Abstract
Background:
Latinos are at higher risk of developing mild cognitive impairment (MCI) and Alzheimer’s dementia than non-Latino Whites. Acculturation factors may influence this risk, yet there are few studies that have examined associations of acculturation, particularly in the context of socioenvironmental and familial factors, and brain health in older Latinos.
Objective:
To examine potential associations between acculturation in context and brain health in older Latinos.
Methods:
Using three previously established composites of acculturation-in-context, (acculturation-related: nativity status, language preference, acculturation scores; contextually-related socioenvironmental: perceived discrimination, loneliness/social isolation, social network size; and familism), and diffusion-tensor imaging (DTI), associations with white matter structural integrity were examined in 92 Latino adults without dementia participating in one of three epidemiological studies of aging. Linear regression models were used to test associations with DTI-derived metrics (fractional anisotropy, FA; trace) as separate outcomes and acculturation composite scores as individual predictors, while adjusting for age, sex, education, scanner, and white matter hyperintensities (voxelwise and total volumes normalized by intracranial volume).
Results:
Higher scores on the socioenvironmental composite were associated with lower FA in two clusters of left-hemisphere connections. Cluster 1 was dominated by both short association pathways connecting frontal regions and projection pathways connecting frontal regions with the thalamus. Cluster 2 was dominated by long association pathways connecting parietal, frontal and temporal regions.
Conclusion:
This study of older Latino adults demonstrated an association between reduced brain white matter integrity and contextually related socioenvironmental experiences known to increase risk of MCI and Alzheimer’s dementia.
Keywords: Latino, Hispanic, Alzheimer’s disease, acculturation, social determinants of health, brain structure, diffusion-tensor imaging
INTRODUCTION
Older Latino/a/x/e or Hispanic (hereinafter referred to as Latino) adults are at increased risk of developing mild cognitive impairment (MCI) [1] and Alzheimer’s disease and related dementias (ADRD) [2] compared to non-Latino Whites and will account for an increasing percentage of the ADRD burden in the US over the coming decades [2]. Various factors influence this disproportionate risk via common impacts on physical/medical (e.g., cardiovascular disease and risk factors) [3-5] and emotional (e.g. depressive symptoms) [6] health, however Latino-specific factors that may contribute to the elevated risk of MCI and ADRD are increasingly being investigated. One particularly salient Latino-specific factor for those living in the United States (US) is acculturation, historically defined as the process of adapting to a new environment and potentially adopting its values and practices [7].
Recently, acculturation – a multi-dimensional construct – has been conceptualized more comprehensively within the context of individual (e.g., language preference, nativity status), socioenvironmental (e.g., social experiences, exposure to discrimination/racism) and family-focused (e.g., familism/family attachment) factors [8-12]. Individual aspects of “acculturation in context” including ethnic identity, familism, and language- as well as social-based acculturation have been shown to influence the physical/medical [13-14], emotional [14-16] and cognitive [17] health of older Latinos, with recent work investigating potential associations of some of these same attributes to brain health [4,17]. Fewer studies exist, however, that systematically assess the acculturation in context framework as it relates to physical/medical and/or cognitive health of older Latinos [9,18] and, to our knowledge, no studies have investigated links between the acculturation in context framework and brain health.
One brain health metric that is known to be sensitive to the lived experience of other minoritized populations, and may prove to be associated with acculturation in context in older Latino adults, is white matter structural integrity. For example, within non-Latino Black adults, associations have been found between perceived discrimination and reduced structural white matter integrity in the form of MRI-derived macrostructural white matter hyperintensities (WMHs) as seen on T2-weighted or FLAIR images [19] as well as microstructural diffusion anisotropy of water molecules on diffusion tensor imaging (DTI) [20-21]. To date, however, no studies that we know of have examined this potential association in older Latinos. This is despite WMH burden being greater in older Latino adults compared to non-Latino Whites [22-23] and DTI-derived FA-profiles of MCI and AD differing between these same groups [24]. Thus, it is feasible that acculturation in context, which takes into consideration such lived experiences as perceived discrimination, may impact white matter integrity in older Latinos.
The current study investigated the associations between three previously established acculturation in context composites [9,18] i.e., acculturation-related (nativity status, language preference, and SASH acculturation scores reflective of US-based acculturation), contextually-related socioenvironmental (higher loneliness/social isolation, smaller social network size, higher perceived discrimination), and familism, and DTI-derived measures (fractional anisotropy [FA] and trace) of white matter integrity. We chose to focus on white matter, and on DTI-derived measures specifically, for two reasons. First, historically, white matter structural integrity has been understudied even though it can have far reaching clinical implications [25], and this is especially true in the Latino population that is generally under-represented in neuroscience research [26]. Second, DTI measures more subtle microstructural morphometric phenomena such as demyelination and axonal injury, as well as other disturbances in diverse cellular mechanisms [27-29] that can occur even in normal-appearing white matter [30-32]. Here we adjusted the DTI regression models for whole brain and voxel-level WMHs to explore whether any potential microstructural effects would remain significant after controlling for alterations in macrostructural morphometry. Based on the few studies that have examined aspects of acculturation and brain gray matter volumes in older Latinos [4,17] and work in other minoritized populations linking select contextually-related socioenvironmental experiences and DTI-derived white matter integrity [20-21], we hypothesize that acculturation- and contextually-related socioenvironmental factors will be associated with white matter integrity. The relationship of familism to brain health has not been previously studied.
MATERIALS AND METHODS
Study Population
Five cohort studies of aging and dementia were reviewed for potential Latino participants: Rush Alzheimer’s Disease Center (RADC) Latino [33] and African American [34] Cores and the Religious Orders Study [35], as well as the Rush Memory and Aging Project [35] and the Minority Aging Research Study [34]. All cohort studies recruit participants without known dementia at baseline determined via a standard clinical evaluation [33,35] and NINCDS/ADRDA diagnostic criteria [36]. Annual evaluations, conducted in participants’ preferred language (English or Spanish), are harmonized across studies, conducted by the same study staff, and include a medical history, neuropsychological testing and neurological evaluation. In 2012, harmonized 3 Tesla (3T) MRI of the brain was added to all cohort studies except the Latino Core which started 3T neuroimaging in 2017. All studies followed the ethical standards set in the 1964 Declaration of Helsinki and its later amendments and were approved by the Institutional Review Board of Rush University Medical Center.
Eligibility for the current analyses required the participant to 1) self-identify as Latino/Hispanic, 2) have successfully completed one 3T MRI session yielding data that passed quality-control, 3) be without dementia at study analytic baseline, i.e., their first MRI session, 4) have simultaneously completed annual clinical and cognitive assessments which included an evaluation of acculturation in context at study analytic baseline. At the time of analysis, 397 self-identified Latinos were alive and active in one of the cohort studies at the start of 3T MRI. Of these, 187 consented to MRI and were eligible for scanning while 210 were either ineligible due to MRI contraindications (e.g., claustrophobia, metal in the body, living out of the geographic area, physical/cognitive/sensory impairments) or refused MRI. Of those 187 who consented, MRI data were not available for 74 participants due to withdrawal from the cohort study (n=5), death (n=8), refusal after initial consent (n=2), pending MRI scheduling (n=55), or pending processing and quality control at the time of these analyses (n=4); this left 113 participants with a first 3T MRI scan. Of these 113, 6 were missing DTI data and 3 were pending DTI post-processing at study analytic baseline, leaving 104 valid DTI scans for analysis. No participants were excluded secondary to a diagnosis of dementia at baseline MRI. Twelve participants were excluded for incomplete data on acculturation in context related variables. This left 92 Latino participants from three of the five parent studies (Latino Core=66; African American Core=2, Rush Memory and Aging Project=24) contributing to the analyses.
Assessment of Acculturation in Context
As previously described [9,18], acculturation in context metrics were chosen to reflect not only acculturation- and contextually-related factors important to the lived experience of Latinos [8,10,11,12,37] but also the National Institute on Aging Health Disparities Research Framework [38]. Unrotated factor loadings derived from principal component analysis [9] led to the creation of three composite scores for acculturation in context based on the following item-level data: 1) acculturation-related composite included nativity status (based on country of origin documented separately for participants [US or non-US] and their parents [neither parent, one parent, or both parents born outside mainland US]), total and domain-specific language- and social-based acculturation scores from the Short Acculturation Scale for Hispanics [39], and participants’ language preference for testing (English or Spanish); 2) contextually-related socioenvironmental composite included scores from the Williams Everyday Discrimination Scale [40], a modified 5-item version of the de Jong-Gierveld Loneliness Scale assessing social isolation [41], and an evaluation of social network size [42]; 3) familism composite was comprised of only one item-level data point, the total score of the 6-item Sabogal Familism Measure [43]. Raw scores from these 10 metrics were converted to standard z-scores using the baseline mean (SD) of the entire sample. Select z-scores were multiplied by −1 to ensure that a higher score reflected higher levels of acculturation to the US or higher exposure to an adverse contextually-related socioenvironmental milieu. Higher familism scores reflected higher levels of identification and attachment of an individual to their family.
Image acquisition
MRI data were collected across two sites on either a 3T Philips MRI scanner and a 3T Siemens scanner using a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence, a 2D T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence, and a 2D spin-echo echo-planar diffusion-weighted sequence. The parameters on the 3T Philips scanner were: for MPRAGE TR=8 ms, TE=3.7 ms, TI=955 ms, flip-angle=8°, field of view=240 mm × 228 mm, 181 sagittal slices, acquired voxel size=1×1×1 mm3, and an acceleration factor of 2 (SENSE); for FLAIR TR=9 s, TE=90 ms, TI=2500 ms, field of view=220 mm × 220 mm, 35 axial slices, acquired voxel size=0.9×1.1×4 mm3, and an acceleration factor of 1.6 (SENSE); for DTI TR=10.7 s, TE=55 ms, field of view=224 mm × 224 mm, 65 axial slices, acquired voxel size=2×2×2 mm3, b=1000 s/mm2 for 40 diffusion directions and 6 b = 0 s/mm2 volumes. The parameters on the 3T Siemens scanner were: for MPRAGE TR=2.3 s, TE=2.98 ms, TI=900 ms, flip-angle=9°, field of view=256 mm × 256 mm, 176 sagittal slices, acquired voxel size=1×1×1 mm3, and an acceleration factor of 2 (GRAPPA); for FLAIR TR=9 s, TE=150 ms, TI=2490 ms, field of view=220 mm × 220 mm, 35 axial slices, acquired voxel size=0.9×0.9×4 mm3, and an acceleration factor of 2 (GRAPPA); for DTI TR=8.1 s, TE=85 ms, field of view=224 mm × 224 mm, 65 axial slices, acquired voxel size=2×2×2 mm3, b=1000 s/mm2 for 40 diffusion directions and 6 b = 0 s/mm2 volumes.
Image processing
Fractional anisotropy (FA) and the trace of the diffusion tensor, two of the most commonly used measures derived from the diffusion tensor [44], were used to characterize white matter structural integrity. Correction of distortions in the diffusion-weighted volumes caused by eddy currents and magnetic field non-uniformities, bulk-motion correction, B-matrix reorientation, tensor fitting, and generation of FA and trace maps were accomplished with TORTOISE (http://www.tortoisedti.org)[45-47]. White matter lesions appearing hyperintense in T2-weighted images (white matter hyperintensities; WMHs) were segmented for each participant based on both MPRAGE and FLAIR data [48] and a mask (0 and 1s) was generated (voxels with WMH were given values of 1). The total volume of WMH was calculated from this mask. The WMH mask of each participant was transformed to the space of the corresponding processed DTI data based on the transformation of the FLAIR image volume to the b = 0 s/mm2 volume.
Statistical Approach
Descriptive summaries of all variables at study baseline were conducted using SAS/STAT software, Version 9.4 of the SAS System for Linux [49]. The association of the three acculturation in context composite scores (acculturation-related, contextually-related socioenvironmental, and familism as separate predictors) with DTI-derived metrics (FA and trace as separate outcomes) was analyzed using tract-based spatial statistics (TBSS) along the white matter skeleton [50]. DTI data from individual participants were non-linearly transformed to the space of the IIT Human Brain Atlas (v.5.0) (www.nitrc.org/projects/iit) [51] using DR-TAMAS. The resulting spatial transformations were then applied to the corresponding FA maps, and local FA maxima were projected onto the IIT Human Brain Atlas (v.5.0) white matter skeleton using TBSS [50]. The same projection parameters were used to project the trace and WMH mask values from the same voxels as the local FA maxima. Linear regression was then used to test the association of each acculturation in context composite with FA along the white matter skeleton (three models; one for each composite), while adjusting for age, sex, and education (given their associations to both predictors and outcomes), as well as scanner, the logarithm base 10 of the total volume of WMHs expressed as a percent of the intracranial volume, and the presence of WMHs at the voxel level. Models were then rerun using the trace of the diffusion tensor along the white matter skeleton as the outcome. The analysis was conducted using FSL Permutation Analysis of Linear Models (PALM) [52], assuming different variances across scanners and using two exchangeability blocks (one per scanner; i.e. permutations occurred only between participants imaged on the same scanner). Statistical inference was based on 1000 permutations of the data, and tail approximation was used to accelerate the analysis [53]. Associations were considered significant at p < 0.05, with Family Wise Error (FWE) rate correction. The Threshold-Free Cluster Enhancement (TFCE) [54] method defined clusters of significance. The regionconnect software (www.nitrc.org/projects/iit) determined the most probable connections passing through clusters showing significant effects, according to the connectivity information contained in the IIT Human Brain Atlas (v.5.0; developed using high angular resolution diffusion imaging probabilistic tractography).
RESULTS
Participant characteristics
Participant characteristics are shown in Table 1. Of the 92 persons in the analytic sample, 83% were female. The mean age at the time of MRI was 73.9 years (SD = 6.5 years). Measures were administered in Spanish for 58% of the sample. The mean education was 11.9 years (SD = 4.8 years), with 18%, 10%, 26% and 46% of the sample having 2-7, 8, 9-12, and over 12 years of education, respectively.
TABLE 1.
Participant Characteristics (N = 92).
| Mean | SD | |
|---|---|---|
| Age (years) | 73.9 | 6.5 |
| Education (years) | 11.9 | 4.8 |
| # Female (%) | 76/92 (83.0%) | |
| MMSE | 27.6 | 2.0 |
| Country of Origin (%) | ||
| US Mainland | 22/92 (23.9%) | |
| Cuba | 1/92 (1.1%) | |
| Ecuador | 4/92 (4.4%) | |
| Honduras | 1/92 (1.1%) | |
| Mexico | 30/92 (32.6%) | |
| Peru | 2/92 (2.2%) | |
| Puerto Rico | 16/92 (17.4%) | |
| Other | 16/92 (17.4%) | |
| WMH (% ICV; log 10) | −0.522 | 0.362 |
| Acculturation in Context * | ||
| Acculturation-related Composite | −1.01 | 1.09 |
| Contextually-related Socioenvironmental Composite | 0.22 | 0.86 |
| Familism | −0.04 | 1.00 |
MMSE = Mini Mental Status Examination.
WMH = white matter hyperintensities, values expressed as % of intracranial volume (ICV) and transformed to logarithm base 10 for use in regression models.
z-scores (Lamar et al. 2021).
Acculturation in Context and DTI
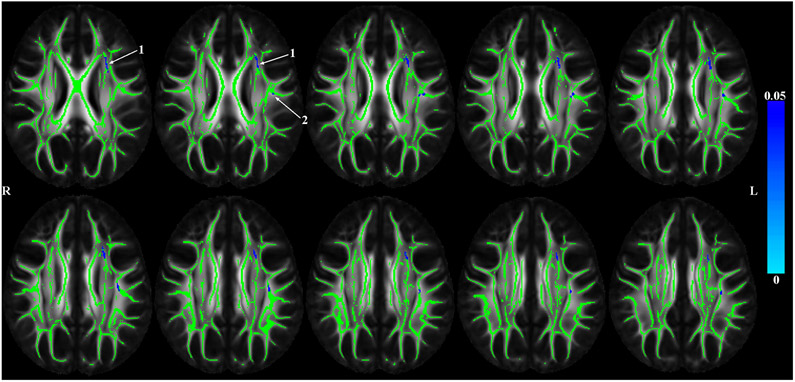
TBSS analyses corrected for age, sex, education, scanner, and WMHs (voxelwise and overall burden) resulted in significant negative associations between the contextually-related socioenvironmental composite and FA. As seen in Figure 1, higher scores on this composite were associated with lower FA in select clusters of the white matter skeleton (Figure 1).
Figure 1.
P-value maps showing (in blue) white matter regions in which higher contextually-related socioenvironmental composite score is associated with lower DTI-derived fractional anisotropy adjusted for age, sex, education, scanner and white matter hyperintensities (WMHs; voxelwise and total volumes normalized by intracranial volume). Results were TFCE and FWE corrected and represented p<0.05. The arrows point to two significant clusters of probable white matter connections associated with the socioenvironmental composite.
We used regionconnect to determine the most probable white matter connections (Table 2) passing through the two clusters with significant associations between the socioenvironmental composite and FA shown in Figure 1. Cluster 1 was dominated by left-hemisphere short association pathways connecting the superior frontal and middle frontal cortices with the basal ganglia, pars opercularis and insula. Cluster 2 was dominated by left-hemisphere long association pathways connecting regions of the parietal (inferior parietal, supramarginal, post-central) and temporal lobes (middle temporal cortex) with regions in the frontal lobe (rostral middle frontal, pre-central, pars opercularis) and insula.
TABLE 2.
List of most probable connections passing through the white matter clusters showing significant associations of the socioenvironmental composite and FA adjusting for age, education, sex, scanner, total volume of WMHs normalized by intracranial volume and the presence of WMH at the voxel level as seen in Figure 1.
| Cluster # | Connection | % |
|---|---|---|
| 1 | L superior frontal and L thalamus proper | 17.8 |
| L parsopercularis and L superior frontal | 13.2 | |
| L caudal middle frontal and L thalamus proper | 4.5 | |
| L rostral middle frontal and L caudate | 4.4 | |
| L caudal middle frontal and L parsopercularis | 4.2 | |
| L superior frontal and L putamen | 4.0 | |
| L superior frontal and L pallidum | 3.7 | |
| L superior frontal and L insula | 3.6 | |
| L caudal middle frontal and L caudate | 3.5 | |
| 2 | L inferior parietal and L parsopercularis | 8.7 |
| L inferior parietal and L precentral | 7.4 | |
| L inferior parietal and L rostral middle frontal | 7.2 | |
| L middle temporal and L precentral | 6.1 | |
| L middle temporal and L rostral middle frontal | 6.0 | |
| L middle temporal and L parsopercularis | 6.0 | |
| L postcentral and L insula | 4.7 | |
| L parsopercularis and L supramarginal | 3.9 | |
| L precentral and L supramarginal | 3.1 | |
| L rostral middle frontal and L supramarginal | 3.1 |
List derived from “regionconnect” tool running on the IIT Human Brain Atlas (v.5.0). The last column shows the % probability that a streamline passing through a voxel of the cluster belongs to a certain connection. Connections with probabilities < 3% not shown.
No associations were found between the acculturation-related composite or the familial composite and FA, or between any of the three composites and trace of the diffusion tensor.
DISCUSSION
This study examined the cross-sectional associations between three acculturation in context composites (acculturation-related, contextually-related socioenvironmental, familism) and DTI-derived white matter structural integrity in a sample of older Latino participants without dementia. The results showed that, after correcting for age and other relevant covariates, higher scores on the contextually-related socioenvironmental composite, reflecting exposure to higher levels of discrimination and social isolation as well as smaller social networks, were associated with lower fractional anisotropy across several exclusively left-hemisphere pathways, involving short association pathways connecting regions within the prefrontal cortex (PFC), pathways connecting the PFC with the basal ganglia and insula, and long association pathways connecting the inferior parietal and middle temporal regions with the PFC and primary motor areas. There were no associations between either the acculturation-related composite or the familism composite with white matter integrity. Taken together, these results suggest that acculturation in context is related to white matter integrity in older Latinos and that negative socioenvironmental acculturative experience may be the most robust associate of compromised white matter integrity in this minoritized population.
The contextually-related socioenvironmental composite used in this study measured three aspects of the lived experience: discrimination, loneliness/social isolation, and social network size. Each experience has been independently associated with reduced white matter integrity [55-57] in the general aging literature (across race and ethnicity). More work is now being done investigating some of these same experiences (e.g., discrimination) within studies exclusive to older non-Latino Black adults and associations have been found with white matter integrity [19-21]. The results of the current study extend the link between these aspects of the lived experience and compromised white matter integrity to older Latino adults and begin to move this field of study beyond prior studies investigating these metrics of lived experience in isolation to considering them as a contextually-related socioenvironmental composite within a larger Latino acculturation in context framework. The findings remained robust even when the diffusion models were adjusted for whole-brain and voxel-wise WMHs, indicating that diffusion alterations associated with aspects of older Latinos’ socioenvironment occur in white matter over and above visible macrostructural changes. It is thus plausible that the presence of socioenvironmental-related white matter diffusion features may signal risk of future brain health disorders, including vessel disease and ADRD neurodegeneration, that are known to drive decline in cognition in older Latinos. Longitudinal studies are needed to explore this possibility.
Older Latinos’ contextually-related socioenvironmental experiences of higher levels of discrimination and loneliness/social isolation, as well as smaller social networks, when taken together as a composite, were associated with reduced white matter integrity in a distributed circuitry that included predominantly prefrontal, parietal, temporal, thalamic, insular and striatal regions. Although the social brain connectome remains to be fully characterized [58-59], these regions are among those known to participate in the varied cognitive and emotional operations that drive social behavior including face processing, behavioral regulation, motor/action comprehension, empathy, language, mentalizing, and mirroring [25,60-61]. Rapid and efficient communication between these regions via healthy white matter pathways is critical for effective and adaptive social function and even mild disruption of connectivity can lead to social dysfunction [25,55,59,62]. In this study, findings emerged solely in the left-hemisphere, consistent with a number of studies that have shown the left-hemisphere to be favored for higher-order social processes that draw on language and on semantic concepts such as the self-other distinction [63] and perceived discrimination [20-21].
The biological mechanisms underlying the association between negative social experiences and brain health are complex and not yet fully understood. Eisenberg and colleagues [64] have suggested that experiences of social disconnection might be processed as a threat to survival, which sets off a “neural alarm system” that signals threat and pain and leads to physiological responses such as HPA axis and sympathetic nervous system (cardiovascular) activation and the concomitant elevation of potentially neurotoxic glucocorticoids and proinflammatory cytokines [65-68]. It is feasible that threat and pain accompanying chronic social disconnection and discrimination experiences related to acculturation of older Latinos could trigger such a cascade of physiological reactions, creating the white matter anomalies evidenced in this study. This compromised connectivity could, in turn, influence emotional regulation and decision-making regarding important health behaviors and increase risk of adverse peripheral health outcomes [21] such as cardiovascular disease and diabetes. These conditions are known to be prevalent in Latinos [13,69], cause further damage to white matter tracts [70-71], and increase susceptibility to MCI and ADRD dementia [72-73]. Longitudinal studies examining biological pathways and their directional links between acculturation-related negative social experiences, behavior, and brain health in Latinos are needed.
White matter tracts are plastic throughout development and respond to behavioral interventions [74-78]. Latinos with negative socioenvironmental acculturative experiences and evidence of microstructural alterations on DTI (an accessible clinical imaging tool) may be candidates for socioenvironmental interventions that could protect or even repair [79] damage to white matter, thus lowering risk of MCI and ADRD. Clinical trials are needed to examine the efficacy of non-pharmacological interventions to reduce the pain of social disconnection [80] and protect brain health. The findings reported here underscore the importance of including older Latinos in these trials, and the potential utility of white matter imaging as a biological outcome.
This study had limitations. First, the small sample size precluded the consideration of important covariates such as presence of mild cognitive impairment, vascular risk factors, and/or depression. As such, the results should be considered foundational in the larger effort to design studies that will increase understanding of the links between acculturation in context and brain health and lead to interventions that improve quality of life and reduce health-related disparities in older Latinos. Second, although the study was cross-sectional and cannot address directionality of effects, the findings demonstrate that the lived social experiences of older Latino adults and white matter integrity are, indeed, associated. Longitudinal studies using larger samples of older Latinos that can accommodate relevant covariates, improve power and understand directionality of effects are sorely needed. Third, the sample was comprised of mostly female participants living in the Chicagoland area, with an average of about 12 years of education, and the findings may not generalize to other categories of older Latino adults across the US and internationally. Finally, although most of the participants in this study were born outside of mainland US, the sample size was too small to examine the impact of country of origin on the results.
Despite these limitations, this study also had strengths. First, imaging studies focused on Latinos are rare and studies attempting to understand the associations of acculturation in context with brain health are even rarer. Second, the participants represented a variety of Latino backgrounds and were recruited from well-characterized cohort studies with fully harmonized methods. Third, controlling for WMHs on a whole-brain and voxel-wise basis allowed the examination of the effects of acculturation in context on white matter diffusion outcomes over and above the effects of macrostructural compromise. Finally, the most probable connections impacted by abnormal white matter structural integrity and linked to contextually-related socioenvironmental experiences were identified and characteristics of the regions connected can be extracted and used in mediation analyses in future studies.
Conclusions
The results of this study suggest that the contextually-related socioenvironmental composite of the acculturation in context framework is linked to brain health in older Latinos. Longitudinal work is needed to determine the directionality of these effects and examine the predictive validity of these associations for future vessel disease and other brain health disorders, including ADRD neurodegeneration, that are known to drive cognitive decline in older Latinos.
ACKNOWLEDGEMENTS
Woojeong Bang, MS, Jennifer Poirier PhD and Alysha Hodges MS provided statistical programming. We are indebted to the altruism of the participants of RADC Latino and Clinical Cores, MARS, MAP and ROS.
FUNDING
This work was supported by the National Institute on Aging (grant numbers R01 AG062711, R01 AG17917, R01 AG022018, R01AG064233, R01AG052200, P30 AG010161, and P30 AG72975), the National Institute of Neurological Disorders and Stroke (UF1NS100599) and by the Illinois Department of Public Health.
Footnotes
CONFLICT OF INTEREST
Konstantinos Arfanakis is an Editorial Board Memory of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review. All other authors report no conflicts of interest.
DATA AVAILABILITY
Information regarding obtaining data from these studies for research use can be found at the RADC Research Resource Sharing Hub (www.radc.rush.edu).
REFERENCES
- [1].Perales-Puchalt J, Gauthreaux K, Shaw A, McGee JL, Teylan MA, Chan KCG, Rascovsky K, Kukull WA, Vidoni ED (2021) Risk of mild cognitive impairment among older adults in the United States by ethnoracial group. Int Psychogeriatr 33, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Matthews KA, Wei X, Gagliotic AH, Holt JB, Croft JB, Mack D, McGuire LC (2019) Racial and ethnic estimates of Alzheimer’s disease related dementias in the United States (2015-2060) in adults aged ≥65 years. Alzheimers Dement, 15,17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Daviglus ML, Pirzada A, Talavera GA (2014) Cardiovascular Disease Risk Factors in the Hispanic/Latino Population: Lessons from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Prog in Cardiovascular Diseases, 57, 230–236. [DOI] [PubMed] [Google Scholar]
- [4].O’Bryant SE, Johnson LA, Barber RC, Braskie M, Christian B, Hall JR, Hazra N, King K, Kothapalli D, Large S, Mason D, Matsiyevskiy E, McColl R, Nandy R, Palmer R, Petersen M, Philips N, Rissman RA, Shi Y, Toga AW, Vintimilla R, Vig R, Zhang F, Yaffe K, HABLE Study Team (2021) The Health & Aging Brain among Latino Elders (HABLE) study methods and participant characteristics. Alzheimer’s Dement, 13, e12202. 10.1002/dad2.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schneiderman N, Llabre M, Cowie CC, Barnhart J, Carnethon M, Gallo LC, Giachello A, Heiss G, Kaplan RC, LaVange LM, Teng Y, Villa-Caballero L, Aviles-Santa ML (2014) Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study of Latinos (HCHS/SOL). Diabetes Care, 37, 2233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Raji MA, Reyes-Ortiz CA, Kuo Y-F, Markides KS, Ottenbacher KJ (2007) Depressive symptoms and cognitive change in older Mexican Americans. J Geriatr Psychiatry Neurol, 20, 145–52. [DOI] [PubMed] [Google Scholar]
- [7].Berry JW 2005) Acculturation: Living successfully in two cultures. Int J Intercultural Relations, 29, 697–712. [Google Scholar]
- [8].Abraido-Lanza AF, Echeverri SE, Florez KR (2016) Latino immigrants, acculturation, and health: Promising new directions in research. Annu Rev Public Health, 37, 219–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lamar M, Barnes LL, Leurgans S, Fleischman DA, Farfel JM, Bennett DA, Marquez DX (2021) Acculturation in Context: The relationship between acculturation and socioenvironmental factors with level of and change in cognition in older Latinos. J Gerontol B Psychol Sci Soc Sci 76, e129–e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lara M, Gamboa C, Kahramanian MI, Morales LS, Bautista DEH (2005) Acculturation and Latino health in the United States: A review of the literature and its sociopolitical context. Annu Rev Pub Health, 26, 367–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lopez-Class M, Castro FG, Ramirez AG (2011) Conceptions of acculturation: A review and statement of critical issues. Social Sci Med, 72, 1555–1562. [DOI] [PubMed] [Google Scholar]
- [12].Schwartz SJ, Unger JB Zamboanga BL, Szapocznik J (2010) Rethinking the concept of acculturation: Implications for theory and research. Am Psychologist, 65, 237–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Concha JB, Kelly K, Mezuk B (2021) Hispanic/Latino ethnic identity and diabetes: An examination of underlying acculturation processes and the Hispanic/Latino health advantage. Health, Education & Behavior,48, 285–294. [DOI] [PubMed] [Google Scholar]
- [14].Gallegos ML, Segrin C (2022) Family connections and the Latino health paradox: Exploring the mediating role of loneliness in the relationships between the Latina/o cultural value of familism and health. Health Commun, 37, 1204–1214. [DOI] [PubMed] [Google Scholar]
- [15].Alcaron RD, Parekh A, Wainberg ML, Duarte CS, Araya R, Oquendo MA (2016) Hispanic immigrants in the USA: social and mental health perspectives. Lancet Psychiatry, 3, 860–70. [DOI] [PubMed] [Google Scholar]
- [16].Torres L (2010) Predicting levels of Latino depression: acculturation, acculturative stress, and coping. Cultur Divers Ethnic Minor Psychol, 16, 256–63. [DOI] [PubMed] [Google Scholar]
- [17].Rodriguez M, Mendoza L, Rodriguez I, Rosselli M, Loewenstein D, Burke S, Orozco A, Duara R (2022) Cultural factors related to neuropsychological performance and brain atrophy among Hispanic older adults with amnestic Mild Cognitive Impairment (aMCI): A pilot study. Applied Neuropsychology:Adult, 29, 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lamar M, Estrella ML, Capuano AW, Leurgans SE, Fleischman DA, Barnes LL, Lange-Maia B, Bennett DA & Marquez DX (2023). A longitudinal study of acculturation in context and cardiovascular health and their effects on cognition among older Latinos. JAHA in press. doi: 10.1161/JAHA.122.027620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zahodne LB, Sharifian N, Kraal AZ, Morris EP, Sol K, Zaheed AB, Meister L, Mayeux R, Schupf N, Manly JJ, Brickman AM (2023) Longitudinal associations between racial discrimination and hippocampal and white matter hyperintensity volumes among older Black adults. Soc Sci Med, 316, 114789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fani N, Harnett NG, Bradley B,Mekawi Y, Powers A, Stevens JS, Ressler KJ, Carter SE (2021) Racial discrimination and white matter microstructure in trauma-exposed black women. Biol Psychiatr, doi.org/ 10.1016/jbiopsych.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Okeke O, Elbasheir A, Carter SE, Powers A, Mekawi Y, Gillespie CF, Schwartz AC, Bradley B, Fani N (2022) Indirect effects of racial discrimination on health outcomes through prefrontal cortical white matter integrity. Biol Psychiat: Cog Neurosci Neuroimage, doi.org/ 10.1016/j.bpsc.2022.05.004. [DOI] [PubMed] [Google Scholar]
- [22].Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, Reitz C, Small SA, Mayeux R, DeCarli C, Brown TR (2008) Brain morphology in older African Americans, Caribbean Hispanics, and Whites from Northern Manhattan. Arch Neurol, 65,1053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wright CB, DeRosa JT, Moon MP, Strobino K, DeCarli C, Cheung YK, Assuras S, Levin B, Stern Y, Sun X, Rundek T, Elkind MSV, Sacco RL (2021) Race/ethnic disparities in mild cognitive impairment and dementia:The Northern Manhattan Study. J Alzheimers Dis, 80, 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hall JR, Johnson LA, Zhang F, Petersen M, Toga AW, Shi Y, Mason D, Rissman RA, Yaffe K, O’Bryant SE, HABLE Study (2021) Using fractional anisotropy imaging to detect MCI and AD among Mexican Americans and non-Hispanic Whites: A HABLE study. Dement Geriatr Cogn Disord, 50, 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Filley CM (2020) Social cognition and white matter: Connectivity and cooperation. Cogn Behav Neurol, 33, 67–75. [DOI] [PubMed] [Google Scholar]
- [26].Quiroz YT, Solis M, Aranda MP, Arbaje AI, Arroyo-Miranda M, Cabrera LY, Carrasquillo MM, Corrada MM, Crivelli L, Diminich ED, Dorsman KA, Gonsales M, Gonzalez HM, Gonzalez-Seda AL, Grinber LT, Guerrero LR, Hill CV, Jimenez-Velazquez IZ, Guerra JJL, Lopera F, Maestre G, Medina LD, O’Bryant S, Penaloz C, Pinzon MM, Mavarez RVPy, Pluim CF, Raman R, Rascovsky K, Rentz DM, Reyes Y, Rosselli M, Tansey MG, Vila-Castelar C, Zuelsdorff M, Carrillo M, Sexton C (2022) Addressing disparities in dementia risk, early detection and care in Latino populations: Highlights from the second Latinos & Alzheimer’s Symposium. Alzheimer’s Dement, 18, 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH Jr (2018) Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Map, 31, 378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chang EH, Argyelan M, Aggarwal M, Chandon T-SS, Karlsgodt KH, Mori S, Malhotra AK (2017) The role of myelination in measures of white matter integrity: Combination of diffusion tensor imaging and two-photon microscopy of CLARITTY intact brains. Neuroimage, 147, 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Parekh MB, Gurjarpadhye AA, Manoukian MAC, Dubnika A, Rajadas J, Inayathullah M (2015) Recent developments in diffusion tensor imaging of brain. Radiol Open J, 1, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].MacLullich AMJ, Ferguson KJ, Reid LM, Deary IJ, Starr JM, Seckl JR, Bastin ME, Wardlaw JM. (2009) Higher systolic blood pressure is associated with increased water diffusivity in normal-appearing white matter. Stroke, 40, 3869–3871. [DOI] [PubMed] [Google Scholar]
- [31].Maillard P, Fletcher E, Harvey D, Carmichael O, Reed B, Mungas D, DeCarli C (2011) White matter hyperintensity penumbra. Stroke, 42, 1917–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vernooij MW, de Groot M, van der Lugt A, Ikram MS, Krestin GP, Hofman A, Niessen WJ, Breteler MMB (2008) White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. NeuroImage,43, 470–477. [DOI] [PubMed] [Google Scholar]
- [33].Marquez DX, Glover CM, Lamar M, Leurgans SE, Shah RC, Barnes LL, Aggarwal NT, Buchman AS, Bennett DA (2020) Representation of older Latinxs in cohort studies at the Rush Alzheimer’s Disease Center. Neuroepidemiology, 54, 404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA (2012) The Minority Aging Research Study: Ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res, 9, 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bennett DA, Buchman AS, Boyle P, Barnes LL, Wilson RS, Schneider JA (2018) Religious Orders Study and Rush Memory and Aging Project J Alzheimers Dis, 64, S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34, 939–44. [DOI] [PubMed] [Google Scholar]
- [37].Abraido-Lanza AF, Armbrister AN, Florez KR, Aguirre AN (2006) Toward a theory-driven model of acculturation in public health research. Am J Public Health, 96, 1342–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hill CV, Perez-Stable EJ, Anderson NA, Bernard MA (2015) The National Institute on Aging Health Disparities Research Framework. Ethn Dis, 25, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marín G. (1987). Development of a short acculturation scale for Hispanics. Hispanic J Beh Sci, 9, 183–205. [Google Scholar]
- [40].Williams DR, Yu Y, Jackson JS, Anderson NB (1997) Racial differences in physical and mental health: Socioeconomic status, stress, and discrimination. J Health Psychol, 2, 335–351. [DOI] [PubMed] [Google Scholar]
- [41].Wilson RS, Krueger RR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, Tang Y, Bennett DA (2007) Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry, 64,234–240. [DOI] [PubMed] [Google Scholar]
- [42].Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS (2006) The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol, 5, 406–412. [DOI] [PubMed] [Google Scholar]
- [43].Sabogal F, Marin G, Otero-Sabogal R (1987) Hispanic familism and acculturation: What changes and what doesn’t? Hisp J of Beh Sci, 9, 397–412. [Google Scholar]
- [44].Alexander AL, Lee JE, Lazar M, Field AS (2007) Diffusion tensor imaging of the brain. Neurotherapeutics, 4, 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson Imaging, 111, 209–219. [DOI] [PubMed] [Google Scholar]
- [46].LeBihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging, 13, 534–546. [DOI] [PubMed] [Google Scholar]
- [47].Pierpaoli C, Walker L, Irfanoglu MO, Barnett A, Batter PJ (2020) TORTOISE: an integrated software package for processing of diffusion MRI data. In: Proceedings of the 18th Annual Meeting ISMRM, Stockholm, Sweden. [Google Scholar]
- [48].Li H, Jiang G, Zhang J, Wang R, Wang Z, Zheng W-S, Menze B (2018) Fully convolutional network ensembles for white matter hyperintensities segmentation in MR images. Neuroimage, 183, 650–665. [DOI] [PubMed] [Google Scholar]
- [49].SAS Institute Inc (2013) SAS/ACCESS® 9.4 Interface to ADABAS: Reference. Cary, NC: SAS Institute Inc. [Google Scholar]
- [50].Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage,31, 1487–1505. [DOI] [PubMed] [Google Scholar]
- [51].Zhang S, Arfanakis K (2018) Evaluation of standardized and study-specific diffusion tensor imaging templates of the adult human brain: Template characteristics, spatial normalization accuracy, and detection of small inter-group FA differences. Neuroimage, 172, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Winkler AM, Webster MA, Vidaurre D, Nichols TE, Smith SM (2015) Multi-level block permutation. Neuroimage, 123, 253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Winkler AM, Ridgway GR, Douaud G, Nichols TE, Smith SM (2016) Faster permutation inference in brain imaging. Neuroimage, 141, 502–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localization in cluster inference. Neuroimage, 44, 83–98. [DOI] [PubMed] [Google Scholar]
- [55].Lam JA, Murray ER, Yu KE, Ramsey M, Nguyen TT, Mishra J, Martis B, Thomas ML, Lee EE (2021) Neurobiology of loneliness: a systematic review. Neuropsychopharmacology, 46, 1873–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Molesworth T, Sheu LK, Cohen S, Gianaros PJ, Verstynen TD (2015) Social network diversity and white matter microstructural integrity in humans. Scan, 10, 1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wassenaar TM, Yaffe K, van der Werf YD, Sexton CE (2019) Associations between modifiable risk factors and white matter of the aging brain: insights from diffusion tensor imaging studies. Neurobiol Ag, 80, 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Porcelli S, Van Der Wee N, Van Der Werff S, Aghajani M Glennon JC, van Heukelum S, Mogavero F, Lobo A, Oliver FJ, Lobo E, Posadas M, Dukart J, Kozak R, Arce E, Ikram A, Vorstman J, Bilderbeck A, Saris I, Kas MJ, Serretti A (2019) Social brain, social dysfunction, and social withdrawal. J Neurosci Biobehav Rev, 97, 10–33. [DOI] [PubMed] [Google Scholar]
- [59].Wang Y, Olson IR (2018) The original social network: White matter and social cognition. Trends Cogn Sci, 22, 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bickart KC, Dickerson BC, Barrett LF (2014b) The amygdala as a hub in brain networks that support social life. Neuropsychologia, 63, 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cacioppo JT, Cacioppo ST, Dulawa W, Palmer AA (2014) Social neuroscience and its potential contribution to psychiatry. World Psychiatry, 13, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kennedy DP, Adolphs R (2012) The social brain in psychiatric and neurological disorders. Trends Cogn Sci, 16, 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Alaca-Lopez D, Smallwood J, Jeffries E, Van Overwalle F, Vogeley K, Mars RB, Turetsky BI, Laird AR, Fox PT, Eickhoff SB, Bzdok D (2018) Computing the social brain connectome across systems and states. Cerebral Cortex, 28, 2207–2232. [DOI] [PubMed] [Google Scholar]
- [64].Eisenberger NI, Cole SW (2012) Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci, 15, 669–674. [DOI] [PubMed] [Google Scholar]
- [65].Alshammari TK, Alghamdi H, Alkhader LF (2020) Analysis of the molecular and behavioral effects of acute social isolation on rats. Beh Brain Res, 377,11219. doi: 10.1016/j.bbr.2019.112191. [DOI] [PubMed] [Google Scholar]
- [66].Bosch JA, de Geus EJC, Carroll D, Goedhard AD, Anane LA, Veldhuizen van Zanten, Helmerhorst EJ, Edwards KM (2009) A general enhancement of autonomic and cortisol responses during social evaluative threat. Psychosom Med, 71, 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cole SW, Hawkley LC, Arevalo JMSung CY, Rose RM, Cacioppo JT (2007) Social regulation of gene expression in human leukocytes. Genome Biol, 8, R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Friedler B, Crapser J, McCullough L (2015) One is the deadliest number: The detrimental effects of social isolation on cerebrovascular diseases and cognition. Acta Neuropathol, 129, 493–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Foti SA, Khambaty T, Birnbaum-Weitzman O, Arguelles W, Penedo F, Giacinto RAE, Gutierrez AP, Gallo LC, Giachello AL, Schneiderman N, Llabre MM (2020) Loneliness, cardiovascular disease, and diabetes prevalence in the Hispanic Community Health Study/Study of Latinos Sociocultural Ancillary Study. J Immigrant Minority Health, 22, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Cox SR, Lyall DM, Ritchie SJ, Bastin ME, Harris MA, Buchannan CR, Fawns-Ritchie C, Barbu MC, de Nooij L, Reus LM, Alloza C, Shen X, Neilson E, Alderson HL, Hunter S, Liewald DC, Whallley HC, McIntosh AM, Lawrie SM, Pell JP, Tucker-Drob EM, Wardlaw JM, Gale CR, Deary IJ (2019) Associations between vascular risk factors and brain MRI indices in UK Biobank. Eur Heart J, 40, 2290–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Porter A, Leckie R, Verstynen T (2018) White matter pathways as both a target and mediator of health behaviors. Ann NY Acad Sci, 1428, 71–88. [DOI] [PubMed] [Google Scholar]
- [72].Gasecka A, Siwik D, Gajewska M, Jaguszewski MJ, Mazurek T, Filipiak KJ, Postula M, Eyileten C (2020) Early biomarkers of neurodegenerative and neurovascular disorders in diabetes. J Clin Med, 9, 2807. doi: 10.3390/jcm9092807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Leszek J, Mikhaylenko EV, Belousov DM, Koutsouraki E, Szczechowiak K, Kobusiak-Prokopowicz M, Mysiak A, Diniz BS, Somasundaram SG, Kirkland CE, Aliev G (2021) The links between cardiovascular diseases and Alzheimer’s Disease. Curr Neuropharmacology, 19, 152–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Colmenares AM, Voss MW, Fanning J, Salerno EA, Gothe NP, Thomas ML, McAuley E, Kramer AF, Burzynska AZ (2021) White matter plasticity in healthy older adults: The effects of aerobic exercise. Neuroimage, 239, 927–937. [DOI] [PubMed] [Google Scholar]
- [75].Huber E, Donnelly PM, Rokem A, Yeatman JD (2018) Rapid and widespread white matter plasticity during an intensive reading intervention. Nat Commun, 9, 2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Schlegel AA, Rudelson JJ, Tse PU (2012) White matter structure changes as adults learn a second language. J Cogn Neurosci, 24, 1664–1670. [DOI] [PubMed] [Google Scholar]
- [77].Scholz J, Klein MC, Behrens, Johansen-Berg H (2009) Training induces changes in white-matter architecture. Nat Neurosci, 12, 1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yotumoto Y, Chang L-H, Ni R, Pierce R, Andersen GJ, Watanabe T, Sasaki Y (2014) White matter in the older brain is more plastic than in the younger brain. Nature Communications, 5, 5504. doi: 10.1038/ncomms6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Liu J, Dietz K, Deloyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nester EJ, Dupree J, Casaccia P (2012) Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci, 15, 1621–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Cacioppo S, Grippo AJ, London S, Goossens L, Cacioppo JT (2015) Loneliness: Clinical import and interventions. Perspect Psychol Sci, 10, 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Information regarding obtaining data from these studies for research use can be found at the RADC Research Resource Sharing Hub (www.radc.rush.edu).