Abstract
Background:
The efficacy of anti-programmed cell death (PD)-1 monotherapy in advanced hepatocellular carcinoma (aHCC) is limited, and combination therapy with lenvatinib and pembrolizumab has shown promising results. However, comparative studies between immune monotherapies and combination therapies are lacking.
Objectives:
To investigate the efficacy and safety of anti-PD-1 monotherapy (PD-1) and anti-PD-1 plus lenvatinib (PD-1 + L) in patients with aHCC to guide clinical treatment decisions.
Design:
A retrospective study was conducted on a cohort of patients with aHCC who received either PD-1 monotherapy or PD-1 + L combination therapy between January 2018 and January 2020.
Methods:
The study retrospectively reviewed the medical records of 94 eligible patients with aHCC, with 39 in the PD-1 group and 55 in the PD-1 + L group. The efficacy outcomes, including objective response rate (ORR), disease control rate (DCR), duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety, were assessed.
Results:
With a median follow-up of 30.1 months, the PD-1 + L group demonstrated a significantly higher ORR (32.7% versus 10.3%, p = 0.013), better DCR (80.0% versus 53.8%, p = 0.012), longer median PFS (10.6 versus 4.4 months, p < 0.001) and longer median OS (18.4 versus 8.5 months, p = 0.013) than PD-1 group. For the responders, the efficacy of the two groups was durable (DOR was 11.6 versus 3.5 months, p = 0.009). Subgroup analyses based on prior tyrosine kinase inhibitor (TKI) treatment and the presence or absence of macrovascular tumor thrombosis or extrahepatic metastases favored the PD-1 + L group. The combination therapy was a good predictor of PFS and OS in multivariate analysis. Grade 3/4 treatment-related adverse events were more common in PD-1 + L group, with higher incidences of hypertension and hand–foot skin reactions.
Conclusions:
PD-1 monotherapy and PD-1 plus lenvatinib combination therapy were well-tolerated in patients with aHCC. PD-1 + L showed significantly better survival benefits than PD-1 monotherapy.
Keywords: anti-PD-1, combination therapy, hepatocellular carcinoma, immune checkpoint inhibitors, lenvatinib
Plain language summary
Understanding the impact of PD-1 and lenvatinib combination therapy on advanced hepatocellular carcinoma
Abstract: This plain language summary provides a description of our research on the combination therapy of PD-1 and lenvatinib for advanced Hepatocellular carcinoma (aHCC). We aimed to investigate the effectiveness and safety of this treatment approach and offer insights for clinical decisions.
Why was this study done?
HCC is a challenging condition to treat, especially in advanced stages. We explored whether the combination of two drugs, PD-1 and lenvatinib, can offer better outcomes for patients with aHCC than PD-1 monotherapy.
What did the researchers do?
We conducted a retrospective study on patients diagnosed with aHCC who received PD-1 alone or PD-1 combined with lenvatinib between January 2018 and January 2020. We analysed the medical records to assess the treatment’s efficacy and safety.
What did the researchers find?
After a median follow-up of 30.1 months, we observed significant improvements in the combination therapy group, with higher response rates, better disease control, longer progression free survival, and more extended overall survival than those in the PD-1 monotherapy group. Responders in the combination group also experienced a longer duration of response.
What do the findings mean?
Our results address the lack of data for real-world clinical experiences regarding anti-PD-1 monotherapy for patients with aHCC compared to immunotherapy plus lenvatinib are lacking. The combination of PD-1 and lenvatinib was more effective and offered better survival benefits for patients with aHCC than PD-1 alone. These results could provide new hope for patients with this challenging condition.
Limitations: The study was conducted at a single centre with relatively few patients. Additionally, most patients had hepatitis B-associated liver cancer, which may limit the generalisability of our findings to other populations.
Conclusions: PD-1 plus lenvatinib is a promising treatment option for patients with aHCC. It is well-tolerated, and its effectiveness surpasses that of PD-1 therapy alone. Our findings could potentially guide clinicians in making treatment decisions for patients with aHCC.
Introduction
Liver cancer ranks as the third-leading cause of tumor-related deaths globally. 1 Hepatocellular carcinoma (HCC) is the most prevalent form of liver cancer, accounting for approximately 90% of all liver cancer cases. The tyrosine kinase inhibitors (TKIs) sorafenib and lenvatinib have been the global frontline standard for decades. 2 However, their efficacy remains unsatisfactory.3,4 The advent of immunotherapy, with the development of immune checkpoint inhibitors (ICIs) such as the PD-1 inhibitors pembrolizumab and nivolumab, has revolutionized the treatment of HCC.5,6 Nonetheless, PD-1 monotherapy demonstrates an objective response rate (ORR) of only approximately 14–17% 7 and definitive real-world clinical data confirming the efficacy of single-agent treatments are lacking.
Clinical trials exploring ICIs in combination with various drugs have shown promising results and are expected to transform the management of advanced HCC (aHCC).8 –11 The FDA has granted accelerated approval for the use of lenvatinib plus pembrolizumab as a first-line therapy for aHCC, based on encouraging findings from the KEYNOTE-524 trial. 10 Notably, despite the favorable efficacy of combination therapy, the incidence of grade 3/4 treatment-related adverse events (TRAEs) has significantly increased (60–70%). 12 Consequently, a subset of patients still receive monotherapy owing to unacceptable combination therapy-associated TRAEs. Both immune monotherapy and combination therapy have demonstrated favorable clinical benefits in appropriate populations.
Although the clinical efficacy of lenvatinib in combination with different PD-1 inhibitors has been reported successively,13 –18 there is relatively little clinical experience regarding anti-PD-1 monotherapy for patients with aHCC compared with immunotherapy plus lenvatinib in real-world cohorts. Therefore, this retrospective study aimed to compare the efficacy and safety of PD-1 inhibitors alone versus lenvatinib plus PD-1 inhibitors for patients with aHCC, providing valuable real-world insights to improve clinical decision-making.
Materials and methods
Patients
This retrospective study was conducted at the Chinese People’s Liberation Army General Hospital and approved by the Ethics Committee of Chinese People’s Liberation Army General Hospital. The data were privacy-compliant, and the requirement for informed consent was waived. The final analysis included 94 eligible patients with aHCC who received either PD-1 monotherapy or PD-1 plus lenvatinib combination therapy at our institution between January 2018 and January 2020. The key inclusion criteria were as follows: (1) age ⩾18 years; (2) pathologically diagnosed with HCC; and (3) presence of baseline imaging showing at least one measurable lesion. Patients were excluded if they were treated by combination of heterogeneous therapies or with incomplete medical data. PD-1 blockade treatment was administered intravenously at standard doses: nivolumab (3 mg/kg every 2 weeks), pembrolizumab (200 mg every 3 weeks), sintilimab (200 mg every 3 weeks), and camrelizumab (200 mg every 3 weeks). For patients who received lenvatinib (Eisai, Co., Ltd., Tokyo, Japan), the dosage was adjusted based on body weight (8 mg for patients <60 kg and 12 mg for patients ⩾60 kg).
Outcome assessment
Before initiating therapy, all clinical and laboratory data were collected. Tumor evaluation was performed using dynamic computed tomography or magnetic resonance imaging every 6 weeks, following the response evaluation criteria for solid tumors (RECIST1.1). Treatment was discontinued in case of disease progression, unacceptable toxicity, death, or any other reasons. Safety assessments and grading were obtained from the patients’ electronic medical records or patient descriptions via follow-up calls.
Statistical analysis
Survival outcomes were estimated using the Kaplan–Meier method with the log-rank test. OS was estimated from the date of the initial treatment to the date of death or last follow-up (censored). PFS was estimated from the first dose of the PD-1 inhibitor to the date of progression or death.
Antitumor responses were categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to the RECIST1.1 criteria. The ORR was defined as the proportion of patients with the best tumor response, CR, and PR. Disease control rate (DCR) was defined as the percentage of patients who achieved CR, PR, or SD. The duration of response (DOR) was evaluated in patients with CR or PR, defined as the time from the first CR or PR to PD or death.
Descriptive statistics were used to summarize demographic data, and categorical data were presented as numbers of patients (percentages). Fisher’s exact test was used for discrete variables, and the Wilcoxon rank-sum test was used for continuous variables. Univariate and multivariate Cox regression analyses were performed to identify independent prognostic factors for OS and PFS. Variables with p < 0.20 in univariable analyses were entered into the multivariate analysis. Statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS version 25.0 (IBM Corp, Armonk, NK).
Results
Baseline characteristics of the patients
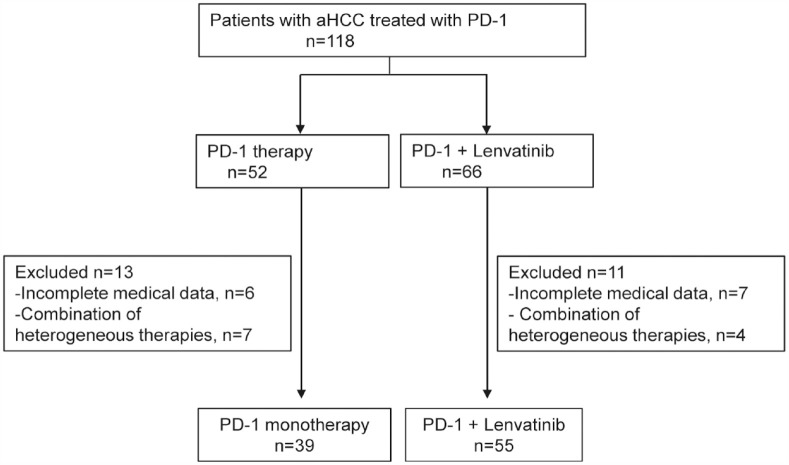
A total of 94 eligible patients with aHCC who received PD-1 inhibitor monotherapy or lenvatinib combination therapy between January 2018 and January 2020 were included (Figure 1).
Figure 1.
Patient selection flow.
aHCC, advanced hepatocellular carcinoma.
Most patients were classified as having Barcelona Clinic Liver Cancer (BCLC) stage C–D (79.5% versus 92.7%, p = 0.068), Eastern Cooperative Oncology Group performance status (ECOG) performance status of 0–1 (79.5% versus 92.7%, p = 0.068), Child–Pugh class A (71.8% versus 76.4%, p = 0.638), and hepatitis B virus (HBV) infection (74.4% versus 85.5%, p = 0.280). Macrovascular tumor thrombosis (MVT) and extrahepatic metastases (EM) did not differ between the two groups. A total of 65 (69.1%) patients were treated in the first-line setting, whereas the other 29 (30.9%) patients had received prior systemic therapy, including TKIs or antiangiogenic agents. In the PD-1 group, 12 patients [sorafenib (n = 10), lenvatinib (n = 1), sorafenib followed by regorafenib (n = 1)] had received TKIs. In the PD-1 + L group, 17 patients [sorafenib (n = 14), regorafenib (n = 2), sorafenib followed by anlotinib (n = 1)] had received TKIs. In the PD-1 group, 20 (51.3%) patients received pembrolizumab, 11 (28.2%) patients received nivolumab, 3 (7.7%) patients received sintilimab, and 5 (12.8%) patients received camrelizumab, whereas 33 (60.0%) patients received pembrolizumab, 11 (20.0%) patients received nivolumab, 6 (10.9%) patients received sintilimab, and 5 (9.1%) patients received camrelizumab in the PD-1 + L group. Additional patient information from our cohort study is shown in Table 1.
Table 1.
Baseline characteristics of the patients.
| Characteristics, n% | All patients, N = 94 | PD-1, N = 39 | PD-1 + L, N = 55 | p value |
|---|---|---|---|---|
| Median age | 56 (50–62) | 59 (51–65) | 54 (49–61) | 0.071 |
| Gender | ||||
| Male | 78 (83.0%) | 32 (82.1%) | 46 (83.6%) | 0.840 |
| Female | 16 (17.0%) | 7 (17.9%) | 9 (16.4%) | |
| Comorbidities | ||||
| Hypertension | 17 (18.1%) | 10 (25.6%) | 7 (12.7%) | 0.173 |
| Diabetes | 15 (16.0%) | 8 (20.5%) | 7 (12.7%) | 0.394 |
| Smoking history | 29 (30.9%) | 10 (25.6%) | 19 (34.6%) | 0.377 |
| Alcohol use | 32 (34.0%) | 10 (25.6%) | 22 (40.0%) | 0.187 |
| ECOG PS | 0.068 | |||
| 0–1 | 82 (87.2%) | 31 (79.5%) | 51 (92.7%) | |
| > 1 | 12 (12.8%) | 8 (20.5%) | 4 (7.3%) | |
| BCLC stage | 0.097 | |||
| B | 12 (12.8%) | 8 (20.5%) | 4 (7.3%) | |
| C | 79 (84.0%) | 29 (74.4%) | 50 (90.9%) | |
| D | 3 (3.2%) | 2 (5.1%) | 1 (1.8%) | |
| Child–Pugh class | ||||
| A | 70 (74.5%) | 28 (71.8%) | 42 (76.4%) | 0.638 |
| B | 24 (25.5%) | 11 (28.2%) | 13 (23.6%) | |
| Etiology | ||||
| HBV infected | 76 (80.9%) | 29 (74.4%) | 47 (85.5%) | 0.280 |
| HCV infected | 3 (3.2%) | 1 (2.6%) | 2 (3.6%) | |
| None | 15 (15.9%) | 9 (23.1%) | 6 (10.9%) | |
| PD-1 agent | ||||
| Pembrolizumab | 53 (56.38%) | 20 (51.3%) | 33 (60.0%) | 0.680 |
| Nivolumab | 22 (23.4%) | 11 (28.2%) | 11 (20.0%) | |
| Sintilimab | 9 (9.6%) | 3 (7.7%) | 6 (10.9%) | |
| Camrelizumab | 10 (10.6%) | 5 (12.8%) | 5 (9.1%) | |
| AFP > 200 ng/ml | ||||
| Yes | 65 (69.1%) | 23 (59.0%) | 42 (76.4%) | 0.112 |
| No | 29 (30.9%) | 16 (41.0%) | 13 (23.6%) | |
| MVT | ||||
| Yes | 48 (51.1%) | 18 (46.2%) | 30 (54.5%) | 0.530 |
| No | 46 (48.9%) | 21 (53.8%) | 25 (45.5%) | |
| EM | ||||
| Yes | 66 (70.2%) | 23 (59.0%) | 43 (78.2%) | 0.066 |
| No | 28 (29.8%) | 16 (41.0%) | 12 (21.8%) | |
| Lines | ||||
| 1 | 65 (69.1%) | 27 (69.2%) | 38 (69.1%) | 0.931 |
| 2 | 25 (26.6%) | 10 (25.7%) | 15 (27.3%) | |
| 3 | 4 (4.3%) | 2 (5.1%) | 2 (3.6%) | |
| Prior treatment | ||||
| TKIs | 29 (30.8.0%) | 12 (30.7%) | 17 (30.9%) | 0.818 |
| TACE | 44 (46.80%) | 21 (53.8%) | 23 (41.8%) | 0.297 |
| Ablation | 26 (27.7%) | 13 (33.3%) | 13 (33.3%) | 0.353 |
| Radiotherapy | 13 (13.8%) | 7 (17.9%) | 6 (10.9%) | 0.374 |
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; EM, extrahepatic metastases; HBV, hepatitis B virus; HCV, hepatitis C virus; MVT, macrovascular tumor thrombosis; TACE, transcatheter arterial chemoembolization; TKIs, tyrosine kinase inhibitors.
Patients were treated with a median of 6 (3–9) cycles in the PD-1 group and 10 (5–17) cycles in the PD-1 + L group. The median duration of lenvatinib treatment for patients in the combination group was 8.60 months (3.86–13.57 months). Subsequent treatments after disease progression following mono-immunotherapy or combination therapy is presented in Supplemental Table S1. The combination group had a greater chance of receiving subsequent treatments.
Efficacy
Response evaluation
During a median follow-up duration of 30.1 months, the best response was evaluated, however, no CR was achieved in the total cohort. In the PD-1 group, 4 patients achieved PR, 17 patients achieved SD, and 18 patients showed PD. In the PD-1 + L group, 18 patients achieved PR, 26 patients achieved SD, and 11 patients showed PD (Table 2). The ORR and DCR were significantly higher in the PD-1 + L group (32.7% versus 10.3%, p = 0.013 and 80.0% versus 53.8%, p = 0.012, respectively). Among the responders, the median DOR in the PD-1 + L group according to RECIST 1.1 was 11.6 months (95% CI 6.7–16.6) compared to 3.5 months (95% CI 0–7.1) in the PD-1 group (p = 0.009), indicating that the response was more persistent in the PD-1 + L group.
Table 2.
Responses to treatment by RECIST 1.1.
| Parameter, n% | Entire cohort, N = 94 | PD-1, N = 39 | PD-1 + L, N = 55 | p value |
|---|---|---|---|---|
| Best overall response, n (%) | 0.007 | |||
| CR | 0 | 0 | 0 | |
| PR | 22 | 4 | 18 | |
| SD | 43 | 17 | 26 | |
| PD | 29 | 18 | 11 | |
| ORR, % | 23.4% | 4 (10.3%) | 18 (32.7%) | 0.013* |
| DCR, % | 69.1% | 21 (53.8%) | 44 (80.0%) | 0.012* |
| DOR, months | 10.1 (5.5–14.6) | 3.5 (0–7.1) | 11.6 (6.7–16.6) | 0.009* |
CR, Complete response; DCR, disease-control rate; DOR, duration of response; ORR, objective response rate; PD, Progressive disease; PR, Partial response; SD, Stable disease.
p < 0.05, statistically significant.
Overall survival and progression-free survival
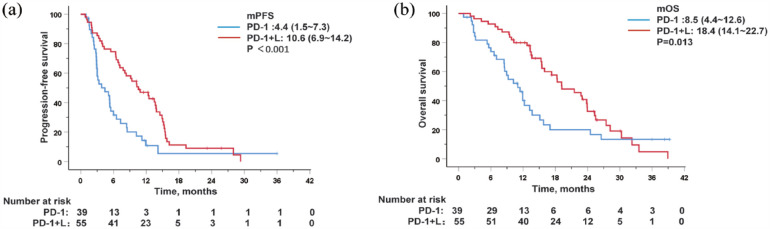
Compared with the PD-1 group, patients in the PD-1 + L group showed notably better survival outcomes. The median PFS (mPFS) was longer in the PD-1 + L group (10.6 months, 95% CI 6.9–14.2) compared to the PD-1 group (4.4 months, 95% CI 1.5–7.3) [p < 0.001; Figure 2(a)]. The 6- and 12-month OS rates in the PD-1 group were 68.5% and 31.8%, respectively, whereas those in the PD-1 + L group were 90.9% and 76.2%, respectively. The median OS was 8.5 months (95% CI 4.4–12.6) in the PD-1 group and 18.4 months (95% CI 14.1–22.7) in the PD-1 + L group [p = 0.013; Figure 2(b)].
Figure 2.
Kaplan–Meier curves for progression-free survival (a) and overall survival (b) in PD-1 and PD-1 + L group.
Survival analysis by prior treatment with TKI
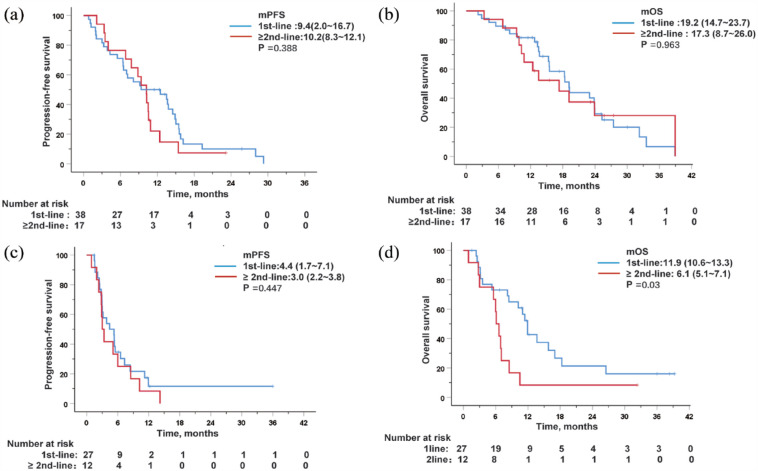
Survival analysis was performed based on prior TKI treatment. In the PD-1 + L group, 38 (69.1%) patients received PD-1 plus lenvatinib as first-line therapy and 17 (30.9%) patients had previously received TKIs. When comparing patients who had received prior TKI treatment with patients treated with first-line therapy, no significant difference was found in mPFS [9.4 months, (95% CI 2.0–16.7) versus 10.2 months, (95% CI 8.3–12.1), p = 0.388], and mOS [19.2 months (95% CI 14.7–23.7) versus 17.3 months (95% CI 8.7–26.0), p = 0.963; Figure 3(a) and (b)]. In the PD-1 group, 12 (30.7%) patients had received prior TKIs, and the mOS of these patients was significantly shorter than that of patients treated with first-line therapy [11.9 months (95% CI 10.6–13.3) versus 6.1 months (95% CI 5.1–7.1), p = 0.030; (Figure 3(d)]. No significant difference was found in mPFS [4.4 months (95% CI 1.7–7.1) versus 3.0 months (95% CI 2.2–3.8), p = 0.447; Figure 3(c)]. These results suggest that combination therapy is still a better option for achieving improved clinical benefits for patients with aHCC who received prior treatment with TKIs.
Figure 3.
Kaplan–Meier curves for progression-free survival and overall survival after stratification by prior treated with TKI or not in patients treated with PD-1 + L (a and b) or PD-1 (c and d).
TKIs, tyrosine Kinase Inhibitors.
Survival analysis stratified by MVT or EM
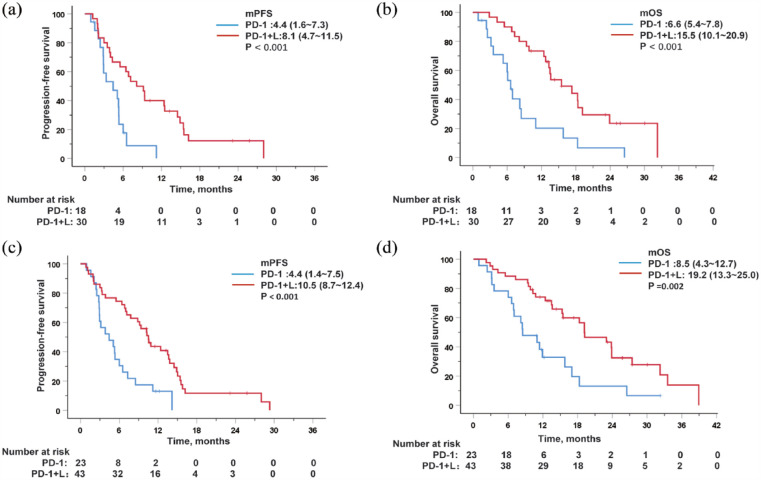
In the subgroup analysis of patients with MVT or EM, the results showed that the median PFS and OS of patients in the PD-1 + L group were significantly longer. Among the patients with MVT, the mOS in the PD-1 + L and PD-1 groups were 15.5 months (95% CI 10.1–20.9) and 6.6 months (95% CI 5.4–7.8), respectively (p < 0.001), and the mPFS was 8.1 months (95% CI 4.7–11.5) in the PD-1 + L group versus 4.4 months (95% CI 1.6–7.3) in the PD-1 group [p < 0.001; Figure 4(a) and (b)]. For patients with EM, a remarkable distinction in median PFS and OS was also observed between the two groups. The PD-1 + L group had a mPFS of 10.5 months (95% CI 8.7–12.4) compared to 4.4 months (95% CI 1.4–7.5) in the PD-1 group (p < 0.001), and a median OS of 19.2 months (95% CI 13.3–25.0) versus 8.5 months (95% CI 4.3–12.7) in the PD-1 group [p = 0.002; Figure 4(c) and (d)]. A trend toward a longer survival benefit was also observed in patients without MVT (Supplemental Figure S1) or EM (Supplemental Figure S2) treated with PD-1+L. These results suggest that combination therapy is superior to immune monotherapy in patients with MVT or EM.
Figure 4.
Kaplan–Meier curves for progression-free survival and overall survival after stratification by the presence of MVT (a and b) or EM (c and d).
EM, extrahepatic metastases; MVT, macrovascular tumor thrombosis.
Prognostic factors associated with OS and PFS
In the univariate analysis of the entire cohort, an ECOG score of 0–1 (HR 3.16, 95% CI 1.58–6.34, p = 0.001), combination therapy (HR 0.82, 95% CI 0.70–0.96, p = 0.015) and the absence of MVT (HR 1.67, 95% CI 1.02–2.73, p = 0.042) were the prognostic factors significantly associated with better OS (Table 3). After incorporating factors with p < 0.2 into the multivariate analysis, ECOG score of 0–1 (HR 2.71, 95% CI 1.33–5.53, p = 0.006), combination therapy (HR 0.81, 95% CI 0.68–0.96, p = 0.013) and MVT (HR 1.71, 95% CI 1.03–2.84, p = 0.038) were still significant independent predictors of better OS.
Table 3.
Univariate and multivariate analyses of factors associated with OS.
| Characteristics | Univariate analysis | p Value | Multivariate analysis | p Value | |||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| Age | ⩽56 versus >56 | 0.99 | 0.98–1.00 | 0.917 | |||
| Gender | Male versus female | 1.11 | 0.57–2.19 | 0.757 | |||
| ECOG | ⩽1 versus >1 | 3.16 | 1.58–6.34 | 0.001* | 2.71 | 1.33–5.53 | 0.006* |
| Lines | 1 versus ⩾2 | 1.35 | 0.82–2.23 | 0.241 | |||
| Therapy | PD-1 versus PD-1 + L | 0.82 | 0.70–0.96 | 0.015* | 0.81 | 0.68–0.96 | 0.013* |
| HBsAg | + versus − | 0.99 | 0.54–1.83 | 0.991 | |||
| EM | NO versus Yes | 0.87 | 0.53–1.48 | 0.645 | |||
| MVT | NO versus Yes | 1.67 | 1.02–2.73 | 0.042* | 1.71 | 1.03–2.84 | 0.038* |
| BCLC stage | B versus C–D | 1.59 | 0.75–3.36 | 0.224 | |||
| AFP ng/mL | <200 versus ⩾200 | 0.94 | 0.57–1.55 | 0.801 | |||
| Child–Pugh Class | A versus B | 1.44 | 0.85–2.42 | 0.174 | 1.5 | 0.87–2.56 | 0.143 |
| PD-1 inhibitors | Pembrolizumab | ||||||
| Nivolumab | 1.26 | 0.59–2.71 | 0.557 | ||||
| Others | 1.01 | 0.58–1.89 | 0.872 | ||||
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; EM, extrahepatic metastases; MVT, macrovascular tumor thrombosis; OS, overall survival.
p < 0.05, statistically significant.
The combination therapy was the only factor associated with PFS in the univariate analysis (HR 0.78, 95% CI 0.67–0.91, p = 0.002) (Table 4). After including prognostic factors with p < 0.2 in the multivariate analysis, the combination therapy still remained the only independent risk factor associated with better PFS (HR 0.80, 95% CI 0.68–0.94, p = 0.006).
Table 4.
Univariate and multivariate analyses of factors associated with PFS.
| Characteristics | Univariate analysis | p Value | Multivariate analysis | p Value | |||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| Age | ⩽56 versus > 56 | 0.99 | 0.97–1.02 | 0.509 | |||
| Gender | Male versus female | 0.92 | 0.52–1.64 | 0.782 | |||
| ECOG | ⩽1 versus >1 | 1.91 | 0.97–3.76 | 0.060 | 1.43 | 0.70–2.86 | 0.331 |
| Lines | 1 versus ⩾2 | 1.31 | 0.82–2.10 | 0.255 | |||
| Therapy | PD-1 versus PD-1 + L | 0.78 | 0.67–0.91 | 0.002* | 0.80 | 0.68–0.94 | 0.006* |
| HBsAg | + versus − | 1.07 | 0.61–1.88 | 0.822 | |||
| EM | NO versus Yes | 0.83 | 0.51–1.33 | 0.436 | |||
| MVT | NO versus Yes | 1.23 | 0.79–1.91 | 0.353 | |||
| BCLC stage | B versus C–D | 1.21 | 0.62–2.37 | 0.573 | |||
| AFP ng/mL | <200 versus ⩾200 | 0.67 | 0.43–1.05 | 0.213 | |||
| Child–Pugh Class | A versus B | 1.11 | 0.67–1.82 | 0.686 | |||
| PD-1 inhibitors | Pembrolizumab | ||||||
| Nivolumab | 1.39 | 0.69–2.78 | 0.355 | ||||
| Others | 1.11 | 0.62–2.00 | 0.732 | ||||
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; EM, extrahepatic metastases; MVT, macrovascular tumor thrombosis; PD, progressive disease; PFS, progression-free survival.
p < 0.05, statistically significant.
Safety profile
TRAEs were reported in 20 (51.3%) patients in the PD-1 group and 45 (81.8%) patients in the PD-1 + L group. The most common TRAEs in the PD-1 monotherapy group were thrombocytopenia (n = 10; 25.6%), fatigue (n = 6; 15.4%), and pruritus (n = 5; 12.8%). Grade 3 TRAEs occurred in 10 patients (25.6%). One patient (2.6%) discontinued treatment with PD-1 inhibitors because of immune-mediated pneumonitis.
Hypertension [3 (7.7%) versus 18 (32.7%), p = 0.005] and hand–foot skin reactions [0 versus 6 (10.9%), p = 0.04] occurred more frequently in the combination group. The incidence of ⩾grade 3 TRAEs was higher in the PD-1 + L group compared to the PD-1 group [32 (58.2%) versus 10 (25.6%), p = 0.003]. The most frequently occurring ⩾grade 3 TRAEs were hypertension (n = 7, 12.7%). Three patients discontinued therapy; one patient had immune-mediated hepatitis, and the other two had severe thrombocytopenia in the PD-1 + L group.
Liver dysfunction, including elevated aspartate aminotransferase and alanine aminotransferase levels, hyperbilirubinemia, and hypoalbuminemia, was reported in 3 (7.7%) patients in the PD-1 group and 11 (20.0%) patients in the PD-1 + L group. Serious liver dysfunction (grade 3 or higher) was observed in one (2.6%) patient in the PD-1 group and 3 (5.5%) patients in the PD-1 + L group (p = 0.639). Most abnormal liver function was mild to moderate, and liver function returned to normal in most patients after the administration of liver protection drugs. No serious TRAEs resulted in patient deaths. Additional details are shown in Table 5.
Table 5.
TRAEs in patients.
| TRAE (n, %) | All patients (N = 94) | PD-1 (N = 39) | PD-1 + L (N = 55) | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| Any grade | Grade ⩾ 3 | Any grade | Grade ⩾ 3 | Any grade | Grade ⩾ 3 | Any grade | Grade ⩾ 3 | |
| Fatigue | 15 (16.0%) | 4 (4.3%) | 6 (15.4%) | 2 (5.1%) | 9 (16.3%) | 2 (3.6%) | 0.898 | 1 |
| Loss of appetite | 11 (11.7%) | 0 | 3 (7.7%) | 0 | 8 (14.5%) | 0 | 0.352 | – |
| Diarrhea | 9 (9.6%) | 1 (1.1%) | 3 (7.7%) | 0 | 6 (10.9%) | 1 (1.8%) | 0.731 | 1 |
| Rash | 14 (14.9%) | 4 (4.3%) | 4 (10.3%) | 1 | 10 (18.1%) | 3 (5.5%) | 0.383 | 0.639 |
| Pruritus | 9 (9.6%) | 0 | 5 (12.8%) | 0 | 4 (7.3%) | 0 | 0.482 | – |
| Pneumonitis | 3 (3.2%) | 2 (2.1%) | 1 (2.6%) | 1 (2.6%) | 2 (3.6%) | 1 (1.8%) | 1 | 1 |
| Hypothyroidism | 4 (4.3%) | 0 | 1 (2.6%) | 0 | 3 (5.5%) | 0 | 0.639 | – |
| Proteinuria | 5 (5.3%) | 1 (1.1%) | 0 | 0 | 5 (9.1%) | 1 (1.8%) | 0.074 | – |
| Hypertension | 21 (22.3%) | 8 (8.5%) | 3 (7.7%) | 1 (2.6%) | 18 (32.7%) | 7 (12.7%) | 0.005* | 0.134 |
| Edema peripheral | 2 (2.1%) | 0 | 0 | 0 | 2 (3.6%) | 0 | 0.509 | – |
| Neutropenia | 18 (19.1%) | 7 (7.4%) | 4 (10.3%) | 1 (2.6%) | 14 (25.5%) | 6 (10.9%) | 0.109 | 0.233 |
| Thrombocytopenia | 31 (33.0%) | 9 (9.6%) | 10 (25.6%) | 3 (7.7%) | 21 (38.2%) | 6 (10.9%) | 0.203 | 0.731 |
| Liver damage | 14 (14.9%) | 4 (4.3%) | 3 (7.7%) | 1 (2.6%) | 11 (20.0%) | 3 (5.5%) | 0.143 | 0.639 |
| Hand–foot–skin reaction | 6 (6.4%) | 2 (2.1%) | 0 | 0 | 6 (10.9%) | 2 (3.6%) | 0.04* | 0.509 |
| Leading to discontinuation | 0 | 4 (4.3%) | 0 | 1 (2.6%) | 0 | 3 (5.5%) | – | 0.639 |
| Leading to death | 0 | 0 | 0 | 0 | 0 | 0 | – | – |
TRAEs, treatment-related adverse events.
p < 0.05, statistically significant.
Discussion
The findings suggested that the combination of PD-1 and lenvatinib strongly improved the ORR, mPFS, and mOS of patients compared to PD-1 monotherapy. The responses of the patients were consistent, and the toxicity profiles were manageable in both groups.
The treatment of aHCC remains challenging. Molecular-targeting drugs, such as sorafenib and lenvatinib, have several limitations, including drug resistance, toxicity or intolerability. Immunotherapy with PD-1/PD-L1 inhibitors, either as monotherapy with nivolumab and pembrolizumab or combination therapy, has become a mainstream treatment modality for aHCC.19,20 In real clinical experience, a certain number of patients still use immune monotherapy. However, studies about first-line immune monotherapy is limited. In the Checkmate 459 trial, the mOS of single-agent nivolumab in the first-line setting was 16.4 months, and it did not significantly improve OS compared to sorafenib (14.7 months). 21 Similarly, KEYNOTE-224 cohort 2 demonstrated monotherapy with pembrolizumab has an ORR of 16%, mPFS of 4 months, and mOS of 17 months. 22 Another small real-world retrospective study by Pramod et al. 23 reported on patients receiving immunotherapy as a first-line systemic treatment for HCC in the United States. However, their study enrolled only 14 patients, and the results showed an ORR of 14.3%, mPFS of 4 months, and mOS of 8 months, leading to an unsatisfactory conclusion. In our study, the mPFS and mOS of patients receiving first-line treatment in the PD-1 group were 4.4 months and 11.9 months, respectively. The real-life experience appears to be lower than data from clinical trials, possibly because most patients with a poor general status, such as Child–Pugh B, ECOG > 1, and BCLC-D, are generally excluded from clinical trials. Patients with autoimmune diseases (ADs) are also excluded from clinical trials and the use of ICIs in patients with ADs in clinical practice should account for the high-risk of disease flares or worsening, severe immune toxicity or diminished efficacy. Increasing studies have evaluated the safety and efficacy of ICIs in these patients, despite an elevated risk of immune-related AEs, the efficacy of immunotherapy is not affected.24 –26 It is necessary to clarify the pathophysiological mechanism underlying immune-related AEs, to find effective biomarkers to predict their occurrence, and to find suitable patients expected to benefit from immunotherapy.
Owing to the limited efficacy of monotherapy, combination trials of immunotherapies with different drugs, including atezolizumab plus bevacizumab, pembrolizumab plus lenvatinib and ipilimumab plus nivolumab are constantly being attempted. In the present study, the ORR, mPFS, and mOS of PD-1 plus lenvatinib were 32.7%, 10.6 months, and 18.4 months, respectively, indicating a marked antitumor benefit. In addition, the clinical benefits of PD-1 plus lenvatinib for systemic therapy-naive and TKI treatment-experienced patients with aHCC were similar. The efficacy of PD-1 monotherapy sharply decreased in TKI treatment-experienced aHCC compared to treatment-naïve aHCC. This finding indicates that PD-1 plus lenvatinib therapy may be preferable for selected patients who have previously received TKI therapy.
Previous clinical trials have excluded patients with high-risk factors. However, our analysis included patients with high-risk factors, including Child–Pugh class B, ECOG > 1, BCLC-D, and MVT or EM, and it showed that PD-1 plus lenvatinib still achieved good efficacy in these populations. In general, patients with MVT, which is common in patients with advanced HCC, have limited treatment options, a higher risk of recurrence, and a dismal prognosis, with OS of only 2–4 months with the best supportive care. 27 The real-life clinical benefits for these patients are moderate, 28 with improved mOS by approximately 2 months. Because patients with MVT are generally excluded from most clinical trials, data regarding immunotherapy in these patients remain limited.29,30 Our real-world experience for patients with MVT revealed that the mOS for PD-1 plus lenvatinib therapy was 15.5 months, providing a promising strategy for this sub-population. Further prospective randomized trials are urgently required to provide effective management strategies for patients with HCC and MVT.
The response of different organs to PD-1 combined with lenvatinib is specific in patients with aHCC,13,30 owing to the heterogeneity of the tumor immune microenvironment in different organs. Li-Chun et al. reported that the corresponding ORRs for liver, lung, lymph node, and other intra-abdominal metastases were 22.4%, 41.2%, 26.3%, and 38.9%, respectively. 31 In our study, in both the PD-1 and PD-1 + L groups, patients with EM tended to have better survival benefits than patients without EM. These findings suggest that extrahepatic lesions have a higher response rate to immunotherapy than intrahepatic lesions. Future long-term follow-up studies with larger numbers of patients are needed to shed more light on this aspect.
Lenvatinib is a multi-targeted small-molecule TKI with stronger activity against Vascular Endothelial Growth Factor Receptor (VEGFR)receptors and the Fibroblast Growth Factor Receptor (FGFR) family, making it is more likely to cause hypertension, proteinuria, and hand–foot skin reactions. 2 In this study, the incidence of TRAEs from combination therapy was higher than that from PD-1 monotherapy, especially concerning hypertension, hand–foot skin reactions, and TRAEs ⩾grade 3. However, the TRAEs were expected and manageable. The combination of lenvatinib and pembrolizumab has shown favorable antitumor activity against various solid cancers, including HCC, endometrial cancer,32,33 renal cancer,34,35 and adrenal cortical carcinoma. 36 While this combined strategy holds promise for bringing long-term survival benefits and new hope for patients with various solid tumors, more research is needed to thoroughly explore its potential. In China, many patients with HCC are economically disadvantaged and tend to choose domestic PD-1 agents over pembrolizumab or nivolumab. Therefore, we included multiple PD-1 inhibitors in combination with lenvatinib. Our findings suggest that lenvatinib combined with various PD-1 agents has similar antitumor effects as lenvatinib combined with pembrolizumab.
Our study had some limitations. First, this was a retrospective study conducted at a single center with a relatively small number of patients. Subgroup analyses were also performed with a small sample size, and the results should be interpreted with caution. Data were obtained from medical records and follow-up visits, accordingly, there may be some inaccuracies in the information, and patients lost to follow-up could not be included in the study, introducing certain biases and limitations. Second, as HBV-associated HCC is more prevalent in China, with 80.9% of the patients in this study having HBV-associated HCC, the results may not be representative of other HCC populations with alcohol consumption and Hepatitis C virus infection. Further prospective and randomized trials are needed to validate our findings. However, this retrospective study is the first to compare the clinical benefits and safety profiles of PD-1 agents alone and PD-1 plus lenvatinib for aHCC. Our findings provide real-world experience with PD-1 monotherapy compared to PD-1 agents plus lenvatinib for patients with aHCC with high-risk factors, regardless of prior exposure to TKI therapy. Both PD-1 monotherapy and combination therapy with lenvatinib could benefit selected patients with aHCC, with a notably higher efficacy for the combination therapy than for PD-1 monotherapy. The toxicity profiles and tolerability of the two therapeutic strategies were manageable. PD-1, in combination with lenvatinib, has remarkable advantages against aHCC and warrants further exploration to identify patients who can benefit appropriately.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359231206274 for Efficacy and safety of anti-PD-1 monotherapy versus anti-PD-1 antibodies plus lenvatinib in patients with advanced hepatocellular carcinoma: a real-world experience by Qingyan Liu, Rong Li, Lingling Li, Gaokun Wang, Shiyu Ji, Xuan Zheng, Xiaodong Jia, Haitao Tao and Yi Hu in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-2-tam-10.1177_17588359231206274 for Efficacy and safety of anti-PD-1 monotherapy versus anti-PD-1 antibodies plus lenvatinib in patients with advanced hepatocellular carcinoma: a real-world experience by Qingyan Liu, Rong Li, Lingling Li, Gaokun Wang, Shiyu Ji, Xuan Zheng, Xiaodong Jia, Haitao Tao and Yi Hu in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors would like to thank Editage (https://app.editage.cn/) for technical editing of the manuscript.
Footnotes
Availability of data and materials: Reasonable requests for datasets used in the analysis in the course of the current study are available from the authors.
ORCID iDs: Qingyan Liu  https://orcid.org/0009-0002-5551-4431
https://orcid.org/0009-0002-5551-4431
Rong Li  https://orcid.org/0009-0000-9591-7214
https://orcid.org/0009-0000-9591-7214
Lingling Li  https://orcid.org/0009-0004-9654-1692
https://orcid.org/0009-0004-9654-1692
Gaokun Wang  https://orcid.org/0009-0006-8174-5179
https://orcid.org/0009-0006-8174-5179
Shiyu Ji  https://orcid.org/0009-0005-5245-1115
https://orcid.org/0009-0005-5245-1115
Xuan Zheng  https://orcid.org/0000-0002-1145-7167
https://orcid.org/0000-0002-1145-7167
Xiaodong Jia  https://orcid.org/0000-0002-2145-6272
https://orcid.org/0000-0002-2145-6272
Haitao Tao  https://orcid.org/0009-0001-1295-8692
https://orcid.org/0009-0001-1295-8692
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Qingyan Liu, Department of Oncology, Fifth Medical Center of the Chinese People’s Liberation Army General Hospital, Beijing, ChinaMedical School of Chinese People’s Liberation Army, Beijing, China.
Rong Li, Department of Health Medicine, Second Medical Center of the Chinese People’s Liberation Army General Hospital, Beijing, China.
Lingling Li, Department of Oncology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Gaokun Wang, Changchun Second Retired Cadre Rest Center of Jilin Provincial Military Region, Changchun, Jilin.
Shiyu Ji, Department of Oncology, Fifth Medical Center of the Chinese People’s Liberation Army General Hospital, Beijing, China.
Xuan Zheng, Department of Oncology, Fifth Medical Center of the Chinese People’s Liberation Army General Hospital, Beijing, China.
Xiaodong Jia, Department of Oncology, Fifth Medical Center of the Chinese People’s Liberation Army General Hospital, 100 West Fourth Ring Road, Fengtai District, Beijing 100039, China.
Haitao Tao, Department of Oncology, Fifth Medical Center of the Chinese People’s Liberation Army General Hospital, 28 Fuxing Road, Haidian District, Beijing 100000, China.
Yi Hu, Department of Oncology, Fifth Medical Center of the Chinese People’s Liberation Army General Hospital, 28 Fuxing Road, Haidian District, Beijing 100000, China.
Declarations
Ethics approval and consent to participate: The study was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital (approval number: S2018-092-01). All patients signed an informed consent form before receiving treatment.
Consent for publication: Not applicable.
Author contributions: Qingyan Liu: Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft.
Rong Li: Data curation; Resources; Visualization.
Lingling Li: Formal analysis; Methodology; Writing – review & editing.
Gaokun Wang: Methodology; Writing – review & editing.
Shiyu Ji: Resources; Writing – review & editing.
Xuan Zheng: Methodology; Writing – review & editing.
Xiaodong Jia: Conceptualization; Visualization; Writing – review & editing.
Haitao Tao: Conceptualization; Data curation; Formal analysis; Methodology; Writing – review & editing.
Yi Hu: Conceptualization; Methodology; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and publication of this article.
The authors declare that there is no conflict of interest.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021; 7: 6. [DOI] [PubMed] [Google Scholar]
- 3. Spinzi G, Paggi S. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med 2008; 359: 2497–2498. [DOI] [PubMed] [Google Scholar]
- 4. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 5. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 6. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (checkmate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sangro B, Sarobe P, Hervás-Stubbs S, et al. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021; 18: 525–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med 2020; 382: 1894–1905. [DOI] [PubMed] [Google Scholar]
- 9. Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. JAMA Oncol 2020; 6: e204564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finn RS, Ikeda M, Zhu AX, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol 2020; 38: 2960–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol 2021; 22: 977–990. [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Jiang M, Zhu J, et al. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed Pharmacother 2020; 132: 110797. [DOI] [PubMed] [Google Scholar]
- 13. Huang C, Zhu XD, Shen YH, et al. Organ specific responses to first-line lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: a retrospective analysis. Biomark Res 2021; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mo DC, Luo PH, Huang SX, et al. Safety and efficacy of pembrolizumab plus lenvatinib versus pembrolizumab and lenvatinib monotherapies in cancers: a systematic review. Int Immunopharmacol 2021; 91: 107281. [DOI] [PubMed] [Google Scholar]
- 15. Wu CJ, Lee PC, Hung YW, et al. Lenvatinib plus pembrolizumab for systemic therapy-naïve and -experienced unresectable hepatocellular carcinoma. Cancer Immunol Immunother 2022; 71: 2631–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Q, Cao M, Yuan G, et al. Lenvatinib Plus camrelizumab vs. lenvatinib monotherapy as first-line treatment for unresectable hepatocellular carcinoma: a multicenter retrospective cohort study. Front Oncol 2022; 12: 809709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schulte N, Li M, Zhan T, et al. Response of advanced HCC to pembrolizumab and lenvatinib combination therapy despite monotherapy failure. Z Gastroenterol 2020; 58: 773–777. [DOI] [PubMed] [Google Scholar]
- 18. Huang X, Xu L, Ma T, et al. Lenvatinib plus immune checkpoint inhibitors improve survival in advanced hepatocellular carcinoma: a retrospective study. Front Oncol 2021; 11: 751159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol 2022; 19: 151–172. [DOI] [PubMed] [Google Scholar]
- 20. Martini G, Ciardiello D, Paragliola F, et al. How immunotherapy has changed the continuum of care in hepatocellular carcinoma. Cancers 2021; 13: 4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (checkmate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2022; 23: 77–90. [DOI] [PubMed] [Google Scholar]
- 22. Verset G, Borbath I, Karwal M, et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: data from the Open-Label, Phase II KEYNOTE-224 trial. Clin Cancer Res 2022; 28: 2547–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaudel P, Mohyuddin GR, Fields-Meehan J. Nivolumab use for first-line management of hepatocellular carcinoma: results of a real-world cohort of patients. Fed Pract 2021; 38: 89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tison A, Garaud S, Chiche L, et al. Immune-checkpoint inhibitor use in patients with cancer and pre-existing autoimmune diseases. Nat Rev Rheumatol 2022; 18: 641–656. [DOI] [PubMed] [Google Scholar]
- 25. Meserve J, Facciorusso A, Holmer AK, et al. Safety and tolerability of immune checkpoint inhibitors in patients with pre-existing inflammatory bowel diseases: a systematic review and meta-analysis. Aliment Pharmacol Ther 2020; 53: 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cai Q, Huo GW, Zhu FY, et al. Safety and efficacy of immune checkpoint inhibitors in advanced cancer patients with autoimmune disease: a meta-analysis. Hum Vaccin Immunother 2022; 18: 2145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu PH, Huo TI, Miksad RA, et al. Hepatocellular carcinoma with portal vein tumor involvement: best management strategies. Semin Liver Dis 2018; 38: 242–251. [DOI] [PubMed] [Google Scholar]
- 28. Cheng AL, Guan Z, Chen Z, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III sorafenib Asia-Pacific trial. Eur J Cancer Care 2012; 48: 1452–1465. [DOI] [PubMed] [Google Scholar]
- 29. Tsai HM, Han MZ, Lin YJ, et al. Real-world outcome of immune checkpoint inhibitors for advanced hepatocellular carcinoma with macrovascular tumor thrombosis. Cancer Immunol Immunother 2021; 70: 1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuo HY, Chiang NJ, Chuang CH, et al. Impact of immune checkpoint inhibitors with or without a combination of tyrosine kinase inhibitors on organ-specific efficacy and macrovascular invasion in advanced hepatocellular carcinoma. Oncol Res Treat 2020; 43: 211–220. [DOI] [PubMed] [Google Scholar]
- 31. Lu LC, Hsu C, Shao YY, et al. Differential organ-specific tumor response to immune checkpoint inhibitors in hepatocellular carcinoma. Liver Cancer 2019; 8: 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 2022; 386: 437–448. [DOI] [PubMed] [Google Scholar]
- 33. Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol 2020; 38: 2981–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee CH, Shah AY, Rasco D, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol 2021; 22: 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021; 384: 1289–1300. [DOI] [PubMed] [Google Scholar]
- 36. Bedrose S, Miller KC, Altameemi L, et al. Combined lenvatinib and pembrolizumab as salvage therapy in advanced adrenal cortical carcinoma. J Immunother Cancer 2020; 8: e001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359231206274 for Efficacy and safety of anti-PD-1 monotherapy versus anti-PD-1 antibodies plus lenvatinib in patients with advanced hepatocellular carcinoma: a real-world experience by Qingyan Liu, Rong Li, Lingling Li, Gaokun Wang, Shiyu Ji, Xuan Zheng, Xiaodong Jia, Haitao Tao and Yi Hu in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-2-tam-10.1177_17588359231206274 for Efficacy and safety of anti-PD-1 monotherapy versus anti-PD-1 antibodies plus lenvatinib in patients with advanced hepatocellular carcinoma: a real-world experience by Qingyan Liu, Rong Li, Lingling Li, Gaokun Wang, Shiyu Ji, Xuan Zheng, Xiaodong Jia, Haitao Tao and Yi Hu in Therapeutic Advances in Medical Oncology