Abstract
Once-weekly therapy with combinations of isoniazid plus a rifamycin was tested in the mouse low-dose aerosol infection model against two strains of Mycobacterium tuberculosis. Combinations of isoniazid and rifalizil and isoniazid and rifapentine were both highly effective. These animal model data thus support the evaluation of these regimens under clinical conditions.
The therapy of disease caused by Mycobacterium tuberculosis is now further complicated by the emergence of increasing drug resistance. One of the factors contributing to this specific problem, and indeed to the therapy of tuberculosis as a whole, is patient compliance (1). To address this issue, the World Health Organization has instigated a program of directly observed therapy (2, 3, 5, 6, 14, 20).
Among approaches to encourage patient compliance are shortening the therapy period (some regimens in the past have required a year or more of daily treatment) and lengthening the period between doses, e.g., instituting once-weekly therapy. This latter approach is particularly attractive in areas of the world, such as Africa, in which patients may be widely dispersed in rural areas away from the nearest clinic (8, 10, 17, 24, 25).
In this study, we used the mouse aerosol infection model (18) to evaluate the combination of isoniazid and the newly available rifamycins (4, 7, 9, 11–13, 15, 16, 19, 21, 22) given once weekly. Combinations of isoniazid with rifapentine (RPT) or rifalizil (RLZ) were the most effective in this model. These data suggest that such regimens are indeed worthy of evaluation in clinical trials.
Female specific-pathogen-free C57BL/6 mice (Jackson Laboratories, Bar Harbor, Maine) were aerogenically infected with M. tuberculosis Erdman (TMC107) or the highly virulent (drug-sensitive) strain CSU93 (23) with a Middlebrook aerosol generation device (Glas-Col Inc., Terre Haute, Ind.). Mice were exposed to a standardized inoculum that reproducibly deposits 50 to 100 bacilli into the lung tissues. The course of the infection was then monitored against time by plating serial dilutions of individual whole-organ homogenates on nutrient 7H11 agar and counting bacterial colony formation 14 to 21 days later after incubation at 37°C in humidified air. RLZ, previously designated KRM-1648, was provided by PathoGenesis Corp., Seattle, Wash.; rifampin (RIF) was purchased from Sigma Chemical Co., St. Louis, Mo. RPT was provided by Hoechst Marion Roussel, Inc., Cincinnati, Ohio; rifabutin (RBT) was provided by Pharmacia Upjohn, Columbus, Ohio. The MICs (of RIF, 0.125 μg; of RPT, 0.03 μg; of RBT, 0.03 μg; and of RLZ, 0.008 μg), determined by plating inocula onto agar containing dilutions of drug, for the two strains were similar. For use in vivo, each drug was initially dissolved in a small volume of ethanol and then further dissolved in 0.05% methylcellulose and 0.04% Tween 80 in distilled water prior to oral gavage. Therapy, started at week 3, was given once weekly for 5 weeks. All drugs were given at a dose of 25 mg/kg of body weight/day.
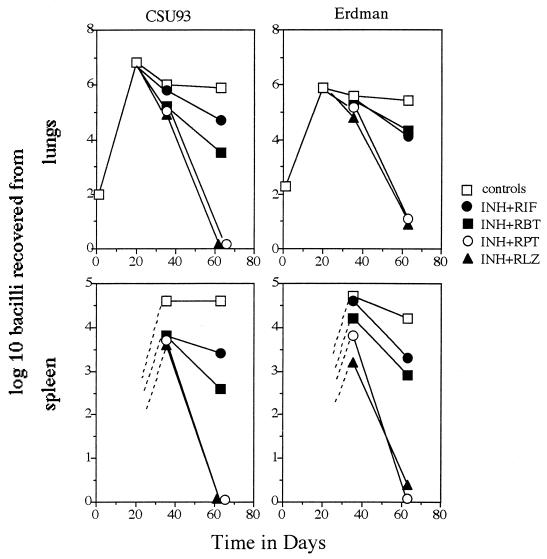
The results of the study are shown in Fig. 1. One week after the 5-week therapy period was discontinued, mice given the isoniazid-RLZ or isoniazid-RPT combination showed clearance in the lungs of the CSU93 strain and only a few detectable Erdman bacilli. Animals treated with isoniazid and RIF or isoniazid and RBT also showed clearance, but to a much lesser degree. A similar pattern of clearance was seen in the spleen. Bacteria in mice disseminate to this organ and can usually be detected a few weeks into infection. Bacteria were detectable in all mice at day 35 (2 weeks into therapy), but these were then cleared in a pattern similar to that seen in the lungs, again with isoniazid-RLZ and isoniazid-RPT being the most-effective drugs. These animal model data therefore support the evaluation of these regimens as intermittent therapy in clinical trials.
FIG. 1.
Growth of M. tuberculosis CSU93 and Erdman following aerosol exposure of C57BL/6 mice and the effects of therapy (given from days 21 to 56). INH, isoniazid. All drugs were given at a dose of 25 mg/kg/day. Data are expressed as mean values (n = 4; standard errors of the mean, omitted for clarity, did not exceed 0.45).
Acknowledgments
This work was supported by NIH programs AI-45239 and AI-45244.
We thank Rick O’Brien for suggesting this study.
REFERENCES
- 1.Buchanan R J. Compliance with tuberculosis drug regimens: incentives and enablers offered by public health departments. Am J Public Health. 1997;87:2014–2017. doi: 10.2105/ajph.87.12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burman W J, Dalton C B, Cohn D L, Butler J R, Reves R R. A cost-effectiveness analysis of directly observed therapy vs self-administered therapy for treatment of tuberculosis. Chest. 1997;112:63–70. doi: 10.1378/chest.112.1.63. [DOI] [PubMed] [Google Scholar]
- 3.Caminero J A, Pavon J M, Rodriguez de Castro F, Diaz F, Julia G, Cayla J A, Cabrera P. Evaluation of a directly observed six months fully intermittent treatment regimen for tuberculosis in patients suspected of poor compliance. Thorax. 1996;51:1130–1133. doi: 10.1136/thx.51.11.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapuis L, Ji B, Truffot-Pernot C, O’Brien R J, Raviglione M C, Grosset J H. Preventive therapy of tuberculosis with rifapentine in immunocompetent and nude mice. Am J Respir Crit Care Med. 1994;150:1355–1362. doi: 10.1164/ajrccm.150.5.7952564. [DOI] [PubMed] [Google Scholar]
- 5.Chaulk C P, Moore-Rice K, Rizzo R, Chaisson R E. Eleven years of community-based directly observed therapy for tuberculosis. JAMA. 1995;274:945–951. [PubMed] [Google Scholar]
- 6.Cohn D L, Catlin B J, Peterson K L, Judson F N, Sbarbaro J A. A 62-dose, 6-month therapy for pulmonary and extrapulmonary tuberculosis. A twice-weekly, directly observed, and cost-effective regimen. Ann Intern Med. 1990;11 2:407–415. doi: 10.7326/0003-4819-76-3-112-6-407. [DOI] [PubMed] [Google Scholar]
- 7.Dhillon J, Mitchison D A. Activity in vitro of rifabutin, FCE 22807, rifapentine, and rifampin against Mycobacterium microti and M. tuberculosis and their penetration into mouse peritoneal macrophages. Am Rev Respir Dis. 1992;145:212–214. doi: 10.1164/ajrccm/145.1.212. [DOI] [PubMed] [Google Scholar]
- 8.Dick J, Clarke M, Tibbs J, Schoeman H. Combating tuberculosis—lessons learnt from a rural community project in the Klein Drakenstein area of the Western Cape. S Afr Med J. 1997;87:1042–1047. [PubMed] [Google Scholar]
- 9.Dickinson J M, Mitchison D A. In vitro observations on the suitability of new rifamycins for the intermittent chemotherapy of tuberculosis. Tubercle. 1987;68:183–193. doi: 10.1016/0041-3879(87)90054-7. [DOI] [PubMed] [Google Scholar]
- 10.Ellis J H, Beyers N, Bester D, Gie R P, Donald P R. Sociological and anthropological factors related to the community management of tuberculosis in the Western Cape communities of Ravensmead and Uitsig. S Afr Med J. 1997;87:1047–1051. [PubMed] [Google Scholar]
- 11.Gonzalez-Montaner L J, Natal S, Yongchaiyud P, Olliaro P. Rifabutin for the treatment of newly-diagnosed pulmonary tuberculosis: a multinational, randomized, comparative study versus rifampicin. Tubercle Lung Dis. 1994;75:341–347. doi: 10.1016/0962-8479(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 12.Heifets L B, Lindholm-Levy P, Flory M A. Bactericidal activity in vitro of various rifamycins against Mycobacterium avium and Mycobacterium tuberculosis. Am Rev Respir Dis. 1990;141:626–630. doi: 10.1164/ajrccm/141.3.626. [DOI] [PubMed] [Google Scholar]
- 13.Hirata T, Saito H, Tomioka H, Sato K, Jidoi J, Hosoe K, Hidaka T. In vitro and in vivo activities of the benzoxazinorifamycin KRM-1648 against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1995;39:2295–2303. doi: 10.1128/aac.39.10.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iseman M D. Directly-observed therapy, patient education and combined drug formulations: complementary, not alternative, strategies in tuberculosis control. Tubercle Lung Dis. 1996;77:101. doi: 10.1016/s0962-8479(96)90020-9. [DOI] [PubMed] [Google Scholar]
- 15.Jabes D, Della Bruna C, Rossi R, Olliaro P. Effectiveness of rifabutin alone or in combination with isoniazid in preventive therapy of mouse tuberculosis. Antimicrob Agents Chemother. 1994;38:2346–2350. doi: 10.1128/aac.38.10.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji B, Truffot-Pernot C, Lacroix C, Raviglione M C, O’Brien R J, Olliaro P, Roscigno G, Grosset J. Effectiveness of rifampin, rifabutin, and rifapentine for preventive therapy of tuberculosis in mice. Am Rev Respir Dis. 1993;148:1541–1546. doi: 10.1164/ajrccm/148.6_Pt_1.1541. [DOI] [PubMed] [Google Scholar]
- 17.Johansson E, Diwan V K, Huong N D, Ahlberg B M. Staff and patient attitudes to tuberculosis and compliance with treatment: an exploratory study in a district in Vietnam. Tubercle Lung Dis. 1996;77:178–183. doi: 10.1016/s0962-8479(96)90035-0. [DOI] [PubMed] [Google Scholar]
- 18.Kelly B P, Furney S K, Jessen M T, Orme I M. Low-dose aerosol infection model for testing drugs for efficacy against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:2809–2812. doi: 10.1128/aac.40.12.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klemens S P, Grossi M A, Cynamon M H. Activity of KRM-1648, a new benzoxazinorifamycin, against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother. 1994;38:2245–2248. doi: 10.1128/aac.38.10.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowak R. WHO calls for action against TB. Science. 1995;267:1763. doi: 10.1126/science.7892595. [DOI] [PubMed] [Google Scholar]
- 21.Orme I M. Antimycobacterial activity in vivo of LM427 (rifabutin) Am Rev Respir Dis. 1988;138:1254–1257. doi: 10.1164/ajrccm/138.5.1254. [DOI] [PubMed] [Google Scholar]
- 22.Pretet S, Lebeaut A, Parrot R, Truffot C, Grosset J, Dinh-Xuan A T. Combined chemotherapy including rifabutin for rifampicin and isoniazid resistant pulmonary tuberculosis. Eur Respir J. 1992;5:680–684. [PubMed] [Google Scholar]
- 23.Valway S E, Sanchez M P C, Shinnick T F, Orme I M, Agerton T, Hoy D, Jones J S, Westmoreland H, Onorato I M. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N Engl J Med. 1998;338:633–639. doi: 10.1056/NEJM199803053381001. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson D, Anderson E, Davies G R, Sturm A W, McAdam K P. Efficacy of twice weekly treatment for tuberculosis given under direct observation in Africa. Trans R Soc Trop Med Hyg. 1997;91:87–89. doi: 10.1016/s0035-9203(97)90407-7. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson D, Davies G R, Connolly C. Directly observed therapy for tuberculosis in rural South Africa, 1991 through 1994. Am J Public Health. 1996;86:1094–1097. doi: 10.2105/ajph.86.8_pt_1.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]