Abstract
Pyroptosis is a programmed necrotic cell death executed by gasdermins, a family of pore-forming proteins. The cleavage of gasdermins by specific proteases enables their pore-forming activity. The activation of the prototype member of the gasdermin family, gasdermin D (GSDMD), is linked to innate immune monitoring by inflammasomes. Additional gasdermins such as GSDMA, GSDMB, GSDMC, and GSDME are activated by inflammasome-independent mechanisms. Pyroptosis is emerging as a key host defense strategy against pathogens. However, excessive pyroptosis causes cytokine storm and detrimental inflammation leading to tissue damage and organ dysfunction. Consequently, dysregulated pyroptotic responses contribute to the pathogenesis of various diseases, including sepsis, atherosclerosis, acute respiratory distress syndrome, and neurodegenerative disorders. This review will discuss the inflammatory consequences of pyroptosis and the mechanisms of pyroptosis-induced tissue damage and disease pathogenesis.
Introduction
Pyroptosis is a lytic form of programmed cell death executed by pore-forming proteins called gasdermins. The gasdermin family comprises GSDMA, GSDMB, GSDMC, GSDMD, GSDME, and GSDMF (PJVK/DFNB59)[1]. Gasdermins have a lipophilic N-terminal domain with an intrinsic pore-forming activity and a C-terminal domain connected via a linker. Under steady state, the C-terminal domain represses the pore-forming activity of the N-terminal domain. Specific proteases activated in response to distinct signals cleave the linker region separating the N- and C-terminal domains and relieving intramolecular inhibition. As a result, the N-terminal domain translocates to the plasma membrane and binds to the acidic phospholipids, such as phosphoinositides, of the cytoplasmic leaflet of the plasma membrane. Subsequently, the N-terminal domain oligomerizes and forms ring-shaped pores on the plasma membrane. The accumulation of gasdermin pores eventually leads to cell lysis.
Inflammasome-mediated pyroptosis
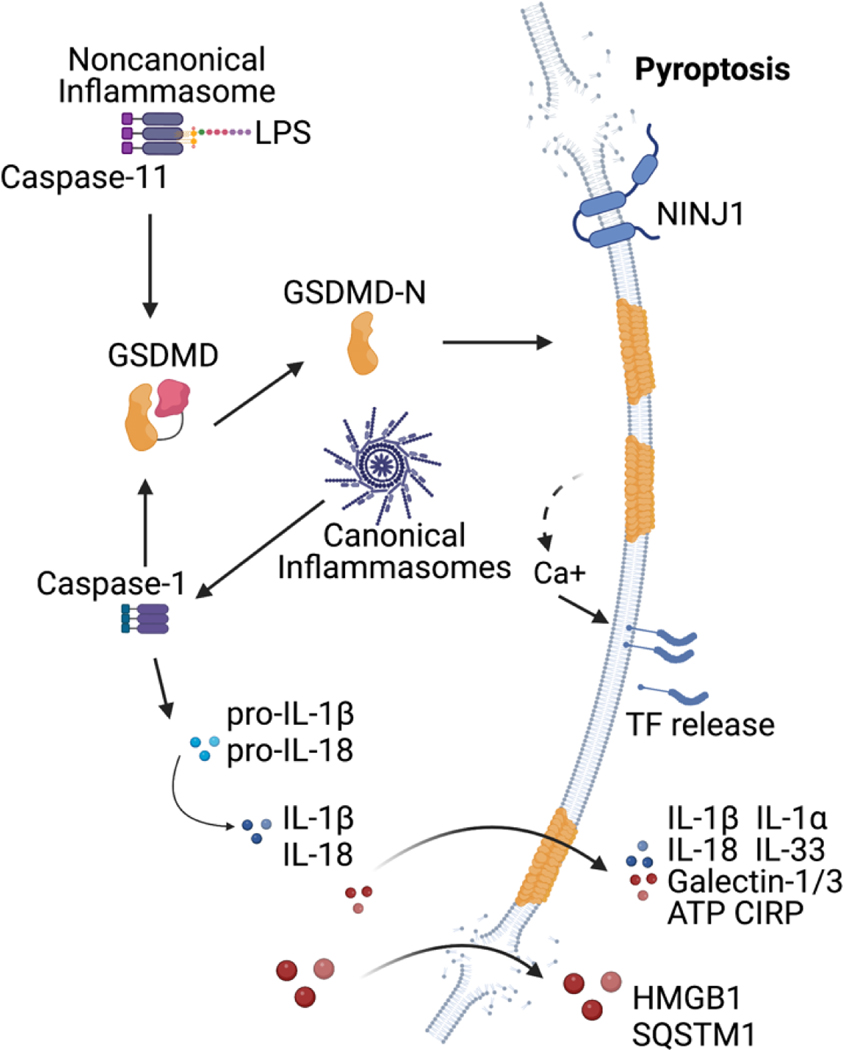
The pattern recognition receptors (PRRs) expressed by immune cells actively survey the extracellular and intracellular compartments for pathogen-associated molecular patterns (PAMPs), pathogen-induced cellular alterations, and damage-associated molecular patterns (DAMPs) [2]. These receptors can elicit an inflammatory response by upregulating proinflammatory cytokines and chemokines and homeostatic responses such as autophagy to remove PAMPs and eliminate damaged organelles. Additionally, specialized innate immune sensors called inflammasomes induce pyroptosis. Inflammasomes are multimolecular complexes consisting of a sensor [like NLR family pyrin domain containing 3 (NLRP3), Absent in Melanoma 2 (AIM2), NLR family CARD domain containing 4 (NLRC4), etc], an adaptor protein-apoptosis-associated speck-like protein containing a CARD (ASC), and an effector protease caspase-1. Inflammasome assembly in response to the cytosolic presence of microbial components or infection-induced cellular perturbations leads to the autoproteolysis of caspase-1 into its active form [3–5]. Caspase-1 mediates the maturation of proforms of IL-1β and IL-18. Importantly, GSDMD has been discovered as the substrate of caspase-1 [6,7]. GSDMD is also a substrate for related caspases, caspase-11 in rodents and caspase-4/5 in humans that function as sensors for gram-negative bacterial lipopolysaccharide (LPS) localized in the cytosol [8–11]. Caspase-1/11/4/5 cleavage of GSDMD liberates the N-terminal domain, which causes plasma membrane perforation (Figure 1) [12–15]. GSDMD pore formation leads to ion influx, osmotic swelling, and terminal cell lysis, the latter was shown to be mediated by a transmembrane protein ninjurin-1 [16]. GSDMD pore formation does not always lead to pyroptosis owing to the efforts of plasma membrane repair pathways. Calcium influx via GSDMD pores activates the translocation of the ESCRT-III complex to the plasma membrane, which removes GSDMD pores into ectosomes, repairing the membrane [17]. Furthermore, gasdermin pores have recently been shown to be repaired by acid sphingomyelinase (ASM) [18]. Following membrane pore formation, calcium influx-induced recruitment of ASM to the plasma membrane via lysosomal exocytosis generates ceramide from sphingomyelin. Ceramide then triggers the internalization of pores via endocytosis leading to membrane repair.
Figure 1. Inflammasome-mediated GSDMD activation and pyroptosis.
Caspase-1 activation by canonical inflammasome complexes or caspase-11-noncanonical inflammasome activation by cytosolic LPS initiates gasdermin-D (GSDMD) cleavage and membrane pore formation. K+ efflux through GSDMD pores also activates NLRP3 inflammasome and caspase-11, which cleaves pro-IL-1β and pro-IL-18 to active cytokines. Pro-inflammatory cytokines like IL-1β, IL-18 and smaller DMAPs/alarmins like ATP, IL-1α, and galectin-1 are released through GSDMD pores. Ca+ influx through GSDMD pores activates tissue factor (TF) and initiates the coagulation cascade. Cells finally undergoes pyroptotic lysis mediated by ninjurin-1 (NINJ-1). The terminal cell rupture facilitates release of larger DAMPs such as HMGB1, LDH and SQSTM1, which cannot pass through GSDMD pore.
Inflammasome-independent activation of gasdermins and pyroptosis
Inflammasome-independent mechanisms of pyroptosis have also been well-documented (Figure 2). While GSDMD is activated by inflammasome-activated caspases 1, 4, 5, and 11, other gasdermins such as GSDME, GSDMC, and GSDMB are activated by different proteases that are responsible for the inflammasome-independent execution of pyroptosis. Caspase-3 activated by supposedly apoptotic signals cleaves GSDME to execute pyroptosis [19]. For instance, chemotherapeutic drugs can activate caspase-3-GSDME cleavage in cancer cells and induce pyroptosis instead of apoptosis [19]. Lymphocytes can also elicit pyroptosis in cancer cells through granzyme B (GzmB)-mediated GSDME cleavage [20]. GzmB also activates caspase-3, amplifying pore formation and pyroptosis [21]. Granzyme A expressed by cytotoxic lymphocytes has been shown to cleave GSDMB leading to pyroptosis [22]. Metabolite dyshomeostasis can also trigger pyroptosis. α-ketoglutarate (α-KG), an essential TCA cycle metabolite, is reduced to L-2HG in an acidic environment resulting in increased ROS levels and oxidation and internalization of death receptor 6 (DR6). The DR6 receptosome formation recruits and activates caspase-8-mediated GSDMC cleavage [23]. Additionally, during helminth infection, STAT6 O-GlcNAcylation activates GSDMC transcription; however, the proteolytic enzyme required for GSDMC cleavage in this context is not clear. GSDMC plays important roles in anti-helminth type 2 immunity [24,25]. Interestingly, GSDMD can also be activated by other proteases. Inhibition of TAK1 by Yersinia YopJ activates RIPK1-caspase-8-mediated GSDMD cleavage and pyroptosis [26,27]. In aging neutrophils, lysosomal membrane permeabilization releases neutrophils elastase into cytosol leading to GSDMD activation and pyroptosis [28]. In addition to the cleavage of gasdermins by cellular proteases, the cysteine protease SpeB of group A Streptococcus cleaves GSDMA leading to pyroptosis in keratinocytes [29,30].
Figure 2. Inflammasome-independent activation of gasdermins and pyroptosis.
Inflammation-independent activation of GSDMA, GSDMB, GSDMC, and GSDME by SpeB, granzyme A, caspase-8, and caspase-3/granzyme B, respectively, also leads to plasma membrane perforation and pyroptosis.
Consequences of pyroptosis
The roles of gasdermin-mediated pyroptosis in various conditions like infection and inflammation, tumor suppression and cardiovascular diseases are emerging. Pyroptosis is emerging as a pivotal host defense strategy; cells dying via pyroptosis not only eliminate replication niches for intracellular bacteria but reportedly encapsulate them with cellular membranes in such a way that enhances their uptake and killing by newly recruited phagocytic cells such as neutrophils [31]. On the other hand, pyroptosis occurring widely without adequate regulation can cause tissue-damaging inflammation. Pyroptosis-induced inflammation has been implicated in many acute and chronic diseases like sepsis, IBD, atherosclerosis, and neurodegenerative diseases [32,33]. How pyroptosis is mediating inflammation and pathology is emerging from an extensive body of recent literature, which is reviewed here focusing on inflammasome-mediated pyroptosis (Figure 3).
Figure 3. Inflammatory and pathological consequences of pyroptosis.
GSDMD pore formation and pyroptosis lead to the release of proinflammatory cytokines, alarmins and DAMPs. These inflammatory molecules act on bystander cells and promote a proinflammatory response. Cell death and exacerbated inflammation can lead to disruption of endothelial barrier in blood vessels and vital organs such as lungs, resulting in leucocyte infiltration. GSDMD pore formation also activates the coagulation cascade and contributes to lethality due to disseminated intravascular coagulation. Pyroptosis in neutrophils and other cells triggers NETosis. Aberrant NETosis and improper NET and DAMP removal can further induce pyroptosis and tissue damage.
Tissue damage by pyroptosis-induced blood clotting
Despite having an important protective role in microbial infections, over-activation of the inflammasome-dependent pyroptotic pathway is known to cause extensive tissue damage via death of host cells [34,35]. In fact, pyroptotic cell death is one of the primary contributors of septic shock in the setting of Gram-negative bacterial infections. Until recently, the mechanisms underlying pyroptosis-induced-lethality in septicemia has remained elusive. Emerging evidence has revealed that extensive pyroptosis leads to the excessive release of tissue factor (TF), that initiates a cascade of blood clotting events or coagulation resulting in eventual death. TF is an well-studied transmembrane glycoprotein that functions as the primary initiator of the coagulation cascade during injury [36]. However, TF has also been implicated in the development of disseminated intravascular coagulation (DIC), a life-threatening blood clotting complication in experimental endotoxemia and in patients with sepsis [37,38].
It has been demonstrated that pyroptosis downstream of canonical or non-canonical inflammasome activation by E. coli T3SS rod protein EprJ or LPS, respectively, is at the epicenter of a dysregulated coagulation cascade exacerbated by TF release [39]. The causative role of pyroptosis in coagulation was confirmed, as mice deficient in GSDMD, but not IL-1β or IL-18 receptors, were protected against inflammasome-induced coagulation and lethality. In keeping with role of caspase-1 and caspase-11 in inflammasome activation and pyroptosis, mice lacking these caspases were also resistant to E. coli-induced coagulation. Furthermore, pyroptosis primarily occurring in macrophages and monocytes contributed to coagulation and death, as macrophage and monocyte depletion protected mice from coagulation and lethality. In fact, pyroptotic macrophages were the main source of TF released into the circulatory system of mice undergoing canonical or non-canonical inflammasome activation. Interestingly, TF from pyroptotic macrophages and monocytes was released via microvesicles triggering DIC and lethality [37]. Genetic deficiency or inhibition of TF using a blocking monoclonal antibody diminished inflammasome-induced coagulation and death.
Another study found that LPS activated the coagulation cascade in mice in a caspase-11-and GSDMD-dependent manner [40]. Activation of the non-canonical inflammasome pathway upon cytosolic LPS exposure licensed GSDMD to form pores in cellular membranes that led to an influx of calcium (Ca2+) ions. The GSDMD-mediated Ca2+ influx resulted in the activation of transmembrane protein 16F (TMEM16F), a calcium-dependent phospholipid scramblase, which further led to the exposure of phosphatidylserine (PS), an essential membrane phospholipid, on the cell surface. PS flip to the outer lipid bilayer in turn increased the pro-coagulant activity of TF to trigger DIC. Interestingly, while PS exposure and TF activation required GSDMD activity, they occurred independent of pyroptotic membrane rupture. This suggests that GSDMD activation and plasma membrane pores in cells in a hyperactive state not only promote IL-1 cytokine release but may persistently trigger coagulation cascade [41,42]. The involvement of the GSDMD-PS-TF signaling in coagulation was also evident in clinically relevant models of sepsis including intraperitoneal E. coli infection and cecum ligation and puncture (CLP) [43]. Furthermore, blood plasma analysis revealed that biomarkers of GSDMD activation such as IL-1α, IL-1β and PS exposure, but not global inflammation markers such as IL-6 and C-reactive protein (CRP), significantly correlated with the development and severity of DIC in septic patients, thereby implicating the importance of these findings in the clinical setting.
More recently [44], it was shown that transmembrane protein 173 (TMEM173) or STING, an endoplasmic reticulum (ER)-resident protein, mostly known for its critical role in the cGAS-DNA sensing pathway [45], is also essential for driving immunocoagulation events during bacterial sepsis. Importantly, the dysregulated coagulation driven by STING was independent of the classical cGAS-STING pathway [46,47] as cGAS−/− mice were not protected from systemic coagulation in a CLP model of polymicrobial sepsis. STING also promoted the release of TF in the circulation during sepsis. Furthermore, STING expressed in the myeloid compartment, but not T cells, was responsible for triggering DIC in mice. Mechanistically, the release of TF and coagulation driven by STING required ER stress-mediated calcium influx into the cytosol. This intracellular calcium signaling was also needed for GSDMD cleavage into its active N-terminal fragment, an event that was necessary to drive TF release and lethal coagulation in sepsis, downstream of STING. In vitro and in vivo experiments utilizing E. coli and S. pneumoniae confirmed that STING-dependent systemic coagulation is active in Gram-negative as well as Gram-positive bacterial infections. Clinical analyses revealed that the STING-GSDMD axis was also active in the peripheral blood mononuclear cells (PMBCs) of septic patients with DIC. Taken together, these studies establish GSDMD-dependent pyroptosis as a key signaling event that connects inflammation and blood clotting during infections and pyroptosis-driven DIC as a critical pathogenic mechanism during sepsis [48].
Pyroptosis-induced loss of vital cells
A key mechanism by which pyroptosis contributes to undesirable outcomes is the loss of critical cell types themselves and consequently their indispensable functions. For instance, an intact endothelial lining of the vasculature is vital for cardiovascular function, oxygen exchange, and tissue health [49,50]. In endotoxemia-induced acute lung injury (ALI), caspase-11 activation mediates pyroptosis in lung endothelial cells [34]. Endothelial cell pyroptosis disrupts the endothelial barrier leading to neutrophil infiltration, lung edema, and release of proinflammatory cytokines, all characteristic features of ALI [51,52]. Conditional deletion of caspase-11 in endothelial cells protected against LPS-induced ALI [34]. Similarly, GSDMD-mediated cytokine release and pyroptosis in vascular endothelial cells play a role in the progression of atherosclerotic plaques. Vascular endothelial cell death and excessive inflammation can disrupt the vascular membrane, leading to T cell and monocyte infiltration [32]. Moreover, inhibiting caspase-1 activation in endothelial cells improved angiogenesis and ischemia progression [53]. Furthermore, excessive GSDMD-mediated pyroptosis in macrophages and monocytes during can be detrimental during sepsis. Intriguingly, caspase-11 activation in intestinal epithelial cells (IEC) also contributes to LPS-driven lethality [50]. Chemotherapeutic drugs can induce pyroptosis in GSDME-expressing cancer cells. However, as GSDME is also expressed in healthy cells, chemotherapy also leads to pyroptosis and loss of vital cells in the gastrointestinal and respiratory tracts and the spleen contributing to chemotherapy-induced toxicity [19].
Inflammation and tissue damage due to pyroptotic cytokines and DAMPs
The release of inflammatory cytokines, alarmins, and DAMPs is a characteristic feature of pyroptosis. These cytokines and DAMPs lack the signaling peptide required for conventional secretion via the ER-golgi network and are released through GSDMD pores or ruptured membrane [54,55]. Although these molecules have a physiological function inside the cell, once outside, they act as a marker of cell damage. PRRs detect extracellular DAMPs and activate several immune pathways leading to the recruitment of immune cells and induction and/or amplification of inflammatory responses.
ATP.
The release of essential cellular components like ATP is tightly regulated but can passively occur when cells undergo stress, injury, and plasma membrane permeabilization membrane, like during pyroptosis. Extracellular ATP can act as a DAMP in an autocrine or paracrine manner and bind to the purinergic receptor P2X7R [56]. As extracellular ATP can promote K+ efflux via P2X7R in macrophages, the ATP-P2X7R signaling activates the NLRP3 inflammasome [57,58]. Importantly, removing extracellular ATP using apyrase significantly reduced cellar disintegration, mitochondrial damage, cytokine storm, and mortality in an LPS-induced inflammation model [59].
CIRP.
Macrophages release a nuclear RNA chaperone, cold-inducible RNA binding protein (CIRP), via caspase-11-mediated GSDMD pores, which act as a DAMP during sepsis [60]. Extracellular CIRP (eCIRP) signals through TLR4/MyD88 and activates NF-ΚB inducing proinflammatory cytokines and chemokines in myeloid and lymphoid cells and amplifying the inflammatory response. In addition, eCIRP can upregulate T cell activation markers such as CD69 and CD25 as well as ICAM-1 on neutrophils leading to increased NETosis. Importantly, eCIRP can induce NLRP3 inflammasome activation and pyroptosis in endothelial cells. Collectively, eCIRP-induced inflammation and endothelial barrier damage are detrimental to sepsis-induced ALI [61].
IL-1 alarmins.
The interleukin-1 family of cytokines plays important roles in inflammation and immunity. IL-1β and IL-18 are translated in an inactive form and require cleavage by caspase-1 to become bioactive. Caspase-4 but not its murine counterpart caspase-11 can also cleave IL-18 [62]. The mature IL-1β and IL-18 are released through GSDMD pores. Other members of the IL-1 family, IL-1α and IL-33, are also released during pyroptosis and act as a DAMP [63]. Human sepsis patients have increased circulating IL-1β [64]. IL-1 and TNF can act in synergy and induce tissue damage, exacerbating the severity of sepsis [65]. IL-1α and IL-1β signal through IL-1R, whereas IL-18 binds to IL-18R, both pathways activating NF-kB via MyD88 and enhancing local inflammation [66]. IL-18 can also regulate T cell and NK cell responses by upregulating IFN-γ production. Elevated IL-18 and IFN-γ are associated with autoimmune diseases like rheumatoid arthritis, type 1 diabetes milieu, psoriasis, Crohn’s disease, and systemic lupus erythematosus [67]. IL-1α and IL-33 have implications in IBD and experimental colitis. IL-33 can limit Th17 responses required to clear the infection and disrupt the intestinal barrier in experimental infectious colitis, enhancing gut permeability [68]. It has recently been shown that GSDMC activation in the intestine during helminth infections leads to the release of IL-33. IL-33 released via GSDMC pores promotes intestinal inflammation and anti-helminth immunity [69]
HMGB1.
HMGB1 is another critical DAMP released by pyroptotic and necrotic cells which can cause tissue damage and organ dysfunction. HMGB1 signaling through TLR4 and RAGE activates NF-kB. However, it can also bind other proinflammatory molecules like DNA, histones, IL-1α, IL-1β etc. and act in synergy [70]. Notably, HMGB1 released by hepatocytes can activate caspase-11 and initiate pyroptosis by delivering LPS to the cytosol through the HMGB1 receptor, RAGE [71]. Moreover, HMGB1-mediated pyroptosis of hepatic macrophages can exacerbate acute liver injury in sepsis [72]. Hence, inhibiting HMGB1 activity by antagonists provides protection in murine sepsis models [73]. Furthermore, HMGB1 increases enterocyte permeability and impairs intestinal barrier function in mice [74]. In a mouse model of colitis, GSDME-dependent pyroptosis in intestinal epithelial cells promoted intestinal inflammation via HMGB1 release [75].
Galectin-1.
Galectin-1 is a member of the β-galactoside binding protein family, which modulates immune response by binding to glycans on membrane glycoproteins. Galectin-1 has been shown to be released during programmed necrotic cell death including pyroptosis and necroptosis [76].
Importantly, extracellular galectin-1 functions as a DAMP by limiting the anti-inflammatory activity of CD45. Furthermore, genetic deficiency of galectin-1 or its antibody-mediated neutralization attenuates inflammation, organ damage, and death during sepsis. Galectin-1 levels were elevated in human sepsis patient serum [77], and more importantly, higher mortality rate was observed in patients with high levels of galectin-1 [78]. Furthermore, serum galectin-1 levels correlated with disease severity in COVID-19 patients and has been proposed as a significant predictor for COVID-19 severity [79]. These reports suggest that galectin-1 can be a biomarker to stratify sepsis and COVID-19 patients. Another related galectin, galectin-3, has also been shown to be released by cells undergoing pyroptosis [80,81].
SQSTM1.
Sequestosome 1 (SQSTM1/p62) is an autophagy receptor that can activate NF-KB via TRAF-6. SQSTM1 is actively secreted upon the activation of TLR4 signaling and can also be released via caspase-11-GSDMD pores by macrophages and monocytes [82]. Extracellular SQSTM1 acts as a DAMP and modulates inflammatory response during sepsis. Extracellular SQSTM1 signals through insulin receptor (INSR), activates NF-ΚB, and promotes metabolic reprogramming and polarization of macrophages to an M1 phenotype. This macrophage polarization promotes inflammation and coagulation during septic shock in mice. Moreover, circulating SQSTM1 is also associated with sepsis severity.
Pyroptosis and cytokine storm
Cytokine storm is a life-threatening hyperinflammatory condition characterized by elevated levels of cytokines and other inflammatory molecules in the bloodstream [83]. Cytokine storm occurring during systemic bacterial, viral, and fungal infections and sepsis are the result of an over-activation of immune and non-immune cells that release excessive amounts of proinflammatory cytokines and chemokines, which in turn recruit additional cytokine-secreting immune cells, resulting in a vicious cycle of exacerbated inflammation. Such uncontrolled inflammation can lead to complications including tissue damage, multiorgan failure, and death. Given that pyroptosis causes the release of proinflammatory cytokines (IL-1β and IL-18) and DAMPs in response to diverse microbial infections, it appears to have a crucial involvement in the induction and aggravation of the cytokine storm during infectious insults. Indeed, multiple lines of evidence suggest that excessive pyroptosis occurring in innate immune cells is likely a key mechanism underlying the development and reinforcement of the cytokine storm [76,84,85]. Mechanistically, DAMPs, IL-1β, and IL-18 released upon pyroptosis trigger the expression of additional proinflammatory cytokines such as IL-6, IL-8, and TNF [76,85–87]. Uncontrolled activation of these pathways during infection can lead to a disproportionate release of these cytokines and consequently result in a cytokine storm.
Interestingly, pyroptosis has recently received much attention for its potential role in the cytokine storm that develops during SARS-CoV-2 infection [88–90]. Viral proteins encoded by SARS-CoV-2, such as the nucleocapsid (N) protein, non-structural protein 6 (NSP6), viroporin ORF3a, envelop (E) protein, and spike (S) protein have all been implicated in the COVID-19-mediated hyperinflammation via engagement of the NLRP3 inflammasome and subsequent pyroptosis [91–95]. Furthermore, the pyroptosis-driven release of LDH, IL-1β, and IL-18 has been implicated in massive inflammation in severe and fatal cases of COVID-19 disease [89]. These studies highlight the significance of the pyroptotic pathway in causing the cytokine storm and promoting a hyperinflammatory state during infections.
Tissue damage by NET formation
Activated neutrophils release neutrophil extracellular traps (NETs) in a process called NETosis. Aberrant NETosis can be detrimental, and a crucial role for NETs in metabolic, autoimmune, and chronic inflammatory diseases is emerging. The NET formation is characterized by the release of chromatin and histones along with antimicrobial granular proteins like myeloperoxidase, neutrophil elastase, and cathepsins [96,97]. GSDMD activation in neutrophils by caspase-11 and other proteases leads to NET formation [98,99]. A recent report suggests that NLRC4-caspase-1 activation during Pseudomonas aeruginosa infection can induce neutrophil pyroptosis and incomplete NETosis [100]. Moreover, pyroptosis in platelets during severe sepsis also triggers NET formation [101].
Neutrophilic contents like histones and mitochondrial DNA released during NETosis act as DAMPs. NET-derived DAMPs activate bystander cells and amplify the inflammatory response. For example, extracellular histone can induce CXCL9 and CXCL10 release from monocytes. Arginine-rich histones can also upregulate adhesion markers on endothelial cells to increase leucocyte migration [102]. Furthermore, histones can activate the NLRP3 inflammasome in dendritic cells and result in IL-1β secretion [103]. Macrophages generally remove NETs; however, delayed phagocytosis and degradation can induce alveolar macrophage pyroptosis in the LPS-induced ALI model [104]. Mechanistically, NET and caspase-1 levels correlate with disease severity and mortality in acute respiratory distress syndrome patients [104]. Another study has shown that NET-derived DAMPs like HMGB1 can induce RAGE-mediated caspase-1 activation and pyroptosis in macrophages during sepsis [105]. Endothelial cells can induce NETs during sepsis and other chronic inflammatory diseases, resulting in vascular damage. [97]. It is evident from all these reports that apart from its role in bacterial killing and clearance, NETs propagate inflammation and tissues damage through NET-associated DAMPs such as histones and DNA.
Therapeutic targeting of pyroptosis-induced inflammation
The involvement of pyroptosis in inflammation and tissue damage raises the possibility of targeting it for therapeutic intervention in numerous diseases including sepsis. In particular, gasdermins, being the final executioner in pyroptosis, has the potential to be exploited for targeted therapy as it has been implicated in the pathogenesis of a host of infectious [12,106–109] as well as non-infectious inflammatory conditions [110–117]. Moreover, GSDMD pore formation is a common culmination of both the canonical as well as the non-canonical inflammasome pathways [14] making it an even more appealing target for controlling inflammasome-driven inflammation. In this regard, several GSDMD inhibitors have been reported in the literature [98,106,111,118–120]. In fact, an FDA-approved drug for alcoholism [121], disulfiram, was recently identified as a direct and potent inhibitor of pyroptosis [122]. Mechanistically, disulfiram inhibits GSDMD pore formation by covalently and selectively modifying the Cys191 residue of human GSDMD [123], thereby suppressing GSDMD pore formation and the subsequent inflammatory effects. More importantly, disulfiram protected mice from LPS-induced sepsis [122,123] and experimental autoimmune encephalomyelitis (EAE) [111]. While these reports highlight the protective effects of pyroptosis inhibition in mouse models of infectious and inflammatory disease, whether these findings translate to therapeutic benefits in patients remains to be tested and warrants clinical investigation.
Conclusion and perspective
Inflammasome signaling and additional pathways induce gasdermin-mediated pyroptosis. Extensive research over the years demonstrated the molecular underpinnings of pyroptosis. Pyroptosis is an immunostimulatory cell death exerting local and systemic effects through the release of inflammatory mediators and the recruitment of immune cells. Depending upon the magnitude and the context of the activation, pyroptosis can be a beneficial or harmful host response. Aberrant pyroptosis is long-known to be detrimental to the host and the underlying mechanisms include loss of vital cell types, coagulopathy, and DAMP-driven uncontrolled inflammation. Still, our understanding of pyroptosis-mediated pathophysiological effects is limited. Particularly, whether and how host initiates tissue repair responses following pyroptosis remains largely unknown. A comprehensive understanding of pyroptosis-mediated effects shaping disease outcomes can pave the way for the development of better therapeutics.
Highlights.
Pyroptosis is a programmed necrotic cell death executed by gasdermins.
Pyroptosis is emerging as a key host defense strategy against pathogens.
Excessive pyroptosis causes cytokine storm and detrimental inflammation.
Acknowledgements
The authors apologize to those investigators whose original papers could not be cited because of the space limitation. The Rathinam laboratory is supported by the US National Institutes of Health (R01AI119015 and R01AI148491).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Broz P, Pelegrín P, Shao F, The gasdermins, a protein family executing cell death and inflammation, Nat Rev Immunol. (2019) 1–15. 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- [2].Kawai T, Akira S, The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors, Nat Immunol. 11 (2010) 373–384. 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- [3].Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A, Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria, Nat Immunol. 11 (2010) 1136–1142. 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rathinam VAK, Fitzgerald KA, Inflammasome Complexes: Emerging Mechanisms and Effector Functions, Cell. 165 (2016) 792–800. 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Broz P, Dixit VM, Inflammasomes: mechanism of assembly, regulation and signalling, Nat Rev Immunol. 16 (2016) 407–420. 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- [6].Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F, Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death, Nature. 526 (2015) 660–665. 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- [7].Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM, Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling, Nature. 526 (2015) 666–671. 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- [8].Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F, Inflammatory caspases are innate immune receptors for intracellular LPS, Nature. 514 (2014) 187–192. 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- [9].Kayagaki N, Warming S, Lamkanfi M, Walle LV, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM, Non-canonical inflammasome activation targets caspase-11, Nature. 479 (2011) 117–121. 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- [10].Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA, Cytoplasmic LPS Activates Caspase-11: Implications in TLR4-Independent Endotoxic Shock, Science. 341 (2013) 1250–1253. 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszyński A, Forsberg LS, Carlson RW, Dixit VM, Noncanonical Inflammasome Activation by Intracellular LPS Independent of TLR4, Science. 341 (2013) 1246–1249. 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- [12].Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM, Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling, Nature. 526 (2015) 666–671. 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- [13].He W, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang Z-H, Zhong C-Q, Han J, Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion, Cell Res. 25 (2015) 1285–1298. 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F, Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death, Nature. 526 (2015) 660–665. 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- [15].Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J, Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores, Nature. 535 (2016) 153–158. 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kayagaki N, Kornfeld OS, Lee BL, Stowe IB, O’Rourke K, Li Q, Sandoval W, Yan D, Kang J, Xu M, Zhang J, Lee WP, McKenzie BS, Ulas G, Payandeh J, Roose-Girma M, Modrusan Z, Reja R, Sagolla M, Webster JD, Cho V, Andrews TD, Morris LX, Miosge LA, Goodnow CC, Bertram EM, Dixit VM, NINJ1 mediates plasma membrane rupture during lytic cell death, Nature. 591 (2021) 131–136. 10.1038/s41586-021-03218-7. [DOI] [PubMed] [Google Scholar]
- [17].Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P, ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation, Science. 362 (2018) 956–960. 10.1126/science.aar7607. [DOI] [PubMed] [Google Scholar]
- [18].Nozaki K, Maltez VI, Rayamajhi M, Tubbs AL, Mitchell JE, Lacey CA, Harvest CK, Li L, Nash WT, Larson HN, McGlaughon BD, Moorman NJ, Brown MG, Whitmire JK, Miao EA, Caspase-7 activates ASM to repair gasdermin and perforin pores, Nature. 606 (2022) 960–967. 10.1038/s41586-022-04825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F, Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin, Nature. 547 (2017) 99–103. 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- [20].Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, Junqueira C, Meza-Sosa KF, Mok TMY, Ansara J, Sengupta S, Yao Y, Wu H, Lieberman J, Gasdermin E suppresses tumour growth by activating anti-tumour immunity., Nature. 579 (2020) 415–420. 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tan Y, Chen Q, Li X, Zeng Z, Xiong W, Li G, Li X, Yang J, Xiang B, Yi M, Pyroptosis: a new paradigm of cell death for fighting against cancer, J Exp Clin Canc Res. 40 (2021) 153. 10.1186/s13046-021-01959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, Wang Y, Li D, Liu W, Zhang Y, Shen L, Han W, Shen L, Ding J, Shao F, Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells, Science. 368 (2020). 10.1126/science.aaz7548. [DOI] [PubMed] [Google Scholar]
- [23].Zhang J, Zhou B, Sun R, Ai Y, Cheng K, Li F, Wang B, Liu F, Jiang Z, Wang W, Zhou D, Chen H, Wu Q, The metabolite α-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8, Cell Res. 31 (2021) 980–997. 10.1038/s41422-021-00506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhao M, Ren K, Xiong X, Xin Y, Zou Y, Maynard JC, Kim A, Battist AP, Koneripalli N, Wang Y, Chen Q, Xin R, Yang C, Huang R, Yu J, Huang Z, Zhang Z, Wang H, Wang D, Xiao Y, Salgado OC, Jarjour NN, Hogquist KA, Revelo XS, Burlingame AL, Gao X, von Moltke J, Lin Z, Ruan H-B, Epithelial STAT6 O-GlcNAcylation drives a concerted anti-helminth alarmin response dependent on tuft cell hyperplasia and Gasdermin C, Immunity. 55 (2022) 623–638.e5. 10.1016/j.immuni.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xi R, Montague J, Lin X, Lu C, Lei W, Tanaka K, Zhang YV, Xu X, Zheng X, Zhou X, Urban JF, Iwatsuki K, Margolskee RF, Matsumoto I, Tizzano M, Li J, Jiang P, Up-regulation of gasdermin C in mouse small intestine is associated with lytic cell death in enterocytes in worm-induced type 2 immunity, Proc National Acad Sci. 118 (2021) e2026307118. 10.1073/pnas.2026307118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA, Berger SB, Gough PJ, Bertin J, Proulx MM, Goguen JD, Kayagaki N, Fitzgerald KA, Lien E, Pathogen blockade of TAK1 triggers caspase-8–dependent cleavage of gasdermin D and cell death, Science. 362 (2018) 1064–1069. 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR, Poltorak A, Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection, Proc National Acad Sci. 115 (2018) E10888–E10897. 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kambara H, Liu F, Zhang X, Liu P, Bajrami B, Teng Y, Zhao L, Zhou S, Yu H, Zhou W, Silberstein LE, Cheng T, Han M, Xu Y, Luo HR, Gasdermin D Exerts Anti-inflammatory Effects by Promoting Neutrophil Death., Cell Reports. 22 (2018) 2924 2936. 10.1016/j.celrep.2018.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].LaRock DL, Johnson AF, Wilde S, Sands JS, Monteiro MP, LaRock CN, Group A Streptococcus induces GSDMA-dependent pyroptosis in keratinocytes, Nature. 605 (2022) 527–531. 10.1038/s41586-022-04717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Deng W, Bai Y, Deng F, Pan Y, Mei S, Zheng Z, Min R, Wu Z, Li W, Miao R, Zhang Z, Kupper TS, Lieberman J, Liu X, Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis, Nature. (2022) 1–7. 10.1038/s41586-021-04384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jorgensen I, Zhang Y, Krantz BA, Miao EA, Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis, J Exp Med. 213 (2016) 2113–2128. 10.1084/jem.20151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang Z, Shi J, Chen L, Fu C, Shi D, Qu H, Role of Pyroptosis and Ferroptosis in the Progression of Atherosclerotic Plaques, Frontiers Cell Dev Biology. 10 (2022) 811196. 10.3389/fcell.2022.811196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang S, Liang Y, Yao J, Li D, Wang L, Role of Pyroptosis in Inflammatory Bowel Disease (IBD): From Gasdermins to DAMPs, Front Pharmacol. 13 (2022) 833588. 10.3389/fphar.2022.833588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cheng KT, Xiong S, Ye Z, Hong Z, Di A, Tsang KM, Gao X, An S, Mittal M, Vogel SM, Miao EA, Rehman J, Malik AB, Caspase-11–mediated endothelial pyroptosis underlies endotoxemia-induced lung injury, J Clin Invest. 127 (2017) 4124–4135. 10.1172/jci94495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G, Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock, Immunity. 43 (2015) 923–932. 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gando S, Levi M, Toh C-H, Disseminated intravascular coagulation, Nat Rev Dis Primers. 2 (2016) 16037. 10.1038/nrdp.2016.37. [DOI] [PubMed] [Google Scholar]
- [37].Grover SP, Mackman N, Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis., Arteriosclerosis Thrombosis Vasc Biology. 38 (2017) 709–725. 10.1161/atvbaha.117.309846. [DOI] [PubMed] [Google Scholar]
- [38].Gando S, Saitoh D, Ogura H, Fujishima S, Mayumi T, Araki T, Ikeda H, Kotani J, Kushimoto S, Miki Y, Shiraishi S, Suzuki K, Suzuki Y, Takeyama N, Takuma K, Tsuruta R, Yamaguchi Y, Yamashita N, Aikawa N, for A JA.M.S.R.S. Group, A multicenter, prospective validation study of the Japanese Association for Acute Medicine disseminated intravascular coagulation scoring system in patients with severe sepsis, Crit Care. 17 (2013) R111. 10.1186/cc12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wu C, Lu W, Zhang Y, Zhang G, Shi X, Hisada Y, Grover SP, Zhang X, Li L, Xiang B, Shi J, Li X-A, Daugherty A, Smyth SS, Kirchhofer D, Shiroishi T, Shao F, Mackman N, Wei Y, Li Z, Inflammasome Activation Triggers Blood Clotting and Host Death through Pyroptosis, Immunity. 50 (2019) 1401–1411.e4. 10.1016/j.immuni.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang X, Cheng X, Tang Y, Qiu X, Wang Y, Kang H, Wu J, Wang Z, Liu Y, Chen F, Xiao X, Mackman N, Billiar TR, Han J, Lu B, Bacterial Endotoxin Activates the Coagulation Cascade through Gasdermin D-Dependent Phosphatidylserine Exposure, Immunity. 51 (2019) 983–996.e6. 10.1016/j.immuni.2019.11.005. [DOI] [PubMed] [Google Scholar]
- [41].Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC, The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages, Immunity. 48 (2018) 35–44.e6. 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zanoni I, Tan Y, Gioia MD, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR, Kagan JC, An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells, Science. 352 (2016) 1232–1236. 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lewis AJ, Seymour CW, Rosengart MR, Current Murine Models of Sepsis, Surg Infect. 17 (2016) 385–393. 10.1089/sur.2016.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang H, Zeng L, Xie M, Liu J, Zhou B, Wu R, Cao L, Kroemer G, Wang H, Billiar TR, Zeh HJ, Kang R, Jiang J, Yu Y, Tang D, TMEM173 Drives Lethal Coagulation in Sepsis, Cell Host Microbe. 27 (2020) 556–570.e6. 10.1016/j.chom.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Burdette DL, Vance RE, STING and the innate immune response to nucleic acids in the cytosol, Nat Immunol. 14 (2013) 19–26. 10.1038/ni.2491. [DOI] [PubMed] [Google Scholar]
- [46].Ishikawa H, Barber GN, STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling, Nature. 455 (2008) 674–678. 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Motwani M, Pesiridis S, Fitzgerald KA, DNA sensing by the cGAS–STING pathway in health and disease, Nat Rev Genet. 20 (2019) 657–674. 10.1038/s41576-019-0151-1. [DOI] [PubMed] [Google Scholar]
- [48].Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent J-L, Sepsis and septic shock, Nat Rev Dis Primers. 2 (2016) 16045. 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kovacs SB, Oh C, Maltez VI, McGlaughon BD, Verma A, Miao EA, Aachoui Y, Neutrophil Caspase-11 Is Essential to Defend against a Cytosol-Invasive Bacterium, Cell Reports. 32 (2020) 107967. 10.1016/j.celrep.2020.107967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kumari P, Russo AJ, Wright SS, Muthupalani S, Rathinam VA, Hierarchical cell-type-specific functions of caspase-11 in LPS shock and antibacterial host defense, Cell Reports. 35 (2021) 109012. 10.1016/j.celrep.2021.109012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Aird WC, The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome, Blood. 101 (2003) 3765–3777. 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- [52].Andonegui G, Bonder CS, Green F, Mullaly SC, Zbytnuik L, Raharjo E, Kubes P, Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs, J Clin Invest. 112 (2003) 1264–1264. 10.1172/jci16510c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lopez-Pastrana J, Ferrer LM, Li Y-F, Xiong X, Xi H, Cueto R, Nelson J, Sha X, Li X, Cannella AL, Imoukhuede PI, Qin X, Choi ET, Wang H, Yang X-F, Inhibition of Caspase-1 Activation in Endothelial Cells Improves Angiogenesis, J Biol Chem. 290 (2015) 17485–17494. 10.1074/jbc.m115.641191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nickel W, Rabouille C, Mechanisms of regulated unconventional protein secretion, Nat Rev Mol Cell Bio. 10 (2009) 148–155. 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- [55].Dimou E, Nickel W, Unconventional mechanisms of eukaryotic protein secretion, Curr Biol. 28 (2018) R406–R410. 10.1016/j.cub.2017.11.074. [DOI] [PubMed] [Google Scholar]
- [56].Junger WG, Immune cell regulation by autocrine purinergic signalling, Nat Rev Immunol. 11 (2011) 201–212. 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Steinberg TH, Silverstein SC, Extracellular ATP4-promotes cation fluxes in the J774 mouse macrophage cell line., J Biol Chem. 262 (1987) 3118–3122. 10.1016/s0021-9258(18)61477-2. [DOI] [PubMed] [Google Scholar]
- [58].Ferrari D, Chiozzi P, Falzoni S, Hanau S, Virgilio FD, Purinergic Modulation of Interleukin-1β Release from Microglial Cells Stimulated with Bacterial Endotoxin, J Exp Medicine. 185 (1997) 579–582. 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cauwels A, Rogge E, Vandendriessche B, Shiva S, Brouckaert P, Extracellular ATP drives systemic inflammation, tissue damage and mortality, Cell Death Dis. 5 (2014) e1102. 10.1038/cddis.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tan C, Reilly B, Jha A, Murao A, Lee Y, Brenner M, Aziz M, Wang P, Active Release of eCIRP via Gasdermin D Channels to Induce Inflammation in Sepsis., J Immunol Baltim Md 1950. 208 (2021) 2184–2195. 10.4049/jimmunol.2101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Aziz M, Brenner M, Wang P, Extracellular CIRP (eCIRP) and inflammation, J Leukocyte Biol. 106 (2019) 133–146. 10.1002/jlb.3mir1118-443r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Knodler LA, Crowley SM, Sham HP, Yang H, Wrande M, Ma C, Ernst RK, Steele-Mortimer O, Celli J, Vallance BA, Noncanonical Inflammasome Activation of Caspase-4/Caspase-11 Mediates Epithelial Defenses against Enteric Bacterial Pathogens, Cell Host Microbe. 16 (2014) 249–256. 10.1016/j.chom.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Man SM, Karki R, Kanneganti T, Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases, Immunol Rev. 277 (2017) 61–75. 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cannon JG, Tompkins RG, Gelfand JA, Michie HR, Stanford GG, van der Meer JWM, Endres S, Lonnemann G, Corsetti J, Chernow B, Wilmore DW, Wolff SM, Burke JF, Dinarello CA, Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever., J Infect Dis. 161 (1990) 79–84. 10.1093/infdis/161.1.79. [DOI] [PubMed] [Google Scholar]
- [65].Movat HZ, Burrowes CE, Cybulsky MI, Dinarello CA, Acute inflammation and a Shwartzman-like reaction induced by interleukin-1 and tumor necrosis factor. Synergistic action of the cytokines in the induction of inflammation and microvascular injury., Am J Pathology. 129 (1987) 463–76. [PMC free article] [PubMed] [Google Scholar]
- [66].Brikos C, Wait R, Begum S, O’Neill LAJ, Saklatvala J, Mass Spectrometric Analysis of the Endogenous Type I Interleukin-1 (IL-1) Receptor Signaling Complex Formed after IL-1 Binding Identifies IL-1RAcP, MyD88, and IRAK-4 as the Stable Components*, Mol Cell Proteomics. 6 (2007) 1551–1559. 10.1074/mcp.m600455-mcp200. [DOI] [PubMed] [Google Scholar]
- [67].Nakamura S, Otani T, Ijiri Y, Motoda R, Kurimoto M, Orita K, IFN-γ-Dependent and -Independent Mechanisms in Adverse Effects Caused by Concomitant Administration of IL-18 and IL-12, J Immunol. 164 (2000) 3330–3336. 10.4049/jimmunol.164.6.3330. [DOI] [PubMed] [Google Scholar]
- [68].Palmieri V, Ebel J-F, Phuong NNT, Klopfleisch R, Vu VP, Adamczyk A, Zöller J, Riedel C, Buer J, Krebs P, Hansen W, Pastille E, Westendorf AM, Interleukin-33 signaling exacerbates experimental infectious colitis by enhancing gut permeability and inhibiting protective Th17 immunity, Mucosal Immunol. 14 (2021) 923–936. 10.1038/s41385-021-00386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhao M, Ren K, Xiong X, Xin Y, Zou Y, Maynard JC, Kim A, Battist AP, Koneripalli N, Wang Y, Chen Q, Xin R, Yang C, Huang R, Yu J, Huang Z, Zhang Z, Wang H, Wang D, Xiao Y, Salgado OC, Jarjour NN, Hogquist KA, Revelo XS, Burlingame AL, Gao X, von Moltke J, Lin Z, Ruan H-B, Epithelial STAT6 O-GlcNAcylation drives a concerted anti-helminth alarmin response dependent on tuft cell hyperplasia and Gasdermin C, Immunity. 55 (2022) 623–638.e5. 10.1016/j.immuni.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yang H, Wang H, Andersson U, Targeting Inflammation Driven by HMGB1, Front Immunol. 11 (2020) 484. 10.3389/fimmu.2020.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X, Zhao X, Liu J, Tang C, Liu Z, Huang Y, Peng H, Xiao L, Tang D, Scott MJ, Wang Q, Liu J, Xiao X, Watkins S, Li J, Yang H, Wang H, Chen F, Tracey KJ, Billiar TR, Lu B, The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis, Immunity. 49 (2018) 740–753.e7. 10.1016/j.immuni.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Huang Y, Zang K, Shang F, Guo S, Gao L, Zhang X, HMGB1 mediates acute liver injury in sepsis through pyroptosis of liver macrophages., Int J Burn Trauma. 10 (2020) 60–67. [PMC free article] [PubMed] [Google Scholar]
- [73].Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ, Reversing established sepsis with antagonists of endogenous high-mobility group box 1, Proc National Acad Sci. 101 (2004) 296–301. 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP, HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice, Gastroenterology. 123 (2002) 790–802. 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- [75].Tan G, Huang C, Chen J, Chen B, Zhi F, Gasdermin-E-mediated pyroptosis participates in the pathogenesis of Crohn’s disease by promoting intestinal inflammation, Cell Reports. 35 (2021) 109265. 10.1016/j.celrep.2021.109265. [DOI] [PubMed] [Google Scholar]
- [76].Russo AJ, Vasudevan SO, Méndez-Huergo SP, Kumari P, Menoret A, Duduskar S, Wang C, Sáez JMP, Fettis MM, Li C, Liu R, Wanchoo A, Chandiran K, Ruan J, Vanaja SK, Bauer M, Sponholz C, Hudalla GA, Vella AT, Zhou B, Deshmukh SD, Rabinovich GA, Rathinam VA, Intracellular immune sensing promotes inflammation via gasdermin D–driven release of a lectin alarmin, Nat Immunol. 22 (2021) 154–165. 10.1038/s41590-020-00844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Russo AJ, Vasudevan SO, Méndez-Huergo SP, Kumari P, Menoret A, Duduskar S, Wang C, Sáez JMP, Fettis MM, Li C, Liu R, Wanchoo A, Chandiran K, Ruan J, Vanaja SK, Bauer M, Sponholz C, Hudalla GA, Vella AT, Zhou B, Deshmukh SD, Rabinovich GA, Rathinam VA, Intracellular immune sensing promotes inflammation via gasdermin D–driven release of a lectin alarmin, Nat Immunol. 22 (2021) 154–165. 10.1038/s41590-020-00844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chou R-H, Tsai C-T, Lu Y-W, Guo J-Y, Lu C-T, Tsai Y-L, Wu C-H, Lin S-J, Lien R-Y, Lu S-F, Yang S-F, Huang P-H, Elevated serum galectin-1 concentrations are associated with increased risks of mortality and acute kidney injury in critically ill patients, Plos One. 16 (2021) e0257558. 10.1371/journal.pone.0257558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Markovic SS, Gajovic N, Jurisevic M, Jovanovic M, Jovicic BP, Arsenijevic N, Mijailovic Z, Jovanovic M, Dolicanin Z, Jovanovic I, Galectin-1 as the new player in staging and prognosis of COVID-19, Sci Rep-Uk. 12 (2022) 1272. 10.1038/s41598-021-04602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen Y, Wang H, Shen J, Deng R, Yao X, Guo Q, Lu A, Sun B, Zhang Y, Meng G, Gasdermin D Drives the Nonexosomal Secretion of Galectin-3, an Insulin Signal Antagonist., J Immunol. 203 (2019) 2712–2723. 10.4049/jimmunol.1900212. [DOI] [PubMed] [Google Scholar]
- [81].Phulphagar K, Kühn LI, Ebner S, Frauenstein A, Swietlik JJ, Rieckmann J, Meissner F, Proteomics reveals distinct mechanisms regulating the release of cytokines and alarmins during pyroptosis, Cell Reports. 34 (2021) 108826. 10.1016/j.celrep.2021.108826. [DOI] [PubMed] [Google Scholar]
- [82].Zhou B, Liu J, Zeng L, Zhu S, Wang H, Billiar TR, Kroemer G, Klionsky DJ, Zeh HJ, Jiang J, Tang D, Kang R, Extracellular SQSTM1 mediates bacterial septic death in mice through insulin receptor signalling, Nat Microbiol. 5 (2020) 1576–1587. 10.1038/s41564-020-00795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Fajgenbaum DC, June CH, Cytokine Storm, New Engl J Medicine. 383 (2020) 2255–2273. 10.1056/nejmra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yan J, Zhang J, Wang Y, Liu H, Sun X, Li A, Cui P, Yu L, Yan X, He Z, Rapidly Inhibiting the Inflammatory Cytokine Storms and Restoring Cellular Homeostasis to Alleviate Sepsis by Blocking Pyroptosis and Mitochondrial Apoptosis Pathways, Adv Sci. 10 (2023) 2207448. 10.1002/advs.202207448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Gong T, Liu L, Jiang W, Zhou R, DAMP-sensing receptors in sterile inflammation and inflammatory diseases, Nat Rev Immunol. 20 (2020) 95–112. 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- [86].Gleeson TA, Nordling E, Kaiser C, Lawrence CB, Brough D, Green JP, Allan SM, Looking into the IL-1 of the storm: are inflammasomes the link between immunothrombosis and hyperinflammation in cytokine storm syndromes?, Discov Immunol. 1 (2022). 10.1093/discim/kyac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lamkanfi M, Sarkar A, Walle LV, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti T-D, Dixit VM, Inflammasome-Dependent Release of the Alarmin HMGB1 in Endotoxemia, J Immunol. 185 (2010) 4385–4392. 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhang J, Wu H, Yao X, Zhang D, Zhou Y, Fu B, Wang W, Li H, Wang Z, Hu Z, Ren Y, Sun R, Tian Z, Bian X, Wei H, Pyroptotic macrophages stimulate the SARS-CoV-2-associated cytokine storm, Cell Mol Immunol. 18 (2021) 1305–1307. 10.1038/s41423-021-00665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Rodrigues TS, de Sá KSG, Ishimoto AY, Becerra A, Oliveira S, Almeida L, Gonçalves AV, Perucello DB, Andrade WA, Castro R, Veras FP, Toller-Kawahisa JE, Nascimento DC, de Lima MHF, Silva CMS, Caetite DB, Martins RB, Castro IA, Pontelli MC, de Barros FC, do Amaral NB, Giannini MC, Bonjorno LP, Lopes MIF, Santana RC, Vilar FC, Auxiliadora-Martins M, Luppino-Assad R, de Almeida SCL, de Oliveira FR, Batah SS, Siyuan L, Benatti MN, Cunha TM, Alves-Filho JC, Cunha FQ, Cunha LD, Frantz FG, Kohlsdorf T, Fabro AT, Arruda E, de Oliveira RDR, Louzada-Junior P, Zamboni DS, Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients, J Exp Med. 218 (2020) e20201707. 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Freeman TL, Swartz TH, Targeting the NLRP3 Inflammasome in Severe COVID-19, Front Immunol. 11 (2020) 1518. 10.3389/fimmu.2020.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Pan P, Shen M, Yu Z, Ge W, Chen K, Tian M, Xiao F, Wang Z, Wang J, Jia Y, Wang W, Wan P, Zhang J, Chen W, Lei Z, Chen X, Luo Z, Zhang Q, Xu M, Li G, Li Y, Wu J, SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation, Nat Commun. 12 (2021) 4664. 10.1038/s41467-021-25015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sun X, Liu Y, Huang Z, Xu W, Hu W, Yi L, Liu Z, Chan H, Zeng J, Liu X, Chen H, Yu J, Chan FKL, Ng SC, Wong SH, Wang MH, Gin T, Joynt GM, Hui DSC, Zou X, Shu Y, Cheng CHK, Fang S, Luo H, Lu J, Chan MTV, Zhang L, Wu WKK, SARS-CoV-2 non-structural protein 6 triggers NLRP3-dependent pyroptosis by targeting ATP6AP1, Cell Death Differ. 29 (2022) 1240–1254. 10.1038/s41418-021-00916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Xu H, Akinyemi IA, Chitre SA, Loeb JC, Lednicky JA, McIntosh MT, Bhaduri-McIntosh S, SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway, Virology. 568 (2022) 13–22. 10.1016/j.virol.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zheng M, Karki R, Williams EP, Yang D, Fitzpatrick E, Vogel P, Jonsson CB, Kanneganti T-D, TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines, Nat Immunol. 22 (2021) 829–838. 10.1038/s41590-021-00937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Theobald SJ, Simonis A, Georgomanolis T, Kreer C, Zehner M, Eisfeld HS, Albert M, Chhen J, Motameny S, Erger F, Fischer J, Malin JJ, Gräb J, Winter S, Pouikli A, David F, Böll B, Koehler P, Vanshylla K, Gruell H, Suárez I, Hallek M, Fätkenheuer G, Jung N, Cornely OA, Lehmann C, Tessarz P, Altmüller J, Nürnberg P, Kashkar H, Klein F, Koch M, Rybniker J, Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19, Embo Mol Med. 13 (2021) e14150. 10.15252/emmm.202114150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A, Neutrophil Extracellular Traps Kill Bacteria, Science. 303 (2004) 1532–1535. 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- [97].Kaplan MJ, Radic M, Neutrophil Extracellular Traps: Double-Edged Swords of Innate Immunity, J Immunol. 189 (2012) 2689–2695. 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sollberger G, Choidas A, Burn GL, Habenberger P, Lucrezia RD, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B, Krüger R, Herzig A, Zychlinsky A, Gasdermin D plays a vital role in the generation of neutrophil extracellular traps, Sci Immunol. 3 (2018). 10.1126/sciimmunol.aar6689. [DOI] [PubMed] [Google Scholar]
- [99].Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, Schroder K, Noncanonical inflammasome signaling elicits gasdermin D–dependent neutrophil extracellular traps, Sci Immunol. 3 (2018). 10.1126/sciimmunol.aar6676. [DOI] [PubMed] [Google Scholar]
- [100].Santoni K, Pericat D, Pinilla M, Bagayoko S, Hessel A, Leon-Icaza S-A, Bellard E, Mazères S, Doz-Deblauwe E, Winter N, Paget C, Girard J-P, Cougoule C, Poincloux R, Lamkanfi M, Lefrançais E, Meunier E, Planès R, Caspase-1-driven neutrophil pyroptosis promotes an incomplete NETosis upon Pseudomonas aeruginosa infection, Biorxiv. (2022) 2021.06.28.450116. 10.1101/2021.06.28.450116. [DOI] [Google Scholar]
- [101].Su M, Chen C, Li S, Li M, Zeng Z, Zhang Y, Xia L, Li X, Zheng D, Lin Q, Fan X, Wen Y, Liu Y, Chen F, Luo W, Bu Y, Qin J, Guo M, Qiu M, Sun L, Liu R, Wang P, Hwa J, Tang WH, Gasdermin D-dependent platelet pyroptosis exacerbates NET formation and inflammation in severe sepsis, Nat Cardiovasc Res. 1 (2022) 732–747. 10.1038/s44161-022-00108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Li X, Ye Y, Peng K, Zeng Z, Chen L, Zeng Y, Histones: The critical players in innate immunity, Front Immunol. 13 (2022) 1030610. 10.3389/fimmu.2022.1030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Allam R, Darisipudi MN, Tschopp J, Anders H, Histones trigger sterile inflammation by activating the NLRP3 inflammasome, Eur. J. Immunol 43 (2013) 3336–3342. 10.1002/eji.201243224. [DOI] [PubMed] [Google Scholar]
- [104].Li H, Li Y, Song C, Hu Y, Dai M, Liu B, Pan P, Neutrophil Extracellular Traps Augmented Alveolar Macrophage Pyroptosis via AIM2 Inflammasome Activation in LPS-Induced ALI/ARDS, J Inflamm Res. 14 (2021) 4839–4858. 10.2147/jir.s321513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Chen L, Zhao Y, Lai D, Zhang P, Yang Y, Li Y, Fei K, Jiang G, Fan J, Neutrophil extracellular traps promote macrophage pyroptosis in sepsis, Cell Death Dis. 9 (2018) 597. 10.1038/s41419-018-0538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, Benson BL, Chirieleison SM, Huang AY, Dubyak GR, Xiao TS, Li X, Abbott DW, Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis, Sci Immunol. 3 (2018). 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kang R, Zeng L, Zhu S, Xie Y, Liu J, Wen Q, Cao L, Xie M, Ran Q, Kroemer G, Wang H, Billiar TR, Jiang J, Tang D, Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis, Cell Host Microbe. 24 (2018) 97–108.e4. 10.1016/j.chom.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ferreira AC, Soares VC, de Azevedo-Quintanilha IG, da S S.Dias G, Fintelman-Rodrigues N, Sacramento CQ, Mattos M, de Freitas CS, Temerozo JR, Teixeira L, Hottz ED, Barreto EA, Pão CRR, Palhinha L, Miranda M, Bou-Habib DC, Bozza FA, Bozza PT, Souza TML, SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes, Cell Death Discov. 7 (2021) 43. 10.1038/s41420-021-00428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ding X, Kambara H, Guo R, Kanneganti A, Acosta-Zaldívar M, Li J, Liu F, Bei T, Qi W, Xie X, Han W, Liu N, Zhang C, Zhang X, Yu H, Zhao L, Ma F, Köhler JR, Luo HR, Inflammasome-mediated GSDMD activation facilitates escape of Candida albicans from macrophages, Nat Commun. 12 (2021) 6699. 10.1038/s41467-021-27034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Jo E-K, Kim JK, Shin D-M, Sasakawa C, Molecular mechanisms regulating NLRP3 inflammasome activation, Cell Mol Immunol. 13 (2016) 148–159. 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Li S, Wu Y, Yang D, Wu C, Ma C, Liu X, Moynagh PN, Wang B, Hu G, Yang S, Gasdermin D in peripheral myeloid cells drives neuroinflammation in experimental autoimmune encephalomyelitis, J Exp Medicine. 216 (2019) 2562–2581. 10.1084/jem.20190377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lu Y, Meng R, Wang X, Xu Y, Tang Y, Wu J, Xue Q, Yu S, Duan M, Shan D, Wang Q, Wang H, Billiar TR, Xiao X, Chen F, Lu B, Caspase-11 signaling enhances graft-versus-host disease, Nat Commun. 10 (2019) 4044. 10.1038/s41467-019-11895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Xu B, Jiang M, Chu Y, Wang W, Chen D, Li X, Zhang Z, Zhang D, Fan D, Nie Y, Shao F, Wu K, Liang J, Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice, J Hepatol. 68 (2018) 773–782. 10.1016/j.jhep.2017.11.040. [DOI] [PubMed] [Google Scholar]
- [114].Li J, Zhao J, Xu M, Li M, Wang B, Qu X, Yu C, Hang H, Xia Q, Wu H, Sun X, Gu J, Kong X, Blocking GSDMD processing in innate immune cells but not in hepatocytes protects hepatic ischemia– reperfusion injury, Cell Death Dis. 11 (2020) 244. 10.1038/s41419-020-2437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Zhang D, Qian J, Zhang P, Li H, Shen H, Li X, Chen G, Gasdermin D serves as a key executioner of pyroptosis in experimental cerebral ischemia and reperfusion model both in vivo and in vitro, J. Neurosci. Res. 97 (2019) 645–660. 10.1002/jnr.24385. [DOI] [PubMed] [Google Scholar]
- [116].Jia C, Zhang J, Chen H, Zhuge Y, Chen H, Qian F, Zhou K, Niu C, Wang F, Qiu H, Wang Z, Xiao J, Rong X, Chu M, Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation, Cell Death Dis. 10 (2019) 778. 10.1038/s41419-019-2021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Yang F, Qin Y, Lv J, Wang Y, Che H, Chen X, Jiang Y, Li A, Sun X, Yue E, Ren L, Li Y, Bai Y, Wang L, Silencing long non-coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy, Cell Death Dis. 9 (2018) 1000. 10.1038/s41419-018-1029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Wang B, Nemmara VV, Wilson R, Jiang Z, Khalighinejad F, Muneeruddin K, Shaffer SA, Dutta R, Ionete C, Pesiridis S, Yang S, Thompson PR, Fitzgerald KA, Succination inactivates gasdermin D and blocks pyroptosis, Science. 369 (2020) 1633–1637. 10.1126/science.abb9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Martín-Sánchez F, Diamond C, Zeitler M, Gomez AI, Baroja-Mazo A, Bagnall J, Spiller D, White M, Daniels MJD, Mortellaro A, Peñalver M, Paszek P, Steringer JP, Nickel W, Brough D, Pelegrín P, Inflammasome-dependent IL-1β release depends upon membrane permeabilisation, Cell Death Differ. 23 (2016) 1219–1231. 10.1038/cdd.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wang Y, Zhu X, Yuan S, Wen S, Liu X, Wang C, Qu Z, Li J, Liu H, Sun L, Liu F, TLR4/NF-κB Signaling Induces GSDMD-Related Pyroptosis in Tubular Cells in Diabetic Kidney Disease, Front Endocrinol. 10 (2019) 603. 10.3389/fendo.2019.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Wright C, Moore RD, Disulfiram treatment of alcoholism, Am J Medicine. 88 (1990) 647–655. 10.1016/0002-9343(90)90534-k. [DOI] [PubMed] [Google Scholar]
- [122].Hu JJ, Liu X, Zhao J, Xia S, Ruan J, Luo X, Kim J, Lieberman J, Wu H, Identification of pyroptosis inhibitors that target a reactive cysteine in gasdermin D, Biorxiv. (2018) 365908. 10.1101/365908. [DOI] [Google Scholar]
- [123].Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, Ruan J, Luo X, Lou X, Bai Y, Wang J, Hollingsworth LR, Magupalli VG, Zhao L, Luo HR, Kim J, Lieberman J, Wu H, FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation, Nat Immunol. 21 (2020) 736–745. 10.1038/s41590-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]