Abstract
Following a request from the European Commission, EFSA was asked to deliver a scientific opinion on the safety of 41 compounds to provide a Herbal flavour and belonging to different chemical groups, when used as sensory additives in feed for all animal species. Fourteen out of the 41 compounds were tested in tolerance studies in chickens for fattening, piglets, cattle for fattening and Atlantic salmon. No adverse effects were observed in the tolerance studies at 10‐fold the intended level. The Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) concluded that the 14 tested compounds were safe for these species at the proposed use level and conclusions were extrapolated to all animal species. For the remaining 27 compounds, read‐across from structurally similar compounds tested in tolerance trials and belonging to the same chemical group was applied. The FEEDAP Panel concluded that these 27 compounds were safe for all animal species at the proposed use level. No safety concern would arise for the consumer and the environment from the use of the 41 compounds up to the maximum proposed use level in feed.
Keywords: sensory additives, flavourings, tolerance studies with mixture of flavourings, herbal flavour, safety, read‐across, environment
1. Introduction
1.1. Background and terms of reference as provided by the requestor
Regulation (EC) No 1831/2003 1 establishes the rules governing the Community authorisation of additives for use in animal nutrition and, in particular, Article 9 defined the term of the authorisation by the Commission.
The applicant, FEFANA asbl, is seeking a Community authorisation of 41 flavourings compounds (undec‐10‐enal, terpineol acetate, borneol, d,l‐isomethone, l‐carvone, (1R)‐1,7,7‐trimethylbicyclo[2.2.1]heptanone, 2 isobornyl acetate, 3‐propylidenephthalide, phenylacetic acid, methyl salicylate, thymol, carvacrol, benzothiazole, terpinolene, isoborneol, trans‐menthone, bornyl acetate, 3‐butylidenephthalide, phenylacetaldehyde, phenethyl acetate, phenethyl phenylacetate, methyl phenylacetate, ethyl phenylacetate, isobutyl phenylacetate, 3‐methylbutyl phenylacetate, 2‐methoxyphenol, 2‐methoxy‐4‐methylphenol, 4‐ethylguaiacol, 2‐methoxy‐4‐vinylphenol, 4‐ethylphenol, 2‐methylphenol, 4‐methylphenol, 2,6‐dimethoxyphenol, phenol, 2,6‐dimethyphenol, 2‐isopropylphenol, benzene‐1,3‐diol, alpha‐phellandrene, alpha‐terpinene, gamma‐terpinene and l‐limonene) as feed additives to be used as flavouring compounds for all animal species (Table 1).
Table 1.
Description of the additives
| Category of additive | Sensory additive |
| Functional group of additives | Flavouring compounds |
| Description | Undec‐10‐enal, terpineol acetate, borneol, d,l‐isomethone, l‐carvone, (1R)‐1,7,7‐trimethylbicyclo[2.2.1]heptanone, 2 isobornyl acetate, 3‐propylidenephthalide, phenylacetic acid, methyl salicylate, thymol, carvacrol, benzothiazole, terpinolene, isoborneol, trans‐menthone, bornyl acetate, 3‐butylidenephthalide, phenylacetaldehyde, phenethyl acetate, phenethyl phenylacetate, methyl phenylacetate, ethyl phenylacetate, isobutyl phenylacetate, 3‐methylbutyl phenylacetate, 2‐methoxyphenol, 2‐methoxy‐4‐methylphenol, 4‐ethylguaiacol, 2‐methoxy‐4‐vinylphenol, 4‐ethylphenol, 2‐methylphenol, 4‐methylphenol, 2,6‐dimethoxyphenol, phenol, 2,6‐dimethyphenol, 2‐isopropylphenol, benzene‐1,3‐diol, alpha‐phellandrene, alpha‐terpinene, gamma‐terpinene and l‐limonene |
| Target animal category | All animal species |
| Applicant | FEFANA asbl |
| Type of request | New opinion |
On 12/07/2016, 13/11/2012, 20/04/2016, 06/03/2012, 07/03/2012, 13/06/2012, 01/02/2023, 08/03/2016 and 10/03/2015, the Panel on Additives and Products or Substances used in Animal Feed of the European Food Safety Authority (“EFSA”), in its opinions on the safety and efficacy of the products, could not conclude on the safety of undec‐10‐enal, terpineol acetate, borneol, d,l‐isomethone, l‐carvone, (1R)‐1,7,7‐trimethylbicyclo[2.2.1]heptanone, 2 isobornyl acetate, 3‐propylidenephthalide, phenylacetic acid, methyl salicylate, thymol, carvacrol, benzothiazole, terpinolene, isoborneol, trans‐menthone, bornyl acetate, 3‐butylidenephthalide, phenylacetaldehyde, phenethyl acetate, phenethyl phenylacetate, methyl phenylacetate, ethyl phenylacetate, isobutyl phenylacetate, 3‐methylbutyl phenylacetate, 2‐methoxyphenol, 2‐methoxy‐4‐methylphenol, 4‐ethylguaiacol, 2‐methoxy‐4‐vinylphenol, 4‐ethylphenol, 2‐methylphenol, 4‐methylphenol, 2,6‐dimethoxyphenol, phenol, 2,6‐dimethyphenol, 2‐isopropylphenol, benzene‐1,3‐diol, alpha‐phellandrene, alpha‐terpinene, gamma‐terpinene, and l‐limonene as feed additives for all animal species due to different aspects related to the safety for human health, animal health or the environment.
The Commission gave the possibility to the applicant to submit supplementary information and data in order to complete the assessment and to allow a revision of the EFSA's opinions concerned. The new data have been received on 15 January 2023.
In view of the above, the Commission asks EFSA to deliver a new opinion on the above‐mentioned 41 flavouring compounds as feed additives for all animal species, based on the supplementary data submitted by the applicant, in accordance with Article 29(1)(a) of Regulation (EC) No 178/2002.
The following table lists: the aspects on which the applicant has submitted information, the species affected, the use level requested and, in the column “TT (c)”, if the tests were performed (T) or if extrapolation is requested (E) (Table 2).
Table 2.
Tolerance Trial (TT) M3 “Herbal”: 14 chemically defined flavourings tested and extrapolation to 27 non‐tested compounds
| CG (a) | EFSA sub‐group (b) | FLAVIS no | FAD number | Name Register name | Requested use level (mg/kg) | TT (c) | Comment Different sections for which data is being submitted (e) |
|---|---|---|---|---|---|---|---|
| CG 04 | C 04 | 05.035 | FAD‐2010‐0041 | Undec‐10‐enal | 5 | T |
Evaluated by EFSA at 1 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 06 | CG06 | 09.830 (d) | FAD‐2010‐0025 | Terpineol acetate | 10 | T |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 10 mg/kg. |
| CG 08 | CG08.1 | 02.016 (d) | FAD‐2010‐0125 | Borneol | 15 | T |
Evaluated by EFSA at 1 mg/kg. Animal safety data, Consumer safety and ERA at 15 mg/kg. |
| CG 08 | CG08.1 | 07.078* | FAD‐2010‐0125 | d,l‐Isomenthone | 5 | T |
Evaluated by EFSA at 0.3–0.5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 08 | CG08.1 | 07.147 (d) | FAD‐2010‐0125 | l‐Carvone | 10 | T |
Evaluated by EFSA at 0.3–0.5 mg/kg. Animal safety data, Consumer safety and ERA at 10 mg/kg. |
| CG 08 | CG08.1 | 07.215 | FAD‐2010‐0125 | (1R)‐1,7,7‐trimethyl bicyclo[2.2.1]heptanone | 5 | T |
Evaluated by EFSA at 0.3–0.5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 08 | CG08.1 | 09.218 | FAD‐2010‐0125 | Isobornyl acetate | 5 | T |
Evaluated by EFSA at 1–5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 11 | CG11.1 | 10.005 | FAD‐2010‐0089 | 3‐Propylidenephthalide | 5 | T |
Evaluated by EFSA at 1 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 15 | CG15 | 08.038 | FAD‐2010‐0027 | Phenylacetic acid | 25 | T |
Evaluated by EFSA at 1–1.5 mg/kg. Animal safety data, Consumer safety and ERA at 25 mg/kg. |
| CG 23 | CG23 | 09.749 (d) | FAD‐2010‐0028 | Methyl salicylate | 50 | T |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 10 mg/kg. |
| CG 25 | CG25 | 04.006 | FAD‐2009‐0050 | Thymol | 125 | T |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 125 mg/kg. |
| CG 25 | CG25 | 04.031 | FAD‐2009‐0050 | Carvacrol | 125 | T |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 125 mg/kg. |
| CG29 | CG29.2 | 15.016 | FAD‐2010‐0410 | Benzothiazole | 0.5 | T |
Evaluated by EFSA at 0.05 mg/kg. Animal safety data, Consumer safety and ERA at 0.5 mg/kg. |
| CG 31 | CG31.1 | 01.005 (d) | FAD‐2010‐0022 | Terpinolene | 14.5 | T |
Evaluated by EFSA at 1–1.5 mg/kg. Animal safety data, Consumer safety and ERA at 14.5 mg/kg. |
| CG 08 | CG08.1 | 02.059 | FAD‐2010‐0125 | Isoborneol | 5 | E |
Evaluated by EFSA at 1–5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 08 | CG08.1 | 07.176 | FAD‐2010‐0125 | trans‐Menthone | 5 | E |
Evaluated by EFSA at 0.3–0.5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 08 | CG08.1 | 09.017 | FAD‐2010‐0125 | Bornyl acetate | 5 | E |
Evaluated by EFSA at 0.3–0.5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 11 | CG11 | 10.024 | FAD‐2010‐0089 | 3‐Butylidenephthalide | 5 | E |
Batch to batch data missing: FEEDAP Panel was unable to perform an assessment for this compound Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 15 | CG15 | 05.030 | FAD‐2010‐0027 | Phenylacetaldehyde | 5 | E |
Evaluated by EFSA at 1–1.5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 15 | CG15 | 09.031 | FAD‐2010‐0027 | Phenethyl acetate | 5 | E |
Evaluated by EFSA at 1–1.5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 15 | CG15 | 09.707 | FAD‐2010‐0027 | Phenethyl phenylacetate | 5 | E |
Evaluated by EFSA at 1–1.5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 15 | CG15 | 09.783 (d) | FAD‐2010‐0027 | Methyl phenylacetate | 5 | E |
Evaluated by EFSA at 1–1.5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 15 | CG15 | 09.784 (d) | FAD‐2010‐0027 | Ethyl phenylacetate | 10 | E |
Evaluated by EFSA at 1–1.5 mg/kg. Animal safety data, Consumer safety and ERA at 10 mg/kg. |
| CG 15 | CG15 | 09.788 (d) | FAD‐2010‐0027 | Isobutyl phenylacetate | 10 | E |
Evaluated by EFSA at 1–1.5 mg/kg. Animal safety data, Consumer safety and ERA at 10 mg/kg. |
| CG 15 | CG15 | 09.789 (d) | FAD‐2010‐0027 | 3‐Methylbutyl phenylacetate | 25 | E |
Evaluated by EFSA at 1–1.5 mg/kg. Animal safety data, Consumer safety and ERA at 25 mg/kg. |
| CG 25 | CG25 | 04.005 | FAD‐2009‐0050 | 2‐Methoxyphenol | 5 | E |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 25 | CG25 | 04.007 | FAD‐2009‐0050 | 2‐Methoxy‐4‐methoxyphenol | 5 | E |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 25 | CG25 | 04.008 | FAD‐2009‐0050 | 4‐Ethylguaiacol | 5 | E |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 25 | CG25 | 04.009 | FAD‐2009‐0050 | 2‐Methoxy‐4‐vinylphenol | 5 | E |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 25 | CG25 | 04.022 | FAD‐2009‐0050 | 4‐Ethylphenol | 5 | E |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 25 | CG25 | 04.027 | FAD‐2009‐0050 | 2‐Methylphenol | 5 | E |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 25 | CG25 | 04.028 | FAD‐2009‐0050 | 4‐Methylphenol | 5 | E |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 25 | CG25 | 04.036 | FAD‐2009‐0050 | 2,6‐Dimethoxyphenol | 5 | E |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 25 | CG25 | 04.041 | FAD‐2009‐0050 | Phenol | 5 | E |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 25 | CG25 | 04.042 | FAD‐2009‐0050 | 2,6‐Dimethyphenol | 5 | E |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 25 | CG25 | 04.044 | FAD‐2009‐0050 | 2‐Isopropylphenol | 5 | E |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 25 | CG25 | 04.047 | FAD‐2009‐0050 | Benzene‐1,3‐diol | 5 | E |
Evaluated by EFSA at 5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 31 | CG31.1 | 01.006 | FAD‐2010‐0022 | alpha‐Phellandrene | 5 | E |
Evaluated by EFSA at 1–1.5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 31 | CG31.1 | 01.019 | FAD‐2010‐0022 | alpha‐Terpinene | 5 | E |
Evaluated by EFSA at 1–1.5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 31 | CG31.1 | 01.020 | FAD‐2010‐0022 | gamma‐Terpinene | 5 | E |
Evaluated by EFSA at 1–1.5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
| CG 31 | CG31.1 | 01.046 | FAD‐2010‐0022 | l‐Limonene | 5 | E |
Evaluated by EFSA at 1–1.5 mg/kg. Animal safety data, Consumer safety and ERA at 5 mg/kg. |
Chemical group for flavouring substances as defined in the Commission Regulation (EC) No 1565/2000.
Chemically defined groups that have been split in separate Opinion (e.g. due to genotoxic concern, or else) are indicated with sub‐numbers.
T: tested in tolerance trial; E: extrapolated safety from representative compounds included in the tolerance trials.
“Requested new feed level” > “FFAC High use level”.
Animal safety data was submitted for all animal species (ruminants (cattle for fattening), piglets and broilers) including marine aquatic species (tolerance trials were performed in Atlantic salmon as representative species for the marine aquatic environment).
d,l‐Isomenthone [07.078] is authorised by Commission Regulation 2018/245 at recommended maximum content of 0.3 mg/kg for pigs and poultry and 0.5 mg/kg for other species ad categories (https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2018.053.01.0087.01.ENG), but has been included in to the tolerance trial at 5 mg/kg complete feed in order to extrapolate safety data derived for this compound to trans‐menthone [07.176].
1.2. Additional information
In the context of the re‐evaluation of feed flavourings, the FEEDAP Panel issued 39 opinions dealing with 568 compounds, including those objects of this evaluation. For about 35% of the compounds assessed, in the absence of data (tolerance studies and/or toxicological studies with the additives under assessment from which a no observed adverse effect level (NOAEL) could be derived) or because of the unsuitability of the available toxicological data, the FEEDAP Panel could not conclude on the safety for target animals of the compounds at the maximum use level proposed by the applicant. The FEEDAP Panel, however, was in each case able to identify a lower safe use level for all animal species, based on the available toxicological information or, more commonly, based on the application of the threshold of toxicological concern (TTC) approach. For these compounds, the FEEDAP Panel also concluded that no safety concern would arise for the consumer or for the environment from the use of these compounds at the identified safe levels in feed. For 49 of the 568 compounds (about 9%), in the absence of specific studies to assess the safety for the user, the FEEDAP Panel could not conclude on the safety for the users when handling the additives. From the current application, this only concerns benzothiazole [15.016].
For a number of substances, the safe use level identified by the FEEDAP Panel was lower than that claimed by the applicant to be typically used in feed and, in some cases, considered by the industry to be too low to allow an effective use as flavouring. The European Commission gave the applicant the possibility to submit complementary information with the aim to demonstrate the safety of the proposed use levels. The applicant recognised that to provide tolerance or toxicological studies for each individual flavouring would not be feasible and would have required a very high number of animals. As an alternative, the applicant proposed the use of tolerance studies designed to test a number of flavouring compounds simultaneously in a mixture, using concentrations which reflected their commercial application and an overdose. The intention was then to conclude on a safe level in feed for each component of the mixture based on their concentration in the mixture and the outcome of the tolerance study.
Four different mixtures (characterised by different olfactory notes, i.e. milky‐vanilla, toasted cereal, herbal and TuttiFrutti) with a total of 68 compounds have been designed to be tested in three major species, chickens for fattening, piglets and cattle for fattening, for a total of 12 tolerance trials. Based on the structural similarity within a chemical group, the applicant also proposed the extrapolation of the conclusions from some of the compounds tested in the tolerance trials to structurally similar compounds belonging to the same chemical group, giving an overall total of 133 compounds. Data on residues in manure samples (excreta from chickens and in faeces and urine from piglets and cattle for fattening) from animals fed the mixture of additives at the maximum recommended use level were also collected to be used in the assessment of the safety for the environment.
As the tolerance studies were started in October 2016, over a 3‐year planning, they were designed to follow the provisions present in the guidance on sensory additives (EFSA FEEDAP Panel, 2012a,b,c,d,e,f,g), which was in place at that time. The FEEDAP Panel exceptionally accepts the approach.
This application deals with the results of tolerance studies made with one of the four mixtures tested and the implications for target animal safety, consumer safety and the environment and it covers the 41 compounds under assessment, belonging to several chemical groups (CGs), namely CG 4, 6, 8, 11, 15, 23, 25, 29 and 31, when used as feed flavourings for all animal species which were assessed by the FEEDAP Panel (EFSA FEEDAP Panel, 2012a,b,c,d,e, 2015, 2016a,b,c).
The list of the 41 flavouring compounds currently authorised for food 3 and feed 4 uses together with the EU Flavour Information System (FLAVIS) number, the chemical group as defined in Commission Regulation (EC) No 1565/2000 5 and the corresponding EFSA opinion is given in Table 3.
Table 3.
Flavourings compounds under assessment, grouped according to the chemical group (CG) as defined in Commission Regulation (EC) No 1565/2000 5 , with indication of the EU Flavour Information System (FLAVIS) number and the corresponding FEEDAP opinion
| CG | Chemical Group | Product (EU register name) | FLAVIS No | FEEDAP opinion, Year |
|---|---|---|---|---|
| 04 | Non‐conjugated and accumulated unsaturated straight‐chain and branched‐chain aliphatic primary alcohols, aldehydes, acids, acetals and esters | Undec‐10‐enal | 05.035 | 2016a |
| 06 | Aliphatic, alicyclic and aromatic saturated and unsaturated tertiary alcohols and esters with esters containing tertiary alcohols ethers | Terpineol acetate | 09.830 | 2012a |
| 08 | Secondary alicyclic saturated and unsaturated alcohols, ketones, ketals and esters with ketals containing alicyclic alcohols or ketones and esters containing secondary alicyclic alcohols | d,l‐Borneol | 02.016 | 2016b |
| d,l‐Isoborneol | 02.059 | |||
| d,l‐Isomenthone (cis‐menthone) | 07.078 | |||
| l‐Carvone | 07.147 | |||
| trans‐Menthone (a) | 07.176 | |||
| (1R)‐1,7,7‐trimethyl bicyclo[2.2.1]heptanone (d‐camphor) | 07.215 | |||
| d,l‐Bornyl acetat (a) | 09.017 | |||
| d,l‐Isobornyl acetate | 09.218 | |||
| 11 | Alicyclic and aromatic lactones | 3‐Propylidenephthalide | 10.005 | 2012b |
| 3‐Butylidenephthalide | 10.024 | |||
| 15 | Phenyl ethyl alcohols, phenylacetic acids, related esters, phenoxyacetic acids and related esters | Phenylacetaldehyde | 05.030 | 2012c |
| Phenylacetic acid | 08.038 | |||
| Phenethyl acetate | 09.031 | |||
| Phenethyl phenylacetate | 09.707 | |||
| Methyl phenylacetate | 09.783 | |||
| Ethyl phenylacetate | 09.784 | |||
| Isobutyl phenylacetate | 09.788 | |||
| 3‐Methylbutyl phenylacetate | 09.789 | |||
| 23 | Benzyl alcohols, aldehydes, acids, esters and acetals | Methyl salicylate | 09.749 | 2012d |
| 25 | Phenol derivatives containing ring‐alkyl, ring‐alkoxy and side chains with an oxygenated functional group | 2‐Methoxyphenol (guaiacol) | 04.005 | 2012e |
| Thymol | 04.006 | |||
| 2‐Methoxy‐4‐methylphenol (creosol) | 04.007 | |||
| 4‐Ethylguaiacol | 04.008 | |||
| 2‐Methoxy‐4‐vinylphenol (4‐vinylguaiacol) | 04.009 | |||
| 4‐Ethylphenol | 04.022 | |||
| 2‐Methylphenol | 04.027 | |||
| 4‐Methylphenol | 04.028 | |||
| Carvacrol | 04.031 | |||
| 2,6‐Dimethoxyphenol | 04.036 | |||
| Phenol | 04.041 | |||
| 2,6‐Dimethylphenol | 04.042 | |||
| 2‐Isopropylphenol | 04.044 | |||
| Benzene‐1,3‐diol (resorcinol) | 04.047 | |||
| 29 | Thiazoles, thiophene and thiazoline | Benzothiazole | 15.016 | 2016c |
| 31 | Aliphatic and aromatic hydrocarbons and acetals containing saturated aldehydes | Terpinolene | 01.005 | 2015 |
| α‐Phellandrene | 01.006 | |||
| α‐Terpinene | 01.019 | |||
| γ‐Terpinene | 01.020 | |||
| l‐Limonene | 01.046 |
trans‐Menthone [07.176]: menthone exists only as trans‐isomer. Referred in the opinion to as menthone.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of supplementary information to previous applications on the same products. 6
The European Union Reference Laboratory (EURL) considered that the conclusions and recommendations reached in the previous assessment regarding the methods used for the control of the chemically defined groups in animal feed are valid and applicable for the current application. 7
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety of 41 flavourings belonging to different chemically defined groups is in line with the principles laid down in Regulation (EC) No 429/2008 8 and the relevant guidance documents: Guidance for the preparation of dossiers for sensory additives (EFSA FEEDAP Panel, 2012f), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012g), Guidance on the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017) and Guidance on the assessment of the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019).
3. Assessment
The additives under assessment are 41 compounds belonging to several chemical groups, namely CG 4, 6, 8, 11, 15, 23, 25, 29 and 31, intended for use as sensory additives (functional group: flavouring compounds) in feed for all animal species.
In previous opinions of the FEEDAP Panel (EFSA FEEDAP Panel 2012a,b,c,d,e, 2015, 2016a,b,c), the 41 compounds under assessment except 3‐butylidenephthalide [10.024] were fully characterised and evaluated for their safety and efficacy as flavouring substances. 3‐Butylidenephthalide was excluded from further assessment because of the absence of data on the purity of the compound (EFSA FEEDAP Panel, 2012b). For the remaining 40 compounds, the FEEDAP Panel could not conclude on the safety for target animals at the maximum use level proposed by the applicant. The Panel, however, was in each case able to identify a safe use level for all animal species, lower than the maximum level proposed by the applicant, based on the available toxicological information or, more commonly, based on the application of the TTC approach. The Panel also concluded that no safety concern would arise for the consumer or the environment from the use of these compounds at the identified safe levels in feed but did not conclude at the maximum use level proposed by the applicant. The majority of the compounds under assessment were considered by the FEEDAP Panel as irritant to skin, eye and the respiratory tract, and as dermal and respiratory sensitisers, based on the material safety data sheets provided by the suppliers.
The applicant has provided new data to address the limitations identified in the previous assessments regarding the characterisation of 3‐butylidenephthalide [10.024] and the safety of the 41 compounds for the target species, the consumer and the environment. The new data submitted consist of analytical data for 3‐butylidenephthalide and tolerance studies in chickens for fattening, piglets, cattle for fattening and Atlantic salmon, performed with a mixture of 14 of the flavourings under assessment. 9 Data on residues in manure samples (excreta from chickens, faeces and urine from piglets and cattle for fattening and faeces from Atlantic salmon) from animals fed the mixture of additives at the maximum recommended use level were also collected to allow the FEEDAP Panel to review its assessment of the safety for the environment. For the remaining 27 compounds under assessment, which were not tested in the tolerance trials, the applicant proposed to extrapolate the conclusions from structurally similar compounds tested in the tolerance studies. For each compound, the applicant provided arguments to demonstrate the safety for the consumer at the proposed use levels. No new data were submitted on the safety for the user.
3.1. Characterisation of 3‐butylidenephthalide
3‐Butylidenephthalide [10.024] belongs to chemical group 11. In its previous assessment, the FEEDAP Panel was unable to perform an assessment of the safety of 3‐butylidenephthalide for the target species, the consumer and the environment because of insufficient purity (EFSA FEEDAP Panel, 2012b).
The applicant has now provided analytical data on three batches of the additive which showed compliance with the proposed specification of 99%, with a content of 3‐butylidenephthalide in the range ■■■■■ 10
The safety of 3‐butilidenephthalide for the target species, the consumer and the environment is assessed in the corresponding sections.
3.2. Conditions of use
The 41 compounds under assessment are intended to be added to feed for all animal species without a withdrawal period. The maximum use levels proposed by the applicant for each compound are shown in Table 4.
Table 4.
Conditions of use for the 41 compounds under assessment: maximum proposed use level in feed for all animal species
| CG | Product (EU register name) | FLAVIS No | All animal species (mg/kg complete feed) |
|---|---|---|---|
| 04 | Undec‐10‐enal | 05.035 | 5 |
| 06 | Terpineol acetate | 09.830 | 10 |
| 08 | d,l‐Borneol | 02.016 | 15 |
| d,l‐Isoborneol | 02.059 | 5 | |
| d,l‐Isomenthone | 07.078 | 5 | |
| l‐Carvone | 07.147 | 10 | |
| Menthone | 07.176 | 5 | |
| d‐Camphor | 07.215 | 5 | |
| d,l‐Bornyl acetate | 09.017 | 5 | |
| d,l‐Isobornyl acetate | 09.218 | 5 | |
| 11 | 3‐Propylidenephthalide | 10.005 | 5 |
| 3‐Butylidenephthalide | 10.024 | 5 | |
| 15 | Phenyl acetaldehyde | 05.030 | 5 |
| Phenylacetic acid | 08.038 | 25 | |
| Phenethyl acetate | 09.031 | 5 | |
| Phenethyl phenylacetate | 09.707 | 5 | |
| Methyl phenylacetate | 09.783 | 10 | |
| Ethyl phenylacetate | 09.784 | 10 | |
| Isobutyl phenylacetate | 09.788 | 10 | |
| 3‐Methylbutyl phenylacetate | 09.789 | 25 | |
| 23 | Methyl salicylate | 09.749 | 50 |
| 25 | 2‐Methoxyphenol | 04.005 | 5 |
| Thymol | 04.006 | 125 | |
| 2‐Methoxy‐4‐methylphenol | 04.007 | 5 | |
| 4‐Ethylguaiacol | 04.008 | 5 | |
| 2‐Methoxy‐4‐vinylphenol | 04.009 | 5 | |
| 4‐Ethylphenol | 04.022 | 5 | |
| 2‐Methylphenol | 04.027 | 5 | |
| 4‐Methylphenol | 04.028 | 5 | |
| Carvacrol | 04.031 | 125 | |
| 2,6‐Dimethoxyphenol | 04.036 | 5 | |
| Phenol | 04.041 | 5 | |
| 2,6‐Dimethylphenol | 04.042 | 5 | |
| 2‐Isopropylphenol | 04.044 | 5 | |
| Resorcinol (benzene‐1,3‐diol) | 04.047 | 5 | |
| 29 | Benzothiazole | 15.016 | 0.5 |
| 31 | Terpinolene | 01.005 | 14.5 |
| α‐Phellandrene | 01.006 | 5 | |
| α‐Terpinene | 01.019 | 5 | |
| γ‐Terpinene | 01.020 | 5 | |
| l‐Limonene | 01.046 | 5 |
3.3. Safety
3.3.1. Safety for the target species
3.3.1.1. Test item and feed preparation
The mixture tested in the tolerance studies is referred as ‘herbal’ and includes 14 flavouring compounds belonging to several chemical groups. The individual components of the mixture, their FLAVIS numbers, the maximum recommended level proposed by the applicant (1×; in kg complete feed) and the two higher levels tested, 3× and 10× (tested in chickens for fattening, weaned piglets and cattle for fattening) or 11× (tested in Atlantic salmon) are described in Table 5.
Table 5.
Individual components of the mixture and intended levels tested in tolerance trials in terrestrial animals (1×, 3× or 10× maximum recommended level) and Atlantic salmon (1×, 3× or 11× maximum recommended level)
| CG | EU register name | FLAVIS No | 1× | 3× | 10× | 11× |
|---|---|---|---|---|---|---|
| mg/kg complete feed | ||||||
| 04 | Undec‐10‐enal | 05.035 | 5 | 15 | 50 | 55 |
| 06 | Terpineol acetate | 09.830 (c) | 10 | 30 | 100 | 110 |
| 08 | d,l‐Borneol | 02.016 (c) | 15 | 45 | 150 | 165 |
| 08 | d,l‐Isomenthone | 07.078 | 5 | 15 | 50 | 55 |
| 08 | l‐Carvone | 07.147 (c) | 10 | 30 | 100 | 110 |
| 08 | d‐Camphor | 07.215 | 5 | 15 | 50 | 55 |
| 08 | d,l‐Isobornyl acetate | 09.218 | 5 | 15 | 50 | 55 |
| 11 | 3‐Propylidenephthalide | 10.005 | 5 | 15 | 50 | 55 |
| 15 | Phenylacetic acid | 08.038 | 25 | 75 | 250 | 275 |
| 23 | Methyl salicylate | 09.749 (c) | 50 | 150 | 500 | 550 |
| 25 | Thymol | 04.006 | 125 | 375 | 1,250 | 1,375 |
| 25 | Carvacrol | 04.031 | 125 | 375 | 1,250 | 1,375 |
| 29 | Benzothiazole | 15.016 | 0.5 | 1.5 | 5 | 5.5 |
| 31 | Terpinolene | 01.005 | 14.5 | 43.5 | 145 | 159.5 |
EU: European Union; FLAVIS No: EU Flavour Information System numbers.
■■■■■
■■■■■
■■■■■ 11 ■■■■■ 12 ■■■■■ 13 ■■■■■
Homogeneity of the test product was tested on 10× maximum recommended level samples (terrestrial animals) at different time intervals, 14 taking 10 individual subsamples and by monitoring carvacrol as a marker. The coefficient of variation ranged between 3.1% and 4.8% in poultry feed, between 2.6% and 5.0% in feed for piglets and between 5.5% and 8.4% in feed for cattle for fattening. No homogeneity data were available for feed for salmon.
3.3.1.2. Tolerance study in chickens for fattening
A total of 800 1‐day‐old male chickens for fattening (Ross 308) were distributed in groups of 25 animals to 32 pens, arranged in eight blocks of four pens each. Pens within each block were randomly allocated into four groups (eight replicates per treatment). 15 Two basal diets (starter up to day 14, and grower from day 15 to 36) based on maize and soyabean meal were either not supplemented (control) or supplemented with the ‘herbal’ mixture to provide 1×, 3× or 10× maximum recommended level per kg feed (confirmed by analysis 16 ). Animals were under study for 36 days; diets were offered ad libitum in mash form.
Mortality and health status were checked daily, and dead animals were necropsied. The average pen body weight and feed intake were recorded on days 1, 14 and 35. The average daily weight gain, average feed intake and feed‐to‐gain ratio were calculated. At the end of the trial, blood samples were taken from two birds per pen for haematology 17 and blood biochemistry 18 analyses (the birds were randomly selected at the beginning of the study). On day 36, two chickens from each pen from the control and 10× treatment groups were killed and subjected to necropsy and gross pathology evaluations. 19 An analysis of variance (ANOVA) was done with the data on a pen basis and considering the treatment and the block as the main effects. Group means were compared with Tukey test. The significance level was set at 0.05.
The birds were in general good health throughout the study. One bird from group 1× died and five birds (2 from the control group and 1 bird from each of the remaining groups) were culled during the study.
The supplementation of the diet of chickens with the herbal mixture at any level for 35 days showed no differences when compared with the control diet in terms of zootechnical performance parameters monitored: final body weight (control group = 1,972 g), daily feed intake (77.8 g), daily gain (55.1 g) and feed to gain ratio (1.41).
The dietary treatment had no effect on the blood haematological profile or any biochemical parameter at the end of the study. Concerning gross pathology, there were no differences observed between treatments in the organs' macroscopic evaluation and weights of the birds necropsied.
The FEEDAP Panel concludes that the components of the herbal mixture are safe for chickens for fattening under the proposed conditions of use with a margin of safety of 10.
3.3.1.3. Tolerance study in weaned piglets
A total of 144 Piétrain × (Landrace × Large White) weaned piglets (ca. 33 days old; average body weight of 8.2 kg) were distributed in groups of four animals (two males and two females) in 36 pens, arranged in nine blocks of four pens (considering both pen location in the room and initial body weight). Pens within each block were randomly allocated to the treatments (9 replicates per treatment). 20 Two basal diets (pre‐starter, up to day 14 of trial and starter, from 15 to 42 day of trial) mainly based on maize and soyabean meal were either not supplemented (control) or supplemented with the herbal mixture to provide: 1×, 3× or 10× maximum recommended level per kg feed (confirmed by analysis 21 ). The experimental feeds were offered ad libitum in mash form for 42 days.
Mortality and health status were checked daily. Piglets were individually weighed on days 1, 14 and 42 of trial. Feed intake was registered per pen on every diet change (days 1 and 14), and average daily gain, average daily feed intake and feed to gain ratio were calculated and corrected for mortality. At the end of the experiment, blood samples were taken from two piglets per pen (one male and one female randomly selected at the beginning of the trial) for haematology 17 and blood biochemistry. 22 On day 42, one piglet from each pen from the control group and the 10× group was killed and subjected to gross pathology evaluations. The experimental unit was the pen for zootechnical performance and the individual animal for blood parameters. The experimental data were analysed by using a generalised linear model, with the diet, block and sex (only for blood data) as fixed effects. Group means were compared with Tukey's test. The significance level was set at 0.05.
Mortality and culling were on average 5.6% and not treatment related. No differences were observed between groups for final body weight (control group = 30.0 kg), daily feed intake (847 g), daily weight gain (520 g) and feed to gain ratio (1.63). The dietary treatment had no effect on the blood haematological profile. Regarding the biochemistry parameters, alanine amino transferase (ALT) concentration was significantly lower in animals receiving 3× and 10× (42 and 41 IU/L) when compared to control pigs (53 IU/L), and creatinine kinase concentration was significantly lower in the 10× group (1,165 IU/L) than in the control (3,646 IU/L). Such findings were considered to have no clinical significance. No macroscopic lesions were observed in the analysed organs in any of the animals necropsied.
The FEEDAP Panel concludes that the components of the herbal mixture are safe for weaned piglets under the proposed conditions of use with a margin of safety of 10.
3.3.1.4. Tolerance study in cattle for fattening
A total of 24 Holstein bulls (180–250 kg body weight) were balanced by body weight, housed in individual pens and randomly allocated into four groups (6 replicates per treatment). A basal concentrate based on maize, barley and beet pulp was either not supplemented (control) or supplemented with the herbal mixture to provide 1×, 3× or 10× maximum recommended level per kg concentrate feed (confirmed by analysis 23 ). The animals were offered ad libitum the concentrate, in mash form and straw for 47 days.
Mortality and health status were checked every day. Animals were weighed on days 1, 21 and 42, while feed intake was registered daily for concentrate and straw. The average daily gain, average daily feed intake and the feed to gain ratio were calculated. Blood samples were taken on days 1 and 42 from all animals for haematology17 and blood biochemistry. 24 Gross pathology 25 was carried out at day 47 on four animals from the control and four from the 10× groups. The experimental data were analysed using a mixed model with repeated measurements including the treatment, time and their interaction as fixed effects, plus the random effect of the pen. Initial body weight was used as a covariate for zootechnical parameters. The significance level was set at 0.05.
The general health of the animals was good throughout the study and no animals died. For the overall period, there were no significant differences between treatments in the final body weight (control group = 284 kg), average daily weight gain (1.74 kg), daily feed intake (6.4 kg dry matter (DM), including both concentrate and straw) and feed to gain ratio (3.67). Regarding the blood haematology and biochemistry data, no differences were observed between control and supplemented groups. Moreover, no relevant macroscopic lesions were observed in the organs analysed at the slaughterhouse.
The study showed no adverse effects when the herbal mixture was added up to 10× the maximum recommended level in the concentrate. As the intake of straw DM was about 6.5% of the total DM intake of the animals, the real exposure to the additive was lower than the one intended in the conditions of use. Considering the intake of straw, the levels tested would correspond to 0.94, 2.80 and 9.12× the maximum recommended level in complete feed. Consequently, the FEEDAP Panel concludes that the components of the herbal mixture are safe for cattle for fattening under the proposed conditions of use with a margin of safety of at least 9.
3.3.1.5. Tolerance study in Atlantic salmon
A total of 396 post‐smolt Atlantic salmon (Salmo salar) (average weight of 245 g) were distributed in 12 fibreglass tanks in a flow through system and the tanks randomly allocated to four groups (representing 3 tanks per treatment; 33 fish per tank). 26
A basal extruded diet based on fish meal, fish oil, wheat gluten and soy protein concentrate was either not supplemented (control) or supplemented with the mixture to provide 1×, 3× or 11× maximum recommended level per kg feed (confirmed by analysis). 27 The experimental diets were offered to fish three times per day in slight excess (10% overfeeding) for 96 days.
Mortality and health status were checked every day. Fish were individually weighed at days 1, 55 and 96 of the trial. Feed intake was registered daily per tank. At the end of the study (day 96), the specific growth rate, thermal growth coefficient, total feed intake and feed‐to‐gain ratio (corrected for mortality) were calculated for the whole experimental period (1–96 days). On day 1 and 96, blood samples were taken from five fish per tank and analysed for haematology 28 and clinical biochemistry. 29 On day 96, 30 fish from the control and 30 from 11× group (10 from each replicated tank) were killed, weighed and necropsied to perform the gross pathology evaluation. 30
The experimental data were statistically analysed with an analysis of variance (ANOVA), using the tank as the experimental unit and the diet as fixed effect. Mean group differences were tested using Tukey's test. The significance level was set at 0.05.
No significant differences in final weight (control group = 794 g), total feed intake per tank (13,590 g), thermal growth coefficient (2.70) and feed to gain ratio (0.75) were observed between supplemented and control groups. The specific growth rate was lower in the 11× group compared to the control (1.28 vs. 1.24%).
No mortality and culling were recorded during the trial and no negative effect on fish overall health was observed with any tested level of the additive. No differences on the blood haematology and biochemistry parameters analysed were seen between groups. Moreover, no relevant macroscopic lesions were observed in the studied organs of fish fed the control or the 11× diet. 31
In the absence of effects on the feed to gain ratio, blood parameters and the gross pathology evaluation, the Panel does not consider the lower specific growth rate at the 11× an adverse effect.
The FEEDAP Panel concludes that the components of the herbal mixture are safe for salmonids under the proposed conditions of use with a margin of safety of 11.
3.3.1.6. Conclusions on the safety for the target species for the compounds tested in the tolerance studies
Based on the tolerance studies in chickens for fattening, weaned piglets, cattle for fattening and salmon, in which no adverse effects were seen at 10×, 10×, >9 × and 11× overdose, respectively, the FEEDAP Panel considers that the 14 compounds are safe for these species at the proposed use level.
As the margin of safety is similar in all species, the conclusions are extrapolated to all animal species for all the 14 compounds tested.
3.3.1.7. Extrapolation of the conclusions of the tolerance studies
For the remaining 27 compounds under assessment not tested in the tolerance trials, namely d,l‐isoborneol [02.059], menthone [07.176], d,l‐bornyl acetate [09.017], 3‐butylidenephthalide [10.024], phenyl acetaldehyde [05.030], phenethyl acetate [09.031], phenethyl phenylacetate [09.707], methyl phenylacetate [09.783], ethyl phenylacetate [09.784], isobutyl phenylacetate [09.788], 3‐methylbutyl phenylacetate [09.789], 2‐methoxyphenol [04.005] 2‐methoxy‐4‐methylphenol [04.007], 4‐ethylguaiacol [04.008], 2‐methoxy‐4‐vinylphenol [04.009], 4‐ethylphenol [04.022], 2‐methylphenol [04.027], 4‐methylphenol [04.028], 2,6‐dimethoxyphenol [04.036], phenol [04.041], 2,6‐dimethylphenol [04.042], 2‐isopropylphenol [04.044], resorcinol (benzene‐1,3‐diol) [04.047], α‐phellandrene [01.006], α‐terpinene [01.019], γ‐terpinene [01.020] and l‐limonene [01.046], the applicant proposed to extrapolate the conclusions for structurally similar compounds tested in the tolerance studies and belonging to the same chemical group.
The proposed conditions of use for the 27 compounds candidate for read‐across are summarised in Table 4.
Read‐across has been widely applied in the risk assessment of food and feed flavourings. Based on considerations related to structural and metabolic similarities, flavourings are grouped into chemical groups as defined in Annex I of Regulation (EC) No 1565/2000 and structural groups named Flavouring Group Evaluation (FGE). According to the guidance on the preparation of dossiers for sensory additives (EFSA FEEDAP Panel, 2012a,b,c,d,e,f,g), ‘The conclusions obtained for an individual flavouring may be extended to other flavourings belonging to the same structural group (e.g. an FGE).’
The application of read‐across within a chemical group is applied on a case‐by‐case basis, considering the structural features, the physico‐chemical properties and the expected reactivity of the compounds under assessment, as discussed in the paragraphs below.
Chemical group 8
The chemical structures of the compounds under assessment belonging to CG 8 are shown in Fig. 1. The applicant proposed to read‐across from d,l‐borneol [02.016] to d,l‐bornyl acetate [09.017], from d,l‐isobornyl acetate [09.218] to d,l‐isoborneol [02.059] and d,l‐isomenthone [07.078] to menthone [07.176]. The FEEDAP Panel considers that the proposal for read‐across is justified by the structural and the expected metabolic similarity within the three groups of compounds, as shown in Figure 1. Target animals have esterases, which split the esters into the corresponding alcohols (d,l‐borneol or d,l‐isoborneol) and acid (acetic acid). For d,l‐isomenthone and menthone, a similar metabolic pathway is expected with the reduction of the ketone and subsequent conjugation with glucuronic acid.
Figure 1.
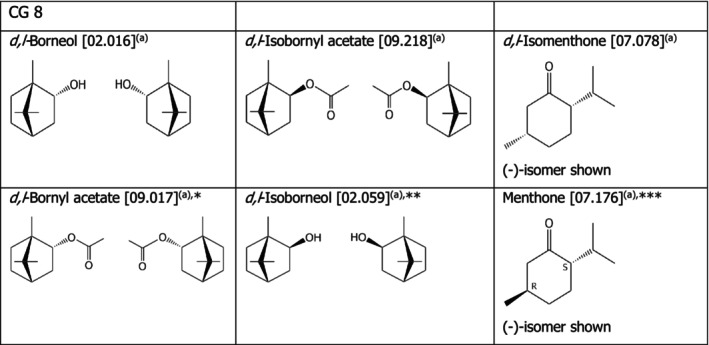
- (a): Racemate. *: Proposed extrapolation from [02.016]; **proposed extrapolation from [09.218]; ***: Proposed extrapolation from [07.078].
Considering that no adverse effects were observed for d,l‐borneol [02.016] and d,l‐isobornyl acetate [09.218] when tested in a mixture of the 14 flavourings, respectively, up to 150 and 50 mg/kg complete feed in the tolerance studies in chickens, piglets, cattle for fattening and Atlantic salmon, and considering the structural similarity of the compounds tested with the compounds candidate for read‐across, the FEEDAP Panel concludes that the use of d,l‐bornyl acetate [09.039] and d,l‐isoborneol [02.059] at 5 mg/kg complete feed is safe for all animal species.
Similarly, considering that no adverse effects were observed for d,l‐isomenthone [07.078] when tested in a mixture of the 14 flavourings in the tolerance studies in chickens, piglets, cattle for fattening and Atlantic salmon up to 50 mg/kg, and considering the structural similarity of the compound tested with menthone [07.176], the FEEDAP Panel concludes that the use of menthone [07.176] at 5 mg/kg complete feed is safe for all animal species.
Chemical group 11
The chemical structures of the two compounds under assessment belonging to CG 11 are shown in Figure 2. The applicant proposed to read‐across from 3‐propylidenephthalide [10.005] to 3‐butylidenephathlide [10.024]. The FEEDAP Panel considers that the proposal for read‐across is justified by the structural and the expected metabolic similarity between the two compounds, as shown in Figure 2. ‘The γ‐lactone, 3‐propylidenephthalide [10.005], is hydrolysed in vivo in mammals to 2‐(2‐hydroxyalkyl)benzoic acid which may be excreted directly, or the side chain oxygenated functional group (alcohol or enolic alcohol) may be oxidised (alcohol) or reduced (enol). The reduced form is subsequently conjugated and excreted. The benzoic acid moiety may conjugate with glycine and be excreted mainly as the hippurate, while the ketone function may be reduced to the corresponding alcohol and excreted as the glucuronic acid conjugate’ (reviewed in EFSA FEEDAP Panel, 2012b). A similar metabolic pathway is expected for 3‐butylidenephathlide [10.024].
Figure 2.
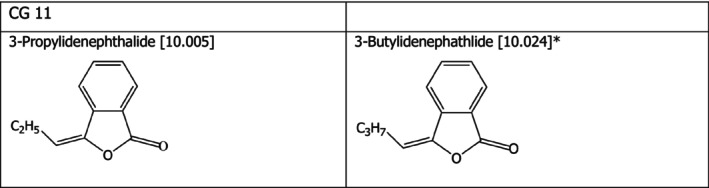
- *: Proposed extrapolation from [10.005].
Considering that no adverse effects were observed for 3‐propylidenephthalide [10.005] when tested in a mixture of the 14 flavouring up to 50 mg/kg in the tolerance studies in chickens, piglets, cattle for fattening and Atlantic salmon, and considering the structural similarity of the compound tested with 3‐butylidenephathlide [10.024], the FEEDAP Panel concludes that the use of 3‐butylidenephathlide [10.024] at 5 mg/kg complete feed is safe for all animal species.
Chemical group 15
The chemical structures of the compounds under assessment belonging to CG 15 are shown in Figure 3. The applicant proposed to read‐across from phenylacetic acid [08.038] to phenyl acetaldehyde [05.030], phenethyl acetate [09.031], phenethyl phenylacetate [09.707], methyl phenylacetate [09.783], ethyl phenylacetate [09.784], isobutyl phenylacetate [09.788], 3‐methylbutyl phenylacetate [09.789]. For all the compounds except phenyl acetaldehyde [05.030], the FEEDAP Panel considers that the proposal for read‐across is justified by the structural and metabolic similarity between the compounds, as shown in Figure 3. Phenethyl and phenylacetate esters are rapidly hydrolysed in vivo to yield 2‐phenylethan‐1‐ol and phenylacetic acid. 2‐Phenylethan‐1‐ol is further oxidised to phenylacetic acid, which is conjugated and excreted primarily in the urine. Therefore, most of the flavouring agents in this group will be hydrolysed and/or oxidised to yield phenylacetic acid that is excreted either free or in conjugated form (reviewed in EFSA FEEDAP Panel, 2012c).
Figure 3.
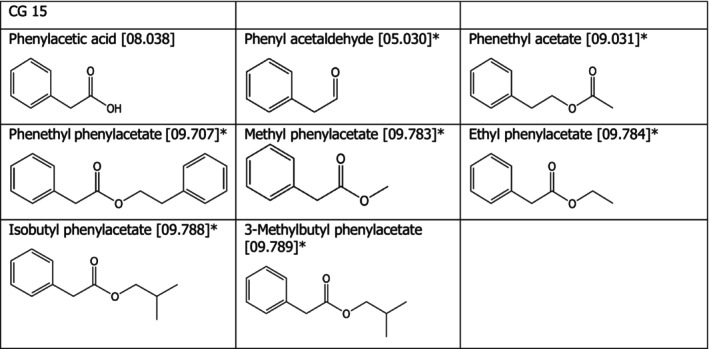
- *: Proposed extrapolation from [08.038]
Considering that no adverse effects were observed for phenylacetic acid [08.038] when tested in a mixture of the 14 flavouring up to 250 mg/kg in the tolerance studies in chickens, piglets, cattle for fattening and Atlantic salmon, and considering the structural similarity between the two compounds, the FEEDAP Panel concludes that the use of phenethyl acetate [09.031], phenethyl phenylacetate [09.707], methyl phenylacetate [09.783], ethyl phenylacetate [09.784], isobutyl phenylacetate [09.788], 3‐methylbutyl phenylacetate [09.789] at 5 mg/kg complete feed is safe for all animal species.
For phenyl acetaldehyde [05.030], the FEEDAP Panel considers that read‐across from acetaldehyde [05.001] would be more appropriate. In the assessment of the safety for the target species of compounds belonging to CG 01 (EFSA FEEDAP Panel, 2013), the Panel concluded that acetaldehyde [05.001] is safe at the proposed use level of 5 mg/kg complete feed for all animal species, based on a NOAEL of 120 mg/kg body weight (bw) per day for acetaldehyde. Based on the same NOAEL, safe concentrations in feed for the target species are derived for phenyl acetaldehyde [05.030] following the EFSA Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017), resulting in concentrations ranging from 13 mg/kg complete feed (chickens for fattening) to 235 mg/kg (ornamental fish). Therefore, it is concluded that phenyl acetaldehyde [05.030] is safe at 5 mg/kg complete feed for all animal species.
Chemical Group 25
The applicant proposed to read‐across from thymol [04.006] and carvacrol [04.031] to 12 compounds belonging to chemical group 25. In its previous assessment (EFSA FEEDAP Panel, 2012e), the FEEDAP Panel already concluded that the use of 2‐methoxyphenol (guaiacol) [04.005], 2‐methoxy‐4‐methylphenol (creosol) [04.007], 4‐ethylguaiacol [04.008], 2‐methoxy‐4‐vinylphenol (4‐vinylguaiacol) [04.009], 4‐ethylphenol [04.022], 2‐methylphenol [04.027], 4‐methylphenol [04.028], 2,6‐dimethoxyphenol [04.036], phenol [04.041], 2,6‐dimethylphenol [04.042], 2‐isopropylphenol [04.044] and resorcinol (benzene‐1,3‐diol) [04.047] at 5 mg/kg complete feed is safe for all animal species. Therefore, there is no need to revise the conclusion of the former assessment based on the new evidence submitted.
Chemical Group 31
The chemical structures of the compounds under assessment belonging to CG 31 are shown in Figure 4. The applicant proposed to read‐across from terpinolene [01.005] to α‐phellandrene [01.006], α‐terpinene [01.019], γ‐terpinene [01.020] and l‐limonene [01.046]. The FEEDAP Panel considers that the proposal for read‐across is justified by the structural and metabolic similarity between the compounds, as shown in Figure 4. After absorption, these hydrocarbons are oxidised to polar oxygenated metabolites by the cytochrome P450 enzymes, alcohol dehydrogenase and aldehyde dehydrogenases. The resulting hydroxylated metabolites may be excreted in conjugated form or undergo further oxidation, yielding more polar metabolites that are also excreted in conjugated form in the urine. If a double bond is present, epoxide intermediates may form and these are rapidly detoxified either by hydrolysis to yield diols, or by conjugation with glutathione (reviewed in EFSA FEEDAP Panel, 2015).
Figure 4.
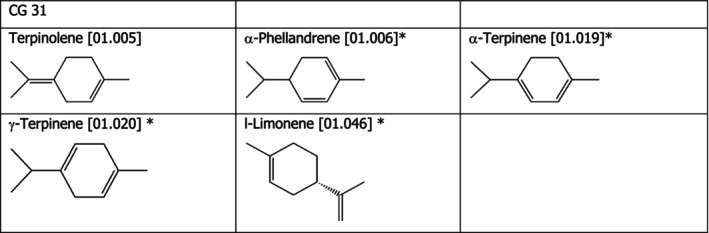
- * proposed extrapolation from [01.005]
The FEEDAP Panel notes that the five compounds under assessment belong to CG 31, subassessment group III as defined in Flavouring Group Evaluation 25 (FGE.25) and FGE.78 (EFSA CEF Panel, 2015a,b). A NOAEL of 250 mg/kg bw per day has been identified for d‐limonene [01.045] in CG 31 (EFSA FEEDAP Panel, 2015) and applied using read‐across to the compounds under assessment, terpinolene [01.005] to α‐phellandrene [01.006], α‐terpinene [01.019], γ‐terpinene [01.020] and l‐limonene [01.046]. The same NOAEL of 250 mg/kg bw per day has been identified for terpineol 32 [02.230] in CG 6 (EFSA FEEDAP Panel, 2012a). Based on the same NOAEL, safe concentrations in feed for the target species are derived for these compounds following the EFSA Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017), resulting in concentrations ranging from 28 mg/kg complete feed (chickens for fattening) and 489 mg/kg (ornamental fish). Therefore, it is concluded that α‐phellandrene [01.006], α‐terpinene [01.019], γ‐terpinene [01.020] and l‐limonene [01.046] are safe at 5 mg/kg complete feed for all animal species.
Considering that no adverse effects were observed for terpinolene [01.005] when tested in a mixture of the 14 flavouring up to 145 mg/kg in the tolerance studies in chickens, piglets, cattle for fattening and Atlantic salmon, and considering the structural similarity between the compound belonging to chemical group 25, the FEEDAP Panel concludes that the use of α‐phellandrene [01.006], α‐terpinene [01.019], γ‐terpinene [01.020] and l‐limonene [01.046] at 5 mg/kg complete feed is safe for all animal species.
3.3.1.8. Conclusions on safety for the target species
Based on the results of the tolerance studies in chickens for fattening, piglets and cattle for fattening, and read‐across from the compounds tested to structurally similar compounds belonging to the same chemical group, the FEEDAP Panel concludes that the 41 compounds are safe for all animal species at the corresponding maximum proposed use level, according to the conditions of use summarised in Table 4.
3.3.2. Safety for the consumer
The safety for the consumer of the 41 compounds used as food flavourings has been already assessed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) and EFSA, as described in the former opinions of the FEEDAP Panel (see Table 3). All the compounds are currently authorised in the EU as food flavourings without limitations, except d‐camphor [07.215].
In its previous assessments of the 41 compounds as feed flavourings, the FEEDAP Panel already concluded that the use of 12 compounds, namely 2‐methoxyphenol (guaiacol) [04.005], 2‐methoxy‐4‐methylphenol (creosol) [04.007], 4‐ethylguaiacol [04.008], 2‐methoxy‐4‐vinylphenol (4‐vinylguaiacol) [04.009], 4‐ethylphenol [04.022], 2‐methylphenol [04.027], 4‐methylphenol [04.028], 2,6‐dimethoxyphenol [04.036], phenol [04.041], 2,6‐dimethylphenol [04.042], 2‐isopropylphenol [04.044] and resorcinol (benzene‐1,3‐diol) [04.047] at 5 mg/kg complete feed is safe for the consumer. Since this is the maximum use level proposed by the applicant in the current dossier, there is no need to revise the conclusion of the former assessment (EFSA FEEDAP Panel, 2012e).
For 28 compounds, the FEEDAP Panel concluded on the safety for the consumer at a lower level than the maximum use level proposed by the applicant for the target species and concluded that no concern would arise for the consumer from the use of the compounds up to those levels which were considered safe for the target species. In the absence of deposition and residue studies of the compounds in farm animals, the conclusions of the former assessments were based on the expected extensive metabolism and excretion of the compounds in the target animals. Based on the same considerations on the ability of the target animals to metabolise and excrete the compounds under assessment, the FEEDAP Panel considers that the use of these flavourings at the higher proposed use levels in feed would not appreciably increase the human exposure to these compounds.
In the absence of data on purity, the FEEDAP Panel was not able to perform an assessment of 3‐butylidenephthalide [10.024] (EFSA FEEDAP Panel, 2012b). Considering the new data submitted on the characterisation of the additive and the structural and metabolic similarity with 3‐propylidenephthalide (see Section 3.3.1.7), the FEEDAP Panel concludes that no safety concern would arise for the consumer from the use of 3‐butylidenephthalide at the proposed use level of 5 mg/kg complete feed.
Overall, the FEEDAP Panel concludes that, no safety concern would arise for the consumer from the use of these 41 compounds up to the maximum proposed use level in feed, according to the conditions of use summarised in Table 4.
3.3.3. Safety for the environment
In its previous assessments, the FEEDAP Panel concluded that the use of the 40 out of the 41 compounds under assessment in animal feed at the maximum safe level for the target species is considered safe for the environment. 3‐Butylidenephthalide [10.024] was excluded from the assessment because of the absence of data on characterisation (EFSA FEEDAP Panel, 2012b).
To support the safety of use levels in feed higher than those considered safe for the environment in the previous assessments, the applicant provided experimental data to revise the conclusions on the safety for the environment for 14 of the compounds under assessment and made a proposal to extrapolate to the remaining 27 compounds.
At the end of the tolerance trials, samples of faeces and urine were collected from animals from the control group and from the group administered with the maximum recommended level (1×). For piglets, faecal samples (2 animals per pen, all pens) and urine (one animal per pen, 2 pens per treatment) were collected at day 42. For cattle for fattening, faeces and pen manure samples were collected at day 42 from all animals and urine samples from two pens per treatment. For chickens for fattenizng, samples of excreta were collected at day 36 (from 1 animal per pen, all pens). For Atlantic salmon (33 animals per tank, all tanks), the faeces were collected at the end of the study (day 96). The concentrations of the 14 components of the mixture were determined in all samples.
For each component, the fraction of the dose considered to be active (FA) was calculated as the ratio between the average concentration in manure (corrected by the concentration in control) and the theoretical concentration of the compounds fed to the animals.
The concentration of the additives in manure from the control group and the group receiving the maximum recommended level (1×) was calculated from the average concentrations of the additives in faeces and urine sample as follow:
where piglet total manure is 84 kg (45 kg dung and 39 kg urine), cattle for fattening total manure is 58 kg (40 kg dung and 18 kg urine) and broiler total manure is 85 kg. 33 The FEEDAP Panel notes that the metabolism study submitted does not comply with the provisions of the FEEDAP guidance to evaluate the safety for the feed additives for the environment (EFSA FEEDAP Panel, 2019). Particularly, the volume of excreta produced was not measured and default values (without a range of variability) were used to calculate the concentration in manure.
The concentrations in manure determined in samples taken at the end of the tolerance studies in poultry, pigs, cattle for fattening and Atlantic salmon are summarised in Table 6.
Table 6.
Concentrations in manure of the 14 compounds tested in tolerance trials with herbal mixture (a)
| CG | EU register name | FLAVIS No | Use level | Manure levels | Conclusion | |||
|---|---|---|---|---|---|---|---|---|
| mg/kg | Poultry | Pigs | Cattle | Salmon | ||||
| % FA | ||||||||
| 04 | Undec‐10‐enal | 05.035 | 5 | 0.45 | – | – | 3.03 | Endogenously produced Extensively metabolised |
| 06 | Terpineol acetate | 09.830 | 10 | 0 | 2.33 | 0.3 | 0 |
Natural occurrence Extensively metabolised |
| 08 | d,l‐Borneol | 02.016 | 15 | 0 | 0.70 | 0.87 | 0 |
Natural occurrence Extensively metabolised |
| 08 | d,l‐Isomenthone | 07.078 | 5 | 2.83 | 0 | 0.72 | 0 |
Natural occurrence Extensively metabolised |
| 08 | l‐Carvone | 07.147 | 10 | 1.24 | 3.43 | 0.40 | 1.15 |
Natural occurrence Extensively metabolised |
| 08 | d‐Camphor | 07.215 | 5 | 2.75 | 0 | 0.01 | 0 | Natural occurrence Extensively metabolised |
| 08 | d,l‐Isobornyl acetate | 09.218 | 5 | 0.90 | 1.84 | 0.19 | 0.12 | Natural occurrence Extensively metabolised |
| 11 | 3‐Propylidenephthalide | 10.005 | 5 | 2.37 | 1.08 | 0.82 | 0 | Extensively metabolised |
| 15 | Phenylacetic acid | 08.038 | 25 | – | – | – | 0 |
Endogenously produced Extensively metabolised |
| 23 | Methyl salicylate | 09.749 | 50 | 0.61 | 0.66 | 0.28 | 0.02 | Natural occurrence Extensively metabolised |
| 25 | Thymol | 04.006 | 125 | 4.92 | 0.80 | 1.72 | 4.02 |
Natural occurrence Extensively metabolised |
| 25 | Carvacrol | 04.031 | 125 | 0.06 | 0.94 | 2.36 | 0.13 |
Natural occurrence Extensively metabolised |
| 29 | Benzothiazole | 15.016 | 0.5 | 0 | 0.69 | 0.95 | 0 |
Natural occurrence Extensively metabolised |
| 31 | Terpinolene | 01.005 | 5 | 0.26 | 0.99 | 0.09 | 1.27 |
Natural occurrence Extensively metabolised |
The concentrations in manure were calculated from the concentrations determined in faeces and urine samples taken at the end of the tolerance studies in pigs and cattle for fattening and in excreta samples taken at the end of the tolerance study in poultry. The concentrations are expressed as the percentage of fraction of the dose considered to be active (%FA)
The analytical results expressed as % FA indicate that all the compounds tested are extensively metabolised in the target species, the fraction in manure being < 5% of the theoretical concentration fed to the animals. The data confirm the hypothesis made by the FEEDAP Panel that compounds belonging to CG 4 and 31 are extensively metabolised in the animals.
Extensive metabolism in all species was also demonstrated for compounds belonging to CG 4, 6, 8, 11, 15, 23, 25 and 29. For all the compounds tested, the concentrations detected in manure of all target species indicate that the compounds are extensively metabolised and a Phase II assessment at the proposed use levels in feed is not required.
For several compounds, terpineol acetate [09.830], d,l‐borneol [02.016], d,l‐isomentone [07.078], l‐carvone [07.147], d‐camphor [07.215], d,l‐isobornyl acetate [09.218], methyl salicylate [09.749], thymol [04.006], carvacrol [04.031], benzothiazole [15.016] and terpinolene [01.005], the applicant provided evidence on the natural occurrence in European plants in concentrations higher than the proposed use level in feed. 34
For the compounds not tested in the tolerance trial, the applicant provided additional information on the natural occurrence and arguments for the read‐across from structurally related compounds tested in the tolerance trials, as summarised in Table 7. Based on the above (natural occurrence and/or extensive metabolism), a Phase II assessment is not required for these compounds at the proposed conditions of use. In addition, the applicant provided data on the natural occurrence of d,l‐isoborneol [02.059], menthone [07.176], d,l‐bornyl acetate [09.017], 3‐butylidenephthalide [10.024], phenyl acetaldehyde [05.030], methyl phenylacetate [09.783], ethyl phenylacetate [09.784], 2‐methoxyphenol [04.005], 2‐methoxy 4‐vinylphenol [04.009], phenol [04.041], α‐phellandrene [01.006], α‐terpinene [01.019], γ‐terpinene [01.020] and l‐limonene [01.046] above the proposed use level in feed. For the 10 compounds belonging to CG 25 non tested in tolerance trials, the FEEDAP Panel already concluded that they are not expected to pose a risk to the environment when used at the level considered safe for the target species (5 mg/kg) (EFSA FEEDAP Panel, 2012e).
Table 7.
Conclusions for the 27 compounds non tested in tolerance trials
| CG | Product (EU register name) | FLAVIS No | Use level (mg/kg) | Conclusion |
|---|---|---|---|---|
| 08 | d,l‐Isoborneol | 02.059 | 5 | Read‐across, natural occurrence |
| Menthone | 07.176 | 5 | Read‐across, natural occurrence | |
| d,l‐Bornyl acetate | 09.017 | 5 | Read‐across, natural occurrence | |
| 11 | 3‐Butylidenephthalide | 10.024 | 5 | Read‐across, extensively metabolised, natural occurrence |
| 15 | Phenyl acetaldehyde | 05.030 | 5 | Read‐across, extensively metabolised, natural occurrence |
| Phenethyl acetate | 09.031 | 5 | Read‐across, extensively metabolised | |
| Phenethyl phenylacetate | 09.707 | 5 | Read‐across, extensively metabolised | |
| Methyl phenylacetate | 09.783 | 10 | Read‐across, extensively metabolised, natural occurrence | |
| Ethyl phenylacetate | 09.784 | 10 | Read‐across, extensively metabolised, natural occurrence | |
| Isobutyl phenylacetate | 09.788 | 10 | Read‐across, extensively metabolised | |
| 3‐Methylbutyl phenylacetate | 09.789 | 25 | Read‐across, extensively metabolised | |
| 25 | 2‐Methoxyphenol | 04.005 | 5 | Read‐across, natural occurrence extensively metabolised |
| 2‐Methoxy‐4‐methylphenol | 04.007 | 5 | Read‐across, extensively metabolised | |
| 4‐Ethylguaiacol | 04.008 | 5 | Read‐across, extensively metabolised | |
| 2‐Methoxy‐4‐vinylphenol | 04.009 | 5 | Read‐across, extensively metabolised, natural occurrence | |
| 4‐Ethylphenol | 04.022 | 5 | Read‐across, extensively metabolised | |
| 2‐Methylphenol | 04.027 | 5 | Read‐across, extensively metabolised | |
| 4‐Methylphenol | 04.028 | 5 | Read‐across, extensively metabolised | |
| 2,6‐Dimethoxyphenol | 04.036 | 5 | Read‐across, extensively metabolised | |
| Phenol | 04.041 | 5 | Read‐across, extensively metabolised, natural occurrence | |
| 2,6‐Dimethylphenol | 04.042 | 5 | Read‐across, extensively metabolised | |
| 2‐Isopropylphenol | 04.044 | 5 | Read‐across, extensively metabolised | |
| Benzene‐1,3‐diol | 04.047 | 5 | Read‐across, extensively metabolised | |
| 31 | α‐Phellandrene | 01.006 | 5 | Read‐across, extensively metabolised, natural occurrence |
| α‐Terpinene | 01.019 | 5 | Read‐across, extensively metabolised, natural occurrence | |
| γ‐Terpinene | 01.020 | 5 | Read‐across, extensively metabolised, natural occurrence | |
| l‐Limonene | 01.046 | 5 | Read‐across, extensively metabolised, natural occurrence |
3.3.3.1. Conclusions on safety for the environment
The FEEDAP Panel concluded that the 41 compounds under assessment are safe for the environment when used in animal feed for all animal species up to the highest proposed use level.
4. Conclusions
The FEEDAP Panel concludes that the 41 flavouring compounds under assessment are safe for all animal species, consumers, and the environment at the following proposed maximum use levels:
| Chemical Group | Product (EU register name) | FLAVIS No | All animal species (mg/kg complete feed) |
|---|---|---|---|
| 04 | Undec‐10‐enal | 05.035 | 5 |
| 06 | Terpineol acetate | 09.830 | 10 |
| 08 | d,l‐Borneol | 02.016 | 15 |
| d,l‐Isoborneol | 02.059 | 5 | |
| d,l‐Isomenthone | 07.078 | 5 | |
| l‐Carvone | 07.147 | 10 | |
| Menthone | 07.176 | 5 | |
| d‐Camphor | 07.215 | 5 | |
| d,l‐Bornyl acetate | 09.017 | 5 | |
| d,l‐Isobornyl acetate | 09.218 | 5 | |
| 11 | 3‐Propylidenephthalide | 10.005 | 5 |
| 3‐Butylidenephthalide | 10.024 | 5 | |
| 15 | Phenyl acetaldehyde | 05.030 | 5 |
| Phenylacetic acid | 08.038 | 25 | |
| Phenethyl acetate | 09.031 | 5 | |
| Phenethyl phenylacetate | 09.707 | 5 | |
| Methyl phenylacetate | 09.783 | 10 | |
| Ethyl phenylacetate | 09.784 | 10 | |
| Isobutyl phenylacetate | 09.788 | 10 | |
| 3‐Methylbutyl phenylacetate | 09.789 | 25 | |
| 23 | Methyl salicylate | 09.749 | 50 |
| 25 | 2‐Methoxyphenol | 04.005 | 5 |
| Thymol | 04.006 | 125 | |
| 2‐Methoxy‐4‐methylphenol | 04.007 | 5 | |
| 4‐Ethylguaiacol | 04.008 | 5 | |
| 2‐Methoxy‐4‐vinylphenol | 04.009 | 5 | |
| 4‐Ethylphenol | 04.022 | 5 | |
| 2‐Methylphenol | 04.027 | 5 | |
| 4‐Methylphenol | 04.028 | 5 | |
| Carvacrol | 04.031 | 125 | |
| 2,6‐Dimethoxyphenol | 04.036 | 5 | |
| Phenol | 04.041 | 5 | |
| 2,6‐Dimethylphenol | 04.042 | 5 | |
| 2‐Isopropylphenol | 04.044 | 5 | |
| Resorcinol (benzene‐1,3‐diol) | 04.047 | 5 | |
| 29 | Benzothiazole | 15.016 | 0.5 |
| 31 | Terpinolene | 01.005 | 14.5 |
| α‐Phellandrene | 01.006 | 5 | |
| α‐Terpinene | 01.019 | 5 | |
| γ‐Terpinene | 01.020 | 5 | |
| l‐Limonene | 01.046 | 5 |
Abbreviations
- ANOVA
Analysis of variance
- CG
chemical group
- DM
dry matter
- EURL
European Union Reference Laboratory
- FA
Active fraction
- FEEDAP
EFSA Scientific Panel on Additives and Products or Substances used in Animal Feed
- FFAC
Feed Flavourings authorisation Consortium of FEFANA (EU Association of Specialty Feed Ingredients and their Mixtures)
- FGE
food group evaluation
- FLAVIS
The EU Flavour Information System
- FL‐no
FLAVIS number
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- NOAEL
no observed adverse effect level
- TTC
threshold of toxicological concern
- WHO
World Health Organization
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis, V ., Azimonti, G ., Bastos, M. L ., Christensen, H ., Dusemund, B ., Durjava, M ., Kouba, M ., López‐Alonso, M ., López Puente, S ., Marcon, F ., Mayo, B ., Pechová, A ., Petkova, M ., Ramos, F ., Villa, R. E ., Woutersen, R ., Brantom, P ., Chesson, A ., … Manini, P . (2023). Safety of 41 flavouring compounds providing a herbal flavour and belonging to different chemical groups for use as feed additives in all animal species (FEFANA asbl). EFSA Journal, 21(10), 1–27. 10.2903/j.efsa.2023.8340
Requestor: European Commission
Question number: EFSA‐Q‐2023‐00030
Panel members: Vasileios Bampidis, Giovanna Azimonti, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Fašmon Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Roberto Edoardo Villa and Ruud Woutersen.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output (in alphabetical order of the last name): Jaume Galobart, Matteo Lorenzo Innocenti and Maria Vittoria Vettori.
Legal notice: The full opinion is published in accordance with Article 10(6) of Regulation (EC) No 1935/2004, and it implements EFSA's decision on confidentiality, in accordance with Article 20 of the said Regulation. Certain technical details have been awarded confidential status by EFSA and consequently withheld from public disclosure by redaction. This opinion may be subject to editing once the confidentiality decision‐making on the additional information received is completed.
Declarations of interest: If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Adopted: 26 September 2023
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Synonym: d‐camphor.
Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1.
European Union Register of Feed Additives pursuant to Regulation (EC) No 1831/2003. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed-eu-reg-comm_register_feed_additives_1831-03.pdf
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96 of the European Parliament and of the Council. OJ L 180, 19.7.2000, p. 8.
FEED dossiers' reference: FAD‐2010‐0041, FAD‐2010‐0025, FAD‐2010‐0125, FAD‐2010‐0089, FAD‐2010‐0027, FAD‐2010‐0028, FAD‐2009‐0050, FAD‐2010‐0410, FAD‐2010‐0022.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0041.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0025.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0125.pdf; https://ec.europa.eu/jrc/si%20tes/jrcsh/files/FinRep-FAD-2010-0089.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0027.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0028.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2009-0050.pdf; https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2010-0022.pdf
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Undec‐10‐enal, terpineol acetate, d,l‐borneol, d,l‐isomenthone, l‐carvone, d‐camphor, d,l‐isobornyl acetate, 3‐propylidenephthalide, phenylacetic acid, methyl salicylate, thymol, carvacrol, benzothiazole and terpinolene.
Technical dossier/Annex_81_CG_11_10_024_CoAs_conf.
Sampling time: day 1, 7, 14, 21 and 28 (chickens for fattening); day 4, 12, 18, 25, 32 and 39 (piglets); day 1, 6, 12, 20, 26, 33 and 40 (cattle for fattening); day 1, 7 and 14 (Atlantic salmon)
Recovery at 1×: ■■■■■ for poultry, ■■■■■ for piglets, ■■■■■ for cattle for fattening, and ■■■■■ for Atlantic salmon.
Recovery at 3×: ■■■■■ for poultry, ■■■■■ for piglets, ■■■■■ for cattle for fattening, and 89–134% for Atlantic salmon.
Sampling time: day 1, 14 and 28 (chickens for fattening); day 21, 25 and 39 (piglets); day 0, 26 and 40 (cattle for fattening).
Technical dossier/Annex_4_TT_M3_Protocol_Poultry_Conf.
Technical dossier/Annex_9_TT_M3_Report_Poultry_Conf/Recovery of intended values: 1×: ■■■■■ 3×: ■■■■■ 10×: ■■■■■.
Total count for erythrocytes, packed cell volume, haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, total and differential counts for leucocytes, platelet counts.
Sodium, potassium, chloride, calcium, phosphate, magnesium, total protein, albumin, globulin, glucose, uric acid, cholesterol, creatinine, bilirubin, acute phase protein, amylase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), gamma‐glutamyl transferase (GGT), alkaline phosphatase (ALP), and creatine kinase.
Liver, spleen, kidneys, adrenal gland, lung, stomach, pancreas, small intestine, colon, caecum, thymus, thyroid gland, intestinal lymph nodes, ovaries/testes and heart.
Technical dossier/Annex_2_TT_M3_Protocol_Piglet_Conf.
Technical dossier/Annex_7_TT_M3_Report_Piglet_Conf/Recovery of intended values: 1×: 109% (43–167%), 3×: 96% (48–108%); 10×: 91% (60–93%).
Sodium, potassium, chloride, calcium, phosphate, magnesium, total protein, albumin, globulin, glucose, uric acid, cholesterol, creatinine, bilirubin, acute phase protein, amylase, alanine aminotransferase (GPT), aspartate aminotransferase (ASAT), lactate dehydrogenase (LDH), gamma‐glutamyltransferase (GGT), alkaline phosphatase (ALP), creatine kinase, prothrombin time and fibrinogen.
Technical dossier/Annex_8_TT_M3_Report_Cattle_Conf/Recovery of intended values: 1×: ■■■■■ 3×: ■■■■■ 10×: ■■■■■.
Alkaline phosphatase (ALP), amylase, gamma‐glutamyl transferase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (ASAT), lactate dehydrogenase (LDH), creatine kinase (CK), calcium, phosphate, magnesium, potassium, sodium, chloride, cholesterol, lactic acid, albumin, total protein, urea, creatinine, glucose, biliary salts.
Liver, lungs, kidneys and spleen.
Technical dossier/Annex_10_TT_M3_Report_Salmon_Conf/Annex_Sin_1_6_TTM3_Salmon_Trials_Reply.
Technical dossier/Annex_10_TT_M3_Report_Salmon_Conf/Recovery of intended values: 1×: ■■■■■ 3×: ■■■■■; 10×: ■■■■■
Haemoglobin and haematocrit.
Alkaline phosphatase (ALP), alanine aminotransferase (ALT), amylase, aspartate aminotransferase (ASAT), calcium, potassium, chloride, sodium, phosphorus, creatin kinase (CK), C‐reactive protein (CRP), glucose, lactate dehydrogenase (LDH), total protein and bilirubin.
Heart, liver, intestine, spleen, kidney, gonads, gills, eye and bone structure.
Annex_18_TT_M3_Salmon_Raw_data.
Terpineol is a mixture of four isomers: α‐terpineol [02.014], a mixture of (R)‐(+)‐α‐terpineol and (S)‐(−)‐α‐terpineol, β‐terpineol, γ‐terpineol and 4‐terpinenol [02.072] (or δ‐terpineol). The specification for terpineol [02.230] covers α‐, β‐, γ and δ‐terpineol. Composition of mixture: 55–75% α‐terpineol, 16–23% γ‐terpineol, 1–10% cis‐β‐terpineol, 1–13% trans‐β‐terpineol and 0–1% δ‐terpineol (EFSA CEF Panel, 2015c) FGE.18Rev 3.
Technical dossier/2023‐01‐14‐Report_EFSA_TT_M3_Herbal_Conf/page 49.
Technical dossier/2023‐01‐14‐Report_EFSA_TT_M3_Herbal_Conf.
References
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2015a. Scientific Opinion on Flavouring Group Evaluation 25, Revision 3 (FGE.25Rev3): Aliphatic hydrocarbons from chemical group 31. EFSA Journal 2015;13(4):4069, 116 pp. 10.2903/j.efsa.2015.4069 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , 2015b. Scientific Opinion on Flavouring Group Evaluation 78, Revision 2 (FGE.78Rev2): Consideration of aliphatic and alicyclic and aromatic hydrocarbons evaluated by JECFA (63rd meeting) structurally related to aliphatic hydrocarbons evaluated by EFSA in FGE.25Rev3. EFSA Journal 2015;13(4):4067, 72 pp. 10.2903/j.efsa.2015.4067 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012a. Scientific opinion on the safety and efficacy of aliphatic, alicyclic, and aromatic saturated and unsaturated tertiary alcohols and esters with esters containing tertiary alcohols ethers (chemical group 6) when used as flavourings for all animal species. EFSA Journal 2012;10(11):2966, 25 pp. 10.2903/j.efsa.2012.2966 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012b. Scientific Opinion on the safety and efficacy of alicyclic and aromatic lactones (chemical group 11) when used as flavourings for all animal species. EFSA Journal 2012;10(3):2622, 14 pp. 10.2903/j.efsa.2012.2622 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012c. Scientific Opinion on the safety and efficacy of phenyl ethyl alcohols, phenylacetic acids, related esters, phenoxyacetic acids and related esters (chemical group 15) when used as flavourings for all animal species. EFSA Journal 2012;10(3):2625, 16 pp. 10.2903/j.efsa.2012.2625 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012d. Scientific Opinion on the safety and efficacy of benzyl alcohols, aldehydes, acids, esters, and acetals (chemical group 23) when used as flavourings for all animal species. EFSA Journal 2012;10(7):2785, 30 pp. 10.2903/j.efsa.2012.2785 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012e. Scientific Opinion on the safety and efficacy of phenol derivatives containing ring‐alkyl, ring‐alkoxy and side‐chains with an oxygenated functional group (chemical group 25) when used as flavourings for all species. EFSA Journal 2012;10(2):2573, 19 pp. 10.2903/j.efsa.2012.2573 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012f. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534, 26 pp. 10.2903/j.efsa.2012.2534 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2012g. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2013. Scientific Opinion on the safety and efficacy of straight‐chain primary aliphatic alcohols/aldehydes/acids, acetals and esters with esters containing saturated alcohols and acetals containing saturated aldehydes (chemical group 01) when used as flavourings for all animal species. EFSA Journal 2013;11(4):3169, 35 pp. 10.2903/j.efsa.2013.3169 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2015. Scientific Opinion on the safety and efficacy of aliphatic and aromatic hydrocarbons (chemical group 31) when used as flavourings for all animal species. EFSA Journal 2015;13(3):4053, 22 pp. 10.2903/j.efsa.2015.4053 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2016a. Scientific opinion on the safety and efficacy of non‐conjugated and accumulated unsaturated straight‐chain and branched‐chain aliphatic primary alcohols, aldehydes, acids, acetals and esters belonging to chemical group 4 when used as flavourings for all animal species. EFSA Journal 2016;14(8):4559, 22 pp. 10.2903/j.efsa.2016.4559 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2016b. Scientific opinion on the safety and efficacy of secondary alicyclic saturated and unsaturated alcohols, ketones, ketals and esters with ketals containing alicyclic alcohols or ketones and esters containing secondary alicyclic alcohols from chemical group 8 when used as flavourings for all animal species. EFSA Journal 2016;14(6):4475, 26 pp. 10.2903/j.efsa.2016.4475 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2016c. Scientific opinion on the safety and efficacy of thiazoles, thiophene and thiazoline belonging to chemical group 29 when used as flavourings for all animal species. EFSA Journal 2016;14(6):4441, 16 pp. 10.2903/j.efsa.2016.4441 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Bastos M, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, de Knecht J, Kolar B, van Beelen P, Padovani L, Tarres‐Call J, Vettori MV and Azimonti G, 2019. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(4):5648, 78 pp. 10.2903/j.efsa.2019.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]


