Abstract
Hydrogen sulfide (H2S) is an important biological mediator across all kingdoms of life and plays intertwined roles in various disciplines, ranging from geochemical cycles to industrial processes. A common need across these broad disciplines is the ability to detect and measure H2S in complex sample environments. This Perspective focuses on key advances and opportunities for H2S detection and quantification that are relevant to chemical biology. Specifically, we focus on methods for H2S detection and quantification most commonly used in biological samples, including activity-based H2S probes, the methylene blue assay, the monobromobimane assay, and H2S-sensitive electrode measurements. Our goal is to help simplify what at first may seem to be an overwhelming array of detection and measurement choices, to articulate the strengths and limitations of individual techniques, and to highlight key unmet needs and opportunities in the field.
Keywords: Hydrogen sulfide, reactive sulfur species, detection, quantification, measurement
Introduction
Hydrogen sulfide (H2S) is a small gaseous molecule that is relevant across a wide range of fields including industry, geochemistry, biology, and chemistry (Figure 1).1−5 H2S is a common byproduct from a variety of industrial processes, including adhesive and fertilizer manufacturing, oil and gas refinement, and wood pulp and paper processing. In addition to its classic malodorous rotten egg smell, high levels of H2S pose health and safety risks to humans and significant corrosion risks to industrial equipment. H2S levels as low as 0.01 ppm in air can be detected by the human nose, with higher concentrations of 20 ppm leading to lung and eye irritation. Further elevated concentrations of ∼300 ppm cause eye damage, and levels of >700 ppm lead to severe complications including respiratory failure and death.6 In addition, olfactory fatigue resulting in the loss of the ability to smell H2S often occurs at ∼100 ppm. To put these levels in perspective, concentrations of 20–200 ppm of H2S are often found in raw gasifier gas in the petroleum industry and can increase depending on factors such as feedstock composition. In pulp and paper manufacturing, flue gas produced from black liquor gasification can contain up to 2000 ppm sulfur content, where H2S is also present and is scrubbed and/or oxidized significantly prior to release.7 Moving away from anthropogenic generation, H2S is a common component of volcanic gases, hot springs, swamps, and hydrothermal vents. Background H2S levels in air typically range from 0.1 and 0.33 ppb, and increased H2S levels of 0.1–0.5 ppm are common in dilute volcanic plumes.8,9 Each of these naturogenic sources provides important reservoirs of reduced sulfur in the global sulfur cycle feeding into processes ranging from sulfide mineralization to various oxidation processes, generating elemental sulfur (S8) and sulfate (SO42–). This geochemical sulfur cycle also intersects with complex microbial sulfur cycling processes involved in anaerobic respiration, ranging from dissimilatory sulfate reduction in which SO42– serves as the terminal electron acceptor in the production of H2S, to sulfur oxidizing chemolithotrophic bacteria that oxidize H2S to support carbon fixation.10−12
Figure 1.
General schematic of broad fields in which H2S plays an important role.
Moving to more complex eukaryotic systems, H2S also plays key roles in signaling and redox homeostasis in fungi, plants, and mammals. Highlighting contemporary roles in mammalian physiology, H2S plays critical roles in hypertension, angiogenesis, neuromodulation, aging, and other pathways—all of which have garnered significant interest as potential biomarkers or targets for pharmacological intervention.4,13,14 Concentrations of free H2S/HS– in mammalian systems have been heavily debated in the literature and significantly refined with improved analytical approaches and techniques, with currently accepted values being in the low to mid-nM range in human serum and plasma. Within each of the diverse fields described above, the fundamental chemistry of H2S and related reactive sulfur species (RSS) provides a foundational platform on which these complex molecular processes can be understood at an atomistic level. More practically, insights into this fundamental chemistry have also been leveraged to provide new and evolving approaches for the detection and quantification of H2S and related species in complex environments.
Mirroring the breadth of environments in which H2S is found, the specific needs for H2S measurement vary significantly and depend on sample type, required detection limit, temporal resolution, and many other factors. For example, in industrial environments where H2S is present or generated, the rapid detection of high H2S levels in air that exceed regulatory or safety limits for human health constitutes a critical component of life safety monitoring. In such environments, the use of electronic H2S sensor devices that monitor H2S levels in the gas phase and alarm when operating exposure or operating limit thresholds are exceeded is commonplace. In addition to safety concerns, H2S measurement is also important in many sectors to ensure product quality. For example, low levels of H2S are present in many food and beverage products, such as wine, beer, milk, cheese, and other foodstuffs,15−18 but higher levels may result in unwanted flavor and aroma profiles or inform on production problems. Depending on the specific product or food matrix, H2S detection can range from quantitative analytical measurement of total or dissolved sulfide to a qualitative sensory panel for detection of “off” odors. Expanding to other environments, H2S measurements are also commonly carried out on environmental and biological samples, often with significantly varied target concentration ranges, matrix types, and accompanying analytical methods. Sample matrix and complexity also further complicate these methods, and there are different needs and requirements for bulk/homogenized samples, versus other applications with intact cells or tissues. In addition, sample storage is also an important consideration based on both the volatility of H2S and its propensity to be oxidized into other RSS. Building from this broad palette of measurement approaches, our goal in this Perspective will be to focus on key advances and opportunities for H2S detection and quantification relevant to chemical biology. Based on the breadth of the field, we will aim to provide representative and selective examples of different chemistry and approaches rather than comprehensive coverage of this rapidly expanding area. Specifically, our goals are to help simplify what at first may seem to be an overwhelming array of detection and measurement choices, to articulate the strengths and limitations of individual techniques, and to highlight key unmet needs and opportunities in the field.
Endogenous H2S Generation and Probing Biological Function
H2S is commonly referred to as a “gasotransmitter” along with nitric oxide (NO) and carbon monoxide (CO), meaning that it is an endogenously produced gaseous molecule that acts on molecular targets at physiologically relevant concentrations within the same organism in which it was generated.19 Both enzymatic and nonenzymatic pathways contribute to endogenous H2S generation, which adds an additional level of complexity to the H2S landscape. Enzymatic H2S generation stems primarily from cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST)/cysteine aminotransferase (CAT), which use homocysteine (Hcy) or cysteine (Cys) to generate H2S (Figure 2).13 H2S can also be formed through nonenzymatic pathways occurring through the reduction of reductant-labile sulfide sources, including polysulfides, persulfides, and thiosulfate. Importantly, these different generation manifolds allow for both tight spatiotemporal H2S regulation through enzymatic synthesis as well as more general H2S modulation by the redox-labile RSS pools in response to different stimuli.20,21 Once generated, H2S is involved in a broad array of biological and physiological processes including vasodilation, neuromodulation, angiogenesis, and cytoprotection from reactive oxygen species.22−27 Highlighting the importance of biological regulation of basal H2S levels, dysregulation of endogenous H2S has been observed in different disease states such as asthma, Alzheimer’s disease, diabetes, cancer, and other pathophysiological conditions.28−32
Figure 2.
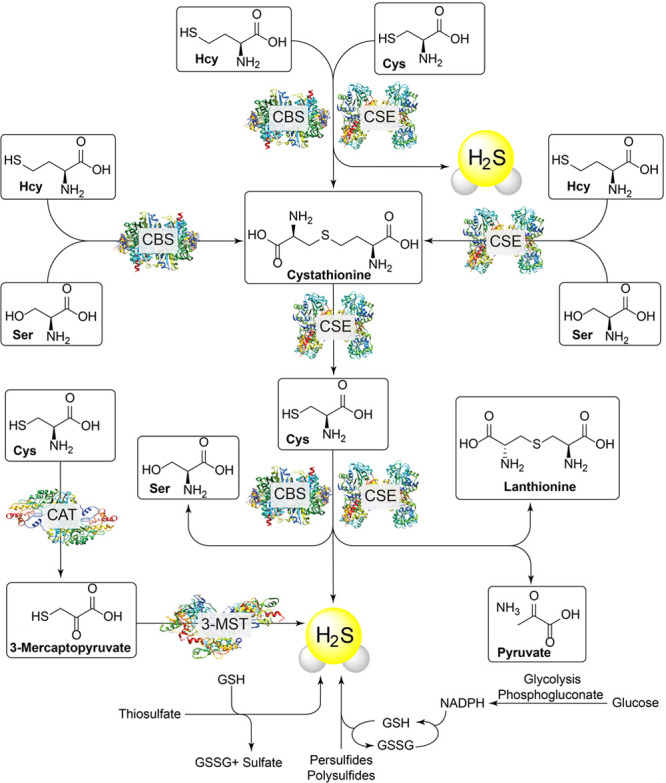
Selected pathways for the generation of endogenous H2S in mammalian systems.
Our understanding of the multifaceted roles of H2S in biology has benefited from the use of different approaches to probe different aspects of RSS biology. For example, the use of enzymatic knock out (KO) models, exogenous delivery, and endogenous detection serve as key investigative pillars to study and support the roles of H2S in chemical biology. CSE knockout mice have served as the most commonly used KO model for H2S investigations,33 although 3-MST and CBS KO models are also available.34,35 While KO models provide insights into how reduced H2S generation impacts specific biological functions, the development of H2S releasing compounds (H2S donors) has provided a complementary set of tools to determine responses to elevated H2S levels, or replacement/rescue of H2S levels in deficient systems.36−38 Importantly, many classes of H2S donors provide a slow release of H2S, which better matches endogenous H2S production, rather than a single bolus of H2S release from sulfide salts like NaSH or Na2S, which often provide a toxicological rather than pharmacological profile. Complementing these tools for H2S modulation, new approaches for H2S detection and quantification have been integral to understanding endogenous H2S regulation in different systems, disease states, and responses to various stimuli. Despite the importance of H2S measurement in biological contexts, the dynamic environment and low levels of H2S present in complex biological media has made H2S detection, measurement, and quantification particularly challenging. In many ways, the expansion of H2S as an important biomolecule has necessitated the need for new approaches for H2S measurement in complex environments, leading to new platforms for both qualitative and quantitative investigation into H2S and adjacent RSS pools.
Basic Properties of H2S and Interactions with Adjacent RSS Pools
H2S is a weak acid, with pKa(1) = 7.0 for the equilibrium H2S ⇌ HS– + H+ and pKa(2) > 14 for the equilibrium HS– ⇌ S2– + H+. These pKa values mean that, at physiological pH, about 80% of H2S is speciated as hydrosulfide (HS–) with the remainder being the diprotic H2S form. Dianionic S2– was previously considered to be part of this speciation at <1%; however, recent studies have shown that S2– is not present in aqueous solutions.39 In general, we will use the term “H2S” to encompass the H2S + HS– speciation found in aqueous samples, unless a specific protonation state is required for clarity. The ability of H2S to readily access two distinct protonation states provides unique reactivity not available to NO or CO. For example, diprotic H2S is more lipophilic, which likely facilitates membrane permeability and translocation across hydrophobic environments. In contrast, HS– is more hydrophilic, which facilitates diffusion in aqueous environments, and is also a potent nucleophile, which enables reactions with other electrophilic RSS. These physicochemical properties of H2S contribute to its fundamental reactivity in biology and also provide the foundation for chemical methods for detection and quantification.
Although the primary focus of this Perspective is on H2S measurement, a basic understanding of adjacent RSS pools is also important for appreciating the complexity and potential pitfalls of different analytical approaches (Figure 3). In general, sulfur oxidation states in biological RSS range from −2 to +6, which highlights the complexity of the RSS redox landscape.40−42 The sulfur atoms in H2S, thiols, and iron–sulfur clusters are the most reduced forms of sulfur and have oxidation states of −2, whereas the sulfur atom in SO42– has an oxidation state of +6. Interspersed between these end points are other important RSS, including species that comprise the redox labile or sulfane sulfur pool. These pools typically contain partially oxidized sulfur in the −1 or 0 oxidation state, which can readily be reduced to H2S by biological reductants. Common examples of such species include organic polysulfides (RS(S)nSR), inorganic polysulfides (HS(S)nSH), and persulfides (RSSH). These partially oxidized sources of sulfur not only offer an important storage pool for redox labile sulfide, but also comprise an important component of RSS signaling and action.43 As a simple example, protein persulfidation, in which a cysteine residue undergoes an oxidative post-translational modification to form cysteine persulfide, is now recognized as an important pathway associated with H2S signaling and modification of enzyme activity.43−45 Moreover, the RSS pool also plays an important role in redox buffering and stress response mechanisms. Readers interested in the intricacies of these biological sulfur pools are referred to a number of contemporary reviews,40,43,46−50 and our intent is to focus on H2S measurement as one piece of this greater puzzle.
Figure 3.
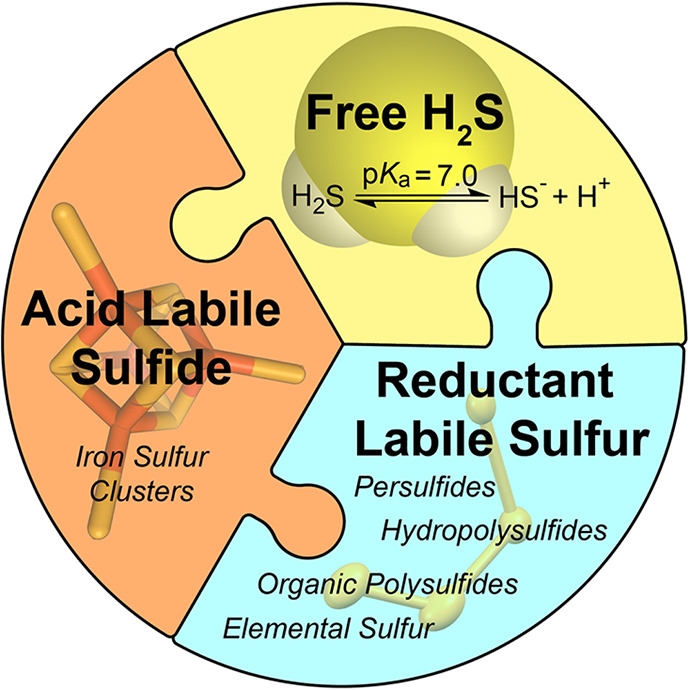
Schematic summary of the different RSS pools.
Common Methods for Detection and Quantification
A wide array of tools for H2S measurement are available, and while this abundance is useful, it also poses the challenge of matching the method with the measurement need. At the most basic level, the sample types, specific environments, experimental requirements, and concentration ranges all contribute to the selection of a specific H2S measurement method. Some of the most common approaches for H2S detection and measurement relevant to chemical biology are listed in Table 1, each of which is described in more detail later in this Perspective.
Table 1. General Summaries of H2S Measurement Approaches Using Fluorescent Probes, Methylene Blue, Monobromobimane, and Electrode Approaches.
| method | detection limit | sample types | benefits | limitations |
|---|---|---|---|---|
| fluorescent probes | probe dependent | aqueous media | tunable emission wavelengths | selectivity, rate dependent on sensing chemistry |
| biological fluids | compatible with live cells and tissues | signal accumulation measurements | ||
| cell and tissue culture | subcellular targeting | difficult for quantification | ||
| in vivo applications | different detection methods available | not reversible | ||
| methylene blue (MB) | ∼2 μM | aqueous media | simple, high-throughput method | limited detection range |
| biological fluids | low-cost reagents and instrumentation | extracts acid-labile sulfide | ||
| cell lysates | adaptable to different applications | potential bleaching by ros | ||
| relatively short analysis time | does not provide real-time measurement | |||
| monobromobimane (mBB) | ∼2 nM | aqueous media | high sensitivity | strict sample preparation and storage needs |
| biological fluids | separation of labile sulfide pools | expensive reagents | ||
| cell lysates | stable trapped sulfide product | does not provide real-time measurement | ||
| use with complex biological samples | time required for sample analysis | |||
| electrode | 5–300 nM | aqueous media | real-time measurement | frequent calibration required |
| biological fluids | reversible responses | sensitive to solution components | ||
| cell lysates | simple experimental setup | reproducibility across devices | ||
| high sensitivity | electrode lifetime |
Perhaps the most common H2S detection method, at least from the number of publications within the past decade, is activity-based fluorescent probes. These probes translate a H2S-specific chemical reaction, often utilizing its nucleophilic,51,52 reductive,53,54 or metallophilic55,56 nature to generate a fluorescent response from a specifically designed fluorogenic system. This approach is not limited to fluorescent reporters and has also been used to develop colorimetric, chemiluminescent, photoacoustic, and other types of reporting mechanisms. Different approaches that covalently trap the sulfur atom from H2S in a specific product, such as in the methylene blue (MB) or monobromobimane (mBB) trapping methods, are also available. The MB assay relies on a H2S-specific reaction to generate the final MB dye, which can be measured and quantified by UV–vis spectroscopy. By contrast, the mBB method labels all sulfhydryl nucleophiles and relies on high-performance liquid chromatography (HPLC) analysis to provide accurate quantification of the fluorescent product derived from H2S. Using different chemistry, a common approach for quantification, particularly with high temporal resolution, is the use of amperometric H2S sensitive electrodes. These electrodes function by coupling H2S oxidation within the electrode to quantifiable signal at the working electrode.57 Each of the methods for H2S detection and quantification outlined above is discussed in greater detail in this Perspective.
One challenge in H2S detection and measurement is that there is not a single “gold standard” method that applies broadly across sample types or disciplines. For example, the simple detection of H2S in the headspace of an experiment can be accomplished using colorimetric test strips, whereas observing H2S generation in response to a specific stimulus in live cells or tissues may require specialized fluorescent probes. By contrast, measuring different basal H2S levels and labile pools of H2S in specific tissues requires complex trapping and analytical protocols, whereas the measurement of quantitative rate data may require H2S-responsive electrodes. Our discussion below will focus on detection approaches using fluorescent probes and also quantification approaches, such as the MB assay, mBB method, and H2S-responsive electrodes. We will highlight the development of these methods and how they have impacted the field with the goal of providing benefits and pitfalls of each method and also highlighting further needed advances in the field.
Detection Approaches
A common approach for H2S detection is the use of activity-based fluorescent probes, many of which can be easily prepared or purchased from commercial suppliers. When used in combination with fluorescence microscopy, such probes enable H2S detection in complex environments, including live cell or tissue culture experiments, with a high spatiotemporal resolution. In general, these probes function by using H2S to remove a protecting group on the chromophore and generate a fluorescent response upon reaction with the analyte. The selectivity of such probes for H2S over other analytes is intrinsically tied to the fundamental H2S-selective chemistry from which each probe is designed and also to the types and concentrations of potentially competing analytes. In addition, different probes have varying response rates and respond differently to specific interferants. Ideally, such probes will have high selectivity for H2S over other thiols, such as Cys or GSH, which are generally found in much higher concentrations (up to 5–10 mM for GSH) than H2S. Careful selectivity investigations for H2S over other RSS, particularly polysulfides and persulfides, have not been investigated in detail across the different classes of activity-based probes and would be a useful addition to the field. In addition, because activity-based fluorescent probes react irreversibly with H2S, they function as chemodosimeters for accumulated signal rather than real-time reversible sensors for analyte fluxes. Although chemical approaches to develop cyclable and analyte-replacement fluorescent probes for H2S have been reported, these have not yet produced reversible responses in biologically relevant complex environments.44,58−61 A recent and innovative application in this area is reversible binding and fluorescent response using a recombinant hemoglobin I system from the clam Lucina pectinata, although this approach has not yet been used broadly.59 In general, turn-on fluorescent probes are useful for visualizing low concentrations of analytes, particularly in response to specific stimuli, but also have limitations for quantification in complex environments due to inherent assumptions of probe loading and homogeneity, as well as difficulties in directly assessing potential interferent concentrations. Some of these limitations can be overcome, however, by the use of ratiometric probes. Such probes provide fluorescent signals at different wavelengths both prior to and after reaction with the analyte, which allow for both cellular uptake and reaction with an analyte to be observed directly. These characteristics address some of the challenges with different probe loadings and homogeneities within a sample for turn-on-based probes, although care must be taken to not exceed the dynamic range of a ratiometric system to facilitate accurate measurement.
The development of activity-based fluorescent probes for H2S has expanded tremendously in the past decade and now encompasses a diverse palette of approaches and probes that respond to H2S in different environments. Our goal is not to cover this area comprehensively but rather to highlight key advances and approaches as they relate to H2S detection and measurement. We refer those interested in recent H2S activity-based probe design to a number of excellent reviews on the topic.62−64 In general, most fluorescent- and colorimetric-activity-based probes for H2S can be grouped into those based on H2S-mediated reduction, addition to electrophiles, and metal precipitation (Figure 4).
Figure 4.
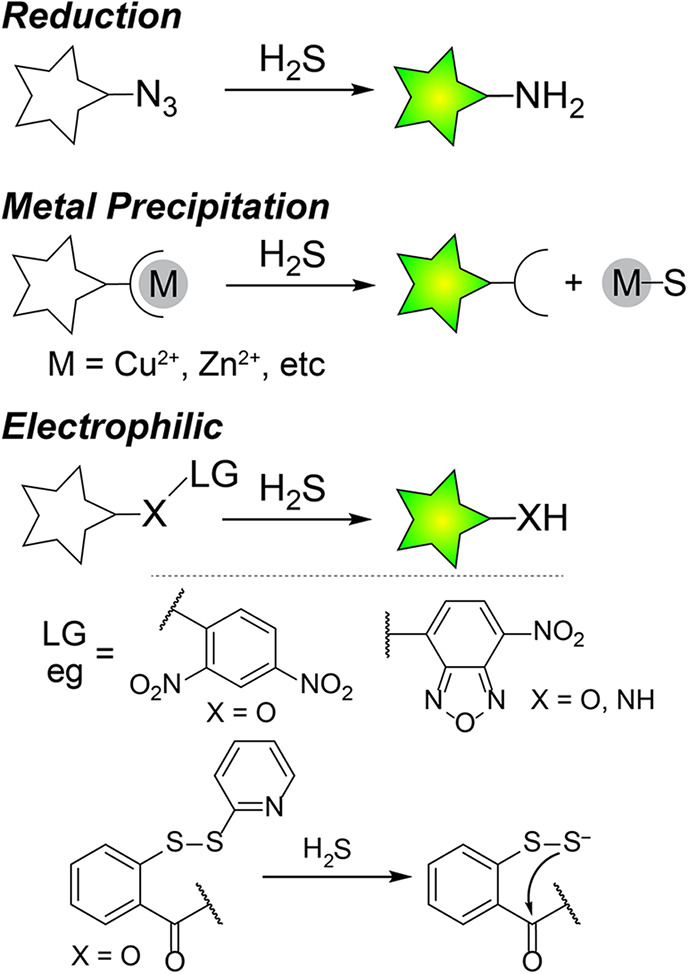
General scheme for the primary approaches for activity-based probes for H2S detection.
One of the most common approaches for H2S fluorescent probe development is the use of H2S-mediated reduction of aryl azides to amines to generate a fluorescence response.53,54,65,66 A major benefit of this approach is that azide-based scaffolds are generally compatible with biological systems and can be easily prepared from the parent amine-based fluorophores. This synthetic ease of incorporation and high biological compatibility are evident from the breadth of papers reporting azide-based probes. This general design simplicity has allowed for a broad palette of probe colors, including near IR (NIR) dyes,65,67−69 targeted probes for subcellular organelles,70−73 and combined approaches with other analytes to generate dual-responsive probes.71,74 In addition, azide based probes are generally selective for H2S over other sulfhydryl containing nucleophiles, although dithiol reductants like dithiothreitol (DTT) or phosphine-based reductants like tris(2-carboxyethyl)phosphine (TCEP) result in rapid azide reduction. Despite the breadth of azide-based probes, specific limitations should be taken into account. For example, many aryl azides are inherently photosensitive and can be photoreduced to the parent amine.75 Such considerations are particularly important for fluorescent imaging experiments in which high intensity excitation light is used, which makes control experiments particularly important for understanding the potential magnitude of photoactivation under the experimental conditions. Similarly, aryl azides can be reduced by certain reductases, which may contribute to background signal in certain contexts.76 The mechanism of H2S-mediated azide reduction requires two equivalents of H2S, proceeds by the initial attack of HS– on the azide, and results in polysulfide formation.77 In general, the rates of H2S-mediated azide reduction are relatively slow (typically requiring up to an hour for full probe activation at reasonable fluorophore and H2S concentrations), although sometimes these rates appear faster in the literature due to the use of high probe and H2S concentrations or by the use of organic cosolvents.
The high nucleophilic character of HS– has also been used to develop different types of electrophilic probes for H2S. Common approaches within this class include SNAr cleavage of electron poor aromatics, disulfide exchange reactions, additions to break conjugation, tandem Michael additions, and nucleophilic addition to conjugated systems.51,52,61,78−86 Selected examples of these approaches are listed in Figure 4. In general, most of these probes function by H2S-mediated cleavage of an electrophilic quenching group, which results in a turn-on response. SNAr cleavage approaches include common 2,4-dinitrophenyl (2,4-DNP) ether and sulfonyl ester motifs,80−82 which react more quickly with the highly nucleophilic HS– than competing thiols, and also nitrobenzofurazan (NBD) motifs that have been optimized for selectivity for H2S over thiols.83−86 A variety of probes based on these electrophilic protecting groups have been developed across a wide array emission wavelengths,87,88 and also with subcellular targeting motifs.81,89,90 One approach to improve the selectivity of electrophilic probes for H2S over competing thiols has been the development of probes with two electrophilic sites.51,78,79 Such probes, which often rely on disulfide exchange and tandem Michael addition, provide a high selectivity for H2S over competing thiols because H2S can participate in two sequential nucleophilic attacks, whereas Cys or GSH cannot. One limitation of this approach is that even though the response is selective for H2S over thiols, other thiols can still react with and consume the probe, meaning that higher concentrations of probe may be needed to overcome basal thiol concentrations, which has an impact on overall cellular thiol levels.
In addition to using the nucleophilic character of H2S to generate an optical response, different probes have also been developed that leverage the metallophilicity of H2S to remove a coordinated metal from a sensor. The most common approach in this class of probes has been to use a metal chelator to bind Cu2+, which results in fluorescence quenching of the fluorophore.55 Upon treatment with H2S, the Cu2+ is removed from the chelator to form CuS and the fluorescence of the system is restored. The use of Cu2+ precipitation is the most common approach in this class of compounds due to the additional paramagnetic quenching from Cu2+, although other metal ions have also been used. A variety of H2S probes based on Cu2+ precipitation have been reported, including probes that function in the NIR, probes with ligated peptides, and probes coupled to lanthanide luminescence chromophores.91−94 Sulfide coordination to metals is also an emerging area in fluorescent probe development although this approach is less prevalent than metal precipitation.95,96 One advantage of the metal precipitation approach is that the response time is significantly faster than that of the reduction or electrophile based approaches. For example, in the initial report of a fluorophore with pendant Cu2+-cyclen complex, addition of NaSH resulted in an almost instant fluorescence response.55 In addition, the selectivity for H2S over other species is often high based on the low solubility of metal sulfides. One of the limitations of this approach is that each of the metal-binding ligands has a specific Ka for different metal ions, meaning that the amount of metal ions bound will depend on the probe concentration. In addition, adding metal ions, even if tightly bound, may have unintended impacts in cellular environments based on the role of different metal ions in different biological pathways. For redox active metals, it is also possible that metal redox state could be modified by cellular reductants or that exchange with labile pools of other metals, such as Zn2+ could occur and modify the fluorescence response.55,97,98
Quantification Approaches
Complementing activity-based probes for H2S detection that primarily provide qualitative measurement data when used in complex environments, a variety of quantitative trapping methods for H2S have also been developed. The goal of these tools is to provide a robust method for accurately measuring the H2S present within a complex sample. Although our goal is to highlight the use of these approaches on biological samples, many of the same examples and approaches can be applied in environmental, food, and beverage samples. In general, our focus in this section will be on quantification approaches within biological samples that rely on direct H2S trapping, followed by analytical measurement. Some of these approaches share fundamental principles similar to those of activity-based probes, but a common difference is that most chemical methods for quantification sequester the sulfur atom from H2S into a chromogenic product, which can be quantified when measured against a calibration curve. In this section, we focus on two commonly used chemical methods for H2S trapping and quantification as well as one electrochemical method.
Methylene Blue Assay
The methylene blue (MB) assay is the most commonly used method for H2S measurement in chemical biology, in part due to its ease of use and low instrumentation requirements for sample analysis. Using a modified method that was first reported in the 1940s,99 the MB assay relies on the reaction of p-dimethylamino aniline with sulfide under acidic conditions in the presence of a FeCl3 catalyst to form the MB dye (Figure 5). Because this method effectively traps the S atom from the sulfide in the MB product, the MB absorbance at the end of the assay is directly proportional to the amount of sulfide trapped by this method. The MB dye has a high extinction coefficient (λmax = 664 nm, ε = 95,000 M–1 cm–1 in water) in an absorbance window that is typically devoid of absorbances from other biological components in solution. One benefit of this method is that it can be used in many types of environments ranging from environmental water samples to H2S donor experiments to cell lysates or other biological media. The MB method can be used to measure H2S levels at different time points from a sample that is generating H2S, or by measuring the total sulfide within a specific sample.
Figure 5.
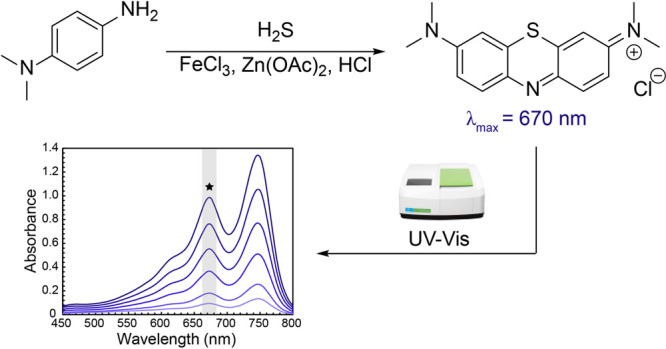
Schematic of the methylene blue method for H2S quantification.
Although the MB method is effective for trapping and measuring sulfide, one drawback of comparing MB data from different reports is that slight modifications used in the general procedure, such as aerobic versus anaerobic incubation, overall incubation time, or sample volume size, can impact overall measurements (vide infra). In theory, proper calibration curves under the exact experimental conditions used for the target measurement can abate these differences, but in many cases sufficient experimental details are not provided to discern small differences in MB method procedures. In brief, the MB assay is typically performed by removing aliquots from a H2S-containing sample and introducing them into cuvettes or a 96-well plate containing a “MB cocktail” solution. In our hands, when analyzing 500 μL sample aliquots we use an equal volume of the “MB cocktail” solution, which is prepared by combining 200 μL of 30 mM FeCl3 in 1.2 M HCl, 200 μL of 20 mM N,N-dimethyl-p-phenylenediamine salt in 7.2 M HCl, and 100 μL of 1% Zn(OAc)2 in water. In general, if differently sized aliquots are used, we recommend using an equal volume of the MB cocktail while keeping the 2:2:1 ratio of the three solution components. After incubation of the MB reaction for 1 h at room temperature while protected from ambient light, the absorbance of the sample is measured by UV–vis spectroscopy, and the H2S concentration is calculated by comparison of the absorbance at 670 nm with a calibration curve generated from NaSH or Na2S run in parallel under identical experimental conditions. Depending on the sample size, this method is applicable to individual cuvette measurements or higher-throughput 96-well plates, although appropriate control experiments should be performed to confirm that H2S volatilization between adjacent wells is not occurring.
An additional benefit of the MB method is its simplicity. Both UV–vis and plate reader instruments are relatively common in analytical or research environments; the required reagents are inexpensive, and the MB cocktail solutions can be stored in an amber bottle protected from light for future use. Despite this simplicity, there are also a number of drawbacks to the MB assay. One key limitation is the relatively modest detection limit of 2 μM,100 which is above the generally accepted mid-nM levels of free H2S in most biological systems. For example, measurements of plasma H2S levels in mice showed that the MB assay is insufficient to differentiate between H2S levels in wildtype and CSE–/– mice.100,101 Despite this limitation, the MB assay has been used broadly to detect and quantify H2S in biological samples. Significant caution should be used, however, when interpreting results of H2S quantification near (or below) the limits of the method, but in general, this method can be useful in measuring differences between samples or accumulation over time. The relatively limited working absorbance range of UV–vis and plate reader spectrophotometers also provides a practical concentration range window that may require sample dilution, depending on the application. A second challenge with using the MB assay in biological applications is that the acidic conditions required for MB formation release sulfide from acid-labile sources, such as iron–sulfur clusters, which may lead to an overestimation of H2S levels. The MB chromophore also has inherent limitations. For example, the primary absorbance used to quantify MB in solution corresponds to the MB dye (MB+), but other species are also present in solution, including the protonated monomer (MB2+), dimer ((MB+)2), and trimer ((MB+)3), which can complicate analysis and highlight both the need for pH control and also a narrow concentration range for accurate quantification.102 In addition, MB can also be reduced by reductants like NADPH to form colorless leucomethylene blue or decomposed by oxidants like H2O2, both of which can have deleterious impacts on H2S measurements using the MB method.103
Monobromobimane Method
A significant advance in H2S quantification, particularly for biological samples, was the development of H2S trapping methods that allowed for measurement by fluorescence rather than absorbance, which significantly improved the detection limit. By adapting common methods for thiol labeling, researchers developed the monobromobimane (mBB) method for sulfide quantification (Figure 6a).100,104 Much like the MB method, the mBB method traps the S atom from H2S in a stable, quantifiable product. Specifically, the mBB method uses an excess of mBB at a basic pH (9.5) to trap H2S as the fluorescent sulfide dibimane (SdB) product. The resultant SdB product can then be quantified by HPLC against an SdB standard, reaching a low detection limit of 2 nM. Importantly, the HPLC analysis step is required, because the mBB also labels other thiols, such as Cys or GSH, to form fluorescent products.
Figure 6.
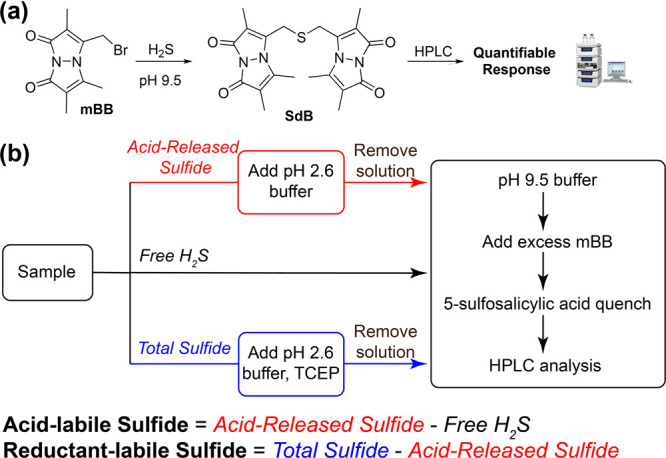
(a) General schematic for the reaction of mBB to form SdB in the presence of H2S. (b) Summary of the workflow for the mBB method for H2S quantification including the general approach for separating sulfide sources from the acid- and reductant-labile sulfur pools.
One important aspect of the mBB method is that workflows have been developed to allow for separation and quantification of free, acid-labile, and reductant-labile sulfide pools from the sample, which provides a significant advance over the MB method (Figure 6b). Detailed procedures for analytical separation of these pools have been published previously and we refer the reader to detailed methods on this approach.101 Summarizing this approach briefly, free H2S can be measured by adding the sample (30 μL) into an amber vial containing 100 mM Tris-HCl buffer (70 μL, pH 9.5, 0.1 mM DTPA), followed by the addition of excess mBB (50 μL, 10 mM in MeCN). After incubating for 30 min, the reaction is quenched with 5-sulfosalicylic acid (50 μL, 200 mM) to acidify the solution, stop the labeling reaction, and to precipitate any remaining soluble proteins. Subsequent HPLC analysis and comparison against a calibration curve with a SdB standard (λex = 390 nm, λem = 475 nm) allows for H2S quantification. Because this method is run at basic pH during the labeling step, acid-labile sulfide is not released or quantified by this method.
To measure the acid-labile sulfide directly, the above procedure is modified to first incubate the sample in acidic phosphate buffer (100 mM, pH 2.6, 0.1 mM DTPA) in a sealed vial to both release any acid-labile H2S and also to volatilize all of the H2S to the headspace. The remaining solution is then removed by syringe, and pH 9.5 buffer is added followed by mBB to trap the H2S as SdB. HPLC analysis of the SdB product provides the total acid-released sulfide, which is the sum of the free and acid-labile H2S concentrations. The acid-labile component can be calculated by measuring the acid-released sulfide (acid-labile + free H2S; red path in Figure 6b) and subtracting the free H2S (black path in Figure 6b) . Similarly, the reductant-labile sulfide can be quantified by measuring the total sulfide (acid-labile + reductant-labile + free H2S; blue path in Figure 6b) and subtracting the acid-released sulfide (acid-labile + free H2S, red path in Figure 6b). To measure the total sulfide, the same procedure is followed for measuring acid-labile sulfide, but in the first incubation step at pH 2.6, 1 mM TCEP is added to fully reduce any sulfane sulfur species to H2S. To accurately separate and measure these three pools, careful attention to sample preparation and storage is needed. Changes in light exposure, pH, and degassed buffer can cause inconsistencies in results.105 Moreover, the presence of trace metals can also impact the results, which can be abated by the storage of samples with diethylenetriaminepentaacetic acid (DTPA) to chelate any free metals.
As highlighted above, a key advance of the mBB approach is both the separation of different sulfide pools and also the low 2 nM detection limit, which has made the mBB method useful for the measurement of H2S in biomedical samples. In addition, this method allows for investigation where sulfide is distributed between different sulfur pools in different sample populations or disease states.106−108 Highlighting a direct comparison with the MB method, the mBB method is sufficiently sensitive for direct measurement, quantification, and differentiation between H2S levels in plasma of wildtype and CSE–/– mice.100,101 The usefulness of the mBB method is reflected in the breadth of applications and complex samples, including blood/plasma, cell and tissue lysates, and other biological materials.
The reagents in the mBB method are significantly more sensitive than those in the MB assay and can be degraded by both light and radical processes. These details significantly increase the sample storage complexity, particularly when sample storage is required prior to measurement. In addition, a sufficient excess of mBB is required to not only increase the rate of SdB formation but also to label any other reactive thiol groups that may be present in solution. When comparing the mBB method to methods such as the MB assay, the mBB method is significantly more cost and infrastructure intensive. The mBB trapping agent is significantly more expensive (∼$3,500/g from Cayman Chemicals) than the N,N-dimethyl-p-phenylenediamine dihydrochloride (∼$6/g from Aldrich) used in the MB assay, making sample size a key consideration when comparing these assays. In addition, HPLC instruments with fluorescence detectors are typically more expensive and more specialized than UV–vis instruments or plate readers and also often require significant method optimization for suitable peak separation and analytical measurements.
Sample preparation and sample uniformity are also important considerations for this method, and small deviations can cause large changes in the measured H2S levels. For example, changing the pH of the mBB labeling reaction from of 9.5 to 8.0 significantly decreases the rate and amount of SdB product formation.109 Additionally, common reagents such as 2-mercaptoethanol and N-ethylmaleimide cannot be present in the sample because they disrupt the SdB forming reaction.100,101 Similarly, mBB solutions, both prior to analysis and during analysis, need to be protected from light to avoid unwanted photodebromination to form bimane and also must be protected from free metal ions, particularly iron, to prevent radical decomposition of mBB. These and other considerations have been investigated in detail in recently published work.105
H2S-Sensitive Electrodes
Unlike the earlier examples that couple an H2S reaction to different chromophores, H2S-sensitive electrodes couple the oxidation of H2S at an electrode with a quantifiable output signal. Importantly, this approach allows for both quantification and real-time measurements. Such real-time feedback is not possible with the MB and mBB methods, both of which require incubation times before analysis. Because of these characteristics, H2S-sensitive electrodes provide additional temporal information that is otherwise unavailable for most activity-based labeling techniques. Commercial H2S electrodes are available from multiple vendors and typically function through proprietary components or designs, although the same general principles apply for detection. In the general electrode design, H2S passes through a silicone membrane surrounding the electrode into a basic solution inside to electrode to form HS–. This membrane provides initial selectivity for H2S and also leverages the differential permeability of H2S and HS–. The HS– trapped within the electrode is then oxidized by [Fe(CN)6]3– to form [Fe(CN)6]4–, and the reoxidation of [Fe(CN)6]4– at the working electrode generates a response signal that corresponds directly to HS– concentration, which can be quantified by comparison to an appropriate calibration curve. H2S electrodes are an attractive option for H2S measurement because they allow for real-time feedback, with both H2S increases and decreases being readily measurable. Under optimized conditions, commercial electrodes report detection limits as low as 5–300 nM. Unlike the MB or mBB quantification methods, H2S-responsive electrodes allow for almost immediate, real-time responses to H2S in solution, making this approach convenient for measuring fast changes or responses to specific stimuli. As mentioned above, the signal reversibility of H2S electrodes provides an additional advantage over the MB, mBB, or fluorescent probe methods and enables the measurement in dynamic systems.
An additional benefit of electrode measurements is that the experimental setup is relatively simple. Because the electrode reports on real-time concentrations, the measurement vial should be closed to outside air both to avoid H2S oxidation and also to limit H2S volatilization and release. This is usually accomplished by using a septum seal with holes for the electrode, grounding wire, and any needed additions by syringe. In our hands, electrode measurements are sensitive to changes in light and temperature, as well as nearby vibrations, which should all be minimized during the course of an experiment. For all electrode measurements, the electrode needs to equilibrate in the measurement solution before aliquots of the H2S-containing sample are added. In addition, solution homogeneity is important, and efficient stirring with a crossed stir bar can help to maintain rapid mixing. Individual injections of the H2S containing sample generate an electrode response, which can be quantified against an H2S calibration curve obtained under identical experimental conditions. In our experience, new calibration curves should be collected on the same day as a quantification experiment to minimize possible external factors influencing the signal readout.
Despite the general ease of use and reversible signal, H2S electrodes also have several drawbacks that may limit their utility in different contexts. For example, most electrodes have minimal tolerance to organic solvents, which may not be a problem for biological samples but may limit their utility for measuring H2S release from synthetic compounds with limited water solubility. In addition, electrode sensitivity typically decreases during the electrode lifetime, which may provide complications if measurements are needed near the lower detection limit of the electrode. Much like the MB and mBB methods, H2S electrodes also require calibration prior to use against NaSH or Na2S standards, which is required frequently due to electrode drift and high electrode sensitivity to components in the solution or pH (vide infra). Additionally, H2S electrodes have a finite lifetime, which in our experience has been ∼1 year, requiring fairly frequent replacement, which can be challenging for longer studies. We have also observed significant variance in electrode performance between electrodes, even from the same manufacturer, which also highlights the importance of frequent calibrations.
Additional Methods
In addition to the above methods described in detail, a number of other approaches have been commonly used to detect or monitor H2S in complex systems and can be particularly useful in different contexts. For example, lead acetate (Pb(OAc)2) test strips provide one of the simplest methods of H2S detection and are frequently used for H2S detection in water samples and in the headspace of bacterial cultures. In such systems, test strips impregnated with Pb(OAc)2 react with H2S, which results in the conversion of colorless Pb(OAc)2 to black lead sulfide (PbS). Benefits of this method include the broad availability of Pb(OAc)2 strips, the low cost of this method, and low infrastructure requirements for measurement.110,111 In some cases, the general range of H2S concentrations (typically in ppm in water) can be estimated based on the color level of the test strip after a specific amount of test time. Related methods have also been used to measure H2S generation in bacterial cultures.112 This approach has also been modified for H2S detection in air by using Pb(OAc)2 cassettes, which can be used to monitor H2S in different industrial environments. In general, this method has been primarily used in environmental testing and bacterial cultures, with few applications in more complex eukaryotic systems.
Sulfide levels can also be measured directly by UV spectrophotometry from the absorbance of HS– from 214–300 nm with a detection limit of ∼0.6 μM.113 This method typically requires spectral deconvolution to remove other absorbances from competing species with known absorbance profiles and subsequent fitting of the data to calculate H2S/HS– concentrations. This method may also have interference from other anions or oxidized sulfur species. In general, this method is best suited at basic pH ranges of 8–9 and for natural water samples rather than samples with high levels of organic materials.
Chromatography methods have also been used for H2S detection and quantification in complex matrices. In particular, gas chromatography (GC) with sulfur selective detectors has been useful for accurate quantification of low levels of H2S as well as other sulfur species. Such detectors include flame photometric detectors (FPD),114 pulsed-flame photometric detectors (PFPD),115 and sulfur chemiluminescent detectors (SCD),116 each of which provides greater sensitivity to sulfur containing molecules than more standard thermal conductivity detectors (TCD) or flame ionization detectors (FID). In general, these methods measure H2S levels in the headspace of a sample, which requires information about the pH-dependent speciation and partitioning of H2S between the liquid and gas phases of the sample. One benefit of GC measurement is that it also allows for simultaneous detection and potential quantification of other volatile sulfur-containing gases in the sample, which has been demonstrated previously in various sample types, including environmental, food, and enzyme-derived systems.117−120
Additionally, chromatographic H2S measurement has been used in tandem with electrophilic labeling approaches to detect and quantify H2S and low molecular weight polysulfides using mass spectrometry (MS). In these approaches, H2S and other sulfhydryl containing species are labeled with an electrophile prior to analysis by liquid or gas chromatographic separation followed by MS measurement. Some labeling agents include mBB,121 ethyl iodoacetate,122N-ethylmaleimide,123 and NBD ethers.124 This approach is also compatible with tandem MS/MS experiments and the use of stable isotope labeled internal standards, which further enhance the analytical value of this approach. In general, the use of chromatography in parallel with tandem MS/MS is attractive due to its low nM detection limits, application to various biological thiols, and use in biological media. In addition, chromatographic approaches for sulfide detection using flow gas dialysis coupled with electrochemical quantification by ion chromatography have been reported.125,126 This method reports good recovery of sample (95–99%) and has been used to measure post-mortem sulfide levels in rat and human brain tissue, although the required gas dialysis pretreatment step increases the complexity of this approach.
Analytical Complexity and Sensitivity
One theme hidden in many methods for H2S measurement is the trade-off between the analytical “cost” and the specificity of the method (Figure 7). For example, methods such as the MB assay have lower infrastructure requirements and can be easily performed in most laboratory environments using inexpensive reagents and common instrumentation. On the other hand, GC or HPLC methods are more sensitive with lower detection limits but require more complex and expensive technology. In general, one singular method is not “perfect”, and the choice of approach should depend not only on experimental availability, but also and more importantly on the specific application in question.
Figure 7.
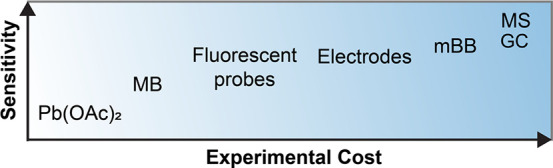
Overview of the trade-off between sensitivity and experimental complexity for common approaches for H2S measurement.
Reproducibility
The methods described above have been used in thousands of studies to detect, measure, and/or quantify H2S. Despite this breadth of usage, a clear growth opportunity for the field is interlab and interfield reproducibility, which requires a critical eye to the outcomes of measurements. A simple example of these challenges can be found in early literature from the biomedical H2S literature that claimed H2S levels of 20–50 μM H2S levels in blood or plasma.127 Although these data were supported by analytical measurements at the time, they were also highly illogical because the H2S detection limit of the human nose is ∼1 μM. More broadly, better stewardship of analytical procedures and experimental details is needed for the field to communicate and work together more cohesively. As analytical approaches change and evolve, knowing precisely how prior work was carried out is crucial for interpreting past results. Based on these needs, our goal here is to highlight a few relatively simple steps that researchers can use to better provide the context in which H2S measurements were made, allow for better interpretations of the results, and improve reproducibility throughout the field.
A simple first step is better standardization of analytical procedures, including how concentration units are reported. Based on the breadth and diversity of experimental requirements for H2S measurement in different systems, it is impossible to have a single uniform method that meets all needs. Because slight modifications to standard methods are common and often necessary in different systems, we strongly advocate that researchers should provide the experimental details of how H2S measurements were performed (including required sample dilution, quantified protein levels, storage times, etc.) rather than referring to methods in prior published work without providing additional details that are specific to current investigation. In addition, we strongly encourage researchers to include a representative H2S calibration curve in the Supporting Information when H2S quantification is reported within a manuscript.
Adding to the above general challenges related to data and experimental stewardship, significant challenges in H2S measurement are tied to its fundamental properties. H2S is volatile and readily oxidized and also participates in broad chemistry, often resulting in irreversible chemistry that consumes or functionalizes this reactive molecule. Based on these sensitivities, buffers and analysis stock solutions should be degassed to remove oxygen, and care should be taken to not introduce dissolved metal ions prior to incubation with H2S-containing samples to prevent unanticipated H2S oxidation. These challenges may not be fully appreciated or adequately accounted for by researchers who are first entering the field, especially based on the large disparity of H2S concentrations reported in the literature in various contexts. In the last section of this Perspective, our goal is to provide selected examples of how seemingly small changes in an analytical procedure can significantly impact outcomes. This is by no means a comprehensive or exhaustive list but rather is meant to illustrate how seemingly innocuous changes can significantly impact reproducibility.
In the previous sections, we highlighted the importance of measuring and providing H2S calibration curves for each of the analytical methods to help improve interfield reproducibility. With a similar goal in mind, we used an H2S-sensitive electrode as an illustrative example to highlight the importance of measuring the calibration curve under the exact experimental conditions being used for analysis. In our hands, H2S electrodes are often more sensitive to different solution components than other measurement approaches; therefore, the magnitude of these differences may scale differently across various analytical techniques. In the first example, we measured the H2S calibration curves at different pH values. Based on the detection chemistry of H2S responsive electrodes, namely that H2S is the species that crosses the electrode membrane, the pH of solution impacts the speciation of H2S versus HS–, meaning that at more acidic pH values more H2S is quantified (Figure 8a).57 Highlighting the impact of pH, even the smaller change from a pH of 7.0 to 7.4 results in a 2-fold change in the slope of the H2S calibration curve. These data highlight the need to not only know the pH of a solution being investigated, but also to accurately replicate this pH when obtaining the calibration data. Although large changes in pH between samples are generally unlikely, changes in other components are much more common. For example, enzymes, amino acids, thiols, or organic solvents may all be present at different levels depending on the experiment being performed. Importantly, the presence of such species also can have significant impacts on H2S-sensitive electrode calibration curve data. As an example of this influence, we demonstrate that even the presence of glutathione (GSH) at levels that would commonly be found in different biological samples significantly affects the slope of electrode calibration curves (Figure 8b).
Figure 8.
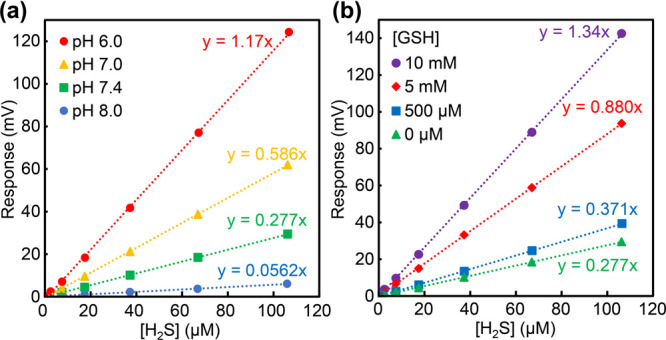
(a) H2S responsive electrode response to different H2S concentrations at pH values from 6.0 to 8.0. (b) H2S responsive electrode response to different H2S concentrations in the presence of different concentrations of GSH.
One common component of most analytical methods for H2S measurement requires either removing sample aliquots during time course experiments or diluting samples to match the concentration range of the analytical technique being used. Unlike many analytes that are targeted in chemical biology, H2S is a gas, which means that the partitioning between the liquid and gas phases is impacted by the amount of headspace in a sample vial. Therefore, changes in the sample headspace can also change H2S concentrations in solution. A practical example of this problem is observed when changes in measured H2S levels occur due to headspace alteration in the experiment. To demonstrate this challenge in the MB method, Figure 9 shows how increasing the headspace volume decreases the amount of H2S quantified in solution due to re-equilibration between the solution and the headspace. In this example, varying amounts of an identical H2S solution were added to three 20 mL vials so that each vial had 0 (A), 10 (B), or 15 (C) mL of headspace and equal H2S concentrations. After incubation for 15 min to allow for equilibration between the solution and the headspace, an aliquot of the solution was removed and analyzed using the MB assay. As shown in Figure 9, this change in headspace results in significant differences of measured H2S in each solution sample with less than half of the initial H2S concentration (A) measured in the vial with the largest headspace (C). Taken together, this simple experiment shows the importance of minimizing changes in headspace during H2S analyses.
Figure 9.
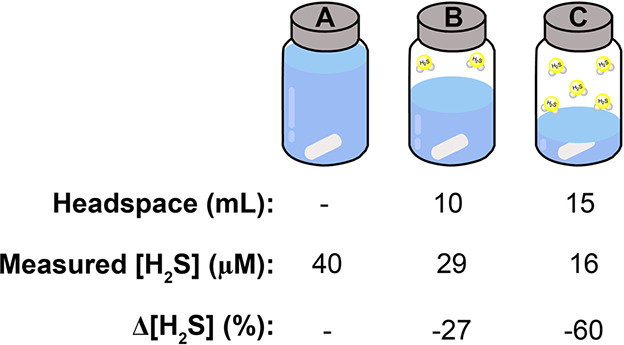
Increasing the sample headspace can impact measured H2S concentrations in solution based on partitioning of H2S between the solution and gas phases of the sample.
Outlook and Needed Advances
In many ways, the sheer number of published reports on H2S detection provides an erroneous view that H2S measurement in biological samples is a “solved problem,” and many opportunities exist to significantly advance the measurement science of this complex analyte. The past decade has seen a rapid evolution of tool and method development that has had major impacts in understanding the biological roles of H2S, including significantly refining the accepted endogenous levels of H2S and related RSS. The quest by many researchers to develop new tools for H2S detection has also advanced the fundamental chemistry of H2S reactivity, which in some cases has translated directly to an increased understanding of the biological reaction chemistry of H2S and related species. Despite these advances, there are still major hurdles that need to be overcome to bridge the gap between innovation and impact and enable further use of these important measurement approaches to address biological problems.
One significant gap in the available toolbox for H2S measurement is associated with the properties of available activity-based probes for H2S detection. For example, reversible indicators that function in complex environments, such as live cells or tissues, remain a key and unmet need. Although the available activity-based chemodosimeter approaches provides valuable tools for in cellulo and in vivo applications, this impact would be exponentially expanded from the development of reversible platforms for detection. Such tools would allow for new insights into the real-time dynamics of H2S generation and consumption akin to what can be investigated using available reversible probes for analytes like Ca2+ or Zn2+. Fast-responding, ratiometric, activity-based probes may help to bridge part of this gap but are still limited by reversibility challenges. In addition, analyte-replacement approaches move toward addressing challenges with analyte consumption, but have limitations for quantification due to the need to be able to stop the replacement or amplification step.
Similarly, having real-time tools that allow for visualization and measurement of changes in RSS speciation would significantly advance our understanding of RSS translocation and trafficking. The development of such tools relies directly on our ability to design platforms that couple reversible H2S/HS– binding to an optical response in the presence of a broad milieu of competing analytes. This challenge is a major one and will require a better understanding of the fundamental molecular recognition that governs reversible H2S/HS– interactions on both organic and inorganic platforms.
More broadly, there is also a significant need to standardize analytical methods that allow for a better comparison of H2S levels across different studies and disciplines. This need will in part depend on coalescence of different subfields around specific measurement approaches and also better stewardship of experimental procedures and data used for different types of measurements. It is unlikely that one specific method will even be appropriate for all sample types, but small advances in measurement approaches that contribute positively to reproducibility are poised to make a significant impact. One example of such an advancement would be new materials that allow for better H2S separation and detection, which could enable higher biological compatibility with electrode measurement approaches. Similarly, labeling reagents that require less careful handling but maintain high H2S detection fidelity across biologically relevant concentration ranges would significantly simplify measurement and reproducibility. More broadly, the advent and refinement of “low tech” H2S measurement approaches that allow for rapid quantification of endogenous H2S levels in biological specimens could revolutionize how H2S is measured in biomedical environments. Furthermore, such approaches would be valuable in bridging the gap between currently available methods and effective point-of-care measurements needed to incorporate H2S or RSS analysis into a more standard panel of biomarkers that can be easily monitored from various biological samples.
Approaches for H2S measurement have seen a resurgence in the past decade due to the increased appreciation for the central and complex roles of H2S in eukaryotic biology. In many ways, this field is rapidly maturing and will benefit from a renewed focus on detection approaches that solve specific unmet challenges with the goal of maximizing impact. As this transition occurs, there are bound to be growing pains and unanticipated wrong turns due to the inherent complexity of the systems being investigated, but we are confident that the fundamental advances from the past decade provide a strong foundation for future growth and innovation. The complexity of these challenges will require future advances to incorporate multidisciplinary approaches that not only focus on the fundamental detection chemistry, but also on the method compatibility with specific environments, matrices, and experimental requirements.
Experimental Details
General Procedure for H2S Calibration Curves
A 10 mM NaSH stock solution was prepared under N2 in deoxygenated PBS buffer (140 mM NaCl, 3 mM KCl, 10 mM phosphate) with a pH of 6.0–8.0 depending on the calibration curve, which was then further diluted to 1.0 mM. A Unisense SULF-500 electrode tip was placed through a split-top septum into 20 mL of degassed PBS buffer (pH 6.0 to 8.0), and the signal was allowed to stabilize. In calibration experiments with GSH, stock solutions of GSH were made in PBS pH 7.4 buffer and added to 20 mL of PBS buffer (pH 7.4), forming final concentrations of 500 μM to 10 mM, and the signal was allowed to stabilize. Finally, additions of 1.0 mM NaSH were added to the vial (5, 10, 20, 40, 60, and 80 μL), allowing for signal stabilization between each addition.
Author Contributions
The manuscript was written through contributions of all authors. CRediT: Haley M. Smith visualization, writing-original draft, writing-review & editing; Michael D Pluth funding acquisition, visualization, writing-original draft, writing-review & editing.
This work was supported by the NIH (R01GM113030 to MDP) and an NSF training grant (DGE- 2022168 to HMS).
The authors declare no competing financial interest.
References
- Marriott R. A.; Pirzadeh P.; Marrugo-Hernandez J. J.; Raval S. Hydrogen sulfide formation in oil and gas. Can. J. Chem. 2016, 94 (4), 406–413. 10.1139/cjc-2015-0425. [DOI] [Google Scholar]
- Mousa H. A.-L. Short-term effects of subchronic low-level hydrogen sulfide exposure on oil field workers. Environ. Health Prev. Med. 2015, 20 (1), 12–17. 10.1007/s12199-014-0415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece G. M. W. B.; Drinker P. Determination and Recording of Carbon Disulfide and Hydrogen Sulfide in the Viscose-Rayon Industry. J. Indust. Hygiene Toxicol. 1940, 22 (9), 416–424. [Google Scholar]
- Kolluru G. K.; Shen X. G.; Bir S. C.; Kevil C. G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide Biol. Chem. 2013, 35, 5–20. 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S. K.; Kim K.-H.; Tang K. A review of sensor-based methods for monitoring hydrogen sulfide. Trends. Analyt. Chem. 2012, 32, 87–99. 10.1016/j.trac.2011.08.008. [DOI] [Google Scholar]
- Hughes M. N.; Centelles M. N.; Moore K. P. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: A review. Free Radic. Biol. Med. 2009, 47 (10), 1346–1353. 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Torres W.; Pansare S. S.; Goodwin J. G. Hot Gas Removal of Tars, Ammonia, and Hydrogen Sulfide from Biomass Gasification Gas. Catal. Rev. 2007, 49 (4), 407–456. 10.1080/01614940701375134. [DOI] [Google Scholar]
- Malone Rubright S. L.; Pearce L. L.; Peterson J. Environmental toxicology of hydrogen sulfide. Nitric Oxide 2017, 71, 1–13. 10.1016/j.niox.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer C.; Francis P.; Burton M.; Maciejewski A. J. H.; Boardman L. Remote measurement of volcanic gases by Fourier transform infrared spectroscopy. Appl. Phys. B: Laser Opt. 1998, 67 (4), 505–515. 10.1007/s003400050536. [DOI] [Google Scholar]
- Frigaard N. U.; Dahl C.. Sulfur Metabolism in Phototrophic Sulfur Bacteria. In Advances in Microbial Physiology, Vol 54; Poole R. K., Ed.; 2009; Vol. 54, pp 103–200. [DOI] [PubMed] [Google Scholar]
- Jorgensen B. B.; Findlay A. J.; Pellerin A. The Biogeochemical Sulfur Cycle of Marine Sediments. Front. Microbiol. 2019, 10, 849. 10.3389/fmicb.2019.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmann J.; van Gemerden H. Microbial interactions involving sulfur bacteria: implications for the ecology and evolution of bacterial communities. FEMS Microbiol. Rev. 2000, 24 (5), 591–599. 10.1111/j.1574-6976.2000.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Wang R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration that Blossomed. Physiol. Rev. 2012, 92 (2), 791–896. 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- Wallace J. L.; Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discovery 2015, 14 (5), 329–345. 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- Thomas E. L.; Reineccius G. A.; DeWaard G. J.; Slinkard M. S. Determination of Hydrogen Sulfide in Heated Milks by Gas-Liquid Chromatographic Headspace Analysis1. J. Dairy Sci. 1976, 59 (11), 1865–1869. 10.3168/jds.S0022-0302(76)84454-2. [DOI] [Google Scholar]
- Ferreira V.; Franco-Luesma E.; Vela E.; López R.; Hernández-Orte P. Elusive Chemistry of Hydrogen Sulfide and Mercaptans in Wine. J. Agric. Food Chem. 2018, 66 (10), 2237–2246. 10.1021/acs.jafc.7b02427. [DOI] [PubMed] [Google Scholar]
- Li H.; Jia S. R.; Zhang W. J. Rapid determination of low-level sulfur compounds in beer by headspace gas chromatography with a pulsed flame photometric detector. J. Am. Soc. Brew. Chem. 2008, 66 (3), 188–191. 10.1094/ASBCJ-2008-0629-01. [DOI] [Google Scholar]
- Kristoffersen T. Interrelationships of Flavor and Chemical Changes in Cheese. J. Dairy Sci. 1967, 50 (3), 279–284. 10.3168/jds.S0022-0302(67)87410-1. [DOI] [Google Scholar]
- Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter?. FASEB J. 2002, 16 (13), 1792–1798. 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- Kimura H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int. 2013, 63 (5), 492–497. 10.1016/j.neuint.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Bhatia M. Hydrogen Sulfide as a Vasodilator. IUBMB Life 2005, 57, 603–606. 10.1080/15216540500217875. [DOI] [PubMed] [Google Scholar]
- Cheng Y. Q.; Ndisang J. F.; Tang G. H.; Cao K.; Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am. J. Physiol. Heart Circ. Physiol. 2004, 287 (5), H2316–H2323. 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- Sun H. J.; Wu Z. Y.; Nie X. W.; Bian J. S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2020, 10, 1568. 10.3389/fphar.2019.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H.; Shibuya N.; Kimura Y. Hydrogen Sulfide Is a Signaling Molecule and a Cytoprotectant. Antioxid. Redox Signal. 2012, 17 (1), 45–57. 10.1089/ars.2011.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Z.; Bian J. S. Hydrogen Sulfide: A Neuromodulator and Neuroprotectant in the Central Nervous System. ACS Chem. Neurosci. 2014, 5 (10), 876–883. 10.1021/cn500185g. [DOI] [PubMed] [Google Scholar]
- Coletta C.; Papapetropoulos A.; Erdelyi K.; Olah G.; Modis K.; Panopoulos P.; Asimakopoulou A.; Gero D.; Sharina I.; Martin E.; Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. U.S.A. 2012, 109 (23), 9161–9166. 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetropoulos A.; Pyriochou A.; Altaany Z.; Yang G. D.; Marazioti A.; Zhou Z. M.; Jeschke M. G.; Branski L. K.; Herndon D. N.; Wang R.; Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 2009, 106 (51), 21972–21977. 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.; Zhang G.; Wondimu T.; Ross B.; Wang R. Hydrogen sulfide and asthma. Exp. Physiol. 2011, 96 (9), 847–852. 10.1113/expphysiol.2011.057448. [DOI] [PubMed] [Google Scholar]
- Giovinazzo D.; Bursac B.; Sbodio J. I.; Nalluru S.; Vignane T.; Snowman A. M.; Albacarys L. M.; Sedlak T. W.; Torregrossa R.; Whiteman M.; Filipovic M. R.; Snyder S. H.; Paul B. D. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. U.S.A. 2021, 118 (4), e2017225118 10.1073/pnas.2017225118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Yang W.; Jia X.; Yang G.; Duridanova D.; Cao K.; Wang R. Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab Invest. 2009, 89 (1), 59–67. 10.1038/labinvest.2008.109. [DOI] [PubMed] [Google Scholar]
- Szabo C. Gasotransmitters in cancer: from pathophysiology to experimental therapy. Nat. Rev. Drug Discovery 2016, 15 (3), 185–203. 10.1038/nrd.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C.; Coletta C.; Chao C.; Modis K.; Szczesny B.; Papapetropoulos A.; Hellmich M. R. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. U.S.A. 2013, 110 (30), 12474–12479. 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G. D.; Wu L. Y.; Jiang B.; Yang W.; Qi J. S.; Cao K.; Meng Q. H.; Mustafa A. K.; Mu W. T.; Zhang S. M.; Snyder S. H.; Wang R. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322 (5901), 587–590. 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara N.; Nagano M.; Ito T.; Shimamura K.; Akimoto T.; Suzuki H. Antioxidant enzyme, 3-mercaptopyruvate sulfurtransferase-knockout mice exhibit increased anxiety-like behaviors: a model for human mercaptolactate-cysteine disulfiduria. Sci. Rep. 2013, 3, 1568. 10.1038/srep01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M.; Osada J.; Aratani Y.; Kluckman K.; Reddick R.; Malinow M. R.; Maeda N. Mice Deficient in Cystathionine Beta-Synthase - Animal-Models for Mild and Severe Homocyst(e)inemia. Proc. Natl. Acad. Sci. U.S.A. 1995, 92 (5), 1585–1589. 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C.; Papapetropoulos A. International Union of Basic and Clinical Pharmacology. CII: Pharmacological Modulation of H2S Levels: H2S Donors and H2S Biosynthesis Inhibitors. Pharmacol. Rev. 2017, 69 (4), 497–564. 10.1124/pr.117.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C. R.; Dillon K. M.; Matson J. B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. 10.1016/j.bcp.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinn C. M.; Cerda M. M.; Pluth M. D. Activatable Small-Molecule Hydrogen Sulfide Donors. Antioxid. Redox Signal. 2020, 32 (2), 96–109. 10.1089/ars.2019.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P. M.; Batka D.; Hefter G.; Königsberger E.; Rowland D. Goodbye to S2– in aqueous solution. Chem. Commun. 2018, 54 (16), 1980–1983. 10.1039/C8CC00187A. [DOI] [PubMed] [Google Scholar]
- Lau N.; Pluth M. D. Reactive sulfur species (RSS): persulfides, polysulfides, potential, and problems. Curr. Opin. Chem. Biol. 2019, 49, 1–8. 10.1016/j.cbpa.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Bailey T. S.; Henthorn H. A.; Pluth M. D. The Intersection of NO and H2S: Persulfides Generate NO from Nitrite through Polysulfide Formation. Inorg. Chem. 2016, 55 (24), 12618–12625. 10.1021/acs.inorgchem.6b01660. [DOI] [PubMed] [Google Scholar]
- Bailey T. S.; Pluth M. D. Reactions of isolated persulfides provide insights into the interplay between H2S and persulfide reactivity. Free Radic. Biol. Med. 2015, 89, 662–667. 10.1016/j.freeradbiomed.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovic M. R.; Zivanovic J.; Alvarez B.; Banerjee R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118 (3), 1253. 10.1021/acs.chemrev.7b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F.; Gao Y.; Wang J.; Sun S. Reversible and selective luminescent determination of ClO–/H2S redox cycle in vitro and in vivo based on a ruthenium trisbipyridyl probe. Analyst 2014, 139 (13), 3324–3329. 10.1039/C4AN00331D. [DOI] [PubMed] [Google Scholar]
- Petrovic D.; Kouroussis E.; Vignane T.; Filipovic M. R. The Role of Protein Persulfidation in Brain Aging and Neurodegeneration. Front. Aging Neurosci. 2021, 13, 674135. 10.3389/fnagi.2021.674135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K.; Akaike T.; Sawa T.; Kumagai Y.; Wink D. A.; Tantillo D. J.; Hobbs A. J.; Nagy P.; Xian M.; Lin J.; Fukuto J. M. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: Implications of their possible biological activity and utility. Free Radic. Biol. Med. 2014, 77, 82–94. 10.1016/j.freeradbiomed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson K. R. H2S and polysulfide metabolism: Conventional and unconventional pathways. Biochem. Pharmacol. 2018, 149, 77–90. 10.1016/j.bcp.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Benchoam D.; Cuevasanta E.; Moller M. N.; Alvarez B.. Persulfides, at the crossroads between hydrogen sulfide and thiols. In Protein Oxidation; Hooper N., Ed.; 2020; Vol. 64, pp 155–168. [DOI] [PubMed] [Google Scholar]
- Kolluru G. K.; Shen X. G.; Kevil C. G. Reactive Sulfur Species A New Redox Player in Cardiovascular Pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2020, 40 (4), 874–884. 10.1161/ATVBAHA.120.314084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl S.; Xian M. Recent development of polysulfides: Chemistry and biological applications. Curr. Opin. Chem. Biol. 2023, 75, 102325. 10.1016/j.cbpa.2023.102325. [DOI] [PubMed] [Google Scholar]
- Liu C.; Pan J.; Li S.; Zhao Y.; Wu L. Y.; Berkman C. E.; Whorton A. R.; Xian M. Capture and Visualization of Hydrogen Sulfide by a Fluorescent Probe. Angew. Chem., Int. Ed. 2011, 50 (44), 10327–10329. 10.1002/anie.201104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y.; Karpus J.; Kabil O.; Zhang S. Y.; Zhu H. L.; Banerjee R.; Zhao J.; He C. Selective fluorescent probes for live-cell monitoring of sulphide. Nat. Commun. 2011, 2, 495. 10.1038/ncomms1506. [DOI] [PubMed] [Google Scholar]
- Lippert A. R.; New E. J.; Chang C. J. Reaction-Based Fluorescent Probes for Selective Imaging of Hydrogen Sulfide in Living Cells. J. Am. Chem. Soc. 2011, 133 (26), 10078–10080. 10.1021/ja203661j. [DOI] [PubMed] [Google Scholar]
- Peng H. J.; Cheng Y. F.; Dai C. F.; King A. L.; Predmore B. L.; Lefer D. J.; Wang B. H. A Fluorescent Probe for Fast and Quantitative Detection of Hydrogen Sulfide in Blood. Angew. Chem., Int. Ed. 2011, 50 (41), 9672–9675. 10.1002/anie.201104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakura K.; Hanaoka K.; Shibuya N.; Mikami Y.; Kimura Y.; Komatsu T.; Ueno T.; Terai T.; Kimura H.; Nagano T. Development of a Highly Selective Fluorescence Probe for Hydrogen Sulfide. J. Am. Chem. Soc. 2011, 133 (45), 18003–18005. 10.1021/ja207851s. [DOI] [PubMed] [Google Scholar]
- Galardon E.; Tomas A.; Roussel P.; Artaud I. New fluorescent zinc complexes: towards specific sensors for hydrogen sulfide in solution. Dalton Trans. 2009, 42, 9126–9130. 10.1039/b907115f. [DOI] [PubMed] [Google Scholar]
- Jeroschewski P.; Steuckart C.; Kühl M. An Amperometric Microsensor for the Determination of H2S in Aquatic Environments. Anal. Chem. 1996, 68 (24), 4351–4357. 10.1021/ac960091b. [DOI] [Google Scholar]
- Steiger A. K.; Pardue S.; Kevil C. G.; Pluth M. D. Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes. J. Am. Chem. Soc. 2016, 138 (23), 7256–7259. 10.1021/jacs.6b03780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac M.; Melet A.; Galardon E. Reversible Detection and Quantification of Hydrogen Sulfide by Fluorescence Using the Hemoglobin I from Lucina pectinata. ACS Sensors 2018, 3 (10), 2138–2144. 10.1021/acssensors.8b00701. [DOI] [PubMed] [Google Scholar]
- Hou F.; Huang L.; Xi P.; Cheng J.; Zhao X.; Xie G.; Shi Y.; Cheng F.; Yao X.; Bai D.; Zeng Z. A Retrievable and Highly Selective Fluorescent Probe for Monitoring Sulfide and Imaging in Living Cells. Inorg. Chem. 2012, 51 (4), 2454–2460. 10.1021/ic2024082. [DOI] [PubMed] [Google Scholar]
- Chen Y. C.; Zhu C. C.; Yang Z. H.; Chen J. J.; He Y. F.; Jiao Y.; He W. J.; Qiu L.; Cen J. J.; Guo Z. J. A Ratiometric Fluorescent Probe for Rapid Detection of Hydrogen Sulfide in Mitochondria. Angew. Chem., Int. Ed. 2013, 52 (6), 1688–1691. 10.1002/anie.201207701. [DOI] [PubMed] [Google Scholar]
- Lin V. S.; Chen W.; Xian M.; Chang C. J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44 (14), 4596–4618. 10.1039/C4CS00298A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Fang Y.; Yan J.; Ren X.; Zheng C.; Wu B.; Wang S.; Li Z.; Hua H.; Wang P.; Li D. Small-molecule fluorescent probes for H2S detection: Advances and perspectives. Trends. Analyt. Chem. 2021, 134, 116117 10.1016/j.trac.2020.116117. [DOI] [Google Scholar]
- Lin V. S.; Chang C. J. Fluorescent probes for sensing and imaging biological hydrogen sulfide. Curr. Opin. Chem. Biol. 2012, 16 (5), 595–601. 10.1016/j.cbpa.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. B. A.; Li P.; Song P.; Wang B. S.; Zhao J. Z.; Han K. L. An ICT-based strategy to a colorimetric and ratiometric fluorescence probe for hydrogen sulfide in living cells. Chem. Commun. 2012, 48 (23), 2852–2854. 10.1039/c2cc17658k. [DOI] [PubMed] [Google Scholar]
- Montoya L. A.; Pluth M. D. Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem. Commun. 2012, 48 (39), 4767–4769. 10.1039/c2cc30730h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T.; Qiang T.; Ren L.; Cheng F.; Wang B.; Li M.; Hu W.; James T. D. Near-infrared fluorescent probe for hydrogen sulfide: high-fidelity ferroptosis evaluation in vivo during stroke. Chem. Sci. 2022, 13 (10), 2992–3001. 10.1039/D1SC05930K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose D. A.; Sakla R.; Sharma N.; Gadiyaram S.; Kaushik R.; Ghosh A. Sensing and Bioimaging of the Gaseous Signaling Molecule Hydrogen Sulfide by Near-Infrared Fluorescent Probes. Acs Sensors 2020, 5 (11), 3365–3391. 10.1021/acssensors.0c02005. [DOI] [PubMed] [Google Scholar]
- Sun W.; Fan J. L.; Hu C.; Cao J. F.; Zhang H.; Xiong X. Q.; Wang J. Y.; Cui S.; Sun S. G.; Peng X. J. A two-photon fluorescent probe with near-infrared emission for hydrogen sulfide imaging in biosystems. Chem. Commun. 2013, 49 (37), 3890–3892. 10.1039/c3cc41244j. [DOI] [PubMed] [Google Scholar]
- Yang S.; Qi Y.; Liu C. H.; Wang Y. J.; Zhao Y. R.; Wang L. L.; Li J. S.; Tan W. H.; Yang R. H. Design of a Simultaneous Target and Location-Activatable Fluorescent Probe for Visualizing Hydrogen Sulfide in Lysosomes. Anal. Chem. 2014, 86 (15), 7508–7515. 10.1021/ac501263d. [DOI] [PubMed] [Google Scholar]
- Chen J. W.; Zhao M. K.; Jiang X. Q.; Sizovs A.; Wang M. C.; Provost C. R.; Huang J.; Wang J. Genetically anchored fluorescent probes for subcellular specific imaging of hydrogen sulfide. Analyst 2016, 141 (4), 1209–1213. 10.1039/C5AN02497H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya L. A.; Pluth M. D. Organelle-Targeted H2S Probes Enable Visualization of the Subcellular Distribution of H2S Donors. Anal. Chem. 2016, 88 (11), 5769–5774. 10.1021/acs.analchem.6b00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. S.; Liang D. W.; Tang X. J. Visualizing Hydrogen Sulfide in Mitochondria and Lysosome of Living Cells and in Tumors of Living Mice with Positively Charged Fluorescent Chemosensors. Anal. Chem. 2016, 88 (18), 9213–9218. 10.1021/acs.analchem.6b02459. [DOI] [PubMed] [Google Scholar]
- Ren M. G.; Li Z. H.; Deng B. B.; Wang L.; Lin W. Y. Single Fluorescent Probe Separately and Continuously Visualize H2S and HClO in Lysosomes with Different Fluorescence Signals. Anal. Chem. 2019, 91 (4), 2932–2938. 10.1021/acs.analchem.8b05116. [DOI] [PubMed] [Google Scholar]
- Hammers M. D.; Taormina M. J.; Cerda M. M.; Montoya L. A.; Seidenkranz D. T.; Parthasarathy R.; Pluth M. D. A Bright Fluorescent Probe for H2S Enables Analyte-Responsive, 3D Imaging in Live Zebrafish Using Light Sheet Fluorescence Microscopy. J. Am. Chem. Soc. 2015, 137 (32), 10216–10223. 10.1021/jacs.5b04196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor L. J.; Mistry I. N.; Collins S. L.; Folkes L. K.; Brown G.; Conway S. J.; Hammond E. M. CYP450 Enzymes Effect Oxygen-Dependent Reduction of Azide-Based Fluorogenic Dyes. ACS Cent. Sci. 2017, 3 (1), 20–30. 10.1021/acscentsci.6b00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn H. A.; Pluth M. D. Mechanistic Insights into the H2S-Mediated Reduction of Aryl Azides Commonly Used in H2S Detection. J. Am. Chem. Soc. 2015, 137 (48), 15330–15336. 10.1021/jacs.5b10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.; Peng B.; Li S.; Park C.-M.; Whorton A. R.; Xian M. Reaction Based Fluorescent Probes for Hydrogen Sulfide. Org. Lett. 2012, 14 (8), 2184–2187. 10.1021/ol3008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B.; Chen W.; Liu C. R.; Rosser E. W.; Pacheco A.; Zhao Y.; Aguilar H. C.; Xian M. Fluorescent Probes Based on Nucleophilic Substitution-Cyclization for Hydrogen Sulfide Detection and Bioimaging. Chem.—Eur. J. 2014, 20 (4), 1010–1016. 10.1002/chem.201303757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. W.; Lin W. Y.; Zheng K. B.; He L. W. A near-infrared fluorescent turn-on probe for fluorescence imaging of hydrogen sulfide in living cells based on thiolysis of dinitrophenyl ether. Chem. Commun. 2012, 48 (85), 10529–10531. 10.1039/c2cc34031c. [DOI] [PubMed] [Google Scholar]
- Liu T. Y.; Xu Z. C.; Spring D. R.; Cui J. N. A Lysosome-Targetable Fluorescent Probe for Imaging Hydrogen Sulfide in Living Cells. Org. Lett. 2013, 15 (9), 2310–2313. 10.1021/ol400973v. [DOI] [PubMed] [Google Scholar]
- Fu L.; Tian F. F.; Lai L.; Liu Y.; Harvey P. D.; Jiang F. L. A ratiometric ″two-in-one″ fluorescent chemodosimeter for fluoride and hydrogen sulfide. Sens. Actuators B: Chem. 2014, 193, 701–707. 10.1016/j.snb.2013.12.038. [DOI] [Google Scholar]
- Montoya L. A.; Pearce T. F.; Hansen R. J.; Zakharov L. N.; Pluth M. D. Development of Selective Colorimetric Probes for Hydrogen Sulfide Based on Nucleophilic Aromatic Substitution. J. Org. Chem. 2013, 78 (13), 6550–6557. 10.1021/jo4008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C.; Zhu Q.; Liu W. W.; Chen W. B.; Xi Z.; Yi L. NBD-based colorimetric and fluorescent turn-on probes for hydrogen sulfide. Org. Biomol. Chem. 2014, 12 (3), 479–485. 10.1039/C3OB41870G. [DOI] [PubMed] [Google Scholar]
- Song F.; Li Z.; Li J.; Wu S.; Qiu X.; Xi Z.; Yi L. Investigation of thiolysis of NBD amines for the development of H2S probes and evaluating the stability of NBD dyes. Org. Biomol. Chem. 2016, 14 (47), 11117–11124. 10.1039/C6OB02354A. [DOI] [PubMed] [Google Scholar]
- Jiang C.; Huang H.; Kang X.; Yang L.; Xi Z.; Sun H.; Pluth M. D.; Yi L. NBD-based synthetic probes for sensing small molecules and proteins: design, sensing mechanisms and biological applications. Chem. Soc. Rev. 2021, 50 (13), 7436–7495. 10.1039/D0CS01096K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Zheng X. E.; Zou F.; Shang Y. G.; Meng W. Q.; Lai E.; Xu Z. C.; Liu Y.; Zhao J. A highly selective and sensitive near-infrared fluorescent probe for imaging of hydrogen sulphide in living cells and mice. Sci. Rep. 2016, 6, 674135. 10.1038/srep18868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S. Y.; Zhou E. B.; Hong J. X.; Feng G. Q. Nitrobenzoxadiazole Ether-Based Near-Infrared Fluorescent Probe with Unexpected High Selectivity for H2S Imaging in Living Cells and Mice. Anal. Chem. 2019, 91 (20), 13136–13142. 10.1021/acs.analchem.9b03383. [DOI] [PubMed] [Google Scholar]
- Pak Y. L.; Li J.; Ko K. C.; Kim G.; Lee J. Y.; Yoon J. Mitochondria-Targeted Reaction-Based Fluorescent Probe for Hydrogen Sulfide. Anal. Chem. 2016, 88 (10), 5476–5481. 10.1021/acs.analchem.6b00956. [DOI] [PubMed] [Google Scholar]
- Cai X. K.; Zhang Z. C.; Dong Y. L.; Hao T. T.; Yi L.; Yang X. A biotin-guided near-infrared fluorescent probe for imaging hydrogen sulfide and differentiating cancer cells. Org. Biomol. Chem. 2023, 21 (2), 332–338. 10.1039/D2OB02034C. [DOI] [PubMed] [Google Scholar]
- Wu H. X.; Krishnakumar S.; Yu J.; Liang D.; Qi H. Y.; Lee Z. W.; Deng L. W.; Huang D. J. Highly Selective and Sensitive Near-Infrared-Fluorescent Probes for the Detection of Cellular Hydrogen Sulfide and the Imaging of H2S in Mice. Chem.—Asian J. 2014, 9 (12), 3604–3611. 10.1002/asia.201402860. [DOI] [PubMed] [Google Scholar]
- Yang X. J.; Shen L. Q.; Bao H. B.; Fang X. X.; Xu J. W.; Zhao Y. X.; Yang W. A tricarbocyanine near-infrared fluorescent probe for sulfide through a copper displacement mechanism. Sens. Actuators B: Chem. 2015, 220, 1361–1367. 10.1016/j.snb.2015.07.057. [DOI] [Google Scholar]
- Wang P.; Wu J.; Di C. X.; Zhou R.; Zhang H.; Su P. R.; Xu C.; Zhou P. P.; Ge Y. S.; Liu D. A.; Liu W. S.; Tang Y. A novel peptide-based fluorescence chemosensor for selective imaging of hydrogen sulfide both in living cells and zebrafish. Biosens. Bioelectron. 2017, 92, 602–609. 10.1016/j.bios.2016.10.050. [DOI] [PubMed] [Google Scholar]
- Tang Z. X.; Song B.; Ma H.; Shi Y. Y.; Yuan J. L. A ratiometric time-gated luminescence probe for hydrogen sulfide based on copper(II)-coupled lanthanide complexes. Anal. Chim. Acta 2019, 1049, 152–160. 10.1016/j.aca.2018.10.048. [DOI] [PubMed] [Google Scholar]
- Strianese M.; Pellecchia C. Metal complexes as fluorescent probes for sensing biologically relevant gas molecules. Coord. Chem. Rev. 2016, 318, 16–28. 10.1016/j.ccr.2016.04.006. [DOI] [Google Scholar]
- Strianese M.; Guarnieri D.; Lamberti M.; Landi A.; Peluso A.; Pellecchia C. Fluorescent salen-type Zn(II) Complexes As Probes for Detecting Hydrogen Sulfide and Its Anion: Bioimaging Applications. Inorg. Chem. 2020, 59 (21), 15977–15986. 10.1021/acs.inorgchem.0c02499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T.; Hirao K. Density functional study of the binding of the cyclen-coordinated M(II) (M = Zn, Cu, Ni) complexes to the DNA base. Why is Zn better to bind?. J. Mol. Struct. Theochem 2002, 581 (1), 203–213. 10.1016/S0166-1280(01)00759-X. [DOI] [Google Scholar]
- Anichini A.; Fabbrizzi L.; Paoletti P.; Clay R. M. Ring size effect on the enthalpies of formation of copper(II) and zinc(II) complexes with 1,4,7,10-tetra-aza-cyclododecane and 1,5,9,12-tetra-aza-cyclo-pentadodecane. Inorg. Chim. Acta 1977, 22, L25–L27. 10.1016/S0020-1693(00)90880-6. [DOI] [Google Scholar]
- Fogo J. K.; Popowsky M. Spectrophotometric Determination of Hydrogen Sulfide. Anal. Chem. 1949, 21 (6), 732–734. 10.1021/ac60030a028. [DOI] [Google Scholar]
- Shen X.; Pattillo C. B.; Pardue S.; Bir S. C.; Wang R.; Kevil C. G. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic. Biol. Med. 2011, 50 (9), 1021–1031. 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X.; Peter E. A.; Bir S.; Wang R.; Kevil C. G. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic. Biol. Med. 2012, 52 (11), 2276–2283. 10.1016/j.freeradbiomed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenens J.; Schoonheydt R. A. Visible Spectroscopy of Methylene Blue on Hectorite, Laponite B, and Barasym in Aqueous Suspension. Clays Clay Miner. 1988, 36 (3), 214–224. 10.1346/CCMN.1988.0360302. [DOI] [Google Scholar]
- Khan I.; Saeed K.; Zekker I.; Zhang B. L.; Hendi A. H.; Ahmad A.; Ahmad S.; Zada N.; Ahmad H.; Shah L. A.; Shah T. R.; Khan I. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14 (2), 242. 10.3390/w14020242. [DOI] [Google Scholar]
- Wintner E. A.; Deckwerth T. L.; Langston W.; Bengtsson A.; Leviten D.; Hill P.; Insko M. A.; Dumpit R.; VandenEkart E.; Toombs C. F.; Szabo C. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br. J. Pharmacol. 2010, 160 (4), 941–957. 10.1111/j.1476-5381.2010.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditroi T.; Nagy A.; Martinelli D.; Rosta A.; Kozich V.; Nagy P. Comprehensive analysis of how experimental parameters affect H2S measurements by the monobromobimane method. Free Radic. Biol. Med. 2019, 136, 146–158. 10.1016/j.freeradbiomed.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Shen X. G.; Carlstrom M.; Borniquel S.; Jadert C.; Kevil C. G.; Lundberg J. O. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic. Biol. Med. 2013, 60, 195–200. 10.1016/j.freeradbiomed.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpal S.; Katikaneni P.; Deshotels M.; Pardue S.; Glawe J.; Shen X. G.; Akkus N.; Modi K.; Bhandari R.; Dominic P.; Reddy P.; Kolluru G. K.; Kevil C. G. Total sulfane sulfur bioavailability reflects ethnic and gender disparities in cardiovascular disease. Redox Biol. 2018, 15, 480–489. 10.1016/j.redox.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna A. F.; Di Nunzio A.; Amoresano A.; Pane F.; Fontanarosa C.; Pucci P.; Vigorito C.; Cirillo G.; Zacchia M.; Trepiccione F.; Ingrosso D. Divergent behavior of hydrogen sulfide pools and of the sulfur metabolite lanthionine, a novel uremic toxin, in dialysis patients. Biochimie 2016, 126, 97–107. 10.1016/j.biochi.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Shen X.; Kolluru G. K.; Yuan S.; Kevil C. G.. Chapter Two - Measurement of H2S In Vivo and In Vitro by the Monobromobimane Method. In Methods in Enzymology; Cadenas E., Packer L., Eds.; Academic Press: 2015; Vol. 554, pp 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderholm A. L.; Findleton C. L.; Kumar G.; Hong Y.; Bisson L. F. Identification of Genes Affecting Hydrogen Sulfide Formation in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2008, 74 (5), 1418–1427. 10.1128/AEM.01758-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washio J.; Sato T.; Koseki T.; Takahashi N. Hydrogen sulfide-producing bacteria in tongue biofilm and their relationship with oral malodour. J. Med. Microbiol. 2005, 54 (9), 889–895. 10.1099/jmm.0.46118-0. [DOI] [PubMed] [Google Scholar]
- Shatalin K.; Shatalina E.; Mironov A.; Nudler E. H2S: A Universal Defense Against Antibiotics in Bacteria. Science 2011, 334 (6058), 986–990. 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- Guenther E. A.; Johnson K. S.; Coale K. H. Direct Ultraviolet Spectrophotometric Determination of Total Sulfide and Iodide in Natural Waters. Anal. Chem. 2001, 73 (14), 3481–3487. 10.1021/ac0013812. [DOI] [PubMed] [Google Scholar]
- Blanchette A. R.; Cooper A. D. Determination of hydrogen sulfide and methyl mercaptan in mouth air at the parts-per-billion level by gas chromatography. Anal. Chem. 1976, 48 (4), 729–731. 10.1021/ac60368a002. [DOI] [PubMed] [Google Scholar]
- Trabue S.; Scoggin K.; Mitloehner F.; Li H.; Burns R.; Xin H. Field sampling method for quantifying volatile sulfur compounds from animal feeding operations. Atmos. Environ. 2008, 42 (14), 3332–3341. 10.1016/j.atmosenv.2007.03.016. [DOI] [Google Scholar]
- Vitvitsky V.; Kabil O.; Banerjee R. High Turnover Rates for Hydrogen Sulfide Allow for Rapid Regulation of Its Tissue Concentrations. Antioxid. Redox Signal. 2012, 17 (1), 22–31. 10.1089/ars.2011.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitvitsky V.; Banerjee R.. H2S Analysis in Biological Samples Using Gas Chromatography with Sulfur Chemiluminescence Detection. In Hydrogen Sulfide in Redox Biology, Pt A; Cadenas E., Packer L., Eds.; 2015; Vol. 554, pp 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H. Performance characterization of the GC/PFPD for H2S, CH3SH, DMS, and DMDS in air. Atmos. Environ. 2005, 39 (12), 2235–2242. 10.1016/j.atmosenv.2004.12.039. [DOI] [Google Scholar]
- Lu X.; Fan C. X.; Shang J. G.; Deng J. C.; Yin H. B. Headspace solid-phase microextraction for the determination of volatile sulfur compounds in odorous hyper-eutrophic freshwater lakes using gas chromatography with flame photometric detection. Microchem. J. 2012, 104, 26–32. 10.1016/j.microc.2012.04.001. [DOI] [Google Scholar]
- Jo S. H.; Kim K. H.; Kim Y. H.; Lee M. H.; Ahn J. H.; Szulejko J. E.; Sohn J. R.; Ryu C. E. Y.; Kim A. Y. H. Study of odor from boiled eggs over time using gas chromatography. Microchem. J. 2013, 110, 517–529. 10.1016/j.microc.2013.05.011. [DOI] [Google Scholar]
- Tan B.; Jin S.; Sun J.; Gu Z.; Sun X.; Zhu Y.; Huo K.; Cao Z.; Yang P.; Xin X.; Liu X.; Pan L.; Qiu F.; Jiang J.; Jia Y.; Ye F.; Xie Y.; Zhu Y. Z. New method for quantification of gasotransmitter hydrogen sulfide in biological matrices by LC-MS/MS. Sci. Rep. 2017, 7 (1), 46278. 10.1038/srep46278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaeb H.; Choucair I.; Wang Z.; Li X. S.; Li L.; Boyd W. C.; Hine C.; Tang W. H. W.; Gogonea V.; Hazen S. L. Stable isotope dilution mass spectrometry quantification of hydrogen sulfide and thiols in biological matrices. Redox Biol. 2022, 55, 102401. 10.1016/j.redox.2022.102401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerot E.; Bagnoud A.; Cicchetti E. Quantification of Hydrogen Sulfide and Methanethiol and the Study of Their Scavenging by Biocides of the Isothiazolone Family. ChemPlusChem. 2014, 79 (1), 77–82. 10.1002/cplu.201300234. [DOI] [PubMed] [Google Scholar]
- Lan L.-A.; Wu S.-Y.; Meng X.-G.; Jiang J.-J.; Zheng M.-Y.; Fan G.-R. A simple liquid chromatography tandem mass spectrometric method for fast detection of hydrogen sulfide based on thiolysis of 7-nitro-2,1,3-benzoxadiazole ether. J. Chromat. A 2020, 1625, 461243. 10.1016/j.chroma.2020.461243. [DOI] [PubMed] [Google Scholar]
- Goodwin L. R.; Francom D.; Urso A.; Dieken F. P. Determination of trace sulfides in turbid waters by gas dialysis/ion chromatography. Anal. Chem. 1988, 60 (3), 216–219. 10.1021/ac00154a006. [DOI] [Google Scholar]
- Goodwin L. R.; Francom D.; Dieken F. P.; Taylor J. D.; Warenycia M. W.; Reiffenstein R. J.; Dowling G. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J. Anal Toxicol 1989, 13 (2), 105–9. 10.1093/jat/13.2.105. [DOI] [PubMed] [Google Scholar]
- Olson K. R. The therapeutic potential of hydrogen sulfide: separating hype from hope. Am. J. Physiol. Regul. 2011, 301 (2), R297–R312. 10.1152/ajpregu.00045.2011. [DOI] [PubMed] [Google Scholar]


