Dear Editor,
Understanding the evolution and survival mechanisms of endangered wild medicinal herbs is crucial for their cultivation, utilization, and conservation. The snow lotus species Saussurea involucrata (Kar. & Kir.) Sch. Bip. (2n = 32) (i.e. the well-known Tianshan snow lotus) which belongs to the eudicot family Asteraceae, is a famous traditional Chinese medicinal herb having anti-inflammatory, antioxidant, and anti-cancer effects; the major bioactive components that exhibit clinical functions in this plant are acacetin, hispidulin, and rutin [1]. S. involucrata grows in rock fissures (Fig. 1A) with elevations of 2400–4100 m in the Tianshan and Altai Mountains, surviving in harsh alpine environments characterized by low temperatures and strong ultraviolet radiation. The growth rate is slow, taking 6–8 years for S. involucrata to go from seed germination to flowering. Due to the distinct habitat, the resources of S. involucrata are rather rare and the species has fallen into endangered status in China due to over-collecting [2].
Figure 1.
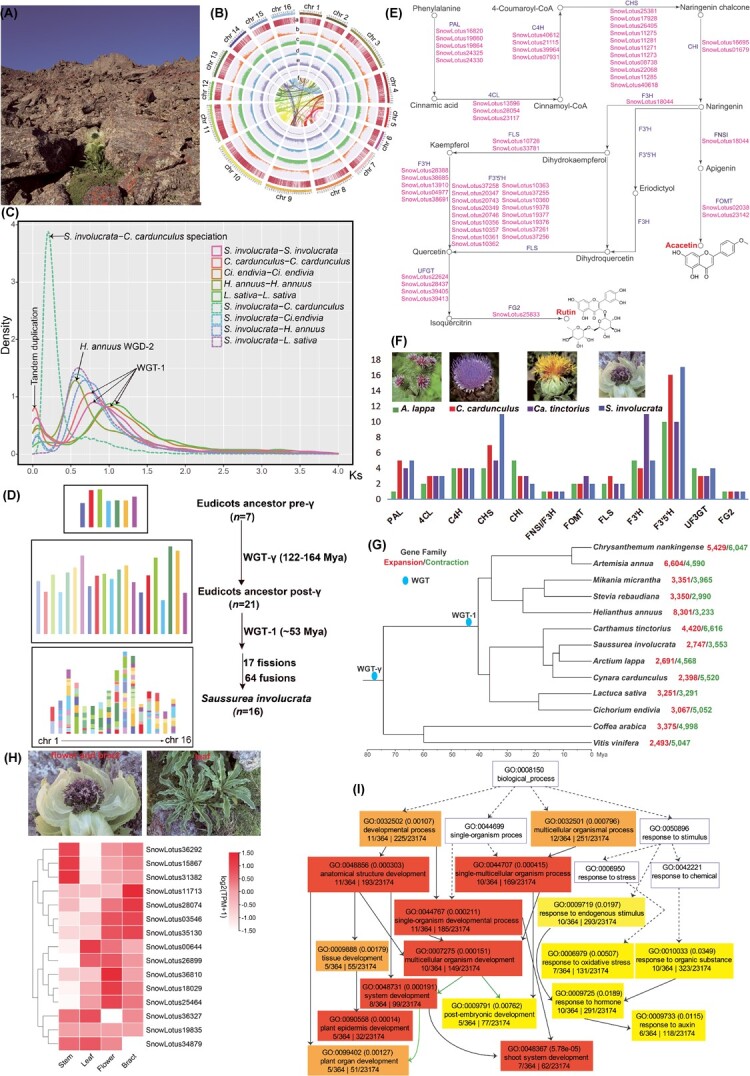
Assembly, annotation, and analyses of the Saussurea involucrata genome. All S. involucrata photographs are taken by Dr. Bing Liu from the Institute of Botany, Chinese Academy of Sciences. (A) Photographs showing habitat of S. involucrata. (B) Genomic landscape of S. involucrata. (f) GC content, (e) Gypsy LTR density, (d) Copia LTR density, (c) total LTR density, (b) gene density, and (a) gene expression in leaf. (C) Distribution of synonymous substitution (Ks) of S. involucrata paralogues, Cynara cardunculus paralogues, Cichorium endivia paralogues, Helianthus annuus paralogues, Lactuca sativa paralogues, S. involucrata-C. cardunculus orthologues, S. involucrata-Ci. endivia orthologues, S. involucrata-H. annuus orthologues, and S. involucrata-L. sativa orthologues. (D) Evolutionary scenario of S. involucrata from the ancestral eudicot karyotype. (E) Predicted candidate genes involved in acacetin and rutin biosynthesis in S. involucrata. (F) Comparison of gene numbers in gene families involved in acacetin and rutin biosynthesis between S. involucrata and three other Asteraceae species. The images of C. cardunculus, Arctium lappa, and Carthamus tinctorius are from Plant Photo Bank of China (PPBC). (G) Phylogenetic analysis among 13 eudicot species, including S. involucrata and 10 other Asteraceae species, with information of expansion/contraction of gene families. (H) Expression profiles of identified genes related to cold resistance in S. involucrata across four tissues: stem, leaf, flower, and bract. (I) Enriched GO terms (highlighted with colors) of genes specifically expressed in S. involucrata bracts.
In the current study, we developed a chromosome-scale genome assembly for S. involucrata (accession BGD2108) by combing PacBio HiFi sequencing and High-throughput chromosome conformation capture (Hi-C) technology. Using HiFiasm [3], we obtained 338 high quality contigs (contig N50 = 90 Mb) with a total assembly size of 2452 Mb (Table 1). In total, we placed 94.7% of the contigs on 16 pseudochromosomes (Table 1; Fig. 1B). The completeness of the S. involucrata genome assembly evaluated by Benchmarking Universal Single Copy Orthologs (BUSCO) [4] showed 98.7% complete matches in the Embryophyta version 10 dataset (Table 1). The S. involucrata genome assembly assessed by Merqury [5] revealed a consensus quality value (QV) of 51.08 (corresponding to a base accuracy of 99.999%) and a completeness rate of 97.23%. Collectively, the above results indicate that the S. involucrata genome assembly is of high quality.
Table 1. Statistics of the Saussurea involucrata genome assembly and annotation.
| Saussurea involucrata | |
|---|---|
| Assembly | |
| Number of contigs | 338 |
| Contig N50 (Mb) | 90 |
| Genome length (Mb) | 2452 |
| Anchor rate (%) | 94.7 |
| BUSCO completeness (%) | 98.7 |
| QV by Mercury | 51.08 |
| Completeness rate by Mercury (%) | 97.23 |
| GC content (%) | 38.58 |
| Annotation | |
| Repetitive sequences (%) | 82.77 |
| Predicted gene models | 44 486 |
| Total functionally annotated | 42 580 |
| Mean exon length (bp) | 275.46 |
| Number of annotated mRNAs | 44 486 |
| Mean mRNA length (bp) | 3387.64 |
The S. involucrata genome assembly contains 82.77% repetitive sequences (2030 Mb), of which long terminal repeats (LTRs) accounted for the largest percentage (40.43%) (Table S1, see online supplementary material). We predicted a total of 44 486 protein-coding genes in the S. involucrata genome assembly, among which 42 580 (95.7%) were functionally annotated (Table 1; Table S2, see online supplementary material).
To infer the evolutionary history of S. involucrata, we performed a genomic comparison of S. involucrata with Cynara cardunculus (artichoke), Cichorium endivia (curly endive), Lactuca sativa (lettuce), and Helianthus annuus (sunflower) as representatives of Asteraceae. The ancestral eudicot karyotype (AEK) consists of 7 (pre-γ AEK) or 21 (post-γ AEK) protochromosomes (γ, i.e. WGT-->-γ, indicates the ancestral whole-genome triplication of the Eudicots) [6, 7]. The distribution of synonymous substitutions per synonymous site (Ks) between collinear gene pairs revealed that S. involucrata only experienced a whole-genome triplication (WGT-1, ~53 mya) event after the WGT-γ event (Fig. 1C and D), which is the same as found in artichoke and lettuce [6, 8]. The syntenic pattern between genomic regions in artichoke and S. involucrata (Figs S1 and S2, see online supplementary material) suggested that at least 64 chromosome fusions and 17 chromosome fissions were necessary for S. involucrata to reach the modern structure of 16 chromosomes (Fig. 1D).
Acacetin and rutin are two predominant bioactive constituents found within S. involucrata [1]. By comparing with homologous genes in Arabidopsis thaliana, we predicted S. involucrata genes encoding each of 13 enzymes in the acacetin and rutin biosynthetic pathway (Fig. 1E): phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxylase (C4H), 4-coumarate CoA ligase (4CL), chalcone synthase (CHS), chalcone isomerase (CHI), flavanone-3-hydroxylase (F3H), flavanone-3′-hydroxylase (F3’H), flavanone-3′,5′-hydroxylase (F3’5’H), flavonol synthase (FLS), flavonol 3-O-glucosyltransferase (UF3GT), flavonol-3-O-glucoside L-rhamnosyltransferase (FG2), flavone synthase I (FNSI), and flavonoid O-methyltransferase (FOMT). Overall, we identified 57 candidate genes involved in acacetin and rutin biosynthesis in S. involucrata (Fig. 1E). Compared with C. cardunculus, Arctium lappa and Carthamus tinctorius, the number of CHS homologs in S. involucrata was increased significantly (Fig. 1F).
The phylogenetic tree using 280 single-copy orthologous genes constructed for S. involucrata and ten other Asteraceae species with Vitis vinifera and Coffea arabica as outgroups revealed the 11 Asteraceae species were clustered into four clades and S. involucrata was close to A. lappa (Fig. 1G). A total of 38 086 gene families were shared by above 13 species. The number of contracted and expanded gene families in S. involucrata were 3553 and 2747, respectively (Fig. 1G). Gene ontology analyses showed that the gene families expanded in S. involucrata are enriched in genes related to DNA integration, recombination, replication, and repair (Fig. S3, see online supplementary material) which are fundamental molecular mechanisms and reveal a potential survival strategy of S. involucrata under severe abiotic stress conditions. Additionally, we identified 539 gene families specific to S. involucrata and found these gene families showed enrichment for genes regulating activity of cysteine-type peptidases (Fig. S4, see online supplementary material) which play significant roles in defense responses against environmental stresses including cold and oxidative stress [9].
S. involucrata is known to have great tolerance to cold stress. By conducting comparative analysis with reported genes functionally related to cold resistance, we identified 15 homologous genes in S. involucrata from protein families, including transcription factor ICE1, calcium-dependent protein kinase 1, dehydrin, stearoyl-acyl-carrier-protein desaturase, fructose-bisphosphate aldolase, late embryogenesis abundant protein, and cold-regulated 413 plasma membrane protein (Table S3, see online supplementary material). We evaluated the expression profiles of these cold stress-resistance genes across four S. involucrata tissues (i.e. stem, leaf, flower, and bract) and found most are highly expressed in flowers and bracts (Fig. 1H).
One striking character of S. involucrata is the inflorescences surrounded by well-developed membranous bracts (Fig. 1H). Based on the transcripts per million (TPM) values of the Illumina sequencing data, gene expression levels in S. involucrata stem, leaf, flower, and bract were evaluated. Finally, we identified 364 genes uniquely expressed in the bracts of S. involucrata. These specifically expressed genes are enriched for genes associated with tissue development, and response to auxin and oxidative stress (Fig. 1I), indicating the bracts may play a critical role for S. involucrata to survive under oxidative stress induced by strong ultraviolet light.
In summary, we present a high-quality assembly of S. involucrata genome, which will be a great resource for the study of this famous traditional Chinese medicinal herb. The results in the present study lay the foundation for future research on the genes related to acacetin and rutin biosynthesis in S. involucrata and provide new insights into understanding the genome evolution and molecular mechanisms underlying abiotic stress tolerance of this species.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (U2003122) and the Second Tibetan Plateau Scientific Expedition and Research (STEP) program (2019QZKK0502). Sequencing service was provided by Bioyi Biotechnology Co., Ltd. Wuhan, China
Author contributions
H.W., H.S., and Y.S. developed the idea and designed the experiment; W.S. collected the plant materials; Y.S., A.Z., and X.Z. performed the statistical analyses; Y.S. and J.B.L. interpreted the results and wrote the manuscript. All authors read, edited, and approved the final manuscript.
Data availability
The raw DNA sequencing reads and the assembled genome of Saussurea involucrata have been submitted to NCBI. The BioProject ID is PRJNA991078, the BioSample ID is SAMN36288184.
Conflict of interest statement
The authors declare no conflicts of interest.
Supplementary data
Supplementary data is available at Horticulture Research online.
Supplementary Material
Contributor Information
Yanxia Sun, CAS Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan 430074, Hubei, China; Center of Conservation Biology, Core Botanical Gardens, Chinese Academy of Sciences, Wuhan 430074, Hubei, China.
Aidi Zhang, CAS Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan 430074, Hubei, China; Center of Economic Botany, Core Botanical Gardens, Chinese Academy of Sciences, Wuhan 430074, Hubei, China.
Jacob B Landis, BTI Computational Biology Center, Boyce Thompson Institute, Ithaca, NY 14853, USA; School of Integrative Plant Science, Section of Plant Biology and the L.H. Bailey Hortorium, Cornell University, Ithaca, NY 14853, USA.
Wei Shi, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Urumchi 830011, Xinjiang, China.
Xiujun Zhang, CAS Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan 430074, Hubei, China; Center of Economic Botany, Core Botanical Gardens, Chinese Academy of Sciences, Wuhan 430074, Hubei, China.
Hang Sun, Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China.
Hengchang Wang, CAS Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan 430074, Hubei, China; Center of Conservation Biology, Core Botanical Gardens, Chinese Academy of Sciences, Wuhan 430074, Hubei, China.
References
- 1. Gong G, Huang J, Yang Yet al. Saussureae involucratae herba (snow lotus): review of chemical compositions and pharmacological properties. Front Pharmacol. 2020;10:1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mu J, Fu Y, Liu Bet al. SiFBA5, a cold-responsive factor from Saussurea involucrata promotes cold resilience and biomass increase in transgenic tomato plants under cold stress. BMC Plant Biol. 2021;21:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng H, Jarvis ED, Fedrigo Oet al. Haplotype-resolved assembly of diploid genomes without parental data. Nat Biotechnol. 2022;40:1332–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simão FA, Waterhouse RM, Ioannidis Pet al. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–2 [DOI] [PubMed] [Google Scholar]
- 5. Rhie A, Walenz BP, Koren Set al. Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020;21:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Badouin H, Gouzy J, Grassa CJet al. The sunflower genome provides insights into oil metabolism, flowering and Asterid evolution. Nature. 2017;546:148–52 [DOI] [PubMed] [Google Scholar]
- 7. Salse J. Ancestors of modern plant crops. Curr Opin Plant Biol. 2016;30:134–42 [DOI] [PubMed] [Google Scholar]
- 8. Liu B, Yan J, Li Wet al. Mikania micrantha genome provides insights into the molecular mechanism of rapid growth. Nat Commun. 2020;11:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fagundes D, Bohn B, Cabreira Cet al. Caspases in plants: metacaspase gene family in plant stress responses. Funct Integr Genomics. 2015;15:639–49 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw DNA sequencing reads and the assembled genome of Saussurea involucrata have been submitted to NCBI. The BioProject ID is PRJNA991078, the BioSample ID is SAMN36288184.


