Abstract
Fatty liver hemorrhagic syndrome is a widespread metabolic disease in laying hens that decreases egg production and even causes death in severe cases. Many traditional Chinese medicine ingredients, such as saikosaponin a (SSa), have been shown to alleviate fatty liver, but the underlying mechanisms remain unclear. In this study, we aimed to explore the alleviation of dietary SSa on excessive hepatic lipid deposition and the interactions between intestinal microbiota and bile acid (BA) in laying hens. Fifty-four 35-wk-old laying hens were randomly allocated into 3 treatment groups with 6 replicates (3 birds per replicate) and fed with a basal diet (CON), high-energy and low-protein diet (HELP), and HELP diet with 30 mg/kg SSa (HELP + SSa). SSa reversed diet-induced egg production rate decrease (P < 0.05). SSa could potently ameliorate HELP-induced accumulation of hepatic cholesterol and liver injury via the increase (P < 0.05) of mRNA expression of BA synthesis gene, such as cholesterol 7 alpha-hydroxylase 1. SSa treatment alleviated gut dysbiosis, especially reducing (P < 0.05) the relative abundance of bile salt hydrolase (BSH)-producing bacteria such as Lactobacillus, Bifidobacterium, and Turicibacter. Ileal BA metabolomic analysis revealed that SSa increased (P < 0.05) the content of tauro-conjugated BAs, mainly taurochenodeoxycholic acid and tauro-α-muricholic acid. The mRNA expression of farnesoid X receptor (FXR) and fibroblast growth factor 19 were decreased (P < 0.05) in intestine, which was associated with increased gene expression of enzymes in the BA synthesis that reduced the levels of cholesterol. Moreover, SSa treatment inhibited intestinal BA reabsorption via decreasing (P < 0.05) the mRNA expression of apical sodium-dependent bile acid transporter. Our findings indicated that SSa reduced liver cholesterol accumulation and alleviated fatty liver in laying hens through microbiota-BA-intestinal FXR crosstalk.
Key words: saikosaponin a, fatty liver, bile acid metabolism, gut microbiota, laying hen
INTRODUCTION
Fatty liver hemorrhagic syndrome (FLHS) is a metabolic disease frequently arising in caged high-production laying hens, that is characterized by lipid metabolic disorders and excessive fat accumulation. FLHS results in dramatic decreases in egg production and accounts for ∼74% of noninfectious mortality in caged laying hens (Shini et al., 2019). The high prevalence exacts a huge economic burden on the layer industry and the effective measurements against FLHS are urgently required. Currently, nutritional modulation with Chinese herbal medicine such as Bupleuri Radix has been implicated in the prevention against lipid metabolism disorders, including FLHS and obesity (Li et al., 2017). Saikosaponin a (SSa) is the major bioactive ingredient in Bupleuri Radix and exerts hepatoprotective activity and beneficial effects on lipid homeostasis (Lim et al., 2021). SSa ameliorated hepatic lipid metabolism disorder in rats by promoting intracellular lipid and cholesterol catabolic-related genes, such as peroxisome proliferator-activated receptor alpha (PPARα), angiotensin II receptor type 1a, and cholesterol 7 alpha-hydroxylase-1 (CYP7a1) (Li et al., 2021; Zheng et al., 2023). However, there exists a knowledge gap regarding the potential effects and underlying mechanisms of SSa on fatty liver in laying hens.
Gut microbiota plays a critical role in maintaining host homeostasis by regulating various physiological processes such as nutrient digestion, immune status, lipid metabolism and metabolic syndrome. Accumulating evidence demonstrated that traditional Chinese herbal drugs can ameliorate the disturbed lipid homeostasis in obesity and FLHS via modulating gut microbiota (Fan et al., 2022; Sun et al., 2022; Zhai et al., 2022). Considering the poor intestinal absorption and bare bioactive compounds in circulating system (Tang et al., 2007), we hypothesize that relief of FLHS associated metabolic disorders by SSa is primarily mediated by regulating gut microbiota. The hypothesis is supported by several studies that gut microbiome is a key target for saponins to regulate lipid metabolism (Liu et al., 2013; Li et al., 2020). Bile acids (BAs) act as a bridge between the intestinal flora and liver via enterohepatic circulation (Schroeder and Backhed, 2016), which participate in the regulation of host lipid metabolism by acting as ligands for liver and gut farnesoid X receptor (FXR) (Jiang et al., 2015). Previous researches have indicated the promotion of liver synthesis and fecal excretion of bile acids in response to SSa treatment, suggesting that SSa may elicit changes in bile acid composition, subsequently participating in host lipid metabolism and favoring the alleviation of lipid accumulation-induced liver injury (Wu et al., 2008; Li et al., 2021). But the exact role of gut microbiota-BA crosstalk in SSa-mediated effect on lipid metabolism regulation in laying hens remains unclear.
In this study, we aimed to investigate the protective effects of SSa on hepatic lipid accumulation induced by high-energy and low-protein (HELP) diet in a laying hen model and determine whether these effects depend on the interactions between gut flora and BAs. Our results provide new evidence for the alleviated effects of SSa on liver lipid metabolism disorder via regulating the BA metabolism and gut microbiota, and would help to identify novel strategies to ameliorate fatty liver.
MATERIALS AND METHODS
The Animal Ethical and Welfare Committee, Northwest A&F University, accepted this study (Approval No. DK2022061).
Experimental Design and Bird's Management
The present trial was performed with 54 laying hens (Jingfen No. 6, Yukou Poultry, Beijing, China), at the age of 35 wk. Birds were randomly assigned to 3 trial groups with 6 replicates (3 birds per replicate), and all cages had the same environmental conditions. The first group (CON) was supplemented with a basal diet; the second group (HELP): high-energy and low-protein diet; the third group (HELP + SSa): high-energy and low-protein diet with SSa (30 mg/kg) (Baoji Earay Bio-tech Company, Xian, China). The corn-soybean basal diet composition was prepared according to the China Feeding Standard of Chicken (NY/T 33-2004). Table 1 gave the composition and nutrient levels of basic and experimental diets. Before the experiment, birds in each group were fed for 2 wk to gradually acclimate the experimental diets. Experiment lasted for 12 wk, during which food and water were provided ad libitum to the hens. Birds were housed in 3-tier battery cages with 3 birds each cage (cage size: 45 cm × 45 cm × 45 cm). Eggs were collected and irregular eggs (broken, soft, and misshapen) were recorded every day. Egg production and weight were recorded daily and feed consumption was recorded every 2 wk during the experimental period.
Table 1.
The composition and nutrient levels of the experimental diets.
| Items (%) | Control | HELP3 diets |
|---|---|---|
| Ingredients | ||
| Corn | 64.15 | 66.25 |
| Soybean meal | 22.70 | 13.85 |
| Ground limestone | 7.50 | 7.50 |
| Soybean oil | 0.65 | 7.40 |
| Premix1 | 5.00 | 5.00 |
| Total | 100.00 | 100.00 |
| Nutrient level2 | ||
| Metabolizable energy (MJ/kg) | 11.20 | 12.96 |
| Crude protein | 15.80 | 12.00 |
| Calcium | 3.78 | 3.75 |
| Total phosphorus | 0.40 | 0.35 |
| Available phosphorus | 0.21 | 0.19 |
| Lysine | 0.76 | 0.53 |
| Methionine | 0.40 | 0.35 |
| Methionine + cysteine | 0.68 | 0.57 |
The premix provided the following per kg of diets: vitamin A 10,000 IU, vitamin D3 1,800 IU, vitamin E 10 IU, vitamin K 10 mg, vitamin B12 1.25 mg, thiamine l mg, riboflavin 4.5 mg, calcium pantothenate 50 mg, niacin 24.5 mg, pyridoxine 5 mg, biotin 1 mg, folic acid 1 mg, choline 500 mg, Mn 60 mg, I 0.4 mg, Fe 80 mg, Cu 8 mg, Se 0.3 mg, Zn 60 mg.
Crude protein, calcium, and total phosphorus were measured values, and the rest were calculated values.
HELP: high-energy and low-protein diet group.
Biochemical Indicators Assay
At the end of the experiment, 1 bird from each replicate were randomly selected for blood sample collection. Serum samples were separated by centrifugation at 3,000 rpm/5 min at 4°C for 10 min and then stored at −80°C for further analysis. The levels of serum total triglyceride (TG), total cholesterol (TC), aspartate aminotransferase (AST), alanine transaminase (ALT), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) was determined by using reagent kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the manufacturer's protocols.
At the end of the experiment, 3 eggs were selected from each replicate and the yolks were separated for the measurement of TC and TG levels. Egg yolks were diluted with ethanol and the TC and TG contents of yolk samples were determined by reagent kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer's instructions.
At the end of the experimental period, 1 layer was randomly selected from each replicate, weighed, euthanized and dissected. The liver and abdominal fat weight were recorded. A small portion of the liver was collected in cryotube and stored at −80°C (<8 wk). The liver samples were mixed with ethanol as 1 g: 9 mL for grinding and the mixture was centrifugated at 12,000 rpm at 4°C for 10 min. The supernatant was collected and the content of liver TG, TC, superoxide dismutase (SOD), and nonesterified fatty acid (NEFA) was determined with a commercial colorimetric kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Histopathological Analysis
Liver samples were fixed with 4% paraformaldehyde solution for 24 h and then embedded in paraffin. Tissue was cutting up into 5 μm thick slices and stained with hematoxylin and eosin (H&E stain). Slides were imaged using an Olympus BX 54/43 microscope with a DP80 Olympus camera (Olympus Corporation, Tokyo, Japan).
Real-Time Quantitative PCR
Ileal mucosa was rinsed with cold sterile phosphate buffer saline and collected with sterile slides. The mucosa samples were snap frozen immediately in liquid nitrogen and then stored at −80°C for RNA extraction. Total RNA was separated from liver and ileum tissue sample with the use of TRIZOL reagent (Tiangen Biotech, Beijing, China) on the basis of the manufacturer's instructions. RNA was reversed into cDNA by using the Evo M-MLV RT Kit (Accurate Biology, Xian, China). Real-time quantitative PCR (RT-qPCR) was performed with the Bio-Rad PCR cycler (Bio-Rad Laboratories, Hercules, CA). The cycle program was as follows: 95°C for 5 min; 40 cycles at 95°C for 5 s, and 60°C for 30 s. Primers of the target genes and reference gene (β-actin) were listed in Table 2. qRT-PCR data were analyzed using the comparative 2−△△Ct method.
Table 2.
Primer sequences for each target gene.
| Gene | Primer sequences (5′–3′) | GeneBank accession number |
|---|---|---|
| FXR | F: GAAAGGACCACACAGCAT | AF492497.1 |
| R: CTCCGTGCCAAGTTTCTA | ||
| FGF19 | F: CCGCCAGCAATTCTTCTA | NM_204674.2 |
| R: GCAGCGTTTGAGTCACTA | ||
| ASBT | F: AAGGCTCGTGGGTTATCA | NM_001319027.1 |
| R: ACGACATCTGCTCCAAGA | ||
| Ibabp | F: GTGGGATGTTTGAGTCAGTG | NM_001277701.1 |
| R: TCTGCTGTTCCTCTGTGA | ||
| FAS | F: CTATCGACACAGCCTGCTCCT | NM_205155.4 |
| R: CAGAATGTTGACCCCTCCTACC | ||
| ACC | F: AATGGCAGCTTTGGAGGTGT | XM_046929960.1 |
| R: TCTGTTTGGGTGGGAGGTG | ||
| LXRα | F: CAAAGGGAATGAATGAGC | XM_046917664.1 |
| R: AGCCGAAGGGCAAACAC | ||
| PPARα | F: ACGGAGTTCCAATCGC | XM_046906400.1 |
| R: AACCCTTACAACCTTCACAA | ||
| PPARγ | F: CGAGGAGTCTTCCAACTC | XM_046925952.1 |
| R: CCTGATGGCATTATGTGA | ||
| HMGCR | F: ATGTCAGGAGTGCGACAACT | XM_046934671.1 |
| R: CGTCCTTCACGACTCTCTCG | ||
| SREBP-1 | F: TGGTGGTGGACGCCGAGAAG | XM_046927256.1 |
| R: GTCGTTGATGGATGAGCGGTAGC | ||
| SREBP-2 | F: CCAAGGAGAGCCTGTACTGC | XM_015289037.4 |
| R: CCCATTGAGTCCAGGAAAGA | ||
| CYP7a1 | F: TGACCCAGCAGAAGGAAACA | NM_001001753.2 |
| R: ACCCAGGTGTTAGGCTGAAA | ||
| β-actin | F: GAGAAATTGTGCGTGACATCA | NM_205518.2 |
| R: CCTGAACCTCTCATTGCCA |
LXRα: liver X receptor α; FXR: farnesoid X receptor; FGF: fibroblast growth factor; CYP7a: cholesterol 7α-hydroxylase; ACC: acetyl-CoA carboxylase; FAS: fatty acid synthetase; SREBP: sterol-regulatory element-binding protein; PPAR: peroxisome proliferator-activated receptor; HMGCR: β-hydroxy-β-methylglutaryl-coenzyme reductase; ASBT: apical sodium-dependent bile acid transporter; Ibabp: ileum bile acid binding protein.
16S rRNA Sequencing and Data Analysis
Ileal digest was collected in a sterile cryopreservation tube and immediately frozen in liquid nitrogen before storage at −80°C for DNA extraction. The DNA was isolated from the ileum content samples using the cetyltrimethylammonium bromide. The PCR amplification of the bacterial 16S rDNA gene V3 to V4 region was carried out using the primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The PCR products were purified with AMPure XT beads (Beckman Coulter Genomics, Danvers, MA), and the resulting library was generated using the Illumina NovaSeq platform (LC-Bio Technology, Hang Zhou, China). Quality filtering on the raw reads were performed under specific filtering conditions to obtain the high-quality clean tags according to the fqtrim (v0.94). According to the SILVA (release 138) classifier, feature abundance was normalized using the relative abundance of each sample. Histogram of linear discriminant analysis (LDA) distribution was designed with the LDA effect size (LEfSe) analysis software (https://www.omicstudio.cn/tool/). Sequencing data are available in the National Center of Biotechnology Information Sequence Read Archive database (accession number: PRJNA904838).
Quantification of Intestinal BAs
The methods of sample preparation and UPLC-MS/MS, as well as specific information for BA standards referred to the reported method (Wang et al., 2020). For BAs quantification, ileum content samples were homogenized and centrifuged to remove the proteins. The solutions were evaporated with nitrogen gas at 37°C and then redissolved in mobile phase. BAs were quantified using an ultra-high-performance liquid chromatography-mass spectrometry system (ExionLC AD UHPLC-QTRAP 6500+, AB SCIEX, Boston, MA). Principal component analysis (PCA) of BAs was designed with the R package (v3.5.0).
Statistical Analysis
SPSS 27 (Ammonk IBM, New York, NY) was employed for analyzing data. Statistical differences between multiple groups were analyzed by 1-way ANOVA. Multiple comparisons were conducted using the Bonferroni method. P < 0.05 was considered statistically significant. Data were presented as mean ± SEM. Graphs were generated using the GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA).
RESULTS
Egg Production and Feed Intake
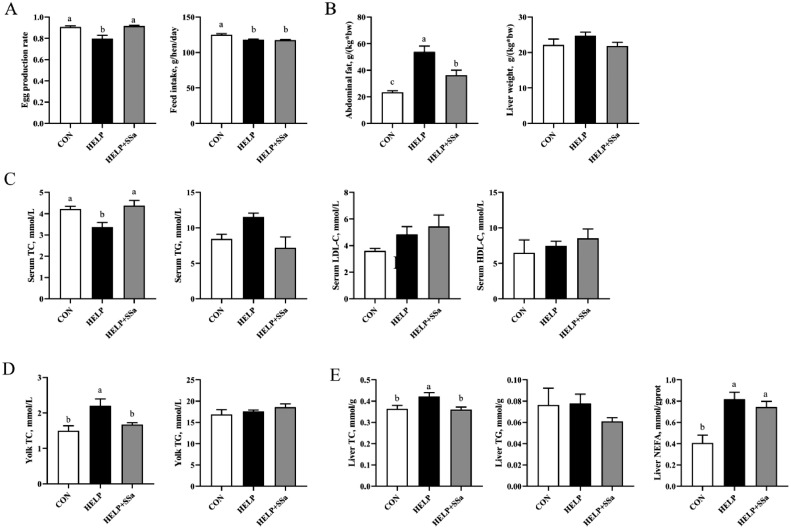
The diet of HELP had a significant decreased (P < 0.05) effect on the egg production rate, which was reversed by SSa supplement (Figure 1A). SSa had no effect (P > 0.05) on feed intake, which was decreased (P < 0.05) by HELP diet compared with CON group (Figure 1A).
Figure 1.
The effect of saikosaponin a on lipid metabolism in laying hens (n = 6). (A) The egg production rate and the feed intake. (B) The rate of abdominal fat and liver. (C) Serum total triglyceride (TG) and total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels. (D) Yolk TG and TC levels. (E) Liver TC, TG, and nonesterified fatty acid (NEFA) content. a,bDifferent letters represent significant differences (P < 0.05). CON, control group; HELP, high-energy and low-protein diet group; HELP + SSa, high-energy and low-protein with saikosaponin a group.
Organ Measurements
Results of liver and abdominal fat weight were presented in Figure 1B. HELP increased (P < 0.05) the abdominal fat rate in laying hens, which was reversed (P < 0.05) by SSa supplement. The HELP and SSa had no significant (P > 0.05) change in liver weight.
Lipid Parameters of Serum, Yolk, and Liver
The yolk TC levels were significantly decreased (P < 0.05) in SSa group (Figure 1D). The HELP and SSa had no significant (P > 0.05) change in yolk TG level. The serum TC levels were increased (P < 0.05) in HELP group, while SSa reversed (P < 0.05) this alteration (Figure 1C). The levels of serum TG, LDL-C, and HDL-C had no significant (P > 0.05) change with the SSa treatment (Figure 1C). The levels of hepatic TC and NEFA were increased (P < 0.05) in HELP group compared with CON group (Figure 1E). SSa treatment showed an inhibited (P < 0.05) regulation (Figure 1E) on hepatic TC level compared with the HELP group, but no effect on (P > 0.05) TG and NEFA levels (Figure 1E).
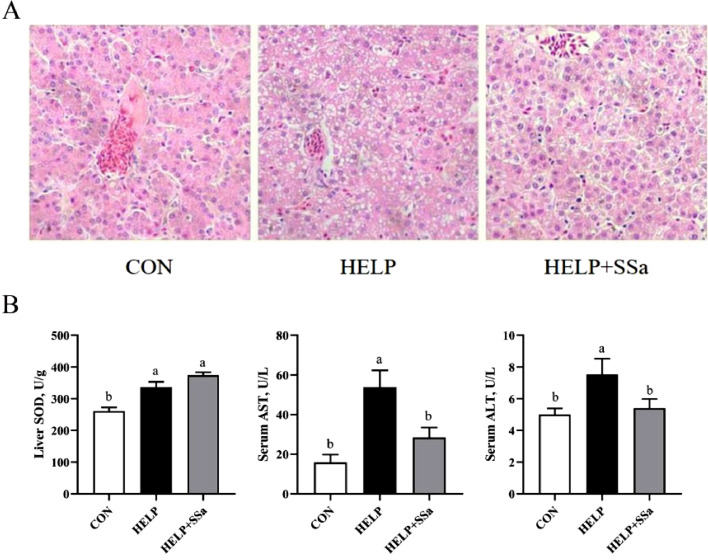
Liver Injury Biochemistry and Morphology
The results of H&E staining showed remarkable steatosis on hepatocytes and hepatocyte ballooning with HELP diet, and SSa reduced fatty droplets and ballooning degeneration (Figure 2A). HELP diet increased (P < 0.05) the level of liver SOD, serum ALT, and serum AST (Figure 2B). SSa decreased (P < 0.05) the serum ALT and AST levels compared with HELP group (Figure 2B).
Figure 2.
Effects of saikosaponin a on liver injury in HELP-challenged laying hens (n = 6). (A) H&E staining of liver section, viewed under a microscope (100×). (B) Liver superoxide dismutase (SOD) activity, serum aspartate aminotransferase (AST) levels, and serum alanine transaminase (ALT) levels. a,bDifferent letters represent significant differences (P < 0.05). CON, control group; HELP, high-energy and low-protein diet group; HELP + SSa, high-energy and low-protein with saikosaponin a group.
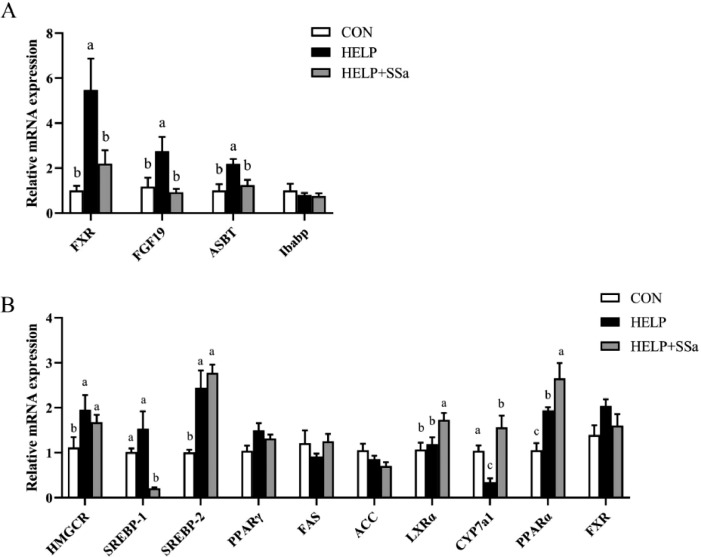
Expression of Genes Related to Lipid Metabolism in Liver and Ileum
As showed in Figure 3A, no significant influences (P > 0.05) were observed on relative mRNA expression of ileal bile acid binding protein (Ibabp) with SSa addition. However, SSa significantly decreased (P < 0.05) the mRNA expression of FXR, fibroblast growth factor (FGF19), and apical sodium-dependent bile acid transporter (ASBT) in ileal mucosa compared with HELP group. HELP diet significantly increased (P < 0.05) the mRNA expression of β-hydroxy-β-methylglutaryl-coenzyme reductase (HMGCR), sterol-regulatory element-binding protein-2 (SREBP-2) and PPARα, and decreased (P < 0.05) the mRNA expression of CYP7a1 in liver (Figure 3B). SSa intervention increased (P < 0.05) the relative mRNA expression of liver X receptor alpha (LXRα), CYP7a1, and PPARα and decreased (P < 0.05) SREBP-1 expression in the liver (Figure 3B).
Figure 3.
Effects of saikosaponin a treatment on the relative mRNA expression in the ileal (A) and liver (B) of laying hens (n = 6). a,bDifferent letters represent significant differences (P < 0.05). CON, control group; HELP, high-energy and low-protein diet group; HELP + SSa, high-energy and low-protein with saikosaponin a group; LXRα: liver X receptor α; FXR: farnesoid X receptor; FGF: fibroblast growth factor; CYP7a: cholesterol 7α-hydroxylase; ACC: acetyl-CoA carboxylase; FAS: fatty acid synthetase; SREBP: sterol-regulatory element-binding protein; PPAR: peroxisome proliferator-activated receptor; HMGCR: β-hydroxy-β-methylglutaryl-coenzyme reductase; ASBT: apical sodium-dependent bile acid transporter; Ibabp: ileum bile acid binding protein.
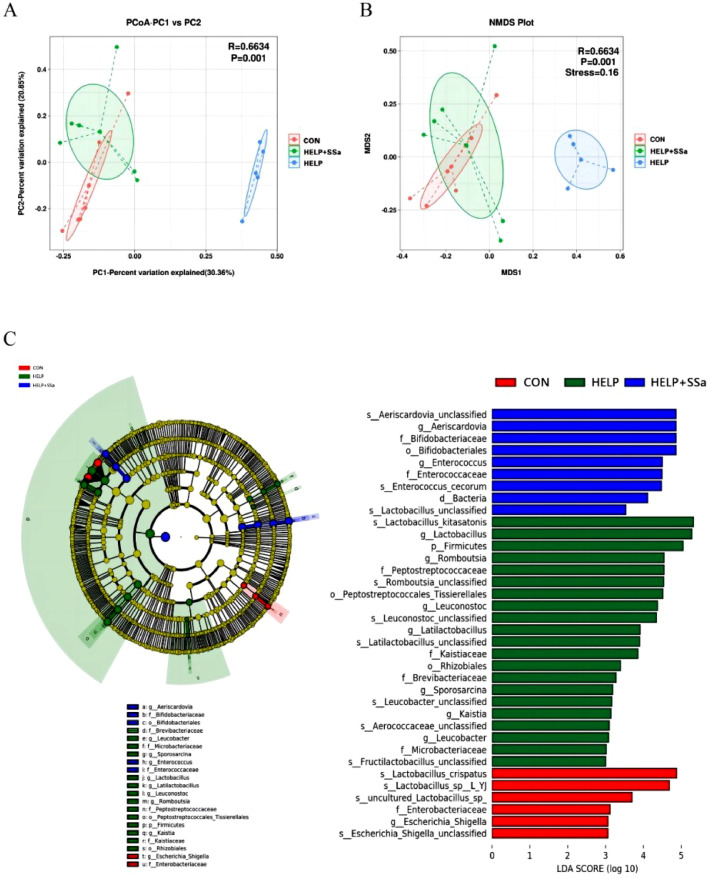
Ileal Microbial Profile
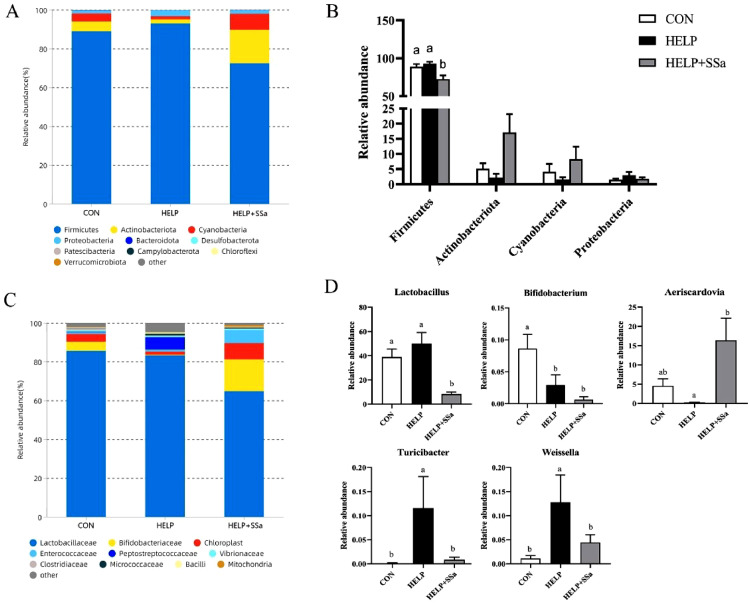
No significant differences (P > 0.05) in species richness (as reflected by Chao1 and pielou_e indices) or alpha-diversity (as reflected by Shannon and Simpson indices) were observed in ileal microbiota at the taxonomic level (Table 3). However, principal co-ordinates analysis results (Figure 4A) and nonmetric multidimensional scaling results (Figure 4B) showed a significant separation (P < 0.05) of ileal microbial communities between HELP and HELP + SSa groups. Firmicutes and Actinobacteria were the dominant phyla in all groups, accounting for more than 70% of the whole ileal microbial communities (Figure 5A). SSa supplementation resulted in a decreased (P < 0.05) relative abundance of Firmicutes (Figure 5B). The dominant classes were Lactobacillaceae and Bifidobacteriaceae (Figure 5C). The relative abundances of Lactobacillaceae, Erysipelotrichaceae, Eggerthellaceae, and Eggerthellaceae were decreased (P < 0.05) by SSa intervention (Figure 5C). At genus level, the abundance of Aeriscardovia abundances was increased (P < 0.05) with SSa addition (Figure 5D). The abundance of Lactobacillus, Weissella, and Turicibacter was decreased (P < 0.05) with SSa addition (Figure 5D). The LEfSe analysis was conducted to identify the relative richness (LDA > 3.0) of bacterial members in the ileum of 3 groups (Figure 4C). Lactobacillus, Romboutsia, and Latilactobacillus were enriched in the HELP, while Aeriscardovia and Enterococcus were enriched in SSa-supplemented group.
Table 3.
The effect of saikosaponin a on α-diversity of ileum microbiota of laying hens.
| Treatments | Shannon | Simpson | Chao1 | Pielou_e |
|---|---|---|---|---|
| CON | 4.30 ± 0.18 | 0.87 ± 0.03 | 174.61 ± 23.14 | 0.59 ± 0.03 |
| HELP | 3.99 ± 0.35 | 0.79 ± 0.05 | 238.81 ± 41.18 | 0.51 ± 0.03 |
| HELP + SSa | 4.59 ± 0.20 | 0.88 ± 0.03 | 190.53 ± 6.89 | 0.61 ± 0.03 |
| P value | 0.246 | 0.195 | 0.211 | 0.056 |
CON, control group; HELP, high-energy and low-protein diet group; HELP + SSa, high-energy and low-protein diet with saikosaponin a group.
Figure 4.
Beta diversity analysis of gut microbiota between the 3 groups. (A) Principal co-ordinates analysis plot. (B) Nonmetric multidimensional scaling plot. (C) The analysis of the nonparametric factors Kruskal-Wallis rank-sum test and LDA discrimination. CON, control group; HELP, high-energy and low-protein diet group; HELP + SSa, high-energy and low-protein with saikosaponin a group.
Figure 5.
Effects of saikosaponin a on the composition of gut microbiota in laying hens (n = 6). (A) Microbiota composition at the phylum level. (B) Relative abundance of major bacteria at the phylum level. (C) Microbiota composition at the family level. (D) Relative abundance of major bacteria at the genus level. a,bDifferent letters represent significant differences (P < 0.05). CON, control group; HELP, high-energy and low-protein diet group; HELP + SSa, high-energy and low-protein with saikosaponin a group.
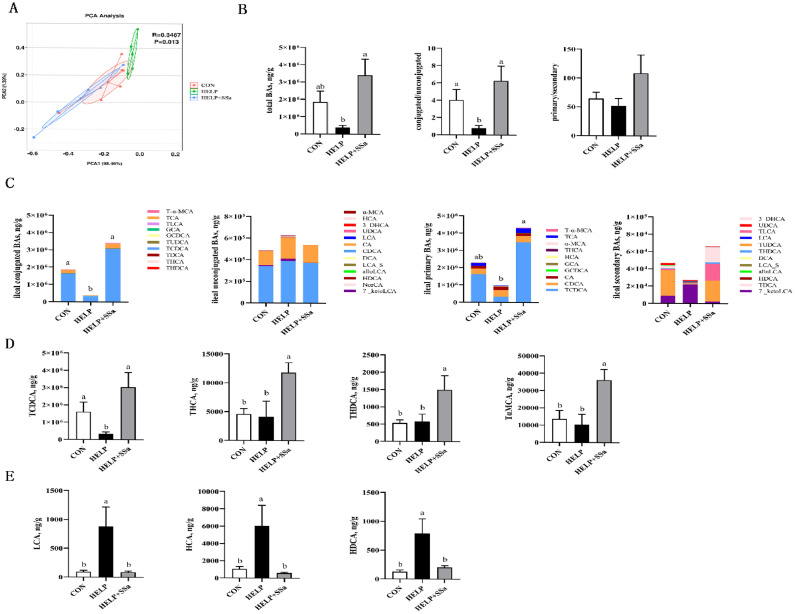
Ileal BA Profile
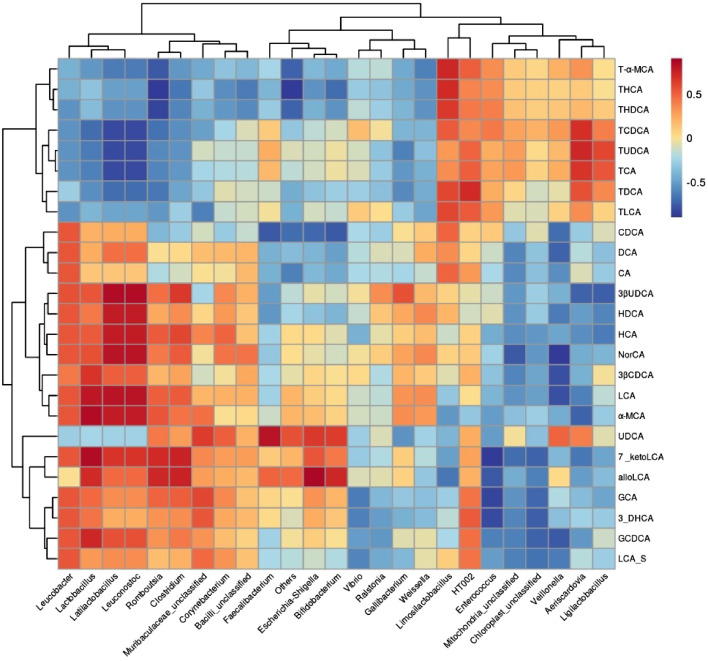
Compared with HELP diet, SSa had a considerable difference in BA structure (Figure 6A). The total BA, conjugated BA, and the conjugated BA to unconjugated BA ratio (Figure 6B) were evidently enhancive (P < 0.05) with SS intervention. The levels of taurohyodeoxycholic acid (THDCA), taurohyocholic acid (THCA), taurochenodeoxycholic acid (TCDCA), and tauro-alpha-muricholic acid (TαMCA) were remarkably elevated (P < 0.05) with SSa supplementation (Figure 6D). SSa decreased the levels of lithocholic acid (LCA), hyodeoxycholic acid (HDCA), and hyocholic acid (HCA) in ileum (P < 0.05). A subsequent Spearman's correlation analysis revealed a negative correlation between conjugated BA levels with Lactobacillus, Ligilactobacillus, and Leuconostoc, whereas FXR agonists, like LCA, HDCA, and HCA, had a positively correlation with Lactobacillus and Ligilactobacillus (Figure 7).
Figure 6.
Effects of saikosaponin a on the composition of bile acids (BAs) in laying hens (n = 6). (A) BAs principal component analysis (PCA) plot; (B) Total BAs content levels, the conjugated/unconjugated BA ratio and primary/secondary BA ratio; (C) The composition of ileal conjugated BAS, unconjugated BAs, primary BAs, and secondary BAs; (D) Some conjugated BA monomer contents in ileal contents; (E) Some unconjugated BA monomer contents in ileal contents. a, bDifferent letters represent significant differences (P < 0.05). CON, control group; HELP, high-energy and low-protein diet group; HELP + SSa, high-energy and low-protein with saikosaponin a group; THDCA, taurohyodeoxycholic acid; THCA, taurohyocholic acid; TDCA, taurodeoxycholic acid; TCDCA, taurochenodeoxycholic acid; TUDCA, tauroursodeoxycholic acid; GCDCA, glycochenodeoxycholic acid; GCA, glycocholic acid; TLCA, taurolithocholic acid; TCA, taurocholic acid; T-α-MCA, tauro-alpha-muricholic acid; 7-ketoLCA, 7-ketolithocholic acid; NorCA, 23-norcholic acid; HDCA, hyodeoxycholic acid; alloLCA, allolithocholic acid; LCA-S, lithocholic acid 3-sulfate; DCA, deoxycholic acid; CDCA, chenodeoxycholic acid; CA, cholic acid; LCA, lithocholic acid; UDCA, ursodeoxycholic acid; 3-DHCA, 3-dehydrocholic acid; HCA, hyocholic acid; α-MCA, alpha-muricholic acid.
Figure 7.
Spearman's correlation analysis between the identified bacterial species and bile acids.
DISCUSSION
FLHS has become one of the most common noninfectious diseases that contribute to a great losses of poultry industry. Considerable evidence indicated that SSa showed great potential in preventing lipid metabolism disorders (Tang et al., 2023), but the effect on FLHS in laying hens remains unclear. In the current study, we found that SSa treatment alleviated diet-induced fatty liver by decreasing cholesterol deposition and ameliorating liver injury. The beneficial effect of SSa was attributed to the decrease of intestinal BSH-producing bacteria abundance and subsequently increase of ileal conjugated BA level. The SSa-induced change of intestinal BA profile was beneficial to the decrease of liver lipid deposition via inhibiting BA receptors expression, such as FXR and ASBT, promoting hepatic BA synthesis and decreasing BA reabsorption.
FLHS is characterized by lipid metabolism disorder and hepatic steatosis which can be induced by HELP diet, a classical research method in laying hens (Rozenboim et al., 2016). In the present study, HELP diet reduced egg production rate and increased lipid droplet area, NEFA level, and cholesterol content of liver, which was consistent with the natural FLHS characteristics. In addition, the elevation of serum AST and ALT levels in HELP group indicated the injury of hepatic cell and the abnormal liver function (Rozenboim et al., 2016). Diet-induced hepatic lipid metabolism disorder could be partly reversed by SSa, as shown by the decrease of liver TC content, yolk TC content and serum AST level. In addition, the increase of serum TC level and decrease of liver TC level implied that the transportation of liver cholesterol into blood would be promoted following SSa treatment. Therefore, SSa addition could relieve HELP diet-induced fatty liver, as indicated by the decrease of liver cholesterol accumulation and alleviation of liver injury.
The excessive lipid deposition contributed to the development of fatty liver and further aggravates lipotoxicity-related hepatocellular injury via the activation of endoplasmic reticulum stress (Musso et al., 2013; Wang et al., 2021). Our data showed that HELP-induced lipid dysregulation was characterized by the promotion of cholesterol synthesis and the suppression of BA synthesis. Cholesterol biosynthesis was under the control of the HMGCR and SREBP-2 (Jo et al., 2011), a transcription factor, which could further activate the expression of genes (including HMGCR) in the cholesterol synthesis pathway (Liu et al., 2012). In this study, there were no significant effects on HELP-induced upregulation of HMGCR and SREBP-2 expression following SSa treatment, possibly indicating that the cholesterol clearance in SSa group is not achieved by inhibiting hepatic cholesterol synthesis. The marked drop in hepatic cholesterol level might be associated with the activated BA synthesis and increased intracellular cholesterol efflux in response to SSa treatment. Cholesterol 7α-hydroxylase, encoded by CYP7a1, represents the rate-limiting step of the cholesterol conversion into BAs (Goodwin et al., 2003). LXR exerts a crucial function in maintaining cholesterol homeostasis through promoting cholesterol efflux and suppressing LDL uptake. Some studies had pointed out that saponins could act as a potential ligand to stimulate LXR expression and CYP7a1 expression (Kawase et al., 2013; Xiong et al., 2021). In the current study, the increased expression of CYP7a1 might be attributed to the upregulation of transcriptional factors LXR and SREBP-1 with SSa supplementation. However, it was reported that SSa could inhibit the maturation of SREBP-1 in a dose-dependent manner but had no effects on its transcription levels (Lim et al., 2021). Therefore, SSa could promote cholesterol excretion and subsequently protect from HELP-induced liver lipid metabolism disorder in laying hens.
Alteration in gut microbiota composition is strongly linked to the progression of FLHS. The HELP-induced gut dysbiosis, such as the increased Firmicutes abundance, would disrupt host energy metabolism and facilitate the occurrence of fatty liver disease (Armougom et al., 2009). SSa addition decreased the relative abundance of Firmicutes in ileum, which might contribute to the relief of liver lipid deposition. Additionally, the abundance of Erysipelotrichaceae, Eggerthellaceae, and Coriobacteriaceae was increased with HELP diet, whereas SSa intervention could reverse these alterations. An excess abundance of Erysipelotrichia would lead to the imbalance of choline metabolism, reflected by choline depletion and high trimethylamine levels in serum, which might be associated with liver fat deposition and a high risk of FLHS development (Vallianou et al., 2021). Eggerthellaceae involved in host lipid metabolism, whose increased abundance was discovered in mice with fatty liver (Chen et al., 2023). Genus Enterorhabdus belonging to Eggerthellacease was believed to inhibit lipogenesis as indicated by the positive correlation with hepatic TG content and SREBP-1 mRNA expression (Duan et al., 2022). Coriobacteriaceae had been considered as a pathobiont because its occurrence had a correlation with increased blood cholesterol level and a series of pathologies such as obesity and FLHS (Romo-Vaquero et al., 2019). Of particular interest was that these bacteria had the ability to synthesize BSH, which was a key player in the microbe-host dialogue functionally regulating host cholesterol and BA metabolism (Joyce et al., 2014). The decreased abundance of these BSH-producing bacteria suggested that low BSH activity might be responsible for changed cholesterol metabolism in the liver. At the genus level, SSa significantly decreased the relative abundance of BSH-producing Lactobacillus, Weissella, and Turicibacter. Previous studies demonstrated that a decrease of Lactobacillus abundance inhibited hepatic lipidosis (Jiang et al., 2015; Cai et al., 2016). Weissella and Turicibacter had been shown to alleviate lipid metabolism dysregulation by producing BSH and regulating the intestinal BA profile (Wu et al., 2020). These results further supported our finding that inhibition of BSH-producing bacteria abundance might be involved in upregulation of cholesterol metabolism, which was consistent with several reports showing the metabolic benefits of inhibiting BSH activity (Huang et al., 2019; Xiong et al., 2021; Fan et al., 2022). There are massive studies supporting the role of low-BSH activity in reducing hepatic lipidosis through the regulation of intestinal BA profile, while some studies reported the benefit of BSH-producing probiotics that exert a serum cholesterol-lowering effect and prevention of hypercholesterolemia (Li et al., 2022; Münzker et al., 2022; Zhang et al., 2023). There is a growing appreciation for the role that the diversity of BSH in the probiotics and their substrate selectivity play in the management of metabolism disease. Differences in substrate specificity might underlie the double-edged effects of BSH on the host metabolism or at least serve as a marker for health (Jarocki et al., 2014). It could be assumed that the intestinal BA profile alteration caused by BSH-producing bacteria might play a pivotal role in SSa protection against diet-induced liver lipid deposition.
BAs are critical regulators in cholesterol homeostasis and lipid metabolism, whose biological characteristics are closely related to their hydrophobic structures. The enrichment of unconjugated BA due to long term high fat diet (HFD) were believed to induce the increased intestinal permeability and hepatic steatosis (Li et al., 2022). The ileal total BA level was elevated, especially conjugated BA, including THDCA, THCA, TCDCA, and TαMCA, following SSa treatment, which might contribute to protection from HELP diets-induced liver lipid accumulation and injury. The alteration of conjugated BA level was mainly due to the change of BSH-producing intestinal bacteria relative abundance. Our study demonstrated that conjugated BA had a negative relationship with the Firmicutes which was the primary phylum on BSH synthesis. Besides, some BSH-producing bacteria species, like Lactobacillus and Latilactobacillus, had a negative relationship with primary conjugated BA. This was consistent with previous reports that increased fecal excretion of BAs was associated with the cholesterol-lowing activity of saikosaponins (Tang et al., 2023). However, it was still unclear that the specific effects of SSa on BA metabolism and its mechanism underlying the alleviation of HELP-induced fatty liver. In the present study, SSa intervention resulted in a shift toward more hydrophilic forms of BAs, like THDCA, together with the decreased HDCA and LCA contents, which might favor the mitigation of BA-mediated hepatotoxicity. The result was consistent with previous study that the increase of THDCA could improve the laying performance of late-phase hens and decrease the serum TC levels by modulating gut microbiota composition (Yang et al., 2022). LCA, the 7α-dehydroxylation product of CDCA, was reported as a potential biomarker for the identification of nonalcoholic fatty liver in type 2 diabetes mellitus patients (Sheng et al., 2022). Similarly, this study indicated that LCA and its stereoisomers had a negative relationship with the relative abundance of Clostridium, the primary 7α-dehydroxylase-producing bacteria. FXR-FGF19 pathway plays an important role in regulating BA synthesis and the pleiotropic activities of endogenous BAs through enterohepatic recycling. As a member of the nuclear receptor superfamily, FXR is highly expressed in the liver and intestine. The intestinal FXR has been reported to be activated in HFD-induced fatty liver (Fiorucci and Distrutti, 2015). In intestine, FXR can be upregulated by BAs in the terminal ileum and further induces the expression of FGF15/19 (Katafuchi and Makishima, 2022). FGF15/19 goes into the liver through portal vein and inhibits CYP7a1 expression and hepatic BA synthesis. Similarly, this study showed that HELP diet increased the expression of intestinal FXR and FGF19, which might be related to the accumulation of hepatic cholesterol. Conjugated BA such as TCDCA, THCA, and TαMCA are identified as naturally occurring FXR antagonists, whereas unconjugated BA including CA, CDCA, and DCA are FXR agonists (Sun et al., 2021). The massive accumulation of taurine conjugated BA in the intestine alleviated diet-induced fatty liver by inhibiting intestinal FXR with the theabrownin treatment (Huang et al., 2019). In our study, SSa inhibited intestinal FXR signaling by decreasing BSH-expression flora and altering ileum BA profile, suggesting that the microbiota-BA-intestinal FXR axis might be the main way of SSa regulation on hepatic cholesterol metabolism. Approximately 95% of intestinal BAs are reabsorbed into enterohepatic circulation by ASBT in the ileum to maintain the size of the BA pool (Al-Dury and Marschall, 2018). ASBT inhibitor had been demonstrated to ameliorate hepatic steatosis by regulating BA metabolism in HFD-fed mice (Rao et al., 2016). In the present work, intestinal ASBT expression was be downregulated by the alterations of ileal BA composition with SSa treatment, which contributed to the excretion of BAs and the decrease of hepatic cholesterol levels. Therefore, SSa might attenuate hepatic cholesterol accumulation by regulating BA synthesis and reabsorption via intestinal microbiota-BA-FXR pathway.
CONCLUSIONS
In summary, SSa reversed diet-induced hepatic cholesterol accumulation and liver injury by reshaping gut microbiota composition and BA profiles. The beneficial effects of SSa were believed to be mediated mainly through the decrease of BSH-carrying bacteria, such as Lactobacillus, and increase of intestinal conjugated BA, especially FXR antagonists like TCDCA and TαMCA, which decreased the hepatic cholesterol accumulation through microbiota-BA-intestinal FXR crosstalk. These findings indicated that SSa might act as an efficient additive to alleviate excessive lipid deposition-induced liver damage, which provided a potential intervention strategy for resolving fatty liver in laying hens.
ACKNOWLEDGMENTS
This study was supported by China Agriculture Research System of MOF and MARA (CARS-40-S20), the National Natural Science Foundation of China (32302776), the Scientific Startup Foundation for Doctors of Northwest A and F University (No. 2452022022), and Agricultural Science and Technology Innovation Project of Shaanxi (K3031222146).
DISCLOSURES
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
REFERENCES
- Al-Dury S., Marschall H.U. Ileal bile acid transporter inhibition for the treatment of chronic constipation, Cholestatic Pruritus, and NASH. Front. Pharmacol. 2018;9:931. doi: 10.3389/fphar.2018.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armougom F., Henry M., Vialettes B., Raccah D., Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and methanogens in anorexic patients. PLoS One. 2009;4:e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Zhang L., Jones R.A., Correll J.B., Hatzakis E., Smith P.B., Gonzalez F.J., Patterson A.D. Antioxidant drug tempol promotes functional metabolic changes in the gut microbiota. J. Proteome Res. 2016;15:563–571. doi: 10.1021/acs.jproteome.5b00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Chen S., Chuang Y., Chiang B. Attenuation of the severity and changes in the microbiota in an animal model of primary biliary cholangitis by FOXP3− regulatory T cells. Clin. Transl. Disc. 2023;3:e187. [Google Scholar]
- Duan R., Huang K., Guan X., Li S., Xia J., Shen M., Sun Z., Yu Z. Tectorigenin ameliorated high-fat diet-induced nonalcoholic fatty liver disease through anti-inflammation and modulating gut microbiota in mice. Food Chem. Toxicol. 2022;164 doi: 10.1016/j.fct.2022.112948. [DOI] [PubMed] [Google Scholar]
- Fan J., Sun J., Li T., Yan X., Jiang Y. Nuciferine prevents hepatic steatosis associated with improving intestinal mucosal integrity, mucus-related microbiota and inhibiting TLR4/MyD88/NF-κB pathway in high-fat induced rats. J. Funct. Foods. 2022;88 [Google Scholar]
- Fiorucci S., Distrutti E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol. Med. 2015;21:702–714. doi: 10.1016/j.molmed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Goodwin B., Watson M.A., Kim H., Miao J., Kemper J.K., Kliewer S.A. Differential regulation of rat and human CYP7A1 by the nuclear oxysterol receptor liver X receptor-alpha. Mol. Endocrinol. 2003;17:386–394. doi: 10.1210/me.2002-0246. [DOI] [PubMed] [Google Scholar]
- Huang F., Zheng X., Ma X., Jiang R., Zhou W., Zhou S., Zhang Y., Lei S., Wang S., Kuang J., Han X., Wei M., You Y., Li M., Li Y., Liang D., Liu J., Chen T., Yan C., Wei R., Rajani C., Shen C., Xie G., Bian Z., Li H., Zhao A., Jia W. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019;10:4971. doi: 10.1038/s41467-019-12896-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarocki P., Podlesny M., Glibowski P., Targonski Z. A new insight into the physiological role of bile salt hydrolase among intestinal bacteria from the genus Bifidobacterium. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Xie C., Li F., Zhang L., Nichols R.G., Krausz K.W., Cai J., Qi Y., Fang Z.Z., Takahashi S., Tanaka N., Desai D., Amin S.G., Albert I., Patterson A.D., Gonzalez F.J. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y., Lee P.C., Sguigna P.V., DeBose-Boyd R.A. Sterol-induced degradation of HMG CoA reductase depends on interplay of two Insigs and two ubiquitin ligases, gp78 and Trc8. Proc. Natl. Acad. Sci. U. S. A. 2011;108:20503–20508. doi: 10.1073/pnas.1112831108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce S.A., MacSharry J., Casey P.G., Kinsella M., Murphy E.F., Shanahan F., Hill C., Gahan C.G. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl. Acad. Sci. U. S. A. 2014;111:7421–7426. doi: 10.1073/pnas.1323599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase A., Yamada A., Gamou Y., Tahara C., Takeshita F., Murata K., Matsuda H., Samukawa K., Iwaki M. Increased effects of ginsenosides on the expression of cholesterol 7α-hydroxylase but not the bile salt export pump are involved in cholesterol metabolism. J. Nat. Med. 2013;67:545–553. doi: 10.1007/s11418-012-0713-4. [DOI] [PubMed] [Google Scholar]
- Li X., Ge J., Li Y., Cai Y., Zheng Q., Huang N., Gu Y., Han Q., Li Y., Sun R., Liu R. Integrative lipidomic and transcriptomic study unravels the therapeutic effects of saikosaponins A and D on non-alcoholic fatty liver disease. Acta Pharm. Sin. B. 2021;11:3527–3541. doi: 10.1016/j.apsb.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Han J., Lv J., Wang S., Qu L., Jiang Y. Saikosaponin a-induced gut microbiota changes attenuate severe acute pancreatitis through the activation of Keap1/Nrf2-ARE antioxidant signaling. Oxid. Med. Cell Longev. 2020;2020 doi: 10.1155/2020/9217219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li X., Lu J., Huang Y., Lv L., Luan Y., Liu R., Sun R. Saikosaponins induced hepatotoxicity in mice via lipid metabolism dysregulation and oxidative stress: a proteomic study. BMC Complement. Altern. Med. 2017;17:219. doi: 10.1186/s12906-017-1733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Xiao Y., Huang Y., Song L., Li M., Ren Z. Lactobacillus gasseri rw2014 ameliorates hyperlipidemia by modulating bile acid metabolism and gut microbiota composition in rats. Nutrients. 2022;14:4945. doi: 10.3390/nu14234945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.H., Lee H.S., Han H.K., Choi C.I. Saikosaponin A and D inhibit adipogenesis via the AMPK and MAPK signaling pathways in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2021;22:11409. doi: 10.3390/ijms222111409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.F., Tang J.J., Li P.S., Shen Y., Li J.G., Miao H.H., Li B.L., Song B.L. Ablation of gp78 in liver improves hyperlipidemia and insulin resistance by inhibiting SREBP to decrease lipid biosynthesis. Cell Metab. 2012;16:213–225. doi: 10.1016/j.cmet.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Liu G., Tian Y., Li G., Xu L., Song R., Zhang Z. Metabolism of saikosaponin a in rats: diverse oxidations on the aglycone moiety in liver and intestine in addition to hydrolysis of glycosidic bonds. Drug Metab. Dispos. 2013;41:622–633. doi: 10.1124/dmd.112.048975. [DOI] [PubMed] [Google Scholar]
- Münzker J., Haase N., Till A., Sucher R., Haange S.B., Nemetschke L., Gnad T., Jäger E., Chen J., Riede S.J., Chakaroun R., Massier L., Kovacs P., Ost M., Rolle-Kampczyk U., Jehmlich N., Weiner J., Heiker J.T., Klöting N., Seeger G., Morawski M., Keitel V., Pfeifer A., von Bergen M., Heeren J., Krügel U., Fenske W.K. Functional changes of the gastric bypass microbiota reactivate thermogenic adipose tissue and systemic glucose control via intestinal FXR-TGR5 crosstalk in diet-induced obesity. Microbiome. 2022;10:96. doi: 10.1186/s40168-022-01264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G., Gambino R., Cassader M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog. Lipid Res. 2013;52:175–191. doi: 10.1016/j.plipres.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Rao A., Kosters A., Mells J.E., Zhang W., Setchell K.D., Amanso A.M., Wynn G.M., Xu T., Keller B.T., Yin H., Banton S., Jones D.P., Wu H., Dawson P.A., Karpen S.J. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aaf4823. 357ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo-Vaquero M., Cortés-Martín A., Loria-Kohen V., Ramírez-de-Molina A., García-Mantrana I., Collado M.C., Espín J.C., Selma M.V. Deciphering the human gut microbiome of urolithin metabotypes: association with enterotypes and potential cardiometabolic health implications. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201800958. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Mahato J., Cohen N.A., Tirosh O. Low protein and high-energy diet: a possible natural cause of fatty liver hemorrhagic syndrome in caged White Leghorn laying hens. Poult. Sci. 2016;95:612–621. doi: 10.3382/ps/pev367. [DOI] [PubMed] [Google Scholar]
- Schroeder B.O., Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016;22:1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- Sheng W., Ji G., Zhang L. The effect of lithocholic acid on the gut-liver axis. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.910493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shini A., Shini S., Bryden W.L. Fatty liver haemorrhagic syndrome occurrence in laying hens: impact of production system. Avian Pathol. 2019;48:25–34. doi: 10.1080/03079457.2018.1538550. [DOI] [PubMed] [Google Scholar]
- Sun J., Fan J., Li T., Yan X., Jiang Y. Nuciferine protects against high-fat diet-induced hepatic steatosis via modulation of gut microbiota and bile acid metabolism in rats. J. Agric. Food Chem. 2022;70:12014–12028. doi: 10.1021/acs.jafc.2c04817. [DOI] [PubMed] [Google Scholar]
- Sun W.L., Li X.Y., Dou H.Y., Wang X.D., Li J.D., Shen L., Ji H.F. Myricetin supplementation decreases hepatic lipid synthesis and inflammation by modulating gut microbiota. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109641. [DOI] [PubMed] [Google Scholar]
- Tang J., Wang L., Shi M., Feng S., Zhang T., Han H. Study on the mechanism of Shuganzhi tablet against nonalcoholic fatty liver disease and lipid regulation effects of its main substances in vitro. J. Ethnopharmacol. 2023;316 doi: 10.1016/j.jep.2023.116780. [DOI] [PubMed] [Google Scholar]
- Tang Y.H., Zhang Y.Y., Zhu H.Y., Huang C.G. A high-performance liquid chromatographic method for saikosaponin a quantification in rat plasma. Biomed. Chromatogr. 2007;21:458–462. doi: 10.1002/bmc.773. [DOI] [PubMed] [Google Scholar]
- Vallianou N., Christodoulatos G.S., Karampela I., Tsilingiris D., Magkos F., Stratigou T., Kounatidis D., Dalamaga M. Understanding the role of the gut microbiome and microbial metabolites in non-alcoholic fatty liver disease: current evidence and perspectives. Biomolecules. 2021;12:56. doi: 10.3390/biom12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Sheng F., Zou L., Xiao J., Li P. Hyperoside attenuates non-alcoholic fatty liver disease in rats via cholesterol metabolism and bile acid metabolism. J. Adv. Res. 2021;34:109–122. doi: 10.1016/j.jare.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.W., Wang J., Zhang H.J., Wu S.G. Supplemental clostridium butyricum modulates lipid metabolism through shaping gut microbiota and bile acid profile of aged laying hens. Front. Microbiol. 2020;11:600. doi: 10.3389/fmicb.2020.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.J., Lin Y.H., Chu C.C., Tsai Y.H., Chao J.C. Curcumin or saikosaponin a improves hepatic antioxidant capacity and protects against CCl4-induced liver injury in rats. J. Med. Food. 2008;11:224–229. doi: 10.1089/jmf.2007.555. [DOI] [PubMed] [Google Scholar]
- Wu M., Yang S., Wang S., Cao Y., Zhao R., Li X., Xing Y., Liu L. Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed apoe-/- mice. Front. Pharmacol. 2020;11:223. doi: 10.3389/fphar.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong F., Zheng Z., Xiao L., Su C., Chen J., Gu X., Tang J., Zhao Y., Luo H., Zha L. Soyasaponin A2 alleviates steatohepatitis possibly through regulating bile acids and gut microbiota in the methionine and choline-deficient (MCD) diet-induced nonalcoholic steatohepatitis (NASH) mice. Mol. Nutr. Food Res. 2021;65 doi: 10.1002/mnfr.202100067. [DOI] [PubMed] [Google Scholar]
- Yang B., Huang S., Zhao G., Ma Q. Dietary supplementation of porcine bile acids improves laying performance, serum lipid metabolism and cecal microbiota in late-phase laying hens. Anim. Nutr. 2022;11:283–292. doi: 10.1016/j.aninu.2022.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y., Zhou W., Yan X., Qiao Y., Guan L., Zhang Z., Liu H., Jiang J., Liu J., Peng L. Astragaloside IV ameliorates diet-induced hepatic steatosis in obese mice by inhibiting intestinal FXR via intestinal flora remodeling. Phytomedicine. 2022;107 doi: 10.1016/j.phymed.2022.154444. [DOI] [PubMed] [Google Scholar]
- Zhang X., Yun Y., Lai Z., Ji S., Yu G., Xie Z., Zhang H., Zhong X., Wang T., Zhang L. Supplemental Clostridium butyricum modulates lipid metabolism by reshaping the gut microbiota composition and bile acid profile in IUGR suckling piglets. J. Anim. Sci. Biotechnol. 2023;14:36. doi: 10.1186/s40104-023-00828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q., Li X., Huang N., Li F., Ge J., Wang D., Sun R., Liu R. Saikosaponins ameliorate hyperlipidemia in rats by enhancing hepatic lipid and cholesterol metabolism. J. Ethnopharmacol. 2023;305 doi: 10.1016/j.jep.2022.116110. [DOI] [PubMed] [Google Scholar]