Abstract
Approximately half of patients with alcohol use disorder report pain and this can be severe during withdrawal. Many questions remain regarding the importance of biological sex, alcohol exposure paradigm, and stimulus modality to the severity of alcohol withdrawal-induced hyperalgesia. To examine the impact of sex and blood alcohol concentration on the time course of the development of mechanical and heat hyperalgesia, we characterized a mouse model of chronic alcohol withdrawal-induced pain in the presence or absence the alcohol dehydrogenase inhibitor, pyrazole. Male and female C57BL/6J mice underwent chronic intermittent ethanol vapor ± pyrazole exposure for 4 weeks, 4 d/wk to induce ethanol dependence. Hind paw sensitivity to the plantar application of mechanical (von Frey filaments) and radiant heat stimuli were measured during weekly observations at 1, 3, 5, 7, 24, and 48 hours after cessation of ethanol exposure. In the presence of pyrazole, males developed mechanical hyperalgesia after the first week of chronic intermittent ethanol vapor exposure, peaking at 48 hours after cessation of ethanol. By contrast, females did not develop mechanical hyperalgesia until the fourth week; this also required pyrazole and did not peak until 48 hours. Heat hyperalgesia was consistently observed only in females exposed to ethanol and pyrazole; this developed after the first weekly session and peaked at 1 hour. We conclude that Chronic alcohol withdrawal-induced pain develops in a sex-, time-, and blood alcohol concentration-dependent manner in C57BL/6J mice.
Perspective:
Alcohol withdrawal-induced pain is a debilitating condition in individuals with AUD. Our study found mice experience alcohol withdrawal-induced pain in a sex and time course specific manor. These findings will aid in elucidating mechanisms of chronic pain and AUD and will help individuals remain abstinent from alcohol.
Keywords: Alcohol use disorder, Pain, pyrazole, Hypersensitivity, Withdrawal, Sex differences
Alcohol use disorder (AUD) is characterized as compulsive intake of alcohol, binge drinking to high levels of intoxication,12,22 and physiological dependence.5,47 Dependence manifests as an alcohol withdrawal syndrome that includes negative emotional states when access to alcohol becomes limited or cut off completely.43 Key negative emotional states that determine the escalation of casual drinking behavior to uncontrolled alcohol consumption are thought to include pain. Pain is a hallmark symptom in 43 to 73% of individuals with AUD, and manifests as increased hyperalgesia during alcohol withdrawal due to sensitized central and peripheral mechanisms.2,23,53,69,81 Conversely, escalation of alcohol consumption contributes to the development of hyperalgesia and chronic pain.61 Individuals with AUD often drink to relieve or prevent alcohol withdrawal-induced pain.20,25,44,53,79 Thus, prevention of withdrawal-induced pain may help individuals with AUD remain abstinent from alcohol. However, a better understanding of the mechanisms that lead to the development and maintenance of pain in AUD patients is needed. To address this gap in knowledge, we developed and characterized a mouse model of Chronic Alcohol Withdrawal Induced Pain (CAWIP). We focused on chronic intermittent ethanol vapor (CIEV) paradigms, “the gold standard” for the study of the physical signs of alcohol dependence.36,37,76
In rodents, repeated cycles of ethanol exposure (in the diet, by oral gavage, or by vapor) and withdrawal are necessary to model alcohol dependence and the development of persistent negative affective states.7,36,41,70,76 Prior studies employing animal models of alcohol dependence have reported mechanical and heat hyperalgesia during the withdrawal period.1,3,17,19,24,31-33,45,65,66,71,72 However, these studies have not systematically considered key factors, including the impact of sex, ethanol exposure duration, time of testing after cessation of ethanol, nor blood alcohol concentration (BAC) on the intensity of pain-like behaviors.
Only a few studies have incorporated females,50,54 despite the fact that females generally exhibit greater distress from painful stimuli, greater sensitivity to experimentally induced pain, and a weaker descending control of pain when compared to males.62,64 In addition, women have a higher risk of exposure to alcohol than men during adolescence, which translates to higher occurrence and severity of AUD during adulthood.28,29 Despite these clinically relevant differences in pain sensitivity and AUD between males and females, previous studies have not examined sex–related differences on mechanical and heat sensitivities caused by chronic alcohol exposure and withdrawal. To address this gap, we evaluated CAWIP in both male and female mice.
We hypothesized that the withdrawal syndrome following cessation of CIEV includes mechanical and heat hyperalgesia in both male and female mice. We predict that sensitivity to mechanical and heat stimuli becomes greater with each subsequent week of CIEV. To do this, we tracked behavior across multiple weekly sessions of CIEV, evaluated multiple modalities of hypersensitivity (both mechanical and heat), and measured hyperalgesia at several timepoints after the initiation of ethanol withdrawal. In addition, we conducted our studies in the presence or absence of the alcohol dehydrogenase inhibitor, pyrazole, which is well known to dramatically increase blood alcohol levels in mice.
Methods
Animal Husbandry
64 male and 64 female C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME) and housed in a temperature (22–25°C) and humidity (30–70%) controlled room on a 12 hours light/dark cycle (lights on at 7:00) with ab libitum access to food and water. C57BL/6J mice were chosen because of their strong preference for ethanol when compared to other inbred strains.67,80 Body weight was recorded twice a week from arrival to euthanasia. Upon arrival, mice acclimated to the facility in their home cage for at least 1 week. Mice remained group-housed for the duration of the study. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Chronic Intermittent Ethanol Exposure (CIEV)
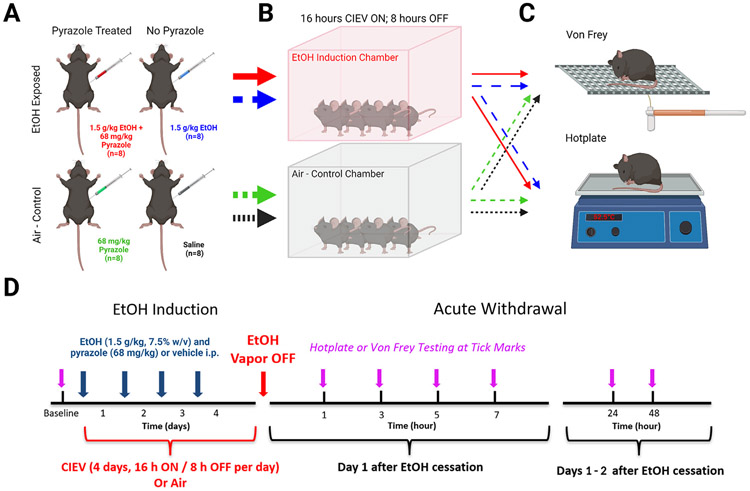
Thirty-two mice of each sex underwent exposure to alcohol vapor as previously described.37 We did not track estrus cycle because its effect on sensitivity to alcohol8 and pain56 is inconsistent, and when reported, is modest at best. Each of the 4 sessions (Monday–Thursday) included 16 hours of exposure to ethanol (17:00–9:00), followed by 8 hours of exposure to ambient room air (9:00–17:00). Thirty-two mice of each sex served as Air-Control (AC) groups and were moved between housing racks and the induction chambers at 9:00 and 17:00 as were the CIEV groups. The temperature and humidity inside the chambers were maintained at 22 to 25°C and 30 to 70%, respectively. Circulating ethanol vapor levels within the chambers were monitored with a custom voltage sensor generously provided by Brian McCool (Wake Forest University). Cohorts were split into 2 main groups: alcohol exposed or air-control. The ethanol exposed group was further divided into 2 groups that received just ethanol (1.5 g/kg in saline, i.p Decon Labs, King of Prussia, PA) or ethanol (1.5 g/kg) with the alcohol dehydrogenase inhibitor pyrazole (68 mg/kg, i.p.; Sigma–Aldrich, P56607–6G; Fig 1A). The air-control group was also further divided into 2 separate groups that received either pyrazole (68 mg/kg in saline) or just saline (Gibco, Waltham, MA; Fig 1A). After pre-CIEV or air-control treatment, mice were immediately placed into the ethanol induction or control chambers (Fig 1B). Three days of behavioral testing (Friday–Sunday) began the morning after the last CIEV session (Fig 1C). This pattern of 4 days of CIEV followed by 3 days of behavioral testing was continued for 4 weeks (Fig 1D).
Figure 1.
Graphical illustration of methods (created with BioRender.com) and timeline used in the current study. (A) Mice were separated into four groups and treated with 1.5 g/kg EtOH + 68 mg /kg pyrazole, 1.5 g/kg EtOH, 68 mg /kg pyrazole, or saline. (B) EtOH treated mice were placed into vapor chambers and exposed to 16 hours of EtOH vapor followed by a period of 8 hours of ambient room air. AC mice were also placed into a different vapor chamber without the presence of EtOH. (C) Four mice from each treatment group were used for either hotplate or von Frey behavioral testing. (D) Schematic representation of the EtOH exposure and behavioral timeline. Mice underwent 5 weeks of CIEV and withdrawal.
Each week, blood alcohol concentrations (BACs) were assessed to ensure our experimental mice were experiencing dependent-like levels of alcohol. Blood was taken via tail vein after the third cycle of CIEV each week. Blood was centrifuged at 2300 x g for 5 minutes and plasma was analyzed with an Analox Alcohol Analyzer (AM1, Analox Instruments, London, UK). Ethanol flow rate into the chamber was adjusted to maintain BACs between 175 and 225 mg/dL in the pyrazole – treated CIEV group. After the final week of behavioral testing, mice underwent a fifth week of CIEV and blood was collected at 1, 3, 5, and 7 hours after removal from the chamber to determine the rate of ethanol clearance during acute withdrawal (Supplemental Fig 1).
Sensory Testing
Mice were acclimated to either the hotplate or von Frey apparatuses in a temperature and light controlled room for 1 hour a day between 9:00 and 11:00 for 3 days, Friday through Sunday. For hotplate acclimation, mice on the first day were placed onto the apparatus at room temperature. On the second and third days, mice were placed onto the apparatus at 52.5°C and latency to response was recorded. Response latency typically decreased during these sessions, but then stabilized before baseline testing. Baseline testing commenced the following Monday before the first CIEV session. Upon removal from the CIEV chamber, mice were moved to the hotplate or von Frey apparatus and were tested after cessation of alcohol vapor at 1, 3, 5, 7, 24, and 48 hours timepoints, as many withdrawal symptoms in rodents occur 24 to 48 hours after cessation of alcohol.43
Hotplate Test
Mice were placed on a 10” x 10” hotplate heated to 52.5°C, enclosed within a 10” x 16” acrylic chamber (Columbus Instruments, Columbus, OH). Upon observation of a jump, or rapid flicking or licking of hind paw, the mouse was immediately removed, and response latency recorded.18,59 If no response was made within 30 seconds after placing mouse onto the hotplate, the animal was removed to avoid lasting tissue injury. Three trials were conducted at 10 minute intervals and averaged.
Von Frey Test
Mice were placed on a wire–mesh rack within individual 4” x 4’ x 14” Plexiglas containers. After an acclimation period of at least 30 minutes, a set of eight monofilaments at logarithmic intervals from 0.008 to 6 grams (0.008, 0.023, 0.07, 0.16, 0.4, 1, 2, and 6 grams; Stoelting, Wood Dale, IL) were applied to the center of the plantar surface of the left hind paw for up to 4 seconds using the up–down method.13 A withdrawal response was defined by rapid retraction of the paw unrelated to normal ambulation. 50% thresholds were calculated using methods previously described.13,21
Statistical Analysis
GraphPad Prism Software version 9.3.0 (La Jolla, CA) was used for graphical presentation and statistical analysis. Mechanical thresholds and heat latency data were analyzed separately. Data were first collapsed across Session (Weeks 1–4) and Time (hours 1–48 hours after cessation of treatment), and then analyzed the main effects of Treatment x Sex with two way ANOVA; significant main effects were followed by post-hoc multiple comparisons. We then evaluated male and female data separately as well as weekly Session 1 to 4 data separately–this enabled Treatment x Time two way repeated measures ANOVA; significant main effects were followed by Sidak’s multiple comparison tests (for data collapsed across Session and Time) or Bonferroni post-hoc analysis (for data segregated by Sex and Session). Mann Whitney tests were used to analyze data between 2 groups at specific timepoints during alcohol withdrawal. Statistical significance was set at P < .05. All data are presented as mean ± SEM. Degrees of freedom F and P values for the Treatment x Time two way repeated measures ANOVA are reported in Supplemental Table 1.
Result
Blood Alcohol Concentrations
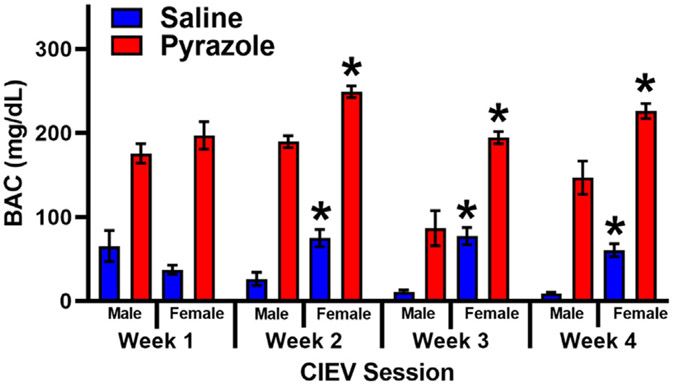
Fig 2 illustrates BAC levels in male and female mice exposed to 4 sessions of CIEV and either Saline or Pyrazole. Two way ANOVA revealed a main effect of Pyrazole (Male: F (3, 54) = 15.85, P < .001; Female: F (2.489, 74.66) = 11.07, P < .001) and Session (Male: F (1, 18) = 91.28, P < .001; Female: F (1, 30) = 252.7, P < .001), with a Pyrazole x Session interaction (Male: F (3, 54) = 5.505, P < .01; Female: F (3, 90) = 5.149, P < .01). Fig 2 illustrates that male and female CIEV + Pyrazole treated groups had a significantly higher BAC when compared to the same sex CIEV + Saline group across all four CIEV Sessions (P < .01 by multiple comparisons). Additionally, two way ANOVA revealed a main effect of Sex (CIEV + Pyrazole: F (1, 24) = 25.25, P < .001, CIEV + Saline: F (1, 24) = 16.16, P < .001) and CIEV Session (CIEV + Saline: F (3, 72) = 19.82, P < .001, CIEV + Saline: F (3, 72) = 1.566, P < .001) with a Sex x CIEV Session interaction (CIEV + Pyrazole: F (3, 72) = 6.215, P < .001, CIEV + Saline: F (3, 72) = 12.16, P < .001) Fig 2 illustrates that females have a significantly higher BAC when compared to males within each treatment group. Thus, CIEV + Pyrazole in both males and females increased BAC.
Figure 2.
BAC during CIEV +/− pyrazole in male and female mice. Male and female mice were exposed to either saline (blue) or pyrazole (red) during the 4 sessions (Weeks 1, 2, 3, 4) of chronic intermittent ethanol vapor (CIEV). Data presented as mean ± SEM. n = 10 males, 16 females per group. BACs from both male and female Pyrazole-treated groups were significantly greater than their same-sex, Saline-treated counterparts (P < .05). *P < .05 female versus male of the respective treatment group and weekly session.
Withdrawal from CIEV ± Pyrazole increases Mechanical and Heat Sensitivity
We first evaluated the data when collapsed (averaged) across Session (Weeks 1–4) and Time (hours 1–48 after cessation of treatment), to focus on the overall effect of withdrawal from ethanol vapor exposure on mechanical and heat sensitivity.
Administration of Pyrazole Alone did not Change Mechanical or Heat Sensitivity
To determine if administration of pyrazole itself influenced mechanical or heat sensitivity, Supplemental Figs 2 and 3 compared the air control groups treated with either pyrazole or saline. Two way ANOVA revealed no differences in either mechanical or heat sensitivity, regardless of sex (P > .05). Therefore, the data of these control groups were combined into 1 group, henceforth referred to as “Air Control,” or “AC.” This AC group was included in subsequent analyses to determine whether withdrawal from CIEV ± pyrazole resulted in sex-dependent changes in mechanical or heat sensitivity (Figs 3-5).
Figure 3.
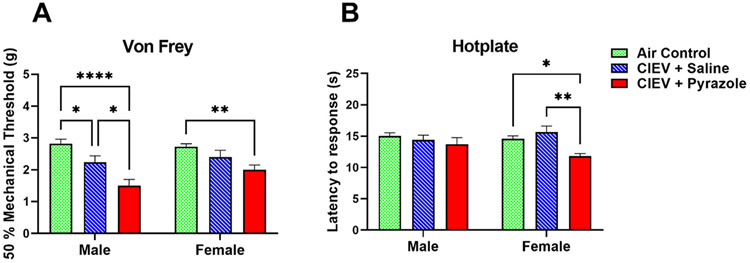
Effect of Chronic Intermittent Ethanol Vapor on von Frey thresholds and hotplate response latencies in males and females. Data were averaged across Session (Weeks 1–4 of CIEV) and Time (Hours 1–48 after cessation of CIEV). A) CIEV + Pyrazole cessation decreased mechanical sensitivity in both sexes. B) CIEV + Pyrazole cessation decreased but heat sensitivity in female but not male mice. All data are presented as mean ± SEM. N = 8 in each CIEV group; N = 16 in Air Control (AC) group. *P < .05 CIEV + pyrazole compared to AC, CIEV + Saline compared to AC, or CIEV + Pyrazole compared to CIEV + Saline; **P < .01 CIEV + Pyrazole compared to AC or CIEV + Pyrazole compared to CIEV + Saline; ****P < .001 CIEV + pyrazole compared to AC.
Figure 5.
Heat response latencies for males (A,C,E,G) and females (B,D,F,H) across the withdrawal period during week 1 (A,B), week 2 (C,D), week 3 (E,F) and week 4 (G, H). Female heat sensitivity begins after 1 week of treatment of CIEV and becomes maximal after four weeks while the males do not develop heat sensitivity after CIEV treatment. All data are presented at mean ± SEM. N = 8 per EtOH exposed group, and 16 per AC group. * P < .05 CIEV + Pyrazole compared to AC; **P < .01 CIEV + Pyrazole compared to AC. #P < .05 CIEV + Pyrazole compared to CIEV + Saline; ###P < .001 CIEV + Pyrazole compared to CIEV + Saline.
Mechanical Hypersensitivity in Both Sexes
Two way ANOVA (Treatment x Sex) revealed a main effect of Treatment (F (2, 58) = 22.82, P < .001). Fig 3A illustrates that CIEV + Pyrazole cessation decreased mechanical sensitivity in each sex, with reductions in von Frey thresholds of 47% in males and 27% in females when compared to the AC groups, and of 36% in males when compared to the CIEV + Saline group (P < .05 by multiple comparisons).
Heat Hypersensitivity Only in Females
In females, two-way ANOVA (Treatment x Sex) revealed a main effect of Treatment (F (2, 58) = 5.84, P < .01). CIEV + Pyrazole withdrawal decreased hotplate response latencies by 19% when compared to the AC group, and by 24% when compared to the CIEV + Saline group (P < .05 by multiple comparisons; Fig 3B). In males, CIEV + Pyrazole did not change hotplate latency (P > .05).
Time Course of CIEV Withdrawal-Induced Mechanical and Heat Hypersensitivity
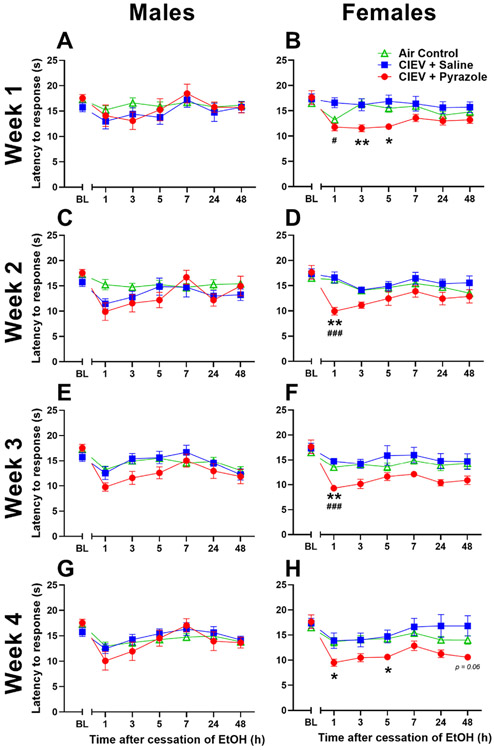
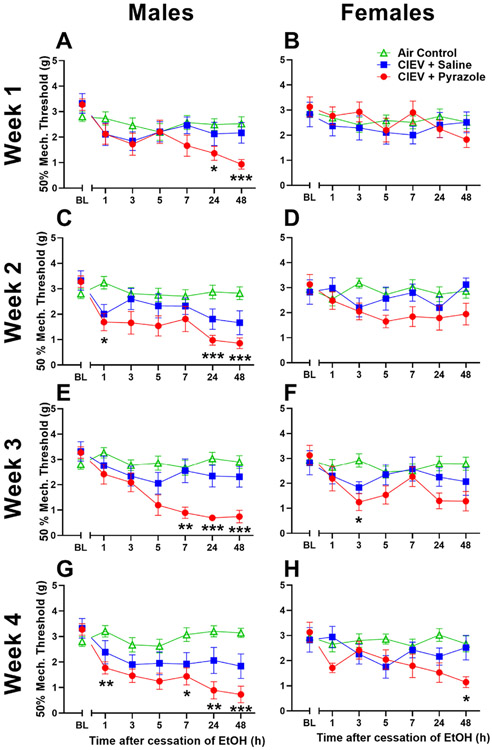
Heat hypersensitivity after CIEV exposure in either male or female mice, but this study was restricted to a single timepoint (28 hour) after the cessation of a single duration (72 hour) of CIEV exposure.54 To fill this gap, we determined the time course of mechanical and heat hypersensitivity at multiple timepoints (1–48 hour) after withdrawal from a multiple number of cycles (1–4 weeks) of exposure to CIEV with a week-by-week analysis (Figs 4 and 5). Statistical results are reported in Supplemental Table 1. Females exhibited mechanical sensitivity at the 48-hours timepoint of the fourth session of CIEV. Furthermore, when timepoints were averaged across each weekly testing session, Supplemental Fig 4 clearly highlights that CIEV induced mechanical hypersensitivity from weeks 1 to 4 in males and from weeks 2 to 4 in females. Also, CIEV induced heat hypersensitivity from weeks 1 to 4 in females, but not in males.
Figure 4.
Mechanical thresholds for males (A,C,E,G) and females (B,D,F,H) across the withdrawal period during week 1 (A,B), week 2 (C,D), week 3 (E,F) and week 4 (G,H). Male mechanical sensitivity begins after one week of treatment of CIEV and becomes maximal after four weeks while the females first experience mechanical sensitivity after the third week of CIEV treatment. All data are presented at mean ± SEM. N = 8 per EtOH exposed group, and 16 per AC group. * P < .05 CIEV + Pyrazole compared to AC; ** P < .01 CIEV + Pyrazole compared to AC; *** P < .001 CIEV + Pyrazole compared to AC.
Mechanical Sensitivity
Week 1
In males, two-way ANOVA (Treatment x Time) revealed a main effect of Time. CIEV + Pyrazole cessation decreased mechanical threshold compared to the AC group (P < .05 by multiple comparisons), a decrease of 64% (Fig 4A). Mechanical thresholds gradually decreased in the CIEV + Pyrazole group at hours 24 and 48 when compared to AC, with a peak effect of 63% at the 48 hours timepoint. In females, no differences were observed (Fig 4B).
Week 2
In males, two way ANOVA revealed main effects of Time, Treatment, and a Time x Treatment interaction, indicating that the effect of ethanol withdrawal was time-dependent. CIEV + Pyrazole cessation gradually decreased mechanical threshold compared to the AC group at hours 1, 24, and 48 during the withdrawal period (P < .05 by multiple comparisons), with a peak effect of 70% at the 48 hours timepoint (Fig 4C). In females, no differences in mechanical thresholds were detected (Fig 4D).
Week 3
In males, two-way ANOVA revealed main effects of Treatment, Time, and a Treatment x Time interaction, indicating that the effect of ethanol withdrawal was time dependent. CIEV + Pyrazole cessation rapidly decreased mechanical threshold compared to the AC group at hours 7, 24, and 48 (P < .05 by multiple comparisons), with a peak effect of 77% at the 24 hours timepoint (Fig 4E). Females exhibited main effects of Treatment and Time, and CIEV + pyrazole decreased mechanical thresholds by 58% during the third hour of withdrawal (Fig 4F).
Week 4
In males, two-way ANOVA revealed a main effect of Treatment, Time, and a Treatment x Time interaction, indicating that the effect of alcohol withdrawal was time dependent. CIEV + Pyrazole cessation rapidly decreased mechanical threshold compared to the AC group at hours 1, 7, 24, and 48 (P < .05 by multiple comparisons), with a peak effect of 77% at the 48 hours timepoint (Fig 4G). In females, we observed main effects of Treatment, Time, and a Treatment x Time interaction, and CIEV + pyrazole gradually decreased mechanical thresholds by 57% when compared to AC at hour 48 during withdrawal (Fig 4H).
Heat Sensitivity
Week 1
In males, no differences were observed (Fig 5A). We found main effects of Time, Treatment, and a Time x Treatment interaction, indicating that the effect of ethanol withdrawal was time-dependent in females (Fig 5B). CIEV + Pyrazole cessation decreased heat latency by an average of 27% when compared to the AC group at hour 3 and 5, with a peak effect of 29% when compared to the CIEV + saline group at hour 1 (P < .05 by multiple comparisons).
Week 2
In males, we report a Treatment x Time interaction, but multiple comparisons test with Bonferroni correction did not confirm differences at specific time points (P-values: h 1: P = .45; h 3: P > .99; h 5: P > .99; h 7: P > .99; h 24: P > .99; h 48: P > .99; Fig 5C). In females, we found a main effect of Treatment and a Treatment x Time interaction, indicating that the effect of ethanol withdrawal was time-dependent (Fig 5D). CIEV + Pyrazole cessation decreased heat response latency by 39% when compared to the AC group during hour 1 of withdrawal, and by 40% as compared to the CIEV + Saline group during hour 1 of withdrawal (P < .05 by multiple comparisons).
Week 3
In males, we found a Treatment x Time interaction that could not be confirmed with multiple comparisons test using the Bonferroni correction (P-values: h 1: P = .10; h 3: P = .85; h 5: P = .98; h 7: P > .99; h 24: P > 0.99; h 48: P > .99; Fig 5E). In females, we found main effects of Treatment and a Treatment x Time interaction. CIEV + Pyrazole cessation decreased heat response latency by 31% as compared to AC and by 37% as compared to CIEV + saline during hour 1 of withdrawal (P < .05 by multiple comparisons; Fig 5F).
Week 4
In males, there was no main effect of CIEV + pyrazole (Fig 5G). In females, we found main effects of Treatment, Time, and a Treatment x Time interaction. CIEV + Pyrazole cessation decreased heat response latency as compared to the AC control group in hours 1 and 5 during withdrawal, with a peak effect of 30% at the 1 hour timepoint. (Fig 5H).
Discussion
Chronic alcohol withdrawal-induced pain (CAWIP) has been studied with a variety of paradigms in rats and mice. We chose a model of chronic intermittent ethanol vapor (CIEV) because it is now recognized as the gold standard in the study of alcohol dependence, allows for precise control of blood alcohol concentration (BAC), and provides reliable and repeatable outcome measures, including mechanical and heat hypersensitivity. Our study establishes the effects of multiple cycles of chronic alcohol vapor exposure and subsequent withdrawal periods on mechanical and heat sensitivity in male and female mice and reveals several important principles in the use of CIEV to study CAWIP. First, we noted that previous studies of alcohol dependence (i.e., alcohol consumption and anxiety-like behaviors upon cessation of alcohol) were restricted to CIEV in the presence of pyrazole.6,40,46,51 To determine whether pyrazole was necessary for other signs of alcohol dependence (in this case, pain-like behaviors upon cessation of alcohol), we included groups that did not receive pyrazole. We found that pyrazole increased the severity of mechanical hyperalgesia and was necessary for the detection of heat hyperalgesia. These results confirm that pyrazole is a necessary component of CIEV protocols to study CAWIP. Second, previous studies were restricted to male rats or mice. We studied both male and female mice, leading to the discovery of 2 clear sex differences: males exhibited greater mechanical hyperalgesia, while only females exhibited heat hyperalgesia. Third, previous studies were restricted to analysis of just 1 session of pain-like behaviors after cessation of CIEV. To determine the developmental time course of CAWIP, we evaluated behavior on four consecutive weeks. We found that mechanical hyperalgesia peaked after 3 weekly cycles of CIEV, and this continued for at least one additional week.
Pyrazole is Necessary for the Full Manifestation of CAWIP
Pyrazole is an alcohol dehydrogenase inhibitor that inhibits the metabolism of alcohol into acetaldehyde. In mouse models of CIEV, pyrazole is required to achieve BACs at levels necessary to achieve dependence.7,41 Pyrazole alone can produce toxic effects in mice such as weight loss and liver necrosis when combined with alcohol.38,49 One group found a strong withdrawal phenotype in CIEV treated mice without the usage of pyrazole, suggesting that pyrazole is not needed in mouse CIEV studies.26 We found that pyrazole substantially increased BACs in the setting of CIEV, while treatment of the AC + Pyrazole group showed no differences in body weight and pain sensitivity when compared to the AC + Saline group in both males and females. These results confirm that pyrazole is a necessary component of CIEV protocols to study CAWIP. Our CIEV + Saline groups achieved significantly lower BACs throughout the experiment when compared to the CIEV + Pyrazole groups (Fig 2).
During equivalent CIEV exposure, BAC was higher in females (200–250 mg/dL) than in males (100–150 mg/dL). This is consistent with previous literature suggesting that women achieve higher BACs than men after consuming the same amount of alcohol.30,57,73 However, our data contrasts with what is seen in rodent studies where males and females achieve the same BAC after a single dose of ethanol.15,34 Despite lower BAC after four weeks of CIEV treatment (males: 147 mg/dL ± 20.0, females: 226 ± 8.9), males exhibited greater mechanical hyperalgesia during alcohol withdrawal. We suggest that males are more sensitive to the development of alcohol dependence in terms of withdrawal-induced mechanical hyperalgesia. By contrast, higher BAC in females was associated with heat hyperalgesia, leading us to speculate that higher BAC is necessary for the development of heat hyperalgesia during alcohol withdrawal.
Males are More Sensitive to Mechanical Stimuli During Withdrawal from CIEV
We discovered that male mice exhibit greater mechanical hyperalgesia than female mice. Our results are consistent with previous reports of mechanical sensitivity after withdrawal from chronic alcohol exposure, albeit restricted to just male mice.1,17,66,72 Male C57BL/6 mice experienced mechanical sensitivity 24 and 48 hours into withdrawal from alcohol administered via oral gavage, and this sensitivity become more pronounced after each subsequent week of alcohol administration.1 This result is similar to those from our study where multiple cycles of CIEV, and withdrawal produced greater mechanical sensitivity. In a IA2BC paradigm, mechanical sensitivity was seen 24 hours into the withdrawal period in male C57BL/6J mice.66,72 We can confirm mechanical sensitivity at the 24 and 48 hours withdrawal period in the CIEV model of alcohol dependence. Little data is available showing mechanical sensitivity in female mice using any model of alcohol dependence, and we are the first to directly compare mechanical thresholds in male and female mice after repeated cycles of CIEV and withdrawal. In a rat model of CAWIP, one study showed decreased mechanical thresholds in female rats 1 week after discontinuation of a nine weeks of alcohol administration via liquid diet.16 However, they did not test for mechanical sensitivity during the critical 24 and 48 hours withdrawal period where physiological signs of dependence are maximal. Overall, multiple cycles of CIEV and withdrawal produced robust mechanical sensitivity in our male mice during the 24 and 48 hours of the withdrawal period, while females experienced mechanical sensitivity during the 48 hours of the withdrawal period.
CIEV Produced Heat Hyperalgesia Only in Female Mice
In females but not males, we found heat hypersensitivity at 1 to 5 hours, but not 7 to 48 hours after the cessation of CIEV exposure. Consistent with our results, heat hypersensitivity was not observed at 28 hours after the cessation of 72 hours of continuous alcohol vapor exposure in females.54 By contrast, one previous study (restricted to males only) indicates that chronic alcohol exposure via intermittent access to 2 bottle choice (IA2BC) produces heat hypersensitivity 24 hours into the withdrawal period.66 This study used radiant heat to stimulate a paw withdrawal response; by contrast, we placed mice on a hotplate and recorded a response to be represented not only by paw lifting, but also licking or jumping. We do not believe that our contrasting data is a result of different testing modalities since both hotplate and Hargreaves both require the integration of supraspinal pain pathways.18 Heat hypersensitivity is seen during withdrawal in male rats subjected to liquid diet, IA2BC, and CIEV models of alcohol dependence.3,19,31,45,45,71 We conclude that only female mice experience heat sensitivity as a result of CIEV and withdrawal. Sex dependent mechanisms in heat nociception could explain why we see heat sensitivity in females and not males in our study.
Maximal Mechanical and Heat Hypersensitivity During Withdrawal Requires Multiple Sessions of CIEV
Previous studies were restricted to analysis of just one session of pain-like behaviors after cessation of CIEV. To determine the number of cycles of CIEV required to generate pain-like behavior during withdrawal, we evaluated behavior on 4 consecutive weeks, using a repeated-measures design that that evaluated multiple time points within a 48 hours withdrawal period. We found that male mice rapidly developed mechanical hypersensitivity after one cycle of CIEV, whereas female mice did not exhibit mechanical hypersensitivity until the latter weeks. Mechanical sensitivity in male mice increased after each subsequent week of CIEV and peaked after three weeks. This pattern reflects what is seen in voluntary drinking studies using CIEV in male mice, where voluntary alcohol drinking increases after each subsequent cycle of CIEV and withdrawal.41,52 However, it took the females three cycles of CIEV to experience mechanical sensitivity initially and did not increase after the subsequent fourth week. We conclude that mechanical hyperalgesia peaked after 3 weekly cycles of CIEV, and this continued for at least one additional week in both sexes.
Overall, heat sensitivity is seen in female mice, but not males, after chronic exposure to CIEV and Pyrazole. Female mice experienced heat sensitivity during early withdrawal timepoints across all 4 weeks. This was not seen in the male counterparts, due to high variability in their behavior. During week 2 and 3, heat sensitivity in the male group is present but was not picked up using conservative statistical methods. Upon further analysis, nonparametric Mann Whitney tests revealed statistical support for potential heat sensitivity in the male CIEV + pyrazole group when compared to AC males during early withdrawal timepoints. Further analysis needs to be conducted with larger sample sizes to account for variability we saw in our male hotplate data. We believe that three to four cycles of CIEV and withdrawal is enough to produce a dependent like phenotype in male and female C57BL/6J mice, as seen through increased mechanical and heat sensitivity.
Dependence on Sex and Sensory Modality of the Time Course of Mechanical and Heat Hyperalgesia after cessation of CIEV
Mechanical
We found that both males and females displayed mechanical sensitivity during withdrawal from CIEV. In males this occurred throughout the 1 to 48 hours duration of testing, while in females this occurred at just 48 hours timepoint). These results are consistent with previous literature (which is restricted to male rodents) indicating that mechanical hypersensitivity develops at later timepoints (>8 hours after cessation of alcohol),1,17,31,50,66,71,72 and point to the novelty of our approach to evaluate sensory thresholds at earlier timepoints (<8 hours) in both male and female mice.
Heat
We found that females displayed heat hypersensitivity 1 to 5 hours after cessation of chronic alcohol administration. We are unable to explain why heat hypersensitivity is seen at such early timepoints, when other signs of the alcohol withdrawal syndrome require 8 hours or more to develop.5,35,40,52 Albeit, we found that the heat hypersensitivity in our female mice normalized by the 24 hours timepoint, which is consistent with reports of the absence of heat hypersensitivity in females when tested at a 28 hours timepoint after 72 hours of continuous alcohol vapor exposure (although this study is the only other one to evaluate pain-like behavior in females, it did not test earlier timepoints).54 In contrast to females, we found that males did not display heat hypersensitivity at any timepoint after cessation of CIEV. These results are inconsistent with previous literature in males indicating that heat hypersensitivity develops after alcohol exposure at later timepoints (>8 hours) in male rodents.3,31,66,71 Future studies are needed to explain these discrepancies in the sex- and time-dependent factors that determine mechanical and heat hypersensitivity after cessation of chronic alcohol administration.
Consideration of Estrous Cycle on Pain and Alcohol Sensitivity
Although some studies suggest that nociception and analgesia vary with stage of estrous cycle in rodents,14,27 most report no correlation with either heat or mechanical sensitivity,39,55,82 indicating that the relationship between estrous cyclicity and pain sensitivity is weak, at best.56
Heightened sensitivity to alcohol occurs with higher estrogen levels during the late follicular phase of the menstrual cycle in women42 and during the diestrus phase in mice and rats.68,75 Although the effects of estrous cycle on alcohol sensitivity are generally modest at best,8 these studies raise the hypothesis that the high estrogen levels associated with diestrus lead to greater alcohol dependence, and thus mechanical and heat hypersensitivity during alcohol withdrawal.
Sex-Differences in Alcohol Analgesia that Underlie CAWIP
A single exposure to alcohol exerts analgesic actions in humans at blood alcohol concentrations greater than 0.08 g/dL.63,74,77 A 2 g/kg dose of alcohol produced antinociception in male rats11,32 and in male and female C57BL/6J mice.58 In addition, the same 2 g/kg dose produced robust antinociception in male C57BL/6J mice but a weaker analgesia in female C57BL/6J mice.78 Similarly, in mice with peripheral nerve injury, reintroduction to alcohol produced greater antinociception in male mice as compared to female mice.9 Thus, rodent studies indicate that males are more susceptible to the antinociceptive effects of alcohol. Thus, alcohol-induced antinociception may have masked the detection of heat hypersensitivity during the early hours after cessation of CIEV in males, when alcohol is still onboard (Supplemental Fig 1). Conversely, being less sensitivity to alcohol antinociception, females responded to the cessation of CIEV with heat hypersensitivity.
Neurological Basis for the Manifestation of CAWIP
Our studies demonstrate a complex interaction between sex, time, and pain modality in the determination of CAWIP. Female mice exhibited less mechanical hyperalgesia than males, yet only females exhibited heat hyperalgesia. These results may suggest that CAWIP in females is initially transmitted along the temperature-sensitive ascending pathways of the spinothalamic tract, while CAWIP in males is predominantly transmitted along the touch-sensitive ascending dorsal column-medial lemniscus tract. Further studies are necessary to determine the circuitry of CAWIP at the synaptic, cellular and systems levels to identify mechanisms underlying sex specific differences in these emergent behaviors.
Mechanism of mechanical and heat hypersensitivity during withdrawal from chronic ethanol exposure are poorly understood. Particularly intriguing is the potential contribution of the central nucleus of the amygdala (CeA). The CeA projects to brainstem areas that contribute to the descending modulation of spinal pain transmission, such as the periaqueductal grey (PAG) and rostral ventral medulla (RVM).4,48 Chronic alcohol decreases GABAergic inhibition from the CeA to PAG and the net effect of this disinhibition is increased activity.3,10 These results might suggest that a descending pain inhibitory CeA→PAG→RVM→dorsal horn pathway60 is inhibited by chronic alcohol exposure; the resulting disinhibition might then contribute to mechanical and heat hypersensitivity upon alcohol withdrawal.3
Conclusion
Our data best illustrates the hourly time course of pain-like behavior during withdrawal, provides a comprehensive description of the week-by-week data and corresponding statistics, and indicates the number of cycles of CIEV required to generate pain-like behavior during withdrawal. This is the first report of sex differences in CIEV withdrawal induced pain. We conclude that male and female mice experience dramatic differences in mechanical and heat hypersensitivity. Future studies will identify key neurochemical systems and brain regions that can contribute to CAWIP and to target these with pharmacological intervention. In summary, we demonstrate that four weeks of CIEV is sufficient to produce mechanical hyperalgesia in both male and female C57BL/6J mice, and heat hyperalgesia in female C57BL/6J mice. The pain associated with chronic alcohol use disorder is incredibly debilitating and disrupts cognitive function, and so it will be imperative to test the affective component of CAWIP, as well as to study the different supraspinal mechanisms by which this occurs.
Supplementary Material
Acknowledgments
This work was supported by NIAAA, NIDA, and NINDS grants AA024836 (SPF), AA020889 (SPF), AA030257 (SPF), AA029942 (AMB), DA037621 (BKT), NS045954 (BKT), NS112632 (BKT), and NS073548 (AJB). We would also like to acknowledge support from Bridging Connections in Addiction Research (BCAR) at the University of Pittsburgh and the Pittsburgh Foundation.
Footnotes
The authors have no conflicts of interest to disclose.
Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jpain.2023.02.024.
References
- 1.Alongkronrusmee D, Chiang T, van Rijn RM: Involvement of delta opioid receptors in alcohol withdrawal-induced mechanical allodynia in male C57BL/6 mice. Drug Alcohol Depend 167:190–198, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apkarian AV, Neugebauer V, Koob G, Edwards S, Levine JD, Ferrari L, Egli M, Regunathan S: Neural mechanisms of pain and alcohol dependence. Pharmacol Biochem Behav 112:34–41, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avegno EM, Lobell TD, Itoga CA, Baynes BB, Whitaker AM, Weera MM, Edwards S, Middleton JW, Gilpin NW: Central amygdala circuits mediate hyperalgesia in alcohol-dependent rats. J Neurosci 38:7761–7773, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannister K, Dickenson AH: Central nervous system targets: Supraspinal mechanisms of analgesia. Neurotherapeutics 17:839–845, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker HC: Alcohol dependence, withdrawal, and relapse. Alcohol Res Health 31:348–361, 2008 [PMC free article] [PubMed] [Google Scholar]
- 6.Becker HC, Diaz-Granados JL, Weathersby RT: Repeated ethanol withdrawal experience increases the severity and duration of subsequent withdrawal seizures in mice. Alcohol 14:319–326, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Becker HC, Lopez MF: Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res 28:1829–1838, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Becker JB, Koob GF: Sex differences in animal models: Focus on addiction. Pharmacol Rev 68:242–263, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilbao A, Leixner S, Wei S, Cantacorps L, Valverde O, Spanagel R: Reduced sensitivity to ethanol and excessive drinking in a mouse model of neuropathic pain. Addict Biol 24:1008–1018, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Cagetti E, Liang J, Spigelman I, Olsen RW: Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABA A receptors. Mol Pharmacol 63:53–64, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Campbell VC, Taylor RE, Tizabi Y: Antinociceptive effects of alcohol and nicotine: involvement of the opioid system. Brain Res 1097:71–77, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Carvalho AF, Heilig M, Perez A, Probst C, Rehm J: Alcohol use disorders. Lancet North Am Ed 394:781–792, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL: Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Craft RM, Mogil JS, Aloisi AM: Sex differences in pain and analgesia: The role of gonadal hormones. Eur J Pain 8:397–411, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Crippens D, White ML, George MA, Jaworski JN, Brunner LJ, Lancaster FE, Gonzales RA: Gender differences in blood levels, but not brain levels, of ethanol in rats. Alcohol Clin Exp Res 23:414–420, 1999 [PubMed] [Google Scholar]
- 16.Cucinello-Ragland JA, Mitchell-Cleveland R, Bradley Trimble W, Urbina AP, Yeh AY, Edwards KN, Molina PE, Simon Peter L, Edwards S: Alcohol amplifies cingulate cortex signaling and facilitates immobilization-induced hyperalgesia in female rats. Neurosci Lett 761:136119, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Logu F, Li Puma S, Landini L, Portelli F, Innocenti A, de Araujo DSM, Janal MN, Patacchini R, Bunnett NW, Geppetti P, Nassini R: Schwann cells expressing nociceptive channel TRPA1 orchestrate ethanol-evoked neuropathic pain in mice. J Clin Invest 129:5424–5441, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deuis JR, Dvorakova LS, Vetter I: Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci 10:284, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dina OA, Barletta J, Chen X, Mutero A, Martin A, Messing RO, Levine JD: Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J Neurosci 20:8614–8619, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ditre JW, Zale EL, LaRowe LR: A reciprocal model of pain and substance use: Transdiagnostic considerations, clinical implications, and future directions. Annu Rev Clin Psychol 15:503–528, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Dixon WJ: Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20:441–462, 1980 [DOI] [PubMed] [Google Scholar]
- 22.Edwards S, Koob GF: Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol 5:393–410, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards S, Vendruscolo LF, Gilpin NW, Wojnar M, Witkiewitz K: Alcohol and pain: a translational review of pre-clinical and clinical findings to inform future treatment strategies. Alcohol Clin Exp Res 44:368–383, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF: Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: Alleviation by CRF1 receptor antagonism. Neuropharmacology 62:1142–1151, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egli M, Koob GF, Edwards S: Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev 36:2179–2192, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenhardt M, Hansson AC, Spanagel R, Bilbao A: Chronic intermittent ethanol exposure in mice leads to an up-regulation of CRH/CRHR1 signaling. Alcohol Clin Exp Res 39:752–762, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Fillingim RB, Ness TJ: Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev 24:485–501, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Foster KT, Hicks BM, Durbin CE, Iacono WG, McGue M: The gender risk-severity paradox for alcohol use disorder from adolescence through young adulthood. Emerg Adulthood 6:375–386, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster KT, Hicks BM, Iacono WG, McGue M: Gender differences in the structure of risk for alcohol use disorder in adolescence and young adulthood. Psychol Med 45:3047–3058, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS: High blood alcohol levels in women. N Engl J Med 322:95–99, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Fu R, Gregor D, Peng Z, Li J, Bekker A, Ye J: Chronic intermittent voluntary alcohol drinking induces hyperalgesia in Sprague-Dawley rats. Int J Physiol Pathophysiol Pharmacol 7:136–144, 2015 [PMC free article] [PubMed] [Google Scholar]
- 32.Gatch MB, Lal H: Effects of ethanol and ethanol withdrawal on nociception in rats. Alcoholism Clin Exp Res 23:328–333, 1999 [PubMed] [Google Scholar]
- 33.Gatch MB, Selvig M: Theophylline blocks ethanol withdrawal-induced hyperalgesia. Alcohol Alcohol 37:313–317, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Gililland KR, Finn DA: The impact of gonadectomy and adrenalectomy on acute withdrawal severity in male and female C57BL/6J and DBA/2J mice following a single high dose of ethanol. Alcohol Clin Exp Res 31:1846–1857, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilpin NW, Richardson HN, Cole M, Koob GF: Vapor inhalation of alcohol in rats. Curr Protoc Neurosci 44:1–19, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN: Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res 33:2113–2123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein DB: Relationship of alcohol dose to intensity of withdrawal signs in mice. J Pharmacol Exp Ther 180:203–215, 1972 [PubMed] [Google Scholar]
- 38.Goldstein DB, Pal N: Alcohol dependence produced in mice by inhalation of ethanol: Grading the withdrawal reaction. Science 172:288–290, 1971 [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez SM, Carrasquillo Y: Estrous cycle and sex differences in referred and visceral sensitivity in rodents. J Pain 20:34–35, 2019 [Google Scholar]
- 40.Griffin WC III, Lopez MF, Becker HC: Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res 33:1893–1900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffin WC III, Lopez MF, Yanke AB, Middaugh LD, Becker HC: Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 201:569–580, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffith AK, Martel MM, Eisenlohr-Moul T, Fillmore MT: Heightened sensitivity to the disinhibiting effect of alcohol in women during the late follicular phase of the menstrual cycle. Exp Clin Psychopharmacol, 2022. 10.1037/pha0000611. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heilig M, Egli M, Crabbe JC, Becker HC: Acute withdrawal, protracted abstinence and negative affect in alcoholism: Are they linked? Addict Biol 15:169–184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakubczyk A, Ilgen M, Kopera M, Krasowska A, Klimkiewicz A, Bohnert A, Blow F, Brower K, Wojnar M: Reductions in physical pain predict lower risk of relapse following alcohol treatment. Drug Alcohol Depend 158:167–171, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang S, Li J, Zuo W, Chen P, Gregor D, Fu R, Han X, Bekker A, Ye J-H: Downregulation of M-channels in lateral habenula mediates hyperalgesia during alcohol withdrawal in rats. Sci Rep 9:2714, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kliethermes CL, Cronise K, Crabbe JC: Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res 28:1012–1019, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Koob GF, Le Moal M: Addiction and the brain antireward system. Annu Rev Psychol 59:29–53, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Kuner R: Central mechanisms of pathological pain. Nat Med 16:1258–1266, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Lelbach WK: Liver cell necrosis in rats after prolonged ethanol ingestion under the influence of an alcohol-dehydrogenase inhibitor. Experientia 25:816–818, 1969 [DOI] [PubMed] [Google Scholar]
- 50.Li J, Chen P, Han X, Zuo W, Mei Q, Bian EY, Umeugo J, Ye J: Differences between male and female rats in alcohol drinking, negative affects and neuronal activity after acute and prolonged abstinence. Int J Physiol Pathophysiol Pharmacol 11:163–176, 2019 [PMC free article] [PubMed] [Google Scholar]
- 51.Littleton JM, Griffiths PJ, Ortiz A: The induction of ethanol dependence and the ethanol withdrawal syndrome: The effects of pyrazole. J Pharm Pharmacol 26:81–91, 1974 [DOI] [PubMed] [Google Scholar]
- 52.Lopez MF, Becker HC: Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 181:688–696, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Maleki N, Tahaney K, Thompson BL, Oscar-Berman M: At the intersection of alcohol use disorder and chronic pain. Neuropsychology 33:795–807, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Metten P, Schlumbohm JP, Huang LC, Greenberg GD, Hack WR, Spence SE, Crabbe JC: An alcohol withdrawal test battery measuring multiple behavioral symptoms in mice. Alcohol 68:19–35, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meziane H, Ouagazzal A-M, Aubert L, Wietrzych M, Krezel W: Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: Implications for phenotyping strategies. Genes, Brain and Behav 6:192–200, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Mogil JS, Chanda ML: The case for the inclusion of female subjects in basic science studies of pain. Pain 117:1–5, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Mumenthaler MS, Taylor JL, O’Hara R, Yesavage JA: Gender differences in moderate drinking effects. Alcohol Res Health 23:55–64, 1999 [PMC free article] [PubMed] [Google Scholar]
- 58.Neddenriep B, Bagdas D, Contreras KM, Ditre JW, Wolstenholme JT, Miles MF, Damaj MI: Pharmacological mechanisms of alcohol analgesic-like properties in mouse models of acute and chronic pain. Neuropharmacology 160:107793, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson TS, Fu W, Donahue RR, Corder GF, Hökfelt T, Wiley RG, Taylor BK: Facilitation of neuropathic pain by the NPY Y1 receptor-expressing subpopulation of excitatory interneurons in the dorsal horn. Sci Rep 9:7248, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ossipov MH, Morimura K, Porreca F: Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 8:143–151, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pahng AR, Edwards S: The convergent neuroscience of affective pain and substance use disorder. Alcohol Res 41:1, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paller CJ, Campbell CM, Edwards RR, Dobs AS: Sex-based differences in pain perception and treatment. Pain Med 10:289–299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perrino AC Jr, Ralevski E, Acampora G, Edgecombe J, Limoncelli D, Petrakis IL: Ethanol and pain sensitivity: Effects in healthy subjects using an acute pain paradigm. Alcohol Clin Exp Res 32:952–958, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Popescu A, LeResche L, Truelove EL, Drangsholt MT: Gender differences in pain modulation by diffuse noxious inhibitory controls: A systematic review. Pain 150:309–318, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Pradhan AA, Tipton AF, Zhang H, Akbari A, Pandey SC: Effect of histone deacetylase inhibitor on ethanol withdrawal-induced hyperalgesia in rats. Int J Neuropsychopharmacolog 22:523–527, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quadir SG, Tanino SM, Rohl CD, Sahn JJ, Yao EJ, Cruz L dos R, Cottone P, Martin SF, Sabino V: The Sigma-2 receptor /transmembrane protein 97 (σ2R/TMEM97) modulator JVW-1034 reduces heavy alcohol drinking and associated pain states in male mice. Neuropharmacology 184:108409, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rhodes JS, Ford MM, Yu C-H, Brown LL, Finn DA, Garland T Jr, Crabbe JC: Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav 6:1–18, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF: Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res 22:1564–1569, 1998 [PubMed] [Google Scholar]
- 69.Robins MT, Heinricher MM, Ryabinin AE: From pleasure to pain, and back again: The intricate relationship between alcohol and nociception. Alcohol Alcohol 54:625–638, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rogers J, Wiener SG, Bloom FE: Long-term ethanol administration methods for rats: Advantages of inhalation over intubation or liquid diets. Behav Neural Biol 27:466–486, 1979 [DOI] [PubMed] [Google Scholar]
- 71.Roltsch Hellard EA, Impastato RA, Gilpin NW: Intracerebral and intra-nasal melanocortin-4 receptor antagonist blocks withdrawal hyperalgesia in alcohol-dependent rats. Addict Biol 22:692–701, 2017 [DOI] [PubMed] [Google Scholar]
- 72.Smith ML, Walcott AT, Heinricher MM, Ryabinin AE: Anterior cingulate cortex contributes to alcohol withdrawal- induced and socially transferred hyperalgesia. eNeuro 4:7–17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taylor JL, Dolhert N, Friedman L, Mumenthaler M, Yesavage JA: Alcohol elimination and simulator performance of male and female aviators: a preliminary report. Aviat Space Environ Med 67:407–413, 1996 [PubMed] [Google Scholar]
- 74.Thompson T, Oram C, Correll CU, Tsermentseli S, Stubbs B: Analgesic effects of alcohol: A systematic review and meta-analysis of controlled experimental studies in healthy participants. J Pain 18:499–510, 2017 [DOI] [PubMed] [Google Scholar]
- 75.Vandegrift BJ, You C, Satta R, Brodie MS, Lasek AW: Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLoS One 12:187698, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vendruscolo LF, Roberts AJ: Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol 48:277–286, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vitus D, Williams MK, Rizk M, Neubert JK, Robinson M, Boissoneault J: Analgesic effects of alcohol in adults with chronic jaw pain. Alcohol Clin Exp Res 46:1515–1524, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.White A, Caillaud M, Carper M, Poklis J, Miles MF, Damaj MI: Thermal antinociceptive responses to alcohol in DBA/2J and C57BL/6J inbred male and female mouse strains. Behav Brain Res 436:114087, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Witkiewitz K, Vowles KE, McCallion E, Frohe T, Kirouac M, Maisto SA: Pain as a predictor of heavy drinking and any drinking lapses in the COMBINE Study and the United Kingdom alcohol treatment trial. Addiction 110:1262–1271, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA: Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol 42:149–160, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zale EL, Maisto SA, Ditre JW: Interrelations between pain and alcohol: An integrative review. Clin Psychol Rev 37:57–71, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao W, Li Q, Ma Y, Wang Z, Fan B, Zhai X, Hu M, Wang Q, Zhang M, Zhang C, Qin Y, Sha S, Gan Z, Ye F, Xia Y, Zhang G, Yang L, Zou S, Xu Z, Xia S, Yu Y, Abdul M, Yang J-X, Cao J-L, Zhou F, Zhang H: Behaviors related to psychiatric disorders and pain perception in C57BL/6J mice during different phases of estrous cycle. Front Neurosci 15:650793, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.