Abstract
Background
KRAS/BRAF mutations (mutKRAS/mutBRAF) are unfavorable prognostic factors for colorectal cancer (CRC) metastases to the liver and lungs. However, their effects on the prognosis for patients with synchronous peritoneal metastasis (S-PM) of CRC after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) are controversial. In the study, we aimed to determine the effects of mutKRAS/mutBRAF on the prognosis for patients with S-PM who received CRS.
Methods
A total of 142 patients diagnosed with S-PM between July 2007 and July 2019 were included in this study. The demographics, mutKRAS/mutBRAF status, overall survival (OS), and progression-free survival (PFS) of the patients were evaluated. The Kaplan–Meier method and log-rank test were used to estimate the difference in survival between groups.
Results
Among 142 patients, 68 (47.9%) showed mutKRAS and 42 (29.5%) showed mutBRAF. The median OS values were 8.4 and 34.3 months for patients with mutBRAF and BRAF wild-type, respectively (P < 0.01). However, KRAS status was not significantly associated with median OS (P = 0.76). Multivariate analysis revealed carcinoembryonic antigen, CRS, HIPEC, and mutBRAF as independent predictors for OS. Based on these findings, a nomogram was constructed. The C-index was 0.789 (95% confidence interval, 0.742–0.836), indicating good predictive ability of the model. Furthermore, the 1- and 2-year survival calibration plots showed good agreement between the predicted and actual OS rates. The area under curves of the 1- and 2-year survival predictions based on the nomogram were 0.807 and 0.682, respectively. Additionally, mutBRAF was significantly associated with lower PFS (P < 0.001).
Conclusions
mutBRAF is an independent prognostic risk factor for S-PM. The established nomogram predicted the OS of patients with CRC having S-PM with high accuracy, indicating its usefulness as a valuable prognostic tool for the designated patient cohort.
Keywords: colorectal cancer, peritoneal metastasis, BRAF, prognosis
Introduction
Colorectal cancer (CRC) is the third most common malignant tumor worldwide, with tumor metastasis and recurrence being the leading cause of death among these patients [1]. The most common site of metastasis in patients with CRC is the liver, accounting for 60% of patients with metastatic CRC (mCRC); peritoneal metastasis (PM) accounts for ∼20% of patients with mCRC [2, 3]. Jayne et al. [4] reported that 5%–10% of newly diagnosed patients with CRC presented with PM, which was referred to as synchronous PM (S-PM). The prognosis for PM was worse than that of isolated distant metastasis at other sites [5]. Moreover, the median overall survival (mOS) was 16.3 months for patients with isolated PM and 24.6 months for those with isolated lung metastasis [6].
Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) is the best treatment option for patients with PM because it may result in long-term survival [7, 8]. Macroscopic tumors in the abdominal cavity are resected in CRS and residual tumors in the abdominal cavity may be killed by the synergistic effect of hyperthermia and chemotherapy in HIPEC. Previous studies have shown that for patients with PM who have undergone CRS with HIPEC, the 5-year survival rate was as high as 40%, with an mOS of ∼40 months [9, 10].
The Ras/Raf/mitogen-activated protein kinase signaling pathway plays a vital role in CRC carcinogenesis [10, 11]. KRAS mutation (mutKRAS) and BRAF mutation (mutBRAF) occur in ∼40% and ∼20% of patients with mCRC, respectively [11, 12]. These mutations are considered unfavorable prognostic factors for liver and lung metastases in patients with CRC [13–15]. Nevertheless, the effects of mutKRAS and mutBRAF on the prognosis for patients with CRC and PM remain controversial. A study has shown that mutKRAS and mutBRAF negatively affect PM [16]. However, other studies have indicated that only mutBRAF is associated with patient prognosis and that the decision for surgery in such patients should be taken with caution [17–19]. Furthermore, no studies have been performed to investigate the effects of mutKRAS and mutBRAF on the prognosis for patients with CRC and S-PM.
In the present study, we determined the effects of mutKRAS and mutBRAF on the prognosis for patients with CRC showing S-PM who underwent CRS. Furthermore, we developed and validated an innovative prognostic nomogram for the mentioned patient cohort.
Patients and methods
Patient selection
Between July 2007 and July 2019, 653 patients were diagnosed with PM at the Sixth Affiliated Hospital, Sun Yat-sen University (Guangzhou, China). We included patients with pathologically confirmed CRC and PM but without liver and lung metastases in this study. The exclusion criteria were as follows: (i) patients without complete clinicopathological and follow-up data; (ii) those who did not undergo CRS; or (iii) those with confirmed CRC and metachronous PM.
Treatment
A multidisciplinary team examined all patients before the surgery. CRS included the resection of primary and invasive peritoneal and visceral tumors as well as complete removal of tumor deposits in the abdominal cavity. Multiorgan resection was performed to achieve complete cytoreduction whenever necessary. Residual lesions were assessed on the basis of the completeness of cytoreduction (CC) score: CC-0, no visible peritoneal tumors after CRS; CC-1, presence of tumor nodules of size <2.5 mm; CC-2, presence of residual tumors of size 2.5–25 mm; and CC-3, presence of tumor nodules of size >25 mm or unresectable tumor nodules anywhere in the abdomen or pelvis. Because the operation involved the resection and anastomosis of the bowel, some patients who underwent CRS received preventive stoma care post-operatively. Furthermore, some patients received HIPEC post-operatively one to three times in a closed manner to kill free tumor cells and prevent tumor recurrence. Chemotherapeutic drugs such as 5-fluorouracil, oxaliplatin, and platinum were mixed with normal saline and heated to 42°C. The perfusion rate was maintained at 100 mL/min during initiation; the temperature of the perfusion fluid was maintained at 42°C for 1 h. The perioperative chemotherapy regimens, comprising mainly 5-fluorouracil, included FOLFOX, FOLFIRI, and XELOX.
Clinicopathological characteristics and follow-up
The clinicopathological characteristics such as age, sex, T category, N category, peritoneal cancer index (PCI), CRS, HIPEC, CC score, preventive stoma, perioperative chemotherapy, tumor location, mutKRAS and mutBRAF status, and histology were considered during the study. The last follow-up was conducted on 20 June 2021. The primary end point was OS, which was defined as the period from the date of initial treatment (chemotherapy or surgical intervention) to the date of death or the last follow-up. The secondary end point was progression-free survival (PFS), which was defined as the time from initial treatment to tumor progression or death because of tumor progression or the last follow-up. The survival curve was plotted by using the Kaplan–Meier method. Univariate analysis was performed to identify prognostic factors associated with OS and PFS. Factors with a P-value of <0.05 were selected for multivariate Cox regression analysis.
Nomogram development and validation
Multivariate Cox regression analysis was performed on variables with a P-value of <0.05 to determine independent prognostic factors. Further, a nomogram was established based on these variables to predict the 1- and 2-year OS in patients with S-PM. The C-index was used to evaluate the predictive accuracy of the nomogram model. Generally, the C-index ranges between 0.5 and 1, with 0.5 being completely random and 1 being completely predictable. Furthermore, a calibration plot was constructed by comparing the 1- and 2-year predicted and actual survival rates. Finally, the predictive performance of the nomogram model was verified by plotting a receiver-operating characteristic (ROC) curve.
Statistical analysis
SPSS (Windows version 25.0) and R (version 4.0.3) software were used for statistical analysis. The R packages “survival,” “rms,” “foreign,” and “survivalROC” were used to establish the prognostic nomogram, calculate the C-index, and plot the calibration and ROC curves, respectively. The chi-square or Fischer’s exact test was performed to compare differences between the categorical variables. The Kaplan–Meier method and log-rank test were used to estimate the difference in OS and PFS. A P-value of <0.05 was considered statistically significant.
Results
Patients’ characteristics and OS
Based on the inclusion and exclusion criteria, 142 patients pathologically diagnosed with CRC and S-PM were included (Figure 1). All patients underwent CRS; 55 patients (38.7%) had a CC score of 0/1 and 87 (61.3%) had a CC score of 2. In addition, 63 patients (44.3%) received HIPEC. Table 1 presents the clinicopathological characteristics and treatment details of the patients. The mOS of all patients was 23.8 months; the 1- and 2-year OS rates were 67.2% and 49.2%, respectively.
Figure 1.
Flow chart of patient selection. CRC = colorectal cancer, S-PM = synchronous peritoneal metastasis, CRS = cytoreductive surgery.
Table 1.
Characteristics of the patients with CRC-SPM
| Variable | No. of patients (%) |
|---|---|
| Age (years) | |
| ≥60 | 59 (41.5) |
| <60 | 83 (58.5) |
| Sex | |
| Male | 76 (53.5) |
| Female | 66 (46.5) |
| T category | |
| T0–3 | 65 (45.7) |
| T4 | 77 (54.3) |
| N category | |
| N0 | 23 (16.1) |
| N1–2 | 119 (83.9) |
| Location of tumor | |
| Right-sided colon | 54 (38.1) |
| Left-sided colon | 69 (48.5) |
| Rectum | 19 (13.4) |
| Obstruction | |
| Yes | 85 (59.8) |
| No | 57 (40.2) |
| PCI | |
| ≥20 | 17 (11.9) |
| <20 | 125 (88.1) |
| Histology | |
| Adenocarcinoma | 102 (71.9) |
| Mucinous adenocarcinoma and signet ring cell carcinoma | 40 (28.1) |
| CRS | |
| CC-0/1 | 55 (38.7) |
| CC-2 | 87 (61.3) |
| HIPEC | |
| Yes | 63 (44.4) |
| No | 79 (55.6) |
| Perioperative chemotherapy | |
| Yes | 98 (69.1) |
| No | 44 (30.9) |
| Preventive stoma | |
| Yes | 42 (29.6) |
| No | 100 (70.4) |
| KRAS status | |
| mutKRAS | 68 (47.9) |
| wtKRAS | 74 (52.1) |
| BRAF status | |
| mutBRAF | 42 (29.5) |
| wtBRAF | 100 (70.5) |
CRS = cytoreductive surgery, CC = completeness of cytoreduction, HIPEC = hyperthermic intraperitoneal chemotherapy, mutKRAS/wtKRAS = KRAS mutation/KRAS wild-type, mutBRAF/wtBRAF = BRAF mutation/BRAF wild-type.
KRAS/BRAF analysis
Among 142 patients, 68 (47.9%) had mutKRAS, 42 (29.5%) had mutBRAF, and 58 (40.8%) possessed a dual wild-type (WT) genotype. mutKRAS tumors were evenly distributed in the left and right colon and the rectum, whereas mutBRAF tumors were mainly located in the right colon (P = 0.060) (Table 2). The mutBRAF and BRAF WT (wtBRAF) groups showed significant differences in the T category, post-operative HIPEC, and tumor location; no significant differences were observed between the mutKRAS and KRAS WT (wtKRAS) groups.
Table 2.
Summary of the demographics of the patients treated with CRS according to the KRAS/BRAF status
| Variable | wtBRAF (n = 100) | mutBRAF (n = 42) | P | wtKRAS (n = 74) | mutKRAS (n = 68) | P |
|---|---|---|---|---|---|---|
| Age (years) | 0.190 | 0.106 | ||||
| ≥60 | 38 (38.0) | 21 (50.0) | 26 (35.1) | 33 (48.5) | ||
| <60 | 62 (62.0) | 21 (50.0) | 48 (64.9) | 35 (51.5) | ||
| Sex | 0.200 | 0.639 | ||||
| Male | 57 (57.0) | 19 (45.2) | 41 (55.4) | 35 (51.5) | ||
| Female | 43 (43.0) | 23 (54.8) | 33 (44.6) | 33 (48.5) | ||
| T category | 0.002 | 0.768 | ||||
| T0–3 | 54 (54.0) | 11 (26.2) | 33 (44.6) | 32 (47.1) | ||
| T4 | 46 (46.0) | 31 (73.8) | 41 (55.4) | 36 (52.9) | ||
| N category | 0.680 | 0.653 | ||||
| N0 | 17 (17.0) | 6 (14.3) | 11 (14.9) | 12 (17.6) | ||
| N1–2 | 83 (83.0) | 36 (85.7) | 63 (85.1) | 56 (82.4) | ||
| CRS | 0.180 | 0.819 | ||||
| CC-0/1 | 43 (43.0) | 12 (28.5) | 28 (37.9) | 27 (39.7) | ||
| CC-2 | 57 (57.0) | 30 (71.5) | 46 (62.1) | 41 (60.3) | ||
| HIPEC | 0.030 | 0.695 | ||||
| Yes | 50 (50.0) | 13 (31.0) | 36 (48.6) | 27 (39.7) | ||
| No | 50 (50.0) | 29 (69.0) | 38 (51.4) | 41 (60.3) | ||
| Perioperative chemotherapy | 0.240 | 0.452 | ||||
| Yes | 72 (72.0) | 26 (62.0) | 49 (66.2) | 49 (72.1) | ||
| No | 28 (28.0) | 16 (38.0) | 25 (33.8) | 19 (27.9) | ||
| Preventive stoma | 0.820 | 0.682 | ||||
| Yes | 29 (29.0) | 13 (31.0) | 23 (31.1) | 19 (27.9) | ||
| No | 71 (71.0) | 29 (69.0) | 51 (68.9) | 49 (72.1) | ||
| Location of tumor | 0.060 | 0.320 | ||||
| Right-sided colon | 43 (71.0) | 26 (62.0) | 33 (44.6) | 36 (52.9) | ||
| Left-sided colon | 43 (43.0) | 11 (26.2) | 30 (40.5) | 24 (35.3) | ||
| Rectum | 14 (14.0) | 5 (11.8) | 11 (14.9) | 8 (11.8) | ||
| PCI | 0.980 | 0.040 | ||||
| ≥20 | 12 (12.0) | 5 (11.8) | 5 (6.8) | 12 (17.6) | ||
| <20 | 88 (88.0) | 37 (88.1) | 69 (93.2) | 56 (82.4) | ||
| Histology | 0.630 | 0.950 | ||||
| Adenocarcinoma | 73 (73.0) | 29 (69.0) | 53 (71.6) | 49 (72.1) | ||
| Mucinous adenocarcinoma and signet ring cell carcinoma | 27 (27.0) | 13 (31.0) | 21 (28.4) | 19 (27.9) |
All values are presented as number of patients followed by percentage in the parentheses.
CRS = cytoreductive surgery, HIPEC = hyperthermic intraperitoneal chemotherapy, mutKRAS/wtKRAS = KRAS mutation/KRAS wild-type, mutBRAF/wtBRAF = BRAF mutation/BRAF wild-type.
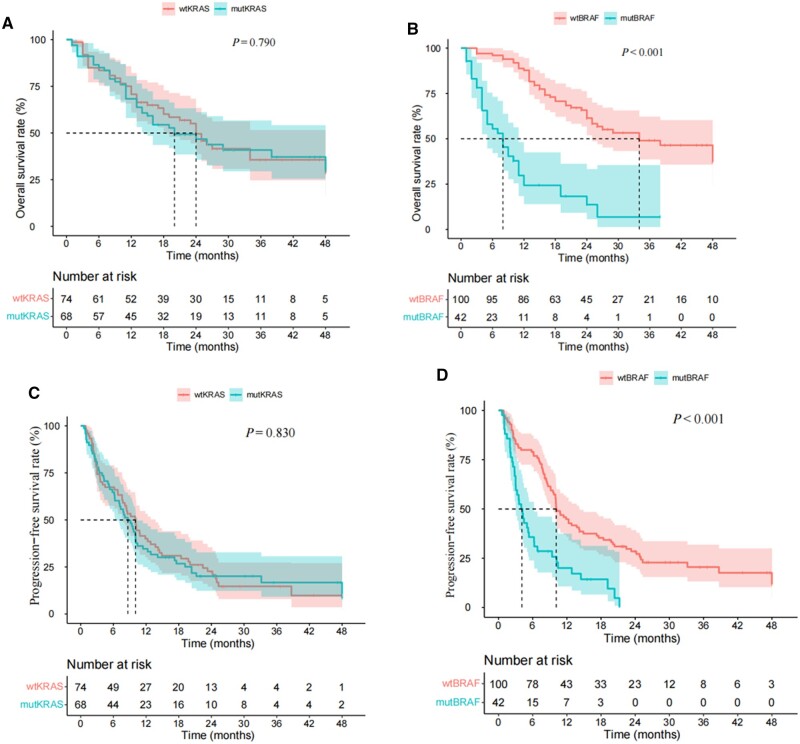
The mOS values were 20.2 and 24.1 months for patients with mutKRAS and wtKRAS, respectively (Figure 2A). No significant difference in the prognosis was observed between the two groups (P = 0.760). The mOS values were 8.4 and 34.3 months for patients with mutBRAF and wtBRAF, respectively (Figure 2B). In contrast to the results for KRAS, patients with mutBRAF had a worse prognosis (P < 0.001).
Figure 2.
The Kaplan–Meier curve of overall survival and progression-free survival for patients with different KRAS and BRAF status. (A) The Kaplan–Meier curve of overall survival for patients with mutKRAS and wtKRAS. (B) The Kaplan–Meier curve of overall survival for patients with mutBRAF and wtBRAF. (C) The Kaplan–Meier curve of progression-free survival for patients with mutKRAS and wtKRAS. (D) The Kaplan–Meier curve of progression-free survival for patients with mutBRAF and wtBRAF. mutKRAS/wtKRAS = KRAS mutation/KRAS wild-type, mutBRAF/wtBRAF = BRAF mutation/BRAF wild-type.
The median PFS for all patients was 9.8 months. The median PFS values were 8.8 and 10.4 months for patients with mutKRAS and wtKRAS, respectively (Figure 2C). No significant difference in the prognosis was observed between the two groups (P = 0.830). The median PFS values were 4.2 and 10.2 months for patients with mutBRAF and wtBRAF, respectively (Figure 2D). In contrast to the results for KRAS, patients with mutBRAF had a worse prognosis (P < 0.001).
Univariate and multivariate Cox analyses
The following factors associated with better outcomes in patients with S-PM CRC were identified by performing univariate Cox analysis: carcinoma embryonic antigen (CEA) of <5.0 ng/mL (P = 0.008), CA199 of <37.0 U/mL (P = 0.008), CC-0/1 (P < 0.001), HIPEC (P = 0.001), and wtBRAF (P < 0.001) (Table 3). The multivariate Cox analysis further revealed CEA of <5.0 ng/mL, CC-0/1, HIPEC, and wtBRAF as independent prognostic factors for OS (Table 3). Thus, these factors were used to construct a nomogram.
Table 3.
Cox univariate and multivariate analyses of the 142 patients with S-PM CRC
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Sex (female) | 1.29 (0.83–2.02) | 0.250 | ||
| CEA (≥5.0 ng/mL) | 1.91 (1.18–3.09) | 0.008 | 1.66 (1.01–2.73) | 0.040 |
| CA199 (≥37.0 U/mL) | 1.82 (1.17–2.86) | 0.008 | 1.19 (0.74–1.91) | 0.450 |
| CA125 (≥35.0 U/mL) | 1.22 (0.88–1.62) | 0.350 | ||
| Location | ||||
| Right-sided colon | Reference | 0.570 | ||
| Left-sided colon | 0.51 (0.31–0.83) | |||
| Rectum | 0.83 (0.44–1.58) | |||
| Obstruction | 1.43 (0.89–2.29) | 0.130 | ||
| CRS (CC-2) | 3.49 (2.02–6.01) | <0.001 | 2.81 (1.59–4.99) | <0.001 |
| HIPEC (Yes) | 0.46 (0.29–0.73) | 0.001 | 0.53 (0.32–0.86) | 0.010 |
| KRAS (mut) | 1.06 (0.68–1.65) | 0.790 | – | |
| BRAF (mut) | 4.32 (2.72–6.85) | <0.001 | 4.35 (2.69–7.01) | <0.001 |
| Category (T4) | 1.52 (0.97–2.38) | 0.060 | ||
| Category (N1–2) | 1.14 (0.82–1.59) | 0.420 | ||
| Histology (mucinous adenocarcinoma and signet ring cell carcinoma) | 1.53 (0.92–2.53) | 0.090 | ||
| PCI (≥20) | 1.21 (0.62–2.35) | 0.600 | ||
CRC = colorectal cancer, S-PM = synchronous peritoneal metastasis, CRS = cytoreductive surgery, HIPEC = hyperthermic intraperitoneal chemotherapy, mutKRAS/wtKRAS = KRAS mutation/KRAS wild-type, mutBRAF/wtBRAF = BRAF mutation/BRAF wild-type.
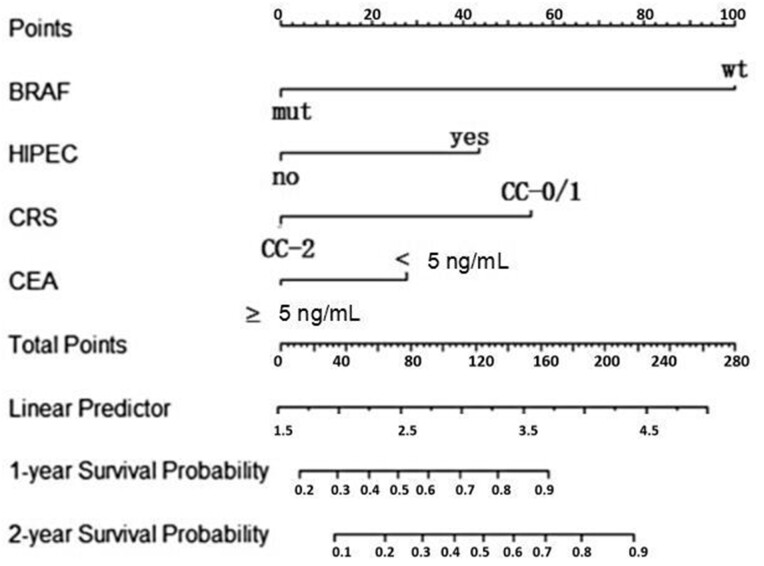
Nomogram construction and validation
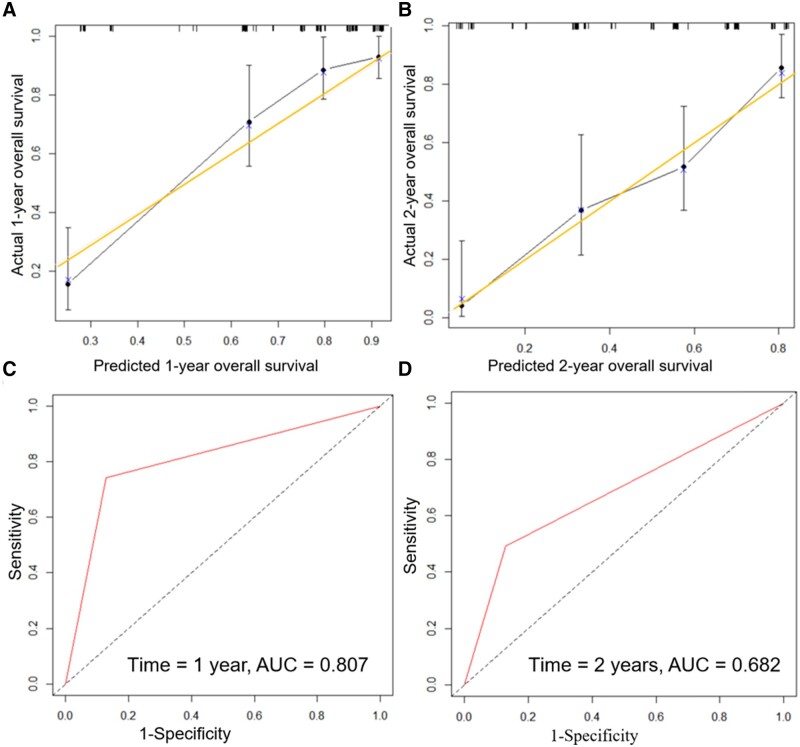
The 1- and 2-year post-operative survival probabilities for patients with S-PM were predicted using the constructed nomogram (Figure 3). To evaluate nomogram performance, we used the C-index to evaluate the ability of the nomogram to predict the 1- and 2-year OS of the patients. The C-index was 0.789 (95% confidence interval [CI], 0.742–0.836), which indicated the good prediction ability of the nomogram model. Furthermore, the 1- and 2-year calibration plots showed good agreement between the predicted and actual survival rates in the training cohort (Figure 4A and B). The area under the curve values for the 1- and 2-year survival predictions on the basis of the constructed nomogram were 0.807 and 0.682, respectively (Figure 4C and D).
Figure 3.
The nomogram of predicting the 1- and 2-year post-operative survival probabilities of patients with synchronous peritoneal metastasis. mut = mutant, wt = wild-type, HIPEC = hyperthermic intraperitoneal chemotherapy, CRS = cytoreductive surgery, CC = completeness of cytoreduction, CEA = carcinoma embryonic antigen.
Figure 4.
The calibration plots and ROC curve of the nomogram. (A) and (B) The 1- and 2-year calibration plots of the nomogram. The horizontal axes display the nomogram-predicted probabilities of overall survival at 1 and 2 years, whereas the vertical axes display the actual overall survival rates at 1 and 2 years. The diagonal line from the lower left to the upper right corner of the plot area is a reference line indicating the ideal prediction. The predictive accuracy of the model was assessed by using an ROC curve. (C) and (D) The AUC values for the 1- and 2-year survival predictions of the nomogram. The larger the AUC value, the better the prediction performance of the nomogram model. ROC curve = receiver-operating characteristic curve, AUC = area under the curve.
Discussion
The present retrospective analysis of 142 patients with S-PM admitted at our hospital showed that the mutKRAS and mutBRAF rates were 47.9% (68/142) and 29.5% (42/142), respectively. mutBRAF was identified as an independent risk factor for OS and PFS, but mutKRAS was not. Furthermore, CEA, CRS, and HIPEC were identified as independent prognostic factors for OS.
Current guidelines and relevant studies have confirmed that CRS, HIPEC, and systemic chemotherapy can considerably improve survival in selective patients with S-PM CRC [9, 20–22]. We found that patients who underwent CRS and received HIPEC had a good survival prognosis (mOS, 30.2 months). However, whether HIPEC is beneficial for patients with PM CRC remains controversial. The PRODIGE 7 trial showed that, compared with CRS alone, CRS + HIPEC provided no additional survival benefits for patients with PM [23]. However, the trial did not distinguish the survival benefits between metachronous and synchronous colorectal PMs. All patients included in the present study showed S-PM. Patients with metachronous PM are more prone to relapse after HIPEC than those with S-PM [24].
CC and PCI scores are the key factors affecting the prognosis for patients who undergo CRS [25, 26]. Herein, CC-0/1 was identified as a prognostic factor for patients who underwent CRS. Sugarbaker [27] reported that the 5-year survival rate for patients with CRC peritoneal metastases with PCI of >20 was 0 and suggested that such patients should receive palliative care. Thus, some researchers consider a PCI of ≤19 as the cut-off value [28, 29]. According to different primary tumor types, some researchers consider a PCI of ≤15 or 17 as the cut-off values for PM in gastric cancer [30, 31]. Nonetheless, the PCI cut-off value remains controversial worldwide. Herein, a PCI of >20 was not significantly associated with patient prognosis, which might be due to the low PCI score (the patients with PCI > 20 accounted for 11.9% of all; the mean PCI score was 6.2 ± 7.4).
Studies have shown mutKRAS and mutBRAF occur in ∼40% and ∼5%–20% of patients with mCRC, respectively [14, 32]. The incidence rates of mutKRAS and mutBRAF in PM CRC have been reported to be 45% and 24%, respectively [16, 18]. In the present study, the occurrence rates of mutKRAS and mutBRAF were slightly higher, reaching 47.9% and 29.5%, respectively. The possible reason is that these patients had S-PM. Moreover, 38.1% of the patients showed right-sided primary tumors, 48.2% showed left-sided tumors, and 13.4% showed rectal tumors. A previous study showed a higher rate of mutBRAF in the right-sided colon than in other sites in patients with CRC [33]. However, we could not determine the relationship between mutKRAS/mutBRAF and tumor location in the present study.
The prognosis for patients with mCRC showing liver and lung metastases is inferior to that for patients with peritoneal metastases [6]. mutKRAS and mutBRAF are unfavorable prognostic factors in patients with CRC showing liver and lung metastases [13, 14]. However, the association of these mutations with PM in patients with CRC remains controversial. Tonello et al. [16] found that, compared with patients harboring WT mutations, those with mutKRAS and mutBRAF exhibited shorter 5-year OS (29.4% and 26.8% vs 51.5%, respectively). Morgan et al. [19] found that mutKRAS was significantly associated with decreased recurrence-free survival, but not with OS. Conversely, Graf et al. [34] found no difference in OS between patients carrying mutKRAS and those with wtKRAS. Furthermore, Larsen et al. [18] found that the OS rates of patients with mutBRAF, mutKRAS, and double-WT were similar. The present study showed mutBRAF as a prognostic risk factor for patients who underwent CRS, whereas mutKRAS was not considered a prognostic factor. In Tonello et al.’s [16] and Larsen et al.’s [18] studies, patients with S-PM and metachronous PM (M-PM) were included. The proportion of patients with S-PM and M-PM accounted for 71.8% and 66.1%, respectively. However, all 142 patients in the present study had S-PM, whereas Morgan et al.’s [19] study included 45 patients with M-PM. Graf et al. [34] included patients with appendiceal primaries and those who received palliative care. These differences in patient conditions may have contributed to the differences in the results.
Studies on primary CRC have shown that the mismatch repair status is crucial in explaining the mutBRAF status, which did not affect OS and disease-free survival in patients with microsatellite instability (MSI) tumors [35, 36]. MSI and mutBRAF are independent adverse prognostic factors for mCRC [36]. Sherman et al. [37] found that, compared with patients with microsatellite stability, patients with PM + MSI showed worse survival. Owing to the retrospective nature of the present study, the mismatch repair status of all patients could not be detected. Furthermore, the data of some patients were missing; thus, the complete data of only 12 patients could be obtained and the relationship between the mismatch repair status and OS was not investigated.
Owing to the higher risk of heterochronous PM recurrence, we focused more on S-PM and KRAS/BRAF, and found that BRAF was significantly associated with OS in patients with S-PM. Meanwhile, we found that patients with mutBRAF had worse PFS than those with mutKRAS. Moreover, the nomogram constructed in this study is probably superior to existing predictive models that do not include any tumor gene status [38–41]. Furthermore, this nomogram exhibits a good predictive ability, with a high C-index. Nonetheless, patients carrying mutBRAF should be more cautious while opting for CRS.
The present study has some limitations. First, the retrospective nature and single-center data used in this study may have contributed to certain biases. Thus, prospective multicenter studies should be conducted to validate the present results. Second, some patients were not included in the study because of the deletion of their genetic statuses. Third, we obtained the BRAF/KRAS statuses from the patients’ pathology reports, which may have contained some errors. For financial reasons, some patients were only tested for a common type of BRAF/KRAS and did not have their entire genome sequenced. Finally, the nomogram established was not externally validated and further studies are warranted.
Conclusions
mutBRAF is an independent prognostic risk factor for S-PM in patients with CRC. In this study, we developed and validated an innovative nomogram to predict OS in patients with S-PM CRC on the basis of the BRAF status, which showed good discriminative ability and high accuracy in predicting the 1- and 2-year OS of patients with CRC having S-PM.
Contributor Information
Zhijie Wu, Department of General Surgery (Colorectal Surgery), The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China.
Xiusen Qin, Department of General Surgery (Colorectal Surgery), The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China.
Yuanxin Zhang, Department of General Surgery (Colorectal Surgery), The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China.
Jian Luo, Department of General Surgery (Colorectal Surgery), The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China.
Rui Luo, Department of General Surgery (Colorectal Surgery), The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China.
Zonglu Cai, Department of General Surgery (Colorectal Surgery), The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China.
Hui Wang, Department of General Surgery (Colorectal Surgery), The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China; Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, P. R. China.
Author Contributions
Study concept and design: Z.W., X.Q., and H.W.; analysis and interpretation of data: Z.W. and X.Q.; drafting of the manuscript: Z.W.; revision of the manuscript: X.Q., Y.Z, J.L, R.L., and H.W.; approval the manuscript: all authors.
Funding
The Sixth Affiliated Hospital,Sun Yat-Sen University Clinical Research 1010 Program [1010CG(2022)-08]. Sun Yat-sen University Clinical Reacher 5010 Program [2017008]. This study was supported by National Key Clinical Discipline and the program of Guangdong Provincial Clinical Research Center for Digestive Diseases [2020B1111170004].
Conflict of Interest
None declared.
References
- 1. Sung H, Ferlay J, Siegel RL. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. Thomassen I, van Gestel YR, Lemmens VE. et al. Incidence, prognosis, and treatment options for patients with synchronous peritoneal carcinomatosis and liver metastases from colorectal origin. Dis Colon Rectum 2013;56:1373–80. [DOI] [PubMed] [Google Scholar]
- 3. van Gestel YR, de Hingh IH, van Herk-Sukel MP. et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol 2014;38:448–54. [DOI] [PubMed] [Google Scholar]
- 4. Jayne DG, Fook S, Loi C. et al. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002;89:1545–50. [DOI] [PubMed] [Google Scholar]
- 5. Bhandare M, Patil P, Pai V. et al. Peritoneal carcinomatosis in colorectal cancers - management perspective needs a change. Clin Colorectal Cancer 2017;16:e1–6. [DOI] [PubMed] [Google Scholar]
- 6. Franko J, Shi Q, Meyers JP. et al. ; Analysis and Research in Cancers of the Digestive System (ARCAD) Group. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol 2016;17:1709–19. [DOI] [PubMed] [Google Scholar]
- 7. Sugarbaker PH. Carcinoma of the colon–prognosis and operative choice. Curr Probl Surg 1981;18:753–802. [DOI] [PubMed] [Google Scholar]
- 8. Verwaal VJ, van Ruth S, de Bree E. et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737–43. [DOI] [PubMed] [Google Scholar]
- 9. Klaver CEL, Wisselink DD, Punt CJA. et al. ; COLOPEC Collaborators Group. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol 2019;4:761–70. [DOI] [PubMed] [Google Scholar]
- 10. Frøysnes IS, Larsen SG, Spasojevic M. et al. Complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastasis in Norway: prognostic factors and oncologic outcome in a national patient cohort. J Surg Oncol 2016;114:222–7. [DOI] [PubMed] [Google Scholar]
- 11. Normanno N, Tejpar S, Morgillo F. et al. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol 2009;6:519–27. [DOI] [PubMed] [Google Scholar]
- 12. Fariña-Sarasqueta A, van Lijnschoten G, Moerland E. et al. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients. Ann Oncol 2010;21:2396–402. [DOI] [PubMed] [Google Scholar]
- 13. Passot G, Kim BJ, Glehen O. et al. ; BIG-RENAPE Working Group. Impact of RAS mutations in metastatic colorectal cancer after potentially curative resection: does site of metastases matter? Ann Surg Oncol 2018;25:179–87. [DOI] [PubMed] [Google Scholar]
- 14. Renaud S, Romain B, Falcoz PE. et al. KRAS and BRAF mutations are prognostic biomarkers in patients undergoing lung metastasectomy of colorectal cancer. Br J Cancer 2015;112:720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Margonis GA, Buettner S, Andreatos N. et al. Association of BRAF mutations with survival and recurrence in surgically treated patients with metastatic colorectal liver cancer. JAMA Surg 2018;153:e180996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tonello M, Baratti D, Sammartino P. et al. Microsatellite and RAS/RAF mutational status as prognostic factors in colorectal peritoneal metastases treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Ann Surg Oncol 2022;29:3405–17. [DOI] [PubMed] [Google Scholar]
- 17. Bhullar D, O'Dwyer S, Wilson M. et al. RAS mutation status should not be used to predict outcome from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases. Ann Surg Oncol 2023;30:792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larsen SG, Goscinski MA, Dueland S. et al. Impact of KRAS, BRAF and microsatellite instability status after cytoreductive surgery and HIPEC in a national cohort of colorectal peritoneal metastasis patients. Br J Cancer 2022;126:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morgan Z, Chow BE, Strong EA. et al. RAS mutation status confers prognostic relevance in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer. J Surg Res 2019;240:130–5. [DOI] [PubMed] [Google Scholar]
- 20. Sánchez-Hidalgo JM, Rodríguez-Ortiz L, Arjona-Sánchez Á et al Colorectal peritoneal metastases: optimal management review. WJG 2019;25:3484–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hashiguchi Y, Muro K, Saito Y. et al. ; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 2020;25:1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baratti D, Kusamura S, Niger M. et al. Colorectal peritoneal metastases treated by perioperative systemic chemotherapy and cytoreductive surgery with or without mitomycin C-based HIPEC: a comparative study using the Peritoneal Surface Disease Severity Score (PSDSS). Ann Surg Oncol 2021;28:3332–42. [DOI] [PubMed] [Google Scholar]
- 23. Quénet F, Elias D, Roca L. et al. ; UNICANCER-GI Group and BIG Renape Group. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:256–66. [DOI] [PubMed] [Google Scholar]
- 24. Hentzen JEKR, Rovers KP, Kuipers H. et al. Impact of synchronous versus metachronous onset of colorectal peritoneal metastases on survival outcomes after Cytoreductive Surgery (CRS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC): a multicenter, retrospective, observational study. Ann Surg Oncol 2019;26:2210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The Chicago Consensus on peritoneal surface malignancies: management of colorectal metastases. Ann Surg Oncol.2020 Jun;27(6):1753–60. [DOI] [PubMed] [Google Scholar]
- 26. Mohamed F, Kallioinen M, Braun M. et al. ; Guideline Committee. Management of colorectal cancer metastases to the liver, lung or peritoneum suitable for curative intent: summary of NICE guidance. Br J Surg 2020;107:943–5. [DOI] [PubMed] [Google Scholar]
- 27. Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol 1999;43(Suppl):S15–25. [DOI] [PubMed] [Google Scholar]
- 28. Chua TC, Morris DL, Esquivel J.. Impact of the peritoneal surface disease severity score on survival in patients with colorectal cancer peritoneal carcinomatosis undergoing complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2010;17:1330–6. [DOI] [PubMed] [Google Scholar]
- 29. Maillet M, Glehen O, Lambert J. et al. ; BIG-RENAPE Working Group. Early postoperative chemotherapy after complete cytoreduction and hyperthermic intraperitoneal chemotherapy for isolated peritoneal carcinomatosis of colon cancer: a multicenter study. Ann Surg Oncol 2016;23:863–9. [DOI] [PubMed] [Google Scholar]
- 30. Swellengrebel HAM, Zoetmulder FAN, Smeenk RM. et al. Quantitative intra-operative assessment of peritoneal carcinomatosis - a comparison of three prognostic tools. Eur J Surg Oncol 2009;35:1078–84. [DOI] [PubMed] [Google Scholar]
- 31. Goéré D, Souadka A, Faron M. et al. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol 2015;22:2958–64. [DOI] [PubMed] [Google Scholar]
- 32. Tol J, Nagtegaal ID, Punt CJA.. BRAF mutation in metastatic colorectal cancer. N Engl J Med 2009;361:98–9. [DOI] [PubMed] [Google Scholar]
- 33. Piawah S, Venook AP.. Targeted therapy for colorectal cancer metastases: a review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer 2019;125:4139–47. [DOI] [PubMed] [Google Scholar]
- 34. Graf W, Cashin PH, Ghanipour L. et al. Prognostic impact of BRAF and KRAS mutation in patients with colorectal and appendiceal peritoneal metastases scheduled for CRS and HIPEC. Ann Surg Oncol 2020;27:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taieb J, Le Malicot K, Shi Q. et al. Prognostic value of BRAF and KRAS mutations in MSI and MSS Stage III colon cancer. J Natl Cancer Inst. 2017;109(5):dwj272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taieb J, Zaanan A, Le Malicot K. et al. Prognostic effect of BRAF and KRAS mutations in patients with stage III colon cancer treated with leucovorin, fluorouracil, and oxaliplatin with or without cetuximab: a post hoc analysis of the PETACC-8 trial. JAMA Oncol 2016;2:643–53. [DOI] [PubMed] [Google Scholar]
- 37. Sherman SK, Schuitevoerder D, Chan CHF. et al. Metastatic colorectal cancers with mismatch repair deficiency result in worse survival regardless of peritoneal metastases. Ann Surg Oncol 2020;27:5074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pelz JOW, Stojadinovic A, Nissan A. et al. Evaluation of a peritoneal surface disease severity score in patients with colon cancer with peritoneal carcinomatosis. J Surg Oncol 2009;99:9–15. [DOI] [PubMed] [Google Scholar]
- 39. Simkens GA, van Oudheusden TR, Nieboer D. et al. Development of a prognostic nomogram for patients with peritoneally metastasized colorectal cancer treated with cytoreductive surgery and HIPEC. Ann Surg Oncol 2016;23:4214–21. [DOI] [PubMed] [Google Scholar]
- 40. Yang Z, Li Y, Qin X. et al. Development and validation of a prognostic nomogram for colorectal cancer patients with synchronous peritoneal metastasis. Front Oncol 2021;11:615321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lv MY, Chen XJ, Chen JG. et al. Nomogram for predicting overall survival time of patients with stage IV colorectal cancer. Gastroenterol Rep (Oxf) 2022;10. [DOI] [PMC free article] [PubMed] [Google Scholar]