INTRODUCTION
Most of the cumulative nutritional deficits in preterm infants occur in the first 2 weeks after birth. Limited enteral nutrition during the first 2 weeks is one of the critical barriers to solving the problem of postnatal growth faltering at term equivalent age among preterm infants. Observational data reveal that critically ill preterm infants rarely receive adequate nutritional intakes during early postnatal life.1 Observational data also indicate that the cumulative nutritional deficits in energy and protein intake that occur soon after birth account for approximately 50% of the decline in weight-for-age observed from birth to hospital discharge2 and may explain the slow growth and increased risk of adverse outcomes observed in preterm infants.1
Meta-analyses of randomized trials designed to establish full enteral nutrition during the first 2 weeks after birth consistently show that early (before or on postnatal day 4)3 and faster (30–40 mL/kg/d) progression of enteral feeding volumes4 increases growth velocity rates from birth to hospital discharge and increases weight-for-age z scores at term equivalent age. In addition, higher volume feedings may allow for catch-up growth beyond the initial postnatal period. Observational data also suggest that enteral nutrition effectively prevents cumulative nutritional deficits in critically ill preterm infants, which may mitigate the risk of late-onset sepsis, bronchopulmonary dysplasia (BPD), neurodevelopmental impairment, and death.1
Despite this collective evidence that early full enteral nutrition reduces the risk of growth faltering and may lower the risk of adverse outcomes, there is marked variability across neonatal units in enteral nutrition practices during the acute phase of illness after birth in preterm infants. The severity of illness and concern for related complications, including spontaneous intestinal perforation (SIP) and necrotizing enterocolitis (NEC), are often cited as the main indications to limit enteral nutrition in these infants.
ENTERAL NUTRITION AND NECROTIZING ENTEROCOLITIS: AN EPIDEMIOLOGIC PERSPECTIVE
Establishing full enteral nutrition soon after birth in preterm infants is difficult without an epidemiologic perspective. Countless reports that enteral feeding is the final event before an infant is diagnosed with NEC are often interpreted as evidence that not offering enteral feeding can prevent NEC. This interpretation may reflect circular reasoning and highlights the importance of establishing differences between association and causation.5 Enteral feeding occurs in 90% of infants diagnosed with NEC, but this report is not proof that exposure to enteral feeding explains 90% of all NEC cases. This finding is evidence that exposure to enteral feeding—which occurs every 2 to 3 h in neonatal units—is common among preterm infants. High exposure to enteral feeding is also observed in infants who never develop NEC. With these high exposure rates to enteral feeding, the occurrence of NEC in proximity to enteral feeding is predictable.
Furthermore, observing that enteral feeding precedes the diagnosis of NEC only establishes temporality in the association. Other criteria such as specificity (ie, enteral feeding is not exclusively associated with NEC), consistency (ie, not all experimental evidence suggests an increased risk of NEC with enteral feeding), or strength (ie, not all infants that receive enteral feeding develop NEC) are needed to establish a causal association.5
Defining the causes of NEC requires a new framework around the principle of multicausality. Like many other complications of prematurity, such as BPD, NEC has multiple causes. Decades of research have proven that enteral feeding is not the only risk factor for NEC. Other risk factors include fetal growth restriction,6 persistent hypoxemia,7 severe anemia,8 and dysbiosis due to frequent exposure to broad-spectrum antibiotics.9 Because these events play a role in the occurrence of NEC, enteral feeding should be considered a component cause along with several others (Fig. 1).
Fig. 1.
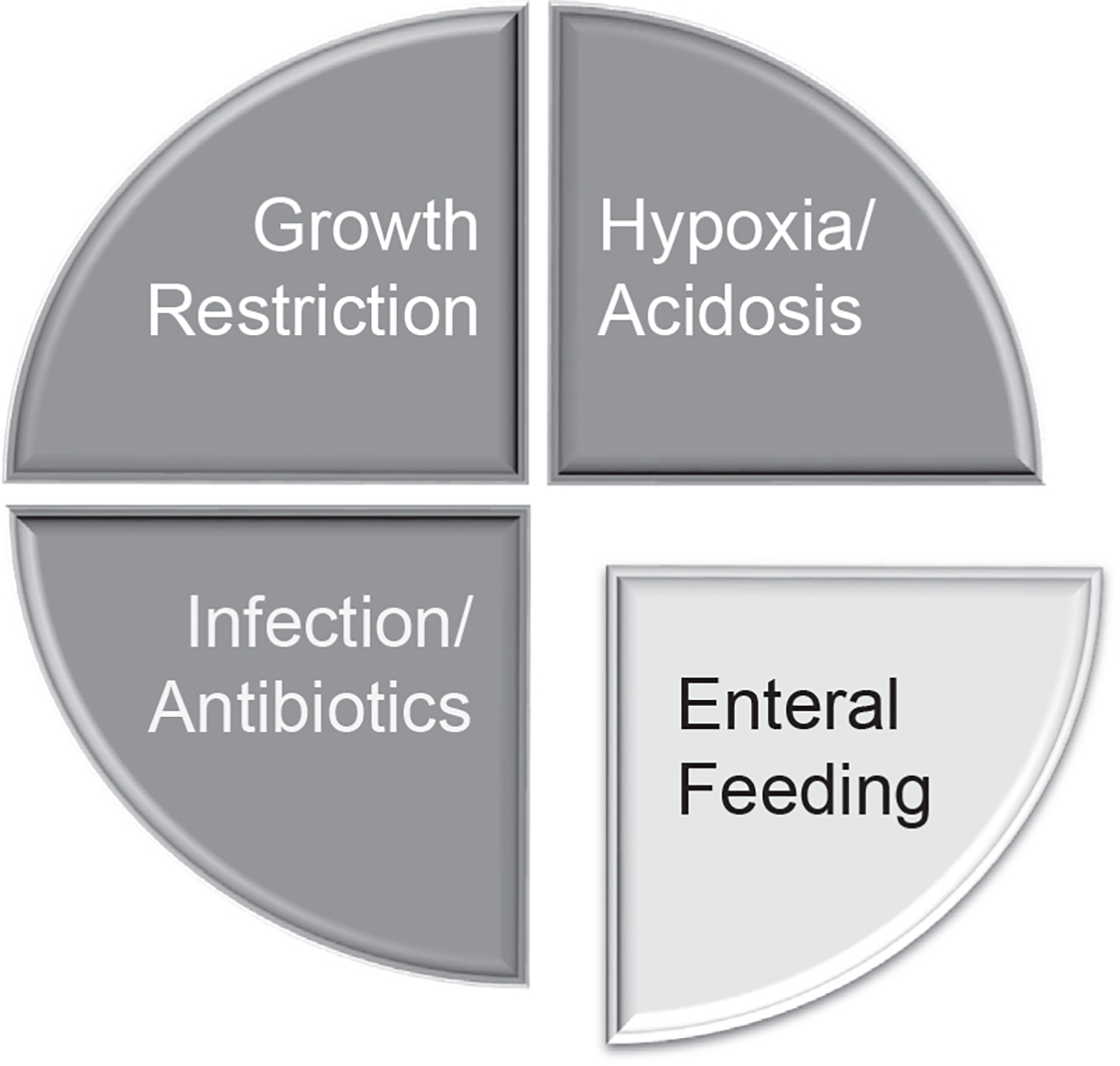
Multicausality in necrotizing enterocolitis.
Multicausality implies that only certain NEC cases can be attributed to enteral feeding, even in clinical scenarios where enteral feeding is the final event before the diagnosis of NEC. With more clinical trials reporting on specific elements of enteral feeding that mitigate the risk of NEC (human milk feeding, arginine, probiotics, and others), the proportion of NEC cases attributed to enteral feeding is decreasing. If this trend continues in subsequent decades, the prevalence of NEC will likely decline, and the proportion of NEC cases attributed to non-feeding factors will likely increase.
As NEC cases decrease, SIP is set to become the most common major intestinal morbidity among preterm infants.10 SIP typically occurs in the first postnatal week when clinicians are initiating enteral feeding. However, early enteral feeding is rarely implicated in the pathogenesis of SIP. The etiology of SIP is also multifactorial and shares some risk factors with NEC including growth restriction and perinatal asphyxia. Bowel ischemia in vulnerable watershed areas, which may occur prenatally or postnatally, is usually the final common pathway leading to bowel perforation. In addition, certain medications are associated with SIP such as the early administration of steroids.10
ENTERAL NUTRITION AND FEEDING INTOLERANCE IN PRETERM INFANTS
The transition from parenteral to enteral nutrition often begins with minimal enteral feeding or trophic feeding (≤24 mL/kg/d), then changes to progressive feeding (increments of feeding volumes usually by 10–35 mL/kg/d each day), and concludes with full enteral feeding (≥120–150 mL/kg/d)11–14 (Fig. 2).
Fig. 2.
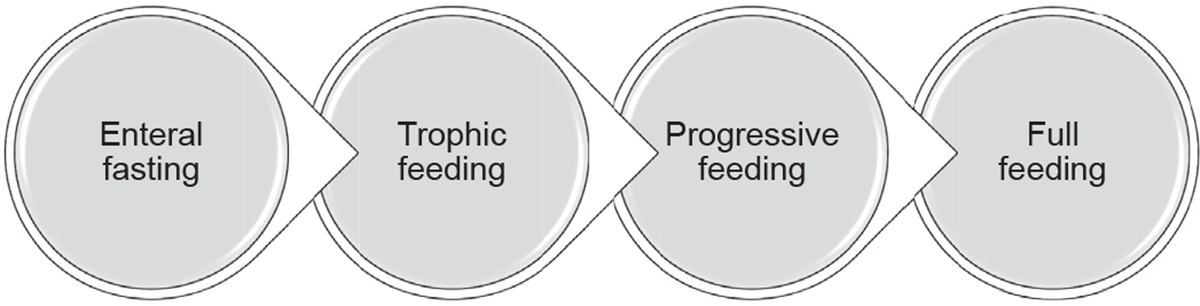
Enteral nutrition in preterm infants.
Development of feeding intolerance is a common symptom of NEC. However, during the transition to full enteral feeding up to 50% of infants experience feeding intolerance.3 Although the inability to absorb and digest human milk or formula in preterm infants is often cited as one of the primary causes,3,15 dysmotility is the most common cause of feeding intolerance. Unlike digestion and absorption, gastrointestinal motility is underdeveloped in most infants born before 28 weeks of gestation16 (Fig. 3).
Fig. 3.
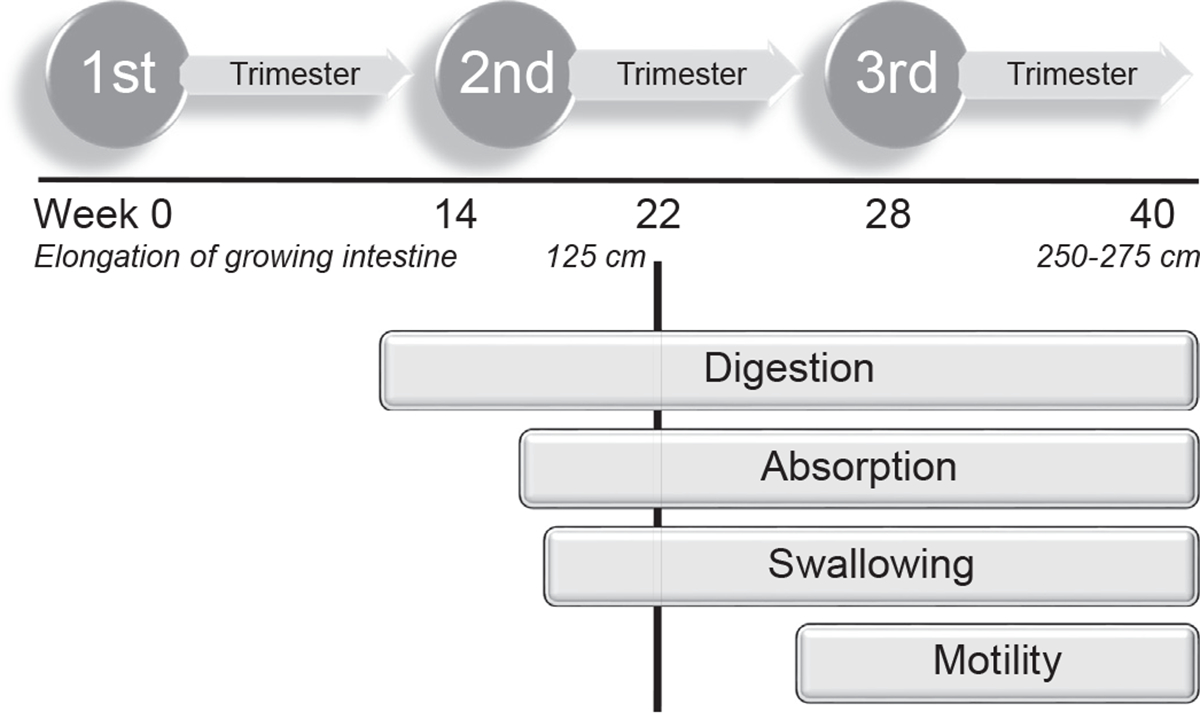
Functional development of the gastrointestinal tract.
High pre-feed gastric residual volume is a common indication to withhold enteral feeding in preterm infants, but a high level of evidence does not support this practice. The routine assessment of gastric residual volumes likely disrupts the natural production of hydrochloric acid in the stomach. Without an acidic gastric environment, preterm infants are at increased risk of bacterial colonization/overgrowth17,18 and feeding intolerance due to the inactivation of the enzyme pepsin that digests protein-containing milk.19 Limited clinical evidence consistently shows that optimizing gastric acidity reduces the risk of gastrointestinal complications. Not checking gastric residual volumes,20,21 re-feeding gastric residual volumes,13 and adding hydrochloric acid to infant formula17 have been associated with a lower risk of feeding intolerance and NEC in randomized clinical trials. Observational studies also reported that avoiding antacid therapy is associated with a lower risk of NEC.22
In settings where the routine assessment of gastric residual volumes is no longer practiced, abdominal distension and emesis are the most common indications to withhold enteral feeding, particularly in preterm infants managed with noninvasive ventilation soon after birth. Although aerophagia and gaseous bowel distension occur in up to 83% of extremely preterm infants managed with noninvasive ventilation,23 there is no evidence from randomized trials that noninvasive ventilation increases the risk of SIP or NEC.24
Routine abdominal radiographs have limited value in assessing feeding intolerance and NEC.23 Several methods, including electrogastrography, abdominal tissue oxygenation using near-infrared spectroscopy, and bowel sound/acoustics, are being evaluated as diagnostic tools to assess feeding intolerance and identify infants at higher risk of intestinal disease.24,25 Until the validity of these methods is sufficient to establish a risk stratification system, standardizing the clinical assessment of feeding intolerance with checklists that include questions about nutrition, motility, ischemia, and sepsis should be considered (Box 1)
Box 1.
Clinical assessment of feeding intolerance
| Nutrition | Has the patient received any formula feeds? Is the infant receiving more than trophic feeds (>25 mL/kg/d)? |
| Motility | Are the bowel sounds hypoactive or absent? Are there visible bowel loops? Is there abdominal distension with discoloration? Has the infant had an increase in abdominal girth? Has the infant had constipation? Has the infant had a bloody stool? |
| Ischemia | Is the infant’s urine output low? Is the infant receiving support with vasopressors? Is the infant’s lactate high? Is the infant showing signs of hypotension? |
| Sepsis | Is the infant experiencing abnormal heart rate characteristics? Has the infant had a relative increase in oxygen requirement? Is the infant having more apneas and bradycardia? |
CLINICAL EVIDENCE FOR FEEDING PROTOCOLS
This section summarizes evidence from randomized clinical trials in a questions and answers format and addresses specific clinical questions frequently discussed during bedside rounds.
Should We Advocate for Enteral Fasting to Reduce the Risk of Necrotizing Enterocolitis?
Enteral fasting was previously considered a protective strategy to mitigate the risk of NEC in critically ill infants25 because enteral feeding can alter splanchnic perfusion, increase the risk of ischemic injury,26 cause osmotic injury to the mucosa, and promote bacterial overgrowth if enteral feeding results in the presence of undigested substrate in the intestinal lumen.27,28 Subsequently, animal and clinical studies revealed a positive linear correlation between small feeding volumes of 12 to 50 mL/kg and gastrointestinal hormones that promote mucosal maturation.29,30
Multiple randomized clinical trials have since confirmed that early initiation of trophic feeding (ie, within the first 96 hours after birth) is a safe alternative to enteral fasting for critically ill preterm infants. In 2013, data from 9 randomized clinical trials of early initiation of trophic feeding with volumes up to 24 mL/kg/d in 754 very low birthweight infants were included in a meta-analysis.31–40 NEC as an outcome was examined in the 9 trials included. Compared to enteral fasting, trophic feeding did not increase the risk of NEC (8% vs. 8%; risk ratio [RR]: 1.07; 95% confidence interval [CI]: 0.67–1.70; N = 748). Eight trials examined the time to establish full enteral feeding. In 3 trials, trophic feeding reduced the time to establish full enteral feeding.33,36,39 Two trials reported data on sepsis. One noted fewer episodes of culture-positive sepsis in the trophic feeding group.36 Mortality did not differ between groups (RR: 0.77; 95% CI: 0.46–1.30). The time to regain birthweight was not shorter in any of the 9 trials included in the meta-analysis.
This meta-analysis detected considerable heterogeneity across trials and included only a few extremely preterm infants. Still, no additional trials on this topic are anticipated. Feeding protocols should not favor a delay in initiation of trophic feeding beyond the first 96 hours after birth, even in critically ill infants with a history of low Apgar scores, acidosis, respiratory distress syndrome, hemodynamic instability, and/or persistent patency of the ductus arteriosus (PDA). Prolonging enteral fasting results in villous atrophy and complicates subsequent feeding. An unmasked trial excluded from all meta-analyses on early enteral feeding compared the effects of late minimal enteral feeding and progressive feeding after prolonged enteral fasting in 144 preterm infants born between 1996 and 2000. Because enteral feeding in both groups started after approximately 10 days of enteral fasting, all infants included in this trial likely had villous atrophy at the time of initiation of enteral feeding. Minimal enteral feeding compared to progressive feeding after prolonged enteral fasting delayed the establishment of full enteral feeding by approximately 13 days, extended hospital stay (mean difference between groups: 11 days), and resulted in a trend for a lower risk of NEC (RR:0.14; CI: 0.02–1.07).41 However, these findings require a cautious interpretation.42 Only 30% of infants in the trial received a human milk diet, and nearly 50% did not benefit from exposure to antenatal steroids. The use of antenatal steroids43 and exclusive human milk feeding with either maternal or donated milk44—both effective strategies that reduce the risk of NEC—have increased substantially over the past 2 decades. Furthermore, late initiation of enteral feeding is now strongly discouraged, and the presence of umbilical catheters or infusions of vasopressor agents are no longer contraindications to initiate enteral feeding.45,46
Should We Promote Enteral Fasting During Patency of the Ductus Arteriosus Treatment or Blood Transfusions?
The evidence favoring enteral fasting to prevent NEC during PDA treatment or blood transfusions is predominantly limited to single-center observational studies with inconsistent results. Although it remains uncertain if enteral fasting can modify the baseline risk of NEC among high-risk infants requiring PDA treatment or blood transfusions, there is evidence from randomized trials that enteral fasting during PDA treatment increases the time to establish full enteral feeding and does not decrease the risk of NEC.47 The potential benefits of enteral fasting to mitigate the risk of NEC during blood transfusions are currently being investigated in a randomized clinical trial.48 However, it remains uncertain if intermittent exposure to blood transfusions transiently increases the risk of NEC.49 Hemoglobin values below 7 g/dL and severity of anemia before a blood transfusion may be associated with a higher risk of NEC rather than the number of blood transfusions.8
Does Extending Enteral Fasting After Nonsurgical or Surgical Necrotizing Enterocolitis Prevent Complications Associated with Necrotizing Enterocolitis?
Observational studies recently questioned the benefits of prolonged enteral fasting after nonsurgical50 and surgical NEC.51 Shortening the duration of enteral fasting by 4 days (from 9 to 5 days) was associated with a 35% decrease in the time to establish full enteral feeding and a reduced need for central venous access after the diagnosis of nonsurgical NEC.50 However, post-NEC strictures were not significantly lower after shortening the duration of enteral fasting (4% vs. 13%).50 Similarly, the early reintroduction of enteral feeding after surgical NEC (≤7 days) was associated with a reduced need for parenteral nutrition 28 days postsurgical intervention in unadjusted analyses. In addition, exposure to early enteral feeding after surgical NEC was not associated with a higher risk of mortality (5% vs. 2%).51 Clinical trials comparing shorter (3–5 days) versus extended (7–10 days) periods of enteral fasting after NEC are warranted.
If trophic feeding prevents villous atrophy, should we extend the duration of trophic feeding to reduce the risk of feeding intolerance and NEC?
By initiating a 3- to 7-day course of trophic feeding within the first 96 hours after birth,11 most clinicians assume that preventing villous atrophy with an extended duration of trophic feeding will reduce the risk of feeding intolerance and NEC in preterm infants.41,52,53 However, data from a recently updated meta-analysis of 14 randomized trials challenge this assumption. In the meta-analysis, early progressive feeding was defined as the introduction of small increments of feeding volumes within the first 96 hours after birth. The trials included in the meta-analysis are summarized in Table 1.
Table 1.
Randomized trials of early progression of enteral feeding1
| Study | Year Study Population | Average GA | Initiation/Progression of Feeding |
|---|---|---|---|
| Ostertag et al,89 | 1986 38 infants with BW < 1500 g | 28 wk (range: 26–32) | Initiation and progression on day 1 vs. day 7 |
| Khayata et al,88 | 1987 12 infants with BW < 1500 g | Initiation and progression on day 2 vs. day 5 | |
| Davey et al,85 | 1994 62 infants with BW < 2000 g in stable condition | 28.5 ± 3 wk | Initiation and progression on day 2 vs. day 5 |
| Karagianni et al,87 | 2010 125 infants with GA 27–34 wk and BW < 10th percentile | 31 wk (IQR: 27–34) | Initiation and progression on day 2 vs. day 7 |
| Perez et al,90 | 2011 239 stable infants with GA 27–32 wk and BW < 1500 g | 30 ± 2 wk | Initiation and progression on day 2 vs. day 5 |
| Leaf et al,55 | 2012 404 infants with GA <35 wk and BW < 10th percentile | 31 ± 2 wk | Initiation and progression on day 2 vs. day 5 |
| Abdelmaaboud et al,81 | 2012 125 infants with GA <37 weeks and BW < 10th percentile | 34 ± 3 wk | Initiation and progression on day 3 vs. day 6 |
| Armanian et al,82 | 2013 82 infants with BW < 1500 g | 30.5 ± 2 wk | Progression on day 4 vs. day 10 |
| Arnon et al,83 | 2013 60 infants with GA <37 weeks and BW < 10th percentile | 32 wk (IQR: 29–34) | Early initiation (<24 h) vs. late initiation |
| Dinerstein et al,86 | 2013 62 infants with BW < 1500 g | 32 wk (IQR: 29–34) | Progression on day 2 vs. day 5 |
| Srinivasan et al,91 | 2017 32 infants with GA < 37 wk and SGA | 32 wk (SD: 2) | Progression on day 2 vs. day 5 |
| Salas et al,56 | 2018 60 infants with GA <28 wk | 26 wk (IQR: 24–28) | Early initiation (<72 h), progression on day 2 vs. day 5 |
| Tewari et al,92 | 2018 62 infants with GA 27–32 wk and SGA | 30 wk (range: 27–32) | Progression on day 1–2 vs. day 5–6 |
| Bozkurt et al,84 | 2020 219 infants with BW < 1251 g | 27 wk (range: 29–34) | Progression on day 2 vs. day 6 |
Abbreviations: BW, birthweight; GA, gestational age; IQR, interquartile range; SGA, small for gestational age.
Compared with delayed progression of feeding (after the first 96 hours), early progressive feeding reduced the time to full enteral feeding by 3 days on average without increasing the risk of NEC (8% vs. 7%; RR: 1.17, 95% CI: 0.80–1.70).3 Six trials included in the meta-analysis also determined the effects of early progressive feeding on feeding intolerance. Data from 581 infants revealed an increased risk of feeding intolerance with early progressive feeding (51% vs. 41%; RR: 1.22, 95% CI: 1.02–1.46). Seven trials reported sepsis data (n = 872). Early progressive feeding compared to delayed progressive feeding reduced the risk of severe infection (22% vs. 31%; RR: 0.70, 95% CI: 0.56–0.87; P = .001). With an absolute risk reduction of 9% in the sepsis outcome, the number needed to treat with early progressive feeding to prevent one case of severe infection is 11.3 Because preventing severe infections is more critical than preventing feeding intolerance among preterm infants, the current evidence supports the early progression of enteral feeding volumes within the first 96 hours after birth and questions the benefits of extending the duration of trophic feeding.
Although several trials in the meta-analysis did not include infants at the highest risk of NEC, the largest randomized trial on early progressive feeding included only highrisk preterm infants with evidence of intrauterine growth restriction (n = 404), and another randomized trial included only high-risk preterm infants 28 weeks of gestation or less (n = 60). They were not powered for significant outcomes, but the results were consistent with the direction of effect for the outcomes reported in the meta-analysis.54–56 These randomized clinical trials in infants considered at the highest risk of NEC due to either ischemia or extreme prematurity reported a potential reduction of culture-proven sepsis after early progression of enteral feeding volumes. Among extremely preterm infants, the risk difference in culture-proven sepsis (10% vs. 27%; P = .18) and culture-proven sepsis or death (13% vs. 27%; P = .20) did not reach statistical significance but favored the early progressive feeding group. The outcome of NEC or death did not differ between groups (27% vs. 20%; P = .56).56
The meta-analysis concluded that the effects of early progression of feeding volumes on long-term growth or neurodevelopment are unknown.3 However, early feeding is associated with a lower risk of nosocomial sepsis,57 mortality, and neurodevelopmental impairment in early childhood.58 Therefore, it is plausible that early progression of enteral feeding volumes—by reducing sepsis—might reduce mortality and neurodevelopmental impairment in later childhood. Additional evidence from randomized trials on this topic is needed.
Does Slow Progression of Enteral Feeding Prevent Necrotizing Enterocolitis?
In randomized trials, slower rates of feeding progression (10–24 mL/kg/d) have been compared with faster rates (25–40 mL/kg/d). A 2021 Cochrane meta-analysis reviewed the cause–effect relationship between feeding progression rates and NEC risk.4 The meta-analysis included 4033 infants from 14 randomized trials. Five trials, including the largest randomized trial,59 enrolled only infants with birthweight less than 1500 g. The remaining trials listed birthweights ranging from less than 1000 g to 1000 to 2000 g as inclusion criteria. One trial included infants with gestational ages between 30 and 34 weeks. Another trial compared 15 mL/kg/d rates versus 35 mL/kg/d rates only in formula-fed infants.60 Five trials did not include infants receiving formula. The remaining trials included infants receiving some human milk.
No significant differences in the risk of NEC were found between infants fed at slower or faster rates (6% vs. 5%; RR: 1.06, 95% CI: 0.83–1.37). Subgroup analyses of extremely low-birthweight infants, small-for-gestational-age infants, and infants with intrauterine growth found similar results, suggesting the generalizability of the findings to these high-risk populations. The effects of faster feeding progression rates on mortality, growth, neurodevelopment, and other comorbidities were also analyzed. Thirteen trials with mortality data did not find a risk reduction in mortality rates with slower feeding progression rates. Ten trials revealed that infants in the slower feeding progression group took longer to regain birthweight, but the largest randomized trial reported no differences in growth at the time of hospital discharge.59 The 2021 meta-analysis also found an increased risk of feeding intolerance in infants fed at slower rates, though the certainty of this evidence was graded as low. Four trials reported longer lengths of stay in the group with slower rates.
The Speed of Increasing milk Feeds Trial (SIFT) contributed approximately 70% of the patients in the meta-analysis and was the only one that reported neurodevelopmental outcomes.59 SIFT was a large, multicenter trial that enrolled 2804 patients across the United Kingdom and the Republic of Ireland. Infants less than 32 weeks of gestation or who had a birthweight of less than 1500 g were eligible and were randomized to receive either slow (18 mL/kg/d) or faster (30 mL/kg/d) feeding progression rates. The primary outcome of the trial was survival without moderate or severe neurodevelopmental disability at 24 months corrected age. Data were available for the primary outcome in 88% of recruited infants. No significant differences in survival or severe or moderate disability were found, but diagnosis of cerebral palsy was more frequent among infants in the faster feeding progression group (5.4% vs. 3.2%; P = .015). Infants in the faster feeding progression group reached full enteral feeding sooner and had fewer days of parenteral nutrition. Neither the analysis with all infants (n = 2793) nor the subgroup analysis with extremely preterm infants (n = 994) found an increased risk of NEC in the faster-feeding progression group.
Does, Early, Full Enteral Nutrition Increase the Risk of Necrotizing Enterocolitis?
As noted previously, randomized clinical trials demonstrated that early and faster progression of enteral feeding volumes does not increase the risk of adverse outcomes and may provide benefits in preterm infants.3,59 With the availability of donated milk and the recognition that intravenous access increases the risk of sepsis, clinical trials have examined early full enteral feedings without requiring intravenous fluids or total parenteral nutrition. So far, 6 randomized clinical trials have been conducted in the past decade, including a combined total of 526 infants.61
In these trials, early full enteral feeding was typically defined as feeding volumes of 60 to 80 mL/kg/d beginning soon after birth. The control group received early progressive feeding with advances of 20 to 30 mL/kg/d until the establishment of full enteral feeding. All of these trials were conducted in resource-limited settings in India, and in 4 of the 6 trials, infants with insufficient maternal milk were supplemented with formula, whereas in 2 trials, donated milk was used to supplement maternal milk. None of the 6 trials included in the meta-analysis of randomized clinical trials masked group assignment.
Early full enteral feeding was associated with a higher weight z-score at discharge compared with early progressive feeding (risk difference [RD]: 0.24; 95% CI: 0.06–0.42). Infants in the early full enteral feeding group also regained birth weight faster (mean difference [MD]: −3 days; 95% CI: −4 to −2 days). In the largest randomized clinical trial of 180 very preterm infants, early full enteral feeding increased growth velocity somewhat (mean difference, 1.2 g/kg/d).62 In the meta-analysis of randomized clinical trials, there was no difference in the rate of feeding intolerance (16% vs. 22%; RR: 0.74; 95% CI: 0.49–1.13; n = 393) or NEC between groups (3% vs. 3%; RR: 0.98; 95% CI: 0.38–2.54; n = 522).61 There was also no difference in the rate of sepsis between groups (7% vs. 9%; RR: 0.72; 95% CI: 0.36–1.46; n = 359). Furthermore, there was no difference in hospital mortality between the groups (RR: 0.78; 95% CI: 0.36–1.70; n = 526). The practice of early full enteral feeding was associated with a decreased length of stay (mean difference, −3 days; 95% CI, −2 to −4 days).
These trials examined shorter-term hospital outcomes, but later growth and neurodevelopmental outcomes were not reported. Overall, these trials included few extremely preterm infants at the highest risk of adverse outcomes in high-income countries. Besides weight at discharge, anthropometric growth measures were not consistently reported in the included trials. Further trials that supplement maternal milk with donated milk in populations at high risk of NEC are recommended before adopting this practice in high-income countries. There are 2 such ongoing randomized clinical trials comparing early full enteral feeding to conventional feeding progression in the United States (E3NACT trial, NCT04337710) and the United Kingdom (FEED1 trial, ISRCTN89654042).
Does Limiting Enteral Intakes to Up 160 mL/kg/d Reduce the Risk of Necrotizing Enterocolitis?
Higher volume feedings are an emerging strategy to improve postnatal growth without increasing the risk of NEC. Minimum volumes of 120 mL/kg/d and maximum feeding volumes of 200 mL/kg/d have been proposed due to the risks of dehydration and overhydration, respectively.63,64 The typical volumes that reduce postnatal growth faltering differ between international guidelines and geographic locations. In North America, feeding volumes of approximately 140 to 160 mL/kg/d of fortified human milk or preterm formula are typical.65 Units in Europe, Australia, and New Zealand are more likely to use 161 to 180 mL/kg/d volumes. Few units in high-income countries with access to safe fortification use volumes above 180 mL/kg/d. However, feeding volumes up to 300 mL/kg/d are used internationally and have been reported to improve postnatal growth without evidence of fluid overload.66
Fluid overload has been associated with multiple adverse outcomes in preterm infants. In a meta-analysis of randomized clinical trials, restrictive fluid volume reduced the risk of patent ductus arteriosus, necrotizing enterocolitis, and intracranial hemorrhage compared with more liberal use of fluid.67 Observational studies also report an association between excessive early fluid administration and adverse pulmonary outcomes, including BPD.68 The proposed mechanism includes maintaining patency of the PDA and pulmonary edema from fluid overload. However, many of the studies of fluid volume reflected the use of early parenteral fluid administration rather than enteral feeding volumes. A randomized clinical trial among 60 preterm infants with bronchopulmonary dysplasia did not demonstrate a benefit of restricting fluids, although the trial was not powered for major neonatal morbidities.69
Several randomized clinical trials have compared higher with usual volume feedings in preterm infants.66,70–72 The usual volume in high-income countries was approximately 140 to 160 mL/kg/d, with a higher volume defined as 180 to 200 mL/kg/d. In contrast, one trial conducted without human milk fortification defined the usual volume as 200 mL/kg/d and the higher volume as 300 mL/kg/d.66 In a meta-analysis of randomized clinical trials, the authors defined usual volume fortified feedings as ≤180 mL/kg/d and higher volume fortified feedings as greater than 180 mL/kg day and included 2 studies.73 The trial comparing 200 versus 300 mL/kg/d was included in a separate analysis of higher volume unfortified feedings that used a cut-off of ≤200 mL/kg/d in the lower group and greater than 200 mL/kg/d for the higher group.
Higher volume feedings increased growth velocity in preterm infants compared with usual volume feedings (MD: 2.6 g/kg/d; 95% CI: 1.4–3.8; n = 271). Other growth measures, including length and head circumference, did not differ statistically between groups, although in the largest study (n = 224) of feeding volumes included in the meta-analysis, linear growth, head circumference, and mid-arm circumference were significantly higher at discharge.70,73 Improvements in postnatal growth failure at discharge did not reach significance (RR: 0.71; 95% CI: 0.50–1.02; n = 271).73 In a secondary analysis of 86 of the 224 participants in the aforementioned higher volume feeding trial, there was no significant difference in the proportion of fat-free mass or percentage of body fat at discharge, suggesting that the increase in growth with higher volume feedings was not simply due to gains in fat.74
Trials of higher volume feedings have not reported increased risks of adverse outcomes. Fluid retention and edema rates did not differ between groups in one trial.71 The largest trial on higher volume feedings found no difference in rates of PDA, BPD, the duration of respiratory support, or length of stay.70 One trial reported a decreased length of stay with higher volume feedings in small for gestational age moderate-late preterm infants.72 Other trials reported no difference in rates of tachypnea or PDA.66 No difference in rates of NEC or feeding intolerance has been attributed to higher volume feedings after the establishment of full enteral feedings.73 Limitations of these trials included the lack of masking and different growth measures used. Significantly, none of the studies were powered for major prematurity outcomes, and most included few extremely preterm infants at the highest risk. Only one study reported neurodevelopmental outcomes, with no difference observed at 12 months of corrected age.71
FUTURE DIRECTIONS
There are multiple evidence-based potentially better nutritional practices that may reduce the time to full enteral feeding, reduce the need for parenteral nutrition, reduce the duration of central venous access, lower the risk of sepsis, and even reduce the length of hospital stay. Lack of masking introduces the possibility of bias and differential misclassification that might be expected to favor the traditional delayed and slower introduction of feedings. However, evidence from randomized clinical trials does not suggest that early progressive feeding, faster feeding rates, early full enteral feeding, or higher-volume feeding increase the risk of NEC. The baseline risk of NEC of approximately 3% to 8% in both groups of the clinical trials discussed above suggests a relatively high residual prevalence of NEC unexplained by feeding interventions and a need to investigate the pathogenesis of NEC further.
Although prolonged enteral fasting and the subsequent development of villous atrophy may have exacerbated the risk of NEC attributed to enteral feeding in earlier feeding trials, the increasing practice of exclusive human milk feeding in the latest trials is likely mitigating this risk. Only human milk feeding compared to formula feeding significantly reduces the risk of NEC. A recently updated meta-analysis found a nearly 50% relative risk reduction of NEC with human milk feeding (from 6.8% to 3.6%).44 There are potential benefits of early and fast progression of enteral feeding, assuming adequate availability of exclusive human milk.75 Early establishment of full enteral feeding using maternal or donated milk could lower the risk of feeding intolerance, NEC, and sepsis. High-risk preterm infants could benefit from an early progression of enteral feeding volumes by 20 to 25 mL/kg/d, ideally within the first 72 hours after birth. Moderate-risk preterm infants could be started on 30 to 40 mL/kg of enteral feeding within the first 24 hours after birth and have their feeding volumes increased daily by 30 to 40 mL/kg/d. Low-risk preterm infants could benefit from starting full enteral feeding volumes of 60 to 80 mL/kg/d within 24 hours after birth, avoiding the need for parenteral nutrition. This risk stratification strategy could decrease the wide variation in feeding practices.
Multicenter trials of early progressive feeding, full early enteral feeding, and higher volume feeding, including infants at the highest risk of adverse outcomes, are warranted. Without these adequately powered multicenter trials assessing longer-term outcomes of feeding practices, clinicians will continue to develop feeding protocols based on observational studies that are more susceptible to bias than randomized trials.76 The need and timing of human milk fortification,77 ideally based on postnatal age rather than volume, will also need further investigation if the goal of early full enteral feeding is achieved with new feeding protocols. Higher enteral protein intake might be required to minimize the risk of cerebral palsy reported in infants exposed to faster feeding rates.59 Because human milk feeding,44 probiotic administration,78 and arginine supplementation79,80 are the only feeding interventions that have shown superiority for the risk reduction of NEC, future trials should consider noninferiority designs to detect significant differences in the outcome of NEC.
SUMMARY
Preterm infants frequently experience malnutrition in the days and weeks after birth which may be preventable. Increasing energy and protein intake could attenuate critical illness, improve growth, and decrease the risk of short-term and long-term morbidity. The central aim of standardized feeding protocols should be the prevention of cumulative nutritional deficits in critically ill infants. Achieving this aim requires a shift in the current standards of care delivered to these infants. Thousands of preterm infants have been randomized to identify potentially better feeding practices that improve growth without increasing the risk of NEC. Although further randomized controlled trials are anticipated to optimize feeding strategies in the most vulnerable extremely preterm populations, there is sufficient data to standardize nutritional practices and improve growth for most very preterm infants.
KEY POINTS.
Some feeding protocols advance enteral nutrition very slowly in preterm infants.
Emerging evidence from randomized clinical trials indicates that slower and later progression of enteral nutrition does not mitigate the risk of necrotizing enterocolitis.
Promoting the early establishment of full enteral nutrition reduces the risk of invasive infections.
Feeding protocols can improve short- and long-term outcomes by decreasing practicevariability and incorporating new clinical evidence favoring the early establishment of full enteral nutrition.
Best practices.
High-risk preterm infants could benefit from an early progression of enteral feeding volumes by 20 to 25 mL/kg/d, ideally within the first 72 hours after birth.
Moderate-risk preterm infants could be started on 30 to 40 mL/kg of enteral feeding within the first 24 hours after birth and have their feeding volumes increased daily by 30 to 40 mL/kg/d.
Low-risk preterm infants could benefit from starting full enteral feeding volumes of 60 to 80 mL/kg/d within 24 hours after birth, avoiding the need for parenteral nutrition.
DISCLOSURE
The authors have no conflicts of interest relevant to this article to disclose. A.A. Salas received honoraria from the Lockwood Group for participation in Mead Johnson advisory board meetings and filed a patent for an instrumented feeding bottle. He is currently supported by the National Institute of Child Health and Human Development, United States (K23HD102554). C.P. Travers is currently supported by the National Heart, Lung, and Blood Institute, United States (K23HL157618).
Abbreviations
- NEC
Necrotizing enterocolitis
- SIP
Spontaneous intestinal perforation
REFERENCES
- 1.Ehrenkranz RA, Das A, Wrage LA, et al. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr Res 2011;69(6):522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics 2001;107(2):270–3. [DOI] [PubMed] [Google Scholar]
- 3.Young L, Oddie SJ, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev 2022;1:CD001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oddie SJ, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev 2021;8:CD001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothman KJ. Epidemiology : an introduction. New York: Oxford University Press; 2002. p. 223, viii. [Google Scholar]
- 6.Kamoji VM, Dorling JS, Manktelow B, et al. Antenatal umbilical Doppler abnormalities: an independent risk factor for early onset neonatal necrotizing enterocolitis in premature infants. Acta Paediatr 2008;97(3):327–31. [DOI] [PubMed] [Google Scholar]
- 7.Askie LM, Darlow BA, Finer N, et al. Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA 2018;319(21):2190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel RM, Knezevic A, Shenvi N, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA 2016;315(9):889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dierikx TH, Deianova N, Groen J, et al. Association between duration of early empiric antibiotics and necrotizing enterocolitis and late-onset sepsis in preterm infants: a multicenter cohort study. Eur J Pediatr 2022;181(10):3715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson JR, Hair A, Clark RH, et al. Spontaneous intestinal perforation (SIP) will soon become the most common form of surgical bowel disease in the extremely low birth weight (ELBW) infant. J Perinatol 2022;42(4):423–9. [DOI] [PubMed] [Google Scholar]
- 11.Salas AA, Kabani N, Travers CP, et al. Short versus extended duration of trophic feeding to reduce time to achieve full enteral feeding in extremely preterm infants: an observational study. Neonatology 2017;112(3):211–6. [DOI] [PubMed] [Google Scholar]
- 12.Klingenberg C, Embleton ND, Jacobs SE, et al. Enteral feeding practices in very preterm infants: an international survey. Arch Dis Child Fetal Neonatal Ed 2012;97(1):F56–61. [DOI] [PubMed] [Google Scholar]
- 13.Salas AA, Cuna A, Bhat R, et al. A randomised trial of re-feeding gastric residuals in preterm infants. Arch Dis Child Fetal Neonatal Ed 2015;100(3):F224–8. [DOI] [PubMed] [Google Scholar]
- 14.Hans DM, Pylipow M, Long JD, et al. Nutritional practices in the neonatal intensive care unit: analysis of a 2006 neonatal nutrition survey. Pediatrics 2009;123(1):51–7. [DOI] [PubMed] [Google Scholar]
- 15.Mank E, Saenz de Pipaon M, Lapillonne A, et al. Efficacy and safety of enteral recombinant human insulin in preterm infants: a randomized clinical trial. JAMA Pediatr 2022;176(5):452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Commare CE, Tappenden KA. Development of the infant intestine: implications for nutrition support. Nutr Clin Pract 2007;22(2):159–73. [DOI] [PubMed] [Google Scholar]
- 17.Carrion V, Egan EA. Prevention of neonatal necrotizing enterocolitis. J Pediatr Gastroenterol Nutr 1990;11(3):317–23. [DOI] [PubMed] [Google Scholar]
- 18.Munkstrup C, Krogfelt KA, Greisen G, et al. Feeding tube practices and the colonisation of the preterm stomach in the first week of life. Dan Med J 2022;69(8). [PubMed] [Google Scholar]
- 19.Neu J Gastrointestinal maturation and implications for infant feeding. Early Hum Dev 2007;83(12):767–75. [DOI] [PubMed] [Google Scholar]
- 20.Parker LA, Weaver M, Murgas Torrazza RJ, et al. Effect of gastric residual evaluation on enteral intake in extremely preterm infants: a randomized clinical trial. JAMA Pediatr 2019;173(6):534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torrazza RM, Parker LA, Li Y, et al. The value of routine evaluation of gastric residuals in very low birth weight infants. J Perinatol 2015;35(1):57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guillet R, Stoll BJ, Cotten CM, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2006;117(2):e137–42. [DOI] [PubMed] [Google Scholar]
- 23.Jaile JC, Levin T, Wung JT, et al. Benign gaseous distension of the bowel in premature infants treated with nasal continuous airway pressure: a study of contributing factors. AJR Am J Roentgenol 1992;158(1):125–7. [DOI] [PubMed] [Google Scholar]
- 24.Salas AA, Carlo WA, Do BT, et al. Growth rates of infants randomized to continuous positive airway pressure or intubation after extremely preterm birth. J Pediatr 2021;237:148–153 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKeown RE, Marsh TD, Amarnath U, et al. Role of delayed feeding and of feeding increments in necrotizing enterocolitis. J Pediatr 1992;121(5 Pt 1):764–70. [DOI] [PubMed] [Google Scholar]
- 26.Maheshwari A, Corbin LL, Schelonka RL. Neonatal necrotizing enterocolitis. Res Rep Neonatol 2011;1:39–53. [Google Scholar]
- 27.Bhatia AM, Feddersen RM, Musemeche CA. The role of luminal nutrients in intestinal injury from mesenteric reperfusion and platelet-activating factor in the developing rat. J Surg Res 1996;63(1):152–6. [DOI] [PubMed] [Google Scholar]
- 28.Hsueh W, Caplan MS, Qu XW, et al. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol 2003;6(1):6–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas A, Bloom SR, Aynsley-Green A. Gut hormones and ‘minimal enteral feeding’. Acta Paediatr Scand 1986;75(5):719–23. [DOI] [PubMed] [Google Scholar]
- 30.Slagle TA, Gross SJ. Effect of early low-volume enteral substrate on subsequent feeding tolerance in very low birth weight infants. J Pediatr 1988;113(3):526–31. [DOI] [PubMed] [Google Scholar]
- 31.Morgan J, Bombell S, McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database Syst Rev 2013;(3):CD000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becerra M, Ambiado S, Kuntsman G, et al. Feeding VLBW infants: effect of early enteral stimulation (EES). Pediatr Res 1996;39:304A. [Google Scholar]
- 33.Dunn L, Hulman S, Weiner J, et al. Beneficial effects of early hypocaloric enteral feeding on neonatal gastrointestinal function: preliminary report of a randomized trial. J Pediatr 1988;112(4):622–9. [DOI] [PubMed] [Google Scholar]
- 34.Meetze W, Valentine C, McGuigan J, et al. Gastrointestinal priming prior to full enteral nutrition in very low birth weight infants. J Pediatr Gastroenterol Nutr 1992;15:163–70. [DOI] [PubMed] [Google Scholar]
- 35.Mosqueda E, Sapiegiene L, Glynn L, et al. The early use of minimal enteral nutrition in extremely low birth weight newborns. J Perinatol 2008;28(4):264–9. [DOI] [PubMed] [Google Scholar]
- 36.McClure RJ, Newell SJ. Randomised controlled study of clinical outcome following trophic feeding. Arch Dis Child Fetal Neonatal Ed 2000;82(1):F29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schanler RJ, Shulman RJ, Lau C, et al. Feeding strategies for premature infants: randomized trial of gastrointestinal priming and tube-feeding method. Pediatrics 1999;103(2):434–9. [DOI] [PubMed] [Google Scholar]
- 38.Saenz de Pipaon M, VanBeek RH, Quero J, et al. Effect of minimal enteral feeding on splanchnic uptake of leucine in the postabsorptive state in preterm infants. Pediatr Res 2003;53(2):281–7. [DOI] [PubMed] [Google Scholar]
- 39.Troche B, Harvey-Wilkes K, Engle WD, et al. Early minimal feedings promote growth in critically ill premature infants. Biol Neonate 1995;67(3):172–81. [DOI] [PubMed] [Google Scholar]
- 40.van Elburg RM, van den Berg A, Bunkers CM, et al. Minimal enteral feeding, fetal blood flow pulsatility, and postnatal intestinal permeability in preterm infants with intrauterine growth retardation. Arch Dis Child Fetal Neonatal Ed 2004;89(4):F293–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berseth CL, Bisquera JA, Paje VU. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2003;111(3):529–34. [DOI] [PubMed] [Google Scholar]
- 42.Engle WD, Lair CS. Early feeding of premature infants questioned. Pediatrics 2004;113(4):931–2. [DOI] [PubMed] [Google Scholar]
- 43.Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015;314(10):1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 2019;7:CD002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiffany KF, Burke BL, Collins-Odoms C, et al. Current practice regarding the enteral feeding of high-risk newborns with umbilical catheters in situ. Pediatrics 2003;112(1 Pt 1):20–3. [DOI] [PubMed] [Google Scholar]
- 46.Havranek T, Johanboeke P, Madramootoo C, et al. Umbilical artery catheters do not affect intestinal blood flow responses to minimal enteral feedings. J Perinatol 2007;27(6):375–9. [DOI] [PubMed] [Google Scholar]
- 47.Clyman R, Wickremasinghe A, Jhaveri N, et al. Enteral feeding during indomethacin and ibuprofen treatment of a patent ductus arteriosus. J Pediatr 2013;163(2):406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gale C, Modi N, Jawad S, et al. The WHEAT pilot trial-WithHolding Enteral feeds Around packed red cell Transfusion to prevent necrotising enterocolitis in preterm neonates: a multicentre, electronic patient record (EPR), randomised controlled point-of-care pilot trial. BMJ Open 2019;9(9):e033543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirpalani H, Bell EF, Hintz SR, et al. Higher or lower hemoglobin transfusion thresholds for preterm infants. N Engl J Med 2020;383(27):2639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel EU, Head WT, Rohrer A, et al. A quality improvement initiative to standardize time to initiation of enteral feeds after non-surgical necrotizing enterocolitis using a consensus-based guideline. J Perinatol 2022;42(4):522–7. [DOI] [PubMed] [Google Scholar]
- 51.Burdall O, Allin B, Ford K, et al. Association between timing of re-introduction of enteral feeding and short-term outcomes following laparotomy for necrotising enterocolitis. J Pediatr Surg 2022;57(7):1331–5. [DOI] [PubMed] [Google Scholar]
- 52.Henderson G, Craig S, Brocklehurst P, et al. Enteral feeding regimens and necrotising enterocolitis in preterm infants: a multicentre case-control study. Arch Dis Child Fetal Neonatal Ed 2009;94(2):F120–3. [DOI] [PubMed] [Google Scholar]
- 53.Jasani B, Patole S. Standardized feeding regimen for reducing necrotizing enterocolitis in preterm infants: an updated systematic review. J Perinatol 2017;37(7):827–33. [DOI] [PubMed] [Google Scholar]
- 54.Kempley S, Gupta N, Linsell L, et al. Feeding infants below 29 weeks’ gestation with abnormal antenatal Doppler: analysis from a randomised trial. Arch Dis Child Fetal Neonatal Ed 2014;99(1):F6–11. [DOI] [PubMed] [Google Scholar]
- 55.Leaf A, Dorling J, Kempley S, et al. Early or delayed enteral feeding for preterm growth-restricted infants: a randomized trial. Pediatrics 2012;129(5):e1260–8. [DOI] [PubMed] [Google Scholar]
- 56.Salas AA, Li P, Parks K, et al. Early progressive feeding in extremely preterm infants: a randomized trial. Am J Clin Nutr 2018;107(3):365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flidel-Rimon O, Friedman S, Lev E, et al. Early enteral feeding and nosocomial sepsis in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2004;89(4):F289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004;292(19):2357–65. [DOI] [PubMed] [Google Scholar]
- 59.Dorling J, Abbott J, Berrington J, et al. Controlled trial of two incremental milk-feeding rates in preterm infants. N Engl J Med 2019;381(15):1434–43. [DOI] [PubMed] [Google Scholar]
- 60.Rayyis SF, Ambalavanan N, Wright L, et al. Randomized trial of “slow” versus “fast” feed advancements on the incidence of necrotizing enterocolitis in very low birth weight infants. J Pediatr 1999;134(3):293–7. [DOI] [PubMed] [Google Scholar]
- 61.Walsh V, Brown JVE, Copperthwaite BR, et al. Early full enteral feeding for preterm or low birth weight infants. Cochrane Database Syst Rev 2020;12:CD013542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nangia S, Vadivel V, Thukral A, et al. Early total enteral feeding versus conventional enteral feeding in stable very-low-birth-weight infants: a randomised controlled trial. Neonatology 2019;115(3):256–62. [DOI] [PubMed] [Google Scholar]
- 63.Agostoni C, Buonocore G, Carnielli VP, et al. Enteral nutrient supply for preterm infants: commentary from the European society of paediatric gastroenterology, hepatology and nutrition committee on nutrition. J Pediatr Gastroenterol Nutr 2010;50(1):85–91. [DOI] [PubMed] [Google Scholar]
- 64.Fusch C, Jochum F. Water, sodium, potassium and chloride. World Rev Nutr Diet 2014;110:99–120. [DOI] [PubMed] [Google Scholar]
- 65.Klingenberg C, Muraas FK, Isaksen CE, et al. Growth and neurodevelopment in very preterm infants receiving a high enteral volume-feeding regimen - a population-based cohort study. J Matern Fetal Neonatal Med 2019;32(10):1664–72. [DOI] [PubMed] [Google Scholar]
- 66.Thomas N, Cherian A, Santhanam S, et al. A randomized control trial comparing two enteral feeding volumes in very low birth weight babies. J Trop Pediatr 2012;58(1):55–8. [DOI] [PubMed] [Google Scholar]
- 67.Bell EF, Warburton D, Stonestreet BS, et al. Effect of fluid administration on the development of symptomatic patent ductus arteriosus and congestive heart failure in premature infants. N Engl J Med 1980;302(11):598–604. [DOI] [PubMed] [Google Scholar]
- 68.Oh W, Poindexter BB, Perritt R, et al. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J Pediatr 2005;147(6):786–90. [DOI] [PubMed] [Google Scholar]
- 69.Fewtrell MS, Adams C, Wilson DC, et al. Randomized trial of high nutrient density formula versus standard formula in chronic lung disease. Acta Paediatr 1997;86(6):577–82. [DOI] [PubMed] [Google Scholar]
- 70.Travers CP, Wang T, Salas AA, et al. Higher- or usual-volume feedings in infants born very preterm: a randomized clinical trial. J Pediatr 2020;224:66–71 e1. [DOI] [PubMed] [Google Scholar]
- 71.Kuschel CA, Evans N, Askie L, et al. A randomized trial of enteral feeding volumes in infants born before 30 weeks’ gestation. J Paediatr Child Health 2000;36(6):581–6. [DOI] [PubMed] [Google Scholar]
- 72.Zecca E, Costa S, Barone G, et al. Proactive enteral nutrition in moderately preterm small for gestational age infants: a randomized clinical trial. J Pediatr 2014;165(6):1135–1139 e1. [DOI] [PubMed] [Google Scholar]
- 73.Abiramalatha T, Thomas N, Thanigainathan S. High versus standard volume enteral feeds to promote growth in preterm or low birth weight infants. Cochrane Database Syst Rev 2021;3:CD012413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salas AA, Travers CP, Jerome ML, et al. Percent body fat content measured by plethysmography in infants randomized to high- or usual-volume feeding after very preterm birth. J Pediatr 2021;230:251–254 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raban S, Santhakumaran S, Keraan Q, et al. A randomised controlled trial of high vs low volume initiation and rapid vs slow advancement of milk feeds in infants with birthweights </= 1000 g in a resource-limited setting. Paediatr Int Child Health 2016;36(4):288–95. [DOI] [PubMed] [Google Scholar]
- 76.Tyson JE, Kennedy KA, Lucke JF, et al. Dilemmas initiating enteral feedings in high risk infants: how can they be resolved? Semin Perinatol 2007;31(2):61–73. [DOI] [PubMed] [Google Scholar]
- 77.Thanigainathan S, Abiramalatha T. Early fortification of human milk versus late fortification to promote growth in preterm infants. Cochrane Database Syst Rev 2020;7:CD013392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sharif S, Meader N, Oddie SJ, et al. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst Rev 2020;10:CD005496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Polycarpou E, Zachaki S, Tsolia M, et al. Enteral L-arginine supplementation for prevention of necrotizing enterocolitis in very low birth weight neonates: a double-blind randomized pilot study of efficacy and safety. JPEN J Parenter Enteral Nutr 2013;37(5):617–22. [DOI] [PubMed] [Google Scholar]
- 80.Amin HJ, Zamora SA, McMillan DD, et al. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J Pediatr 2002;140(4):425–31. [DOI] [PubMed] [Google Scholar]
- 81.Abdelmaaboud M, Mohammed A. A randomized controlled trial on early versus late minimal enteral feeding in preterm growth-restricted neonates with abnormal antenatal Doppler studies. J Neonatal Perinatal Med 2012;5(2):1–8. [DOI] [PubMed] [Google Scholar]
- 82.Armanian AM, Mirbod SM, Kazemipour S, Hassanzade A. Comparison of prolonged low volume milk and routine volume milk on incidence of necrotizing enterocolitis in very low birth weight neonates. Pak J Med Sci, 2013;29(1 Suppl):312–316. [Google Scholar]
- 83.Arnon S, Sulam D, Konikoff F, et al. Very early feeding in stable small for gestational age preterm infants: a randomized clinical trial. J Pediatr 2013;89(4):388–93. [DOI] [PubMed] [Google Scholar]
- 84.Bozkurt O, Alyamac Dizdar E, Bidev D, et al. Prolonged minimal enteral nutrition versus early feeding advancements in preterm infants with birth weight ≤1250 g: a prospective randomized trial. J Matern Fetal Neonatal Med 2020;25:1–7. [DOI] [PubMed] [Google Scholar]
- 85.Davey AM, Wagner CL, Cox C, Kendig JW. Feeding premature infants while low umbilical artery catheters are in place: a prospective, randomized trial. J Pediatr 1994;124(5 Pt 1):795–9. [DOI] [PubMed] [Google Scholar]
- 86.Dinerstein A, Nieto RM, Solana CL, et al. Early minimal enteral feeding with human milk in very low birth weight infants (VLBW): when to start? Pediatric Academic Societies Annual Meeting 2013;1514:579. [Google Scholar]
- 87.Karagianni P, Briana DD, Mitsiakos G, et al. Early versus delayed minimal enteral feeding and risk for necrotizing enterocolitis in preterm growth-restricted infants with abnormal antenatal Doppler results. Am J Perinatol 2010;27(5):367–73. [DOI] [PubMed] [Google Scholar]
- 88.Khayata S, Gutcher G, Bamberger J, Heimler R. Early versus late feeding of low birth weight (LBW) infants: effect on growth and hyperbilirubinemia. Pediatr Res 1987;21:431A. [Google Scholar]
- 89.Ostertag SG, LaGamma EF, Reisen CE, Ferrentino FL. Early enteral feeding does not affect the incidence of necrotizing enterocolitis. Pediatrics 1986;77(3):275–80. [PubMed] [Google Scholar]
- 90.Pérez LA, Pradilla GL, Díaz G, Bayter SM. Necrotising enterocolitis among preterm newborns with early feeding. Biomédica 2011;31(4):485–91. [DOI] [PubMed] [Google Scholar]
- 91.Srinivasan A, Nanavati RN, Kabra NS. Comparison of feeding regimens in preterm neonates with abnormal antenatal Doppler: a randomised controlled trial. Glob J Res Anal 2017;6(4):44–7. [Google Scholar]
- 92.Tewari VV, Dubey SK, Kumar R, et al. Early versus late enteral feeding in preterm intrauterine growth restricted neonates with antenatal doppler abnormalities: an open-label randomized trial. J Trop Pediatr 2018;64(1):4–14. [DOI] [PubMed] [Google Scholar]


