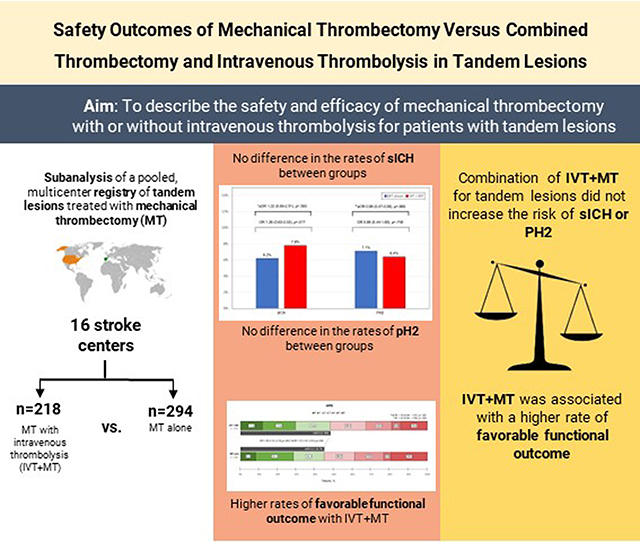
Abstract
BACKGROUND:
We aimed to describe the safety and efficacy of mechanical thrombectomy (MT) with or without intravenous thrombolysis (IVT) for patients with tandem lesions and whether using intraprocedural antiplatelet therapy influences MT’s safety with IVT treatment.
METHODS:
This is a subanalysis of a pooled, multicenter cohort of patients with acute anterior circulation tandem lesions treated with MT from 16 stroke centers between January 2015 and December 2020. Primary outcomes included symptomatic intracranial hemorrhage (sICH) and parenchymal hematoma type 2. Additional outcomes included hemorrhagic transformation, successful reperfusion (modified Thrombolysis in Cerebral Infarction score 2b-3), complete reperfusion (modified Thrombolysis in Cerebral Infarction score 3), favorable functional outcome (90-day modified Rankin Scale score 0–2), excellent functional outcome (90-day modified Rankin Scale score 0–1), in-hospital mortality, and 90-day mortality.
RESULTS:
Of 691 patients, 512 were included (218 underwent IVT+MT and 294 MT alone). There was no difference in the risk of sICH (adjusted odds ratio [aOR], 1.22 [95% CI, 0.60–2.51]; P=0.583), parenchymal hematoma type 2 (aOR, 0.99 [95% CI, 0.47–2.08]; P=0.985), and hemorrhagic transformation (aOR, 0.95 [95% CI, 0.62–1.46]; P=0.817) between the IVT+MT and MT alone groups after adjusting for confounders. Administration of IVT was associated with an increased risk of sICH in patients who received intravenous antiplatelet therapy (aOR, 3.04 [95% CI, 0.99–9.37]; P=0.05). The IVT+MT group had higher odds of a 90-day modified Rankin Scale score 0 to 2 (aOR, 1.72 [95% CI, 1.01–2.91]; P=0.04). The odds of successful reperfusion, complete reperfusion, 90-day modified Rankin Scale score 0 to 1, in-hospital mortality, or 90-day mortality did not differ between the IVT+MT versus MT alone groups.
CONCLUSIONS:
Our study showed that the combination of IVT with MT for tandem lesions did not increase the overall risk of sICH, parenchymal hematoma type 2, or overall hemorrhagic transformation independently of the cervical revascularization technique used. However, intraprocedural intravenous antiplatelet therapy during acute stent implantation might be associated with an increased risk of sICH in patients who received IVT before MT. Importantly, IVT+MT treatment was associated with a higher rate of favorable functional outcomes at 90 days.
Keywords: intracranial hemorrhage, reperfusion, stroke, thrombectomy
Graphical Abstract
Tandem lesions (TLs) account for about 10% to 20% of large vessel occlusion (LVO) strokes.1–5 Mechanical thrombectomy (MT) has demonstrated a safe and effective profile for treating TLs.6–9 However, the optimal acute cervical endovascular approaches to optimize outcomes, including angioplasty alone versus angioplasty and stenting, are currently under investigation.6,10,11 Furthermore, the role of intravenous thrombolysis (IVT) in combination with different treatment options is incompletely defined.
In an international survey performed by our group for treating TLs, the use of IVT+MT was controversial.12 Antiplatelet therapy (APT) during endovascular treatment for TLs may be safe and associated with lower mortality.13 However, when coadministered with IVT in patients undergoing acute carotid stenting, there was significant concern regarding the increased risk of hemorrhagic complications.14 Furthermore, TLs are considered a predictor of poor reperfusion after IVT alone due to underlying atherosclerosis that may impede successful reperfusion.15,16
In acute LVO stroke, most of the current randomized clinical trials failed to prove the noninferiority of MT alone versus IVT+MT.1,3–5,17,18 In addition, recent meta-analyses have suggested a beneficial effect of IVT+MT over MT alone.19,20 That said, the most recent guidelines from the European Stroke Organization-European Society for Minimally Invasive Neurological Therapy and Society of Vascular and Interventional Neurology still recommend that patients should receive IVT in addition to MT if eligible.21,22 In TLs, recent pooled analyses including the TITAN (Thrombectomy in Tandem Lesions) and Endovascular Treatment in Ischemic Stroke registries suggested that IVT before MT may increase the odds of favorable functional outcome without increasing the risk of hemorrhagic complications.23 Moreover, results from the German Stroke Registry-Endovascular Treatment study showed that the use of IVT in TL was an independent predictor of successful reperfusion (modified Thrombolysis in Cerebral Infarction score of 2b-3) in patients treated with MT.9
Hence, in this study, we sought to evaluate the safety and efficacy of MT with or without IVT for patients presenting with acute TLs. In addition, we assessed whether the use of intraprocedural APT was associated with a greater risk of clinically relevant intracranial hemorrhage when added to IVT.
METHODS
Study Population
We used a pooled, multicenter cohort registry for the study. Patient eligibility and methods of collaboration have been reported previously.24 Briefly, the study included adult patients with TL treated with MT within 24 hours after stroke onset from 16 stroke centers (15 hospitals in the United States and 1 in Spain) between January 2015 and December 2020. TL was defined as an intracranial LVO (petrous, sigmoid, or terminus segment of the internal carotid artery (ICA) or M1 or proximal M2 segment of the middle cerebral artery) with concomitant extracranial ICA stenosis ≥70% according to NASCET (North American Symptomatic Carotid Endarterectomy Trial).25 Patients were divided into 2 groups: (1) IVT+MT group (patients treated with IVT before MT) and (2) MT alone group (patients who did not receive IVT before MT). Treatment with IVT was determined at the discretion of the treating clinician. All intracranial occlusions were treated using a stent-retriever and contact aspiration catheters. The endovascular and medical therapeutic interventions were performed according to the protocol of each institution under conscious sedation or general anesthesia and at the discretion of the neurointerventionalists. The study was approved under the waiver of informed consent by the local institutional review board at each participating center. This study follows the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines (Supplemental Material).26 Data will be available upon reasonable request.
We classified the intraprocedural APTs into 4 categories depending on the intraprocedural APT regimen used during MT: no intraprocedural APTs, single oral APTs (aspirin, clopidogrel, or ticagrelor), dual oral APT (aspirin+clopidogrel or aspirin+ticagrelor), or intravenous APT (IV-APT) with or without oral APT (GP IIb/IIIa [glycoprotein IIb/IIIa] inhibitor alone; IIb/IIIa and single oral; IIb/IIIa and dual oral; cangrelor and single oral; cangrelor and dual oral).
Outcome Measures
The primary outcomes of the study were symptomatic intracranial hemorrhage (sICH) and parenchymal hematoma type 2 (PH2). sICH was defined by the ECASS-3 (European Collaborative Acute Stroke Study) criteria as any type of intracranial hemorrhage on follow-up imaging between 22 and 36 hours and 7 days after stroke onset and an increase of ≥4 points on the National Institutes of Health Stroke Scale from baseline or the lowest value within 7 days, or mortality.27 The clinical evaluation was done by the stroke team at each site. We also assessed the rate of ischemic infarct hemorrhagic transformation (HT) defined as no hemorrhage, petechial hemorrhage (hemorrhagic infarction type 1 [H1] and type 2 [H2]), and parenchymal hematoma (parenchymal hematoma type 1 [PH1] and type 2 [PH2]), according to the Heidelberg Bleeding Classification.28
The secondary outcomes included successful (modified Thrombolysis in Cerebral Infarction 2b-3) or complete reperfusion (modified Thrombolysis in Cerebral Infarction 3), favorable functional outcome at discharge (discharge modified Rankin Scale score 0–2), favorable functional outcome at 90 days (90-day modified Rankin Scale score 0–2), excellent functional outcome at 90 days (90-day modified Rankin Scale score 0–1), in-hospital mortality, and mortality at 90 days.
Statistical Analysis
Descriptive statistics were used to summarize continuous and categorical variables. We reported counts and percentages for categorical variables and means (SD) or medians (interquartile range [IQR]) for continuous variables. Shapiro-Wilk test and histograms were used to assess the normality of distributions. For the univariable analysis, we used the Student t tests or Wilcoxon rank-sum test for continuous variables and χ2 or Fisher exact test for categorical variables, as needed.
To evaluate the safety and efficacy outcomes between the 2 patient groups, we performed multivariable logistic regression. Details on the model selection, candidate variables, interaction terms, and subgroup analyses are presented in the Supplemental Methods. The odds ratios (ORs) and 95% CIs for the effect size of each group were computed. We also performed sensitivity analysis by time window within 0 to 6 hours from last known well (LKW)-to-arterial puncture (early window) for all the safety and efficacy outcomes and by type of admission on primary admission patients for favorable functional outcomes. The 6 hours cutoff point was selected with the goal to include a larger proportion of eligible patients for IVT according to the current guidelines.29,30
All the statistical analyses were considered significant at a 2-sided alpha level of ≤0.05. We used R statistical package (version 4.1.3, R Foundation for Statistical Computing, Vienna, Austria) for the analysis. Data will be made available upon reasonable request from the corresponding author.
RESULTS
Of the 691 patients from the registry, 179 were excluded (Figure 1). Of the 512 patients included, 218 were in the IVT+MT group, and 294 were in the MT alone group. The demographic data and baseline characteristics of the 2 groups are presented in the Table. Patients in the IVT+MT group had a lower rate of hypertension (67.9% versus 77.4%; P=0.016) and history of antiplatelet medications (28.7% versus 37.6%; P=0.037) than those in the MT alone group. Furthermore, patients in the IVT+MT group had a higher median admission National Institutes of Health Stroke Scale (17 versus 15; P=0.034), a higher median Alberta Stroke Program Early Computed Tomography Score (9 versus 8; P=0.02), a lower median number of MT passes (1 versus 2; P=0.01), higher rate of first-pass effect (67.4% versus 55.5%; P=0.007), a lower median time from LKW-to-reperfusion (305 versus 662; P<0.001), and a lower median time from LKW-to-arterial puncture (245 versus 603 minutes; P<0.001) than those in the MT alone group. In addition, 323 (63.7%) patients underwent ICA stenting, 192 (65.8%) from the MT alone group, and 131 (60.9%) from the IVT+MT group (P=0.264). Finally, 259 (50.6%) patients were treated within the early window, with a higher proportion of patients treated in the early window receiving IVT (78.9% versus 29.6%; P<0.001).
Figure 1. Flow diagram of patients included in the study.
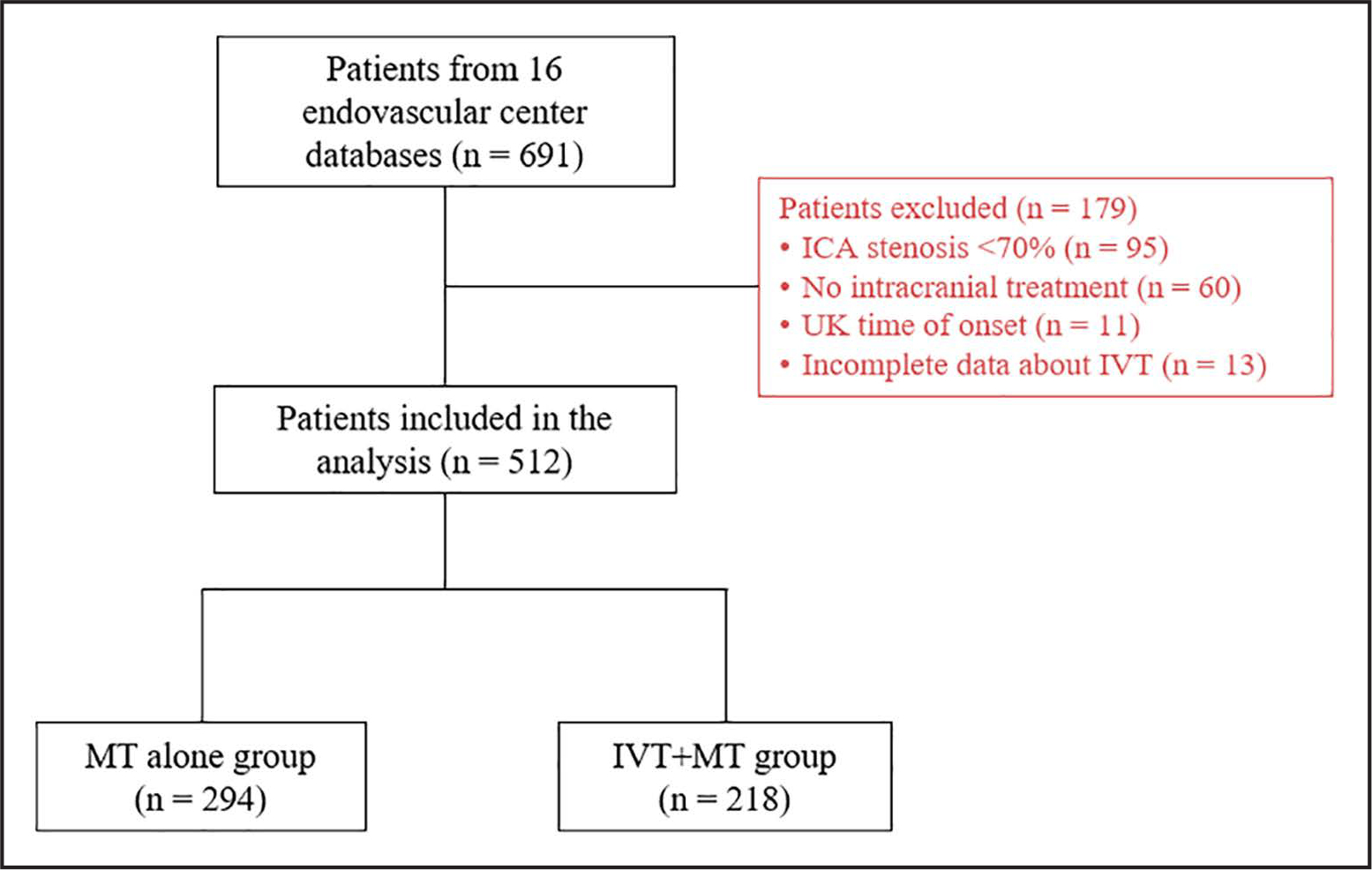
ICA indicates internal carotid artery; IVT, intravenous thrombolysis; LKW, last known well; MT, mechanical thrombectomy; and UK, unknown.
Table.
Baseline and Treatment Characteristics of Patients With Endovascularly Treated Tandem Lesions According to the Use of Intravenous Thrombolysis
| Total (N=512) | MT alone (N=294) | IVT+MT (N=218) | P value | |
|---|---|---|---|---|
| Age, y; median (IQR) | 68 (59–76) | 68 (61–75.7) | 66 (58–75.8) | 0.113 |
| Females, N (%) | 154 (30.1) | 91 (31) | 63 (28.9) | 0.616 |
| White race, N (%) | 322 (63.4) | 186 (64.1) | 136 (62.4) | 0.685 |
| Medical history, N (%) | ||||
| Hypertension | 374 (73.3) | 226 (77.4) | 148 (67.9) | 0.016* |
| Hyperlipidemia | 229 (45.1) | 138 (47.4) | 91 (41.9) | 0.219 |
| Diabetes | 137 (26.9) | 82 (28.1) | 55 (25.3) | 0.491 |
| Atrial fibrillation | 63 (12.4) | 43 (14.7) | 20 (9.2) | 0.062 |
| Current smoker | 118 (23.3) | 69 (24) | 49 (22.5) | 0.891 |
| Previous stroke/TIA | 77 (15.1) | 49 (16.8) | 28 (12.9) | 0.227 |
| Coronary artery disease | 96 (18.8) | 60 (20.5) | 36 (16.5) | 0.257 |
| Prior antiplatelets, N (%) | 171 (33.8) | 109 (37.6) | 62 (28.7) | 0.037* |
| Transfer from outside institution, N (%) | 279 (55.7) | 156 (54.7) | 123 (56.9) | 0.622 |
| Admission glucose (mg/dL), median (IQR) | 125 (107–154) | 125 (107–153) | 125 (107–154) | 0.691 |
| Baseline mRS, N (%) | 0.708 | |||
| 0 | 401 (80) | 230 (79.6) | 171 (80.7) | |
| 1 | 39 (7.8) | 21 (7.3) | 18 (8.5) | |
| 2 | 35 (7) | 21 (7.3) | 14 (6.6) | |
| 3 | 20 (4) | 14 (4.8) | 6 (2.8) | |
| 4 | 5 (1) | 2 (0.7) | 3 (1.4) | |
| 5 | 1 (0.2) | 1 (0.3) | 0 (0) | |
| Admission NIHSS, median (IQR) | 16 (11–20) | 15 (10–20) | 17 (12–20) | 0.034* |
| ASPECTS, median (IQR) | 8 (7–9) | 8 (7–9) | 9 (7–10) | 0.002* |
| Cervical ICA lesion cause, N (%) | 0.201 | |||
| Atherosclerosis | 402 (78.8) | 236 (80.8) | 166 (76.1) | |
| Dissection | 52 (10.2) | 28 (9.5) | 24 (11) | |
| Other† | 56 (10.9) | 28 (9.5) | 28 (12.8) | |
| Intracranial occlusion location, N (%) | 0.063 | |||
| ICA | 87 (27.2) | 58 (30.9) | 29 (22) | |
| M1 | 187 (58.4) | 109 (58) | 78 (59.1) | |
| M2 | 46 (14.4) | 21 (11.2) | 25 (18.9) | |
| Cervical ICA complete occlusion, N (%) | 280 (54.7) | 167 (56.8) | 113 (51.8) | 0.264 |
| General anesthesia, N (%) | 203 (39.6) | 118 (40.1) | 85 (39) | 0.793 |
| First-line technique, N (%) | 0.379 | |||
| Combination | 267 (53.3) | 149 (51.7) | 118 (55.4) | |
| Aspiration device | 150 (29.9) | 85 (29.5) | 65 (30.5) | |
| Stent-retriever | 84 (16.8) | 54 (18.8) | 30 (14.1) | |
| IA tPA, N (%) | 18 (3.6) | 10 (3.5) | 8 (3.8) | 0.884 |
| Number of passes, median (IQR) | 2 (1–3) | 2 (1–3) | 1 (1–2.5)) | 0.001* |
| First-pass effect, N (%) | 301 (60.7) | 156 (55.5) | 145 (67.4) | 0.007*, |
| ICA stenting, N (%) | 323 (63.7) | 192 (65.8) | 131 (60.9) | 0.264 |
| Intraprocedural heparin, N (%) | 183 (35.7) | 104 (35.4) | 79 (36.2) | 0.840 |
| Intraprocedural antiplatelets. N (%) | 0.152 | |||
| Dual oral | 148 (28.9) | 87 (29.6) | 61 (28) | |
| Any intravenous | 173 (33.8) | 107 (36.4) | 66 (30.3) | |
| None | 87 (17) | 41 (13.9) | 46 (21.1) | |
| Single oral | 104 (20.3) | 59 (20.1) | 45 (20.6) | |
| Time metrics, median (IQR) | ||||
| LKW-to-reperfusion, min | 447 (286–765) | 662 (422–978) | 305 (228–404) | <0.001* |
| LKW-to-arterial puncture, min | 375 (217–706) | 603 (356–904) | 245 (168–349) | <0.001* |
| Window, N (%) | <0.001* | |||
| Early | 259 (50.6) | 87 (29.6) | 172 (78.9) | |
| Late | 226 (44.1) | 182 (66.2) | 44 (20.2) | |
| Very late | 27 (5.3) | 25 (8.5) | 2 (0.9) | |
ASPECTS indicates Alberta Stroke Program Early Computed Tomography Score; IA tPA, intra-arterial tissue-type plasminogen activator; ICA, internal carotid artery; IQR, interquartile range; IVT, intravenous thrombolysis; LKW, last known well; mRS, modified Rankin Scale; MT, mechanical thrombectomy; NIHSS, National Institutes of Health Stroke Scale; and TIA, transitory ischemic attack.
P value<0.05.
No dissection or plaque was found after ICA occlusion was revascularized.
Primary Outcomes
Compared with the MT alone group, the IVT+MT group had a higher rate of sICH, but this difference was not significant (7.8% versus 6.2%, P=0.470). After adjusting for the selected covariates, the difference in the risk of sICH between the 2 groups remained nonsignificant (unadjusted OR, 1.26 [95% CI, 0.63–2.53]; P=0.517; adjusted OR [aOR], 1.22 [95% CI, 0.60–2.51]; P=0.583; Figure 2A).
Figure 2. Symptomatic intracranial hemorrhage (sICH) and parenchymal hematoma type 2 (PH2) in patients treated with mechanical thrombectomy (MT) alone and intravenous thrombolysis (IVT)+MT.
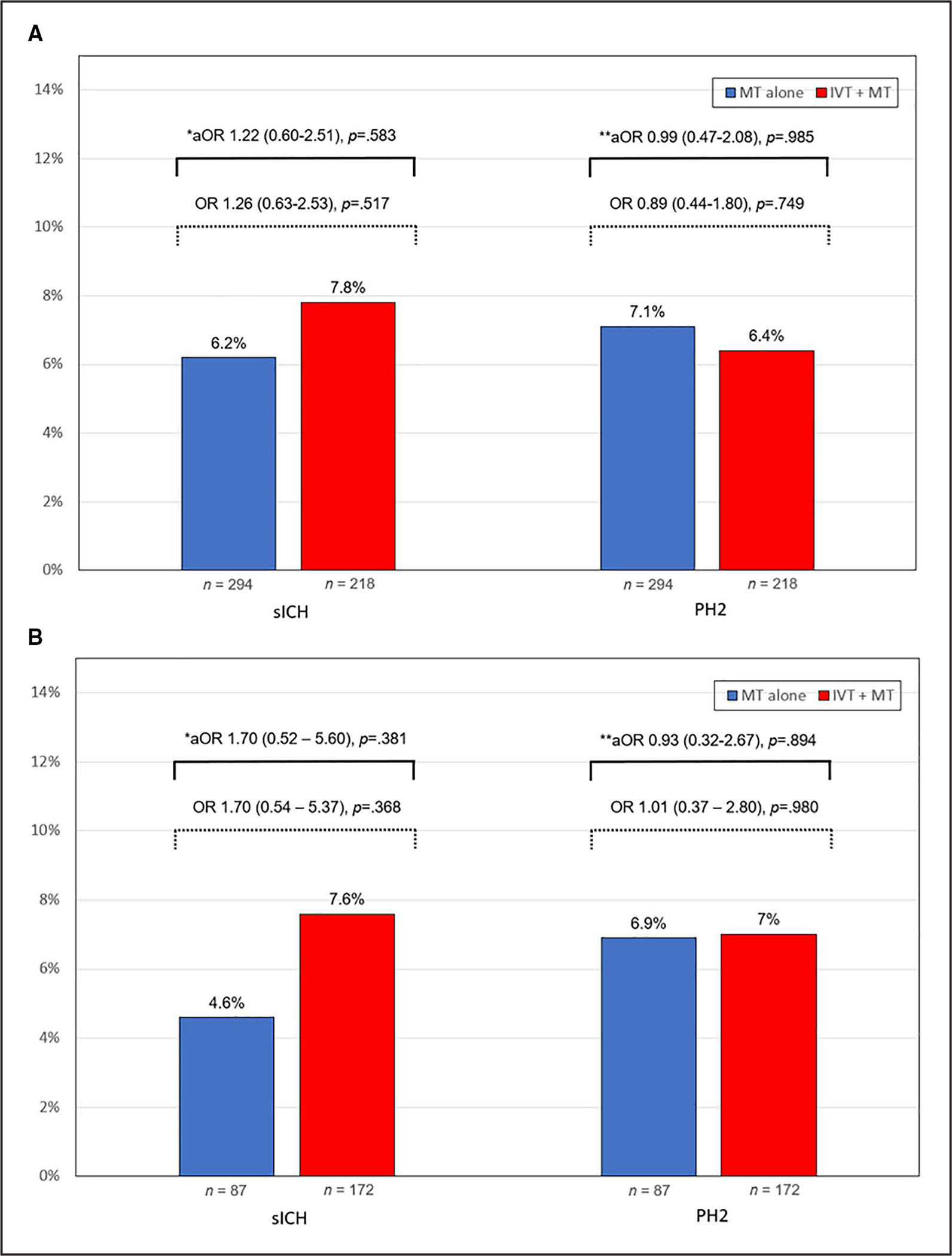
Bar chart illustrating the results for the primary analysis (A) and the sensitivity analysis for the early window (0–6 hours; B). *Adjusted for internal carotid artery (ICA) stenting, number of passes, modified Thrombolysis in Cerebral Infarction 2b-3. **Adjusted for ICA stenting, age, hypertension, Alberta Stroke Program Early Computed Tomography Score.
We assessed for any effect modification of the use of intraprocedural APT on the relationship between IVT and sICH by comparing models with and without an interaction term. This did not show a differential effect between patients who received intraprocedural APT and those who did not (x2=5.66, df=3, p-het=0.129; Figure 3). However, when we explored this interaction by each intraprocedural APT category (Figure S1), a significant level was observed only in patients who received IV-APT (Pinteraction=0.042; Table S1). In the sensitivity analysis, IVT increased the risk of sICH in patients treated with IV-APT therapy (aOR, 3.04 [95% CI, 0.99–9.37]; P=0.05). The effect of IV-APT on the increased risk of sICH with IVT appears related to the use of GP IIb/IIIa inhibitors (Figure S2).
Figure 3. Comparisons in symptomatic intracranial hemorrhage according to the use of intravenous thrombolysis (IVT) in prespecified subgroups.
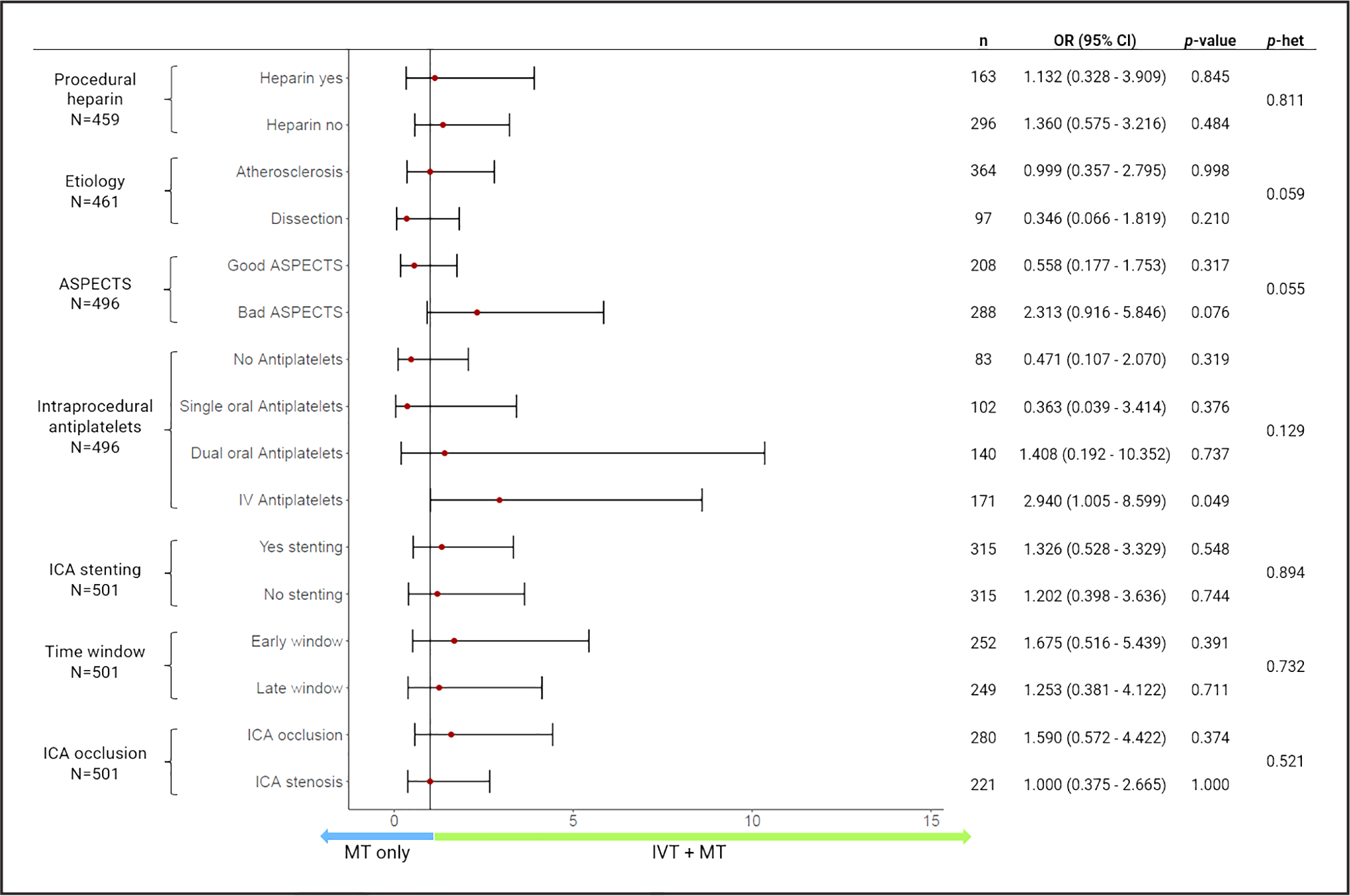
Adjusted for number of passes, modified Thrombolysis in Cerebral Infarction. p-het: P value for test of interactions. ASPECTS indicates Alberta Stroke Program Early Computed Tomography Score; ICA, internal carotid artery; MT, mechanical thrombectomy; OR, odds ratio for sICH; and p-het, P value of heterogeneity.
There were no significant differences in the rates of PH2 between the 2 groups (IVT+MT: 6.4% versus MT alone: 7.1%, P=0.749). After adjusting for confounders, the difference remained nonsignificant (unadjusted OR, 0.89 [95% CI, 0.44–1.80]; P=0.749; aOR, 0.99 [95% CI, 0.47–2.08]; P=0.985; Figure 2A). The comparison between the models with and without an interaction term to assess the effect of intraprocedural APT on the relationship between PH2 and IVT showed no differential effects (x2=3.58, df=3, p-het=0.31). In addition, the interaction by each intraprocedural APT category was also nonsignificant (Figure S3; Table S2). In the sensitivity analysis, the use of IVT did not increase the risk of PH2 with any of the intraprocedural APTs.
With respect to HT, the risk was not significantly different between the MT alone and the IVT+MT groups (aOR, 0.95 [95% CI, 0.62–1.46]; P=0.817; Figure S4). The interaction evaluation showed no significant effect between intraprocedural APT and IVT for HT (IVT×dual oral APT Pinteraction=0.189; IVT×single oral APT Pinteraction=0.264; IVT×IV-APT Pinteraction=0.681). In the sensitivity analyses, the use of IVT did not increase the risk of HT with any of the intraprocedural APTs.
Secondary Outcomes
At 90 days, there was a higher, albeit not statistically significant, rate of favorable functional outcome in the IVT+MT group compared with the MT alone group (48% versus 44.7%, P=0.481). After adjusting for covariates, the IVT+MT group had higher odds of a favorable functional outcome (unadjusted OR, 1.75 [95% CI, 1.02–2.18]; P=0.043; aOR, 1.72 [95% CI, 1.01–2.91]; P=0.045; Figure 4A). In addition, we observed a trend toward increased odds of a favorable outcome in primary admission patients treated with IVT (aOR, 1.84 [95% CI, 0.97–3.55]; P=0.06). In subgroup analyses, there was heterogeneity across intraprocedural APTs and ICA stenting, with stronger odds of favorable outcomes in patients treated with ICA stenting and in those treated with dual oral APTs (Figure 5).
Figure 4. Shift analysis of modified Rankin Scale (mRS) at 90 days in patients treated with mechanical thrombectomy (MT) alone and intravenous thrombolysis (IVT)+MT.
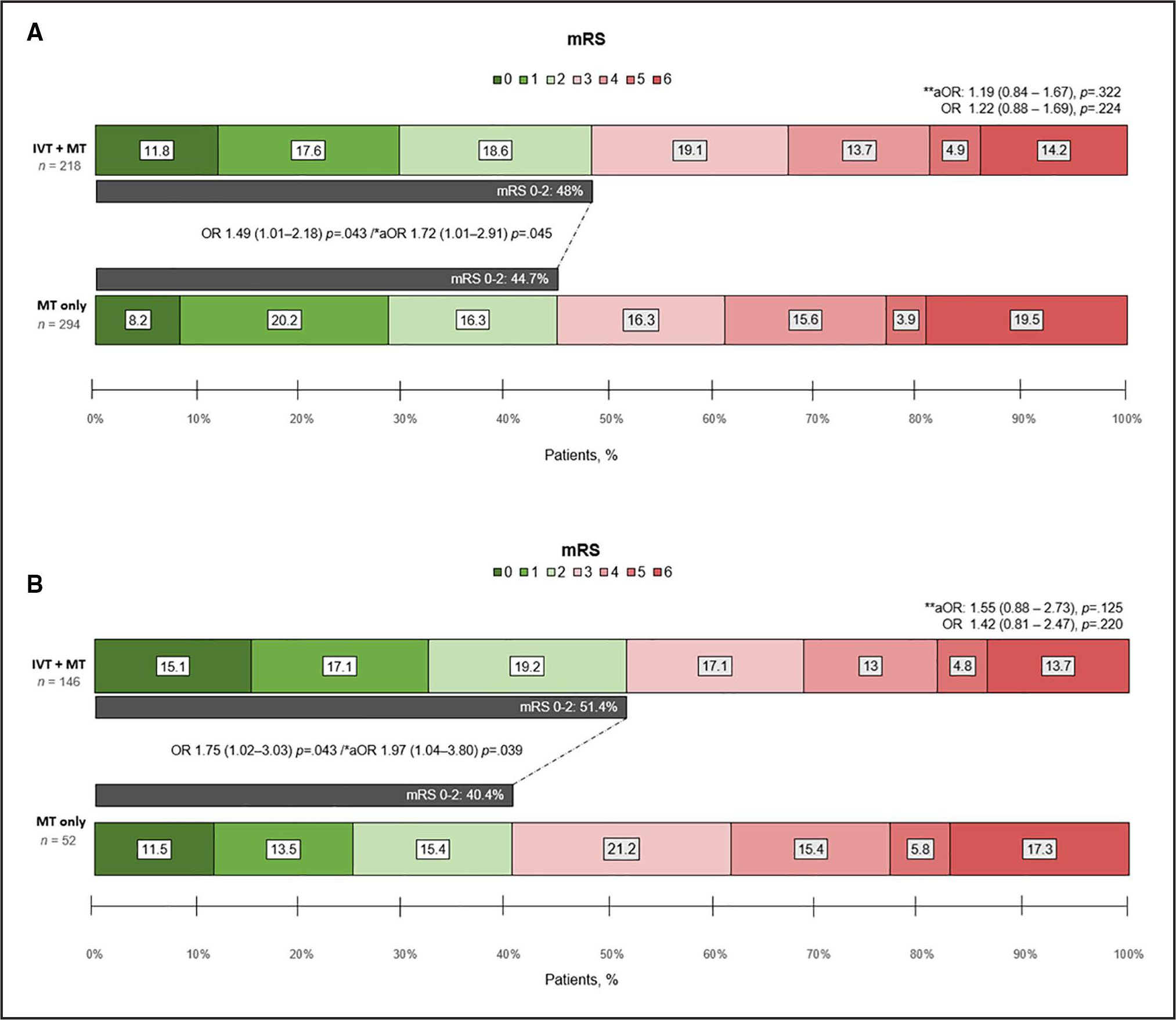
Bar chart depicting the results of the entire cohort (A) and the sensitivity analysis for the early window (0–6 hours; B). aOR indicates adjusted odds ratio; IVT, intravenous thrombolysis; MT, mechanical thrombectomy; and OR, odds ratio. *Categorized mRS score 0 to 2 vs 3 to 6. Adjusted for age, National Institutes of Health Stroke Scale (NIHSS), type of anesthesia, successful reperfusion, internal carotid artery stenting, symptomatic intracranial hemorrhage, and postprocedural antiplatelet therapy. **Multinomial model. Adjusted for age, NIHSS, type of anesthesia, successful reperfusion, internal carotid artery stenting, symptomatic intracranial hemorrhage, and postprocedural antiplatelet therapy.
Figure 5. Comparisons of favorable outcomes at 90 days according to the use of intravenous thrombolysis (IVT) in prespecified subgroups.
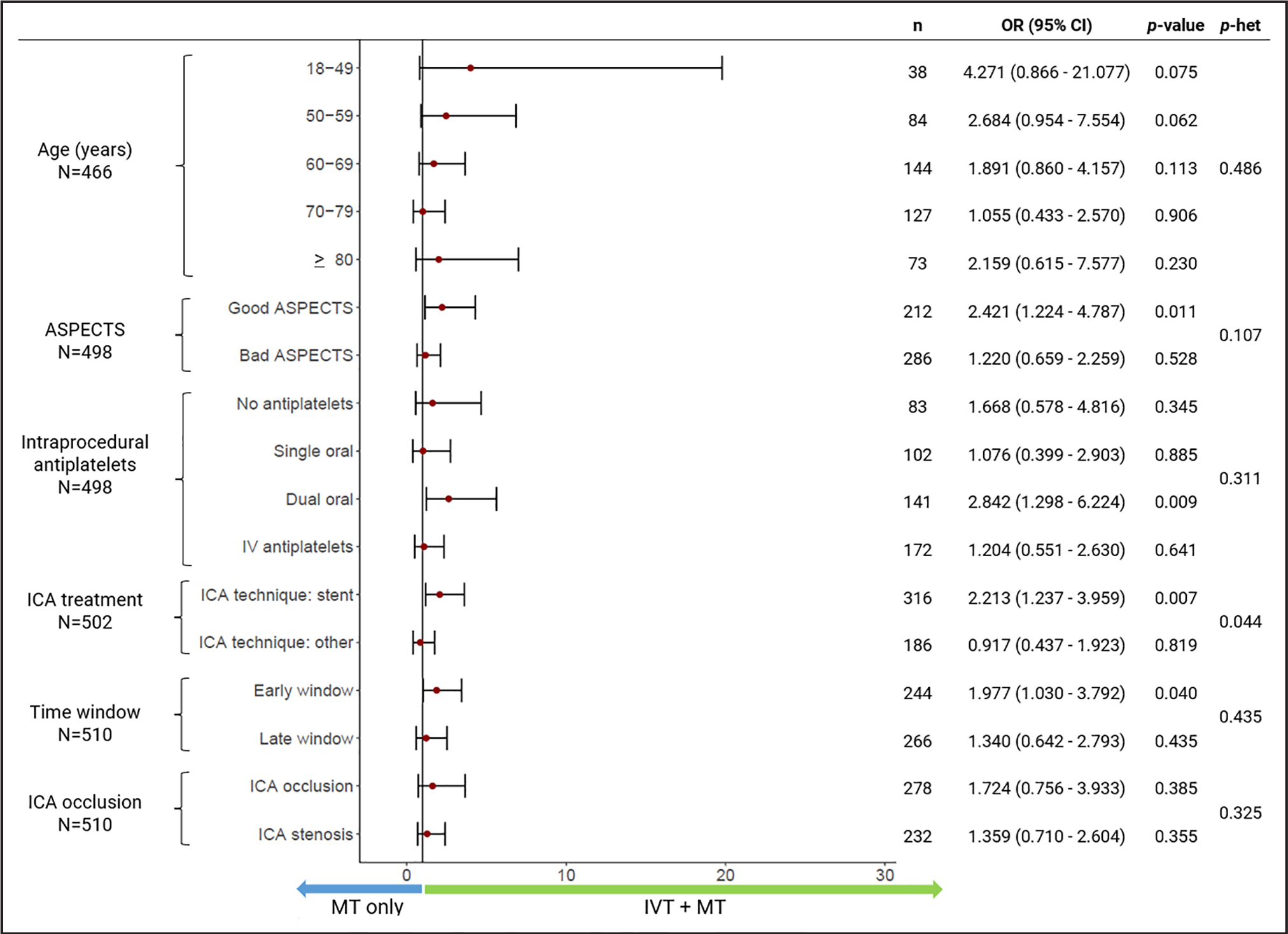
ASPECTS indicates Alberta Stroke Program Early Computed Tomography Score; ICA, internal carotid artery; MT, mechanical thrombectomy; OR, odds ratio of favorable outcome at 90 days; and p-het, P value of heterogeneity.
The odds of successful reperfusion (aOR, 0.95 [95% CI, 0.47–1.93]; P=0.895) and excellent reperfusion (aOR, 0.72 [95% CI, 0.46–1.12]; P=0.141) were not different between patients treated with IVT+MT versus MT alone (Table S3). There was no significant interaction between intraprocedural APT and IVT for successful reperfusion (Pinteraction=0.232); however, there was a significant interaction between IV-APT use with IVT for the outcome of excellent reperfusion (Pnteraction=0.019). In the sensitivity analysis, the use of IVT did not increase the odds of achieving excellent reperfusion with any of the intraprocedural APTs.
In the multivariable analysis, no association was found for in-hospital mortality (aOR, 0.99 [95% CI, 0.44–2.21]; P=0.977) and 90-day mortality (aOR, 0.98 [95% CI, 0.44–2.18]; P=0.956) with the use of IVT prior MT (Table S3).
Early Window Sensitivity Analysis
The early window included only patients with LKW-to-arterial puncture time <6 hours. Similar to the primary analysis, we found no significant differences in the rates of sICH (aOR, 1.70 [95% CI, 0.52–5.60]; P=0.381), PH2 (aOR, 0.93 [95% CI, 0.32–2.67]; P=0.894; Figure 2B), and HT (aOR, 1.33 [95% CI, 0.70–2.52]; P=0.382). The IVT+MT group showed greater odds of a favorable functional outcome at 90 days (aOR, 1.97 [95% CI, 1.04–3.80]; P=0.039; Figure 4B). Finally, there were no differences in additional outcomes (Table S3).
DISCUSSION
Our study compared the safety and efficacy of IVT+MT versus MT alone in patients presenting with acute TLs. We found that (1) the administration of IVT before MT was safe and was not associated with an increased risk of sICH, PH2, or HT; (2) treatment with IVT plus MT was associated with a higher rate of favorable functional outcome at 90 days, especially in TL-patients treated within the 6-hour time window; and (3) the use of intraprocedural IV-APT was associated with an increased risk of sICH in patients who received IVT, but this did not translate into worse clinical outcomes (Figure 5).
The role of IVT in patients with LVO stroke eligible for MT has been a subject of debate. Theoretically, adding IVT may contribute to achieving early reperfusion of the ischemic territory before MT,16,31–33 increase reperfusion rates with fewer recanalization attempts,34 and may improve outcomes in patients with failed MT reperfusion attempts.35 However, the theoretical risk of distal clot embolization to locations not amenable to MT36 and intracranial hemorrhage, the potential delays for arterial puncture, and its elevated cost are considerable disadvantages.37,38
TLs are often excluded and, therefore, underrepresented in clinical trials. As a result, the level of evidence to evaluate the safety of the use of IVT+MT is limited. A pooled analysis of the TITAN and Endovascular Treatment in Ischemic Stroke registries found that bridging therapy did not increase the risk of sICH or PH2, as per our findings.23 They reported a rate of 7.5% for sICH and 5.6% for PH2 in their IVT+MT group, which is comparable with our 7.8% rate for sICH and 6.4% for PH2. Furthermore, our rate of sICH was similar to the rates reported in the 6 randomized clinical trials of bridging therapy in acute LVO, which ranged from 4.7% to 7.8%.1,3–5,17,18 In our study, we also found that the IVT+MT did not increase the risk of HT, which is relevant considering that IVT may promote HT through fibrinolytic and immune mechanisms.39 This finding was consistent in our population independently of the underlying cause, baseline Alberta Stroke Program Early Computed Tomography Score, use of stenting, complete cervical occlusion, or presentation time.
APT has been reported safe, with a low risk for intracranial hemorrhage in patients with TL.14 Its administration primarily occurs intraprocedural to prevent an acute in-stent thrombosis and subsequent restenosis of the cervical segment when a cervical ICA stenting is pursued.40 Zhu et al13 found that IVT before MT did not lead to a significant association with APT (aspirin, clopidogrel, or glycoprotein IIb/IIIa receptor antagonist) and hemorrhagic and procedural complications in patients with TL. Similarly, Anadani et al did not find evidence of heterogeneity in treatment effect sizes according to prior IVT for the risk of hemorrhagic complications. They concluded that ICA stenting was safe in patients with previous IVT.7 According to the ARTIS trial (Antiplatelet Therapy in Combination With rt-PA Thrombolysis in Ischemic Stroke) results, early therapy with intravenous aspirin may increase the risk of hemorrhagic complications in patients who have already received IVT.41 Nevertheless, high-level data are required to guide management decisions regarding the use of intraprocedural APTs and cervical ICA stenting. Currently, 2 ongoing randomized clinical trials (TITAN trial [https://www.clinicaltrials.gov; Unique identifier: NCT03978988]; EASI-TOC trial [Endovascular Acute Stroke Intervention - Tandem Occlusion Trial; https://www.clinicaltrials.gov; Unique identifier: NCT04261478]) aim to assess the safety and efficacy of ICA stenting using different antiplatelet regimens, which will provide us with important insight into the safety of periprocedural antiplatelets.
In our study, we did not find an overall IVT-by-intraprocedural APT interaction. However, when we explored the independent interaction at each of the APT categories, we found that intraprocedural IV-APT (which included GP IIb/IIIa inhibitors and cangrelor) seemed to increase the risk of sICH in patients treated with IVT+MT. Similarly, Stampfl et al42 attributed the high ICH rate (16.6%) to intravenous APT with tirofiban, considering that most of their patients (92%) were treated with IVT before ICA stenting. Also, Heck and Brown found that intravenous APT with abciximab after acute ICA stenting may be associated with higher rates of sICH (31%) in TLs,43 and 1 matched cohort analysis reported a higher rate of parenchymal hemorrhage in patients with TLs and those concomitantly treated with eptifibatide.44 The ATILA project (Aspirin Versus Tirofiban in Endovascular Treatment for Patients With Acute Ischemic Stroke due to Tandem Lesion project; NCT05225961) is a multicenter phase IV randomized clinical trial aimed at determining the safety and efficacy of intravenous tirofiban versus intravenous aspirin in patients with TL treated with MT. The results of this trial will be helpful in understanding the safety of tirofiban in TL. In addition, we observed that the use of GP IIb/IIIa inhibitors was associated with all the hemorrhage cases related to IVT compared with cangrelor (Figure S1). However, the small sample sizes in these subgroups are a limitation for drawing a definite conclusion.
We did not find an association between the use of IVT and successful reperfusion when evaluating our entire TL population. These results are in contrast with the recently published literature that evaluated TL presenting within 8 hours after symptom onset.23 However, when evaluating patients in the early window after adjusting for confounders, IVT was associated with higher odds of functional independence at 90 days. Interestingly, the different effects of IVT on successful reperfusion and functional independence reflect the heterogeneity and chronicity of the occlusions, which may introduce further complexity in the treatment of TLs due to collateral circulation. More importantly, the advantages of pretreatment with IVT, such as clot decomposition and recanalization of microvasculature, might have an additional beneficial effect independently of proximal vessel recanalization.45 In fact, the recently reported CHOICE trial (Chemical Optimization of Cerebral Embolectomy) has shown that the addition of intra-arterial tissue-type plasminogen activator was associated with better functional outcomes at 90 days without any significant differences in the rates of reperfusion or sICH.46
This study has several limitations. First, there is a potential selection bias due to the retrospective nature of its design. Second, the patient selection was determined according to the clinical evaluation of each center and neuro-interventionalist and, therefore, lacks randomization. Third, the clinical and imaging data were self-adjudicated by independent investigators at each center without external control or core imaging laboratory adjudication. Fourth, relevant safety outcomes such as postprocedural and 24-hour extracranial stent patency were not available and, therefore, we could not assess the role of the APT regimen during (and after) MT in-stent patency. Fifth, the significant results from our interaction analysis must be interpreted with caution due to the fact that the overall interaction term (p-het) was not significant, the small number of events in each APT category, and the potential lack of power of the tests. Finally, the predictive models should be interpreted with caution considering the limited sample size and the potential risk of confounding by measured and unmeasured variables.
CONCLUSIONS
Our study shows that IVT+MT for TL cases did not increase the overall risk of sICH, PH2, or overall HT independently of the cervical revascularization technique used. However, the use of intraprocedural IV-APT during stent implantation may be associated with an increased risk of sICH in patients who received IVT before MT. Importantly, treatment with IVT+MT was associated with a higher rate of favorable functional outcomes at 90 days, especially in patients within the 6-hour window. Prospective studies are warranted for confirmation.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- aOR
adjusted odds ratio
- APT
antiplatelet therapy
- ECASS-3
European Collaborative Acute Stroke Study
- HT
hemorrhagic transformation
- ICA
internal carotid artery
- IQR
interquartile range
- IVT
intravenous thrombolysis
- LVO
large-vessel occlusion
- MT
mechanical thrombectomy
- NASCET
North American Symptomatic Carotid Endarterectomy Trial
- PH2
parenchymal hematoma type 2
- sICH
symptomatic intracranial hemorrhage
- TITAN
Thrombectomy in Tandem Lesions
- TL
tandem lesion
Footnotes
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.123.042966.
For Sources of Funding and Disclosures, see page XXX.
This manuscript was sent to Emmanuel Touzé, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Disclosures
A.E. Hassan is a consultant/speaker at Medtronic, Microvention, Stryker, Penumbra, Cerenovus, Genentech, GE Healthcare, Scientia, Balt, vizai, Insera therapeutics, Proximie, NeuroVasc, NovaSignal, Vesalio, Rapid Medical, Imperative Care and Galaxy Therapeutics; principal investigator for COMPLETE study—Penumbra, LVO SYNCHRONISE—vizai, Millipede Stroke Trial—Perfuze, RESCUE—ICAD, Medtronic; steering committee/publication committee member for SELECT, DAWN, SELECT 2, EXPEDITE II, EMBOLISE, CLEAR, ENVI, DELPHI, DISTALS. Dr Divani performed fundings at the University of New Mexico Center for Brain Recovery and Repair Center of Biomedical Research Excellence through Grant Number (NIH P20GM109089, Pilot PI), W81XWH-17-2-0053 (PI), 1R21NS130423-01 (PI). Dr Ribo is consultant at aptaTargets, Anaconda Biomed, Philips, Medtronic, Cerenovus, Vesalio, and Rapid Pulse outside the submitted work. Dr Abraham is a consultant at Penumbra Inc, Qapel, Stryker Corporation. Dr Fifi is a consultant at Cerenovus, Stryker Corporation, Microvention Inc; received stock from Cerebrotech, Imperative Care, Sime&Cure; and received grants from viz AI. Data and Safety Monitoring: MIVI. Dr Yoo is a consultant for Johnson & Johnson Medical Devices & Diagnostics Group—Latin America, LLC, Nicolab, Penumbra Inc, Philips, Vesalio, ZOLL Circulation Inc; received grants from Genetech, USA Inc, Johnson & Johnson Medical Devices & Diagnostics Group—Latin America, LLC, Medtronic, Penumbra Inc, Stryker; Employment at HCA Healthcare; received stock from Insera, Nicolab; performed data and safety monitoring at National Institutes of Health. Dr Mokin is a consultant at Johnson & Johnson Medical Devices & Diagnostics Group—Latin America, LLC, Medtronic, MicroVention Inc, received stock from Bendit Technology, BrainQ, Serenity medical, Synchrone. Dr Yavagal is a consultant at Athersys, Gravity Medical Technology, Johnson & Johnson Health Care Systems Inc, Medtronic USA Inc, Poseydon, Stryker Corporation, Vascular Dynamics; received stock from Athersys, Poseydon, Rapid Medical. Dr Jovin is a consultant at Contego Medical Inc received stock from Anaconda, Freeox Biotech, Galaxy, Kandu, Methinks, Route92, vizai. Grant: Medtronicm, USA, Inc, Stryker Corporation; performed data and safety monitoring at Johnson & Johnson, Cerenovus. Dr Sheth is a consultant at vizAI, Penumbra, Imperative Care; received grants from NIH, vizAI; and took ownership for Motif Neuroscience (not related to this article). Dr Ortega-Gutierrez received grants from NIH-NINDS (R01NS127114-01, R03NS126804), Stryker, Medtronics, Microvention, Penumbra, IschemiaView, vizai, and Siemens; he is a consultant at Medtronic and Stryker Neurovascular. The other authors report no conflicts.
Presented in part at the International Stroke Conference, Dallas, TX, February 8–10, 2023.
Contributor Information
Aaron Rodriguez-Calienes, Department of Neurology, University of Iowa Hospitals and Clinics, Iowa City; Department of Neuroscience, Clinical Effectiveness and Public Health Research Group, Universidad Científica del Sur, Lima, Peru.
Milagros Galecio-Castillo, Department of Neurology, University of Iowa Hospitals and Clinics, Iowa City.
Mudassir Farooqui, Department of Neurology, University of Iowa Hospitals and Clinics, Iowa City.
Ameer E. Hassan, Department of Neurology, Valley Baptist Medical Center/University of Texas Rio Grande Valley, Harlingen, TX.
Mouhammad A. Jumaa, Department of Neurology, ProMedica Toledo Hospital, OH.
Afshin A. Divani, Department of Neurology, University of New Mexico Health Science Center, Albuquerque.
Marc Ribo, Department of Neurology, Hospital Vall d’Hebron, Barcelona, Spain.
Michael Abraham, Department of Neurology, University of Kansas Medical Center.
Nils H. Petersen, Department of Neurology, Yale University School of Medicine, New Haven, CT.
Johanna Fifi, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York.
Waldo R. Guerrero, Department of Neurology and Brain Repair, University of South Florida, Tampa.
Amer M. Malik, Department of Neurology, University of Miami Miller School of Medicine, FL.
James E. Siegler, Cooper Neurological Institute, Cooper University Hospital, Camden, NJ; Cooper Medical School of Rowan University, Candem, NJ.
Thanh N. Nguyen, Department of Neurology, Boston Medical Center.
Albert J. Yoo, Texas Stroke Institute, Dallas-Fort Worth.
Guillermo Linares, Department of Neurology, Saint Louis University, St. Louis, MO.
Nazli Janjua, Asia Pacific Comprehensive Stroke Institute, Pomona Valley Hospital Medical Center, CA.
Darko Quispe-Orozco, Department of Neurology, University of Iowa Hospitals and Clinics, Iowa City.
Wondwossen G. Tekle, Department of Neurology, Valley Baptist Medical Center/University of Texas Rio Grande Valley, Harlingen, TX.
Hisham Alhajala, Department of Neurology, ProMedica Toledo Hospital, OH.
Asad Ikram, Department of Neurology, University of New Mexico Health Science Center, Albuquerque.
Federica Rizzo, Department of Neurology, Hospital Vall d’Hebron, Barcelona, Spain.
Abid Qureshi, Department of Neurology, University of Kansas Medical Center.
Liza Begunova, Department of Neurology, Yale University School of Medicine, New Haven, CT.
Stavros Matsouka, Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York.
Nicholas Vigilante, Cooper Neurological Institute, Cooper University Hospital, Camden, NJ.
Sergio Salazar-Marioni, Department of Neurology, UT Health McGovern Medical School, Houston, TX.
Mohamad Abdalkader, Department of Neurology, Boston Medical Center.
Weston Gordon, Department of Neurology, Saint Louis University, St. Louis, MO.
Jazba Soomro, Texas Stroke Institute, Dallas-Fort Worth.
Charoskon Turabova, Asia Pacific Comprehensive Stroke Institute, Pomona Valley Hospital Medical Center, CA.
Juan Vivanco-Suarez, Department of Neurology, University of Iowa Hospitals and Clinics, Iowa City.
Maxim Mokin, Department of Neurology and Brain Repair, University of South Florida, Tampa.
Dileep R. Yavagal, Department of Neurology, University of Miami Miller School of Medicine, FL.
Tudor Jovin, Cooper Neurological Institute, Cooper University Hospital, Camden, NJ.
Sunil Sheth, Department of Neurology, UT Health McGovern Medical School, Houston, TX.
Santiago Ortega-Gutierrez, Department of Neurology, Neurosurgery & Radiology, University of Iowa Hospitals and Clinics, Iowa City.
REFERENCES
- 1.Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, Peng Y, Han H, Wang J, Wang S, et al. ; DIRECT-MT Investigators. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382:1981–1993. doi: 10.1056/NEJMoa2001123 [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/nejmoa1414905 [DOI] [PubMed] [Google Scholar]
- 3.LeCouffe NE, Kappelhof M, Treurniet KM, Rinkel LA, Bruggeman AE, Berkhemer OA, Wolff L, van Voorst H, Tolhuisen ML, Dippel DWJ, et al. ; MR CLEAN–NO IV Investigators. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021;385:1833–1844. doi: 10.1056/NEJMoa2107727 [DOI] [PubMed] [Google Scholar]
- 4.Fischer U, Kaesmacher J, Strbian D, Eker O, Cognard C, Plattner PS, Bütikofer L, Mordasini P, Deppeler S, Pereira VM, et al. ; SWIFT DIRECT Collaborators. Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: an open-label, blinded-outcome, randomised non-inferiority trial. Lancet. 2022;400:104–115. doi: 10.1016/S0140-6736(22)00537-2 [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, Matsumaru Y, Takeuchi M, Morimoto M, Kanazawa R, Takayama Y, Kamiya Y, Shigeta K, Okubo S, Hayakawa M, et al. ; SKIP Study Investigators. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA. 2021;325:244–253. doi: 10.1001/jama.2020.23522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assis Z, Menon BK, Goyal M, Demchuk AM, Shankar J, Rempel JL, Roy D, Poppe AY, Yang V, Lum C, et al. ; ESCAPE Trialists. Acute ischemic stroke with tandem lesions: technical endovascular management and clinical outcomes from the ESCAPE trial. J Neurointerv Surg. 2018;10:429–433. doi: 10.1136/neurintsurg-2017-013316 [DOI] [PubMed] [Google Scholar]
- 7.Anadani M, Marnat G, Consoli A, Papanagiotou P, Nogueira RG, Siddiqui A, Ribo M, Spiotta AM, Bourcier R, Kyheng M, et al. ; TITAN and ETIS Registry Investigators. Endovascular therapy of anterior circulation tandem occlusions. Stroke. 2021;52:3097–3105. doi: 10.1161/STROKEAHA.120.033032 [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, et al. ; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 9.Feil K, Herzberg M, Dorn F, Tiedt S, Küpper C, Thunstedt DC, Papanagiotou P, Meyer L, Kastrup A, Dimitriadis K, et al. ; GSR investigators†. Tandem lesions in anterior circulation stroke. Stroke. 2021;52:1265–1275. doi: 10.1161/STROKEAHA.120.031797 [DOI] [PubMed] [Google Scholar]
- 10.Rangel-Castilla L, Rajah GB, Shakir HJ, Shallwani H, Gandhi S, Davies JM, Snyder KV, Levy EI, Siddiqui AH. Management of acute ischemic stroke due to tandem occlusion: should endovascular recanalization of the extracranial or intracranial occlusive lesion be done first? Neurosurg Focus. 2017;42:E16. doi: 10.3171/2017.1.FOCUS16500 [DOI] [PubMed] [Google Scholar]
- 11.Wilson MP, Murad MH, Krings T, Pereira VM, O’Kelly C, Rempel J, Hilditch CA, Brinjikji W. Management of tandem occlusions in acute ischemic stroke – intracranial versus extracranial first and extracranial stenting versus angioplasty alone: a systematic review and meta-analysis. J Neurointerv Surg. 2018;10:721–728. doi: 10.1136/neurintsurg-2017-013707 [DOI] [PubMed] [Google Scholar]
- 12.Zevallos CB, Farooqui M, Quispe-Orozco D, Mendez-Ruiz A, Patterson M, Below K, Martins SO, Mansour OY, Mont’Alverne F, Nguyen TN, et al. Proximal Internal Carotid artery Acute Stroke Secondary to tandem Occlusions (PICASSO) international survey. J Neurointerv Surg. 2021;13:1106–1110. doi: 10.1136/neurintsurg-2020-017025 [DOI] [PubMed] [Google Scholar]
- 13.Zhu F, Anadani M, Labreuche J, Spiotta A, Turjman F, Piotin M, Steglich-Arnholm H, Holtmannspötter M, Taschner C, Eiden S, et al. ; TITAN Investigators. Impact of antiplatelet therapy during endovascular therapy for tandem occlusions. Stroke. 2020;51:1522–1529. doi: 10.1161/STROKEAHA.119.028231 [DOI] [PubMed] [Google Scholar]
- 14.Papanagiotou P, Haussen DC, Turjman F, Labreuche J, Piotin M, Kastrup A, Steglich-Arnholm H, Holtmannspötter M, Taschner C, Eiden S, et al. ; TITAN Investigators. Carotid stenting with antithrombotic agents and intracranial thrombectomy leads to the highest recanalization rate in patients with acute stroke with tandem lesions. JACC Cardiovasc Interv. 2018;11:1290–1299. doi: 10.1016/j.jcin.2018.05.036 [DOI] [PubMed] [Google Scholar]
- 15.Rubiera M, Ribo M, Delgado-Mederos R, Santamarina E, Delgado P, Montaner J, Alvarez-Sabín J, Molina CA. Tandem internal carotid artery/middle cerebral artery occlusion. Stroke. 2006;37:2301–2305. doi: 10.1161/01.STR.0000237070.80133.1d [DOI] [PubMed] [Google Scholar]
- 16.Seners P, Turc G, Maïer B, Mas JL, Oppenheim C, Baron JC. Incidence and predictors of early recanalization after intravenous thrombolysis: a systematic review and meta-analysis. Stroke. 2016;47:2409–2412. doi: 10.1161/STROKEAHA.116.014181 [DOI] [PubMed] [Google Scholar]
- 17.Zi W, Qiu Z, Li F, Sang H, Wu D, Luo W, Liu S, Yuan J, Song J, Shi Z, et al. ; DEVT Trial Investigators. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA. 2021;325:234–243. doi: 10.1001/jama.2020.23523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell PJ, Yan B, Churilov L, Dowling RJ, Bush SJ, Bivard A, Huo XC, Wang G, Zhang SY, Ton MD, et al. Endovascular thrombectomy versus standard bridging thrombolytic with endovascular thrombectomy within 4.5 h of stroke onset: an open-label, blinded-endpoint, randomised non-inferiority trial. Lancet. 2022;400:116–125. doi: 10.1016/s0140-6736(22)00564-5 [DOI] [PubMed] [Google Scholar]
- 19.Wang R, Li S, Hao L, Wang Z, Ge Z, Yang S. A meta-analysis of intravenous thrombolysis versus bridging therapy for ischemic stroke. Medicine (Baltim). 2022;101:e30879. doi: 10.1097/MD.0000000000030879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghaith HS, Elfil M, Gabra MD, Nawar AA, Abd-Alkhaleq MS, Hamam KM, Aboelnasr LE, Elgezery EA, Osman MH, Elsayed H, et al. Intravenous thrombolysis before mechanical thrombectomy for acute ischemic stroke due to large vessel occlusion; should we cross that bridge? A systematic review and meta-analysis of 36,123 patients. Neurol Sci. 2022;43:6243–6269. doi: 10.1007/s10072-022-06283-6 [DOI] [PubMed] [Google Scholar]
- 21.Turc G, Tsivgoulis G, Audebert HJ, Boogaarts H, Bhogal P, De Marchis GM, Fonseca AC, Khatri P, Mazighi M, Pérez de la Ossa N, et al. European Stroke Organisation (ESO)-European Society for Minimally Invasive Neurological Therapy (ESMINT) expedited recommendation on indication for intravenous thrombolysis before mechanical thrombectomy in patients with acute ischemic stroke and anterior circulation large vessel occlusion. J Neurointerv Surg. 2022;14:209. doi: 10.1136/neurintsurg-2021-018589 [DOI] [PubMed] [Google Scholar]
- 22.Masoud HE, de Havenon A, Castonguay AC, Asif KS, Nguyen TN, Mehta B, Abdalkader M, Gutierrez SO, Leslie-Mazwi TM, Mansour OY, et al. 2022 brief practice update on intravenous thrombolysis before thrombectomy in patients with large vessel occlusion acute ischemic stroke: a statement from society of vascular and interventional neurology guidelines and practice standards (GAPS) committee. Stroke Vasc Interv Neurol. 2022;2:e000276. doi: 10.1161/SVIN.121.000276 [DOI] [Google Scholar]
- 23.Anadani M, Marnat G, Consoli A, Papanagiotou P, Nogueira RG, Spiotta AM, Bourcier R, Kyheng M, Labreuche J, Siddiqui AH, et al. ; TITAN (Thrombectomy In TANdem lesions) Investigators. Endovascular therapy with or without intravenous thrombolysis in acute stroke with tandem occlusion. J NeuroInterv Surg. 2022;14:314–320. doi: 10.1136/neurintsurg-2020-017202 [DOI] [PubMed] [Google Scholar]
- 24.Farooqui M, Hassan A, Jumaa M, Quispe-Orozco D, Petersen N, Divani A, Ribo M, Abraham M, Fifi JT, Guerrero W, et al. Acute carotid artery stenting for the management of large vessel occlusion ischemic stroke patients with tandem occlusions. JAMA Network Open. 2023;6:e230736. doi: 10.1001/jamanetworkopen.2023.0736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson GG, Eliasziw M, Barr HWK, Clagett GP, Barnes RW, Wallace MC, Taylor DW, Haynes RB, Finan JW, Hachinski VC, et al. The North American symptomatic carotid endarterectomy trial. Stroke. 1999;30:1751–1758. doi: 10.1161/01.str.30.9.1751 [DOI] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 27.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. ; ECASS Investigators. Thrombolysis with alteplase 3 to 45 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 28.von Kummer R, Broderick JP, Campbell BCV, Demchuk A, Goyal M, Hill MD, Treurniet KM, Majoie CBLM, Marquering HA, Mazya MV, et al. The Heidelberg bleeding classification. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 29.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 30.Berge E, Whiteley W, Audebert H, De Marchis GM, Fonseca AC, Padiglioni C, de la Ossa NP, Strbian D, Tsivgoulis G, Turc G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6:I–LXII. doi: 10.1177/2396987321989865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desilles JP, Loyau S, Syvannarath V, Gonzalez-Valcarcel J, Cantier M, Louedec L, Lapergue B, Amarenco P, Ajzenberg N, Jandrot-Perrus M, et al. Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke. 2015;46:3241–3248. doi: 10.1161/STROKEAHA.115.010721 [DOI] [PubMed] [Google Scholar]
- 32.Tsivgoulis G, Katsanos AH, Schellinger PD, Köhrmann M, Varelas P, Magoufis G, Paciaroni M, Caso V, Alexandrov AW, Gurol E, et al. Successful reperfusion with intravenous thrombolysis preceding mechanical thrombectomy in large-vessel occlusions. Stroke. 2018;49:232–235. doi: 10.1161/STROKEAHA.117.019261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ospel JM, Singh N, Almekhlafi MA, Menon BK, Butt A, Poppe AY, Jadhav A, Silver FL, Shah R, Dowlatshahi D, et al. Early recanalization with alteplase in stroke because of large vessel occlusion in the ESCAPE trial. Stroke. 2021;52:304–307. doi: 10.1161/STROKEAHA.120.031591 [DOI] [PubMed] [Google Scholar]
- 34.Fischer U, Kaesmacher J, Molina CA, Selim MH, Alexandrov AV, Tsivgoulis G. Primary thrombectomy in tPA (tissue-type plasminogen activator) eligible stroke patients with proximal intracranial occlusions. Stroke. 2018;49:265–269. doi: 10.1161/STROKEAHA.117.018564 [DOI] [PubMed] [Google Scholar]
- 35.Rozes C, Maier B, Gory B, Bourcier R, Kyheng M, Labreuche J, Consoli A, Mazighi M, Blanc R, Caroff J, et al. ; ETIS Registry Investigators. Influence of prior intravenous thrombolysis on outcome after failed mechanical thrombectomy: ETIS registry analysis. J Neurointerv Surg. 2022;14:688–692. doi: 10.1136/neurintsurg-2021-017867 [DOI] [PubMed] [Google Scholar]
- 36.Ohara T, Menon BK, Al-Ajlan FS, Horn M, Najm M, Al-Sultan A, Puig J, Dowlatshahi D, Sanz AIC, Sohn S-I, et al. Thrombus migration and fragmentation after intravenous alteplase treatment. Stroke. 2021;52:203–212. doi: 10.1161/STROKEAHA.120.029292 [DOI] [PubMed] [Google Scholar]
- 37.Fischer U, Kaesmacher J, Mendes Pereira V, Chapot R, Siddiqui AH, Froehler MT, Cognard C, Furlan AJ, Saver JL, Gralla J. Direct mechanical thrombectomy versus combined intravenous and mechanical thrombectomy in large-artery anterior circulation stroke: a topical review. Stroke. 2017;48:2912–2918. doi: 10.1161/STROKEAHA.117.017208 [DOI] [PubMed] [Google Scholar]
- 38.Ospel JM, McDonough R, Kunz WG, Goyal M. Is concurrent intravenous alteplase in patients undergoing endovascular treatment for large vessel occlusion stroke cost-effective even if the cost of alteplase is only US$1? J Neurointerv Surg. 2022;14:568–572. doi: 10.1136/neurintsurg-2021-017817 [DOI] [PubMed] [Google Scholar]
- 39.Spronk E, Sykes G, Falcione S, Munsterman D, Joy T, Kamtchum-Tatuene J, Jickling GC. Hemorrhagic transformation in ischemic stroke and the role of inflammation. Front Neurol. 2021;12:661955. doi: 10.3389/fneur.2021.661955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paciaroni M, Bogousslavsky J. Antithrombotic therapy in carotid artery stenosis: an update. Eur Neurol. 2015;73:51–56. doi: 10.1159/000367988 [DOI] [PubMed] [Google Scholar]
- 41.Zinkstok SM, Roos YB; ARTIS investigators. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012;380:731–737. doi: 10.1016/S0140-6736(12)60949-0 [DOI] [PubMed] [Google Scholar]
- 42.Stampfl S, Ringleb PA, Möhlenbruch M, Hametner C, Herweh C, Pham M, Bösel J, Haehnel S, Bendszus M, Rohde S. Emergency cervical internal carotid artery stenting in combination with intracranial thrombectomy in acute stroke. AJNR Am J Neuroradiol. 2014;35:741–746. doi: 10.3174/ajnr.A3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heck DV, Brown MD. Carotid stenting and intracranial thrombectomy for treatment of acute stroke due to tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage. J NeuroInterv Surg. 2015;7:170–175. doi: 10.1136/neurintsurg-2014-011224 [DOI] [PubMed] [Google Scholar]
- 44.Rana A, Yu S, Reid-Herrera S, Kamen S, Hunter K, Shaikh H, Jovin T, Thon OR, Patel P, Siegler JE, et al. Eptifibatide use in ischemic stroke patients undergoing endovascular thrombectomy: a matched cohort analysis. Front Neurol. 2022;13:939215. doi: 10.3389/fneur.2022.939215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molina CA, Montaner J, Arenillas JF, Ribo M, Rubiera M, Alvarez-Sabín J. Differential pattern of tissue plasminogen activator-induced proximal middle cerebral artery recanalization among stroke subtypes. Stroke. 2004;35:486–490. doi: 10.1161/01.STR.0000110219.67054.BF [DOI] [PubMed] [Google Scholar]
- 46.Renú A, Millán M, San Román L, Blasco J, Martí-Fàbregas J, Terceño M, Amaro S, Serena J, Urra X, Laredo C, et al. ; CHOICE Investigators. Effect of intra-arterial alteplase vs placebo following successful thrombectomy on functional outcomes in patients with large vessel occlusion acute ischemic stroke: the CHOICE randomized clinical trial. JAMA. 2022;327:826–835. doi: 10.1001/jama.2022.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


