Abstract
In the great Persian Empire, pomegranate (Punica granatum L.) had a wide reputation for use both as an herbal medicine and nutritious food. It was also a symbol of peace and love according to Achaemenid limestones in the great Persia. This paper aims to review the traditional uses of pomegranate in Persian and Islamic traditional medicine and have thorough and current information regarding the pharmacology and phytochemistry of this valuable plant for practical use and further research. Relevant information about P. granatum was collected from scientific publishers and databases including Elsevier, Wiley, PubMed, and Google Scholar between 1950 and 2022. The traditional knowledge was extracted from Persian and Islamic traditional textbooks. Based on traditional textbooks, pomegranate has beneficial effects on diseases related to gastrointestinal, upper and lower respiratory, visual, and reproductive systems. In addition, pomegranate and its preparations have been prescribed for treating metabolic disorders, skin problems, and wounds as well as dental protection. Preclinical and clinical evidence supports many therapeutic potentials of pomegranate in traditional medicine. Its therapeutic effects are mostly attributed to its polyphenols. The knowledge in Persian and Islamic traditional textbooks about pomegranate and its preparations can be used as a guide for further preclinical and mainly clinical studies to discover the therapeutic potential of this valuable plant.
Key Words: Ellagic acid, Ethnopharmacology, Persian traditional medicine, Pomegranate, Punica granatum
Introduction
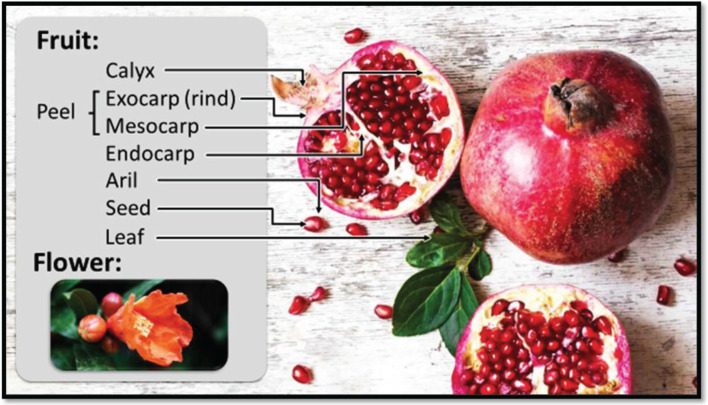
Pomegranate (Punica granatum L.), belonging to the Lythraceae family, is a historic fruit that is indigenous to Central Asia and may be found in places like the Middle East, Iran, and Turkmenistan to northern India (1). P. granatum (Figure 1) is a fruit-bearing shrub or a small tree that grows up to 501507 m with very diverse varieties (2). Pomegranate and its components were found to have powerful anti-oxidant, anti-inflammatory, antifungal, antibacterial, and antimicrobial effects, according to studies conducted in both in vitro and in vivo over the past few decades (1). In addition, some animal studies have shown that pomegranate may have anti-hypertensive and antiproliferative properties (3). Furthermore, pomegranate juice or extracts have been shown in multiple pre-clinical and clinical trials to have positive benefits on a number of disorders, including respiratory diseases (4), digestive problems (5), neurodegenerative diseases (6, 7), metabolic disorders (8, 9), cancer (3, 10), osteoarthritis (11), skin problems (11), etc.
Figure 1.
Different parts of Punica granatum (©Nutritionfacts.org)
Traditionally, pomegranate and its products have been used for the treatment of several health problems. In Islamic and Iranian Traditional Medicines (ITM), this valuable plant was used by traditional physicians in various preparations and diverse application forms for a wide variety of illnesses. They used different parts of the plant mainly fruit peels, fruit juice, and flowers for health problems such as skin diseases, reproductive problems, gastrointestinal disorders, infectious diseases, and respiratory problems (12-19). As mentioned by ITM physicians, most of the beneficial properties of pomegranate are due to its astringent effect. Modern medicine relates this astringency to the presence of phenolic compounds including tannins. Many available pomegranate formulations in the market have been prepared according to traditional knowledge. For instance, anti-aging and cosmetic products from pomegranate are available in the market under many brand names.
Although pharmacological and clinical studies have confirmed several therapeutic effects of pomegranate products and pomegranate constituents, by looking into the uses of this plant in traditional texts, many applications can still be extracted. Nevertheless, there are still many potential applications in the traditional references that can be useful for possible formulations. To the best of our knowledge, there is no similar article discussing the traditional uses of pomegranates in Islamic and Persian medicine. Thus, in the current study, we aimed to have a comprehensive review of the application of pomegranate in ITM and compare it with modern medicine. A recent example of overlapping the traditional and modern uses of this plant is Covid-19. ITM physicians prescribed pomegranate for cough and cold, and as an antimicrobial agent. Interestingly, according to research, P. granatum and the polyphenolic components it contains may be useful against Covid-19 (20).
Phytochemistry
Depending on the cultivar, growing area, maturity, cultivation technique, climate, and storage conditions, different portions of the pomegranate have distinct chemical compositions. Although in each part of the plant, a group of specialized metabolites may dominate, the most important and biologically active constituents are polyphenols and tannins. For instance, pomegranate polyphenols particularly the anthocyanins might have significant effects against metabolic syndrome (21). Nevertheless, pomegranate includes several kinds of phytochemicals, including flavonoids, alkaloids, both condensed tannins (proanthocyanidins, are polymers formed by the condensation of flavans), and hydrolyzable tannins (ellagitannins and gallotannins) and organic acids (Table 1) (22).
Table 1.
Some of the main phytochemicals reported from Punica granatum
Polyphenols
Pomegranate is a rich source of polyphenols including phenolic acids, anthocyanins, and hydrolyzable tannins. Several hyphenated analytical methods such as Ultra high-performance liquid chromatography-mass spectrometry (UHPLC-MSn) have been used to examine the polyphenol profiles of different pomegranate sections (23, 24).
Ellagitannins
Ellagitannins are polymeric compounds that frequently have various amounts of galloyl and hexahydroxydiphenoyl units attached to glucose. One of the metabolite categories in pomegranate that has been the subject of much research is ellagitannins. These substances, which are converted to urolithins in the digestive system, are the primary bioactive phytochemicals in pomegranate juice (22). Ellagitannins vary from gallotannins in that their galloyl groups are connected by C-C bonds. Additionally, gallotannins do not often form macrocycles, but ellagitannins do (25).
Gallotannins
Pomegranate contains some gallotannins. Gallotannins are a variant of hydrolyzable tannins. Gallotannins are polymers created when the hydroxyl group of a polyol carbohydrate, such as glucose, esterifies and bonds with gallic acid, a polyphenol monomer. For instance, 1,3,4-trigalloylglucose may be found in the leaves of pomegranates. Weak acids or bases hydrolyze this type of tannin to create glucose and phenolic acids (22, 25).
Lignans
Pomegranate has been shown to contain a variety of lignans and phenylpropanoidic metabolites with estrogenic action (22, 26, 27).
Phenolic acid derivatives
Phenolic acids including chlorogenic, caffeic, syringic, sinapic, p-coumaric, ferulic, vanillic, ellagic, gallic, and cinnamic acids have been identified in pomegranate peel (22, 28).
Flavonoids
Flavonoids belonging to different subclasses including, flavan-3-ols like (+)-catechin, (−)-epicatechin, and (+)-gallocatechin, flavonols, flavanones, dihydrochalcones, and flavones have been identified in pomegranate (22).
Organic acids
Citric and malic acids are among the organic acids identified from different parts of pomegranate (22).
Anthocyanins
Pomegranate juice’s crimson hue is a result of anthocyanins. Pomegranate anthocyanins such as cyanidin, pelargonidin, and delphinidin have been found to be conjugated with one or two hexose sugars (22).
Alkaloids
The three main alkaloids found in the stem and root barks of pomegranates are pelletierine, pseudopelletierine, and N-methylpelletierine (29). Sedridine, 2-(2′-hydroxypropyl)-∆1piperideine,2-(2′-propenyl)-∆1piperideine, norpseudopelletierine, and the pyrrolidine alkaloids (with a five-membered N-containing ring) hygrine and norhygrine have also been found in root barks of pomegranate at small amounts. In addition to the alkaloids that build up in root and stem barks of the plant, N-(2′,5′-dihydroxyphenyl) pyridinium chloride has been identified in pomegranate leaves, and a pyrrolidine-type alkaloid punigratane (2,5-diheptyl-N-methylpyrrolidine) has been recently characterized in pomegranate fruit peel (22).
Pomegranate in Iran and ancient Persia
Pomegranate production and exports from Iran rank among the highest in the world (71). Pomegranate, known as “Anar” in Persian, grows widely and is cultivated throughout Iran. As a common food, Iranians use pomegranate fruit juice and paste in many dishes including sour chicken, Fesenjan, and Lavashak. Iranian culture has long used the pomegranate as a symbol. For instance, Isfandiyar, a legendary Persian warrior, gains invincibility by eating a pomegranate. The Persian phalanx’s spears were decorated with golden pomegranates, according to Herodotus’ “The Persian War.” (72). Interestingly, the blossom of pomegranate (Golnar and Gole-e-anar) is the symbol of peace, love, and kindness in Iranian culture. In a Persian Achaemenid limestone bas-relief (Figure 2), a flower can be seen in the hand of the king (maybe Darius I or Xerxes) as a symbol of peace. It is interesting to note that the blossom of P. granatum var. pleniflora (Golnar-e Farsi) is known as Golnar and the flowers of other varieties are called Gole-e-anar. Persian Golnar is a plant that is grown for ornamentation and produces unproductive blossoms (Figure 3).
Figure 2.

A Persian Achaemenid limestone bas-relief. (©Memranet.com)
Figure 3.
Blossoms of Punica granatum
Male unproductive blossoms (left) vs female productive blossoms (right)(©Mesarbustes.fr)
Pomegranate in Islamic Traditional Medicine (ITM)
Two kinds of pomegranates are described in ITM textbooks namely wild and cultivated ones with two distinct tastes sweet and sour (12). However, some ITM scientists believed that there are four tastes: astringent (un-ripped pomegranate), sweet, sour, and sour-sweet. Pomegranate’s temperament differs by its type; the sweet one is cold and moist in the first degree, and the sour type is cold and dry in the second degree. The sour-sweet pomegranates, tend to be moderately dry and cold (for more information about the humors you can see Bone and Mills (73)). Sweet pomegranate is good for cold people because it makes their stomachs warm, but harmful for people with acute fever. Sour-sweet pomegranates are good for people with moderate temperament.
From a traditional point of view, pomegranate, as a low-calorie fruit, is generally an astringent, drying, and cooling agent. The most reported medicinal activities for pomegranate are attributed to its astringent property that stimulates the contraction of bodily tissues; often used to soothe the skin and stop bleeding. All types of pomegranates are astringent, however, depending on the types (sweet, sour, and sour-sweet), the benefit of each is according to the prevailing taste (14, 19). It is believed that the astringency and drying properties of the pomegranate seeds are more than the juice and of the peel more than both and interestingly of the un-blossomed flowers (that fall from the trees) much more than all the latter (19). The pomegranate seed is more drying than its juice, but the peel and fleshy mesocarp are more drying than the seed. The drying power of the flower of pomegranate (known as Golnar in Persian) is like peel and fleshy mesocarp. Golnar is used as a highly astringent agent in many traditional prescriptions such as treatment of the wounds (14, 17, 19). Moreover, the astringency property of all parts of sour pomegranate is more than the sweet ones (19, 74). A point that should be considered is that when talking about the seeds of pomegranate (known as Nardoon in Persian) in ITM, it means the sun-dried seeds with the arils not the seeds as a waste or byproduct (12-15, 19, 74-76).
Interestingly, almost all parts of the pomegranate, including, fruits, seeds, peel, leaves, bark, root, flowers (ripped and un-ripped), and even the calyx and stamens (pomegranate’s crown) are used medicinally in ITM. In the following paragraphs, all the mentioned medicinal applications of pomegranate in major ITM textbooks will be discussed briefly and its applications in modern medicine will be cited in detail.
Gastrointestinal system
Pomegranate is believed to be useful for stomach and intestines (17, 18, 74). In some ITM textbooks, it is mentioned that while sweet pomegranate has beneficial effects on the digestive system, sour pomegranate is harmful to the stomach and scrapes the bowels. Therefore, it should be eaten with sweeteners like Halva Ardeh (a kind of Iranian sweet made of sesame and sugar) or honey (13, 15). Avicenna also believed that while the sweet and sour-sweet pomegranates are useful for the stomach, the sour one is not good. He emphasized that the benefits of sweet pomegranate for the stomach are even more than apples and quince (17). In Menhaj: if old people want to eat a sour pomegranate, they should mix it with Balang jam (a kind of jam made from the peel of Citrus medica) (15). Al-Aghraz: both sweet and sour pomegranates are described to remove stomach heat; however sweet pomegranate is useful for the stomach and sour one is harmful (18). On the contrary, in many ITM textbooks such as Al-Jamee, sour pomegranate is also described to have beneficial effects for inflamed stomachs (14). Al-Jamee: both sour and sweet pomegranates if extracted along with their fleshy mesocarp and mixed with red sugar are stomach-strengthening agents (14). The probable point is that in Al-Jamee, sour pomegranate is prescribed with its mesocarp which has astringent properties, so it would tan the stomach. Also, Hakim Mohammad Momen Tonekaboni believed that the extract of both sweet and sour pomegranates along with their fleshy mesocarp would strengthen the stomach (75).
Razi believed that if the seeds of sour pomegranate (sun-dried seeds) are used in food, it would prevent the flow of excess humors to the stomach and intestines (14, 19). And elsewhere in his book, it is said that pomegranate juice has the property of preventing the flow of wastes into the stomach and intestines. It is also useful for treating a fever that causes diarrhea.
In many of the studied references, an anti-parasitic activity is described for pomegranate (18, 75). Razi in his book Al-Hawi has mentioned: “Pomegranate root skin when cooked with rock candy is useful for treating abdominal worms and removing Taenia eggs” (19). Ibn Beytar in his book Al-Jamee Le-Mofradaat al- Adwiah wal- Aghḏiyah (Comprehensive book in Simple Drugs and Foods): Administration of pomegranate peel in patients with intestinal worms and drinking high-temperature water after that would remove the parasites (14). Hakim Mohammad Momen Tonekaboni also believed that the administration of a drink prepared from the milled pomegranate skin in warm water is a certain cure for patients with intestinal worms (14, 75).
In many ITM textbooks, the beneficial properties of pomegranate for healing intestinal wounds, its anti-diarrhea, and its stomach-strengthening activities are mentioned. It has been written in Al-Hawi that edible use of pomegranate calyx, tends to tan the stomach and dry digestive wounds (19). Purging an infusion prepared from pomegranate in combination with Oryza sativa (rice) and barley to the digestive tract has been prescribed for the treatment of diarrhea and intestinal wounds (14, 75). Sitting in a bowl containing an infusion of pomegranate is also recommended for healing intestinal wounds, particularly wounds in the lower parts of the intestines (19). Golnar is also mentioned to be useful for treating these kinds of wounds. Pomegranate seed extract, especially sour pomegranate, has been used for anal wounds and hemorrhoids when cooked with honey (19). If a peeled sour pomegranate along with its mesocarp is milled in a stone mortar and squeezed, then half of the obtained extract is mixed with 10 parts of red sugar and applied, it might cause laxative effects (15). The oral use of sour pomegranate is useful for bile diarrhea and nausea (19). Roasted pomegranate seed flour is a stomach-strengthening and anti-diarrhea agent (19). Pomegranate paste also strengthens the stomach by tanning it (19). Administration of 17-25 pills (pepper seed size) prepared from the skin of sour pomegranate cooked with concentrated vinegar would heal diarrhea, and stomach, intestinal, and anal wounds (14, 75). Administration of a mixture prepared of three to seven unripe pomegranate buds, some acacia leaf, and a little white cumin (milled in a stone mortar) for three to seven consecutive days would heal infants and children with diarrhea (the number of pomegranate buds depends on the child’s temperament, age, and physical strength) (13). In addition, preparation was made from the flowers of pomegranate for the improvement of hernia (19).
Precautions
The sweet pomegranate produces little heat and flatus so it is not recommended for people with a warm temperament like people with acute fever (19). Razi in his book (Eliminating the Harms of Foods): “Sweet pomegranates cause a bit of bloating. Eating after a meal will lower the food from the stomach and need no adjustment because the bloating will quickly subside. But sour pomegranates have a longer stopping time in the stomach, causing bloating and severely cooling the liver, especially if used continuously. It is more harmful to cold people because it cools their liver and prevents the liver from absorbing food and thereby causes diarrhea”.
In other countries
Chinese and Mexican populations have historically used pomegranate exocarp to cure gastrointestinal conditions like diarrhea, dysentery, and stomachaches (77, 78). The antidiarrheal activities of punicalagin, corilagin, and ellagic acid (found in ethyl acetate fraction) alone or in combination have been confirmed (78). In India, fruit juice is traditionally used to treat dysentery by mixing it with warm water twice daily and giving it to anemic people as a tonic. Ash produced by burning seeds has a styptic quality. Fruits are consumed in their natural form to treat jaundice and strengthen the heart. Bark powder is used as an astringent with a spoonful (79). Moreover, fruit is a good source of iron in Pakistan. The fruit of the plant is consumed to treat iron deficiency. Its bark is used to treat nasal congestion. When the fruit’s epicarp is dried, it is administered to cattle to cure diarrhea (80). Traditional Thai herbal medicine for treating diarrhea or bloody mucous diarrhea contains pomegranate pericarp. Studies were conducted on the formulation’s antibacterial activity against microorganisms known to cause diarrhea, such as Staphylococcus aureus, Vibrio cholera, and Vibrio parahaemolyticus. Except for V. cholera, all of the bacterial pathogens examined demonstrated inhibitory zones (6.3-14.8 mm) in response to the P. granatum extracts (ethanol and water)(81).
Evidence from modern medicine (Gastrointestinal system)
Decoctions and extracts from different parts of P. granatum fruit have been shown to alleviate gastrointestinal diseases such as diarrhea, gastrointestinal tumors, gastrointestinal infections such as infections caused by Helicobacter pylori, and inflammatory disorders. The studies consisted of in vitro, in vivo, and clinical studies (Table 2). Despite promising pharmacological activities observed in preclinical studies, there are still very few human clinical trials that study these effects. Hence, to thoroughly explore the benefits of pomegranate derivatives on the human gastrointestinal system and their safety, further clinical trials need to be done.
Table 2.
Therapeutic potential of Punica granatum on the gastrointestinal system
| Model | Activity | Plant part/compd. | Study design | Mechanism | Ref. |
|---|---|---|---|---|---|
| Bacterial | Antibacterial properties against enteropathogens | Fruit exocarp (aqueous and methanol extracts) |
Antibacterial activity against two E. coli species, two Shigella sonnei species, two Shigella flexneri species, and two Salmonella species | Both extracts were active against all microorganisms with inhibition percentage ranging from 36 to 100%; MIC values ranging from 1 to 4 mg/ml |
(77) |
| Bacterial | Antibacterial activity against enterohaemorrhagic E. coli | Fruit shell (aqueous extract) |
E. coli O157:H7, O26:H11, O111:NM and O22; Paper disc agar diffusion method | Growth inhibition; MIC= 0.19mg/ml and MBC= 0.39 mg/ml |
(82) |
| Bacterial | Antibacterial activity against H. pylori | PPE (methanol extract) |
Disc-diffusion method; H. pylori strains; 100 µg/disc plant extract, standard = 8 µg/disc Metronidazole | Growth inhibition; Inhibition zone diameter= 39±3.4 mm |
(83) |
| Bacterial | Antibacterial effect against H. pylori | PPE ethanol extract | Twelve H. pylori clinical isolates; Disk diffusion method; Two-fold agar dilution method | ↓Urease activity; IC50= 6 mg/ml |
(84) |
| Protozoal | Antiprotozoal activity | Fruit exocarp (methanol extract) |
Susceptibility assay; Subculture method; Entamoeba histolytica strain HM1-IMSS and Giardia lamblia isolated IMSS:1090:1; Concentrations (2.5–200 µg/ml); Positive controls=metronidazole and emetine, control=culture medium plus trophozoites and DMSO, blank=culture medium | Active against Entamoeba histolytica; IC50= 29.5 µg/ml |
(85) |
| Cellular | Chemopreventive against colon cancer | Urolithin-A | In vitro (colon cancer cells), and ex vivo in adenoma and normal mucosa from Pirc rats | ↓COX-2 protein expression; ↑C-CASP-3 expression |
(86) |
| Cellular | Antiproliferative in colon cancer cells | PJ | Human colon carcinoma cell lines HT-29 | ↑miR-126; ↓VEGF, IGF and pPI3K, AKT |
(87) |
| Cellular | Anti-inflammatory effect | Pomegranate beverage (polyphenolics) |
Lipopolysaccharide (LPS)-treated CCD-18Co colon-myofibroblastic cells | ↓Ki-67 proliferative index and miR-145; ↓TNF-α, IL-1β, COX-2, and iNOS, p70S6K1 and HIF1α |
(88) |
| Cellular | Suppressing cancer progression and tumor angiogenesis of pancreatic and colon cancer | Pomegranate fruit extract | Human pancreatic cancer (Suit-2) and colon (colo205); Chick chorioallantoic membrane (CAM) cancer implant model | ↓Tumor weight and Hb concentrations, FGF2 expression | (89) |
| Animal | Anti-cancer effect | PPE (ethanol extract) | HPLC for determining EA in samples. MTT assay, apoptosis and scratch assay, gelatin zymography, and quantitative RT-PCR to determine the anti-cancer properties, immunosuppressed C57BL/6 mice carrying human gastric adenocarcinoma cell line | ↓P53, BAX, APAF1, BCL2, iNOS, NF-κB, IL-8 and TNF-α; ↑Cancer cell death |
(90) |
| Animal | Antidiarrheal effect | PPE (aqueous extract; ethyl acetate fraction) | BALB/c mice and Wister rats; orally; 100, 200, 400 mg/kg; 4 hr; in vivo castor oil-induced diarrhea and ex vivo ileum tissues | ↓Diarrhea by inhibiting gastrointestinal transmission and intestinal juice accumulation; Protects against intestinal epithelium injury induced by castor oil |
(78) |
| Animal | Antidiarrheal effect | PPE (aqueous extract) | Adult albino rats; Intraperitoneal injection; 100, 200, 300, and 400 mg/kg; Standard=loperamide; 1 ml of castor oil orally; 5 hr | ↓Diarrhea in a dose-dependent manner by inhibiting intestinal motility and intestinal fluid accumulation; IC50= 174±4 mg/kg |
(91) |
| Animal | Anti-inflammatory activity on the gastrointestinal tract | PPE (aqueous extract) | In vitro (Caco-2 cells) and ex vivo (porcine colonic tissue explants) | ↓Pro-inflammatory cytokines (IL-1A, IL-6 and CXCL8) | (92) |
| Animal | Antisecretory activity on cholera toxin-induced intestinal secretion | Fruit exocarp (methanol and aqueous extract) |
Rat jejunal loops model; Male Sprague–Dawley rats; 300 mg/kg orally; Standard=loperamide | Inhibition of intestinal secretion by 55.9±3.6% in methanol and 19.1±6.9 % in aqueous extract | (93) |
| Animal | Inhibition of gastric mucosal injury | Fruit rind (methanol extract) |
Male Wistar rats; 250 mg/kg and 500 mg/kg plant extract; Standard=ranitidine 400 mg/kg; Aspirin- and ethanol-induced gastric ulceration | ↓Lipid peroxidation levels; ↑Glutathione levels; Inhibition of aspirin-induced gastric ulcer (22.37-74.21%) Inhibition of EtOH-induced gastric ulcer by 21.95 and 63.41% |
(94) |
| Animal | Reduction in inflammation and ulceration scores in intestinal colitis | Pomegranate beverage (polyphenolics) |
Dextran sodium sulfate (DSS)-induced colitis in Sprague–Dawley rats | Protection against DSS-induced colon inflammation and ulceration (50% and 66.7%, P=0.05 and 0.045, respectively) | (88) |
| Animal | Inhibition of diarrhea and gastrointestinal transit (GIT) | Fruits (Gabsi and Garsi varieties); (Juice, total extract, and methanol extract) |
Male Wistar rats; Castor oil-induced diarrhea and charcoal meal test (10% charcoal in 5% gum Arabic); Standard antidiarrheal drug=diaretyl (10 mg/kg, p.o.) | ↓Diarrhea dose-dependently and GIT dose-dependently; The Garsi variety was more effective |
(95) |
| Animal | Chemopreventive against colorectal cancer | Mesocarp (decoction) | Pirc rats; Orally; 6 weeks | ↓Mucin-depleted foci, as CRC biomarkers (P=0.02); ↑Apoptosis (P<0.01) |
(86) |
| Animal | Protective effect in Crohn’s disease | Ellagic acid | Male Wistar rats; Induction of colitis by trinitrobenzene sulfonic acid (TNBS); Ellagic acid (10–20 mg/kg p.o. administered by gavage 48, 24, and 1 hr prior to the induction of colitis and 24 hr later | ↑Mucus production in goblet cells; ↓Neutrophil infiltration and COX-2 and iNOS overexpression, activation of p38, JNK, and ERK1/2 MAPKs |
(96) |
| Animal | Antioxidant against azoxymethane-induced colon cancer | PPE | Male Sprague-Dawley rats; Chow diet and normal tap water; 2 intraperitoneal injections of AOM dissolved in physiological saline once a week (15 mg/kg) for 2 weeks | ↑Glutathione and TAC levels in colonic mucosal tissue | (97) |
| Animal | Suppressing azoxymethane-induced colorectal aberrant crypt foci and inflammation | PJ | Male Sprague-Dawley rats, received PJ (2504.74 mg gallic acid equivalents/l), intraperitoneal injection of AOM 15 mg/kg for 2 weeks | ↑miR-126; ↓COX-2, iNOS, NF-κB (p65) and VCAM-1, IGF and PI3K, AKT, mTOR mRNA, and protein level |
(87) |
| Animal | Anti-inflammatory activity in the acute and chronic colitis | Ellagic acid | Female BALB/c and C57BL/6 mice; Dextran sulfate sodium-induced colitis; 100 mg/d/mouse ellagic acid administered orally | ↓COX-2, iNOS, NF-κB, IL-6, TNF-α, and IFN-γ | (98) |
| Animal | Reducing colitis-induced visceral pain | Pomegranate mesocarp decoction; Polysaccharides; Ellagitannins | Male Sprague–Dawley rats; 2,4-dinitrobenzenesulfonic acid-induced colitis; Pomegranate whole decoction (300 mg/kg), polysaccharides (300 mg/kg), and ellagitannins (45 mg/kg) orally for 14 days | ↓Mast cells and density of collagen fibers in the mucosal stroma | (99) |
| Animal | Antibacterial effect against H. pylori | PPE (ethanol extracts) |
Female Wistar rats, inoculated by gavage 1 ml/rat with H. pylori suspension of 9 McFarland twice daily, treated after 7 weeks with PPE 50 mg/kg | ↓Urease activity | (84) |
| Clinical | Changes in gene expression in colon tissues from colorectal cancer patients | Pomegranate extract | RCT; Programmed colonoscopy (n=2501), older than 18 years and confirmed CRC diagnosis | ↓CDKN1A, EGFR, TYMs, CD44, CTNNB1 | (100) |
1 Pomegranate peel extract; 2 Pomegranate juice
Respiratory system
Sweet pomegranate is useful for chronic coughing, the roughness of the throat, and chest pain and acts like a mucus-softening agent (12, 14-16, 19, 76). It is mentioned in Al-Hawi that when soaked in alum and rainwater, pomegranate is useful for the throat and lungs (19). Also, Avicenna believed when pomegranate seeds are mixed with rain water, it would be beneficial for the lower respiratory system (17). Studying the Islamic traditional textbooks showed that pomegranate is highly recommended for dry coughing (16). For instance, Ibn Beytar prescribed a mixture of pomegranate with the oil of sweet violet (Viola odorata) for dry cough (14). This beneficial effect is also mentioned by many other ITM physicians like Ibn Nafis Qarshi (16), Dawoud Antaki (12), and Ghasani (76). On the other hand, sour pomegranate is harmful to the respiratory system and it hurts the lungs and throat (15).
There is a tried prescription in several ITM textbooks for the treatment of chronic and dry coughing: “It would be a certain cure for chest pain and coughing when the pomegranate’s head is pierced and repeatedly filled with sweet almond or viola oil (to the extent its capacity allows), then put on the fire to absorb the oil; drinking the obtained extract would completely remove coughing”. It is also mentioned that drinking PJ with sugar, starch, Arabic gum, and almond oil has the same effect (13, 14, 74, 75).
Evidence from modern medicine (respiratory system)
Different preparations from P. granatum and their major components such as EGCG have been reported to reduce the severity of respiratory system problems (Table 3). Some studies have evaluated the efficacy of P. granatum during the recent pandemic (101). In recent years, P. granatum fruit has been used to treat and prevent a variety of respiratory illnesses. Pomegranate fruit, juice, extract, peel powder, and oil have been shown in in vitro and in vivo studies to have positive effects on a variety of respiratory conditions, including asthma, lung fibrosis, lung cancer, chronic obstructive pulmonary disease (COPD), and alveolar inflammation by modulating a number of different mechanisms, including anti-proliferative, anti-oxidant, anti-microbial, anti-viral, anti-inflammatory, anti-cancer, and anti-tumorigenic effects. Nevertheless, pomegranate has been used in a limited number of clinical trials as an intervention for various respiratory disorders (4). Consequently, to confirm the efficacy of this natural fruit for the prevention and treatment of lung-related disorders, either alone or in combination with other medicines, well-designed human clinical studies are advised.
Table 3.
Therapeutic potential of Punica granatum on the respiratory system
| Model | Activity | Plant part/Compd. | Study design | Mechanism | Ref. |
|---|---|---|---|---|---|
| Simulation | Antiviral activity against SARS-Cov-2 | PPE; Punicalagin; Punicalin; Urolithin A | Molecular docking simulation through Yasara Structure software based on the AutoDockLGA algorithm and AMBER03 force field; Molecular dynamics simulation in LARMD server | ↓SARS-CoV-2 S-glycoprotein binding ability to ACE2 receptor PPE MIC= 0.06 mg/ml Punicalin MIC= 0.14 mg/ml |
(102) |
| Bacterial | Antimicrobial activity against Mycobacterium tuberculosis and b-lactamase-producing Klebsiella pneumoniae | Fruit compounds: caffeic acid and ellagic acid; EGCG and quercetin |
Double-disc synergy; Phenotypic confirmatory test for ESBL detection; Modified Hodge test | PPE: antimycobacterial activity; MIC 64–1024 mg/ml |
(103) |
| PJ: antimycobacterial activity; MIC 256 -41024 mg/ml | |||||
| EGCG and quercetin: antitubercular and antibacterial; MIC 32–256 mg/ml; MIC 64–56 mg/ml | |||||
| Caffeic acid and ellagic acid: antitubercular and antibacterial activity; MIC 64–512 mg/ml | |||||
| Viral | Antiviral activities against human respiratory syncytial virus (RSV) | Fruit cortex (aqueous extract) |
Cytopathic effect reduction assay | Anti-RSV activity IC50= 62.5 µg/ml |
(104) |
| Viral | Antiviral activity against SARS-Cov-2 | PPE | ABTS assay for antioxidant effects; SARS-CoV2 inhibitor screening assay kit; Three extract concentrations ranging from 0.04 mg/ml to 1 mg/ml; 3CL protease assay (0.04 and 0.2 mg/ml) | ↓SARS-CoV-2 S-glycoprotein binding ability to ACE2 receptor and Activity of the virus 3CL protease | (20) |
| Viral | Antiviral activity against SARS-Cov-2 | Punicalagin | 3CL protease assay kit (BPS Bioscience) | ↓Activity of the virus 3CL protease; IC50= 6.192 μg/ml |
(105) |
| Cellular | Reduction in lung inflammation | PPE (Aqueous extract) |
In vitro: human neutrophils | ↓LPS-induced lung inflammation and myeloperoxidase activity | (106) |
| Cellular | Non-small cell lung carcinoma treatment | Leaves extract | Non-small cell lung carcinoma cell line A549, H1299 and mouse Lewis lung carcinoma cell line LL/2; Cell viability and colony formation assay; Wound-healing migration assay; Cell cycle and apoptosis analysis by flow cytometry; Mitochondrial membrane potential (∆Ym) assay; Detection of ROS | Arresting cell cycle progression in G2/M; Blocked H1299 cell migration and invasion; ↓Metalloproteinase 2 and 9 expressions, ROS and ∆Ym; IC50= 47 μg/ml |
(107) |
| Cellular | Induced cell cycle arrest and apoptosis in human lung Adeno carcinoma A549 Cells | PJ | A549 cells treated with PJ (2% (v/v)) at several time exposures (0–72 hr); Quantification of apoptosis by Annexing V Labeling; Caspase-3, -8 and -9 analyses; MMP analyses | Induced cell cycle arrest at G0/G1 and apoptosis through intrinsic pathway; Loss of MMP; Release of cytochrome c in the cytosol; Activation of caspase-3 and -9 | (108) |
| Cellular | Suppress microsomal prostaglandin E synthase-1 expression and induce apoptosis lung cancer | Ellagitannins; Leaves extract | A549 cells were incubated either with or without 10 ng/ ml IL-1β; 10 μM granatin A, granatin B, or geraniin for 24 hr; Enzyme immunoassay; TUNEL assay | ↓mPGES-1 expression without affecting COX-2, TNF-α, inducible nitric oxide synthase, anti-apoptotic factor; B-cell chronic lymphocytic leukemia/lymphoma 2 | (43) |
| Cellular | Anticancer activity | Fruit extracts of immature pomegranate | Human lung H1299 adenocarcinoma cells; 100 μg/ml of extracts from different fruit matrices | Induction of caspase-3 activity | (109) |
| Animal | Reduction in lung inflammation | PPE (Aqueous extract) |
Male BalbC mice; intraperitoneal injection of 200 mg/kg extract; LPS-induced lung inflammation (5 µg intratracheal LPS instillation) | ↓LPS-induced lung inflammation and myeloperoxidase activity | (106) |
| Animal | Alleviating asthma | Leaves (ethanol extract/microencapsulated extract) |
Female BALB/c mice; Ovalbumin-induced asthma; Encapsulated extract (10 mg/ml, 25 µl per nostril)/ non-encapsulated pomegranate extract (20 mg/Kg, 25 µl per nostril); Intranasal instillation; Standard=dexamethasone | ↓Leukocytes' (eosinophils) recruitment to bronchoalveolar fluid, IL-1β, and IL-5 in the lungs | (110) |
| Animal | Bronchospasmolytic effects | PPE (Aqueous extracts) |
Isolated guinea pig trachea chains; Contractions induced by acetylcholine or histamine; 10 mg/ml plant extract | ↓Force of contraction by histamine by 30-70% | (111) |
| Animal | Protection against acute lung injury | Alloyl-hexahydroxydiphenoyl (HHDP)-glucose (isolated from leaves) |
Male BALB/c mice; intra-tracheal lipopolysaccharide (LPS)-induced acute lung injury; galloyl-HHDP-glucose (5, 50, and 100 mg/Kg); standard= dexamethasone at 5 mg/Kg | ↓TNF-α, IL-6, and IL-1β gene expression and protein levels, lung inflammation, and cell accumulation | (112) |
| Animal | Protection against acute lung injury | Punicalagin | RAW 264.7 murine macrophage cell line; Immunocytochemical analysis | ↓TNF-α, IL-1β, IL-6, protein concentration and myeloperoxidase activity; Suppressing p38 MAPKs and NF-κB pathways |
(113) |
| Animal | Protection against acute lung injury | Punicalagin | Male BALB/c mice with acute respiratory distress syndrome induced by intranasal instillation of LPS (20 mg/kg); Treated with punicalagin (12.5, 25, and 50 mg/kg) 1 hr prior to LPS exposure; Control=dexamethasone (5 mg/kg) | ↓TNF-α, IL-1β, IL-6, macrophage and neutrophil infiltration, myeloperoxidase activity, TLR4 expression and NF-κB activation pathways | (114) |
1 Pomegranate peel extract; 2 Epigallocatechin Gallate; 3 Pomegranate juice
Skin and wound healing properties
One of the most mentioned properties of pomegranates in ITM textbooks is their wound-healing activity (12). Since the flowers, buds, and calyx of pomegranates are the most astringent parts, administration of them has been strongly recommended for the treatment of wounds and injuries, as well as removing scars. However, other pomegranate parts and preparations such as its juice and peels have also been used for this purpose. Razi in his book Al-Hawi has mentioned: “Due to its highly astringent, drying and cooling properties, Golnar when applied to wounds or scratches, quickly heals ulcers and blocks bleeding” (19). Administration of an ointment prepared by grinding pomegranate flowers or buds and mixing them with honey on smallpox scars and other wounds for several consecutive days would eliminate the scars (14). In many textbooks, the burned flower or calyx has been mentioned to have such activity (15-17). Moreover, sweet pomegranate extract benefits wounds and infections when cooked in a copper dish (14).
Pomegranate has also been prescribed for other skin problems such as paronychia, scabies, thrush, erysipelas, and pruritus (12-14, 16, 75). For instance, both sour and sweet pomegranate juice along with their fleshy mesocarp concentrated in a copper pot have been prescribed for treating paronychia and scabies (75).
Evidence from modern medicine (Skin and wound)
Pomegranate is known to minimize photoaging and chronological skin aging by different anti-oxidant and anti-inflammatory mechanisms. These effects are mainly due to the presence of potent polyphenols (ellagitannins and ellagic acid) (115). Pomegranate also has presented four effects considered important to the treatment of skin and soft tissue infections (antimicrobial, anti-oxidant, anti‐inflammatory, and healing) (116). Pomegranate whole fruit is considered to have protective activity against chemical-induced and ultraviolet (UV) radiation-mediated cutaneous damage, including carcinogenesis (117).
The studies that have been done on this topic consist of in vitro, in vivo, and clinical studies (Table 4). These studies mostly show anti-aging, UV radiation protection, wound healing, and skin-lightening effects of pomegranate extracts. Most of the clinical studies have been conducted on the anti-aging effect of this plant’s extracts, and they concluded that pomegranate extracts can alleviate the skin aging process in humans.
Table 4.
Therapeutic potential of Punica granatum on skin and wound
| Model | Activity | Plant part/compd. | Study design | Mechanism | Ref. |
|---|---|---|---|---|---|
| Bacterial | Antimicrobial and antivirulence activities against Pseudomonas aeruginosa | PPE (Aqueous extract) |
P. aeruginosa collected from burn wound cultures | ↓Bacterial gelatinase activity by 40.28±2.35%; ↓Lecithinase activity by 53.84±4.89%; MIC= 1.4 mg/ml |
(118) |
| Cellular | Protection against skin photoaging induced by UVB irradiation | Rind, seed, and fruit (methanol extracts) | Normal human dermal fibroblasts; Irradiated with UVB (170 mJ/cm2) for 1 min. | ↑Procollagen type I and MMP-1 expression (especially by rind extract); ↑Collagen synthesis |
(119) |
| Cellular | Inhibition of melanin content | Fruit (ethanol extract) |
Melan-a melanocyte cells; Melanin inhibition assay; Fruit extract standardized to 20% punicalagin; at concentrations of 50 µg/ml and 100 µg/ml | ↓Melanin content by 40% (50 µg/ml) and 60% (100 µg/ml) | (120) |
| Cellular | Anti-tyrosinase activity | Rind (methanol extract) |
Murine melanoma B16 F0 cells; Positive control=Kojic acid (1%); Extract concentrations (200–500 mg/ml) | Tyrosinase inhibition at 500 μg/ml= 82.3±5% | (121) |
| Cellular | Inhibition of melanin production | Fruit (ethanol extract) |
Testing Salvia hispanica (chia) seed extract and P. granatum fruit extract (20% punicalagin), in combination or alone; Melanin inhibition assay: Melan-a cells, positive control=phenylthiourea 60 μg/ml; Tyrosinase enzyme assay: enzyme extracted from B16 melanoma cells, positive control=kojic acid; Melanogenesis-related gene expression (RT-PCR) analysis: Melan-a cells, positive control=ascorbic acid-2-glucoside | ↓Melanin content (1:1 ratio of the combination of extracts showed maximum efficacy); Not effectively inhibiting tyrosinase activity; ↓Tyrosine, Tyrp1 and Mc1r expression |
(122) |
| Cellular | Protection against oxidative stress | Pomegranate extract | Human keratinocyte HaCaT cells treated with extract for 24 hr; Methylglyoxal-induced cytotoxicity, | ↓Methylglyoxal-DNA adducts, reactive oxygen species; ↑Cell adhesion, migration, and wound healing capacity |
(123) |
| Animal | Chemopreventive agent against skin cancer | Seed oil | Female CD1 mice; Skin cancer initiated with topical exposure of 7,12-dimethylbenzanthracene and with biweekly promotion using 12-O-tetradecanoylphorbol-13-acetate (TPA); Pretreated with 5% pomegranate seed oil | ↓Tumor incidence (P=0.05) and multiplicity; ↓TPA-induced ornithine decarboxylase (ODC) activity (17% reduction) |
(124) |
| Animal | Cutaneous wound healing | Pomegranate peel polyphenols (PPP) gel | Adult alloxan-induced diabetic Wistar rats; Polyphenol mass fraction in PPP gel=30%; | ↓Wound closure; ↑Fibroblast infiltration, collagen regeneration, vascularization, and epithelialization in the wound area; ↑Hydroxyproline, NO production, and NO synthase activities; ↑Transforming growth factor-β1 (TGF-β1), vascular endothelial growth factor (VEGF), and epidermal growth factor (EGF) expressions |
(119) |
| Animal | Wound healing activity | PPE (methanol extract) |
Wistar rats; Water-soluble gel of extract; Negative control=no gel, positive control=blank gel, commercial preparation=silver sulfadiazine; Treatment with 2.5% and 5.0% gel | ↑Wound healing by 55.8% at 2.5% and 59.5% at 5.0% (on day 15); ↑Hydroxyproline content up to 34.5% (2.5%), and 48.5% (5.0%) compared with the negative control; ↑Hydroxyproline 24.1% (2.5%) and 38.0% (5.0%) compared with the positive control |
(125) |
| Animal | Wound healing activity | Male abortive flowers (ethanol extract) |
Male Wistar rats; reference standard (nitrofurazone ointment); topical treatment (extract ointment 200 mg/kg/day); Control (simple ointment); 16 days | ↓Wound size, inflammation cells, collagen fibers, and re-epithelization | (126) |
| Animal | Anti-inflammatory activity on the skin | Rind (aqueous extract); Total pomegranate tannins (TPT) |
Porcine skin mounted in Franz diffusion cells; Test solutions (1 ml): pomegranate rind extract (PRE) (consisted of 20% w/w punicalagin), PRE + ZnSO4, ZnSO4, TPT, tannin-free fraction (TFF) and control=phthalate buffer pH 4.5; Analysis of COX-2 by SDS PAGE and western blotting and Immunohistochemical (IHC) | TPT and PRE and PRE + ZnSO4: ↓COX-2 expression (after 6 hr and up to 24 hr); No anti-inflammatory activity by ZnSO4 and TFF and control |
(127) |
| Animal | Antiaging effect on UVB-induced skin | Pomegranate juice concentrated powder (PCP) | Female SKH‑1 hairless mice; 6 groups: Intact vehicle control‑unexposed; UVB control‑UVB exposed (mice received vehicle control and UVB exposure); PCS 2 ml/kg ‑UVB exposed; PCP 100 mg/kg‑UVB exposed; PCP 200 mg/kg‑UVB exposed; and PCP 400 mg/kg‑UVB exposed; UVB irradiation three times a week for 15 weeks at 0.18 J/cm2; PCS (contained 2.31 mg/g ellagic acid) | Anti-apoptotic effects; Skin matrix metalloproteinase activity inhibition; ↓Wrinkles induced by photoaging; ↑Skin water contents, collagen type I, and hyaluronan contents; ↓IL‑1β; Inhibition of glutathione depletion |
(128) |
| Animal | Wound healing activity | Whole fruit extract standardized with 40% ellagic acid | Male albino rats (Rattus norvegicus); Treatment of incised wounds with topical ointment of pomegranate extract at 2.5, 5, and 7.5% concentrations or Betadine® ointment, twice a day for 7 and 14 days | ↑Wound healing in the application of 7.5% pomegranate extract ointment for 14 days; ↑Collagen deposition; ↓Polymorphonuclear neutrophils infiltration in wound area; ↑Angiogenesis |
(129) |
| Animal | Wound healing activity | PPE | Inbred Sprague–Dawley male rats; excision wound model; Control group: petroleum jelly, experimental group: PPE (100 mg/kg) +petroleum jelly, standard group: mupirocin ointment (100 mg/kg); 15 days | Wound contraction of 95% (P<0.01) by day 15 in the experimental group; ↑Hydroxyproline content (P<0.05) |
(130) |
| Animal | Anti-psoriasis activity | Punicalagin | Male BALB/c mice (6–8 weeks of age), imiquimod (IMQ)-induced psoriasis; Five groups: Control group, IMQ group, IMQ + DEX group (DEX, dexamethasone cream, positive drug for psoriasis), IMQ + Vehicle group, IMQ + PUN group (25 mg/kg PUN) | Inhibiting NF-κB-mediated IL-1β transcription and caspase-1-regulated IL-1β secretion | (131) |
| Clinical | Anti-aging activity | Arils (methanol extract) |
12 human volunteers aged from 35 to 60 y/o, divided into 2 groups, 2 times daily topical application for a month, one group received a placebo | ↓Free radicals | (132) |
| Clinical | Anti-aging activity | Fermented pomegranate extract | Double-blind, 35–55y/o, 40 healthy subjects, half on placebo, drink 50 ml daily; Another 40 subjects, half on placebo, apply 3 ml serum over their face 2 times daily for 4 weeks | ↓Free radicals; ↑Skin moisture, brightness, and elasticity |
(133) |
| Clinical | Skin lightening activity | PPE | Split-face, randomized, double-blind, placebo-controlled test of 30 subjects divided into 2 groups; Applied the preparation on half of their face | ↓Free radicals; Tyrosinase and TRP-2 inhibition |
(134) |
| Clinical | Protection against radiation-induced skin and mucosal changes | Whole fruit extract | Prospective, clinical, double-blind, case-control study; 60 patients (30 cases and 30 controls) undergoing radiotherapy for head and neck cancer; 12 months; 2 capsules/day for 6–7 weeks; each capsule containing 300 mg extract (40% polyphenols and 27% punicalagin) | ↓Severity of radiation-induced mucositis and dermatitis (P<0.0001) | (135) |
| Clinical | Prevention or improvement of skin changes associated with striae | Seed oil | An interventional, nonrandomized study; 20 healthy females; 21–48 years old: 10 with striae albae at the hip level and 10 with no stretchmarks; Measurements of skin hydration level, skin elasticity, and thickness of the dermis at the beginning and after 3 and 6 weeks; Application of oil-in-water cream containing plants once daily | ↑Dermis thickness, hydration, and elasticity values. | (136) |
| Clinical | Reduce photodamage from UVB irradiation | POMx® (Pomegranate extract); PJ |
A parallel, three-arm, open-label RCT; n=72, female, 30–45 years, with Fitzpatrick skin type II-IV, 3 groups: 1–1000 mg of pomegranate extract (PomX), 2- oz of PJ, 3- placebo; 12 weeks; determination of minimal erythema dose (MED) and melanin index by cutometer; | ↑MED; ↓Melanin formation |
(137) |
| Clinical | Improvements in several biophysical properties, wrinkles, and shifts in the skin microbiome | Pomella® (Pomegranate extract) |
Prospective, double-blind placebo-controlled study, healthy males and females aged 25–55 years; 4 weeks; BTBP 3D Clarity Pro® wrinkle assessment, SkinColorCatch® facial erythema index, and melanin index assessment | ↓Wrinkle severity (P<0.01), forehead sebum excretion rate (P=0.14), facial transepidermal water loss (P<0.05) | (138) |
Reproductive system
It is believed that sour pomegranate because of its cold and dry temperament may decrease the libido and the production of semen so it is better to be served with agents with a hot temperament like ginger jam, strong wine, and Shorba (a kind of soup containing garlic and aromatic spices)(74, 75).
Administration of pomegranate preparations such as its juice and paste has been repeatedly prescribed in ITM for decreasing nausea during pregnancy. Tonekaboni has mentioned in his book Tohfah: “Eating the flour obtained from dried seeds of pomegranate is useful for pregnant women who are willing to eat soil and mud” (75).
Pomegranate is believed to be useful for treating uterine wounds, infections, and inflammations (14, 19). Ibn Beytar has written in Al-Jamee: “Using a purged extract obtained from boiled PJ in combination with dill would heal the chronic infections in the uterine (14). He also added that sitting in a decoction from pomegranate peels has the same activity (14). And Razi said in Al-Hawi: “Sitting in a decoction prepared from the seeds of sour pomegranate is useful for the treatment of infections and inflammations in women’s reproductive system” (19). There is also a similar healing property for the flowers of pomegranate (Golnar) (19).
Pomegranate peel, due to its astringency and drying properties, benefits women with extra bleeding in their menstrual period. Tonekaboni prescribed sitting in a decoction of pomegranate peels in these circumstances (75).
Evidence from modern medicine
One of the main mechanisms of action for pomegranate against diseases related to the reproductive system is anti-oxidant activity. Age-related sexual dysfunctions are exacerbated by etiological variables such as organ damage, degenerative illnesses, and the strains of modern life. According to studies, pomegranate can reduce ROS activity in the testis and other organs. Sperms’ plasma membrane is mostly composed of unsaturated fatty acids. It is hence especially vulnerable to oxidative damage. Spermatozoa membranes’ lipid matrix is destroyed by lipid peroxidation, which is also linked to impairments in membrane integrity and loss of motility (139). Thus, pomegranate and its derivatives can inhibit free radicals via their anti-oxidant activities (140), improve sexual dysfunctions, and ameliorate oxidative stress and aging-induced related damages (Table 5). According to Table 5, most of these studies were in vivo and there is a need for more clinical studies to survey the effects of P. granatum and its derivatives on the reproductive system.
Table 5.
Therapeutic potential of Punica granatum on the reproductive system
| Model | Activity | Plant part/compd. | Study design | Mechanism | Ref. |
|---|---|---|---|---|---|
| Viral | Antiviral activity against HSV 1 and 2 | Freeze‑dried juice powder (FP); Aqueous extract (AE); Peels powder (PP) |
HSV‑1 clinical strain and HSV‑2 ATCC G strain; Control=acyclovir; Cytopathic effect inhibition assay; MTT assay | Anti-HSV activity; Anti HSV 1 IC50: FP= 30.6 µg/ml; AE= 15.8 µg/ml; PP= 250 µg/ml; Anti HSV 2 IC50: FP= 18.14 µg/ml; AE= 17.6 µg/ml; PP= 185 µg/ml |
(141) |
| Cellular | Anti-Ovarian cancer | Punicalagin | Human A2780 ovarian cells; Cell Counting Kit-8 assay; Flow cytometry analysis; Protein expression measured by western blot analysis; Wound healing assay | Arresting G1/S phase transition; ↑BAX, TIMP-2 and -3; ↓β-Catenin signaling pathway; activity, Bcl-2, invasion capability and activities of MMP-2 and MMP-9 |
(142) |
| Animal | Improvement of sperm quality | PJ | 28 adult male Wistar rats, divided into 4 groups; Group 1: 1 ml distilled water (control), group 2: 0.25 ml PJ plus 0.75 ml distilled water, group 3: 0.50 ml PJ plus 0.50 ml distilled water, group 4: 1 ml PJ, daily for 7 weeks | ↑Spermatogenic cell density, epididymal sperm concentration, and sperm motility; ↓ROS |
(140) |
| Animal | Reverses the deleterious effect produced by lead acetate (LA) | Pericarp ethanol extract | 30 adult male Holtzman rats; 5 groups: distilled water (control group), LA (lead acetate), LA with EEP (ethanol extract of pomegranate), LA with ascorbic acid (positive control), and EEP alone, respectively; 35 days | Protects the stages of mitosis (stages IX–XI), meiosis (stage XIV), and spermiation (stage VIII) in the spermatogenic cycle | (143) |
| Animal | Increases sex hormones | PPE (methanol extract); PJ |
18 adult male albino rats; 3 groups: control, 200 mg/kg PPE, 3 ml/kg juice, administered for 21 days; Testis indexed after | ↑Testosterone, FSH, LH, endogenous testicular antioxidant enzymes; ↓Lipid peroxidation and nitric oxide formation in testes |
(139) |
| Animal | Protection of testes against carbon tetrachloride intoxication | PJ | 28 Wistar albino male rats; 4 groups: control, CCl4, PJ and PJ+CCl4, CCl4 (2 ml/kg) administered via the intraperitoneal route once a week for ten weeks; PJ via drinking water 2 weeks before and concurrent with CCl4 | ↑Testosterone, FSH, LH, endogenous testicular antioxidant enzymes ↓Lipid peroxidation and nitric oxide formation in testes |
(144) |
| Animal | Sperm improvement in testicular torsion-detorsion | PJ | 21 male Wistar albino rats, 3 groups, control, ischemia/reperfusion (I/R), and PJ+I/R group (0.4 ml/day PJ orally over a period of eight weeks prior to surgery) | ↑Spermatid, spermatocyte and spermatogonia concentrations; ↓Superoxide dismutase and malondialdehyde |
(145) |
| Animal | Protection against 3G radiation-induced reproductive toxicity | PJ | Adult male Wistar rats, 5 groups, control, sham-exposed, 3G exposed, 3G exposed + juice and only juice groups, 3G exposure: 2 hr/day for 45 days, 6 days a week, vector signal generator model (VSG25A) | ↑Sperm count, motility, and viability; ↓Oxidative parameters |
(146) |
| Clinical | Improvement of erectile dysfunction | PJ | Double-blind RCT; Sixty sexually active, healthy males aged 21–70 years with a history of ED for at least 3 months duration, ED Score of 17–25 on the IIEF questionnaire, drink 8 ounces of juice every day for 28 days | ↑NO activity in vascular endothelial cells | (147) |
| Clinical | Anti-hemorrhagic activity against heavy menstrual bleeding | Flower | Double-blind RCT; In comparison with oral tranexamic acid, 123 eligible patients (20 to 49 years), non-anemic (hemoglobin (Hb) of≥10.5g/dl), had no history of previous thromboembolic disorders, chronic illnesses, or other diseases known to interfere with menstrual bleeding | ↓Duration of bleeding and menstrual blood loss | (148) |
Mouth and teeth
Again, because of its drying and astringent properties, pomegranate (particularly its flowers) has had traditional applications for mouth and teeth problems such as repairing loose teeth, oral ulcers, toothache, gum bleeding, and improving gum health (14, 19, 75, 76). A mouthwash from Golnar has been used to stop bleeding gums and repair loose teeth (19, 75). Heravi has prescribed Golnar for the treatment of mouth ulcers and infections as well as bad breath (74). Tonekaboni even suggested PJ for the treatment of malignant mouth ulcers (75). Sour pomegranate extract is useful for infectious mouth ulcers (14). Pomegranate seed extract, especially sour pomegranate, has been used for oral wounds when cooked with honey (19). Keeping PJ in the mouth has been used to strengthen the gums and heal malignant mouth ulcers (75). Interestingly, Heravi believed that pomegranate blunts the tooth because of its astringent properties, and added that sour pomegranate destroys the tooth (74).
Evidence from modern medicine (mouth and teeth)
The efficacy of pomegranate has been evaluated for the treatment of mouth and teeth problems. The studies show that pomegranate is effective for a range of mouth and teeth diseases such as aphthae and chronic periodontitis (149). Mouthwash containing pomegranate extract may help prevent dental plaque and tartar buildup by stifling the activity of the bacteria that produce plaque and reducing their capacity to cling to the tooth structure. In addition to reducing oxidative stress in the oral cavity, flavonoids, one of the active components of pomegranates, are thought to prevent gingivitis through a variety of other processes (150).
The anti-microbial activities of pomegranate have been confirmed by several in vitro, animal, and clinical studies. For instance, in a study, pomegranate has shown interferences with biofilm formation against antibiotic-resistant bacterial strains and quorum sensing among biofilm microorganisms (151). Studies have revealed that pomegranate is effective against a wide range of oral pathogens including, Streptococcus salivarius, S. sanguis, S. mitis, Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Prevotella intermedia (151).
In a study on the effectiveness of Achyranthes aspera, 0.2% aqueous chlorhexidine gluconate, and P. granatum mouthwashes on salivary S. mutans count in children, chlorhexidine showed better results than the other two. However, all showed a statistically significant reduction of S. mutans count and plaque index after 7 days (152). However, in another RCT on thirty children aged 6-12 years old, the activity of PPE was investigated against this pathogen and no statistically significant difference was observed between PPE and chlorhexidine as a positive control (Table 6).
Table 6.
Therapeutic potential of Punica granatum on mouth and teeth
| Model | Activity | Plant part/compd. | Study design | Mechanism | Ref. |
|---|---|---|---|---|---|
| Bacterial | Antimicrobial activity against Streptococcus mutans | Pericarp extract | Disc inhibition zone method and broth dilution assay; Control=chlorhexidine 0.2% | Growth inhibition; MIC= 50 mg/ml |
(153) |
| Bacterial | Antifungal efficacy on Candida albicans | PPE (aqueous decoction) |
Disc diffusion method; The zones of microbial (cultures of Candida albicans) inhibition at 18 and 24 hr | Antifungal activity (P<0.01); Inhibition zone 18.8 mm after 24h and 22 mm after 48 h |
(154) |
| Bacterial | Antimicrobial inhibition as a mouthwash | PPE (methanol extract) |
Agar well method (n=3); Incubated at 37 °C for 24 hr; Repeated 3 times; Bacterial strains: Staphylococcus mutants, Staphylococcus aureus, E. coli, Klebsiella pneumonia, Streptococcus gaseous, and Streptococcus faecalis; 25%, 50%, and 75% methanol extract; Control=chlorhexidine gluconate mouthwash | The 50% and 75% extracts exhibited the highest inhibition on S. mutants | (155) |
| Bacterial | Antibacterial activity against dental caries and gingivitis S. mutans | PPE (hot and cold aqueous extracts) |
Agar disk diffusion method; S. mutans was isolated from fifty-five patients; 10 μl of aqueous peels extract or 0.2% chlorhexidine (standard); incubated at 37 °C for 24 hr | Inhibition zones: hot (31 mm), cold (27 mm), chlorhexidine (25 mm) | (156) |
| Bacterial | Antibacterial activity against Enterococcus faecalis and Candida albicans in root canal dressing | PPE (ethanol extract) |
Inocula of 5.0 × 105 CFU/ml E. faecalis and 1.0 × 103 CFU/ml of C. albicans | ↓Cell viability, biofilm formation; C. Albicans MIC= 62.50 µg/ml; E. faecalis MIC= 15.62 µg/ml |
(157) |
| Cellular | Anti-cancer activity against oral cancer | PPE (ethanol extract) |
KB 3-1 oncogenic cell culture; MTT assay; Extract concentrations of 50, 100, 150, 200, 250, 300, and 350 µg | ↓Cell viability | (158) |
| Animal | Antifungal activity against oral candidiasis | PPE (methanol extract) |
Male Wistar rats immunosuppressed with cyclosporine (40 mg/kg/d) and hydrocortisone acetate (500 μg/kg/d) except the control group; Induction of oral candidiasis via the oral administration of Candida albicans on the palate and tongue; extract 125, 250, and 500 μg/ml/kg and nystatin 100000 U/ml/kg by gavage daily; 15 days | ↓Growth of C. albicans 100% cure in all the doses after 15 days; Most effective Conc.: 500 μg/ml after 5 days |
(159) |
| Clinical | Antimicrobial prophylactic activity on oral bacteria | Fresh fruit juice | 20 patients; A group received 0.2% chlorhexidine mouthwash (10 ml), the other group was given freshly prepared PJ as a mouthwash (75 ml for 2 min); salivary sample pre-rinse and after 15 min | ↓Colony-forming units by pomegranate mouthwash (51.1%) and chlorhexidine mouthwash (71.3%) | (160) |
| Clinical | Gingival bleeding reduction | Fruit (ethanol extract) |
Double-blind, interventional, experimental, longitudinal, and prospective RCT, with an inductive approach; N= 55, 18-56 years old; Control=chlorhexidine 0.12% solution mouthwash; Study groups: pomegranate extract mouthwash (twice daily, 1 min long, with 10 ml of the solution); Ainamo and Bay gingival bleeding index (GBI) on 0, 7, and 15 days |
↓Gingival bleeding index (P<0.001) | (161) |
| Clinical | Reduction of gingivitis risk | PomElla® (pomegranate extract) | Single-blinded RCT; 19–25 years old, n=32, split evenly among both genders; 4 weeks; saliva sample donation before and after the experiment; randomly assigned pomegranate extract PomElla® dissolved in water or placebo: cornstarch in water (three times a day for 1 min per rinse) | ↓Total protein (P<0.01), aspartate aminotransferase activity (P<0.005), α-Glucosidase activity (P<0.05); ↑Antioxidant enzyme ceruloplasmin activities (P<0.05), radical scavenging capacity (P<0.05) |
(162) |
| Clinical | Antibacterial activity against S. mutans | Fruit (mouthwash) |
A single-blinded, parallel-group RCT; n=20; experimental group (I): P. granatum and controlled group (II): chlorhexidine mouthwash; plaque samples evaluated for S. mutans at baseline and 15th day | ↓Mean plaque both groups (group I P=0.043 and group II P=0.047); No significant difference between the two groups at 7th day |
(163) |
| Clinical | Treatment of minor recurrent aphthous stomatitis |
P. granatum flowers (pleniflora, sweet Alak, and Saveh black varieties) (ethanol and aqueous extracts) |
Double-blind method; n=210 (69 F and 141 M); experimental group: alcoholic or water extracts (both 10% in 100 ml), negative control: received nothing; using mouthwash for 10 min, four times a day, for 10 days; measuring the size of lesions at days 1,2,4,6,8, and 10, The pain satisfactory degree was recorded | ↓Entire-time of complete treatment; ↑Patients’ satisfactory |
(164) |
| Clinical | Antimicrobial activity against S. mutans | Pericarp extract | RCT; 30 children, 6-12 years; Control=chlorhexidine 0.2%; Samples collected before and after mouth rinse | ↓Salivary S. mutans count; No statistically significant difference between PPE and chlorhexidine |
(153) |
Eyes, ears, and nose
Administration of a mixture of concentrated PJ, particularly sour pomegranate, with honey has been used for earache and deep nasal wounds (19, 75). Ibn-e-Jazlah has prescribed a mixture of sweet PJ with honey for earache and sour one for treating pterygium (15). Ibn Beytar and Tonekaboni believed that the administration of eardrop prepared by mixing pomegranate with rose oil (Rosa damascene) would heal the earache (14, 75).
ITM scientists did benefit from pomegranate for the treatment of eye problems. Antaki has mentioned: “Concentrated PJ (by the sun or by heating in a copper pan) sharpens the eyesight and is useful for the treatment of epiphora and pannus” (12). Some ancient scholars believed that eating three pomegranate calyxes protects the eye from conjunctivitis for up to a year. If the sweet PJ is put in a glass container (Gharooreh) in front of the sun to make it thick and then applied in the form of kohl (a traditional kind of mascara), it would improve the vision; the older the mixture becomes, the better it yields result (19).
Evidence from modern medicine (Eyes, ears, and nose)
Both in vitro and in vivo studies on P. granatum have indicated the protective effect of multiple preparations of this plant on retinal cell injury and ototoxicity (Table 7). However, the scope and variety of studies on this aspect are limited and clinical trials are lacking. Thus, further studies on pomegranate activities, particularly its anti-oxidant properties that have the ability to diminish or reverse age-related ocular degeneration, are needed.
Table 7.
Therapeutic potential of Punica granatum on eyes, ears, and nose
| Model | Activity | Plant part/compd. | Study design | Mechanism | Ref. |
|---|---|---|---|---|---|
| Cellular | Protecting human retinal pigment epithelium (RPE) cells from photo-oxidative stress | Punicalagin | Human RPE cell line (ARPE-19); Exposed to UV-A radiation for 1, 3, and 5 hr; ARPE-19 cells were pre-treated with punicalagin (24 h); ROS, BAX, and BCL-2 detection | Activating Nrf2/HO-1 signaling pathway; ↓Apoptosis and BAX/BCL-2 ratio; Antagonizing the decrease in cell viability and reduced high levels of ROS |
(165) |
| Cellular | Anti-cataract activity | Leaves (methanol extract) |
Glucose-induced cataract model in goat lenses; Positive control=quercetin (500 μg/ml); Leaves extract concentrations: 250, 500, 1000 μg/ml); 72 hr | Aldose reductase inhibition; ↓Oxidative stress; ↑Antioxidant defense system; IC50= 83.55 ± 3.92 μg/ml |
(166) |
| Animal | Protection on experimental ischemia/reperfusion (I/R) retinal injury | Pomegranate extract | Male albino rats; Groups I and II (sham-operated and received saline or extract, respectively), groups III and IV (I/R) rat models with prior administration of saline or 250 mg/kg/day extract, respectively | PMG prevented I/R-induced retinal damage; ↑Nuclear factor erythroid 2-related factor 2 (Nrf2) immunoreactivity; ↓NO |
(167) |
| Animal | Protection against amikacin-induced ototoxicity | PPE (≥98% ellagic acid) |
BALB/c mice; Control group: physiological saline (100 μl/day) via gavage; Amikacin (AMK) group: intraperitoneally received AMK intramuscular injection at 500 mg/kg/day for 15 consecutive days; PPE plus AMK group: hypodermic injection for AMK at 500 mg/kg/day for 15 consecutive days and PPE (34 mg/kg, 100 μl/day) via gavage for 5 days prior to AMK injection and for 15 days concomitantly with AMK injections; PPE group: PPE via gavage for 20 days; auditory brainstem response (ABR) was recorded 1 day before and 15 days after AMK treatment | Regulating the MAPK/FoxO3a signaling pathway in the cochlea; IC50= 45 μg/ml |
(168) |
Metabolic disorders
In ITM textbooks, it has been widely emphasized that pomegranates are useful for the treatment of metabolic diseases (13, 75). It is mentioned in many books that PJ imparts a rosy-colored appearance to the face (12, 13, 75), implying its beneficial effects on the metabolic system. The effects of pomegranate on metabolic disorders can be tracked in two main organs discussed in the following paragraphs. In our previous publication, the beneficial effects of pomegranate on different components of metabolic disorders were thoroughly discussed (9).
Liver
ITM scientists claim that pomegranate is a cooling agent for the liver (12, 14, 74) so it can decrease the extra heat in this organ in pathogenic conditions (14). For instance, PJ has been recommended for removing the harmful effects of alcohol from the liver. Pomegranate is claimed to act as an antidote for alcohol-induced hepatotoxicity (14, 19). Many ITM researchers have mentioned that pomegranate can remove the heat in the liver caused by eating too much wine (19). However, pomegranates should be used with caution in people with cold temperaments (14). In addition, all types of pomegranate have been prescribed for treating jaundice (12).
Evidence from modern medicine
Studies (in vitro and in vivo) have revealed that pomegranate extracts from the peels, flowers, juice, and seeds can control lipid metabolism in metabolic disorder-related illnesses like atherosclerosis, nonalcoholic fatty liver disease, and type 2 diabetes, preventing the onset of these conditions (9, 169). For instance, in high-fat-fed rats, pomegranate vinegar may control lipid metabolism and lessen liver damage (170).
The beneficial activities of pomegranate extracts and preparations have been evaluated in a range of liver-related disorders. As an example, an alcoholic extract from the flowers of P. granatum has been found to abrogate ferric nitrilotriacetate-induced hepatotoxicity in mice (171). PPE may stop liver fibrosis in biliary-obstructed rats by reducing oxidative stress or increasing endogenous anti-oxidant levels (172). Pomegranate extract has also shown protective effects such as lowering triglyceride and cholesterol content of cells, normalizing the expression of pro-inflammatory cytokines, and improving mitochondrial complex activity in obesity-associated nonalcoholic fatty liver disease (1).
In addition, different nanoformulations have been engineered for improving the activity of pomegranate on liver disorders. For example, silver nanoparticles biosynthesized using P. granatum leaves have shown improved antidiabetic potential in vitro (173). Through boosting fatty acid consumption in hepatocytes, pomegranate-derived omega-5 nanoemulsion might reduce hepatic steatosis in mice fed a high-fat diet (174).
Heart and cardiovascular system
In ITM, pomegranate (especially sour ones) has been described as a hematopoietic (14, 19), anti-palpitation (14, 15, 17, 18, 75), and vasodilatory agent (12, 17, 19, 74). Avicenna in his book Cardiac Medications: “All types of pomegranates including, sour, sweet, and sour-sweet ones are useful for the treatment of palpitation”. It is written in Al-Hawi and many other ITM textbooks that sour pomegranate acts like a polish for the heart and has the ability to burnish the cardiovascular system (14-16, 19, 76); accordingly, it can be deduced that pomegranate has anti-atherosclerosis activity (17, 74-76). Sour pomegranate prevents the flow of waste materials to the body (15) and quenches the excitation of yellow bile, black bile, and blood humor (14, 74, 75). Razi in Al-Hawi has mentioned: The pomegranate paste particularly the one concentrated in a copper pan is used to remove the excess amount of these humors (19).
Evidence from modern medicine
Pomegranate and its derivatives have been found to have protective effects on the cardiovascular system, according to several in vivo and clinical investigations. These vasculoprotective actions include lowering oxidative stress, improving macrophage, endothelial cell, and platelet function, decreasing blood glucose levels, lowering lipid oxidation, and having vasodilatory effects in addition to lowering blood pressure by inhibiting ACE activity (175). For instance, P. granatum flower extract could diminish cardiac fibrosis in Zucker diabetic fatty rats by modulation of cardiac endothelin-1 and NF-κB pathways (176). It could also protect isoproterenol-treated rats against myocardial damage by acting as a free radical scavenger and conserving the endogenous anti-oxidant system (177). A study showed how pomegranate, prickly pear, and apple juice vinegar can protect against swelling, hypertrophy, and fibrosis in obesity-related heart damage (178). Moreover, in individuals with unstable angina, PJ may lessen the frequency, onset, and length of angina pectoris attacks (179). In addition, according to the literature, pomegranate has the ability to reduce blood pressure. For example, the data from a meta-analysis evaluating 8 RCTs showed that PJ can significantly decrease both systolic and diastolic blood pressure levels (180).
Other traditional systems of medicine around the world
Table 8 shows the medicinal application of pomegranate in different countries around the world. Most of the usages mentioned in the table are similar to the prescriptions in ITM textbooks showing the knowledge transfer from Persia to other parts of the world. Similarly, different preparations from all parts of pomegranate including leaves, seeds, fruits, and peels are mentioned. Pomegranate is a well-known medicinal plant in Indian traditional systems of medicine including Ayurveda and Unani medicine.
Table 8.
Application of pomegranate in other countries
| Country | Parts used | Mode of preparation/ administration | Uses | Ref. |
|---|---|---|---|---|
| Algeria | Barks | Decoction, infusion, poultice, cooked | Colon ailments, wounds, stomach ulcers, diarrhea, cough | (181) |
| Peels | Infusion, powder | Aphthae (as a mouthwash), diarrhea, and digestive problems | (149) | |
| Brazil | Fruits, fruit peel, and seed | Decoction, maceration, syrup | Diarrhea, female infection, general infection, worms, cancer, myoma, depurative, diabetes, flu, stomach pain, gastritis, indigestion, ulcer, rheumatism, ovarian cyst, vaginal discharge, uterine infection, vaginal infection, uterine inflammation, wound healing, throat infection, throat inflammation | (182) |
| Fruit, bark | Mouthwash, gargle | Acute respiratory infections in children; sore throat | (183) | |
| India | Seeds | Botanical mixtures | Kidney and urinary disorders | (184) |
| Leaves | Infusion | Cuts and wounds | (185) | |
| Whole fruit | Paste (external) | Snake bites, scabies, salivation with nausea, antifertility, skin diseases | (186) | |
| Pakistan | Leaves/ fruit | Powder/ raw | Skin diseases, dysentery/ astringent, blood purifier, laxative, whooping cough, anthelmintic, diarrhea | (187) |
| Fruits | - | Whooping cough | (188) | |
| Seeds | Powder/ oral | Abdominal worms of children | (189) | |
| Palestine | Fruit/ peel | Squeeze and apply; mill the peel, soak in warm water, and apply |
Hair and scalp treatments | (190) |
| Mexico | Pericarp | Syrup | Dental caries; gum diseases; aphthous ulcers; mouth sores | (191) |
| Morocco | Bark | Decoction | Diabetes, dermocosmetology, digestive system disorders, cardiac and renal diseases | (192) |
| Turkey | Fruit juice | Pressing/ oral | Diabetes | (193) |
| Yemen | Fruit/ peel | Decoction | Oral: Gastrointestinal ailments: stomach ulcer, diarrhea, ascariasis, tapeworm Respiratory tract diseases: asthma |
(194) |
| Paste | Dermal diseases: face pimples |
Conclusion
Pomegranate fruits, leaves, blossoms, and seeds have all been used to cure several ailments in Persian and Islamic traditional medicines. However, pomegranate fruit juice and peel have received far more attention than the other parts. By comparing the ITM findings to modern medical evidence, we can deduce that a significant part of ITM findings is confirmed by modern medicine (195-197). For instance, the anti-bacterial activity of pomegranate in treating wounds, and infections such as respiratory, mouth, teeth, gastrointestinal, and uterine infections were well established in ITM books. Another instance of this conformity is the anti-oxidant property of pomegranate which is useful in the treatment of inflammation, skin aging, atherosclerosis, and metabolic disorders.
Though some of these traditional medical practices have been studied by modern medicine, many are still in their very early stages to be investigated. Although the main bioactive constituents of pomegranate preparations are polyphenols, there are yet insufficient data on the identification and isolation of the bioactive chemicals that are responsible for the biological activities. In addition, more preclinical and clinical research is demanded to confirm the other traditional applications, find the mechanisms of action, and evaluate the efficacy and safety. Overall, the potential of this valuable fruit and functional food can be fully realized via examination of the variety and interrelation of pomegranate phytochemicals as well as preclinical and clinical studies of their bioactivities.
Authors’ Contributions
M A designed the study; Z B and M M collected data and drafted the manuscript; M A and M M discussed the results and strategy; SA E and M A supervised the study; M A and SA E approved of the final version to be published.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to thank the authorities in Mashhad University of Medical Sciences (MUMS) Research council, School of pharmacy, Mashhad for their support.
References
- 1.Zou X, Yan C, Shi Y, Cao K, Xu J, Wang X, et al. Mitochondrial dysfunction in obesity-associated nonalcoholic fatty liver disease: The protective effects of pomegranate with its active component punicalagin. Antioxid Redox Signal. 2014;21:1557–1570. doi: 10.1089/ars.2013.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland D, Hatib K, Bar-Ya’akov I. Pomegranate: Botany, horticulture, breeding. Hortic Rev. 2009;35:127–191. [Google Scholar]
- 3.Sharma K, Kesharwani P, Prajapati SK, Jain A, Jain D, Mody N, et al. An insight into anticancer bioactives from Punica granatum (pomegranate) Anti-Cancer Agents Med Chem. 2022;22:694–702. doi: 10.2174/1871520621666210726143553. [DOI] [PubMed] [Google Scholar]
- 4.Shaikh SB, Bhandary YP. Therapeutic properties of Punica granatum L (pomegranate) and its applications in lung-based diseases: A detailed review. J Food Biochem. 2021;45:e13684. doi: 10.1111/jfbc.13684. [DOI] [PubMed] [Google Scholar]
- 5.Alkhatib M, Fayad C, Badran A, Hamade K, Daou A, Baydoun E, et al. Preventive and therapeutic effects of Punica granatum (Pomegranate) in respiratory and digestive diseases: A review. Appl Sci. 2022;12:12326–12344. [Google Scholar]
- 6.Alami M, Benchagra L, Boulbaroud S, Ramchoun M, Khalil A, Fulop T, et al. Pomegranate (Punica granatum L ) attenuates neuroinflammation involved in neurodegenerative diseases. Foods. 2022;11:2570–2588. doi: 10.3390/foods11172570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George N, AbuKhader M, Al Balushi K, Al Sabahi B, Khan SA. An insight into the neuroprotective effects and molecular targets of pomegranate (Punica granatum) against Alzheimer’s disease. Nutr Neurosci. 2022:1–22. doi: 10.1080/1028415X.2022.2121092. [DOI] [PubMed] [Google Scholar]
- 8.Laurindo LF, Barbalho SM, Marquess AR, Grecco AIS, Goulart RA, Tofano RJ, et al. Pomegranate (Punica granatum L ) and metabolic syndrome risk factors and outcomes: A systematic review of clinical studies. Nutrients. 2022;14:1665–1682. doi: 10.3390/nu14081665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akaberi M, Boghrati Z, Sahebkar A, Emami SA. Therapeutic potential of pomegranate in metabolic disorders. In: Sahebkar A, editor. Natural Products and Human Diseases: Pharmacology, Molecular Targets, and Therapeutic Benefits. Cham: Springer International Publishing; 2021. pp. 421–440. [Google Scholar]
- 10.Johari AS, Zulkepli NA, Sarmoko S. Pomegranate (Punica granatum) derived phytochemical actions towards prostate cancer. Sci Eng Health Stud. 2022;16:1–8. [Google Scholar]
- 11.Mahdavi AM, Javadivala Z. Systematic review of the effects of pomegranate (Punica granatum) on osteoarthritis. Health Promot Perspect. 2021;11:411–425. doi: 10.34172/hpp.2021.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antaki D. Shams a’ddin A . Beirut:: Dar-al-Kotob al-ilmiyah; 2000. Tazkere Oulol-albab (Memorandum book) [Google Scholar]
- 13.Aqili Khorasani MH. Makhzan al-Adwiah (Drug Treasure). Reprinted from a copy which was printed in Calcutta dated in 1844. Tehran: Enqelab-e Eslami Publishing and Educational Organization; 1992. [Google Scholar]
- 14.Ibn Beytar Z. Al-Jamee Le-Mofradaat al- Adwiah wal- Aghziyah (Comprehensive Book in Simple Drugs and Foods) Beirut: Dar- Al-Kotob Al-ilmiyah; 2001. [Google Scholar]
- 15.Ibn Jazlah Y. Minhaj al-bayan fima yasta miluhu al-insan (The methodology of the statement in what a person uses) Cairo, Egypt: Jamiat al-duwal al-arabia, Al-monzamat al-arabia liltarbiat-e-wal-thaqafat-waleulumu, Maehad almakhtutat alearabiati: 2010. [Google Scholar]
- 16.Ibn Nafis Qarshi AD. In: Al-Mujaz fi’l-Tibb (A Commentary on Ibn Sina’s Canon) Azbavi A, editor. Cairo: Ihyaa al-Torath al-Islami: 2001. [Google Scholar]
- 17.Ibn Sina A. Al-Qanun fi’l-Tibb (Canon of Medicine) New Delhi: I.H.M.M.R. Printing Press; 1987. [Google Scholar]
- 18.Jorjani SE. Al-Aghraz al-Tibbiah wal Mabaheth al Alaiiah (Medical goals and Allaii’s discussions) Tehran: The Iranian Culture Foundation; 1966. [Google Scholar]
- 19.Razi MZ. In: Al-Hawi fi’l-Tibb (Comprehensive book of medicine) Khan A, editor. Hyderabad: Osmania Oriental Publications Bureau; 1968. [Google Scholar]
- 20.Tito A, Colantuono A, Pirone L, Pedone E, Intartaglia D, Giamundo G, et al. Pomegranate peel extract as an inhibitor of SARS-CoV-2 spike binding to human ACE2 receptor (in vitro): A promising source of novel antiviral drugs. Front Chem. 2021;9:638187. doi: 10.3389/fchem.2021.638187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naseri R, Farzaei F, Haratipour P, Nabavi SF, Habtemariam S, Farzaei MH, et al. Anthocyanins in the management of metabolic syndrome: A pharmacological and biopharmaceutical review. Front Pharmacol. 2018;9:1310. doi: 10.3389/fphar.2018.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu S, Tian L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (Punica granatum) Molecules. 2017;22:1606. doi: 10.3390/molecules22101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mena P, Calani L, Dall’Asta C, Galaverna G, Garcia-Viguera C, Bruni R, et al. Rapid and comprehensive evaluation of (poly)phenolic compounds in pomegranate (Punica granatum L juice by UHPLC-MSn. Molecules. 2012;17:14821–14840. doi: 10.3390/molecules171214821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdulla R, Mansur S, Lai H, Ubul A, Sun G, Huang G, et al. Qualitative analysis of polyphenols in macroporous resin pretreated pomegranate husk extract by HPLC-QTOF-MS. Phytochem Anal. 2017;28:465–473. doi: 10.1002/pca.2695. [DOI] [PubMed] [Google Scholar]
- 25.Jourdes M, Pouységu L, Deffieux D, Teissedre PL, Quideau S. Hydrolyzable tannins: Gallotannins and ellagitannins. In: Ramawat KG, editor. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013. pp. 1975–2010. [Google Scholar]
- 26.Ito H, Li P, Koreishi M, Nagatomo A, Nishida N, Yoshida T. Ellagitannin oligomers and a neolignan from pomegranate arils and their inhibitory effects on the formation of advanced glycation end products. Food Chem. 2014;152:323–330. doi: 10.1016/j.foodchem.2013.11.160. [DOI] [PubMed] [Google Scholar]
- 27.Yuan T, Wan C, Ma H, Seeram NP. New phenolics from the flowers of Punica granatum and their in vitro α-glucosidase inhibitory activities. Planta Med. 2013;79:1674–1679. doi: 10.1055/s-0033-1350925. [DOI] [PubMed] [Google Scholar]
- 28.Poyrazoğlu E, Gökmen V, Artιk N. Organic acids and phenolic compounds in pomegranates (Punica granatum L ) grown in Turkey. J Food Compos Anal. 2002;15:567–575. [Google Scholar]
- 29.NeuhÖFer H, Witte L, Gorunovic M, Czygan FC. Alkaloids in the bark of Punica granatum L (Pomegranate) from Yugoslavia. Pharmazie. 1993;48:389–391. [Google Scholar]
- 30.Cope AC, Dryden HL, Overberger CG, D’Addieco AA. Preparation of glutaraldehyde and pseudopelletierine. J Am Chem Soc. 1951;73:3416–3418. [Google Scholar]
- 31.Lazny R, Wolosewicz K, Zielinska P, Urbanczyk-Lipkowska Z, Kalicki P. Diastereo- and enantioselective aldol reaction of granatanone (pseudopelletierine) Tetrahedron. 2011;67:9433–9439. [Google Scholar]
- 32.Albrecht M, Gjikaj M, Schmidt A. Intermolecular interactions of punicin derivatives. Tetrahedron. 2010;66:7149–7154. [Google Scholar]
- 33.Rafiq Z, Narasimhan S, Vennila R, Vaidyanathan R. Punigratane, a novel pyrrolidine alkaloid from Punica granatum rind with putative efflux inhibition activity. Nat Prod Res. 2016;30:2682–2687. doi: 10.1080/14786419.2016.1146883. [DOI] [PubMed] [Google Scholar]
- 34.Roberts MF, Cromwell BT, Webster DE. The occurrence of 2-(2-propenyl)-Δ1-piperideine in the leaves of pomegranate (Punica granatum ) Phytochemistry. 1967;6:711–717. [Google Scholar]
- 35.Nawwar MAM, Hussein SAM, Merfort I. Leaf phenolics of Punica granatum. Phytochemistry. 1994;37:1175–1177. [Google Scholar]
- 37.Abe H, Imai H, Ogura D, Horino Y. Synthesis of lactonized valoneoyl group-containing ellagitannins, oenothein C and cornusiin B. Heterocycles. 2020;101:524–535. [Google Scholar]
- 38.Grimshaw J, Haworth RD. Flavogallol. 1956;( J Chem Soc):4225–4232. [Google Scholar]
- 39.El-Toumy SA, Rauwald HW. Two ellagitannins from Punica granatum heartwood. Phytochemistry. 2002;61:971–974. doi: 10.1016/s0031-9422(02)00435-1. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt OT, Fickert W. Flavogallol, ein Baustein der Gerbstoffe der Granatapfel-Schalen. Z Naturforsch B. 1958;13:136. [Google Scholar]
- 41.Reddy MK, Gupta SK, Jacob MR, Khan SI, Ferreira D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007;73:461–467. doi: 10.1055/s-2007-967167. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka T, Nonaka G, Nishioka I. Tannins and related compounds Isolation and characterization of novel ellagitannins, punicacorteins A, B, C and D, and punigluconin from the bark of Punica granatum L. Chem Pharm Bull. 1986;34:656–663. [Google Scholar]
- 43.Toda K, Ueyama M, Tanaka S, Tsukayama I, Mega T, Konoike Y, et al. Ellagitannins from Punica granatum leaves suppress microsomal prostaglandin E synthase-1 expression and induce lung cancer cells to undergo apoptosis. Biosci Biotechnol Biochem. 2020;84:757–763. doi: 10.1080/09168451.2019.1706442. [DOI] [PubMed] [Google Scholar]
- 44.Nawwar MAM, Hussein SAM, Merfort I. NMR spectral analysis of polyphenols from Punica granatum. Phytochemistry. 1994;36:793–798. [Google Scholar]
- 45.Yuan T, Ding Y, Wan C, Li L, Xu J, Liu K, et al. Antidiabetic ellagitannins from pomegranate flowers: Inhibition of α-glucosidase and lipogenic gene expression. Org Lett. 2012;14:5358–5361. doi: 10.1021/ol302548c. [DOI] [PubMed] [Google Scholar]
- 46.Kang B, Kim CY, Hwang J, Jo K, Kim S, Suh HJ, et al. Punicalagin, a pomegranate-derived ellagitannin, suppresses obesity and obesity-induced inflammatory responses via the Nrf2/Keap1 signaling pathway. Mol Nutr Food Res. 2019;63:1900574. doi: 10.1002/mnfr.201900574. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Cao Y, Chen J, Qin H, Yang L. A new possible mechanism by which punicalagin protects against liver injury induced by type 2 diabetes mellitus: Upregulation of autophagy via the Akt/FoxO3a signaling pathway. J Agric Food Chem. 2019;67:13948–13959. doi: 10.1021/acs.jafc.9b05910. [DOI] [PubMed] [Google Scholar]
- 48.Mayer W, Görner A, Andrä K. Punicalagin und Punicalin, zwei Gerbstoffe aus den Schalen der Granatäpfel. Liebigs Ann. 1977;1977:1976–1986. [Google Scholar]
- 49.Wang Y, Zhang H, Liang H, Yuan Q. Purification, antioxidant activity and protein-precipitating capacity of punicalin from pomegranate husk. Food Chem. 2013;138:437–443. doi: 10.1016/j.foodchem.2012.10.092. [DOI] [PubMed] [Google Scholar]
- 50.El-Toumy SA, Rauwald HW. Two new ellagic acid rhamnosides from Punica granatum heartwood. Planta Med. 2003;69:682–684. doi: 10.1055/s-2003-41107. [DOI] [PubMed] [Google Scholar]
- 51.Larrosa M, Gonzalez-Sarrias A, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. 2006;54:1611–1620. doi: 10.1021/jf0527403. [DOI] [PubMed] [Google Scholar]
- 52.Sharma M, Li L, Celver J, Killian C, Kovoor A, Seeram NP. Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin A, on Wnt signaling. J Agric Food Chem. 2010;58:3965–3969. doi: 10.1021/jf902857v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hussein SAM, Barakat HH, Merfort I, Nawwar MAM. Tannins from the leaves of Punica granatum. Phytochemistry. 1997;45:819–823. [Google Scholar]
- 54.Tantray MA, Akbar S, Khan R, Tariq KA, Shawl AS. Humarain: A new dimeric gallic acid glycoside from Punica granatum L bark. Fitoterapia. 2009;80:223–225. doi: 10.1016/j.fitote.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Mazza G, Miniati E. Anthocyanins in fruits, vegetables, and grains. first ed. Boca Raton: CRC Press; 1993. [Google Scholar]
- 56.Singh B, Singh JP, Kaur A, Singh N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L ) peel: A review. Food Chem. 2018;261:75–86. doi: 10.1016/j.foodchem.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 57.Srivastava R, Chauhan D, Chauhan JS. A flavonoid diglycoside from Punica granatum. Indian J Chem B. 2001;40:170–172. [Google Scholar]
- 58.Reddy NP, Reddy BAK, Gunasekar D, Blond A, Bodo B, Murthy MM. Flavonoids from Limnophila indica. Phytochemistry. 2007;68:636–639. doi: 10.1016/j.phytochem.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 59.Bagri P, Ali M, Sultana S, Aeri V. New flavonoids from Punica granatum flowers. Chem Nat Compd. 2010;46:201–204. [Google Scholar]
- 60.Ali M, Sharma N. Phytochemical investigation of the flowers of Punica granatum. Indian J Chem B. 2006;45:1681–1685. [Google Scholar]
- 61.Guo G, He XW, Tzi BN. Pomegranin, an antifungal peptide from pomegranate peels. Protein Pept Lett. 2009;16:82–85. doi: 10.2174/092986609787049330. [DOI] [PubMed] [Google Scholar]
- 62.Bagri P, Ali M, Sultana S, Aeri V. New sterol esters from the flowers of Punica granatum Linn. J Asian Nat Prod Res. 2009;11:710–715. doi: 10.1080/10286020903004291. [DOI] [PubMed] [Google Scholar]
- 63.Xie Y, Morikawa T, Ninomiya K, Imura K, Muraoka O, Yuan D, et al. Medicinal flowers XXIII New taraxastane-type triterpene punicanolic acid, with tumor necrosis factor-α inhibitory activity from the flowers of Punica granatum. Chem Pharm Bull. 2008;56:1628–1631. doi: 10.1248/cpb.56.1628. [DOI] [PubMed] [Google Scholar]
- 64.Jiang HZ, Ma QY, Fan HJ, Liang WJ, Huang SZ, Dai HF, et al. Fatty acid synthase inhibitors isolated from Punica granatum L. J Braz Chem Soc. 2012;23:889–893. [Google Scholar]
- 65.Kho YL, Jung W, Kwon D, Kim JH. Identification of estrone in pomegranate (Punica granatum) extracts by liquid chromatography-tandem mass spectrometry. Food Sci Biotechnol. 2010;19:809–813. [Google Scholar]
- 66.Jabbar Z, Ali M. A new lanostanyl diglucuronoside from the flowers of Punica granatum L. Int Res J Pharm . 2011 [Google Scholar]
- 67.Yusuph M, Mann J. A triglyceride from Punica granatum. Phytochemistry. 1997;44:1391–1392. [Google Scholar]
- 68.Fatope MO, Al Burtomani SK, Takeda Y. Monoacylglycerol from Punica granatum seed oil. J Agric Food Chem. 2002;50:357–360. doi: 10.1021/jf010711w. [DOI] [PubMed] [Google Scholar]
- 69.Khajebishak Y, Payahoo L, Alivand M, Alipour B. Punicic acid: A potential compound of pomegranate seed oil in Type 2 diabetes mellitus management. J Cell Physiol. 2019;234:2112–2120. doi: 10.1002/jcp.27556. [DOI] [PubMed] [Google Scholar]
- 70.Wang RF, Xie WD, Zhang Z, Xing DM, Ding Y, Wang W, et al. Bioactive compounds from the seeds of Punica granatum (pomegranate) J Nat Prod. 2004;67:2096–2098. doi: 10.1021/np0498051. [DOI] [PubMed] [Google Scholar]
- 71.UNECE. New UNECE standard will boost international trade in pomegranate 2022 [cited 2022 2 September] Available from: https://unece.org/sustainable-development/press/new-unece-standard-will-boost-international-trade-pomegranate.
- 72.Langley P. Why a pomegranate? Br Med J (Clin res ed,) 2000;321:1153–1154. doi: 10.1136/bmj.321.7269.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bone K, Mills S. Principles and practice of phytotherapy: Modern Herbal Medicine. second ed. Churchill Livingstone; 2013. [Google Scholar]
- 74.Heravi M. In: Al-Abniyah an Haqayeq al-Adwiyah (Basics of Realities on Drugs) Bahmanyar A, editor. Tehran: Tehran University Publications; 1967. [Google Scholar]
- 75.Tonekaboni MM. Tohfat al-Momenin (Rarity of the faithful) Tehran: Mostafavi Press; 1959. [Google Scholar]
- 76.Ghasani AM. Hadiqat al-Azhar fi Mahiyyat al-ushb wa al-uqqar. Beirut, Lebanon: Dar al-Gharb al- Islami; 1985. [Google Scholar]
- 77.Alanis AD, Calzada F, Cervantes JA, Torres J, Ceballos GM. Antibacterial properties of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J Ethnopharmacol. 2005;100:153–157. doi: 10.1016/j.jep.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 78.Zhao SS, Ma DX, Zhu Y, Zhao JH, Zhang Y, Chen JQ, et al. Antidiarrheal effect of bioactivity-guided fractions and bioactive components of pomegranate (Punica granatum L ) peels. Neurogastroenterol Motil. 2018;30:e13364. doi: 10.1111/nmo.13364. [DOI] [PubMed] [Google Scholar]
- 79.Rao PK, Hasan SS, Bhellum BL, Manhas RK. Ethnomedicinal plants of Kathua district, J&K, India. J Ethnopharmacol. 2015;171:12–27. doi: 10.1016/j.jep.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 80.Aziz MA, Adnan M, Khan AH, Rehman AU, Jan R, Khan J. Ethno-medicinal survey of important plants practiced by indigenous community at Ladha subdivision, South Waziristan agency, Pakistan. J Ethnobiol Ethnomed. 2016;12:53–66. doi: 10.1186/s13002-016-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Limsuwan S, Jarukitsakul S, Issuriya A, Chusri S, Joycharat N, Jaisamut P, et al. Thai herbal formulation ‘Ya-Pit-Samut-Noi’: Its antibacterial activities, effects on bacterial virulence factors and in vivo acute toxicity. J Ethnopharmacol. 2020;259:112975. doi: 10.1016/j.jep.2020.112975. [DOI] [PubMed] [Google Scholar]
- 82.Voravuthikunchai S, Lortheeranuwat A, Jeeju W, Sririrak T, Phongpaichit S, Supawita T. Effective medicinal plants against enterohaemorrhagic Escherichia coli O157: H7. J Ethnopharmacol. 2004;94:49–54. doi: 10.1016/j.jep.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 83.Hajimahmoodi M, Shams-Ardakani M, Saniee P, Siavoshi F, Mehrabani M, Hosseinzadeh H, et al. In vitro antibacterial activity of some Iranian medicinal plant extracts against Helicobacter pylori. Nat Prod Res. 2011;25:1059–1066. doi: 10.1080/14786419.2010.501763. [DOI] [PubMed] [Google Scholar]
- 84.Mayyas A, Abu-Sini M, Amr R, Akasheh RT, Zalloum W, Khdair A, et al. Novel in vitro and in vivo anti-Helicobacter pylori effects of pomegranate peel ethanol extract. Vet World. 2021;14:120–128. doi: 10.14202/vetworld.2021.120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calzada F, Yépez-Mulia L, Aguilar A. In vitro susceptibility of Entamoeba histolytica and Giardia lamblia to plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J Ethnopharmacol. 2006;108:367–370. doi: 10.1016/j.jep.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 86.Tortora K, Femia AP, Romagnoli A, Sineo I, Khatib M, Mulinacci N, et al. Pomegranate by-products in colorectal cancer chemoprevention: Effects in apc-mutated Pirc rats and mechanistic studies in vitro and ex vivo. Mol Nutr Food Res. 2018;62:28948694. doi: 10.1002/mnfr.201700401. [DOI] [PubMed] [Google Scholar]
- 87.Banerjee N, Kim H, Talcott S, Mertens-Talcott S. Pomegranate polyphenolics suppressed azoxymethane-induced colorectal aberrant crypt foci and inflammation: Possible role of miR-126/VCAM-1 and miR-126/PI3K/ AKT/mTOR. Carcinogenesis. 2013;34:2814–2822. doi: 10.1093/carcin/bgt295. [DOI] [PubMed] [Google Scholar]
- 88.Kim H, Banerjee N, Sirven MA, Minamoto Y, Markel ME, Suchodolski JS, et al. Pomegranate polyphenolics reduce inflammation and ulceration in intestinal colitis-involvement of the miR-145/p70S6K1/HIF1α axis in vivo and in vitro. J Nutr Biochem. 2017;43:107–115. doi: 10.1016/j.jnutbio.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 89.Sudha T, Mousa DS, El-Far AH, Mousa SA. Pomegranate (Punica granatum) fruit extract suppresses cancer progression and tumor angiogenesis of pancreatic and colon cancer in chick chorioallantoic membrane model. Nutr Cancer. 2021;73:1350–1356. doi: 10.1080/01635581.2020.1800768. [DOI] [PubMed] [Google Scholar]
- 90.Cheshomi H, Bahrami AR, Rafatpanah H, Matin MM. The effects of ellagic acid and other pomegranate (Punica granatum L ) derivatives on human gastric cancer AGS cells. Hum Exp Toxicol. 2022;41:9603271211064534. doi: 10.1177/09603271211064534. [DOI] [PubMed] [Google Scholar]
- 91.Qnais EY, Elokda AS, Abu Ghalyun YY, Abdulla FA. Antidiarrheal activity of the aqueous extract of Punica granatum (pomegranate) peels. Pharm Biol. 2007;45:715–720. [Google Scholar]
- 92.Mastrogiovanni F, Mukhopadhya A, Lacetera N, Ryan MT, Romani A, Bernini R, et al. Anti-inflammatory effects of pomegranate peel extracts on in vitro human intestinal caco-2 cells and ex vivo porcine colonic tissue explants. Nutrients. 2019;11:548–563. doi: 10.3390/nu11030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Velázquez C, Calzada F, Torres J, González F, Ceballos G. Antisecretory activity of plants used to treat gastrointestinal disorders in Mexico. J Ethnopharmacol. 2006;103:66–70. doi: 10.1016/j.jep.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 94.Ajaikumar KB, Asheef M, Babu BH, Padikkala J. The inhibition of gastric mucosal injury by Punica granatum L (pomegranate) methanolic extract. J Ethnopharmacol. 2005;96:171–176. doi: 10.1016/j.jep.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 95.Souli A, Sebai H, Rtibi K, Chehimi L, Sakly M, Amri M, et al. Inhibitory effects of two varieties of Tunisian pomegranate (Punica granatum L ) extracts on gastrointestinal transit in rat. J Med Food. 2015;18:1007–1012. doi: 10.1089/jmf.2014.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosillo MA, Sanchez-Hidalgo M, Cárdeno A, de la Lastra CA. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem Pharmacol. 2011;82:737–745. doi: 10.1016/j.bcp.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 97.Waly MI, Ali A, Guizani N, Al-Rawahi AS, Farooq SA, Rahman MS. Pomegranate (Punica granatum) peel extract efficacy as a dietary antioxidant against azoxymethane-induced colon cancer in rat. Asian Pac J Cancer Prev. 2012;13:4051–4055. doi: 10.7314/apjcp.2012.13.8.4051. [DOI] [PubMed] [Google Scholar]
- 98.Marín M, María Giner R, Ríos JL, Carmen Recio M. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J Ethnopharmacol. 2013;150:925–934. doi: 10.1016/j.jep.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 99.Parisio C, Lucarini E, Micheli L, Toti A, Khatib M, Mulinacci N, et al. Pomegranate mesocarp against colitis-induced visceral pain in rats: Effects of a decoction and its fractions. Int J Mol Sci. 2020;21:1–16. doi: 10.3390/ijms21124304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nuñez-Sánchez MA, González-Sarrías A, García-Villalba R, Monedero-Saiz T, García-Talavera NV, Gómez-Sánchez MB, et al. Gene expression changes in colon tissues from colorectal cancer patients following the intake of an ellagitannin-containing pomegranate extract: a randomized clinical trial. J Nutr Biochem. 2017;42:126–133. doi: 10.1016/j.jnutbio.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 101.Nikhat S, Fazil M. Overview of Covid-19; its prevention and management in the light of Unani medicine. Sci Total Environ. 2020;728:138859. doi: 10.1016/j.scitotenv.2020.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suručić R, Tubić B, Stojiljković MP, Djuric DM, Travar M, Grabež M, et al. Computational study of pomegranate peel extract polyphenols as potential inhibitors of SARS-CoV-2 virus internalization. Mol Cell Biochem. 2021;476:1179–1193. doi: 10.1007/s11010-020-03981-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dey D, Ray R, Hazra B. Antimicrobial activity of pomegranate fruit constituents against drug-resistant Mycobacterium tuberculosis and β-lactamase producing Klebsiella pneumoniae. Pharm Biol. 2015;53:1474–1480. doi: 10.3109/13880209.2014.986687. [DOI] [PubMed] [Google Scholar]
- 104.Li Y, Ooi LSM, Wang H, But PPH, Ooi VEC. Antiviral activities of medicinal herbs traditionally used in southern mainland China. Phytother Res. 2004;18:718–722. doi: 10.1002/ptr.1518. [DOI] [PubMed] [Google Scholar]
- 105.Saadh MJ, Almaaytah AM, Alaraj M, Dababneh MF, Sa’Adeh I, Aldalaen SM, et al. Punicalagin and zinc (II) ions inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Eur Rev Med Pharmacol Sci. 2021;25:3908–3913. doi: 10.26355/eurrev_202105_25958. [DOI] [PubMed] [Google Scholar]
- 106.Bachoual R, Talmoudi W, Boussetta T, Braut F, El-Benna J. An aqueous pomegranate peel extract inhibits neutrophil myeloperoxidase in vitro and attenuates lung inflammation in mice. Food Chem Toxicol. 2011;49:1224–1228. doi: 10.1016/j.fct.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 107.Li Y, Yang F, Zheng W, Hu M, Wang J, Ma S, et al. Punica granatum (pomegranate) leaves extract induces apoptosis through mitochondrial intrinsic pathway and inhibits migration and invasion in non-small cell lung cancer in vitro. Biomed Pharmacother. 2016;80:227–235. doi: 10.1016/j.biopha.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 108.Ghani RA, Malek NNNA, Ashari NSM, Abdullah N. Pomegranate juice induced cell cycle arrest and apoptosis via mitochondrial pathway in human lung aden carcinoma A549 cells. Int J Eng Technol. 2018;7:287–292. [Google Scholar]
- 109.Russo V, Continella A, Drago C, Gentile A, Malfa SL, Leotta CG, et al. Secondary metabolic profiles and anticancer actions from fruit extracts of immature pomegranates. PLoS ONE. 2021;16:e0255831. doi: 10.1371/journal.pone.0255831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Oliveira JFF, Garreto DV, da Silva MCP, Fortes TS, de Oliveira RB, Nascimento FRF, et al. Therapeutic potential of biodegradable microparticles containing Punica granatum L (pomegranate) in murine model of asthma. Inflamm Res. 2013;62:971–980. doi: 10.1007/s00011-013-0659-3. [DOI] [PubMed] [Google Scholar]
- 111.Mans DRA, Toelsie JR, Oedairadjsingh K, Magali I, Soekhoe R, Bipat R. Evaluation of Surinamese medicinal plants for their potential bronchospasmolytic effects in isolated guinea pig tracheal chains. Res J Med Plant. 2015;9:14–23. [Google Scholar]
- 112.Pinheiro AC, Mendes ARS, Neves M, Prado C, Santana FP, Roncon, et al. Galloyl-Hexahydroxydiphenoyl (HHDP)-glucose isolated from Punica granatum L leaves protects against lipopolysaccharide (LPS)-induced acute lung injury in BALB/c mice. Front Immunol. 2019;10:1978–1989. doi: 10.3389/fimmu.2019.01978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guan S, Wang Z, Huang Y, Huang G, Guan Y, Jiang W, et al. Punicalagin exhibits negative regulatory effects on LPS-induced acute lung injury. Eur Food Res Technol. 2014;239:837–845. [Google Scholar]
- 114.Peng J, Wei D, Fu Z, Li D, Tan Y, Xu T, et al. Punicalagin ameliorates lipopolysaccharide-induced acute respiratory distress syndrome in mice. Inflammation. 2015;38:493–499. doi: 10.1007/s10753-014-9955-5. [DOI] [PubMed] [Google Scholar]
- 115.Davinelli S, Bertoglio JC, Polimeni A, Scapagnini G. Cytoprotective polyphenols against chronological skin aging and cutaneous photodamage. Curr Pharm Des. 2018;24:99–105. doi: 10.2174/1381612823666171109102426. [DOI] [PubMed] [Google Scholar]
- 116.Amparo TR, Seibert JB, Vieira PM, Teixeira LFM, dos Santos ODH, de Souza GHB. Herbal medicines to the treatment of skin and soft tissue infections: advantages of the multi-targets action. Phytother Res. 2020;34:94–103. doi: 10.1002/ptr.6519. [DOI] [PubMed] [Google Scholar]
- 117.Mintie CA, Singh CK, Ahmad N. Whole fruit phytochemicals combating skin damage and carcinogenesis. Transl Oncol. 2020;13:146–156. doi: 10.1016/j.tranon.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zam W, Khaddour A. Antibacterial and antivirulence effects of Syrian native sweet pomegranate (Punica granatum) peel extracts against P aeruginosa provided from burn wounds. Curr Enzym Inhib. 2018;14:141–145. [Google Scholar]
- 119.Huan Y, Peng K, Wang Q, GU Z, LU Y, Jun Z, et al. Effect of pomegranate peel polyphenol gel on cutaneous wound healing in alloxan-induced diabetic rats. Chin Med J (Engl) 2013;126:1700–1706. [PubMed] [Google Scholar]
- 120.Rana J, Diwakar G, Saito L, Scholten JD, Mulder T. Inhibition of melanin content by punicalagins in the super fruit pomegranate (Punica granatum) J Cosmet Sci. 2013;64:445–453. [PubMed] [Google Scholar]
- 121.Vidyalakshmi S, Sahithya D. Preliminary screening of selected plant extracts for anti-tyrosinase activity. J Nat Remedies. 2016;16:18–21. [Google Scholar]
- 122.Diwakar G, Rana J, Saito L, Vredeveld D, Zemaitis D, Scholten J. Inhibitory effect of a novel combination of Salvia hispanica (chia) seed and Punica granatum (pomegranate) fruit extracts on melanin production. Fitoterapia. 2014;97:164–171. doi: 10.1016/j.fitote.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 123.Guo H, Liu C, Tang Q, Li D, Wan Y, Li JH, et al. Pomegranate (Punica granatum) extract and its polyphenols reduce the formation of methylglyoxal-DNA adducts and protect human keratinocytes against methylglyoxal-induced oxidative stress. J Funct Foods. 2021;83:104564. [Google Scholar]
- 124.Hora JJ, Maydew ER, Lansky EP, Dwivedi C. Chemopreventive effects of pomegranate seed oil on skin tumor development in CD1 mice. J Med Food. 2003;6:157–161. doi: 10.1089/10966200360716553. [DOI] [PubMed] [Google Scholar]
- 125.Murthy KN, Reddy VK, Veigas JM, Murthy UD. Study on wound healing activity of Punica granatum peel. J Med Food. 2004;7:256–259. doi: 10.1089/1096620041224111. [DOI] [PubMed] [Google Scholar]
- 126.Pirbalouti AG, Koohpayeh A, Karimi I. The wound healing activity of flower extracts of Punica granatum and Achillea kellalensis in Wistar rats. Acta Pol Pharm. 2010;67:107–110. [PubMed] [Google Scholar]
- 127.Houston DMJ, Bugert J, Denyer SP, Heard CM. Anti-inflammatory activity of Punica granatum L (Pomegranate) rind extracts applied topically to ex vivo skin. Eur J Pharm Biopharm. 2017;112:30–37. doi: 10.1016/j.ejpb.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 128.Kang SJ, Choi BR, Kim SH, Yi HY, Park HR, Song CH, et al. Beneficial effects of dried pomegranate juice concentrated powder on ultraviolet B-induced skin photoaging in hairless mice. Exp Ther Med. 2017;14:1023–1036. doi: 10.3892/etm.2017.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yuniarti WM, Primarizky H, Lukiswanto BS. The activity of pomegranate extract standardized 40% ellagic acid during the healing process of incision wounds in albino rats (Rattus norvegicus) Vet World. 2018;11:321–326. doi: 10.14202/vetworld.2018.321-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nayak SB, Rodrigues V, Maharaj S, Bhogadi VS. Wound healing activity of the fruit skin of Punica granatum. J Med Food. 2013;16:857–861. doi: 10.1089/jmf.2012.0229. [DOI] [PubMed] [Google Scholar]
- 131.Tang L, Li T, Zhang B, Zhang Z, Sun X, Zhu Y, et al. Punicalagin alleviates psoriasis by inhibiting NF-κB-mediated IL-1β transcription and caspase-1-regulated IL-1β secretion. Front Pharmacol. 2022;13:817526. doi: 10.3389/fphar.2022.817526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abdellatif AAH, Alawadh SH, Bouazzaoui A, Alhowail AH, Mohammed HA. Anthocyanins rich pomegranate cream as a topical formulation with anti-aging activity. J Dermatolog Treat. 2021;32:983–990. doi: 10.1080/09546634.2020.1721418. [DOI] [PubMed] [Google Scholar]
- 133.Chan LP, Tseng YP, Liu C, Liang CH. Fermented pomegranate extracts protect against oxidative stress and aging of skin. J Cosmet Dermatol. 2021;21:2236–2245. doi: 10.1111/jocd.14379. [DOI] [PubMed] [Google Scholar]
- 134.Kanlayavattanakul M, Chongnativisit W, Chaikul P, Lourith N. Phenolic-rich pomegranate peel extract: In vitro, cellular, and in vivo activities for skin hyperpigmentation treatment. Planta Med. 2020;86:749–759. doi: 10.1055/a-1170-7785. [DOI] [PubMed] [Google Scholar]
- 135.Thotambailu AM, Bhandary BSK, Sharmila KP. Protective effect of Punica granatum extract in head and neck cancer patients undergoing radiotherapy. Indian J Otolaryngol Head Neck Surg. 2019;71:318–320. doi: 10.1007/s12070-018-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bogdan C, Iurian S, Tomuta I, Moldovan M. Improvement of skin condition in striae distensae: Development, characterization and clinical efficacy of a cosmetic product containing Punica granatum seed oil and Croton lechleri resin extract. Drug Des Devel Ther. 2017;11:521–531. doi: 10.2147/DDDT.S128470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Henning SM, Yang J, Lee R, Huang J, Hsu M, Thames G, et al. Pomegranate juice and extract consumption increases the resistance to UVB-induced erythema and changes the skin microbiome in healthy women: A randomized controlled trial. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-50926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chakkalakal M, Nadora D, Gahoonia N, Dumont A, Burney W, Pan A, et al. Prospective randomized double-blind placebo-controlled study of oral pomegranate extract on skin wrinkles, biophysical features, and the gut-skin axis. J Clin Med. 2022;11:6724–6736. doi: 10.3390/jcm11226724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dkhil MA, Al-Quraishy S, Moneim AEA. Effect of pomegranate (Punica granatum L juice and methanolic peel extract on testis of male rats. Pakistan J Zool. 2013;45:1343–1349. [Google Scholar]
- 140.Türk G, Sönmez M, Aydin M, Yüce A, Gür S, Yüksel M, et al. Effects of pomegranate juice consumption on sperm quality, spermatogenic cell density, antioxidant activity and testosterone level in male rats. Clin Nutr. 2008;27:289–296. doi: 10.1016/j.clnu.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 141.Jadhav P, Kapoor N, Thomas B, Lal H, Kshirsagar N. Antiviral potential of selected Indian medicinal (ayurvedic) plants against herpes simplex virus 1 and 2. N Am J Med Sci. 2012;4:641–647. doi: 10.4103/1947-2714.104316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tang JM, Min J, Li BS, Hong SS, Liu C, Hu M, et al. Therapeutic effects of punicalagin against ovarian carcinoma cells in association with β-catenin signaling inhibition. Int J Gynecol Cancer. 2016;26:1557–1563. doi: 10.1097/IGC.0000000000000805. [DOI] [PubMed] [Google Scholar]
- 143.Leiva KP, Rubio J, Peralta F, Gonzales GF. Effect of Punica granatum (pomegranate) on sperm production in male rats treated with lead acetate. Toxicol Mech Methods. 2011;21:495–502. doi: 10.3109/15376516.2011.555789. [DOI] [PubMed] [Google Scholar]
- 144.Al-Olayan EM, El-Khadragy MF, Metwally DM, Abdel Moneim AE. Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complement Altern Med. 2014;14:164–171. doi: 10.1186/1472-6882-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Atilgan D, Parlaktas B, Uluocak N, Gencten Y, Erdemir F, Ozyurt H, et al. Pomegranate (Punica granatum) juice reduces oxidative injury and improves sperm concentration in a rat model of testicular torsion-detorsion. Exp Ther Med. 2014;8:478–482. doi: 10.3892/etm.2014.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gautam R, Priyadarshini E, Nirala JP, Meena R, Rajamani P. Modulatory effects of Punica granatum L juice against 2115 MHz (3G) radiation-induced reproductive toxicity in male Wistar rat. Environ Sci Pollut Res. 2021;28:54756–54765. doi: 10.1007/s11356-021-14378-4. [DOI] [PubMed] [Google Scholar]
- 147.Forest CP, Padma-Nathan H, Liker HR. Efficacy and safety of pomegranate juice on improvement of erectile dysfunction in male patients with mild to moderate erectile dysfunction: A randomized, placebo-controlled, double-blind, crossover study. Int J Impot Res. 2007;19:564–567. doi: 10.1038/sj.ijir.3901570. [DOI] [PubMed] [Google Scholar]
- 148.Goshtasebi A, Mazari Z, Behboudi Gandevani S, Naseri M. Anti-hemorrhagic activity of Punica granatum L flower (Persian Golnar) against heavy menstrual bleeding of endometrial origin: a double-blind, randomized controlled trial. Med J Islam Repub Iran. 2015;29:199–199. [PMC free article] [PubMed] [Google Scholar]
- 149.Miara MD, Bendif H, Rebbas K, Rabah B, Hammou MA, Maggi F. Medicinal plants and their traditional uses in the highland region of Bordj Bou Arreridj (Northeast Algeria) J Herb Med. 2019;16:100262. [Google Scholar]
- 150.Renuka S, Muralidharan NP. Comparison in benefits of herbal mouthwashes with chlorhexidine mouthwash: A review. Asian J Pharm Clin Res. 2017;10:3–7. [Google Scholar]
- 151.Karygianni L, Al-Ahmad A, Argyropoulou A, Hellwig E, Anderson AC, Skaltsounis AL. Natural antimicrobials and oral microorganisms: a systematic review on herbal interventions for the eradication of multispecies oral biofilms. Front Microbiol. 2016;6:1529. doi: 10.3389/fmicb.2015.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bansal A, Marwah N, Nigam AG, Goenka P, Goel D. Effect of Achyranthes aspera, 0 2% aqueous chlorhexidine gluconate and Punica granatum oral rinse on the levels of salivary Streptococcus mutans in 8 to 12 years old children. J Contemp Dent Pract. 2015;16:903–909. doi: 10.5005/jp-journals-10024-1779. [DOI] [PubMed] [Google Scholar]
- 153.Pinni J, Sankar Avula JS, Mukthineni S, Bandi S, Gokul T. Antimicrobial activity of pomegranate (Punica granatum) pericarp extract against Streptococcus mutans- A source for natural mouth rinse: An in-vitro and in-vivo study. Biomed Pharmacol J. 2018;11:2025–2030. [Google Scholar]
- 154.Pai MBH, Prashant GM, Murlikrishna KS, Shivakumar KM, Chandu GN. Antifungal efficacy of Punica granatum, Acacia nilotica, Cuminum cyminum and Foeniculum vulgare on Candida albicans: An in vitro study. Indian J Dent Res. 2010;21:334–336. doi: 10.4103/0970-9290.70792. [DOI] [PubMed] [Google Scholar]
- 155.Al-Obaidi D, Saja M, Afnan I. In vivo antimicrobial inhibition of Punica granatum extracts as mouthwash. Russ Open Med J. 2017;6:e0403. [Google Scholar]
- 156.Hussain MS, Wafaa SH, Altamemi S. Antibacterial activity of pomegranate peels aqueous extractions on dental caries and gingivitis Streptococcus mutans in compared with 0 2% chlorhexidine. Drug Invent Today. 2019;11:61–64. [Google Scholar]
- 157.Sousa MN, Macedo AT, Ferreira GF, Furtado HLA, Pinheiro AC, Lima-Neto LG, et al. Hydroalcoholic leaf extract of Punica granatum, alone and in combination with calcium hydroxide, is effective against mono- and polymicrobial biofilms of Enterococcus faecalis and Candida albicans. Antibiotics. 2022;11:584–594. doi: 10.3390/antibiotics11050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jesse Joel T, Suluvoy JK, Varghese J. Evaluation of the secondary metabolites of the waste pomegranate rind and its cytotoxicity against oral cancer (KB 3-1) J Pure Appl Microbiol. 2019;13:1667–1672. [Google Scholar]
- 159.Bassiri-Jahromi S. In vivo comparative evaluation of the pomegranate (Punica granatum) peel extract as an alternative agent to nystatin against oral candidiasis. Iran J Med Sci. 2018;43:296–304. [PMC free article] [PubMed] [Google Scholar]
- 160.Kritivasan S, Nazar N, Muralidharan NP. Antimicrobial prophylactic activity of pomegranate juice on oral bacteria as a pre-procedural rinse. Drug Invent Today. 2018;10:3443–3445. [Google Scholar]
- 161.Batista ALA, Lins RDAU, de Souza Coelho R, do Nascimento Barbosa D, Belém NM, Celestino FJA. Clinical efficacy analysis of the mouth rinsing with pomegranate and chamomile plant extracts in the gingival bleeding reduction. Complement Ther Clin Pract. 2014;20:93–98. doi: 10.1016/j.ctcp.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 162.DiSilvestro RA, DiSilvestro DJ, DiSilvestro DJ. Pomegranate extract mouth rinsing effects on saliva measures relevant to gingivitis risk. Phytother Res. 2009;23:1123–1127. doi: 10.1002/ptr.2759. [DOI] [PubMed] [Google Scholar]
- 163.Srilekha M, Jayashri P. Comparing the antimicrobial effectiveness of Punica granatum and chlorhexidine-containing mouthwash: A single-blind randomized clinical trial. Drug Invent Today. 2018;10:1544–1549. [Google Scholar]
- 164.Gavanji S, Larki B, Bakhtari A. The effect of extract of Punica granatum var pleniflora for treatment of minor recurrent aphthous stomatitis. Integr Med Res. 2014;3:83–90. doi: 10.1016/j.imr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Clementi ME, Sampaolese B, Sciandra F, Tringali G. Punicalagin protects human retinal pigment epithelium cells from ultraviolet radiation-induced oxidative damage by activating Nrf2/HO-1 signaling pathway and reducing apoptosis. Antioxidants. 2020;9:473–486. doi: 10.3390/antiox9060473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mestry SN, Juvekar AR. Aldose reductase inhibitory potential and anti-cataract activity of Punica granatum L leaves against glucose-induced cataractogenesis in goat eye lens. Orient Pharm Exp Med. 2017;17:277–284. [Google Scholar]
- 167.Hashem HE, Abd El-Haleem MR, Amer MG, Bor’i A. Pomegranate protective effect on experimental ischemia/reperfusion retinal injury in rats (histological and biochemical study) Ultrastruct Pathol. 2017;41:346–357. doi: 10.1080/01913123.2017.1346737. [DOI] [PubMed] [Google Scholar]
- 168.Liu S, Zhang X, Sun M, Xu T, Wang A. FoxO3a plays a key role in the protective effects of pomegranate peel extract against amikacin-induced ototoxicity. Int J Mol Med. 2017;40:175–181. doi: 10.3892/ijmm.2017.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hou C, Zhang W, Li J, Du L, Lv O, Zhao S, et al. Beneficial effects of pomegranate on lipid metabolism in metabolic disorders. Mol Nutr Food Res. 2019;63:e1800773. doi: 10.1002/mnfr.201800773. [DOI] [PubMed] [Google Scholar]
- 170.Bouazza A, Bitam A, Amiali M, Bounihi A, Yargui L, Koceir EA. Effect of fruit vinegars on liver damage and oxidative stress in high-fat-fed rats. Pharm Biol. 2016;54:260–265. doi: 10.3109/13880209.2015.1031910. [DOI] [PubMed] [Google Scholar]
- 171.Kaur G, Jabbar Z, Athar M, Alam MS. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem Toxicol. 2006;44:984–993. doi: 10.1016/j.fct.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 172.Toklu HZ, Dumlu MU, Sehirli Ö, Ercan F, Gedik N, Gökmen V, et al. Pomegranate peel extract prevents liver fibrosis in biliary-obstructed rats. J Pharm Pharmacol. 2007;59:1287–1295. doi: 10.1211/jpp.59.9.0014. [DOI] [PubMed] [Google Scholar]
- 173.Saratale RG, Shin HS, Kumar G, Benelli G, Kim DS, Saratale GD. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2) Artif Cells Nanomed Biotechnol. 2018;46:211–222. doi: 10.1080/21691401.2017.1337031. [DOI] [PubMed] [Google Scholar]
- 174.Zamora-López K, Noriega LG, Estanes-Hernández A, Escalona-Nández I, Tobón-Cornejo S, Tovar AR, et al. Punica granatum derived omega-5 nanoemulsion improves hepatic steatosis in mice fed a high fat diet by increasing fatty acid utilization in hepatocytes. Sci Rep. 2020;10:15229. doi: 10.1038/s41598-020-71878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang D, Özen C, Abu-Reidah IM, Chigurupati S, Patra JK, Horbanczuk JO, et al. Vasculoprotective effects of pomegranate (Punica granatum L ) Front Pharmacol. 2018;9:544. doi: 10.3389/fphar.2018.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huang THW, Yang Q, Harada M, Li GQ, Yamahara J, Roufogalis BD, et al. Pomegranate flower extract diminishes cardiac fibrosis in zucker diabetic fatty rats: Modulation of cardiac endothelin-1 and nuclear factor-kappaB pathways. J Cardiovasc Pharmacol. 2005;46:856–862. doi: 10.1097/01.fjc.0000190489.85058.7e. [DOI] [PubMed] [Google Scholar]
- 177.Jadeja RN, Thounaojam MC, Patel DK, Devkar RV, Ramachandran AV. Pomegranate (Punica granatum L ) juice supplementation attenuates isoproterenol-induced cardiac necrosis in rats. Cardiovasc Toxicol. 2010;10:174–180. doi: 10.1007/s12012-010-9076-9. [DOI] [PubMed] [Google Scholar]
- 178.Bounihi A, Bitam A, Bouazza A, Yargui L, Koceir EA. Fruit vinegars attenuate cardiac injury via anti-inflammatory and anti-adiposity actions in high-fat diet-induced obese rats. Pharm Biol. 2017;55:43–52. doi: 10.1080/13880209.2016.1226369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Razani Z, Dastani M, Kazerani HR. Cardioprotective effects of pomegranate (Punica granatum) juice in patients with ischemic heart disease. Phytother Res. 2017;31:1731–1738. doi: 10.1002/ptr.5901. [DOI] [PubMed] [Google Scholar]
- 180.Sahebkar A, Ferri C, Giorgini P, Bo S, Nachtigal P, Grassi D. Effects of pomegranate juice on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2017;115:149–161. doi: 10.1016/j.phrs.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 181.Senouci F, Ababou A, Chouieb M. Ethnobotanical survey of the medicinal plants used in the southern mediterranean Case study: The region of bissa (northeastern Dahra mountains, Algeria) Pharmacogn J. 2019;11:647–659. [Google Scholar]
- 182.Ribeiro RV, Bieski IGC, Balogun SO, Martins DTO. Ethnobotanical study of medicinal plants used by Ribeirinhos in the North Araguaia microregion, Mato Grosso, Brazil. J Ethnopharmacol. 2017;205:69–102. doi: 10.1016/j.jep.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 183.Lemos ICS, de Araújo Delmondes G, dos Santos ADF, Santos ES, de Oliveira DR, de Figueiredo PRL, et al. Ethnobiological survey of plants and animals used for the treatment of acute respiratory infections in children of a traditional community in the municipality of barbalha, ceará, Brazil. Afr J Tradit Complement Altern Med. 2016;13:166–175. doi: 10.21010/ajtcam.v13i4.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ballabh B, Chaurasia OP, Ahmed Z, Singh SB. Traditional medicinal plants of cold desert Ladakh—used against kidney and urinary disorders. J Ethnopharmacol. 2008;118:331–339. doi: 10.1016/j.jep.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 185.Wagh VV, Jain AK. Ethnopharmacological survey of plants used by the Bhil and Bhilala ethnic community in dermatological disorders in Western Madhya Pradesh, India. J Herb Med. 2020;19:100234. [Google Scholar]
- 186.Prabu M, Kumuthakalavalli R. Folk remedies of medicinal plants for snake bites, scorpion stings and dog bites in Eastern Ghats of Kolli Hills, Tamil Nadu, India. Int J Res Ayurveda Pharm. 2012;3:696–700. [Google Scholar]
- 187.Rashid S, Ahmad M, Zafar M, Sultana S, Ayub M, Khan MA, et al. Ethnobotanical survey of medicinally important shrubs and trees of Himalayan region of Azad Jammu and Kashmir, Pakistan. J Ethnopharmacol. 2015;166:340–351. doi: 10.1016/j.jep.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 188.Haq F, Ahmad H, Alam M. Traditional uses of medicinal plants of Nandiar Khuwarr catchment (District Battagram), Pakistan. J Med Plants Res. 2011;5:39–48. [Google Scholar]
- 189.Ahmed M, Khan MA, Zafar M, Sultana S. Treatment of common ailments by plant-based remedies among the people of district Attock (Punjab) of Northern Pakistan. Afr J Tradit Complement Altern Med. 2007;4:112–120. doi: 10.4314/ajtcam.v4i1.31201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zaid AN, Jaradat NA, Eid AM, Al Zabadi H, Alkaiyat A, Darwish SA. Ethnopharmacological survey of home remedies used for treatment of hair and scalp and their methods of preparation in the West Bank-Palestine. BMC Complement Altern Med. 2017;17:355–370. doi: 10.1186/s12906-017-1858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rosas-Piñón Y, Mejía A, Díaz-Ruiz G, Aguilar MI, Sánchez-Nieto S, Rivero-Cruz JF. Ethnobotanical survey and antibacterial activity of plants used in the Altiplane region of Mexico for the treatment of oral cavity infections. J Ethnopharmacol. 2012;141:860–865. doi: 10.1016/j.jep.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 192.Bouyahya A, Abrini J, Et-Touys A, Bakri Y, Dakka N. Indigenous knowledge of the use of medicinal plants in the North-West of Morocco and their biological activities. Eur J Integr Med. 2017;13:9–25. [Google Scholar]
- 193.Polat R, Satıl F. An ethnobotanical survey of medicinal plants in Edremit Gulf (Balıkesir–Turkey) J Ethnopharmacol. 2012;139:626–641. doi: 10.1016/j.jep.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 194.Al-Fatimi M. Ethnobotanical survey of medicinal plants in central Abyan governorate, Yemen. J Ethnopharmacol. 2019;241:111973. doi: 10.1016/j.jep.2019.111973. [DOI] [PubMed] [Google Scholar]
- 195.Yayla M, Cetin D, Adali Y, Aksu Kilicle P, Toktay E. Potential therapeutic effect of pomegranate seed oil on ovarian ischemia/reperfusion injury in rats. Iran J Basic Med Sci. 2018;21:1262–1268. doi: 10.22038/ijbms.2018.30149.7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ahmadiankia N. Molecular targets of pomegranate (Punica granatum) in preventing cancer metastasis. Iran J Basic Med Sci. 2019;22:977–988. doi: 10.22038/ijbms.2019.34653.8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dana N, Javanmard SH, Rafiee L. Role of peroxisome proliferator-activated receptor alpha and gamma in antiangiogenic effect of pomegranate peel extract. Iran J Basic Med Sci. 2016;19:106–110. [PMC free article] [PubMed] [Google Scholar]