelet count | <100,000 ≥100,000 |
55 252 |
17.9 82.1 |
| Cr | ≤1.1 >1.1 |
275 24 |
92 8 |
| AST(SGOT) | <2x elevated ≥2x elevated |
263 42 |
86.2 13.8 |
| ALT(SGPT) | <2x elevated ≥2x elevated |
285 18 |
94.1 5.9 |
Note: Cr creatinine, SD standard deviation, AST Aspartate transaminase, ALT alanine transaminase.
Results of research questions
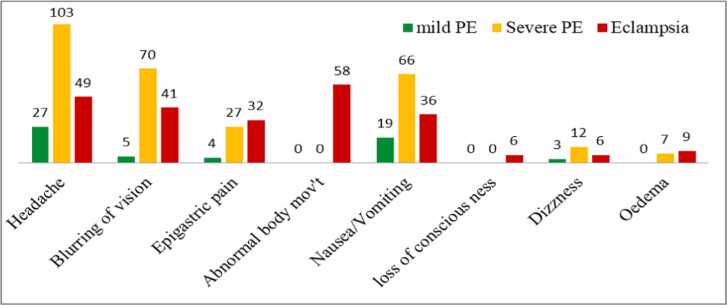
The most common complaint for 179 mothers (57.6 %) in this study was headache, followed by blurring of vision for 116 mothers (37.3 %) with preeclampsia and eclampsia (Fig. 1).
Fig. 1.
Chief complaints of the study subjects at the presentation.
Maternal and perinatal management outcomes for mothers
In this study, among 311 mothers, the majority (49.8 %) had preeclampsia with a severe feature. The remaining cases were eclampsia, while 98 (31.5 %) were preeclampsia without severity features. One fourth (78, or 25.1 %) of mothers develop complications, of which HELLP syndrome accounted for 27 (34.6 %), aspiration pneumonia 33 (42.3 %), acute renal injury 4 (5.2 %), and others 13 (16.9 %). A total of 62 (19.9 %) cases stayed in the hospital for 7 days, with a mean of 6 – 6.5 days. Eclampsia accounts for 63 % of mothers who have a negative pregnancy outcome. Of the total births 171(55 %) were males (Table 3).
Table 3.
Pattern of maternal and perinatal management outcomes according to disease, North Ethiopia 2021.
| Variables | Category | Mild PE | Severe PE | Eclampsia |
|---|---|---|---|---|
| Onset of labor | Spontaneous Induction Immediate C/S |
53(50 %) 9(25.7 %) 36(21.2 %) |
48(45.3 %) 20(57.1 %) 87(51.2 %) |
5(4.7 %) 6(17.1 %) 47(27.6 %) |
| Mode of delivery | SVD Instrument C/S |
54(45.4 %) 4(57.1 %) 40(21.6 %) |
59(49.6 %) 3(42.9 %) 93(50.3 %) |
6(5 %) 52(28.1 %) |
| Maternal Complication | Yes No |
10(12.8 %) 93(39.1 %) |
22(28.2 %) 133(55.9 %) |
46(59 %) 12(5 %) |
| Hospital length of stay | <7days ≥7days |
90(36.1 %) 8(12.9 %) |
116(46.6 %) 39(62.9 %) |
43(17.3 %) 15(24.2 %) |
| Maternal status of discharge | Alive at discharge Died |
98(31.7 %) -------- |
155(50.2 %) -------- |
56(18.1 %) 2(100 %) |
| Outcome of delivery | Alive birth Still birth |
98(35 %) --------- |
132(47.1 %) 23(74.2 %) |
50(17.9 %) 8(25.8 %) |
| Sex of fetus | Male Female |
56(32.7 %) 45(32 %) |
87(51 %) 68(48.2 %) |
28(16.3 %) 28(20 %) |
| APGAR at 1st minute | <7 ≥7 |
27(26 %) 71(38.8 %) |
54(52 %) 85(46.4 %) |
23(22 %) 27(14.8 %) |
| APGAR at 5th minute | <7 ≥7 |
2(12.5 %) 95(35.2 %) |
8(50 %) 132(49 %) |
6(37.5 %) 43(15.9 %) |
| NICU admission | Yes No |
2(8.3 %) 96(37.5 %) |
17(70.8 %) 115(44.9 %) |
5(20.8 %) 45(17.6 %) |
| Neonatal death | Yes No |
--------- 98(34.8 %) |
4(57.1 %) 134(47.5 %) |
3(42.9 %) 50(17.7 %) |
| Maternal outcome | Unfavorable Favorable |
5(6.8 %) 93(39.1 %) |
22(30.1 %) 133(55.9 %) |
46(63 %) 12(5 %) |
| Perinatal outcome | Unfavorable Favorable |
--------- 98(35.9 %) |
27(71.1 %) 128(46.9 %) |
11(28.9 %) 47(17.2 %) |
Note: NICU neonatal intensive care unit, APGAR Appearance, Pulse, Grimace, Activity and Respiration, PE preeclampsia, SVD spontaneous vaginal delivery, CS cesarean section.
Factors associated with the maternal outcome of women with preeclampsia and eclampsia
The association between the dependent and independent variables using Fisher's exact test showed that, epigastric pain and blurred vision were observed more frequently in unfavorable maternal outcomes (p < 0.001, p = 0.005). The majority of patients with two time’s elevated aspartate transaminase (AST) and platelet count 100,000 had unfavorable maternal outcomes (P 0.001). In cases where antihypertensive drugs were used, 31.1 % had an unfavorable outcome (p 0.001), and the higher occurrence of an unfavorable maternal outcome was reported among 16 (66.7 %) of mothers whose creatinine value was greater than 1.1 mg/dl (p 0.001) (Table 4).
Table 4.
Factors associated with the maternal outcome of women with preeclampsia and eclampsia in Ethiopia in 2021.
| Maternal outcomes |
||||
|---|---|---|---|---|
| Predictors | Category | Unfavorable n (%) | Favorable n (%) | P –value |
| Age of mothers | <=20 21–34 >=35 |
17(41.5) 51(24.2) 10(16.9) |
24(58.5) 160(75.8) 49(83.1) |
0.02 |
| Residence | Gondar city Out of Gondar |
32(18.4) 46(33.6) |
142(81.6) 91(66.4) |
0.002 |
| Gravidity | Primigravida Multigravida |
52(31.7) 26(17.7) |
112(68.3) 121(82.3) |
0.007 |
| Epigastric | Yes No |
33(52.4) 45(18.1) |
30(47.6) 203(81.9) |
<0.001 |
| Blurring of vision | Yes No |
40(34.5) 38(19.5) |
76(65.5) 157(80.5) |
0.005 |
| Diagnosis | Mild PE Severe PE Eclampsia |
10(10.2) 22(14.2) 46(79.3) |
88(89.8) 133(85.8) 12(20.7) |
<0.001 |
| Mode of delivery | SVD Instrumental C/S |
11(9.6) 8(53.3) 59(32.6) |
104(90.4) 7(46.7) 122(67.4) |
<0.001 |
| Days of hospitalization | <7 days >=7 days |
44(17.7) 34(54.8) |
205(82.3) 28(45.2) |
<0.001 |
| AST(SGOT) | <2x elevated >2x elevated |
43(16.3) 33(78.6) |
220(83.7) 9(21.4) |
<0.001 |
| PLT | <100 × 103 ≥100 × 103 |
32(58.2) 45(17.9) |
23(41.8) 207(82.1) |
<0.001 |
PLT Platelet, AST Aspartate transaminase, SVD Spontaneous Vaginal Delivery.
Significant at p-value < 0.05.
Discussion
Of the 311 study participants, more than half (53 %) of mothers have illiterate, nearly half (49.8 %) had preeclampsia with severe features. Eclampsia accounted for 18.6 % of females in the study setting. This is higher than some review studies and global reports [2], [9]. In this study, the majority of the women who attend the delivery service were referred (77.2 %), and 27.5 % of them were terminated unfavorably. However, according to a study conducted in Kenya's capital, Nairobi, only 20 % of cases were referred [16]. This could be due to the accessibility of high-quality care services in their surroundings. The source of referral was a significantly associated predictor of maternal outcome in this study (p = 0.002), with being referred from a primary hospital having more unfavorable outcomes than other referral institutions. This may be due to late decision making for the referral. Regarding the presenting signs and symptoms, headache (57.6 %) was the most common chief complaint and statistically significant factor (p = 0.02). which was harmonious, 70 % in Bangladesh, 46.2 % in Iran, 26.8 % in Egypt, and 43 % in Tanzania [17], [18], [19] was the most chief compliant and predictors of maternal outcome. In this study, two women died as a result of eclampsia. The finding was inconsistent with a study in Egypt which report no deaths [18].
This could be explained by the referral of high-risk mothers from the large denominator population and other institutional factors. This discrepancy might be the effect of time-related quality improvement in the EMONC service and due to variation in the study period, sample size, and setting. Complications occurred in 46 (79.34 %) of eclampsia patients, 14.2 % of severe PE patients, and 9.2 % of mild PE patients. The observed complications were pulmonary edema (3.9 %), aspiration pneumonia (42.9 %), AKI (4.2 %), HELLP (35.1 %), PPH (3.9 %), psychotic disorder (2.5 %), uterine rupture (2.5 %), and C/S site infection (10.4 %). A result of this study showed 31 (10 %), 24 (8.6 %), and 7 (2.5 %) of deliveries were complicated by stillbirth, NICU admission, and neonatal death, respectively. This is higher than a study conducted in Egypt [18]. This discrepancy may be attributed to time, sample size, the EmONC service, and other study design factors. According to the analysis output, gestational age, DBP at admission, the severity of the disease, birth weight, and Apgar score at the 5th minute were factors that had a strong and significant association with perinatal outcome (p 0.001). This finding is consistent with the study conducted in Nigeria [20].
Limitation of the study
The limitations of our study include the retrospective and cross-sectional nature of the study design, which did not allow inferences to be drawn regarding the causal relationship among variables.
Conclusion and recommendations
The findings of this study showed that a considerable proportion of women had unfavorable maternal and perinatal outcomes. In addition, the study showed that several factors had a significant association with the occurrence of an unfavourable outcome, and more than 77 % of patients were referred from different health care institutions. However, 27.5 % and 14 % of referred women experienced unfavourable maternal and perinatal outcomes, respectively, and the majority of mothers had less than four ANC follow-ups, which is below the WHO recommendation.
It is recommended to intervene in three areas to improve the maternal and perinatal outcomes of preeclampsia and eclampsia. Coordinate community-based health education for mothers to improve their awareness regarding maternal health service usage and early signs and symptoms of preeclampsia. Improving mothers’ awareness regarding early signs and symptoms of preeclampsia requires strict vital sign monitoring, timely and frequent laboratory updates, and medication administration.
Ethical approval and consent to participants
The ethical review board of the College of Health Sciences at Addis Ababa University approved this study. No. 2139 edu.net for ethical approval). Certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Funding
No funding sources are available yet.
Authors’ contribution
TA developed the proposal, analyzed the data, and wrote and interpreted the results. AA as well as Professor AA drafted the manuscript, revised the proposal, checked the data, and revised the manuscript. OA and DE drafted and revised the manuscript. The authors have read and approved the final manuscript.
Declaration of Competing Interest
The author declared that no competing interest exist.
Acknowledgements
The authors are grateful to the data collectors, emergency coordinators, and all study participants for their contributions to the study's success.
Contributor Information
Ousman Adal, Email: adalousman5@gmail.com.
Destaw Endeshaw, Email: destawendeshaw@gmail.com.
Data availability
The data that support the findings of this study are available upon reasonable request from the corresponding authors.
References
- 1.Moser M. Working group report on high blood pressure in pregnancy. J Clin Hypertens. 2001;3(2):75–88. doi: 10.1111/j.1524-6175.2001.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ag Mersha, Tm Abegaz, Ma Seid. Maternal and perinatal outcomes of hypertensive disorders of pregnancy in ethiopia: systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19(1):1–12. doi: 10.1186/s12884-019-2617-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roccella Ej. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):1–22. [PubMed] [Google Scholar]
- 4.Landon Mb Gh . Gabbe’s obstetrics: normal and problem pregnancies 7th edition, 7; 2016.
- 5.American College of Obstetricians, Task Force on Hypertension in Pregnancy. Hypertension In Pregnancy. Report of The American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. in: obstetrics and gynecology; 2013. [DOI] [PubMed]
- 6.Mohammedseid Si, Megersa Tn, Kumbi S., Biset M. Maternal outcomes of pre-eclampsia In an Ethiopian Gynecologic Hospital. Ann Med Health Sci Res. 2017;7:16–21. [Google Scholar]
- 7.Federal Ministry of Health(Fmoh) Management protocol on selected obstetrics topics. Academia. 2010;5 1–228 P. [Google Scholar]
- 8.Jj Noubiap, Jj Bigna, Uf Nyaga, Am Jingi, Ad Kaze, Jr Nansseu, et al. The burden of hypertensive disorders of pregnancy In Africa: a systematic review and meta-analysis. J Clin Hypertens. 2019;21(4):479–488. doi: 10.1111/jch.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abalos E., Cuesta C., Carroli G., Qureshi Z., Widmer M., Vogel Jp, et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal Outcomes: a secondary analysis of the world health organization multicountry survey on maternal and newborn health. Bjog. 2014;121(Suppl):14–24. doi: 10.1111/1471-0528.12629. [DOI] [PubMed] [Google Scholar]
- 10.Who. Maternal mortality evidance brief. matern mortal; 2019;
- 11.Rana S., Lemoine E., Granger J., Karumanchi Sa. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–1112. doi: 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- 12.Mustafa R., Ahmed S., Gupta A., Venuto Rc.A. Comprehensive review of hypertension in pregnancy. J Pregnancy. 2012:2012. doi: 10.1155/2012/105918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaym A., Bailey P., Pearson L., Admasu K., Gebrehiwot Y. Disease burden due to pre-eclampsia/eclampsia and the Ethiopian health system’s response. Int J Gynecol Obstet. 2011;115(1):112–116. doi: 10.1016/j.ijgo.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Say L., Chou D., Gemmill A., Tunçalp Ö., Moller Ab, Daniels J., et al. Global causes of maternal death: a who systematic analysis. Lancet Glob Health. 2014;2(6):323–333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 15.Meazaw Mw, Chojenta C., Muluneh Md, Loxton D. Systematic and meta-analysis of factors associated with preeclampsia and eclampsia in Sub-Saharan Africa. Plos One. 2020 doi: 10.1371/journal.pone.0237600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Id Cn, Id Go, Pooja S., Ogutu O., Osoti A., Warren Ce. Clinical presentation and outcomes of pre- eclampsia and eclampsia at a National Hospital, Kenya: a retrospective cohort study;2020. p.1–15. [DOI] [PMC free article] [PubMed]
- 17.Mooij R., Lugumila J., Mwashambwa My, Mwampagatwa Ih, Van Dillen J., Stekelenburg J., et al. Characteristics and outcomes of patients with eclampsia and severe pre-eclampsia in a rural hospital in Western Tanzania: a retrospective medical record study. BMC Pregnancy Childbirth. 2015:1–7. doi: 10.1186/s12884-015-0649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahran A., Fares H., Elkhateeb R., Ibrahim M., Bahaa H., Sanad A., et al. Risk factors and outcome of patients with eclampsia at a tertiary hospital in Egypt. BMC Pregnancy Childbirth. 2017:1–7. doi: 10.1186/s12884-017-1619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aggarwal Rs, Mishra V.V., Jasani Af, Gumber M. Of kidney diseases and transplantation renal data from Asia – Africa acute renal failure in pregnancy: our experience. Saudi J Kidney Dis Transpl. 2014;25(2) doi: 10.4103/1319-2442.128621. 450–5. [DOI] [PubMed] [Google Scholar]
- 20.Abera N., Id A., Demissie Bw. Perinatal outcomes of hypertensive disorders in pregnancy at a referral hospital, Southern Ethiopia. PLos One. 2019:1–10. doi: 10.1371/journal.pone.0213240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding authors.