Abstract
Introduction:
Customized, patient-specific interbody cages have been used in the treatment of spinal neoplasia, degenerative disease, infection, congenital anomalies, and trauma. However, to date, their use has been limited to a single spinal level, and the utility of customized spinal implants in multiple spinal levels remains unclear. In addition, limited studies exist that compare outcomes following fusion and decompression surgery using customized implants to traditional, standard implants.
Case Report:
We present two cases. Case 1 consists of a multilevel deformity surgery from L3-S1 ALIF and T10-Pelvis PSF in a patient with a congenital scoliosis (CS) using customized implants on multiple spinal levels. We compare Case 1 to Case 2, in which a patient underwent a lumbar decompression and fusion for CS using standard titanium implants. While the patient in Case 1 reported improved back pain and independent ambulation at 1-year post-operative and required no revision surgery, the patient in Case 2 required revision surgery 2 years post-operative due to pseudoarthrosis.
Conclusion:
CS with right wedge hemivertebrae may be treated with customized implants on multiple spinal levels, and customized implants may provide benefit standard implants.
Keywords: Spine, hemivertebrae, customized implants
Learning Point of the Article:
Customized implants are effective in the treatment of congenital scoliosis with hemivertebrae and may provide benefit over standard implants
Introduction
Surgery for patients with congenital spine malformations such as hemivertebrae poses a challenge for surgeons. Zhang et al. reported revision surgeries in 10.7% of congenital scoliosis (CS) cases undergoing posterior hemivertebrae resection with transpedicular instrumentation due to the pedicle fractures, instrumentation failures, proximal junctional kyphosis, and delayed wound union [1]. In a study analyzing factors causing failure of primary surgery in CS patients with single hemivertebra undergoing posterior spinal fusion, Shi et al. found that implant failure is the driving factor in 53.1% of cases [2]. Furthermore, the overall incidence of postoperative complications is reported to be between 13% at 1-year follow-up and 30% at 5-year follow-up for adults undergoing correction surgery for scoliosis [3, 4].
Patient-specific, customized interbody cage spinal implants may be a key technology to improve surgical efficiency and patient outcomes. Studies evaluating the efficacy and outcomes of customized spinal implants are largely limited to case reports, and larger randomized controlled studies are still needed to evaluate their efficacy compared to traditional implants. However, preliminary data suggest customized spinal implants may help to expedite fusion and functional improvement [5]. This is likely due to increased surface area contact between vertebral segments. In a study evaluating the physiological movement of complete ovine spinal cord segments fused with 3D implants, Loenen et al. found that increased bone contact in titanium cages might facilitate increased early segmental stability by direct osseointegration of the cage at the vertebral endplate [6].
Recent case reports demonstrate the use of customized 3D interbody cages in the treatment of spinal neoplasia [7, 8, 9], degenerative disease [10, 11], infection [12, 13], congenital anomaly [14, 15], and trauma [16]. However, while the aforementioned congenital spinal surgeries were each treated with a single 3D implant, to date, there have been no instances of congenital deformity surgery treated with 3D custom implants in multiple spinal levels. Here, we describe a patient with a history of CS and right wedge hemivertebra that presented with chronic bilateral low back pain, lower extremity pain, and radicular symptoms who was successfully treated using 3D-printed customized interbody cages in multiple spinal levels. To demonstrate the potential benefit and unique ability of custom patient-specific implants, we compare that case to a separate case with a different patient with CS that underwent surgical correction using traditional implants, however, had a failure requiring a revision.
Case Report
Case 1
A 50-year-old female with CS presented with a near lifelong history of lower back pain and left lower extremity radicular symptoms without history of trauma or inciting event. She reported constant pain which was worse with activity including bending, lifting, and twisting. Extensive conservative management including anti-inflammatories, analgesics, muscle relaxants, physical therapy, and epidural steroid injections failed to provide meaningful improvement. The patient opted to proceed with surgical intervention.
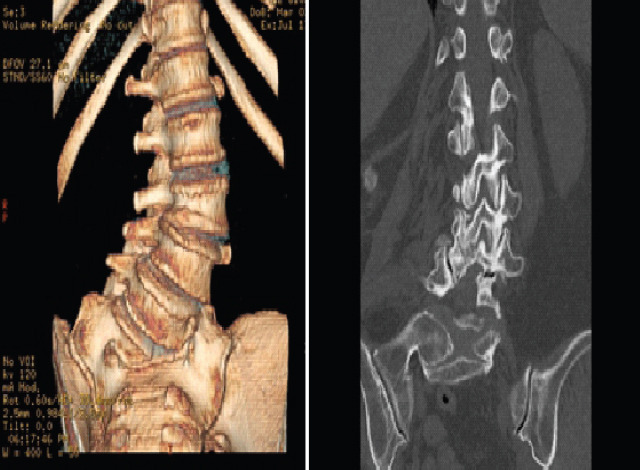
Pre-operative extension radiographs of the lumbar spine (Fig. 1) and computed tomography (CT) imaging (Fig. 2) demonstrated thoracolumbar curve measuring approximately 50° from T11-L5, a right hemivertebrae at L5, a 9 mm of lateral listhesis at L3-4, 15° of lumbar lordosis, and asymmetric disc height loss from L1-S1.
Figure 1.
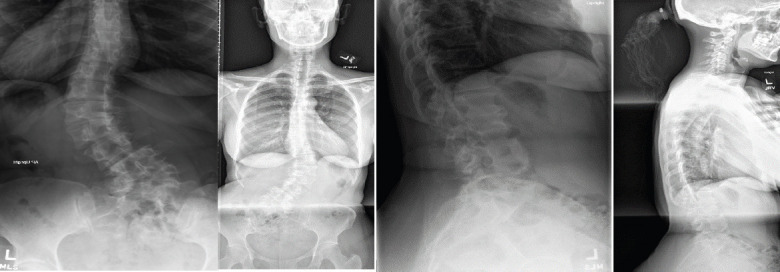
Pre-operative coronal (left) and lateral (right) radiographs. Radiographs demonstrate a 50° thoracolumbar curve from T11-L5, a right L5 hemivertebrae, lateral listhesis at L3-4, 29° of lumbar lordosis and truncal shift to the left.
Figure 2.
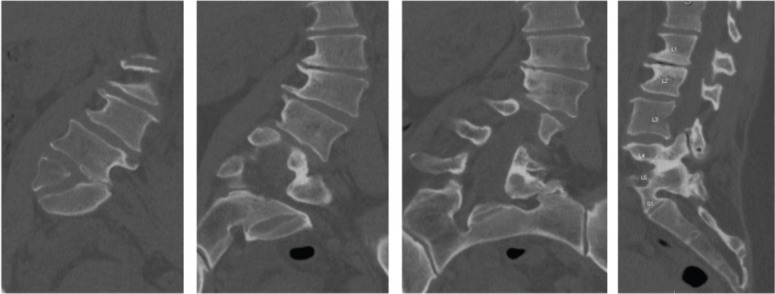
Pre-operative CT. CT demonstrates a 9 mm of lateral listhesis at L3-4, 15° of lumbar lordosis and asymmetric disc height loss from L1-S1.
Pre-operative planning was performed through the Aprevo software (Carlsmed, Carlsbad, CA) and a 3D reconstruction was performed. Customized 3D printed titanium implants were computationally constructed using pre-operative CT images (Figs. 3 and 4). The patient underwent a staged procedure. In Stage 1 (Fig. 5), the patient underwent an L4-S1 posterior column osteotomy in the prone position to mobilize the L4-S1 segment, following which a L3-4 and L-S1 ALIF was performed. Stage 2 of the surgery was performed 2 days later, in which a lateral lumbar interbody fusion using the customized interbodies was performed at L1-2 and L2-3 followed by posterior spinal fusion at T10-Pelvis. The patient had an uncomplicated hospital stay and discharged to a short-term rehabilitation facility.
Figure 3.
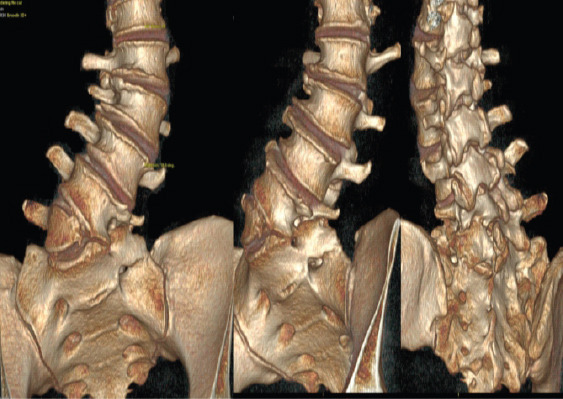
Pre-operative computationally constructed anatomy using CT scans. The images demonstrate a 9 mm of lateral listhesis at L3-4, 15 degrees of lumbar lordosis and asymmetric disc height loss from L1-S1.
Figure 4.
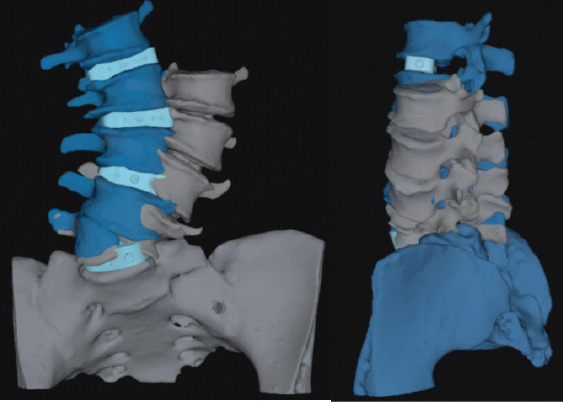
Pre-operative computationally constructed implants. Demonstrates are implants at L1-S1 (light blue) and expected corrected vertebral body position (dark blue).
Figure 5.
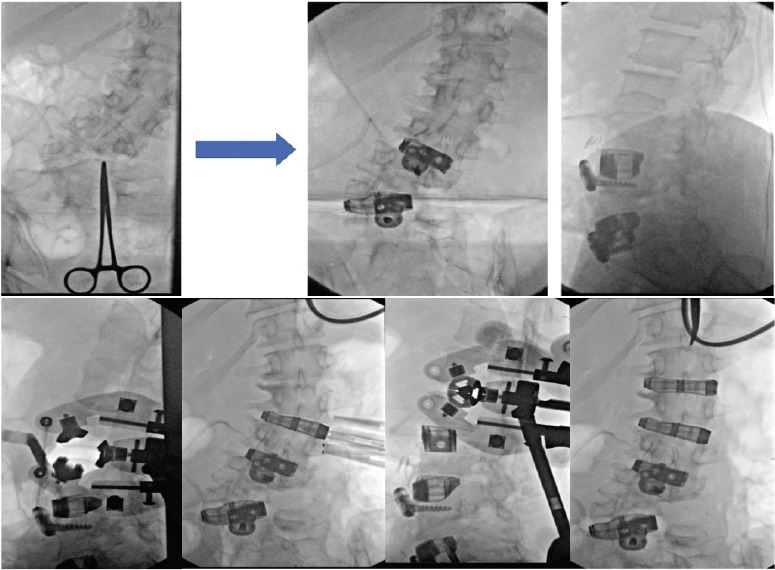
Stage 1 of surgery (top): L4-S1 osteotomy in the prone position to mobilize the L4-S1 segment, followed by L3-4 and L-S1 ALIF. Stage 2 of surgery (bottom): Lateral lumbar interbody fusion using customized interbodies at L1-2 and L2-3 followed by posterior spinal fusion at T10-Pelvis.
At 7-week postoperative, the patient noted significant improvement in her pre-operative back pain and radicular symptoms. She reported a significant improvement in her posture and alignment. At 1-year postoperative, the patient was ambulating independently and able to perform her activities of daily living without any significant difficulty. Overall, the patient had improved pain and movement when compared to her pre-operative baseline. Fig. 6 demonstrates radiographs at 1-year follow-up compared to pre-operative radiographs. This 1-year follow-up was her last follow-up, where she reported resolved pre-operative pain, ambulation without difficulty, and back to her normal workday job.
Figure 6.
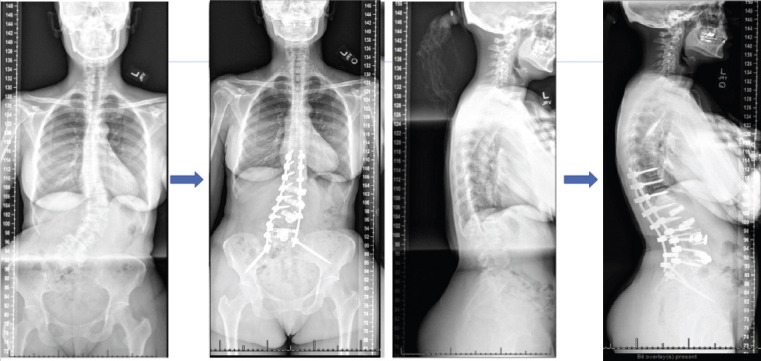
Coronal pre-operative radiographs (left). Arrows point to post-op radiographs at 1-year follow-up. Lateral pre-operative radiographs (right). Arrows pointing to post-op radiographs taken at 1-year follow-up.
Case 2
A 64-year-old woman with a long history of lower back pain and radicular pain presented with worsening lumbar pain over the preceding several months despite extensive physical therapy and epidural steroid injections. Neurologic examination revealed 4/5 motor grade in bilateral EHL but was otherwise intact. The patient opted to proceed with surgical intervention. Pre-operative radiographs (Fig. 7) and CT scan (Fig. 8) demonstrated CS with malformation of the lumbosacral junction, a dynamic spondylolisthesis of L4-5 with stenosis, and multilevel spondylosis with degenerative changes.
Figure 7.
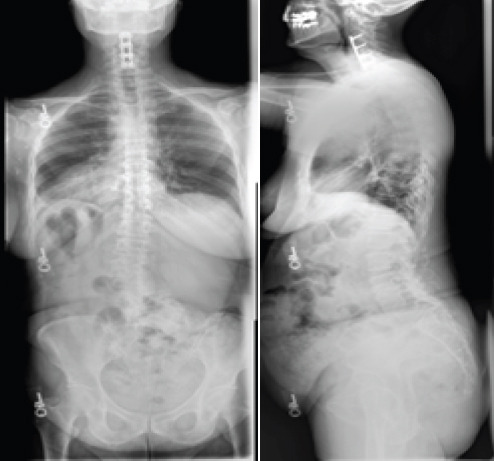
Pre-operative coronal (left) and lateral (right) radiographs. Both demonstrate congenital scoliosis with the malformation of the lumbosacral junction, a dynamic spondylolisthesis of L4-5 with stenosis, and multilevel spondylosis with degenerative changes.
Figure 8.
Pre-operative computationally constructed anatomy (left) using CT scans (right). These images demonstrate congenital scoliosis with the malformation of the lumbosacral junction, a dynamic spondylolisthesis of L4-5 with stenosis, and multilevel spondylosis with degenerative changes.
The index operation consisted of TLIF at L4-5, and T10-pelvis PSF using a standard posterior interbody titanium implant at L4-L5. Cobalt chromium rods were utilized in the index surgery.
1 year postoperatively, she developed a pseudoarthrosis at L4-5 along with bilateral rod fractures at the lumbosacral junction, leading to lumbosacral pain, along with progressive sacroiliac joint dysfunction (Fig. 9). She underwent revision posterior spinal fusion consisting of revision iliac fixation, additional iliac fixation with an accessory rod on fractional curve side, and bilateral sacroiliac joint stabilization and fusion (Fig. 10). Following surgery, she reported resolution of back and leg pain and markedly improved function compared to before initial index surgery,
Figure 9.
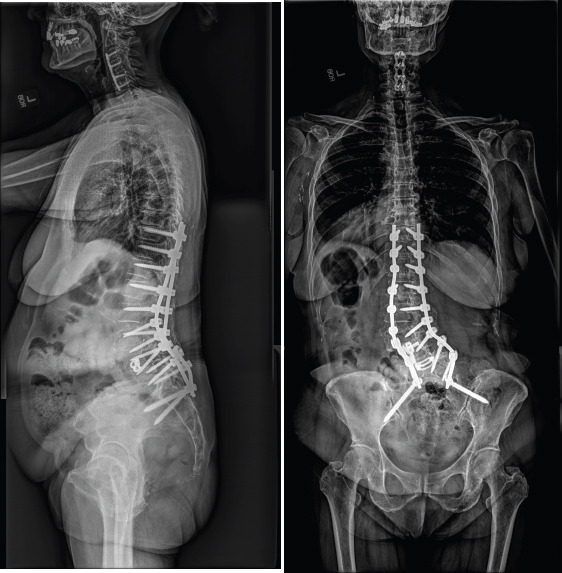
Coronal (left) and lateral (right) radiographs 1-years post-operative. These images demonstrate pseudoarthrosis at L4-5 along with bilateral rod fractures at the lumbosacral junction.
Figure 10.
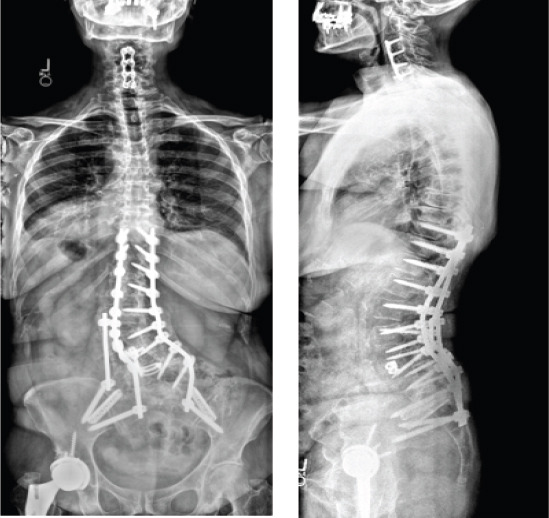
Post-operative revision coronal (left) and lateral (right) radiographs. These images were acquired following revision posterior spinal fusion consisting of revision iliac fixation, additional iliac fixation with an accessory rod on fractional curve side, and bilateral sacroiliac joint stabilization and fusion.
Discussion
The objectives of this study were to (1) illustrate an example of successful surgical treatment of a patient with hemivertebrae using custom patient-specific implants at multiple spinal levels and (2) compare this case a to a similar case that utilized standard implants.
The patient in Case 1 suffered from chronic low back pain due to CS and a right wedge vertebra. Following lumbar decompression and fusion using customized 3D-printed implants, the patient reported significant improvement in her pain and posture (Fig. 6). Given the anatomic complexity of these patients, the development and utilization of customized implants allowed for an excellent endplate apposition without the need for extensive bony resection, which would not be possible with standard interbodies. To our knowledge, this is the first reported case demonstrating the treatment of CS using 3D-printed personalized spinal implants for multiple spinal levels.
Studies evaluating the efficacy of personalized spinal implants are largely limited to case studies. Two of these studies reported the use of these implants in the treatment of congenital spinal disorders. Mobbs et al. [14] presented a 52-year-old woman with an 18-month history of back pain. Similar to our patient, their patient was found to have a congenital hemivertebra at L5 (Figs. 1 and 2) with degenerative changes that was treated with lumbar decompression and fusion with a personalized spinal implant. However, while only the L4/L5 segment was fused in Mobbs et al. case, the patient in our case underwent L3-S1 ALIF and posterior instrumentation T10-Pelvis (Figs. 4 and 5), demonstrating increased severity and unique complexity in our case. In another study [15], a 34-year-old man presented with a 3-year history of bilateral L5 radiculopathy caused by bilateral L5 pars defect, L5/S1 degenerative disc disease, and severe foraminal stenosis. Anterior lumbar interbody fusion surgery was performed with custom 3D interbodies. Again, our case differed from this case in complexity of procedure and medical history as we performed a multilevel deformity surgery using multilevel customized implants.
To address our second objective, we compared Case 1 to Case 2, where the patient underwent an index lumbar decompression and fusion for CS (Figs. 7 and 8) using standard titanium implants. At 2-year post-operative, the patient in Case 2 presented with worsening sacroiliac pain with the evidence of rod fracture and pseudoarthrosis at L4-L5 (Figs. 9). Revision surgery resulted in improved pain and mobility (Figs. 10). Development of pseudoarthrosis and the need for revision surgery in Case 2 may be explained by the decreased surface area available for fusion, decreased contact of the implant with the bone, thus decreased on-growth and through-growth resulting in decreased osteointegration, and stability as compared to Case 1, which utilized custom implants.
While larger, randomized, controlled studies are still needed to gain knowledge pertaining to long-term outcomes in comparison to traditional, non-customized implants, existing biomechanical and animal investigations of customized implants suggest they will provide improved outcomes and increased surgical safety and efficiency. In a study evaluating the biomechanical properties of titanium cages in cervical spine surgery, Fengbin et al. found that implants with increased end plate contact surface area resulted in decreased loss of height of fused segments, lower rate of subsidence, and lower scores of neck pain [16]. During an en bloc resection of L5 vertebral body with single lesion, Mobbs et al. compared reconstruction using a 3D patient-specific implant and an off-the-shelf implant at the same spinal level intraoperatively. The 3D patient-specific implant resulted in decreased time to implant, reduced radiographs required to determine the position of implant, improved end plate fit, and more uniform loading compared to the off-the-shelf implant [5]. From a safety perspective, 3D-implants seem to provide benefit over off-the-shelf implants as they avoid osteotomies required with off-the-shelf implants, thus decreasing operative time and blood loss, as well as allow for pre-planned screw trajectories which decrease the chance of damage to blood vessels, nerves, and the spinal cord [17]. The utility of 3D customized implants in allowing surgeons to operate on native anatomy will be safer, quicker, and result in increased osteointegration and bone/implant interface.
Conclusion
Our study suggests (1) customized interbody implants can be used to successfully treat patients with symptomatic congenital spinal deformities in addition to congenital spinal deformities involving multiple spinal levels and (2) that customized implants may provide benefit over traditional standard implants. In the complex, high-risk surgery involving multiple spinal levels in our patient with congenital aberrant anatomy, 3D customized implants proved to be safe, effective, and led to quality patient outcomes. While our study is a case report, further well-designed studies such as randomized controlled trials with larger sample sizes are needed to verify our results.
Clinical Message.
To our knowledge, this is the first reported case of a patient presenting with chronic pain due to CS and wedge vertebrae who underwent multilevel spinal lumbar interbody fusion surgery with 3D-printed customized implants. We demonstrate that customized implants are effective in the treatment of CS with hemivertebrae and may provide benefit over standard implants.
Biography




Footnotes
Conflict of Interest: Nil
Source of Support: Nil
Consent: The authors confirm that informed consent was obtained from the patient for publication of this case report
References
- 1.Zhang J, Shengru W, Qiu G, Yu B, Yipeng W, Luk KD. The efficacy and complications of posterior hemivertebra resection. Eur Spine J. 2011;20:1692–702. doi: 10.1007/s00586-011-1710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi BL, Li Y, Zhu ZZ, Liu WY, Liu Z, Sun X, et al. Failed primary surgery in congenital scoliosis caused by a single hemivertebra:Reasons and revision strategies. Orthop Surg. 2022;14:349–55. doi: 10.1111/os.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyrölä K, Kautiainen H, Pekkanen L, Mäkelä P, Kiviranta I, Häkkinen A. Long-term clinical and radiographic outcomes and patient satisfaction after adult spinal deformity correction. Scand J Surg. 2019;108:343–51. doi: 10.1177/1457496918812201. [DOI] [PubMed] [Google Scholar]
- 4.Sugawara R, Takeshita K, Inomata Y, Arai Y, Takaso M, Takahashi J, et al. The Japanese scoliosis society morbidity and mortality survey in 2014:The complication trends of spinal deformity surgery from 2012 to 2014. Spine Surg Relat Res. 20193:214–21. doi: 10.22603/ssrr.2018-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mobbs RJ, Choy WJ, Wilson P, McEvoy A, Phan K, Parr WC. L5 En-bloc vertebrectomy with customized reconstructive implant:Comparison of patient-specific versus off-the-shelf implant. World Neurosurg. 2018;112:94–100. doi: 10.1016/j.wneu.2018.01.078. [DOI] [PubMed] [Google Scholar]
- 6.Loenen AC, Peters MJ, Bevers RT, Schaffrath C, van Haver E, Cuijpers VM, et al. Early bone ingrowth and segmental stability of a trussed titanium cage versus a polyether ether ketone cage in an ovine lumbar interbody fusion model. Spine J. 2022;22:174–82. doi: 10.1016/j.spinee.2021.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Xu N, Wei F, Liu X, Jiang L, Cai H, Li Z, et al. Reconstruction of the upper cervical spine using a personalized 3D-printed vertebral body in an adolescent with ewing sarcoma. Spine (Phila Pa 1976) 2016;41:E50–4. doi: 10.1097/BRS.0000000000001179. [DOI] [PubMed] [Google Scholar]
- 8.Choy WJ, Mobbs RJ, Wilcox B, Phan S, Phan K, Sutterlin CE., 3rd Reconstruction of thoracic spine using a personalized 3D-printed vertebral body in adolescent with T9 primary bone tumor. World Neurosurg. 2017;105:1032–e13-7. doi: 10.1016/j.wneu.2017.05.133. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Wang Y, Zhao Y, Liu J, Xiao S, Mao K. Multilevel 3D printing implant for reconstructing cervical spine with metastatic papillary thyroid carcinoma. Spine (Phila Pa 1976) 2017;42:E1326–30. doi: 10.1097/BRS.0000000000002229. [DOI] [PubMed] [Google Scholar]
- 10.Thayaparan GK, Owbridge MG, Thompson RG, D'Urso PS. Designing patient-specific solutions using biomodelling and 3D-printing for revision lumbar spine surgery. Eur Spine J. 2019;28(Suppl 2):18–24. doi: 10.1007/s00586-018-5684-z. [DOI] [PubMed] [Google Scholar]
- 11.Phan K, Sgro A, Maharaj MM, D'Urso P, Mobbs RJ. Application of a 3D custom printed patient specific spinal implant for C1/2 arthrodesis. J Spine Surg. 2016;2:314–8. doi: 10.21037/jss.2016.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung KS, Shin DA, Kim KN, Ha Y, Yoon DH, Yi S. Vertebral reconstruction with customized 3-dimensional-printed spine implant replacing large vertebral defect with 3-year follow-up. World Neurosurg. 2019;126:90–5. doi: 10.1016/j.wneu.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YW, Deng L, Zhang XX, Yu XL, Ai ZZ, Mei YX, et al. Three-dimensional printing-assisted cervical anterior bilateral pedicle screw fixation of artificial vertebral body for cervical tuberculosis. World Neurosurg. 2019;127:25–30. doi: 10.1016/j.wneu.2019.03.238. [DOI] [PubMed] [Google Scholar]
- 14.Mobbs RJ, Coughlan M, Thompson R, Sutterlin CE, Phan K. The utility of 3D printing for surgical planning and patient-specific implant design for complex spinal pathologies:Case report. J Neurosurg Spine. 2017;26:513–8. doi: 10.3171/2016.9.SPINE16371. [DOI] [PubMed] [Google Scholar]
- 15.Mobbs RJ, Parr WC, Choy WJ, McEvoy A, Walsh WR, Phan K. Anterior lumbar interbody fusion using a personalized approach:Is custom the future of implants for anterior lumbar interbody fusion surgery?World Neurosurg. 2019;124:452–458.e1. doi: 10.1016/j.wneu.2018.12.144. [DOI] [PubMed] [Google Scholar]
- 16.Fengbin Y, Jinhao M, Xinyuan L, Xinwei W, Yu C, Deyu C. Evaluation of a new type of titanium mesh cage versus the traditional titanium mesh cage for single-level, anterior cervical corpectomy and fusion. Eur Spine J. 2013;22:2891–6. doi: 10.1007/s00586-013-2976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izatt MT, Thorpe PL, Thompson RG, D'Urso PS, Adam CJ, Earwaker JW, et al. The use of physical biomodelling in complex spinal surgery. Eur Spine J. 2007;16:1507–18. doi: 10.1007/s00586-006-0289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


