Abstract
Wounds are skin tissue damage due to trauma. Many factors inhibit the wound healing phase (hemostasis, inflammation, proliferation, and alteration), such as oxygenation, contamination/infection, age, effects of injury, sex hormones, stress, diabetes, obesity, drugs, alcoholism, smoking, nutrition, hemostasis, debridement, and closing time. Cellulose is the most abundant biopolymer in nature which is promising as the main matrix of wound dressings because of its good structure and mechanical stability, moisturizes the area around the wound, absorbs excess exudate, can form elastic gels with the characteristics of bio-responsiveness, biocompatibility, low toxicity, biodegradability, and structural similarity with the extracellular matrix (ECM). The addition of active ingredients as a model drug helps accelerate wound healing through antimicrobial and antioxidant mechanisms. Three-dimensional (3D) bioprinting technology can print cellulose as a bioink to produce wound dressings with complex structures mimicking ECM. The 3D printed cellulose-based wound dressings are a promising application in modern wound care. This article reviews the use of 3D printed cellulose as an ideal wound dressing and their properties, including mechanical properties, permeability aspect, absorption ability, ability to retain and provide moisture, biodegradation, antimicrobial property, and biocompatibility. The applications of 3D printed cellulose in the management of chronic wounds, burns, and painful wounds are also discussed.
Keywords: cellulose, wound dressing, 3D-printed, 3D-bioprinting
Introduction
Skin tissue damage, better known as wounds, is a health problem requiring special treatment. Wound infection can occur due to improper cleaning, debridement, dressing techniques, and exposure to contamination.1 In addition, many factors hinder the wound healing phase (hemostasis, inflammation, proliferation, and remodeling), such as oxygenation, infections, age and sex hormones, stress, diabetes, obesity, drugs, alcoholism, smoking, and nutrition. A better understanding of this effect on repair can lead to therapies that improve wound healing and treat damaged scar tissue.2 Prevention and treatment measures include disease-specific approaches, moisture-resistant dressings, and additional topical therapies to promote healing.3
Three-dimensional (3D) printing technology is widely used in various applications due to its advantages in sophisticated geometric fabrication and low-cost production. Besides offering speed, accuracy, and flexibility, these modern fabrication techniques also pose critical challenges in developing materials suitable for the biomedical industry.4 Addictive manufacturing technology based on 3D bioprinting offers potential as a promising technology in wound care. The technology uses bioink, which is biocompatible with living cells by mimicking a natural extracellular matrix (ECM). This approach makes it possible to print both natural and synthetic polymer-based wound dressings, which have complementary advantages and disadvantages in composite form. Biopolymers as bioinks tend to be more profitable in nontoxicity, nonantigenicity, inertness, bio-adhesiveness, biocompatibility, biodegradability, and adequate hemostasis than synthetic polymers.5
Collagen and alginate-based natural polymers have been widely used as wound dressings by taking advantage of their properties. Alginate has a high absorption capacity. Chitosan has essential characteristics for treating wounds, including its binding, antifungal, bactericidal properties and permeability to oxygen.6 Collagen is biocompatible to help regenerate epithelium better than silicone-based materials. However, these dressings have a higher cost, adherence to wounds, limited absorption of exudate, and residue deposition at the wound site.7 In addition, gauze dressings or bandages that are cheap and easy to find are limited in providing moisture, do not have antimicrobial properties, repeat use, are difficult to remove, and cause a traumatic effect on the wound. This dry dressing only excels in the aspect of exudate absorption.
Cellulose is a biopolymer with abundant natural availability, both from plants and microbes. Various aspects of cellulose hydrogel 3D printing with extrusion techniques, including hydrogel rheology, fiber entanglement, fiber alignment, gelation, printability, shape accuracy, cell viability, and processing parameters, have been discussed by Wang et al.8 In addition, viscoelastic ink from nanocellulose suspension makes it possible to formulate and design 3D object printing.9
Cellulose as a 3D printed bioink in tissue engineering (particularly wound dressing products) has tremendous potential. Cellulose tends to be easier to modify to obtain the desired physicochemical properties. In addition, the printing capability of the nanocellulose hydrogels due to their shear-thinning behavior and supporting living cells enables 3D bioprinting using nanocellulose. Recent developments have tremendous potential for 3D printing wound dressings.10 Using cellulose as a wound dressing material can save costs compared to other polymers.
Selection of the appropriate wound dressing is critical in promoting optimal wound healing, symptom/risk management, and patient comfort factors during dressing. Wound-related factors, such as the amount of wound exudate, pain management, risk of infection, and type/condition of the wound, should be considered in selecting the type and dressing material for the ideal dressing. In modern wound care, dressings must create a moist environment for the wound, absorb excess exudate, be biocompatible, antibacterial, and have good physical properties. Because of that, 3D bioprinting technology expects to engineer biomaterials into adequate 3D wound dressings for the growth of new scar tissue.
This article reviews using cellulosic materials as bioinks to produce ideal 3D wound dressings, covering mechanical aspects, permeability, absorption of excess exudate, ability to retain and contribute moisture, biodegradation properties, antimicrobial properties, and biocompatibility. This review also briefly reviews the application of cellulose-based 3D wound dressings for chronic wounds, painful wounds, and burns. Finally, this review expects to develop new knowledge about bioprinting technology in producing the ideal wound dressing.
Ideal Wound Dressing
The wound healing mechanism is dynamic and complex by creating environmental conditions appropriate to the wound. Wounds are disturbances in the skin's continuity or mucosa's epithelial layer due to physical or thermal damage.11 Injuries can cause significant morbidity and mortality, as well as medical costs.
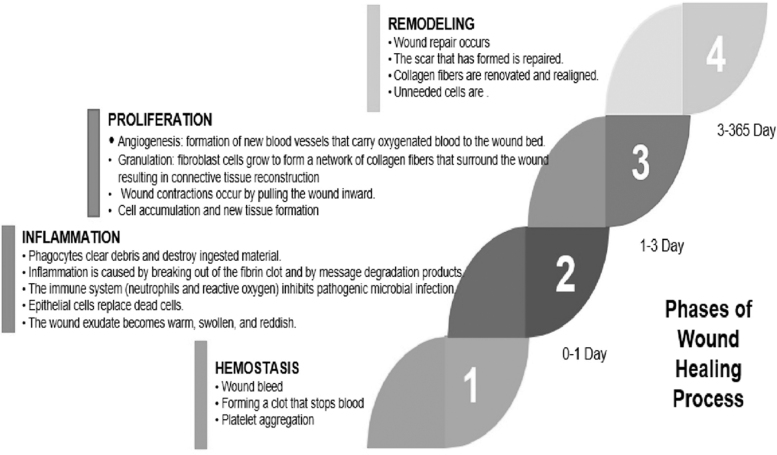
Proper wound care management is essential to accelerate wound healing and cost-efficiency. Wound healing approaches are currently based on cultured autografts, allografts, and epithelial autografts.12 According to the World Health Organization (WHO),13 factors that affect the duration of healing and the potential for wound infection include (1) Patients (age, disease, the effect of injury on healing), (2) Wounds (injured organ or tissue, degree, and nature of the injury, contamination or injury infection, the time between injury and treatment), and (3) Local factors (hemostasis and debridement, time to closure). The wound healing process generally consists of the phases of homeostasis, inflammation, proliferation, and remodeling,5,14,15 as shown in Figure 1.
FIG. 1.
Phases of the wound healing process.
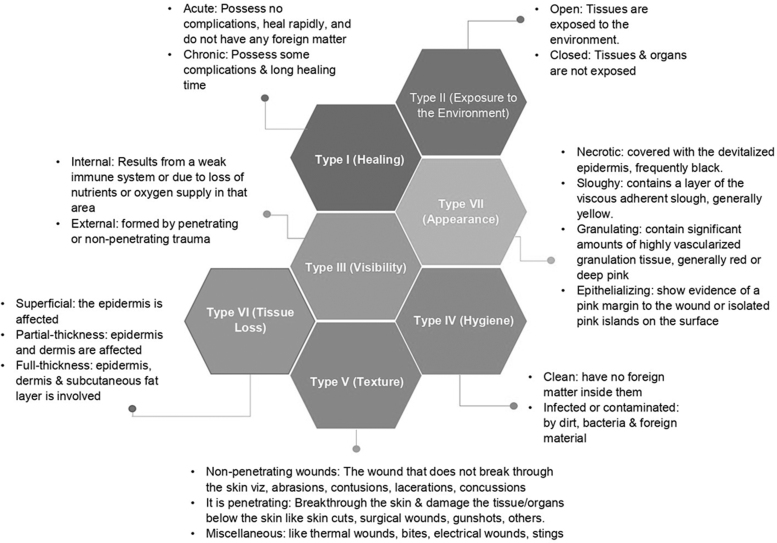
Wound dressings that can retain moisture and topical therapy prevent, treat, and accelerate wound healing.3 Sharma et al15 classified wounds into seven types, as shown in Figure 2. The ideal wound dressing accelerates wound healing, reduces the loss of protein, electrolytes, and fluid from wounds, and helps minimize pain and infection.16 Therefore, it is imperative to assess or pay attention to the mechanism of injury, risk of contamination, injury to deeper structures, damage to the underlying nerve or tissue, any deficit in perfusion, tetanus status, disability, and the amount of tissue lost.17
FIG. 2.
Classification of wound dressings.
Significant advances in wound dressing technology have resulted in many alternative dressings for wound care. This condition depends on the condition and type of wound. Bandages do not have standard requirements. However, wound dressings should support wound care management by maximizing closed wounds' healing benefits and minimizing tissue disturbance (physical and chemical).18 The requirements for ideal wound dressings are11,19–23:
-
(1)
Sterile, nontoxic, clean, nonallergenic: free from toxic chemicals or irritants that come out of the dressing.
-
(2)
Microorganism barrier: prevents the transmission of microorganisms into or outside the wound to protect the wound from infection.
-
(3)
No foreign matter: does not release nonbiodegradable materials and other objects to the wound bed.
-
(4)
Maintain wound moisture: keep the wound and skin margins in an optimal state of hydration (balanced water).
-
(5)
Minimal wound disturbance: placement in situ offers an uninterrupted environment (minimal tissue movement or dressing change).
-
(6)
Protect tissue: protect the wound bed and skin around the wound.
-
(7)
Prevents drying and minimal tissue trauma: minimizes wound pain during application or removal.
-
(8)
Maintaining tissue temperature and pH: treating wounds at optimal temperature and pH to increase blood flow to the wound bed and epidermal migration.
-
(9)
Permeability to gases between the injured tissue and the environment.
-
(10)
Reasonable cost.
-
(11)
Thermal insulation.
-
(12)
Can conform to wound shape and easy to use or elastic.
-
(13)
Absorbs excess exudate.
-
(14)
Biocompatible.
-
(15)
Biodegradable.
Bioink for 3D Bioprinting
3D bioprinting technology makes printed complex structures at high speed and resolution. This technology can produce scaffold that fits the desired design and is practical—lots of polymers are used as 3D printing bioinks. However, the lack of physical-biochemical properties of biomaterials that match the product's characteristics to print is a significant obstacle that limits 3D bioprinting in producing biocompatible products. Biopolymers from natural materials are eco-friendly, promising in printing medical products with attention to sustainability aspects.
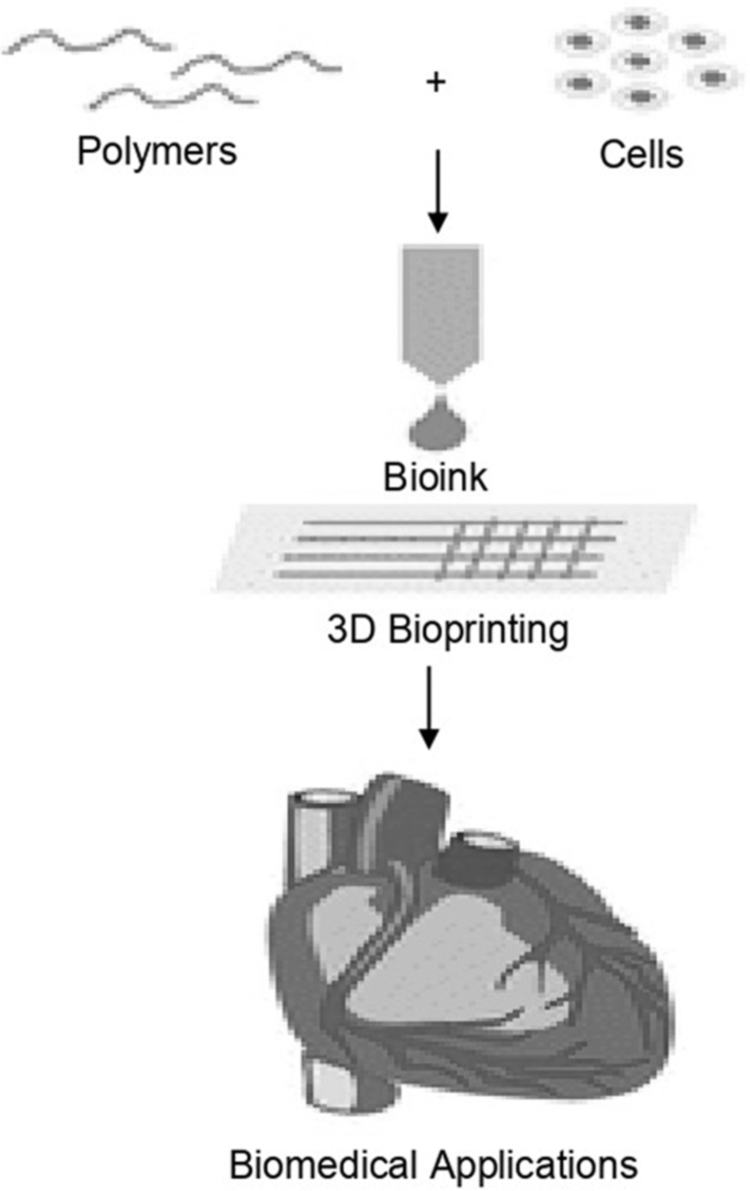
A wound dressing is a medical textile to protect and heal chronic or acute wounds. 3D bioprinting technology can produce wound dressings similar to the physiological conditions of natural skin tissue in vitro. The term “bioink” is a mixture of cells, biomaterials, and bioactive molecules that make printed materials (Fig. 3).24 Meanwhile, “bioprinting” uses bioink as ink, in which cells and other biological materials are stored in a spatially controllable pattern to create living tissues and organs.25 In this context, 3D bioprinting produces a scaffold with a 3D structure for living cells' growth.
FIG. 3.
Bioink definition scheme and bioprinting (Modified from Donderwinkel et al24).
Bioprinting is an additive manufacturing technology, one of its applications to produce medical devices in wound healing applications. Various natural biomaterials have been widely developed and researched as wound healing bioink through 3D printing of wound dressings, for example, cellulose, collagen, alginate, chitosan, chitin, and others. However, biomaterial-based wound dressings need to pay attention to better measure to reconstruct the original skin's function and structure because each biomaterial has different characteristics when used for tissue engineering.26
Cellulose 3D Printed as Wound Dressing
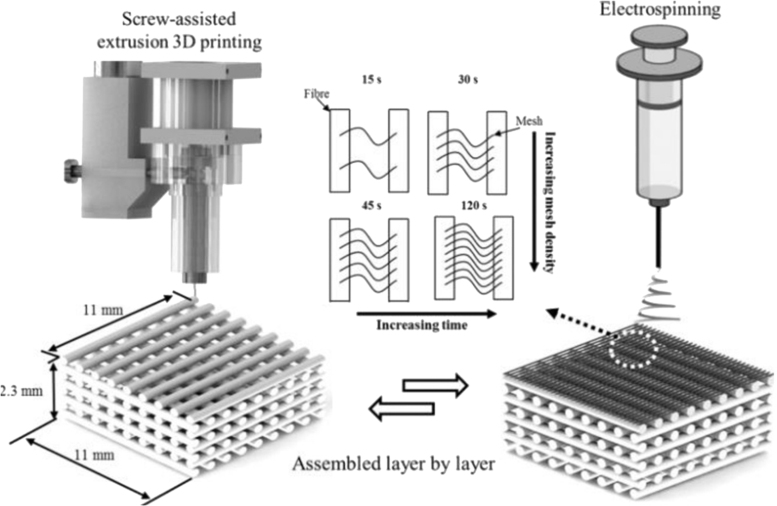
The 3D printing is rapidly emerging as a new technology with a wide variety of biomedical applications, generally based on the suitability of biopolymers to be extruded through a nozzle to create layer-by-layer 3D structures.27 Electrospinning and 3D printing are widely used to produce tissue engineering scaffolds. Both methods have advantages and disadvantages. In the electrospinning method, it is difficult for cells to grow into the nanostructures of the electrospun scaffold. In addition, 3D printed scaffolds have a low print resolution.28 In this case, it is essential to pay attention to the design of the pores to facilitate the development of skin cells. The combination of 3D printing and electrospinning allows the creation of multiscale structures (Fig. 4). As a result, cell proliferation will increase in the scaffolds and aligned cells with elongated morphology in the pores of the imprinted microfibers. In addition, increasing the number of mesh layers increased cell proliferation, migration, and adhesion.
FIG. 4.
Schematic of a double-scale scaffold 3D printing and electrospinning fabrication process.29 3D, three-dimensional.
Incorporating low-density, aligned fibers in 3D-printed scaffolds is a promising development in multiscale hierarchical scaffolds where cell alignment can be desired.29 In vivo trials of bioprinted skin constructions have revealed promising results in wound healing tested on animal or human models. Wound dressings are often a problem in wound healing management. Physiological conditions and the extent of the wound pose a challenge in using the desired dressing. 3D bioprinting technology expects to develop renewable and sustainable biomaterial-based wound dressings according to the patient's wound demands and conditions.
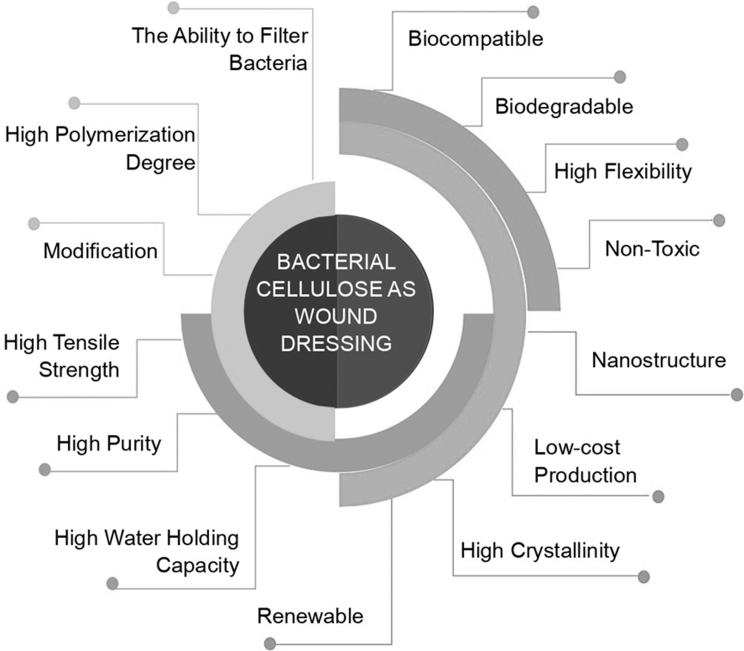
The advantage of using biopolymers for wound dressings is their nontoxicity, nonantigenicity, inertness, bio-adhesiveness, biocompatibility, biodegradability, hemostatic effects, and antimicrobial properties.5 Cellulose is a naturally abundant biopolymer to be developed into 3D biomedical devices. Alginate, gelatin, agarose, hyaluronic acid, fibrin, and chitosan wound dressings provide an excellent ECM mimetic environment and biocompatibility. However, these biopolymers do not have the other uniqueness of cellulosic polymers, such as shear thinning, mechanical strength, and structural shrinkage.30 The combination of various biopolymers expects to produce 3D printed hydrogel composites with ideal bioink properties for wound dressings. There are some reasons for using bacterial cellulose (Fig. 5) and wood cellulose as a wound dressing31–35:
FIG. 5.
The properties of bacterial cellulose as a wound dressing.
-
(1)
Good mechanical structure and stability.
-
(2)
Allows the production of transparent films.
-
(3)
Provides a moist wound healing environment.
-
(4)
Can form elastic gels with bioresponsive characteristics.
-
(5)
Low toxicity.
-
(6)
Biocompatibility.
-
(7)
Biodegradability.
-
(8)
Structural similarity to the ECM.
-
(9)
Low-cost and easy availability.
Material selection is one of the most critical factors in 3D bioprinting, which depends on the product application. In this case, the material must be bio-inert or bio-active, nontoxic, and the mechanical properties must meet the application requirements.36 The crystallinity and nanostructure can produce high ink viscosity and hydrogel strength even at low concentrations. In addition, the ability of hydrogel formation is influenced by the number of OH groups contained in cellulose. Therefore, each type of cellulose will produce different 3D scaffold properties.
Bacterial cellulose is a biopolymer produced from bacteria with a nanometer structure, showing promising wound dressing applications. Compared to plant cellulose, bacterial cellulose has high purity, high porosity, permeability to liquids and gases, high liquid absorption, good mechanical properties, and biocompatibility properties.37 Bacterial cellulose-based hydrogels are the most efficient material for healing complex wounds because they provide moisture. Bacterial cellulose composite with alginate is known to have better water retention properties and provides a smooth dressing exchange.38 Rees et al31 used TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl radical)-mediated oxidation nanocellulose and a combination of carboxymethylation and periodate oxidation. Both types of cellulose do not support bacterial growth. The fiber's small dimensions can produce materials with good rheological properties as 3D printed bioinks. 3D nanocellulose with open porosity can carry and release antimicrobial components.
Cellulose nanofibrils have many hydroxyl groups on the surface of the fibrils leading to strong hydrogen interactions. These interactions occur between the fibrils leading to agglomeration of the fibrils and interactions with water molecules. These interactions provide viscoelastic and shear thinning properties for 3D printing.39 The viscoelastic properties help maintain the structural integrity of the cellulose nanofibril hydrogel after losing water completely on freeze-drying.40 The use of TEMPO-mediated oxidized cellulose nanofibrils (TOCNF) has several benefits, including improved compatibility and adhesion to other matrices (such as hydrophobic thermoplastics). The reaction can be carried out in the water and under mild conditions and has a high anionic charge density on the fibril surface. Incorporating divalent Ca2+ and Mg2+ in deprotonated TOCNF is suitable for 3D printing ink printability and viscoelastic properties.41 In addition, the easy collapse of cellulose nanofibril-based 3D scaffolds after drying can be overcome by the addition of carbon nanotubes to produce conductive cellulose.40
Nanocrystalline cellulose-based hydrogel ink has easier orientation under shearing than nanofibril cellulose-based ink. Nanostructure orientation and nanoscale pore wall roughness in 3D printed hydrogel scaffolds are suitable for cell interaction and growth.42 Nanocrystalline cellulose is stiffer than nanofibril cellulose and bacterial cellulose, containing interconnected fibers. Nanocrystalline cellulose with a carboxyl functional group (charge density 0.5 mmol/g) helps to cross-link. Nanocrystalline cellulose provides favorable rheological properties for 3D printing. The 3D-printed scaffolds were sequentially cross-linked using covalent and ionic reactions resulting in a dimensionally stable hydrogel scaffold with a pore size of 80–2125 μm and a nanoscale pore wall roughness favorable for cell interactions.42
Different types of cellulose will also affect the surface chemical properties, including functional groups and charge.43 When the surface is modified with sulfate esters, nanocrystalline cellulose will have a low viscosity.44 However, adding a water-soluble nanofiller (e.g., alginate) can be a rheological modifier. As a result, a viscoelastic system with a storage modulus (G′) will be higher than a loss modulus (G″), thereby guaranteeing printability.45 Meanwhile, bacterial nanocellulose behaves like “floc” in suspension and is oriented to shear conditions. This condition is associated with interactions between adjacent particles at high concentrations.46 The dispersion of bacterial nanocellulose fibrils exhibited shear-thinning behavior and higher viscosity than nanocrystalline cellulose suspensions at the same concentration. Carboxymethyl oxidized cellulose nanofibrils and TEMPO also exhibit stable hydrogels with strong viscoelastic modulus even at low concentrations of about 1% wt. This effect is due to electrostatic repulsion between negatively charged carboxylate groups (–COO−) on the cellulose nanofibrils.43
Titan Ag 200 is a wound dressing made of nanosilver as antibacterial with sodium carboxymethyl cellulose fiber and cellulose reinforcement fiber as a matrix that has been approved by the FDA.47 Ionic silver released into the wound dressing on contact with wound exudate or blood has an antibacterial effect on wound bacteria retained in the dressing, preventing their colonization. In addition, the dressing structure remains intact through the formation of a gel. Therefore, debris and bacteria absorbed in the wound exudate and remaining in the dressing can be removed when the dressing is changed.
In addition, Titan Ag 200 assists in maintaining a moist wound environment, supports autolytic debridement, and protects wound edges and surrounding skin from maceration. In this case, Titan Ag 200 is used for the management of (1) Wounds with moderate to severe exudate, (2) Burns of partial thickness, (3) Foot ulcers, pressure ulcers, and diabetic ulcers, (4) Surgical wounds (e.g., postoperative, which is allowed to heal by secondary intent and donor site/graft), (5) Traumatic wounds (e.g., abrasions and lacerations), and (6) Wounds that bleed easily such as wounds that have been mechanically or surgically debrided or donor sites. Through medical tests, Titan Ag 200 (containing 40% cellulose) has also been compared with a similar product, namely Aquacel Ag Extra, which contains 18% cellulose,47 with the performance comparisons summarized in Table 1.
Table 1.
Comparison of the Performance of Titan Ag 200 with Aquacel Ag Extra
| Performance criteria | Aquacel Ag Extra | Titan Ag 200 | Comparison |
|---|---|---|---|
| Mode of action | Releases silver ions on contact with wound exudate. Absorbs wound exudate and forms a gel that traps debris and bacteria in the dressing. |
Releases silver ions on contact with wound exudate. Silver ions are released into the wound dressing but not into the wound bed. Absorbs wound exudate and forms a gel that traps debris and bacteria in the dressing. |
Equivalent |
| Silver Releasea | 0.021–0.031 mg/10 cm2/24 h | 0.025–0.030 mg/10 cm2/24 h | Statistical analysis showed that the difference in silver release yield for 7 days was not significant. The device is therefore considered to have an equivalent silver release. |
| Absorbencyb | 24 g/100 cm2 | 30 g/100 cm2 | Equivalent |
| Antibacterial activityc | Assumed to meet the criteria of a >4 log reduction. | Log reduction for various bacteria at corresponding timepoints >4. | Eligible: Antibacterial activity remained above log 4 reduction. |
| Wet tensile strengthd | 15.9 N/cm | 3–5.9 N/cm | Equivalent |
| Biocompatibilitye | Assumed to pass the requirements of applicable ISO 10993 tests. | Comprehensive biocompatibility testing confirmed that the device raises no safety concerns. | Meets Titan Ag 200 biocompatibility requirements according to ISO 10993-1. |
Profile was similar to that of the predicate device in simulated wound fluid over 7 days.
According to British Pharmacopoeia, Test Method for absorbency of Alginate dressings/Surgical dressings, European Standard EN13726-1 March 2002, and product consistently meet specifications.
According to AATCC, 100 was found to consistently meet the requirement of a log 4 reduction compared to a control.
According to British Pharmacopoeia meets the specifications set for this product and displays a strength better than the predicate device.
According to ISO 10993-1-2009 and the following concluded. Testing included: (1) Toxicological Risk Assessment according to ISO 10993-17, (2) Acute systemic toxicity according to ISO 10993-11, (3) Subacute Systemic Toxicity according to ISO 10993, (4) Irritation studies according to ISO 10993-10, (5) Cytotoxicity according to ISO 10993-5, (6) Sensitization according to ISO 10993-10, (7) Material mediated pyrogenicity ISO 10993-11, and (8) Genotoxicity according to ISO 10993-3.
Source: Food Drug Administration (FDA).47
Mechanical properties
In nanocomposite applications, cellulose is widely used as a composite reinforcement agent. The dressing must be durable, stress-resistant, flexible, supple, and elastic. This property is needed to reduce the trauma effect on skin tissue when changing dressings. In addition, the dressing must have good tensile properties, which can withstand the body's pressure exerted by different contoured parts. Good adherence can reduce pain, facilitate decontamination, prevent peripheral passage into the wound by bacteria, and increase tissue binding.48
Using cellulose in concentrations ranging from 2% to 10% gives bioink shear depletion, mechanical strength, and water retention properties.30 Cross-linking is a standard method to improve mechanical properties, but it can reduce biocompatibility.49 3D printing makes it unique to create hydrogel composites suitable for pore size, shape, and structure tissue regeneration applications. Sultan and Mathew42 studied 3D printed hydrogels' physical characteristics from cellulose nanocrystals as reinforcing agents. This porous scaffold is a double cross-penetrating polymer network of sodium alginate and gelatin hydrogel inks. Mechanical properties of 3D printed hydrogel scaffolding are required for soft scaffolding (∼6 kPa), which facilitates chondrogenesis or a more rigid scaffold for bone regeneration (∼16 kPa) with high cytoskeleton stress. Huang and Dean also studied a porous cellulose tissue scaffold for tissue engineering.50 In practice, microscale 3D tissue scaffolding using cellulose acetate produces various pore sizes ranging from 99 to 608 μm. Furthermore, the composite modulus elasticity decrease is inverse to the increase in porosity.
Cellulose nanocrystals contain more than 2000 photoactive groups as highly efficient initiators for radical polymerization, cross-linkers, and nanofillers in 3D hydrogel printing. 3D structured objects printed with mono-functional methacrylate showed superior swelling capacity and enhanced mechanical properties.51 Jiang et al52 examined the mechanical properties of high-strength 3D printed hydrogels to produce 3D scaffolding with high structural complexity and design flexibility in the network engineering field. 3D printed hydrogels were produced from dialdehyde cellulose nanocrystals and gelatin as a bioink. The optimal breaking strength of 4:8 (dialdehyde cellulose nanocrystals: gelatin) is almost 41.3 times greater than gelatin hydrogels. Cross-linked scaffolding with adjustable porosity was successfully obtained as a 3D bioink for tissue repair applications. Hydrogel from nanocellulose and alginate through hydrogel ion cross-linking using calcium ions improves the material's mechanical performance.53
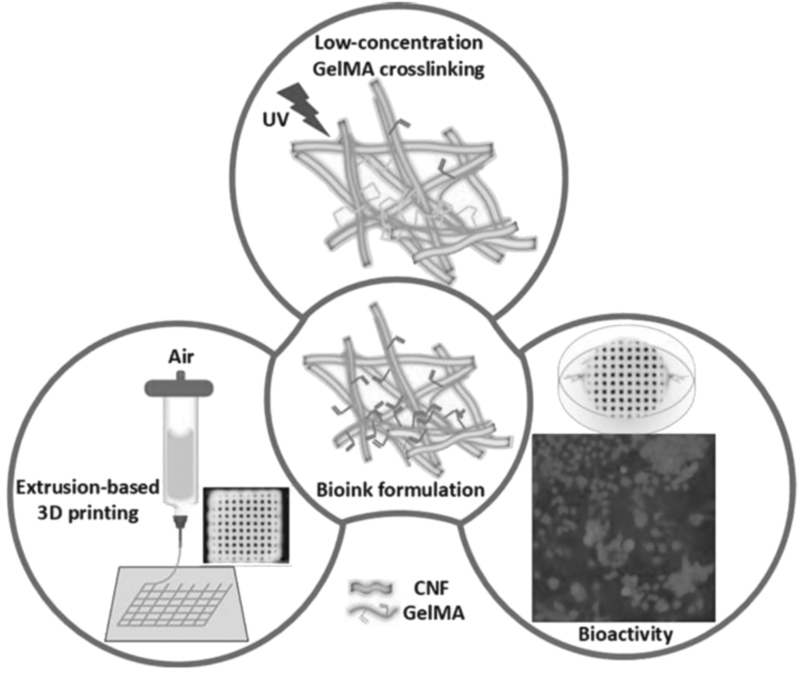
Xu et al54 studied 3D printed nanocellulose hydrogels' mechanical characteristics for wound healing applications. 3D printed hydrogel scaffolding uses in situ Ca2+ cross-linking approach and chemical cross-linking (using 1,4-butanediol diglycidyl ether). 3D printed scaffolding containing 1% nanocellulose has mechanical strength, adjusted from 3 to 8 kPa. Bacterial nanocellulose was also applied to improve structural resolution and enhance the mechanical properties of silk fibroin and gelatin composite hydrogel scaffolding. 3D printed hydrogel of TEMPO nanofibril cellulose (1% w/v) with the addition of ultraviolet cross-linking of methacrylate gelatin (<1% w/v) (Fig. 6) showed a strong interaction between the two biopolymers and good structural stability. The scaffolding's mechanical strength is set in the range of 2.5–5 kPa, and formulations at low concentrations of both materials show great potential in 3D printing for wound healing applications.32 3D printed composite scaffolding with outstanding mechanical properties and hierarchical pore structure holds promise for further tissue engineering applications.55
FIG. 6.
Schematic of a 3D printing hydrogel production based on nanocellulose and gelatin methacrylate.32
Permeability aspects
Moisture permeability and gas permeability of wound dressing films are essential to maintain wound comfort and aid in the healing process.48 3D printed wound dressings are designed to absorb fluids and transfer fluid from the wound/skin surface toward the dressing's permeable layer. Some wound fluid is lost to the atmosphere by evaporation as a water vapor transmission process to increase the dressing's fluid handling capacity. This process's success depends on the proportion of fluid released by the absorbed or lost.56
Permeability characteristics affect the survival of the living cell by absorbing oxygen and nutrients in sufficient quantities. These properties are very beneficial for engineered tissue's optimal development and survival. However, in some cases, the transportation of nutrients and oxygen is often insufficient to support all the cells incorporated into the tissue. In addition, vascular constructs are not readily constructed after implementing engineered tissues in the body. In overcoming this problem, biomaterials used as wound dressings through 3D bioprinting technology must also be selective in the transportation of oxygen and nutrients.57 Perhaps, the design of the scaffold pores also influences this aspect.
Several studies have examined the permeability aspects of cellulose-based wound dressings by 3D printing technology. The wound dressings made of cellulose and mesoporous silica particles exhibit water vapor permeability and swelling properties of the composite membrane, which meet the main requirements of wound dressings.58 Osorio et al59 investigated a 3D composite wound dressing consisting of bacterial cellulose fibrils and polyvinyl alcohol through physical cross-linking. The use of bacterial cellulose for 3D printing is known to have high permeability to liquids and gases.37 Polyvinyl alcohol inserted in bacterial cellulose tubes can increase permeability to water.60 Combining aloe vera gel (30% v/v) with bacterial cellulose also increases water vapor permeability,61 thus showing a promising application of 3D printed wound dressings. Hydrogels from bacterial cellulose and acrylic acid have water vapor permeability in the range of 2035–2666 g/m−2·day−1.62
Absorption ability
One of the main reasons for using cellulose as a 3D printed wound dressing material is its ability to absorb high fluids. Exudates must be managed effectively to create an optimally moist environment to promote wound healing and protect the surrounding skin from maceration risk.56 The clinical requirements for wound dressings' performance are becoming more complicated, but there remains a need for continued exudate management efficiency. Measuring the dressing's fluid handling capacity is essential, considering the absorption capacity and the ability to withstand the absorbed fluid under external pressure. This performance has substantial implications for efficacy and related patient outcomes.63 Poor exudate management increases patient morbidity and costs to health facilities.56
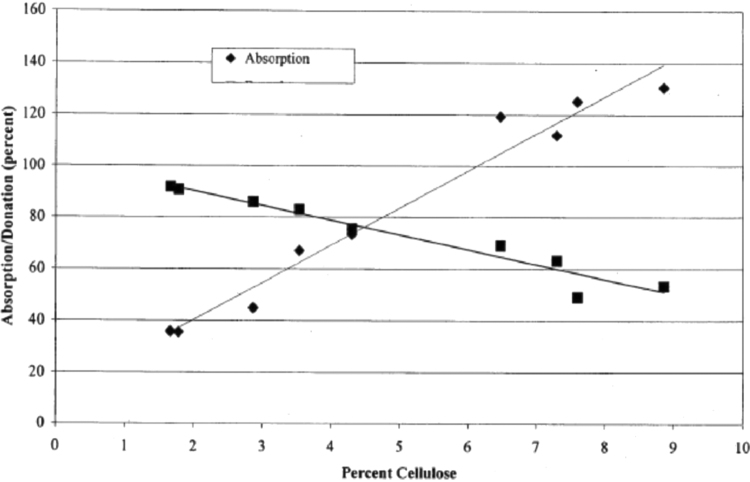
The ability to absorb fluids is due to cellulose's hydrophilic nature because the high concentration of OH in the cellulose structure is such that the percentage of cellulose affects the liquid's absorption ability (Fig. 7). At the nanometer structure, nanocellulose tends to have higher liquid absorption properties than on a larger scale. The small cellulose structure causes a large specific surface area to increase the liquid's binding capacity. For example, a layer of cotton gauze added with microbial cellulose (nanometer structure) increases water absorption and filterability by more than 30%.64 Bacterial cellulose composites and polyvinyl alcohol also have a high water-holding capacity.59 The hydrogel can swell and absorb fluid in its 3D tissue.65 Carboxymethyl groups' presence in carboxymethyl cellulose can absorb wound fluid in the fiber structure.66 Therefore, nanocellulose and carboxymethyl cellulose hold great promise in producing 3D printed wound dressings to absorb excess wound fluid.
FIG. 7.
Effect of percent cellulose on the ability of absorption and fluid donation.7
The method of preparation of bacterial cellulose also influences its absorption properties. Rehydration of bacterial cellulose will be significantly reduced after drying due to entanglement and disruption of the cellulose polymer chains so that the potential for repeated water absorbance is impaired. Meftahi et al67 solved this problem by immersing the bacterial cellulose pellicle in a citric acid solution (as a connecting agent). According to the Brunauer-Emmett-Teller analysis, bacterial cellulose's surface area associated with citric acid (20% w/v with catalyst) was 87.5 times greater than pure bacterial cellulose. The swelling water rate was also significantly increased for the bacterial cellulose associated with citric acid than pure cellulose. This method can increase the absorption ability of cellulose-based 3D printed wound dressings. Nanocellulose and alginate-based 3D printed hydrogels can also absorb water in humid conditions, showing potential applications such as wound dressings.53 In 3D printing cellulose, it is also necessary to consider the pores' size to hold the wound fluid.
Espinosa et al68 examined the addition of alginate to 3D printed cellulose hydrogel's absorption capacity based on TEMPO cellulose nanofibrils for wound dressing applications. Alginate is incorporated into the formulation to facilitate ionic cross-linking with calcium chloride. Nanocellulose-based aerogel exhibits excellent water absorption capabilities, although the incorporation of alginate and cross-linking with CaCl2 causes shrinkage of the 3D mold construction. The hydrophilicity of cellulose and the high specific surface area (14.5 mm2/mm3) of the sponge have a high absorption of simulated wound fluid (about 210%) in the case of freeze-dried cellulose sponges.69
Maintenance and donation of humidity
In modern wound care, the wound condition is maintained in a moist condition, as wet wound dressings can accelerate the wound healing process compared to dry dressings.22 A moist environment can prevent dehydration, increase angiogenesis and collagen synthesis, and increase damage to dead tissue and fibrin. Wound healing in moist conditions under controlled hydration conditions is associated with various mechanisms, namely facilitating the migration of epidermal cells, accelerating epithelialization, and supporting the presence of proteinases and growth factors. In addition, humid conditions can stimulate keratinocyte proliferation and fibroblast growth and support the esthetics and quality of wounds in clinical aspects. In contrast, wound care in dry conditions only affects dead tissue and fibrin and the inflammatory and proliferative phase duration. In addition, dry dressings can cause pain in the wound because they are difficult to remove.70
Cellulose shows promising applications for producing 3D wound dressings that retain moisture. Cellulose will absorb excess exudate to wet the dressing when wearing a bandage. In this condition, the dressing creates a moist environment around the wound. However, in the case of dry wounds, dry wound dressings cannot create a moist atmosphere. In this case, cellulose hydrogel-based dressings play an essential role by donating a certain amount of fluid to the dry wound area to moisten the wound. Besides, cellulose hydrogel-based wound dressings must also have the ability to absorb excess exudate by measuring the degree of hydrogel swelling. The gel moisture-holding capacity of wood nanocellulose fibers was relatively higher (∼7500%) than commercially available wound dressings (2500%). This result suggests that nanofiber cellulosic materials promise a 3D printed dressing material to manage wounds with moderate to high exudate amounts.71 The effect of the percentage of cellulose on fluid donation is shown in Figure 7.
Biodegradation of cellulose
The biodegradable aspect is an essential criterion in selecting 3D printed bioink materials for wound dressing applications. The ideal bioink should degrade in vivo with controlled time and speed and create a similar biological environment for engineered tissue growth. In addition, the degraded product must be nontoxic to the cells incorporated in the tissue.57 In the case of 3D printed wound dressing applications, it is necessary to consider incorporating rigid polymers in bioink, as micro-carriers degrade more slowly than hydrogels and release harmful substances to cells.57
The biodegradability of cellulose in vitro and in vivo was studied by Czaja et al.72 Oxidation of γ-irradiated bacteria cellulose sheets is rapidly rehydrated in aqueous liquids. In vitro studies show that the resorbable membrane's degradation occurs in two main phases: an initial rapid degradation of about 70–80% followed by a slower degradation of an additional 5–10%, ultimately decreasing, leaving a small amount of nonabsorbable material. The prototypes showed marked degradation at all times, with the fastest occurring in the first 2–4 weeks through in vivo studies.
Biodegradation processes vary widely due to hydrolytic or enzymatic labile components in bioink.57 Cellulose biodegradation occurs through the enzymatic hydrolysis mechanism carried out by cellulolytic microorganisms. In this case, these microbes produce endoglycanase, cellobiohydrolase, and β-glucosidase enzymes in catalyzing cellulose depolymerization.73 Cellulose biodegradation is not inhibited in the nano-size range, especially in the crystalline form, although the microbes and pathways involved may differ.74
Antimicrobial study
The dressing functions to form a biocompatible protective layer with the wound to prevent pathogenic bacteria from entering. There are two mechanisms to protect wounds from pathogenic microbes: utilizing the nanometer structure and adding antimicrobial agents. Cellulose does not contain antimicrobial properties, so it needs to be composited with natural active ingredients or biocompatible metals with wound tissue. However, the addition of curcumin in microsphere gelatin scaffolding, porous collagen, and cellulose nanocrystals showed microbial properties and helped heal infected burns.75 Nanocrystalline cellulose films containing curcumin also significantly inhibited growth in three gram-positive bacteria, two gram-negative bacteria, and one yeast in the Hohenstein test in a diabetic wound healing application.76 Hydrogel hydroxypropyl-β-cyclodextrin-loaded-bacterial cellulose-containing curcumin exhibits hemocompatibility and cytocompatibility anti-staphylococci and antioxidants in wound healing.77 Mohanty and Sahoo78 have also discussed the wound healing characteristics of curcumin.
Liyaskina et al79 made cellulose-alginate-based wound dressings and the addition of the antimicrobial agent tetracycline hydrochloride and have high antibiotic activity against S. aureus. Bergonzi et al80 tested a 3D printed alginate scaffold and nanocrystalline cellulose by adding silver nanoparticles to promote cytotoxic and antimicrobial effects to S. aureus and P. aeruginosa with a minimum inhibitory concentration of 10 mg/mL. Gutierrez et al81 also added copper nano to a 3D printed alginate and bacterial cellulose hydrogel by cross-linking with calcium ions, then an ionic exchange with copper ions and ionic cross-linking with copper ions. After being reduced with sodium borohydride (NaBH4), the 3D structure showed antimicrobial behavior against E. coli and S. aureus strains. The addition of antimicrobial metals (copper and silver) to 3D-printed wound dressings also showed solid bactericidal properties.82
Wu and Hong83 studied the interaction of silver-ethylene nanoparticles to develop a 3D printed hydrogel. In this case, the active ingredient's wound dressing is used to produce the super porous antibacterial polyacrylamide/hydroxypropyl methylcellulose hydrogel. The organometallic complex can control the release of silver nanoparticles to balance the nanosilver cross-linked hydrogel's cytocompatibility and antibacterial activities. In vivo experiments demonstrated that a silver nanoparticle cross-linked super porous hydrogel dressing promotes the healing of infected wounds and inhibits scar tissue formation. Cellulose membranes and mesoporous silica particles were also developed as pathogenic antimicrobial wound dressings with a continuous release function.58 The silica coating strategy with CaCO3 helps obtain a cellulose-silica composite membrane with a sustained release profile (chloramphenicol as a drug model) and intense antibacterial activity against S. aureus and E. coli. ZnO nanoparticles in 3D printed alginate hydrogels are also antibacterial against S. epidermidis as an antibacterial dressing for chronic wounds.84
Weishaupt et al85 combine antimicrobial peptides (AMP) and cellulose-binding peptides (CBP) to produce wound dressings based on bacterial cellulose and electro-plant cellulose. The results showed that the material had antibacterial activity against S. aureus (log4 reduction) and P. aeruginosa (log1 reduction). AMP affect bacteria through various modes of action, thereby reducing the evolutionary pressure for antibiotic resistance. Laçin et al86 also use ampicillin as an additional drug for 2,3 dialdehyde bacterial cellulose in inhibiting the growth of E. coli and S. aureus. The ɛ-poly-l-Lysine (ɛ-PLL) peptide can also be combined with wound dressing materials.
In practice, Fursatz et al87 developed a functionalization method of carboxymethyl cellulose and peptides using carbodiimides. This functionalization does not affect cytocompatibility to human fibroblasts and antibacterial properties against S. epidermidis. Furthermore, Tavakolian et al88 studied carboxyl-modified cellulose hydrogels and ɛ-PLL as a base material for wound dressings. The results showed that the antibacterial hydrogel could kill about 99% of the bacteria (S. aureus and P. aeruginosa) exposed after 3 h. Another study showing potential antimicrobial agents for wound dressings is shown in Table 2.
Table 2.
Researched Antimicrobial Agents for Potential Wound Healing Combined with Three-Dimensional Printed Cellulose in a Wound Dressing Application
| Antimicrobial agent | Microbial target | Effect | Refs. |
|---|---|---|---|
| Grapefruit seed extract nanoparticles | S. aureus, E. coli | It shows excellent against E. coli compared to S. aureus and has potential for wound healing applications. | 89 |
| AMP (AMP Tet213) | E. coli, MRSA, S. aureus | Has antimicrobial activity in reducing the growth of these microbes. | 90 |
| Curcumin | — | Shows higher antimicrobial activity and the most massive reduction in bacterial colonies than composites without the addition of curcumin. | 91 |
| CCPs | S. aureus, E. coli | The combination of CCPs further enhances wound healing and antimicrobial activity. | 92 |
| Nanosilver | S. aureus, E. coli | It is bactericidal against S. aureus and E. coli and has a good blood clotting ability for wound healing applications. | 93 |
| Polyhexamethylene biguanide | — | A controlled diffusion membrane provides a long-term antimicrobial effect for wound healing. | 94 |
| Nanosilver | — | Superior properties and synergistic antibacterial effect by combining chitosan with silver nanoparticle. | 95 |
| Silver | P. aeruginosa, S. aureus | The silver-containing composites showed more significant bactericidal activity, consistently achieving complete killings for P. aeruginosa and >99.99% kill for S. aureus. | 96 |
| Silver sulfadiazine | P. aeruginosa, S. aureus | Polyelectrolyte complex wound dressings containing silver sulfadiazine can protect the wound surface from bacterial invasion and effectively suppress bacterial proliferation. | 97 |
| Silver sulfadiazine | P. aeruginosa, E. coli, S. aureus | The silver sulfadiazine showed bacterial activity against P. aeruginosa, E. coli, and S. aureus through disc diffusion testing. | 98 |
| Silver nanoparticle | P. aeruginosa, S. aureus, E. coli, MRSA | The silver nanoparticle was most potent against P. aeruginosa, followed by S. aureus, E. coli, and MRSA. | 99 |
| Polyethylenimine | S. aureus, P. aeruginosa | The material has vigorous activity with S. aureus and P. aeruginosa. | 100 |
| Ciprofloxacin HCl | E. coli K12-MG1655, S. typhimurium, V. vulnificus CMCP6, S. aureus ATCC 25923, B. subtilis | Shows good bactericidal against gram-positive and gram-negative bacteria. | 101 |
| Amoxicillin | S. aureus, E. coli | Shows effective antibacterial performance. | 102 |
| Honey | S. aureus, E. coli | Disc diffusion and dynamic contact tests proved the nanofibers' antibacterial activity loaded with honey against these two bacteria. | 103 |
| Elicriso italic, chamomile blue, cinnamon, lavender, tea tree, peppermint, eucalyptus, lemongrass, lemon oils | E. coli, C. albicans | The essential oil-enriched alginate film resists microbial growth for applying wound dressings, protection, and disinfection of medical devices. | 104 |
| Copper nanoparticles | A. baumannii | It has antimicrobial properties through processes that may be associated with contact killing. | 105 |
| Copper-containing mesoporous bioactive glass | E. coli | Nanocellulose and copper composites showed an inhibitory effect on E. coli. | 106 |
| Au nanoclusters | E. coli, S. mutans | Au and nanocellulose films showed high antibacterial in E. coli and S. mutans in vitro and in vivo to promote healing of chronically infected wounds. | 107 |
| Tannic acid, MgCl2 | S. aureus, E. coli, P. aeruginosa | Tannic acid, MgCl2, and bacterial cellulose composites showed potent against S. aureus, E. coli, and P. aeruginosa, reducing the formation of S. aureus and P. aeruginosa biofilms after 24 h of incubation by 80% and ∼87%, respectively. | 108 |
| Deacetylated acemannan extracted from Aloe vera leaves. | S. aureus, E. coli | The cotton cellulose dressing and aloe vera extract showed significant inhibitory effects against S. aureus and E. coli at 70.2% and 72.4%, respectively. | 109 |
| Coffee | MRSA | Powder robusta coffee has a strong inhibition zone. | 110 |
| Zinc oxide | E. coli, P. aeruginosa, S. aureus, C. freundii | Cellulose-zinc oxide bacterial nanocomposites each showed inhibitory activity against E. coli (90%), P. aeruginosa (87.4%), S. aureus (94.3%), and C. freundii (90.9%). | 111 |
AMP, antimicrobial peptides; CCPs, cyclodextrin/propolis extract inclusion complexes; MRSA, methicillin-resistant S. aureus.
Biocompatibility study
Cells in the human body are available in the ECM 3D structure. The materials used in 3D bioprinting must be biocompatible with cells to accelerate tissue formation, organs, adhesion, and cell proliferation. In addition, stem cells will differentiate into some types of cells to build different tissues. Therefore, selecting the type of biomaterial for which cells will grow must be cytocompatible by supporting cell growth, attachment, proliferation, and migration without causing side effects such as severe inflammation, immunological rejection, or genotoxicity.57 The level of toxicity of nanocellulose has been studied by Roman.112
Cellulose, either plant cellulose or bacterial cellulose, and their derivatives have been known to exhibit good biocompatibility for tissue engineering applications, as summarized in Table 3. Cellulose can maintain high flexibility in shape and structure, provides the ability to adapt to surface biochemistry, displays a high degree of biocompatibility, exhibits vascularity, and is widely available and easy to manufacture.113 Modulevsky et al114 used apple native hypanthium tissue to examine the biocompatibility of subcutaneous grown cellulose in wild-type immunocompetent mice. Histological analysis showed no immune response around the dermis tissue and active fibroblast migration in the cellulose scaffold after 8 weeks and the new collagen ECM deposition. Other results also indicated active vascular formation in the scaffold as pro-angiogenic properties of the original scaffold. Overall, the original cellulose scaffolding is biocompatible based on the direct approach to producing 3D printed wound dressings. Other cellulose biocompatibility studies are summarized in Table 3.
Table 3.
Study of Cellulose Biocompatibility for Medical Applications
| Type of cellulose | Material lain | Cell model | Result | Refs. |
|---|---|---|---|---|
| Hydroxyethylcellulose | Ethylene glycol diglycidyl ether, soy protein isolate | Red blood cells, L929 cells | L929 cells can stick to almost any surface and enter the interior. In addition, there were no significant side effects of whole blood anticoagulation, in vitro breakdown, and in vitro bioactivity. | 115 |
| Bacterial nanocellulose | — | Human auricular cartilage | The compacted nano bacterial cellulose hydrogels are noncytotoxic and cause minimal foreign body response. | 116 |
| 2,3 Dialdehyde bacterial cellulose | — | Keratinocytes and fibroblasts | Cellulosic membranes can stimulate keratinocytes and fibroblast cell proliferation due to increased surface area than nondegradable ones. However, bandages do not have an immunostimulating effect. | 86 |
| Bacterial cellulose | Acrylic acid | Fibroblasts | Hydrogel-based materials can increase human epidermal keratinocytes and human skin fibroblasts through in vivo studies using athymic mice. | 117 |
| Wood nanocellulose | — | Keratinocytes and fibroblasts | Nanocellulosic hydrogels can support the proliferation of fibroblasts and keratinocytes and stimulate wound healing without causing adverse local tissue effects. | 118 |
| Cotton fiber | Copper | Embryonic fibroblast stem cells | Potential as metal-based wound care and against pathogenic bacterial infections. | 105 |
| Nanofibrillated cellulose | Copper-containing mesoporous bioactive glass | Fibroblast 3T3 | A dose-dependent on Cu2+ cytotoxicity in 3T3 fibroblasts and a biologically critical Cu2+ level below 10 mg/L are suggested for survival and growth of 3T3 fibroblasts. In addition, the incorporation of copper into the cellulose matrix increases the pro-angiogenic potential of the biocomposite. | 106 |
| Bacterial cellulose | Polyethylene glycol | Fibroblast 3T3 | Cells incubated for 48 h were able to form cell adhesions and proliferation. | 119 |
| Bacterial cellulose | Acrylic acid | Fibroblast skin cells | The hydrogel is nonirritant, nonallergic, nontoxic to primary human dermal fibroblast skin cells with a viability >88% and is biocompatible with blood with a low hemolytic index. | 62 |
| Bacterial cellulose | Poly(3-hydroxybutyrate-co-4-hydroxybutyrate), trifluoroacetic acid | CHL fibroblast cells | Cells incubated with a composite scaffold for 48 h were able to form cell adhesion and proliferation, which showed better biocompatibility than a scaffold without bacterial cellulose. | 120 |
| TEMPO bacterial cellulose | Silver nanoparticle | — | The combination of TEMPO and nanosilver bacterial cellulose showed high biocompatibility according to the results of the in vitro cytotoxicity test (cell viability >95% after 48 h of incubation). | 46 |
| Carboxymethyl cellulose | Nano ZnO, poly(vinyl alcohol) | — | In vitro healing and cell viability results indicate sample biocompatibility and nontoxicity and an excellent ability to heal and protect wounds. | 121 |
| Cellulose fiber | Deacetylated acemannan extracted from Aloe vera leaves | HepG2 cells | Composites have high biocompatibility and increase cell viability. | 109 |
| Wood nanocellulose fibers | — | Normal human dermal fibroblasts and human epidermal keratinocyte | Nanocellulose aerogels decreased metabolic activity by fibroblasts and keratinocytes but showed no significant cell death. Cytokine profiles showed no induction of the 27 cytokines tested after exposure to nanofiber cellulose. | 71 |
| Bacterial cellulose | Dextran | Dextran-modified bacterial cellulose hydrogels increased cell proliferation without cytotoxicity compared to unmodified bacterial cellulose (BC), accelerated wound healing, and facilitated skin maturation. | 122 |
CHL, Chinese Hamster Lung; TEMPO, 2,2,6,6-tetramethylpiperidine-1-oxyl radical.
Nanocellulose is a suitable material for cell culture by exploiting its shear thinning behavior. Ajdary et al123 printed a low-substitution rate of nanocellulose acetate aqueous suspension for direct ink writing. Significantly lower acetate nanofibrils are required to provide a more porous structure. Nanocellulose acetate produces a dimensionally stable monolithic scaffold that supports drying and wetting. A cardiac myoblast cell model demonstrated attachment, proliferation, and survival for 21 days.
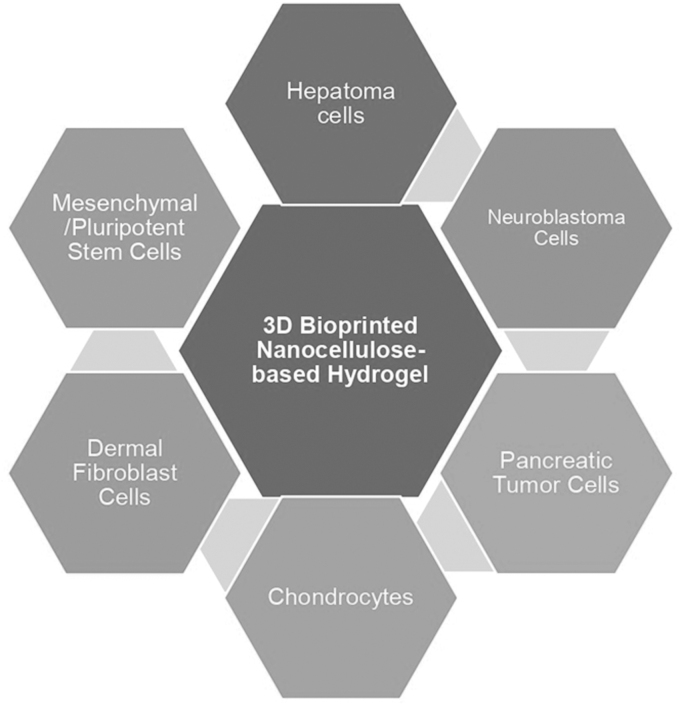
Cellulose-based 3D printing technology for wound dressing applications has recently attracted much interest. The scaffold's biological activity relies on making high-resolution patterns with the orientation and scale of the fibers. Altun et al124 printed a 3D scaffold from bacterial cellulose and polycaprolactone using electrohydrodynamic bioprinting techniques. This scaffold exhibits biocompatibility using cell adhesion and proliferation in vitro. The applications of nanocellulose in skin tissue engineering and wound healing, including the potential for cell growth, drug delivery system, transparency, sensory, cytotoxicity, and immunogenicity, have been discussed by Bacakova et al.125 The 3D-printed nanocellulose-based hydrogels also impact the development of chondrocytes, neuroblastoma cells, dermal fibroblast cells, pancreatic tumor cells, hepatoma cells, and mesenchymal/pluripotent stem cells,73 as shown in Figure 8.
FIG. 8.
Cell survival and proliferation in a bioprinted 3D nanocellulose-based hydrogel (Adopted from Athukoralalage et al73).
Xu et al32 formulated a 3D printed hydrogel from oxidized cellulose nanofibrils (1% w/v) with methacrylate gelatin (<1% w/v). Tests conducted on 3T3 fibroblast cell cultures revealed that the hydrogel has noncytotoxic features and is biocompatible as 3D printing inks. Methacrylate gelatin, incorporated in the cellulose nanofiber (CNF) hydrogel, promotes fibroblast proliferation. 3D printed nanocellulose hydrogels that cross-link with Ca2+ and 1,4-butanediol diglycidyl ether also support fibroblast cell proliferation as hydrogel stiffness increases. Cellulose-based membranes with the addition of AMP and CBP show cytocompatibility properties in human fibroblast cultures.85 Furthermore, hydrogels based on carboxy-modified cellulose and ɛ-PLL also showed high biocompatibility to NIH/3T3 fibroblasts.88
3D printed thin films produced from alginate and carboxymethyl cellulose have been tested to evaluate the effect of growth factors in wound healing applications. The addition of growth factors in the film did not affect the film formation and morphology. Cell viability increased significantly with the addition of growth factors.126 Furthermore, Chinga-Carrasco27 conducted 3D printed fiber nanocellulose testing from bagasse against L929 fibroblast cells. The addition of alginate and Ca2+ causes significant dimensional changes in the 3D print construction. Cell tests showed that bagasse cellulose did not exhibit cytotoxic potential, thus offering the potential for a personalized wound dressing device. The cytotoxicity effect of silver nanoparticles on alginate and nanocellulose-based 3D-printed hydrogels was also studied in hepatocellular carcinoma (HepG2) cell cultures.80
The approach that needs to be considered in producing 3D printed wound dressings is that the cells' survival is greatly influenced by extrusion pressure and shear forces in the molding process. Li et al127 studied the ability of superabsorbent 3D printed scaffolding based on nanocellulose and alginate with porous structures to connect the pores attached to the surface of the scaffold. The scaffold shows excellent ability to absorb cell suspension and deposit cells onto 3D printed scaffolding, more uniform cell distribution, and provide a better microenvironment for cells.
3D-Printed Cellulose in Wound Healing Applications
Chronic wound healing
Wound infections, biofilm formation, amputations, and high medical expenses are often problems in chronic wound healing. Chronic wounds will reach epidemic proportions worldwide due to an aging population and an increasing incidence of diabetes.128 Chronic wounds, including venous ulcers, diabetic ulcers, pressure ulcers, and arterial insufficiency ulcers, are among those that are difficult to treat. Conventional wound care can sometimes lead to suboptimal wound healing and significant morbidity and mortality.129 3D-printed hydrogel-based wound dressings with infectious antimicrobials provide a promising solution.
A personalized approach to treating chronic wounds in diabetes is unavoidable. Cell-based therapy involves designing a 3D cell scaffold bioconstruction obtained by presenting a drug-loaded scaffold with undifferentiated cells to achieve de novo functional tissue in situ.130 Treatment of skin wounds is not only about treating skin tissue but also includes an assessment of individual health, nutrition, comorbidity, and activity level. In this context, chronic wound healing by 3D printed wound dressings requires the design and control of certain drugs added to the composite. This mechanism aims to monitor in real-time without causing interference during chronic wound healing.37
There is a simple method for blending 3D printed cellulose or its composites with natural active ingredients to promote chronic wound healing. First, 3D printed cellulose can be immersed in a solution of the active ingredient to trap the active ingredient with a solvent's help. Next, the solvent will evaporate to leave the active ingredient in the 3D-printed cellulose. Chemical reduction using silver salts, silver nitrate (AgNO3), and a reducing agent, sodium borohydride (NaBH4), can also be used to insert nanosilver in 3D printed cellulose membranes as chronic wound dressings.131
Diabetic patients with foot ulcers show a 150-fold increased risk of amputation due to microbial infection. The natural active ingredients combined with 3D printed cellulose show promising chronic wound prevention potential. Tong et al76 used cotton cellulose nanocrystals (159 nm in size) as a medium for delivering antimicrobials for diabetic wound dressings. In in vivo testing using a diabetic mouse model, curcumin-containing films significantly reduced wound size on day 7 with topical application of curcumin-containing films. The wound is healed by closure by the epithelial tissue and hair that begins to grow. This film significantly enhances hair follicles and sebaceous glands in the skin and can improve diabetic wound healing applications.
Topical coffee powder also showed healing activity in wounds of type 2 diabetes mellitus (90 cases), postamputation wounds in Buerger's disease (15 cases), autoimmune (1 case of juvenile rheumatoid arthritis), burn (6 cases), cellulitis (6 cases), venous malformation (10 cases), and deep femoral soft tissue wound (2 cases) in the human study model.110 Using lignin and its derivatives as an antimicrobial agent combined with bacterial cellulose and coniferyl alcohol shows a promising alternative for chronic wound healing.132
Solway et al133 compared wound healing speed in diabetic foot ulcers using bacterial cellulose wound dressings or Xeroform™ Petrolatum gauze. Wound dressings made of bacterial cellulose can increase wound healing speed and shorten the epithelialization time of diabetic ulcers. Bacterial cellulose also showed decreased depth in chronic varicose ulcers over 120 days without toxicity.134
Kanjou et al135 used modified bacterial cellulose by replacing the fermentation medium with hyaluronic acid, chondroitin sulfate, and cross-linking with sodium alginate and calcium chloride. In vivo testing used an evaluation model—a 60-year-old geriatric patient with diabetic wound healing. 3D printed bacterial cellulose membranes can be applied to cure diabetic ulcers. The development of bacterial cellulose bio-nano composites and magnetic nanoparticles (magnetite) for efficient chronic wound healing in human adipose-derived stem cells in cellular morphology, viability, proliferation, and the cytotoxic potential of scaffolding was studied by Galateanu et al.130 Cellulose acetate/gelatin electro spin also potency for diabetic foot ulcers.136
Painful wounds
Pain can cause a delay in wound healing. Cellulosic wound dressings have been known to reduce pain in wounds compared to textile dressings during removal.137 Foster and Moore (2016) that compared the capabilities of modern cellulose-based fiber dressings with traditional ribbon gauze and proflavin dressings used intra- and postoperatively to evaluate the effects of pain reduction, improved treatment quality, and patient satisfaction.138 The results showed that the pain level upon removal in the cellulose-based fiber bandage group's replacement was significantly reduced (p = 0.002). In addition, many patients preferred using cellulose dressings when the first dressing change was performed at home without analgesia compared to the band gauze group. Polyhexanide dressings containing bio-cellulose dressings also reduce wound pain.139
Maver et al140 combined the nonsteroidal anti-inflammatory drugs, diclofenac sodium (DCS), and local lidocaine (LID) anesthetics with carboxymethyl cellulose 3D and carboxymethyl cellulose electrospun wound dressings. This material is used as a suitable therapeutic solution for painless wound healing. Both carboxymethyl cellulose materials are biocompatible with human skin cells. Drug release is also influenced by the method of preparing wound dressings. Maver et al141 also developed a two-layer pain-relieving wound dressing made of carboxymethylcellulose and polyethylene oxide. These dressings are made by adding pain relievers, namely the nonsteroidal anti-inflammatory diclofenac and local anesthetic lidocaine.
Burns wound healing
Burns of varying thickness require long healing times, extensive size, pain, and repeated injuries due to dressing changes. Therefore, burn injuries are a high risk of morbidity and mortality. In addition, the wound healing process is complex and requires different cells.142
Huang et al143 developed a hydrogel for healing irregular burns to overcome this problem. Hydrogels are produced from natural polymers, namely water-soluble carboxymethyl chitosan and dialdehyde-modified cellulose nanocrystals (DACNC), cross-linked by dynamic Schiff-base between amines from carboxymethyl chitosan and aldehydes from DACNC. Hydrogels showed excellent biocompatibility when tested against normal adult human primary skin fibroblasts and supported cell growth. This study shows that hydrogel can accelerate the healing of partial-thickness burns, relieve pain during dressing changes, and prevent scar formation. Furthermore, Piatkowski et al139 also evaluated the clinical efficacy of polyhexanide containing bio-cellulose dressings compared with silver-sulfadiazine cream in sixty partial-thickness burn patients. As a result, polyhexanides containing bio-cellulose dressings significantly reduced pain (p < 0.01) and faster treated partial-thickness burns than silver-sulfadiazine cream.
The use of bacterial cellulose also shows practical applications for healing burns. Muangman et al144 reported that the microbial cellulose dressing, Nanocell (Thai Nano Cellulose Co. Ltd., Bangkok, Thailand), showed the progress of burn healing to full epithelialization of the face observed for 2 weeks. The dressing is applied once to the face without changing the other dressing. The patient did not show any irritation or allergic reactions during the treatment period, and the wound swab culture did not show any bacterial contamination. This innovative material provides an alternative dressing for superficial partial-thickness burns. In addition, Mohamad et al117 used bacterial cellulose and acrylic acid to improve burn wound healing.
Loh et al145 studied the ability to deliver human epidermal keratinocytes and dermal fibroblasts from bacterial cellulose hydrogels/acrylic acid as wound dressings to partial-thickness burns. In vitro studies have shown that bacterial cellulose/acrylic acid hydrogels have excellent cell adhesion, maintain cell viability with limited migration, and allow cell transfer. In vivo evaluation of wound closure, histological, immunohistochemical, and transmission electron microscopy revealed that hydrogel alone and hydrogel with cells accelerated wound healing compared with untreated controls. This study suggests a potential application of bacterial cellulose/acrylic acid with two functions: a cell carrier and a dressing. In addition, Brassolatti et al142 used pure bacterial cellulose and the addition of lidocaine to full-thickness burns. Using bacterial cellulose demonstrated histological compatibility patterns, mild inflammatory infiltration, better collagen fibers, and mild immunostaining against COX-2 and MMP-9. Thus, bacterial cellulose-based biomaterials can optimize burn to heal.
The addition of active ingredients also supports the healing of infected burns. Guo et al75 made wound dressing scaffolding from curcumin, microspherical gelatin, porous collagen, and cellulose nanocrystals. The scaffold has a high porosity, porous, and extended curcumin release profile, has antibacterial properties, prevents inflammation, and promotes healing of burn infections with full-thickness in mouse models. The addition of curcumin also supports its wound healing properties because it has antioxidant, anti-inflammatory, antimicrobial, and anticarcinogenic activities. Bacterial cellulose nanocomposites and bacterial zinc oxide also demonstrated potential burn dressing materials.118 Based on the explanation above, cellulose can be made through 3D printing technology to treat burns.
Future Directions
Cellulose wound dressing
Cellulose is a biopolymer that shows tremendous potential in the biomedical sector because of its properties: low price, abundant availability, good physicochemical properties, biodegradability, and biocompatibility. Cellulose has demonstrated its biocompatibility properties for tissue engineering applications through low cytotoxicity, can mimic extracellular matrices, and supports cell and tissue development. Various studies using cellulosic materials have succeeded in printing wound dressing designs in accelerating wound healing through biocompatibility studies. However, not many studies have shown that cellulose-based wound dressings meet the requirements for ideal wound dressings.
From the physical aspect, the ideal wound dressing must create a moist atmosphere around the wound, have good mechanical properties (elastic, easy to remove, flexible), permeability to water and gas, and absorb excess exudate. In addition, the ideal wound dressing does not cause trauma to the skin tissue that hinders the wound healing mechanism during dressing changes. Chemically, wound dressings must be clean, sterile, and not toxic to skin cells and tissues. These physicochemical characteristics, coupled with antimicrobial agents, are beneficial in supporting the processes of homeostasis, inflammation, proliferation, and remodeling.
Each type of cellulose contains different functional groups and charges, thus affecting the characteristics of the resulting 3D printed wound dressing. For example, nanocrystalline cellulose contains crystalline parts, which help improve scaffolds' physical and mechanical properties, in contrast to cellulose nanofibrils composed of woven cellulose. However, it will be brittle after the drying process, so binding agents or ions will be added to create a sturdy scaffold. This mechanism is essential for modifying the properties of cellulose both to improve its physicochemical properties and biocompatibility, as well as its ability to bind and regulate drug release.
In addition, the two types of nanocellulose are produced by different methods. Nanocrystalline cellulose is produced by chemical methods, while nanofibril cellulose is produced using mechanical equipment such as ultrafine grinding and ultrasonication. There are considerations for selecting the nanocellulose isolation method related to the cost efficiency and toxicity of the scaffold. In this case, it is necessary to ensure that no chemical residues are toxic to cell growth.
Besides that, microbial resistance is often a significant problem in chronic wound management. Therefore, cellulose-based wound dressings must contain antimicrobial agents without causing microbial resistance properties. In selecting a wound dressing antimicrobial agent, it is also necessary to consider the availability, cost, methods of obtaining active constituents (extraction, isolation, use of solvents), toxicity, age, and storage method in vivo and in vitro testing. Antimicrobial agents based on natural ingredients are expected to be a study in the future because of their abundant availability, generally containing antioxidants and antibacterials. In the future, this aspect is essential to pay attention to in 3D printing cellulose with antimicrobial agents without risking antibiotic resistance.
Wound dressing based cellulose 3D printed
3D printing technology is additive manufacturing using the concept of artificial intelligence through 3D design software. 3D printing technology can increase the effectiveness and efficiency of human performance in designing 3D products. One does not need to spend time, effort, and thought producing products with consistent results. With computer-aided design (CAD), one can change the product design's size proportionally according to the consumer's wishes. Thus, 3D printing technology facilitates social work in various fields.
In designing 3D printed cellulose scaffolding as wound dressings, it is necessary to pay attention to the scaffold's size and pore structure. The scaffold's porous structure dramatically affects the scaffold's physical, chemical, and biocompatibility properties. For example, in conditions of large pores, it may influence stability, elasticity, mechanical structure, excess exudate holding capacity, easy removal without causing pain and wound trauma, and water and gas permeability. It may also be influential in vivo and vitro studies, such as the ability to grow cells and tissues, release antimicrobial agents/agents, and wound healing time. Therefore, 3D printed wound dressings are expected to have a design that meets ideal dressing requirements for treating certain wounds.
The presence of temperature-sensitive active ingredients contributes to the antimicrobial and antioxidant properties. When using 3D printer devices, researchers must ensure that the actives are not damaged during the extrusion process at high temperatures. Clever high temperatures may reduce the performance of (highly-priced) peptides as model drugs, thus impacting their cost efficiency and effectiveness. Therefore, it is crucial to choose the type of 3D printer that matches the characteristics of the drug and cellulose models.
Conclusions
Cellulose is an inexpensive, abundant, biocompatible biopolymer with skin cells and tissues. Due to its unique properties, cellulose has been extensively researched and has shown potential as an ideal wound dressing. To produce a biocompatible wound dressing, it is essential to pay attention to the chemical characteristics of the cellulose and the scaffold design. Cellulose-based 3D printing technology as ideal wound dressings is a renewable and promising innovation for biomedical applications.
Author Disclosure Statement
There are no conflicts to declare.
Funding Information
This work was supported by the Ministry of Research and Technology/National Research and Innovation Agency (Indonesia) and IPB University (Program Bantuan Pembentukan Klaster Kelompok Keilmuan number: 9077/IT3.L1/PT.01.03/P/B/2021).
References
- 1. Hiro ME, Pierpont YN, Ko F, et al. Comparative evaluation of silver-containing antimicrobial dressings on in vitro and in vivo processes of wound healing. ePlasty 2012;12:e48. [PMC free article] [PubMed] [Google Scholar]
- 2. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–229; doi: 10.1177/0022034509359125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Powers JG, Higham C, Broussard K, et al. Wound healing and treating wounds: Chronic wound care and management. J Am Acad Dermatol 2016;74:607–625; doi: 10.1016/j.jaad.2015.08.070 [DOI] [PubMed] [Google Scholar]
- 4. Palaganas NB, Mangadlao JD, de Leon ACC, et al. 3D printing of photocurable cellulose nanocrystal composite for fabrication of complex architectures via stereolithography. ACS Appl Mater Interfaces 2017;9:34314–34324; doi: 10.1021/acsami.7b09223 [DOI] [PubMed] [Google Scholar]
- 5. Alven S, Nqoro X, Aderibigbe BA. Polymer-based materials loaded with curcumin for wound healing applications. Polymers 2020;12(10):2286; doi: 10.3390/polym12102286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bano I, Arshad M, Yasin T, et al. Chitosan: A potential biopolymer for wound management. Int J Biol Macromol 2017;102:380–383; doi: 10.1016/j.ijbiomac.2017.04.047 [DOI] [PubMed] [Google Scholar]
- 7. Hoon R, Oster GA, Damien C, et al. Microbial Cellulose Wound Dressing for Treating Chronic Wound. US 2005/0019380 A1, 2005. Available from: https://patents.google.com/patent/US20050019380A1/en?oq=US +2005%2f0019380+A1 [Last accessed: November 10, 2020].
- 8. Wang Q, Sun J, Yao Q, et al. 3D printing with cellulose materials. Cellulose 2018;25:4275–4301; doi: 10.1007/s10570-018-1888-y [DOI] [Google Scholar]
- 9. Siqueira G, Kokkinis D, Libanori R, et al. Cellulose nanocrystal inks for 3D printing of textured cellular architectures. Adv Funct Mater 2017;27:1604619; doi: 10.1002/adfm.201604619 [DOI] [Google Scholar]
- 10. Sultan S, Siqueira G, Zimmermann T, et al. 3D printing of nano-cellulosic biomaterials for medical applications. Curr Opin Biomed Eng 2017;2:29–34; doi: 10.1016/j.cobme.2017.06.002 [DOI] [Google Scholar]
- 11. Dhivya S, Padma VV, Santhini E. Wound dressings—A review. Biomedicine 2015;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dreifke MB, Jayasuriya AA, Jayasuriya AC. Current wound healing procedures and potential care. Mater Sci Eng C 2015;48:651–662; doi: 10.1016/j.msec.2014.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization (WHO). Wound Management. 2009. Available from: www.who.int/surgery/publications/WoundManagement.pdf [Last accessed: November 10, 2020].
- 14. Aduba DC, Yang H. Polysaccharide fabrication platforms and biocompatibility assessment as candidate wound dressing materials. Bioengineering 2017;4(1):1; doi: 10.3390/bioengineering4010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma S, Dua A, Malik A. Third generation materials for wound dressings. Int J Pharm Sci Res 2017;5(6):2113–2124; doi: 10.13040/IJPSR.0975-8232 [DOI] [Google Scholar]
- 16. Sarabahi S. Recent advances in topical wound care. Indian J Plast Surg 2012;45:379–387. doi: 10.4103/0970-0358.101321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Britto EJ, Nezwek TA, Robins M.. Wound Dressings. StatPearls Publishing: Treasure Island, FL; 2020. [PubMed] [Google Scholar]
- 18. Rippon M, Davies P, White R. Taking the trauma out of wound care: The importance of undisturbed healing. J Wound Care 2013;21(8):359–360; doi: 10.12968/jowc.2012.21.8.359 [DOI] [PubMed] [Google Scholar]
- 19. White R. Wound dressings and other topical treatment modalities in bioburden control. J Wound Care 2011;20(9):431–439; doi: 10.12968/jowc.2011.20.9.431 [DOI] [PubMed] [Google Scholar]
- 20. Thomas S. The Role of Dressings in the Treatment of Moisture-Related Skin Damage. World Wide Wounds 2008. Available from: www.worldwidewounds.com/2008/march/Thomas/Maceration-and-the-role-of-dressings.html#:~:text=Local%20treatment%20of%20moisture%2Drelated,provide%20protection%20to%20periwound%20skin [Last accessed: November 11, 2020].
- 21. Dissemond J, Augustin M, Eming SA, et al. Modern wound care—Practical aspects of non-interventional topical treatment of patients with chronic wounds. Ger Hist 2014;12:541–554; doi: 10.1111/ddg.12351 [DOI] [PubMed] [Google Scholar]
- 22. Ghomi ER, Khalili S, Khorasani SN, et al. Wound dressings: Current advances and future directions. J Appl Polym Sci 2019;136:47738; doi: 10.1002/app.47738 [DOI] [Google Scholar]
- 23. Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Caref 2014;3:511–529; doi: 10.1089/wound.2012.0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donderwinkel I, van Hest JCM, Cameron NR. Bio-inks for 3D bioprinting: Recent advances and future prospects. Polym Chem 2017;8:4451–4471; doi: 10.1039/C7PY00826K [DOI] [Google Scholar]
- 25. Hospodiuk M, Dey M, Sosnoski D, et al. The bioink: A comprehensive review on bioprintable materials. Biotechnol Adv 2017;35:217–239; doi: 10.1016/j.biotechadv.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 26. He P, Zhao J, Zhang J, et al. Bioprinting of skin constructs for wound healing. Burns Trauma 2018;6:5; doi: 10.1186/s41038-017-0104-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chinga-Carrasco G. Potential and limitations of nanocelluloses as components in biocomposite inks for three-dimensional bioprinting and for biomedical devices. Biomacromolecules 2018;19:701–711; doi: 10.1021/acs.biomac.8b00053 [DOI] [PubMed] [Google Scholar]
- 28. Yu Y, Hua S, Yang M, et al. Fabrication and characterization of electrospinning/3D printing bone tissue engineering scaffold. RSC Adv 2016;6:110557–110565; doi: 10.1039/C6RA17718B [DOI] [Google Scholar]
- 29. Vyas C, Ates G, Aslan E, et al. Three-dimensional printing and electrospinning dual-scale polycaprolactone scaffolds with low-density and oriented fibers to promote cell alignment. 3D Print Addit Manuf 2020;7:105–113; doi: 10.1089/3dp.2019.0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Badhe RV, Nipate SS. 5—Cellulosic materials as bioinks for 3D printing applications. In: du Toit LC, Kumar P, Choonara YE, Pillay V (eds.). Advanced 3D-Printed Systems and Nanosystems for Drug Delivery and Tissue Engineering. Woodhead Publishing Series in Biomaterials. The University of Witwatersrand, Johannesburg, South Africa: Elsevier; 2020; pp. 109–137; doi: 10.1016/B978-0-12-818471-4.00005-4 [DOI] [Google Scholar]
- 31. Rees A, Powell LC, Chinga-Carrasco G, et al. 3D bioprinting of carboxymethylated-periodate oxidized nanocellulose constructs for wound dressing applications. BioMed Res Int 2015;2015:925757; doi: 10.1155/2015/925757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu W, Molino BZ, Cheng F, et al. On low-concentration inks formulated by nanocellulose assisted with gelatin methacrylate (GelMA) for 3D printing toward wound healing application. ACS Appl Mater Interfaces 2019;11:8838–8848; doi: 10.1021/acsami.8b21268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gopi S, Balakrishnan P, Chandradhara D, et al. General scenarios of cellulose and its use in the biomedical field. Mater Today Chem 2019;13:59–78; doi: 10.1016/j.mtchem.2019.04.012 [DOI] [Google Scholar]
- 34. Halib N, Perrone F, Cemazar M, et al. Potential applications of nanocellulose-containing materials in the biomedical field. Materials 2017;10(8):977; doi: 10.3390/ma10080977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piras CC, Fernández-Prieto S, De Borggraeve WM. Nanocellulosic materials as bioinks for 3D bioprinting. ACS Biomater Sci Eng 2020;5:1988–1992; doi: 10.1039/C7BM00510E [DOI] [PubMed] [Google Scholar]
- 36. Jiang X, Huang Y, Cheng Y, et al. Effects of lyophilization on the release profiles of 3D printed delivery systems fabricated with carboxymethyl cellulose hydrogel. Polymers 2021;13(5):749; doi: 10.3390/polym13050749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Portela R, Leal CR, Almeida PL, et al. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb Biotechnol 2019;12:586–610; doi: 10.1111/1751-7915.13392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sulaeva I, Hettegger H, Bergen A, et al. Fabrication of bacterial cellulose-based wound dressings with improved performance by impregnation with alginate. Mater Sci Eng C 2020;110:110619; doi: 10.1016/j.msec.2019.110619 [DOI] [PubMed] [Google Scholar]
- 39. Heggset EB, Strand BL, Sundby KW, et al. Viscoelastic properties of nanocellulose based inks for 3D printing and mechanical properties of CNF/alginate biocomposite gels. Cellulose 2018;26:581–595; doi: 10.1007/s10570-018-2142-3 [DOI] [Google Scholar]
- 40. Håkansson KMO, Henriksson IC, Vázquez CDLP, et al. Solidification of 3D printed nanofibril hydrogels into functional 3D cellulose structures. Adv Mater Technol 2016;1:1600096; doi: 10.1002/admt.201600096 [DOI] [Google Scholar]
- 41. Mietner JB, Jiang X, Edlund U, et al. 3D printing of a bio-based ink made of cross-linked cellulose nanofibrils with various metal cations. Sci Rep 2021;11:6461; doi: 10.1038/s41598-021-85865-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sultan S, Mathew AP. 3D printed scaffolds with gradient porosity based on a cellulose nanocrystal hydrogel. Nanoscale 2018;10:4421–4431; doi: 10.1039/C7NR08966J [DOI] [PubMed] [Google Scholar]
- 43. Wang X, Wang Q, Xu C. Nanocellulose-based inks for 3D bioprinting: Key aspects in research development and challenging perspectives in applications—A mini review. Bioengineering 2020;7(2):40; doi: 10.3390/bioengineering7020040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hubbe MA, Tayeb P, Joyce M, et al. Rheology of nanocellulose-rich aqueous suspensions: A review. Bioresources 2017;12(4):9556–9661. [Google Scholar]
- 45. Wu CN, Fuh SC, Lin SP, et al. TEMPO-oxidized bacterial cellulose pellicle with silver nanoparticles for wound dressing. Biomacromolecules 2018;19:544–554; doi: 10.1021/acs.biomac.7b01660 [DOI] [PubMed] [Google Scholar]
- 46. Tsalagkas D, Dimic-Misic K, Gane P, et al. Rheological Behaviour of Sonochemically Prepared Bacterial Cellulose Aqueous Dispersions. In: 6th Symposium on Industrial Engineering 2015. Available from: https://www.researchgate.net/publication/283724688_RHEOLOGICAL_BEHAVIOUR_OF_SONOCHEMICALLY_PREPARED_BACTERIAL_CELLULOSE_AQUEOUS_DISPERSIONS [Last accessed: November 15, 2020].
- 47. Food Drug Administration (FDA). 2018. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf17/K173844.pdf [Last accessed: November 15, 2020].
- 48. Khan TA, Peh KK, Ch'ng HS. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J Pharm Pharm Sci 2000;3:303–311. [PubMed] [Google Scholar]
- 49. Tavakoli J. Physico-mechanical, morphological and biomedical properties of a novel natural wound dressing material. J Mech Behav Biomed Mater 2017;65:373–382; doi: 10.1016/j.jmbbm.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 50. Huang H, Dean D. 3-D printed porous cellulose acetate tissue scaffolds for additive manufacturing. Addit Manuf 2020;31:100927; doi: 10.1016/j.addma.2019.100927 [DOI] [Google Scholar]
- 51. Wang J, Chiappone A, Roppolo I, et al. All-in-one cellulose nanocrystals for 3D printing of nanocomposite hydrogels. Chem Asian J 2017;57:2353–2356; doi: 10.1002/anie.201710951 [DOI] [PubMed] [Google Scholar]
- 52. Jiang Y, Zhou J, Yang Z, et al. Dialdehyde cellulose nanocrystal/gelatin hydrogel optimized for 3D printing applications. J Mater Sci 2018;53:11883–11900; doi: 10.1007/s10853-018-2407-0 [DOI] [Google Scholar]
- 53. Leppiniemi J, Lahtinen P, Paajanen A, et al. 3D-printable bioactivated nanocellulose–alginate hydrogels. ACS Appl Mater Interfaces 2017;9:21959–21970; doi: 10.1021/acsami.7b02756 [DOI] [PubMed] [Google Scholar]
- 54. Xu C, Molino BZ, Wang X, et al. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J Mater Chem B 2018;6:7066–7075; doi: 10.1039/C8TB01757C [DOI] [PubMed] [Google Scholar]
- 55. Huang L, Du X, Fan S, et al. Bacterial cellulose nanofibers promote stress and fidelity of 3D-printed silk based hydrogel scaffold with hierarchical pores. Carbohydr Polym 2019;221:146–156; doi: 10.1016/j.carbpol.2019.05.080 [DOI] [PubMed] [Google Scholar]
- 56. White RJ, Cutting KF. Modern Exudate Management: A Review of Wound Treatments. World Wide Wounds. 2006. Available from: www.worldwidewounds.com/2006/september/White/Modern-Exudate-Mgt.html [Last accessed: November 17, 2020].
- 57. Badhe RV, Godse A, Ahinkar A. Biomaterials in 3D printing: A special emphasis on nanocellulose. Indian J Pharm Educ Res 2020;54:526–540. [Google Scholar]
- 58. Shen Z, Cai N, Xue Y, et al. Silica-cellulose membrane with CaCO3-aided processing for wound dressing application. Polymers 2019;11:808; doi: 10.3390/polym11050808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Osorio M, Velásquez-Cock J, Restrepo LM, et al. Bioactive 3D-shaped wound dressings synthesized from bacterial cellulose: Effect on cell adhesion of polyvinyl alcohol integrated in situ. Int J Polym Sci 2017;2017:1–10; doi: 10.1155/2017/3728485 [DOI] [Google Scholar]
- 60. Gorgieva S. Bacterial cellulose as a versatile platform for research and development of biomedical materials. Processes 2020;8:624; doi: 10.3390/pr8050624 [DOI] [Google Scholar]
- 61. Saibuatong O, Phisalaphong M. Novo aloe vera–bacterial cellulose composite film from biosynthesis. Carbohydr Polym 2010;79:455–460; doi: 10.1016/j.carbpol.2009.08.039 [DOI] [Google Scholar]
- 62. Mohamad N, Buang F, Lazim AM, et al. Characterization and biocompatibility evaluation of bacterial cellulose-based wound dressing hydrogel: Effect of electron beam irradiation doses and concentration of acrylic acid. J Biomed Mater Res Part B Appl Biomater 2016;105:2553–2564; doi: 10.1002/jbm.b.33776 [DOI] [PubMed] [Google Scholar]
- 63. Cutting KF, Westgate SJ. Super-absorbent dressings: How do they perform in vitro? Br J Nurs 2014;21(20):14., 16–19; doi: 10.12968/bjon.2012.21.Sup20.S14 [DOI] [PubMed] [Google Scholar]
- 64. Meftahi A, Khajavi R, Rashidi A, et al. The effects of cotton gauze coating with microbial cellulose. Cellulose 2010;17:199–204; doi: 10.1007/s10570-009-9377-y [DOI] [Google Scholar]
- 65. Bashari A, Shirvan AR, Shakeri M. Cellulose-based hydrogels for personal care products. Polym Adv Technol 2018;29:2853–2867; doi: 10.1002/pat.4290 [DOI] [Google Scholar]
- 66. Yi-min Q. Carboxymethyl cellulose fibers used for wound dressings. Text Res J 2006. Available from: http://en.cnki.com.cn/Article_en/CJFDTotal-FZXB200607026.htm [Last accessed: November 18, 2020].
- 67. Meftahi A, Khajavi R, Rashidi A, et al. Preventing the collapse of 3D bacterial cellulose network via citric acid. J Nanostructure Chem 2018;8:311–320; doi: 10.1007/s40097-018-0275-4 [DOI] [Google Scholar]
- 68. Espinosa E, Filgueira D, Rodríguez A, et al. Nanocellulose-based inks—Effect of alginate content on the water absorption of 3D printed constructs. Bioengineering 2019;6:65; doi: 10.3390/bioengineering6030065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gustaite S, Kazlauske J, Bobokalonov J, et al. Characterization of cellulose based sponges for wound dressings. Colloids Surf A Physicochem Eng Asp 2015;480:336–342; doi: 10.1016/j.colsurfa.2014.08.022 [DOI] [Google Scholar]
- 70. Junker JPE, Kamel RA, Caterson EJ, et al. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments. Adv Wound Caref 2013;2:348–356; doi: 10.1089/wound.2012.0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nordli HR, Chinga-Carrasco G, Mari Rokstad AM, et al. Producing ultrapure wood cellulose nanofibrils and evaluating the cytotoxicity using human skin cells. Carbohydr Polym 2016;150:65–73; doi: 10.1016/j.carbpol.2016.04.094 [DOI] [PubMed] [Google Scholar]
- 72. Czaja W, Kyryliouk D, DePaula CA, et al. Oxidation of γ-irradiated microbial cellulose results in bioresorbable, highly conformable biomaterial. J Appl Polym Sci 2013;131:1–12; doi: 10.1002/app.39995 [DOI] [Google Scholar]
- 73. Athukoralalage SS, Balu R, Dutta NK, et al. 3D bioprinted nanocellulose-based hydrogels for tissue engineering applications: A brief review. Polymers 2019;11:137–146; doi: 10.3390/polym11050898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Singh G, Chandoha-Lee C, Zhang W, et al. Biodegradation of nanocrystalline cellulose by two environmentally-relevant consortia. Water Res 2016;104:137–146; doi: 10.1016/j.watres.2016.07.073 [DOI] [PubMed] [Google Scholar]
- 75. Guo R, Lan Y, Xue W, et al. Collagen-cellulose nanocrystal scaffolds containing curcumin-loaded microspheres on infected full-thickness burns repair. J Tissue Eng Regen Med 2017;11:3544–3555; doi: 10.1002/term.2272 [DOI] [PubMed] [Google Scholar]
- 76. Tong WY, Abdullah AYK, Rozman NAS, et al. Antimicrobial wound dressing film utilizing cellulose nanocrystal as drug delivery system for curcumin. Cellulose 2018;25:631–638; doi: 10.1007/s10570-017-1562-9 [DOI] [Google Scholar]
- 77. Gupta A, Keddie DJ, Kannappan V, et al. Production and characterisation of bacterial cellulose hydrogels loaded with curcumin encapsulated in cyclodextrins as wound dressings. Eur Polym J 2019;118:437–450; doi: 10.1016/j.eurpolymj.2019.06.018 [DOI] [Google Scholar]
- 78. Mohanty C, Sahoo SK. Curcumin and its topical formulations for wound healing applications. Drug Discov Today 2017;22:1582–1592; doi: 10.1016/j.drudis.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 79. Liyaskina EV, Revin VV, Paramonova EN, et al. Bacterial cellulose/alginate nanocomposite for antimicrobial wound dressing. KnE Energy 2018;3(2):202–211; doi: 10.18502/ken.v3i2.1814 [DOI] [Google Scholar]
- 80. Bergonzi C, Remaggi G, Graiff C, et al. Three-dimensional (3D) printed silver nanoparticles/alginate/nanocrystalline cellulose hydrogels: Study of the antimicrobial and cytotoxicity efficacy. Nanomaterials 2020;10(5):844; doi: 10.3390/nano10050844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gutierrez E, Burdiles PA, Quero F, et al. 3D printing of antimicrobial alginate/bacterial-cellulose composite hydrogels by incorporating copper nanostructures. ACS Biomater Sci Eng 2019;5:6290–6299; doi: 10.1021/acsbiomaterials.9b01048 [DOI] [PubMed] [Google Scholar]
- 82. Muwaffak Z, Goyanes A, Clark V, et al. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int J Pharm 2017;527:161–170; doi: 10.1016/j.ijpharm.2017.04.077 [DOI] [PubMed] [Google Scholar]
- 83. Wu Z, Hong Y. Combination of the silver–ethylene interaction and 3D printing to develop antibacterial superporous hydrogels for wound management. ACS Appl Mater Interfaces 2019;11:33734–33747; doi: 10.1021/acsami.9b14090 [DOI] [PubMed] [Google Scholar]
- 84. Cleetus CM, Primo FA, Fregoso G, et al. Alginate hydrogels with embedded ZnO nanoparticles for wound healing therapy. Int J Nanomedicine 2020;15:5097–5111; doi: 10.2147/IJN.S255937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Weishaupt R, Zünd JN, Heuberger L, et al. Antibacterial, cytocompatible, sustainably sourced: Cellulose membranes with bifunctional peptides for advanced wound dressings. Adv Healthc Mater 2020;9:1–13; doi: 10.1002/adhm.201901850 [DOI] [PubMed] [Google Scholar]
- 86. Laçin NT, Kaplan E, Demirbilek M, et al. In vitro evaluation of biocompatibility and immunocompatibility of 2,3 dialdehyde cellulose hydrogel membranes for wound healing. J Biomater Tissue Eng 2017;7:822–828; doi: 10.1166/jbt.2017.1649 [DOI] [Google Scholar]
- 87. Fursatz M, Skog M, Sivler P, et al. Functionalization of bacterial cellulose wound dressings with the antimicrobial peptide ɛ-poly-L-Lysine. Biomed Mater 2018;13:1–11; doi: 10.1088/1748-605X/AA9486 [DOI] [PubMed] [Google Scholar]
- 88. Tavakolian M, Munguia-Lopez JG, Valiei A, et al. Highly absorbent antibacterial and biofilm-disrupting hydrogels from cellulose for wound dressing applications. ACS Appl Mater Interfaces 2020;12:39991–40001; doi: 10.1021/acsami.0c08784 [DOI] [PubMed] [Google Scholar]
- 89. Koneru A, Dharmalingam K, Anandalakshmi R. Cellulose based nanocomposite hydrogel films consisting of sodium carboxymethylcellulose–grapefruit seed extract nanoparticles for potential wound healing applications. Int J Biol Macromol 2020;148:833–842; doi: 10.1016/j.ijbiomac.2020.01.018 [DOI] [PubMed] [Google Scholar]
- 90. Lin Z, Wu T, Wang W, et al. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int J Biol Macromol 2017;140:330–342; doi: 10.1016/j.ijbiomac.2019.08.087 [DOI] [PubMed] [Google Scholar]
- 91. Sadeghianmaryan A, Yazdanpanah Z, Soltani YA, et al. Curcumin-loaded electrospun polycaprolactone/montmorillonite nanocomposite: Wound dressing application with anti-bacterial and low cell toxicity properties. J Biomater Sci Polym Ed 2020;31:169–187; doi: 10.1080/09205063.2019.1680928 [DOI] [PubMed] [Google Scholar]
- 92. Andriotis EG, Eleftheriadis GK, Karavasili C, et al. Development of bio-active patches based on pectin for the treatment of ulcers and wounds using 3D-bioprinting technology. Pharmaceutics 2020;12:56; doi: 10.3390/pharmaceutics12010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Madhumathi K, Kumar PTS, Abhilash S, et al. Development of novel chitin/nanosilver composite scaffolds for wound dressing applications. J Mater Sci Mater Med 2010;21:807–813; doi: 10.1007/s10856-009-3877-z [DOI] [PubMed] [Google Scholar]
- 94. Liu X, Lin T, Gao Y, et al. Antimicrobial electrospun nanofibers of cellulose acetate and polyester urethane composite for wound dressing. J Biomed Mater Res Part B 2012;100B:1556–1565; doi: 10.1002/jbm.b.32724 [DOI] [PubMed] [Google Scholar]
- 95. Abdelgawad AM, Hudson SM, Rojas OJ. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr Polym 2014;100:166–178; doi: 10.1016/j.carbpol.2012.12.043 [DOI] [PubMed] [Google Scholar]
- 96. Ong SY, Wu J, Moochhala SM, et al. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008;29:4323–4332; doi: 10.1016/j.biomaterials.2008.07.034 [DOI] [PubMed] [Google Scholar]
- 97. Kim HJ, Lee HC, Oh JS, et al. Polyelectrolyte complex composed of chitosan and sodium alginate for wound dressing application. J Biomater Sci Polym Ed 2012;10:543–556; doi: 10.1163/156856299X00478 [DOI] [PubMed] [Google Scholar]
- 98. Luan J, Wu J, Zheng Y, et al. Impregnation of silver sulfadiazine into bacterial cellulose for antimicrobial and biocompatible wound dressing. Biomed Mater 2012;7:1–11; doi: 10.1088/1748-6041/7/6/065006 [DOI] [PubMed] [Google Scholar]
- 99. Rujitanaroj P, Pimpha N, Supaphol P. Wound-dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008;49:4723–4732; doi: 10.1016/j.polymer.2008.08.021 [DOI] [Google Scholar]
- 100. Çalamak S, Erdoğdu C, Özalp M, et al. Silk fibroin based antibacterial bionanotextiles as wound dressing materials. Mater Sci Eng C 2014;43:11–20; doi: 10.1016/j.msec.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 101. Unnithan AR, Barakat NAM, Pichiah PBT, et al. Wound-dressing materials with antibacterial activity from electrospun polyurethane–dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr Polym 2012;90:1786–1793; doi: 10.1016/j.carbpol.2012.07.071 [DOI] [PubMed] [Google Scholar]
- 102. Qu J, Zhao X, Liang Y, et al. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem Eng J 2019;362:548–560; doi: 10.1016/j.cej.2019.01.028 [DOI] [Google Scholar]
- 103. Tang Y, Lan X, Liang C, et al. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing. Carbohydr Polym 2019;219:113–120; doi: 10.1016/j.carbpol.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 104. Liakos I, Rizzello L, Scurr DJ, et al. All-natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. Int J Pharm 2014;463:137–145; doi: 10.1016/j.ijpharm.2013.10.046 [DOI] [PubMed] [Google Scholar]
- 105. Cady NC, Behnke JL, Strickland AD. Copper-based nanostructured coatings on natural cellulose: Nanocomposites exhibiting rapid and efficient inhibition of a multi-drug resistant wound pathogen, A. baumannii, and mammalian cell biocompatibility in vitro. Adv Funct Mater 2011;21:2506–2514; doi: 10.1002/adfm.201100123 [DOI] [Google Scholar]
- 106. Wang X, Cheng F, Liu J, et al. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: Biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater 2016;46:286–298; doi: 10.1016/j.actbio.2016.09.021 [DOI] [PubMed] [Google Scholar]
- 107. Wang P, Yin B, Dong H, et al. Coupling biocompatible Au nanoclusters and cellulose nanofibrils to prepare the antibacterial nanocomposite films. Front Bioeng Biotechnol 2020;8:986; doi: 10.3389/fbioe.2020.00986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang ZY, Sun Y, Zheng YD, et al. A biocompatible bacterial cellulose/tannic acid composite with antibacterial and anti-biofilm activities for biomedical applications. Mater Sci Eng C 2020;106:1–10; doi: 10.1016/j.msec.2019.110249 [DOI] [PubMed] [Google Scholar]
- 109. Salah F, Ghoul YE, Alminderej FM, et al. Development, characterization, and biological assessment of biocompatible cellulosic wound dressing grafted Aloe vera bioactive polysaccharide. Cellulose 2019;26:4957–4970; doi: 10.1007/s10570-019-02419-8 [DOI] [Google Scholar]
- 110. Yuwono HS. The new paradigm of wound management using coffee powder. Scand J Surg 2014;2(2):25–29. [Google Scholar]
- 111. Khalid A, Khan R, Islam MU, et al. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr Polym 2017;164:214–221; doi: 10.1016/j.carbpol.2017.01.061 [DOI] [PubMed] [Google Scholar]
- 112. Roman M. Toxicity of cellulose nanocrystals: A review. Ind Biotechnol (New Rochelle N Y) 2015;11:25–33; doi: 10.1089/ind.2014.0024 [DOI] [Google Scholar]
- 113. Hickey RJ, Modulevsky DJ, Cuerrier CM, et al. Customizing the shape and microenvironment biochemistry of biocompatible macroscopic plant-derived cellulose scaffolds. ACS Biomater Sci Eng 2018;4:3726–3736; doi: 10.1021/acsbiomaterials.8b00178 [DOI] [PubMed] [Google Scholar]
- 114. Modulevsky DJ, Cuerrier CM, Pelling AE. Biocompatibility of subcutaneously implanted plant-derived cellulose biomaterials. PLoS One 2016;11:1–19; doi: 10.1371/journal.pone.0157894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhao Y, He M, Jin H, et al. Construction of highly biocompatible hydroxyethyl cellulose/soy protein isolate composite sponges for tissue engineering. Chem Eng J 2018;341:402–413; doi: 10.1016/j.cej.2018.02.046 [DOI] [Google Scholar]
- 116. Ávila HM, Schwarz S, Feldmann EM, et al. Biocompatibility evaluation of densified bacterial nanocellulose hydrogel as an implant material for auricular cartilage regeneration. Appl Microbiol Biotechnol 2014;98:7423–7435; doi: 10.1007/s00253-014-5819-z [DOI] [PubMed] [Google Scholar]
- 117. Mohamad N, Loh EYX, Fauzi MhB, et al. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv Transl Res 2019;9:444–452; doi: 10.1007/s13346-017-0475-3 [DOI] [PubMed] [Google Scholar]
- 118. Basu A, Celma G, Strømme M, et al. In vitro and in vivo evaluation of the wound healing properties of nanofibrillated cellulose hydrogels. ACS Appl Bio Mater 2018;1:1853–1863; doi: 10.1021/acsabm.8b00370 [DOI] [PubMed] [Google Scholar]
- 119. Cai Z, Kim J. Bacterial cellulose/poly(ethylene glycol) composite: Characterization and first evaluation of biocompatibility. Cellulose 2010;17:83–91; doi: 10.1007/s10570-009-9362-5 [DOI] [Google Scholar]
- 120. Zhijiang C, Chengwei H, Guang Y. Poly(3-hydroxubutyrate-co-4-hydroxubutyrate)/bacterial cellulose composite porous scaffold: Preparation, characterization and biocompatibility evaluation. Carbohydr Polym 2012;87:1073–1080; doi: 10.1016/j.carbpol.2011.08.037 [DOI] [Google Scholar]
- 121. Joorabloo A, Khorasani MT, Adeli H, et al. Fabrication of heparinized nano ZnO/poly(vinylalcohol)/carboxymethyl cellulose bionanocomposite hydrogels using artificial neural network for wound dressing application. J Ind Eng Chem 2020;70:253–263; doi: 10.1016/j.jiec.2018.10.022 [DOI] [Google Scholar]
- 122. Lin SP, Kung HN, Tsai YS, et al. Novel dextran modified bacterial cellulose hydrogel accelerating cutaneous wound healing. Cellulose 2017;24:4927–4937; doi: 10.1007/s10570-017-1448-x [DOI] [Google Scholar]
- 123. Ajdary R, Huan S, Ezazi NZ, et al. Acetylated nanocellulose for single-component bioinks and cell proliferation on 3D-printed scaffolds. Biomacromolecules 2019;20:2770–2778; doi: 10.1021/acs.biomac.9b00527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Altun E, Ekren N, Kuruca SE, et al. Cell studies on electrohydrodynamic (EHD)-3D-bioprinted bacterial cellulose\polycaprolactone scaffolds for tissue engineering. Mater Lett 2019;234:163–167; doi: 10.1016/j.matlet.2018.09.085 [DOI] [Google Scholar]
- 125. Bacakova L, Pajorova J, Bacakova M, et al. Versatile application of nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019;9:1–39; doi: 10.3390/nano9020164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Maver U, Gradišnik L, Smrke DM, et al. Impact of growth factors on wound healing in polysaccharide blend thin films. Appl Surf Sci 2019;489:485–493; doi: 10.1016/j.apsusc.2019.06.054 [DOI] [Google Scholar]
- 127. Li Z, Ramos A, Li MC, et al. Improvement of cell deposition by self-absorbent capability of freeze-dried 3D-bioprinted scaffolds derived from cellulose material-alginate hydrogels. Biomed Phys Eng Express 2020;6:1–12; doi: 10.1088/2057-1976/ab8fc6 [DOI] [PubMed] [Google Scholar]
- 128. Cereceres S, Lan Z, Bryan L, et al. Bactericidal activity of 3D-printed hydrogel dressing loaded with gallium maltolate. APL Bioeng 2019;3(2):1–12; doi: 10.1063/1.5088801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Nicholas MN, Yeung J. Current status and future of skin substitutes for chronic wound healing. J Cutan Med Surg 2016;21:23–30; doi: 10.1177/1203475416664037 [DOI] [PubMed] [Google Scholar]
- 130. Galateanu B, Bunea MC, Stanescu P, et al. In vitro studies of bacterial cellulose and magnetic nanoparticles smart nanocomposites for efficient chronic wounds healing. Stem Cells Int 2015;2015:1–10; doi: 10.1155/2015/195096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jung R, Kim Y, Kim HS, et al. Antimicrobial properties of hydrated cellulose membranes with silver nanoparticles. J Biomater Sci Polym Ed 2012;20:311–324; doi: 10.1163/156856209X412182 [DOI] [PubMed] [Google Scholar]
- 132. Zmejkoski D, Spasojević D, Orlovska I, et al. Bacterial cellulose-lignin composite hydrogel as a promising agent in chronic wound healing. Int J Biol Macromol 2018;118:494–503; doi: 10.1016/j.ijbiomac.2018.06.067 [DOI] [PubMed] [Google Scholar]
- 133. Solway DR, Clark WA, Levinson DJ. A parallel open-label trial to evaluate microbial cellulose wound dressing in the treatment of diabetic foot ulcers. Int Wound J 2010;8:69–73; doi: 10.1111/j.1742-481X.2010.00750.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cavalcanti LM, Pinto FCM, De Oliveira GM, et al. Efficacy of bacterial cellulose membrane for the treatment of lower limbs chronic varicose ulcers: A randomized and controlled trial. Rev Col Bras Cir 2017;44:72–80; doi: 10.1590/0100-69912017001011 [DOI] [PubMed] [Google Scholar]
- 135. Kanjou MM, Abdulhakim H, de Olyveira GM, et al. 3-D print celulose nanoskin: Future diabetic wound healing. J biomater nanobiotechnol 2019;10:190–195; doi: 10.4236/jbnb.2019.104011 [DOI] [Google Scholar]
- 136. Samadian H, Zamiri S, Ehterami A, et al. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in vivo studies. Sci Rep 2020;10:1–12; doi: 10.1038/s41598-020-65268-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Pinho E, Soares G. Functionalization of cotton cellulose for improved wound healing. J Mater Chem B 2016;6:1887–1898; doi: 10.1039/C8TB00052B [DOI] [PubMed] [Google Scholar]
- 138. Foster L, Moore P. The application of a cellulose-based fibre dressing in surgical wounds. J Wound Care 2016;6(10); doi: 10.12968/jowc.1997.6.10.469 [DOI] [PubMed] [Google Scholar]
- 139. Piatkowski A, Drummer N, Andriessen A, et al. Randomized controlled single center study comparing a polyhexanide containing bio-cellulose dressing with silver sulfadiazine cream in partial-thickness dermal burns. Burns 2011;37:800–804; doi: 10.1016/j.burns.2011.01.027 [DOI] [PubMed] [Google Scholar]
- 140. Maver T, Smrke DM, Kurečič M, et al. Combining 3D printing and electrospinning for preparation of pain-relieving wound-dressing materials. J Solgel Sci Technol 2018;88:33–48; doi: 10.1007/s10971-018-4630-1 [DOI] [Google Scholar]
- 141. Maver T, Kurečič M, Smrke DM, et al. Electrospun nanofibrous CMC/PEO as a part of an effective pain-relieving wound dressing. J Solgel Sci Technol 2016;79:475–486; doi: 10.1007/s10971-015-3888-9 [DOI] [Google Scholar]
- 142. Brassolatti P, Kido HW, Bossini PS, et al. Bacterial cellulose membrane used as biological dressings on third-degree burns in rats. Biomed Mater Eng 2018;29:29–42. [DOI] [PubMed] [Google Scholar]
- 143. Huang W, Wang Y, Huang Z, et al. On-demand dissolvable self-healing hydrogel based on carboxymethyl chitosan and cellulose nanocrystal for deep partial thickness burn wound healing. ACS Appl Mater Interfaces 2018;10:41076–41088; doi: 10.1021/acsami.8b14526 [DOI] [PubMed] [Google Scholar]
- 144. Muangman P, Opasanon S, Suwanchot S, et al. Efficiency of microbial cellulose dressing in partial-thickness burn wounds. J Am Col Certif Wound Spec 2011;3:16–19; doi: 10.1016/j.jcws.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Loh EYX, Mohamad N, Fauzi MB, et al. Development of a bacterial cellulose-based hydrogel cell carrier containing keratinocytes and fibroblasts for full-thickness wound healing. Sci Rep 2018;8:1–12; doi: 10.1038/s41598-018-21174-7 [DOI] [PMC free article] [PubMed] [Google Scholar]