Abstract
Nerve guidance conduits (NGCs) are an essential solution for peripheral nerve repair and regeneration in tissue engineering and medicine. However, the ability of current NGCs is limited to repairing longer nerve gap (i.e., >20 mm) because it cannot meet the following two conditions simultaneously: (1) directional guidance of the axial high-density channels and (2) regenerative stimulation of the extracellular matrix secreted by Schwann cells (SCs). Therefore, we propose a multi-material 3D bioprinting process to fabricate multi-channel nerve guide conduits (MNGCs) containing SCs. In the article, cell-laden methacrylate gelatin (GelMA) was used as the bulk material of MNGCs. To improve the printing accuracy of the axial channels and the survival rate of SCs, we systematically optimized the printing temperature parameter based on hydrogel printability analysis. The multi-material bioprinting technology was used to realize the alternate printing of supporting gelatin and cell-laden GelMA. Then, the high-accuracy channels were fabricated through the UV cross-linking of GelMA and the dissolving technique of gelatin. The SCs distributed around the channels with a high survival rate, and the cell survival rate maintained above 90%. In general, the study on multi-material 3D printing was carried out from the fabricating technology and material analysis, which will provide a potential solution for the fabrication of MNGCs containing SCs.
Keywords: 3D bioprinting, GelMA, printability analysis, multi-channel nerve guidance conduits, Schwann cells
Introduction
Peripheral nerve injury is one of the most common clinical diseases.1,2 When the nerve gap is long (i.e., >20 mm), it needs autograft of nerve or nerve guide conduits (NGCs) for auxiliary repair.3,4 Although autograft is the highest standard of nerve repair, the donor area will also have the problem of nerve function injury.5 NGCs have become the most reliable alternative to nerve autograft.6 At present, the typical NGCs can be classified into many types. The representative structures are hollow design, multi-channel design, and NGCs with fillers.7,8 The NGCs of hollow design have some problems, such as the thin wall, easy collapse, unable to guide the cell to migration effectively, and the regenerated axons will disperse.9,10 In addition, the NGCs with fillers have poor permeability, and cannot achieve the efficient transport of nutrients and metabolism.11,12
The multi-channel nerve guide conduits (MNGCs) are the most suitable method for repairing the long nerve gap. Compared with the other NGCs, the MNGCs have more channels and smaller apertures, which can facilitate the directional alignment of neurons.6,13–15 Moreover, the MNGCs also have superior tensile, compressive, and bending stiffness, which can play a role in protecting the injured nerve.16 The microchannel in the MNGCs can not only provide a microenvironment for nerve growth, but also increase the surface area of nutrient transport and Schwann cells (SCs) attachment, which play a certain role in guiding the nerve cells growth and migration.17–19 Therefore, the design and fabrication of MNGCs have become a research focus in the field of tissue engineering.
In the past 20 years, the MNGCs have obtained rapid development. Among the early researchers for the fabrication of MNGCs, Moore et al. fabricated NGCs with intraluminal channels using poly(lactic-co-glycolic acid) and the distribution of SCs in the MNGCs was further studied. The effect of MNGCs and SCs on axon regeneration was confirmed by vivo experiments in adult rats, which indicated the direction for the systematic study of MNGCs.20 Johnson et al. designed and manufactured the NGCs with bifurcation structure based on 3D printing technology. The function of the NGCs in the regeneration of the complex nerve gap was verified by animal experiments.21 Zhu et al. printed a kind of transparent NGCs with high resolution and the motor function and sensation of the limbs could be recovered by implanting the NGCs.22,23
In addition Ye et al. used digital light processing technology to print the MNGCs of methacrylate gelatin (GelMA). The role of MNGCs in cell survival, proliferation, and migration was verified by PC12 cell implantation in vitro. The potential application value of GelMA MNGCs in the recovery of peripheral nerve gap was pointed out.24 Tao fabricated the NGCs of GelMA by 3D printing technology. The NGCs provided a good physical microenvironment for the growth of axons.25 The role of SCs in the process of nerve repair had also been fully proved. The SCs could facilitate myelination and release nerve growth factor, which had a significant effect on nerve repair.26 The nerve repair process can be effectively driven through the unique capacity of SCs to dedifferentiate into cells.27
It can be seen from the above studies that the NGCs and SCs play a key role in promoting nerve repair, being the most reliable alternative to nerve autograft.
At present, 3D printing has become the most favorable molding process for the fabrication of MNGCs and its technology is mainly divided into extrusion-based printing (EBP), inkjet-based printing (IBP), and stereo lithography appearance (SLA).28
As an EBP method, fused deposition modeling has a single material, no cell and low molding accuracy for the MNGCs. And the material is single and does not contain cells. When the hydrogel concentration is low, the EBP of cells can be realized, but the printing accuracy is too poor to form a high-density channel structure. When IBP is used to fabricate NGCs, the mechanical properties can be enhanced by composite printing of a variety of materials, but high-density channels cannot be well constructed. The SLA method can fabricate structures with complex shape and high accuracy, but the production cost is obviously high. Moreover, the single material of the SLA cannot meet the needs of cell printing and mechanical properties.
At present, there are many studies on the fabrication of NGCs, and the axial channel and SCs play an important role in the repair of long nerve gap. However, it is still impossible to fabricate the MNGCs containing SCs by printing of single material, and it has become a current challenge. Therefore, we propose a multi-material 3D bioprinting process to fabricate a MNGCs containing SCs based on EBP method. Gelatin and GelMA have excellent biocompatibility and are suitable for cell printing and 3D culture. Gelatin material plays a supporting role when the cell-laden GelMA is printed. The printability and viscosity of the GelMA were analyzed in terms of the temperature sensitivity. In the printable area of GelMA, the printing temperature parameter was optimized systematically to reduce the shear force in the cell-laden GelMA.
The multi-material printing technology was used to realize the alternate printing of supporting gelatin and cell-laden GelMA. Then, the high-accuracy channels were fabricated through the UV cross-linking of GelMA and the dissolving technique of gelatin. These channels and SCs have advantages in nerve repair for growth and migration of nerve cells. In addition, we also explored the printing of the bearing layer outside the MNGCs. The mechanical properties of MNGCs was enhanced by the formation of covalent cross-linking with double bonds of N-(2-hydroxy) acrylamide (HEAA) and GelMA. In a word, the multi-material 3D bioprinting technology will provide a potential solution for the fabrication of MNGCs containing SCs.
Materials and Methods
Materials preparation
GelMA was prepared by using an established method.29 The lyophilized GelMA was packaged and stored at 4°C. GelMA hydrogel with a concentration of 1%, 3%, 5%, 7%, and 9% (w/v) was prepared by dissolving lyophilized GelMA in PBS at 37°C and the photoinitiator (LAP; Sigma-Aldrich) was added to the solution at a concentration of 0.1% (w/v). GelMA/HEAA hydrogel with concentration of 5%/5%, 7%/7%, and 9%/9% (w/v) was prepared by dissolving HEAA (7646-67-5; TCI) in PBS and then dissolving lyophilized GelMA in the HEAA solutions at 37°C. After solution preparation, the solutions were centrifuged (2000 r/min), filtrated (0.22 μm), and stored away from light at 4°C.
Before the cell-laden GelMA preparation, the biological safety cabinet was sterilized with UV irradiation. After the number of cells was counted, the centrifuged cells were evenly mixed with liquid GelMA at 30°C using a transfer pipet and the concentration of SCs was 106 mL−1.
SCs (national collection of authenticated cell cultures) were cultured in DMEM medium (12800017; GIBCO) supplemented with 10% (v/v) high-quality fetal bovine serum, 100 μg/mL streptomycin (Sigma-Aldrich), 100 U/mL penicillin (Sigma-Aldrich), and 1.5 g/L NaHCO3.
The MNGCs were cultured in the medium, and cut into discs of 2 mm after 1 and 4 days, respectively. After cleaning the culture medium, the discs were stained with LIVE/DEAD® Viability/(Thermo Fisher) for 30 min.
The SCs in the discs were observed and shot by Light Sheet Microscope (MuVi-SPIM-CS; LUXENDO), and the number of surviving and dead cells was counted by ImageJ software. Our study was approved by Institutional Review Board.
Material properties
The rheological properties of GelMA hydrogel (1%, 3%, 5%, 7%, and 9%) were measured by rheometer (MARS 60; HAAKE). At an initial temperature of 28°C, the temperature drop rate of the rheometer was controlled to 1°C/min. The storage modulus (G′) and loss modulus (G″) were measured under the oscillation mode and the oscillation frequency was 1 Hz. And the viscosity was measured under the rotation mode and the shear rate was 10 s−1. The extrusion state of GelMA hydrogel (1%, 3%, 5%, 7%, and 9%) was observed by high-speed camera (dimax HS; PCO) under different temperature conditions. The printability of the GelMA hydrogel was analyzed by the extrusion state at the needle.
Fabrication technology
In the experiment, a self-developed high-precision 3D bioprinter (SIA bioprinter PRO) was used and the precision of the printhead was 50 μm. In the temperature control system, both the substrate and the printhead had the function of independent temperature control. The temperature adjustment range of the substrate and printhead was −10°C to 60°C and the temperature control accuracy was ±0.1°C, which can meet the printing requirement of various materials at different temperatures. By controlling the temperature of the printheads, the rheological state of the hydrogel was adjusted to achieve pregel printing. The printing parameters are shown in Table 1.
Table 1.
Printing Parameters
| Printing speed | 10 mm/s |
| Nozzle diameter | 150 μm |
| Printing spacing | 150 μm |
| Printing layer thickness | 150 μm |
| Filling rate | 164% |
| Pre-extrusion volume | 0.4 μL |
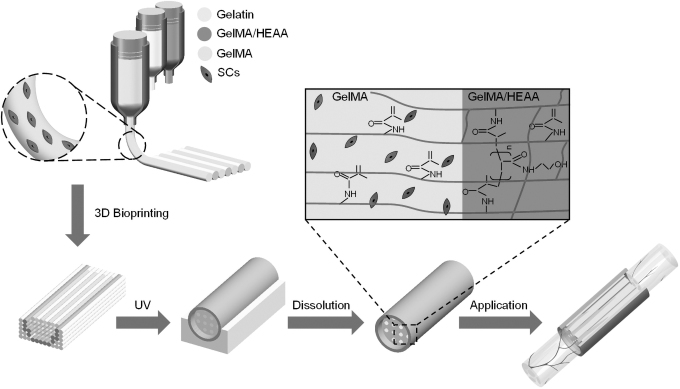
Then the whole structure was irradiated by 365 nm UV for 45 s to realize the cross-linking of GelMA material. The power of ultraviolet light power is 18 W. The cross-linked structure was placed in culture medium at 37°C, and the gelatin was dissolved to form the MNGCs. The inner layer of the MNGCs was GelMA containing SCs, and the outside bearing layer was GelMA/HEAA, where the HEAA can form covalent bond with GelMA (Fig. 1).
FIG. 1.
The schematic of the MNGCs fabrication based on the multi-material 3D bioprinting. MNGCs, multi-channel nerve guide conduits.
Structure design
The geometric size of the MNGCs was Φ 4.2 × 30 mm, and a bearing layer with a thickness of 0.2 mm was printing. The bearing layer was fixed on the surface of the MNGCs through the cross-linking of GelMA, which is expected to increase mechanical properties. The diameter and spacing of inner channels were set to 150 and 105 μm respectively. The self-developed software BiopDesigner was used to configure printing parameters and generate G-code.
Quantitative evaluation
The cross sections of the MNGCs were observed by the microscope (Nikon DMI 8), and the diameter and spacing of the MNGCs were measured by software ImageJ. To analyze the surface area for nerve cells to attach, the channel area ratio in the MNGCs was calculated by the formula (1):30
| (1) |
where Si (mm2) is the area of single channel, i is the number of channels, S0 is the total area of MNGCs.
The MNGCs were frozen with liquid nitrogen and dried in a vacuum freeze dryer for 12 h. After conductive coating on the cross sections of the MNGCs, the pore structure was observed by scanning electron microscope (EVO MA 10; Zeiss). The lyophilized MNGCs were weighed and then immersed in PBS solution for 24 h before weighing again. The porosity of MNGCs can be expressed by the formula (2):20
| (2) |
where MW is the mass of MNGCs after PBS immersion, M0 is the mass of MNGCs after PBS drying, ρ is the density of PBS solution, and Vi is the volume of the channel.
The mechanical properties of MNGCs were tested by texture analyzer (CT3 Texture Analyzer; Brookfield) and three samples were tested for each group. During the test, the MNGCs were cut into 15 mm length to ensure that the actual measured length was more than two times of the diameter. The trigger point load of texture analyzer was 0.008 N, and the test speed was 0.08 mm/s. In the process of axial stretching, the stress–strain data of MNGCs were recorded and fitted linearly. The tensile modulus was calculated by intercepting the strain in the range of 0–10%.
Results
Material printability analysis
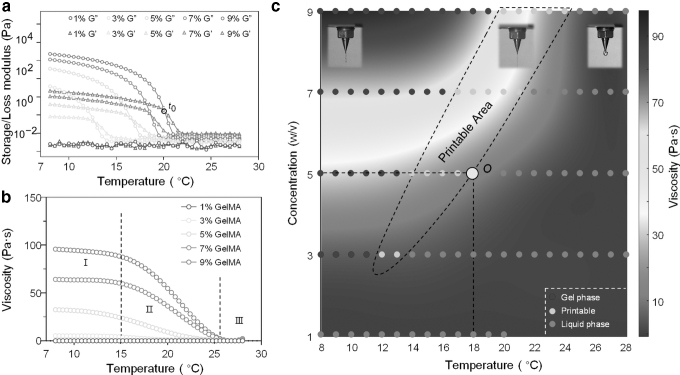
The storage modulus (G′) and loss modulus (G″) of GelMA were measured by rheometer (Fig. 2a). Compared with the G,″ the change of G′ with temperature was obvious, and there was an intersection point t0 in the process of G′ and G″ rising. The temperature of the intersecting point t0 was regarded as the theoretical gel temperature of GelMA. The viscosity curve was divided into three stages based on the speed of the change (Fig. 2b).
FIG. 2.
Characterization of the GelMA. (a) Effect of temperature on storage modulus (G′) and loss modulus (G″) of GelMA. (b) Viscosity as a function of temperature. (c) Coupling diagram of the viscosity rainbow graph and printable area. GelMA, methacrylate gelatin.
When the concentration of GelMA was 1% and 3%, the viscosity was almost not affected by temperature and always close to zero compared with GelMA of the other concentrations. For other concentrations of GelMA, the viscosity changed a little in stage I during the temperature rising process. The “viscosity platform” provided a stability property for the bioprinting in stage I. The viscosity of GelMA decreased rapidly with the increase of temperature in stage II, and the GelMA of higher concentration showed the faster the viscosity decreased. In stage Ⅲ, all concentrations of GelMA had a viscosity close to zero in the current coordinate system.
By fitting the function of GelMA viscosity with temperature, the rainbow diagram was established to describe the effects of concentration and temperature on viscosity (Fig. 2c). In Figure 2c, the red and blue represent the lower and higher viscosity of GelMA, respectively. To verify the accuracy of the theoretical gel temperature, the high-speed camera was used to analyze the printability of GelMA. Through the temperature control system of the printhead, the temperature of GelMA in the printhead was changed to adjust its extrusion state. When the printing temperature was far below (i.e., <t0 − 5) the GelMA theoretical gel temperature, the overall distortion of the extruded GelMA occurs.
When the printing temperature was much higher than (i.e., >t0 + 5) the gel temperature of GelMA, the extruded GelMA formed into the droplet state. The blue dots and the red dots represent GelMA as the gel and liquid state, respectively. The yellow dot is the printable state of GelMA (Fig. 2c). Therefore, when the concentration of GelMA was 5%, the viscosity of point “O” was the lowest in the printable area. Based on the above analysis, the printing temperatures of 5%, 7%, and 9% were set to 18°C, 21°C, and 24°C respectively. We printed other complexed structure to validate the printability and fidelity of GelMA based on temperature analysis (Supplementary Fig. S1).
Structural analysis
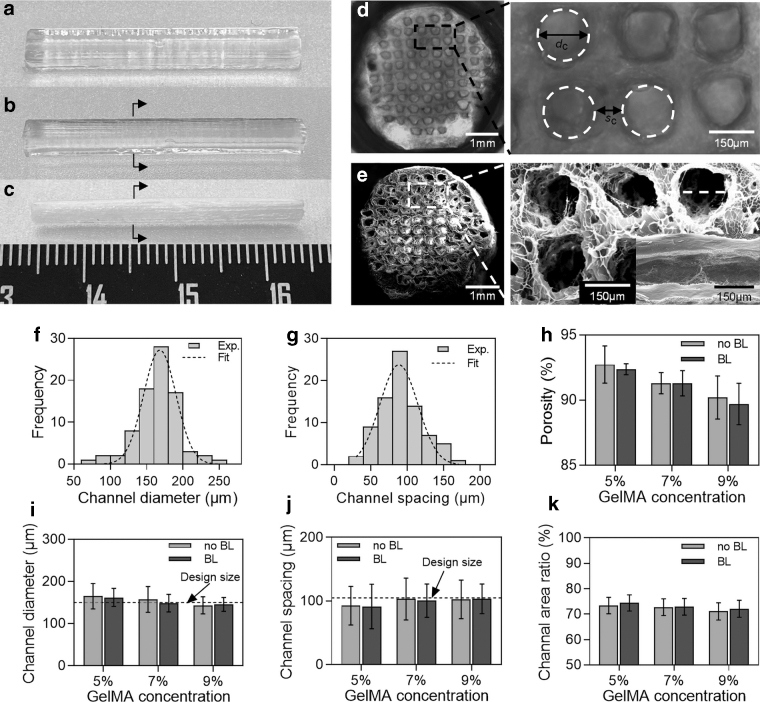
It was observed that the structure had high integrity in low-temperature gel, and there was no inclination or deformation (Fig. 3a). After the structure was cross-linked under UV light, the gelatin that could not be cross-linked by UV light gradually dissolved in culture medium at 37°C. The surface would be smooth after removing gelatin from the structure, and there was no collapse (Fig. 3b). After supporting gelatin dissolving, the printed MNGCs have been saturated with water molecules, so as to achieve the “non-swelling” characteristic. When the MNGCs were lyophilized at low temperature, the whole structure remained stable without large deformation (Fig. 3c).
FIG. 3.
The structural characterizations of the MNGCs. (a–c) The optical images of MNGCs after UV cross-linking, supporting gelatin dissolving and lyophilizing. (d, e) The cross section and local view of MNGCs after gelatin dissolution and lyophilizing. (f, g) Frequency distribution of channel diameter (dc) and channel spacing (sc) (5% GelMA, 5%/5% GelMA/HEAA). (h–k) The quantification of the porosity, channel diameter, channel spacing, and channel area ratio by GelMA concentration. HEAA, N-(2-hydroxy) acrylamide.
Then, we performed swelling experiments on lyophilized MNGCs, which approached the swelling equilibrium after immersion in deionized water for 1 h (Supplementary Fig. S2). By observing the cross section of the MNGCs, a highly unified multi-channel structure was formed inside the conduit (6.1 mm−2). It could be seen from the cross-section view that the channels had good roundness and the structure around the channels was smooth and complete (Fig. 3d). The structure of the MNGCs after lyophilizing was still intact with a clear pore and high connectivity (Fig. 3e).
According to the statistics of the channel diameter (dc) and channel spacing (sc) inside the MNGCs, the trend of normal distribution of channel diameter and spacing could be seen (Fig. 3f, g). The MNGCs were divided into two groups: without bearing layer (no BL) and with BL.
On the whole, when the concentration of GelMA was 5%, the average diameter and spacing of channel had large errors compared with the design size, and the errors decreased with the increase of GelMA concentration. When the concentration was 7% and 9%, the error of channel diameter and spacing was significantly reduced (Fig. 3i, j). This may be due to the weak bonding strength of GelMA at low concentration, and the GelMA on the wall of the channel falls off with the dissolution of gelatin, resulting in a larger channel diameter. With the increase of GelMA concentration, the bonding strength of the MNGCs will also be improved, which reduces the shedding of the GelMA and improves the quality of the channels. This indicates that GelMA can achieve high precision microchannel fabrication by bioprinting.
The PBS solution sublimates after lyophilizing, and then forms the pore characteristics in the MNGCs. It can be seen from the figure that with the increase of GelMA concentration, the porosity of MNGCs decreased gradually. When GelMA concentration was 5% and 7%, the porosity was >90% (Fig. 3h). The GelMA concentration and bearing layer had no obvious effect on the channel area ratio (about 74.5%) of MNGCs (Fig. 3k). The porosity and channel and area ratio can promote the growth of cells, and it is also helpful for the removal of the inside support gelatin.
Mechanical analysis
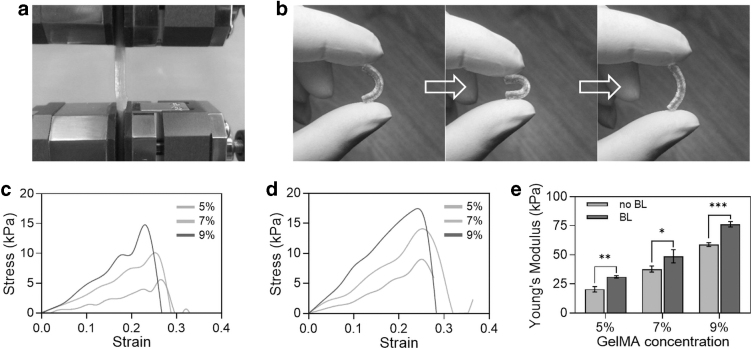
In the application of MNGCs, their stress forms were mainly tension and bending at both sides, so the tensile and bending properties were analyzed.31 After bending the MNGCs (5% GelMA, 5%/10% GelMA/HEAA) for 180° and then releasing one side, it could completely recover to the previous shape without any damage. At this time, the MNGCs remained at high strength and flexibility (Fig. 4b).
FIG. 4.
The mechanical characterizations of the MNGCs. (a) Mechanical property test of the MNGCs. (b) The excellent flexibility properties of the MNGCs after bending and releasing. (c, d) The stress–strain curve of the MNGCs without and with bearing layer. (e) The quantification of the Young's Modulus by GelMA concentration.
The mechanical properties of GelMA in the bearing layer were enhanced through the covalent cross-linking of double bonds in HEAA. It can be seen from the stress–strain curve that the curve with a bearing layer of HEAA was more stable, and the maximum strain can basically reach 30% (Fig. 4c, d). In addition, the Young's modulus of MNGCs with bearing layer increased significantly, and it would increase by >28% when the thickness of bearing layer was only 0.2 mm. With the increase of GelMA concentration, the Young's modulus of MNGCs increased gradually. When the concentration was 9%, the Young's modulus could reach 100 kPa (Fig. 4e).
Cell survival rate
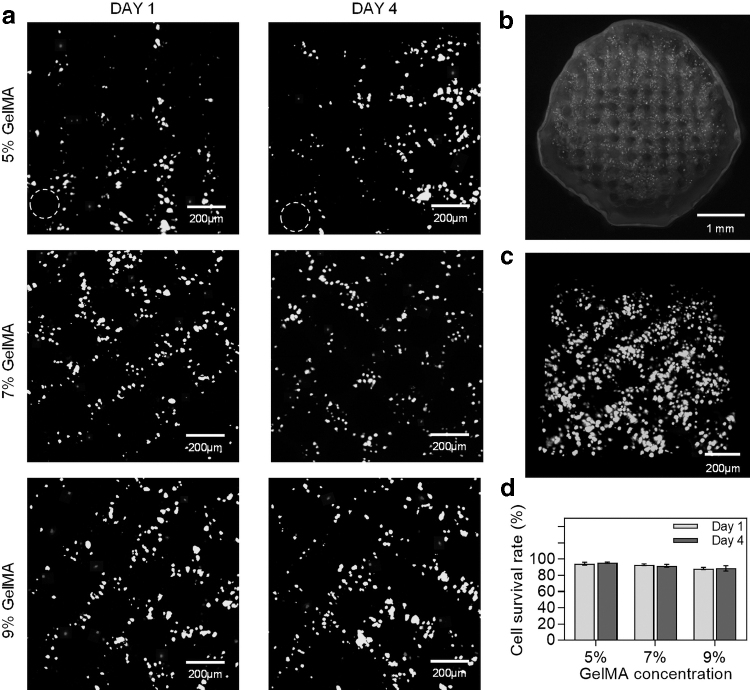
The MNGCs were cultured in the medium for 24 h, and the survival rate of the SCs was analyzed by the number of living and dead cells. It can be seen that the SCs had a high survival rate after printing. With the increase of GelMA concentration, the number of SCs increased gradually, and the distribution of SCs around the channel became more obvious (Fig. 5a). The MNGCs had a clear interface between the bearing layer and the matrix layer (Fig. 5b), which meant that the HEAA in the bearing layer would not have any effect on the SCs.
FIG. 5.
The effect of GelMA concentration on the cell survival rate. (a) Live/Dead staining of GelMA printing with a concentration of 5%, 7%, and 9% (living cells were depicted in green and dead cells in red, 10 × ). (b) Overall diagram of MNGCs with the concentration of 7%. (c) The 3D local view of MNGCs. (d) The quantification of the survival rate of SCs. SCs, Schwann cells.
The MNGCs were cut to a thickness of 500 μm for observation. There were almost no SCs in the channels, and high concentration SCs were distributed evenly around the channels (Fig. 5c). With the increase of GelMA concentration, the cell survival rate decreased slightly, and the structure of bearing layer had no obvious effect on the cell survival rate. When the concentration of GelMA was 5% and 7%, the cell survival rate was nearly 95%. After 4 days of culture, the SCs still maintained a high survival rate (Fig. 5d).
Discussion
In this article, we mainly explore the multi-material 3D bioprinting technology, and propose a novel idea for the fabrication of MNGCs with GelMA hydrogel. We systematically analyzed the extrusion state from two aspects of theory and experiment. For the GelMA material of cell loading, it is necessary to measure the viscosity at different temperatures. The increase of GelMA viscosity will cause SCs to be subjected to greater shear stress, which will damage the cytomembrane and reduce the survival rate of cells.32,33 For this reason, we need to reduce the viscosity of GelMA as much as possible in a printable state.
The channels inside the MNGCs play an important role in guiding nerve cells migration, which is one of the key factors for repairing long nerve gap.34 The high-density channels can be observed from the internal cross-section view, and a good unblocked effect is formed inside the channel, which indicates that gelatin plays a key role in maintaining the integrity of channel shape in printing. In addition, the clear pore inside the MNGCs is also conducive to the transport of nutrients and the discharge of metabolism (Fig. 3e). With the increase of porosity, the permeability of MNGCs will also be enhanced, which is conducive to the penetration of external nutrients and the mutual transmission of signals between nerves.
More importantly, the MNGCs of GelMA can be effectively degraded by enzymes.35–37 MNGCs can be gradually removed by degradation in the process of nerve repair and regeneration. The mass of MNGCs gradually decreases with the increase of degradation time in the enzyme solution. After 11 days, the degradation rate of MNGCs with 5% GelMA is 100% (Supplementary Fig. S3). However, the matching between nerve repair and conduit degradation needs to be further studied. In addition, the cross-linked bearing layer enhances the mechanical properties of the MNGCs after cross-linking.
SCs can release nutritional factors, which have great significance for growth and migration of nerve cells.38 In this article, the survival rate of SCs is about 90% and 95% when printed GelMA concentration is 9% and 5%, respectively. This indicates that the higher GelMA concentration is a factor in cell death. Owing to the lack of culture medium and oxygen in GelMA hydrogel, the cells will gradually die with the increase of printing time (Supplementary Fig. S4). Moreover, the cell survival rate will also be affected by the shear rate.32,33,39 Therefore, shortening printing time and increasing nozzle diameter are effective methods to improve cell survival rate. Due to the limitations of experimental conditions, we did not study the cell morphology/function/differentiation. We think that the study of viability is enough to support the fabrication of nerve conduit containing cells.
With the increase of GelMA concentration, the number of SCs will increase and the cell survival rate will decrease slightly. The increase of the number of SCs in the same thickness indicates that the cross-linking strength of the GelMA is strengthened with the increase of the concentration. It also proves that the GelMA/SCs fall off with the dissolution of gelatin when the concentration is 5%, resulting in the decrease of the cells number.
The current advantages of GelMA MNGCs are the high-density channels and high cell survival rate. These factors will promote nerve repair.40,41 However, GelMA has poor mechanical properties, which limits the use of the MNGCs. When we designed the bearing layer of GelMA/HEAA, it could improve the performance of MNGCs, which was a good improvement direction. Due to the lack of other conditions, we did not study the migration of nerve cells in the MNGCs. Although there are still limitations in terms of nerve repair, 3D bioprinting technology of hydrogel materials has potential value for tissue construction in the future.
Conclusion
In summary, we realized the alternate printing of supporting material and cell-laden material by multi-material printing technology. Then we fabricated the MNGCs containing SCs through the UV cross-linking of GelMA and the dissolving technique of gelatin. We systematically optimized the printing temperature parameter by analyzing the printability of GelMA. The results showed that high-density channels (6.1 mm−2) were formed inside and the average error of channel diameter and spacing was about 5%. A large number of SCs were distributed around the channels, and the cell survival rate was >90%. This work provides a potential solution to fabricate MNGCs for tissue engineering.
Supplementary Material
Authors’ Contributions
L.Z. and X.Z. designed the project. L.Z. completed the design of the experiment and the writing of the article. H.Z. completed the printing experiments. H.W. developed the software BiopDesigner. K.G. cultured the cells. H.Z. and S.L. completed the mechanical structure design of printhead. S.L. and F.G. completed the hardware design of printer. Z.Y. offered suggestions for printing. X.L. supplied the materials for the experiment. All authors commented on the article.
Ethical Approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was financially supported by the National Key Research and Development Project (Grant No. 2020YFB1313100), The Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA16020803), the National Natural Science Foundation of China (Grant No. 51875557), the Research Equipment Development Program of the Chinese Academy of Sciences (Grant Nos. YZ201545, YJKYYQ20170042, YJKYYQ20190045), the National High Technology Research and Development Program of China (863 Program, Grant No. 2015AA020312), and the National Key Research and Development Program of China (Grant No. 2017YFC1104900), Fundation of State Key Laboratory of Robotics (Grant No. 2017-Z16).
Supplementary Material
References
- 1. Lim E, Nakanishi S T, Hoghooghi V, et al. AlphaB-crystallin regulates remyelination after peripheral nerve injury. Proc Natl Acad Sci U S A 2017;114:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daly W, Yao L, Zeugolis D, et al. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface 2012;9:202–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li R, Liu Z, Pan Y, et al. Peripheral nerve injuries treatment: A systematic review. Cell Biochem Biophys 2014;68:449–454. [DOI] [PubMed] [Google Scholar]
- 4. Johnson EO, Soucacos PN. Nerve repair: Experimental and clinical evaluation of biodegradable artificial nerve guides. Injury 2008;39:30–36. [DOI] [PubMed] [Google Scholar]
- 5. Onode E, Uemura T, Takamatsu K, et al. Bioabsorbable nerve conduits three-dimensionally coated with human induced pluripotent stem cell-derived neural stem/progenitor cells promote peripheral nerve regeneration in rats. Sci Rep 2021;11:4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muheremu A, Ao Q. Past, present, and future of nerve conduits in the treatment of peripheral nerve injury. Biomed Res Int 2015;2015:237507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanjairaj V. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomaterialia 2020;106:54–69. [DOI] [PubMed] [Google Scholar]
- 8. Yao L, Billiar KL, Windebank AJ, et al. Multichanneled collagen conduits for peripheral nerve regeneration: Design, fabrication, and characterization. Tissue Eng Part C Methods 2010;16:1585–1596. [DOI] [PubMed] [Google Scholar]
- 9. Apsite I, Constante G, Dulle M, et al. 4D Biofabrication of fibrous artificial nerve graft for neuron regeneration. Biofabrication 2020;12:035027. [DOI] [PubMed] [Google Scholar]
- 10. Yao L, Ruiter G, Wang H, et al. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials 2010;31:5789–5797. [DOI] [PubMed] [Google Scholar]
- 11. Mosahebi A, Fuller P, Wiberg M, et al. Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp Neurol 2002;173:213–223. [DOI] [PubMed] [Google Scholar]
- 12. Park SC, Oh SH, Seo TB, et al. Ultrasound-stimulated peripheral nerve regeneration within asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. J Biomed Mater Res B Appl Biomater 2010;94B:359–366. [DOI] [PubMed] [Google Scholar]
- 13. Chalfoun C T, Wirth G A, Evans G. Tissue engineered nerve constructs: Where do we stand? J Cell Mol Med 2006;10:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gu X S, Ding F, Williams D F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 2014;35:6143–6156. [DOI] [PubMed] [Google Scholar]
- 15. Koh HS, Yong T, Teo WE, et al. In vivo study of novel nanofibrous intra-luminal guidance channels to promote nerve regeneration. J Neural Eng 2010;7:046003. [DOI] [PubMed] [Google Scholar]
- 16. Yang Y, Laporte LD, Rives CB, et al. Neurotrophin releasing single and multiple lumen nerve conduits. J Controll Release 2005;104:433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeffries EM, Wang YD. Biomimetic micropatterned multi-channel nerve guides by templated electrospinning. Biotechnol Bioeng 2012;109:1571–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sierpinski P, Garrett J, Ma J, et al. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 2008;29:118–128. [DOI] [PubMed] [Google Scholar]
- 19. Owens CM, Marga F, Forgacs G, et al. Biofabrication and testing of a fully cellular nerve graft. Biofabrication 2013;5:045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore MJ, Friedman JA, Lewellyn EB, et al. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials 2006;27:419–429. [DOI] [PubMed] [Google Scholar]
- 21. Johnson BN, Lancaster KZ, Zhen GH, et al. 3D printed anatomical nerve regeneration pathways. Adv Funct Mater 2015;25:6205–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu W, Tringale KR, Woller SA, et al. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits. Mater Today 2018;21:951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suri S, Han LH, Zhang WD, et al. Solid freeform fabrication of designer scaffolds of hyaluronic acid for nerve tissue engineering. Biomed Microdevices 2011;13:983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ye WS, Li HB, Yu K, et al. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater Des 2020;192:108757. [Google Scholar]
- 25. Tao J, Zhang JM, Du T, et al. Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomaterialia 2019;90:49–59. [DOI] [PubMed] [Google Scholar]
- 26. Huang L, Gao J, Wang H, et al. Fabrication of 3D scaffolds displaying biochemical gradients along longitudinally oriented microchannels for neural tissue engineering. ACS Appl Mater interfaces 2020;12:48380–48394. [DOI] [PubMed] [Google Scholar]
- 27. Wu P, Tong Z, Luo L, et al. Comprehensive strategy of conduit guidance combined with VEGF producing Schwann cells accelerates peripheral nerve repair. Bioact Mater 2021;6:3515–3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang H, Guo K, Zhang L, et al. Valve-based consecutive bioprinting method for multimaterial tissue-like constructs with controllable interfaces. Biofabrication 2021;13:035001. [DOI] [PubMed] [Google Scholar]
- 29. Van D, Bogdanov B, Rooze ND, et al. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000;1:31–38. [DOI] [PubMed] [Google Scholar]
- 30. Jeffries EM, Wang YD. Incorporation of parallel electrospun fibers for improved topographical guidance in 3D nerve guides. Biofabrication 2013;5:035015. [DOI] [PubMed] [Google Scholar]
- 31. Sridharan R, Reilly RB, Buckley CT. Decellularized grafts with axially aligned channels for peripheral nerve regeneration. J Mech Behav Biomed Mater 2015;41:124–135. [DOI] [PubMed] [Google Scholar]
- 32. Ruberu K, Senadeera M, Rana S, et al. Coupling machine learning with 3D bioprinting to fast track optimisation of extrusion printing. Appl Mater Today 2021;22:100914. [Google Scholar]
- 33. Yin J, Yan M, Wang Y, et al. 3D bioprinting of low concentration cell-laden gelatin methacrylate (GelMA) bioinks with two-step crosslinking strategy. ACS Appl Mater Interfaces 2018;7b16059. [DOI] [PubMed] [Google Scholar]
- 34. Stewart CE, Kan C, Stewart BR, et al. Machine intelligence for nerve conduit design and production. J Biol Eng 2020;14:25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Niu X, Ferracci G, Lin M, et al. Highly substituted decoupled gelatin methacrylamide free of hydrolabile methacrylate impurities: An optimum choice for long-term stability and good cytocompatibility. Int J Biol Macromol 2020;167:479–490. [DOI] [PubMed] [Google Scholar]
- 36. Benton JA, Deforest CA, Vivekanandan V, et al. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng A 2009;15:3221–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klotz BJ, Gawlitta D, Rosenberg A, et al. Gelatin-methacryloyl hydrogels: Towards biofabrication-based tissue repair. Trends Biotechnol 2016;34:394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen JL, Huang D, Wang L, et al. 3D bioprinted multiscale composite scaffolds based on gelatin methacryloyl (GelMA)/chitosan microspheres as a modular bioink for enhancing 3D neurite outgrowth and elongation. J Colloid Interface Sci 2020;574:162–173. [DOI] [PubMed] [Google Scholar]
- 39. Zhao Y, Li Y, Mao S, et al. The influence of printing parameters on cell survival rate and printability in microextrusion-based 3D cell printing technology. Biofabrication 2015;7:045002. [DOI] [PubMed] [Google Scholar]
- 40. Kang J, Hwang J Y, Huh M, et al. Porous poly(3-hydroxybutyrate) scaffolds prepared by non-solvent-induced phase separation for tissue engineering. Macromol Res 2020;28:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sun B, Zhou ZF, Tong W, et al. Development of nanofiber sponges-containing nerve guidance conduit for peripheral nerve regeneration in vivo. ACS Appl Mater Interfaces 2017;9:26684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.