Abstract
We tested 16 erythromycin-resistant clinical isolates of S. aureus, recovered from patients hospitalized in the United States from 1958 to 1969, for the presence of ermA, ermB, and ermC by using PCR. Fifteen of 16 isolates contained at least one copy of ermA; the remaining isolate, which was also clindamycin resistant, contained ermB. Eight of the 15 isolates harboring ermA, all of which were inducible, contained a single copy of the gene in the chromosome, while the remaining seven isolates had two copies of the gene. ermB was plasmid encoded and mediated constitutive resistance to erythromycin.
Erythromycin resistance genes are widely disseminated among many species of bacteria; over a dozen resistance determinants have been described (1, 11, 34). In Staphylococcus aureus, erythromycin resistance is usually due either to ribosomal modification by 23S rRNA methylases mediated primarily by ermA, ermB, or ermC or to active efflux of the antimicrobial agent by an ATP-dependent pump mediated by msrA (10, 14, 27, 29). ermA is most often harbored on the transposon Tn554, which also encodes spectinomycin resistance (8, 18, 22, 33), while ermB is often associated with transposon Tn551 and the penicillinase plasmid, pI258 (21, 28). The ermC gene, which appears to be rare in strains isolated prior to 1970, is normally located on small plasmids ranging in size from 2.4 to 5 kb (12, 13, 27). All of the erm determinants confer cross-resistance to macrolides, lincosamides, and streptogramin B agents (MLSB phenotype) (1, 11, 19).
Several reports have characterized erythromycin resistance determinants in S. aureus isolates recovered prior to 1970 in Denmark (35, 36) or during later periods in the United States or the United Kingdom (11, 15, 33), however, no reports to our knowledge describe the epidemiology of erythromycin resistance in S. aureus isolates from the United States in the period immediately after erythromycin was introduced into clinical practice. Therefore, the goal of this study was to characterize the genes encoding erythromycin resistance in strains of S. aureus isolated from patients in the United States from 1958 to 1969.
Eighteen isolates of S. aureus obtained from patients in the United States prior to 1970 were recovered from freezers at the Centers for Disease Control and Prevention. The organisms were identified by using standard biochemicals (17); bacteriophage results were those previously determined at the Centers for Disease Control and Prevention (3, 4). Antimicrobial susceptibility testing was performed by broth microdilution using cation-adjusted Mueller-Hinton (MH) broth (Becton Dickinson Microbiology Systems, Cockeysville, Md.) (26). Spectinomycin susceptibility was tested by dispensing 5 μl of a 0.5 McFarland standard suspension of the isolate onto MH agar plates containing 300 μg of spectinomycin per ml (33).
Two of the S. aureus isolates were resistant to penicillin and tetracycline only, while 15 were resistant to erythromycin, penicillin, spectinomycin, and tetracycline (Table 1). The remaining isolate (isolate 65-20) manifested high-level erythromycin and clindamycin resistance in addition to penicillin and tetracycline resistance; this was the only erythromycin-resistant isolate that was susceptible to 300 μg of spectinomycin per ml. All 18 isolates were susceptible to oxacillin.
TABLE 1.
Characteristics of erythromycin-resistant isolates
| Strain | Isolationa
|
Gene/expression |
ermA insertsb
|
Resistance patternc | ||
|---|---|---|---|---|---|---|
| Yr | State | No. | Size (kb) | |||
| 58-362 | 1958 | Georgia | 0 | Pn Tc | ||
| 58-431 | 1958 | Georgia | 0 | Pn Tc | ||
| 58-424 | 1958 | Georgia | ermA/inducible | 1 | 6.0 | Er Pn Sp Tc |
| 58-434 | 1958 | Georgia | ermA/inducible | 1 | 6.0 | Er Pn Sp Tc |
| 58-480 | 1958 | Georgia | ermA/inducible | 2 | 6.0–9.7 | Er Pn Sp Tc |
| 58-488 | 1958 | Georgia | ermA/inducible | 1 | 6.0 | Er Pn Sp Tc |
| 58-490 | 1958 | Georgia | ermA/inducible | 2 | 6.0–9.7 | Er Pn Sp Tc |
| 65-8 | 1965 | Georgia | ermA/inducible | 1 | 6.0 | Er Pn Sp Tc |
| 65-20 | 1965 | Georgia | ermB/constitutive | 0 | Cc Er Pn Tc | |
| 65-1119 | 1965 | Alabama | ermA/inducible | 1 | 6.0 | Er Pn Sp Tc |
| 65-1322 | 1965 | West Virginia | ermA/inducible | 1 | 6.0 | Er Pn Sp Tc |
| 66-1752 | 1966 | Arkansas | ermA/inducible | 1 | 6.0 | Er Pn Sp Tc |
| 67-43 | 1967 | West Virginia | ermA/inducible | 1 | 6.0 | Er Pn Sp Tc |
| 67-331 | 1967 | California | ermA/inducible | 2 | 6.0–9.7 | Er Pn Sp Tc |
| 68-81 | 1968 | New York | ermA/inducible | 2 | 6.0–9.7 | Er Pn Sp Tc |
| 68-397 | 1968 | Texas | ermA/inducible | 2 | 6.0–7.0 | Er Pn Sp Tc |
| 69-172 | 1969 | California | ermA/inducible | 2 | 6.0–9.7 | Er Pn Sp Tc |
| 69-412 | 1969 | Delaware | ermA/inducible | 2 | 6.0–9.7 | Er Pn Sp Tc |
Isolates from Georgia are presumed to be from the investigation reported in reference 24.
Size of the EcoRI fragments that hybridized with the ermA DNA probe.
Cc, clindamycin; Er, erythromycin; Pn, penicillin; Tc, tetracycline; Sp, spectinomycin.
The induction of erythromycin resistance was tested by disk diffusion by using standard erythromycin (15 μg) and clindamycin (2 μg) disks (25) as described by Jenssen et al. (15). All of the isolates except 65-20 showed blunting of the clindamycin zones in the disk screening test, indicating induction. Strains 65-20, RN1551 (constitutive ermA control; provided by Gregory Stone, Abbott Park, Ill.), and RN11 (ermB) (28) were clindamycin resistant. To confirm the disk results, strains were induced in MH broth with erythromycin for 90 min and then tested for erythromycin resistance by agar dilution on MH agar with a final inoculum size of 5 × 104 CFU/spot. The control strain S. aureus ATCC 29213 remained susceptible to erythromycin (MIC, <0.5 μg/ml), while strain 1206 (inducible strain containing ermA; provided by Bernard Weisblum, Madison, Wis.) and the four representative isolates 65-8, 65-1322, 68-397, and 58-480 showed an increase in erythromycin MIC of five or more doubling dilutions, rising from 2 to >64 μg/ml.
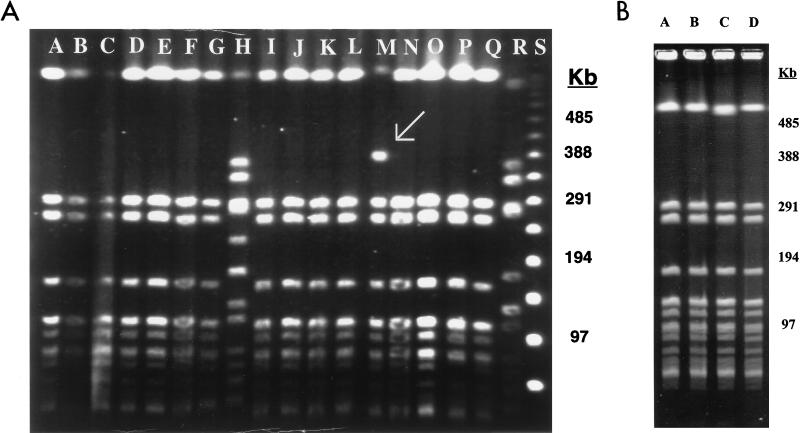
Pulsed-field gel electrophoresis (PFGE) of genomic DNA after digestion in situ with SmaI (3, 32) was performed to determine the genetic relatedness of the isolates. The PFGE patterns of all of the isolates were indistinguishable (Fig. 1) with the exception of isolate 67-331, which showed an additional band at approximately 400 kb (Fig. 1A, lane M). All of the isolates, including the erythromycin-susceptible isolates, belonged to the 80/81 bacteriophage group, including those from 1958 which were presumed to be from nosocomial outbreaks (23, 24). Three isolates, 65-1119, 65-1322, and 67-43, were typed as belonging to the 52/52A/80/81 complex, which is included in the 80/81 bacteriophage complex.
FIG. 1.
(A) Analysis of SmaI restriction fragments of erythromycin-resistant isolates of S. aureus analyzed by PFGE. Lane A, 58-424; lane B, 58-434; lane C, 58-480; lane D, 58-488; lane E, 58-490; lane F, 65-8; lane G, 65-20 (ermB); lane H, 8325 (erm-negative control); lane I, 65-1119; lane J, 65-1322; lane K, 65-1752; lane L, 67-43; lane M, 67-331, lane N, 68-81; lane O, 68-397; lane P, 69-172; lane Q, 69-412; lane R, RN3944 (ermA control); lane S, lambda ladder. (B) Analysis of SmaI restriction fragments of erythromycin-susceptible and erythromycin-resistant isolates of S. aureus analyzed by PFGE. Lane A, 58-362 (erythromycin susceptible); lane B, 58-424 (erythromycin resistant); lane C, 58-431 (erythromycin susceptible); lane D, 58-434 (erythromycin resistant).
Using the PCR primers for ermA, ermB, and ermC described by Sutcliffe et al. (30, 31), we identified ermA in all 15 erythromycin-resistant isolates that showed an inducible pattern of expression (Table 1). The ermB determinant was present in the remaining isolate (65-20), which showed high-level erythromycin and clindamycin resistance that was constitutively expressed. The ermC gene was not present in any of the isolates tested. We did not test for the presence of msrA or other erythromycin resistance genes.
DNA-DNA hybridization studies of total genomic DNA digested with the restriction enzyme EcoRI were performed with an ermA-specific probe labeled with digoxigenin (5, 36). Since EcoRI does not cleave the DNA probe used for detection of ermA, each band observed after hybridization represents one insert of ermA. These assays demonstrated that 8 of 15 isolates harboring ermA contained a single insert on an EcoRI fragment of 6.0 kb (Table 1). The other seven isolates contained two chromosomal inserts, one of which was always on a 6.0-kb fragment, and the others were located on fragments of either 9.7 kb (six isolates) or 7.0 kb (one isolate). The presence of multiple inserts was confirmed in repeated experiments, ruling out the possibility of partial digestion products. Conversely, ermB was located on a 28-kb plasmid on an EcoRI fragment of approximately 13 kb as determined by agarose gel electrophoresis (data not shown). This is consistent with the size of the EcoRI fragment of pI258 that encodes ermB, which is 12.8 kb.
Although resistance to erythromycin in S. aureus was reported shortly after this antimicrobial agent was introduced into clinical practice in the 1950s (7, 16), reports of erythromycin resistance in S. aureus isolates belonging to the 52/52A/80/81 complex, previously known as the 80/81 complex, are rare (2, 9, 20, 23, 24). Exactly how these strains acquired erythromycin resistance is unknown, but transposons are likely to have played a role since the ermA genes were all located in the chromosome and ermA is known to be associated with Tn554 (8, 33). Although Tn554 has a single, specific insertion site in the chromosome, secondary sites have been identified (18), and patterns similar to those reported here have been noted by Westh et al. (36). Our data, although limited, support the assertion of Westh et al. that ermA was the major erythromycin resistance determinant in S. aureus prior to 1970. However, based on recent reports, it appears that ermC has replaced ermA as the most prevalent erythromycin resistance determinant in S. aureus in Denmark and elsewhere (11, 15, 33, 35).
Given the variability of the phage typing patterns, the effect of lysogeny on PFGE patterns, and the 12-year time period over which the isolates were collected, we expected to observe significant variation in the PFGE profiles of the erythromycin-resistant isolates, particularly since oxacillin-susceptible isolates of S. aureus have been shown to have a multitude of PFGE patterns (6). However, all of the isolates, including the erythromycin-susceptible isolates, had virtually identical PFGE patterns. This, and the varying patterns of the ermA chromosomal insertions, suggest that transposons carrying ermA entered this S. aureus clone on multiple occasions. Although transduction cannot be ruled out as an additional mechanism of gene spread, we feel that this explanation is less likely given the specificity of the Tn554 insertion sites (18).
Eady et al. reported that human isolates of S. aureus containing ermB are rare (11). Thus, we were surprised to find this gene among our isolates. The first ermB gene was identified on transposon Tn551 on plasmid pI258 and was subsequently detected on a variety of other plasmids (35). Why ermB has not been more widely disseminated among S. aureus strains remains unclear.
In summary, we have shown that erythromycin-resistant isolates of S. aureus belonging to the 52/52A/80/81 complex recovered from 1958 to 1969 from patients in the United States can harbor either an ermA or ermB gene. Although the mode of entry of the resistance gene into the S. aureus isolates is unclear, these isolates appear to have acquired resistance on multiple occasions in multiple locations in the United States, suggesting that the selective pressure to develop resistance was greater during this early period of antimicrobial use than previously appreciated (1, 8, 34).
Acknowledgments
We thank Sigrid McAllister for identification of the isolates, Bertha Hill for assistance with the susceptibility testing studies, Christine Steward and Tammy Bannerman for assistance with strain typing, and Kamile Rasheed for helpful discussions.
REFERENCES
- 1.Arthur M, Brisson-Noel A, Courvalin P. Origin and evolution of genes specifying resistance to macrolide, lincosamide and streptogramin antibiotics: data and hypotheses. J Antimicrob Chemother. 1987;20:783–802. doi: 10.1093/jac/20.6.783. [DOI] [PubMed] [Google Scholar]
- 2.Asheshov E H, Rippon J E. Changes in typing pattern of phage-type 80 staphylococci. J Gen Microbiol. 1959;20:634–643. doi: 10.1099/00221287-20-3-634. [DOI] [PubMed] [Google Scholar]
- 3.Bannerman T L, Hancock G A, Tenover F C, Miller J M. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J Clin Microbiol. 1995;33:551–555. doi: 10.1128/jcm.33.3.551-555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair J E, Williams R E O. Phage typing of staphylococci. Bull W H O. 1961;24:771–778. [PMC free article] [PubMed] [Google Scholar]
- 5.Boehringer Manheim Corporation. The Genius System user’s guide for filter hybridization. Indianapolis, Ind: Boehringer Manheim Corporation; 1995. [Google Scholar]
- 6.Carles-Nurit M J, Christophle B, Broche S, Gouby A, Bouziges N, Ramuz M. DNA polymorphisms in methicillin-susceptible and methicillin-resistant strains of Staphylococcus aureus. J Clin Microbiol. 1992;30:2092–2096. doi: 10.1128/jcm.30.8.2092-2096.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chabbert Y. Antagonisme in vitro entre l’erythromycine et la spiramycine. Ann Inst Pasteur (Paris) 1956;90:787–790. [PubMed] [Google Scholar]
- 8.Chikramane S G, Matthews P R, Noble W C. Tn554 inserts in methicillin-resistant Staphylococcus aureus from Australia and England: comparison with an American methicillin-resistant group. J Gen Microbiol. 1991;137:1303–1307. doi: 10.1099/00221287-137-6-1303. [DOI] [PubMed] [Google Scholar]
- 9.Comptois R D. Changes in the phage-typing patterns of staphylococci following lysogenization with a related group of Staphylococcus bacteriophages. Can J Microbiol. 1960;6:491–502. doi: 10.1139/m60-057. [DOI] [PubMed] [Google Scholar]
- 10.Duval, J. 1985. Evolution and epidemiology of MLS resistance. J. Antimicrob. Chemother. 16(Suppl. A):137–149. [DOI] [PubMed]
- 11.Eady E A, Ross J, Tipper J L, Walters C E, Cove J H, Noble W C. Distribution of genes encoding erythromycin ribosomal methylases and an erythromycin efflux pump in epidemiologically distinct groups of staphylococci. J Antimicrob Chemother. 1993;31:211–217. doi: 10.1093/jac/31.2.211. [DOI] [PubMed] [Google Scholar]
- 12.Horinouchi S, Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibiotics. J Bacteriol. 1982;150:804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iordanescu S. Three distinct plasmids originating in the same Staphylococcus aureus strain. Arch Rheum Pathol Exp Microbiol. 1976;35:111–118. [PubMed] [Google Scholar]
- 14.Janosy L, Nakajima Y, Hashimoto H. Characterization of plasmids that confer inducible resistance to 14-membered macrolides and streptogramin B type antibiotics in Staphylococcus aureus. Microbiol Immunol. 1990;34:723–735. doi: 10.1111/j.1348-0421.1990.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 15.Jenssen W D, Thakker-Varia S, Dubin D T, Weinstein M P. Prevalence of macrolides-lincosamides-streptogramin B resistance and erm gene classes among clinical strains of staphylococci and streptococci. Antimicrob Agents Chemother. 1987;31:883–888. doi: 10.1128/aac.31.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones W F, Jr, Nichols R L, Finland M. Development of resistance and cross-resistance in vitro to erythromycin, carbomycin, oleandomycin, and streptogramin. Proc Soc Exp Biol Med. 1956;93:388–391. doi: 10.3181/00379727-93-22766. [DOI] [PubMed] [Google Scholar]
- 17.Kloos W E, Bannerman T L. Staphylococcus and Micrococcus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 282–298. [Google Scholar]
- 18.Krolewski J J, Murphy E, Novick R P, Rush M G. Site-specificity of the chromosomal insertion of Staphylococcus aureus transposon Tn554. J Mol Biol. 1981;152:19–33. doi: 10.1016/0022-2836(81)90093-0. [DOI] [PubMed] [Google Scholar]
- 19.Lampson B L, Parisi J T. Naturally occurring Staphylococcus epidermidis plasmids expressing constitutive macrolide-lincosamide-streptogramin B resistance contains a deleted attenuator. J Bacteriol. 1986;166:479–483. doi: 10.1128/jb.166.2.479-483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Light I J, Atherton H D, Sutherland J M. Decreased colonization of newborn infants with Staphylococcus aureus 80/81: Cincinnati General Hospital, 1960–1972. J Infect Dis. 1975;131:281–285. doi: 10.1093/infdis/131.3.281. [DOI] [PubMed] [Google Scholar]
- 21.Mitsuhashi S, Morimura M, Kono K, Oshima H. Elimination of drug resistance of Staphylococcus aureus by treatment with acriflavine. J Bacteriol. 1963;86:162–164. doi: 10.1128/jb.86.1.162-164.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy E. Inhibition of Tn554 transposition: deletion analysis. Plasmid. 1983;10:260–269. doi: 10.1016/0147-619x(83)90040-9. [DOI] [PubMed] [Google Scholar]
- 23.Nahmias A, Sakurai N, Blumberg R, Doege A, Sulzer C. The Staphylococcus “80/81 complex”: epidemiological and laboratory observations. J Infect Dis. 1961;109:211–222. doi: 10.1093/infdis/109.3.211. [DOI] [PubMed] [Google Scholar]
- 24.Nahmias A J, Godwin J T, Updyke E L, Hopkins W A. Postsurgical staphylococci infections. JAMA. 1960;174:1269–1275. doi: 10.1001/jama.1960.03030100037009. [DOI] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. 6th ed. 17, no. 1. Approved standard M2-A6. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. 17, no. 2. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 27.Novick, R. P., and E. Murphy. 1985. MLS-resistance determinants in Staphylococcus aureus and their molecular evolution. J. Antimicrob. Chemother. 16(Suppl. A):101–110. [DOI] [PubMed]
- 28.Novick R P, Murphy E, Gryczan T J, Baron E, Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979;2:109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- 29.Ross J L, Eady E A, Cove J H, Cunliffe W J, Baumberg S, Wootton J C. Inducible erythromycin resistance in staphylococci is encoded by a member of ATP-binding transport super-gene family. Mol Microbiol. 1990;4:1207–1214. doi: 10.1111/j.1365-2958.1990.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 30.Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L. Detection of erythromycin-resistant determinants by PCR. Antimicrob Agents Chemother. 1996;40:2562–2566. doi: 10.1128/aac.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thakker-Varia S, Jenssen W D, Moon-McDermott L, Weinstein M P, Dubin D T. Molecular epidemiology of macrolides-lincosamides-streptogramin B resistance in Staphylococcus aureus and coagulase-negative staphylococci. Antimicrob Agents Chemother. 1987;31:735–743. doi: 10.1128/aac.31.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westh H, Hougaard D M, Vuust J, Rosdahl V. erm genes in erythromycin-resistant Staphylococcus aureus and coagulase-negative staphylococci. APMIS. 1995;103:225–232. [PubMed] [Google Scholar]
- 36.Westh H, Hougaard D M, Vuust J, Rosdahl V T. Prevalence of erm gene classes in Staphylococcus aureus strains isolated between 1959 and 1988. Antimicrob Agents Chemother. 1995;39:369–373. doi: 10.1128/aac.39.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]