Abstract
Respiratory syncytial virus (RSV) can lead to serious disease in infants, and no approved RSV vaccine is available for infants. This first in-human clinical trial evaluated a single dose of BLB201, a PIV5-vectored RSV vaccine administrated via intranasal route, for safety and immunogenicity in RSV-seropositive healthy adults (33 to 75 years old). No severe adverse events (SAEs) were reported. Solicited local and systemic AEs were reported by <50% of participants and were mostly mild in intensity. Vaccine virus shedding was detected in 17% of participants. Nasal RSV-specific immunoglobulin A responses were detected in 48%, the highest level observed in adults among all intranasal RSV vaccines evaluated in humans. RSV-neutralizing antibodies titers in serum rose ≥1.5-fold. Peripheral blood RSV F–specific CD4+ and CD8+ T cells increased from ≤0.06% at baseline to ≥0.26 and 0.4% after vaccination, respectively, in >93% participants. The safety and immunogenicity profile of BLB201 in RSV-seropositive adults supports the further clinical development of BLB201.
Intranasal RSV vaccine based on kennel cough vaccine component PIV5 is safe and immunogenic in humans.
INTRODUCTION
Respiratory syncytial virus (RSV) is the leading global cause of morbidity and mortality in infants due to infection-associated bronchiolitis and pneumonia, especially in those with underlying cardiopulmonary disease (1–3). The global yearly incidence of RSV-associated acute respiratory tract infections is estimated at 33 million children aged <5 years, leading to 3 million hospitalizations and up to 60 to 200 thousand deaths (4). RSV also causes substantial disease burden in the elderly (5, 6). In the United States, RSV is responsible for approximately 60 to 160 thousand hospitalizations and 6 to 13 thousand deaths annually in adults ≥65 years of age (7, 8). The high burden of disease caused by RSV and its economic costs makes RSV a high priority target for vaccine development.
RSV causes both upper and lower respiratory diseases. Recently, RSV prefusion F (preF) protein–based vaccines have been shown to be effective in reducing symptoms of RSV-associated lower respiratory tract disease in elderly (aged ≥60 years) in clinical trials and received approval for elderly (9–12). Still, there is no RSV vaccine that prevents upper and/or lower respiratory tract infection/diseases. Furthermore, the clinical development of RSV vaccines for infants and young children has not led to a vaccine product yet. Because of concerns for enhanced RSV diseases in children with inactivated RSV vaccine, those development programs have primarily used intranasally delivered live-attenuated RSV vaccines and viral-vectored vaccines (13). While immune correlates of protection for RSV infection are not clear, nasal antibody (Ab), especially nasal RSV-specific immunoglobulin A (IgA), is thought to be critical for protection against RSV infection (14), leading to the focus on intranasal approaches for RSV vaccine development, especially for infants and children. In clinical evaluations in phase 1 and 2 trials in infants who were RSV naïve (RSV seronegative), some of the attenuated RSV vaccines have been shown to promote RSV-specific Ab and cell-mediated immunity (CMI) (13, 15–17). Although the safety profiles of these vaccines have been generally acceptable, the challenge with the live-attenuated RSV vaccines has been to identify a favorable balance between immunogenicity and safety (13, 15). Additional hurdle for attenuated RSV vaccine includes maternal anti-RSV Ab and long-acting RSV-neutralizing antibodies (nAbs) that have been approved for use in infants. A bovine parainfluenza virus 3 (bPIV3)–vectored RSV vaccine was evaluated in adults and infants (18, 19) but was abandoned because of the genetic instability of the vaccine. An intranasal chimpanzee adenovirus (ChAd)–vectored vaccine, PanAd3-RSV, has also been evaluated in adults as part of a prime-boost regimen in combination with intramuscular vaccination (20, 21). A single dose of intranasal PanAd3-RSV immunization was well tolerated in humans but did not generate detectable immune responses to RSV in adults.
BLB201 is a live viral-vectored candidate RSV vaccine based on a parainfluenza virus 5 (PIV5) encoding the RSV F antigen. PIV5 is a negative-sense RNA virus and replicates in the cytoplasm of infected cells without a DNA phase in its life cycle. PIV5 is a component of the kennel cough vaccine commonly administered by intranasal route in dogs. Vaccinated dogs can shed virus up to 5 days after dosing, exposing humans to PIV5. However, although the vaccine has been used for 50 years, no known human illness has been reported from human exposure to vaccinated dogs (22). PIV5 infects many cell types using sialic acid receptors with minimal cytopathic effect, allowing it to infect mucosal cells in vivo. Intranasal PIV5-vectored vaccines have been effective against respiratory pathogens including influenza viruses, Middle East respiratory syndrome coronavirus, and severe acute respiratory syndrome coronavirus 2 in animal models via intranasal immunization (23–29). In preclinical studies, a single intranasal dose of PIV5-vectored RSV vaccine induced RSV F–specific Ab and CMI in mice, cotton rats, and African green monkeys and protected against RSV challenge (30–32). Here, we report the first in-human clinical study of PIV5-vectored RSV vaccine BLB201 in RSV-seropositive healthy adults in the United States.
RESULTS
Participants and study conduct
A total of 30 subjects were enrolled in this phase 1 trial, and all received a single intranasal dose of BLB201 at 107.5 plaque forming units (PFU). The vaccine was administrated as a 0.25-ml spray to each nostril (total volume of 0.5 ml) using a MAD Nasal Intranasal Mucosal Atomization Device (Teleflex MAD300). The mean ages in group 1 (planned participant ages 18 to 59 years and actual enrollee ages 33 to 59 years) and group 2 (planned participant ages 60 to 75 years and actual enrollee ages 61 to 75 years) were 45 and 67 years, respectively (Table 1). The majority of participants were female (70%). Most participants in the two groups were white (73 and 80% in groups 1 and 2, respectively). All except one participant completed the study, and this participant in group 1 was lost to follow-up after day 7.
Table 1. Trial cohort demographics.
| Group | Group 1 | Group 2 |
|---|---|---|
| N | 15* | 15 |
| Mean age (range) | 44.9 (33, 59) | 66.8 (61, 75) |
| Male/female | 4/11 | 5/10 |
| Race/ethnicity | 11 white (73%), others-Black/African American and American Indian | 12 white (80%), others-Black/African American and Asian |
*One participant dropped out of the study on day 8 (lost to follow-up).
Safety
After intranasal immunization with Teleflex MAD300, the safety and tolerability of BLB201 were monitored for 6 months. Participants recorded information of local and systemic adverse events (AEs) for 7 days after vaccination (solicited local and systematic events) and unsolicited AEs for 28 days using a memory aid. No serious AE (SAE) assessed as related to vaccine was reported, and no symptomatic RSV infection was reported during the 6-month trial period. In the 1-week period after vaccination, all solicited local AEs that were reported were mild in intensity (grade 1; Table 2). Runny nose was the most common reaction (in 7 of 15 participants in each age group). Sore throat and cough were reported by 4 of 15 and 3 of 15 participants, respectively, in group 1 and by 1 of 15 participants for each symptom in group 2. Within 1 hour after vaccination, post-nasal drip was reported by four participants and difficulty in concentration by one participant.
Table 2. Summary of local and systemic reactogenicity and AEs.
Grading scale: Grade 1 = mild (awareness of a symptom but the symptom is easily tolerated); grade 2 = moderate (discomfort enough to cause interference with usual activity); grade 3 = severe (incapacitating; unable to perform usual activities; requires absenteeism from work or bed rest). AE, adverse event.
| Group | Group 1 | Group 2 | |
|---|---|---|---|
| Solicited local AE (days 1–8): Number (and percentage) of subjects reporting grade 1 events (no grade 2 or grade 3 events reported) | |||
| Grade 1 | Grade 1 | ||
| Runny nose/nasal congestion | 7 (47%) | 7 (47%) | |
| Sore throat | 4 (27%) | 1 (7%) | |
| Cough | 3 (20%) | 1 (7%) | |
| Solicited systematic AE (days 1–8): Proportion of subjects reporting grades 1 and 2 (no grade 3 events reported). Only grade 1 AE reported in group 1. | |||
| Grade 1 | All Grades | Grade 2 | |
| Fever | 0 | 0 | 0 |
| Headache | 2 (13%) | 5 (33%) | 3 (20%) |
| Myalgia | 1 (7%) | 5 (33%) | 2 (13%) |
| Fatigue | 5 (33%) | 2 (13%) | 1 (7%) |
| Chills | 0 | 1 (7%) | 0 |
| Nausea/Vomiting | 0 | 1 (7%) | 0 |
| Breathing discomfort | 0 | 1 (7%) | 0 |
| Unsolicited AE (assessed as possibly related to vaccine): Only grade 1 AEs reported. | |||
| Difficult concentration for 1 hour after dosing | 1 (7%) | 0 | |
| Post-nasal drip after dosing | 4 (27%) | 0 | |
| Brain fog | 0 | 1 (7%) | |
In group 1, all solicited systemic AEs reported by 5 of 15 participants were mild in intensity and included fatigue, headache, and myalgia being reported by 5 of 15, 2 of 15, and 1 of 15 participants, respectively. In group 2, the solicited systemic AEs reported by 8 of 15 participants were mild or moderate in intensity and included fatigue, headache, and myalgia being reported by 2 of 15, 5 of 15, and 5 of 15 participants, respectively, as well as chills, nausea/vomiting, and breathing discomfort being reported by 1 of 15 for each symptom. The single episode of breathing discomfort was mild and resolved within 2 days after vaccination. No solicited fever was reported in either group. Throughout the 6-month study period, five medically attended AEs were reported by four participants (all in group 2), none of which was assessed as being related to study vaccine.
Vaccine virus shedding and genetic stability
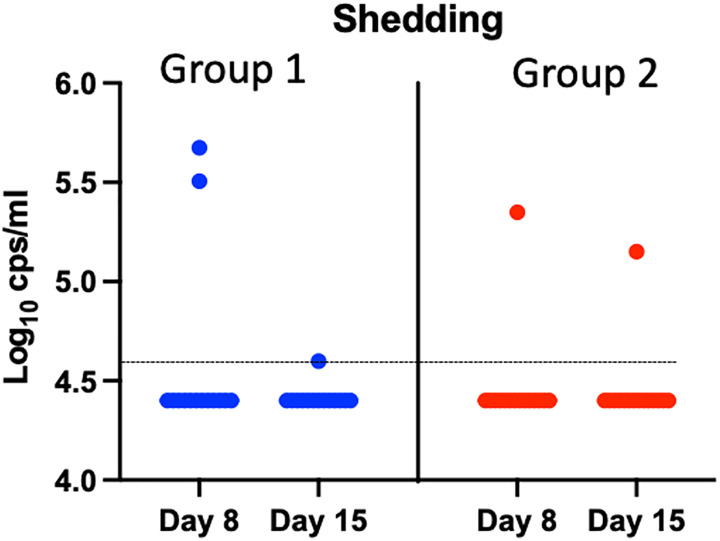
At day 1 before participants were immunized, no vaccine virus genomic RNA was detected as expected [i.e., below the lower limit of detection (LOD) by reverse transcription quantitative polymerase chain reaction (RT-qPCR)] in the nasal swabs of any participant (Fig. 1). At either 1 or 2 weeks after vaccination, the vaccine virus genomic RNA was detected in the nasal swabs of five participants: three participants in group 1 and two participants in group 2. For two participants in which vaccine virus genomic RNA was detected at 2 weeks after vaccination, the vaccine vector was no longer detected at 4 or 8 weeks after vaccination. The sequences of the BLB201 were successfully obtained in four of five positive samples, and they matched the original sequence for the inserted RSV F gene and the flanking regions. This suggested that BLB201 remained genetically stable after vaccination, and shedding of the vaccine virus lasted <4 weeks after vaccination.
Fig. 1. Vaccine-virus shedding. PIV5 viral genomic RNA detection by RTqPCR in individual BLB201 recipients at days 8 and 15.
The dotted line indicates the LOD at 4.6 log10 copies/ml.
Immunogenicity
Serum Ab titers
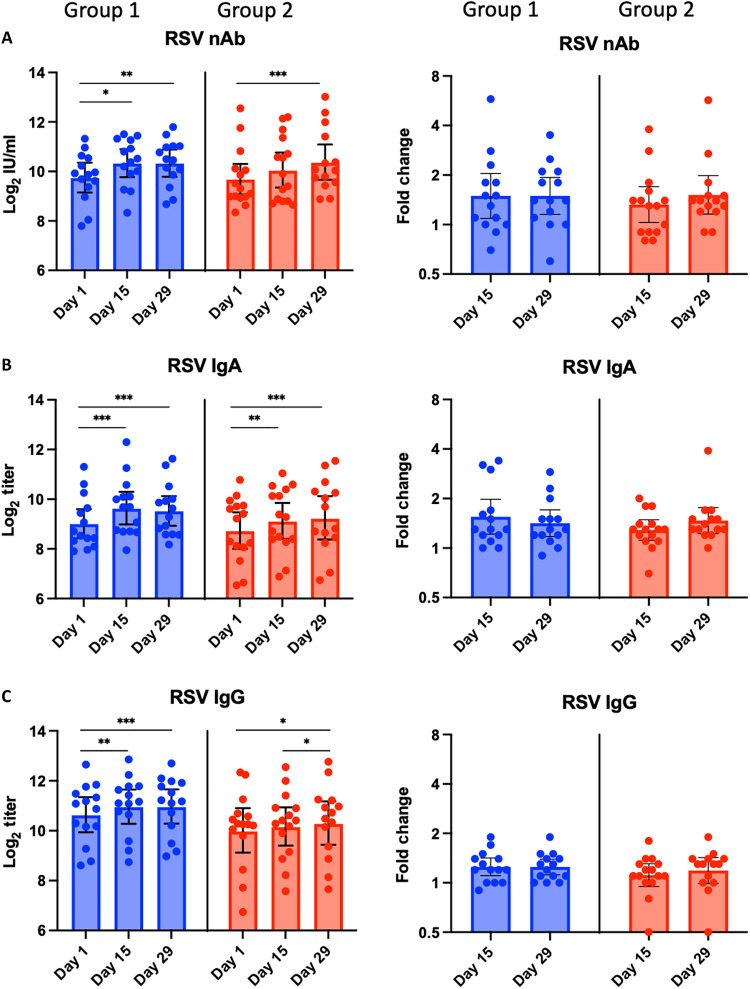
All participants were seropositive for RSV nAbs at baseline, and the titer changes are presented in Fig. 2 and fig. S1A. In group 1, the nAb geometric mean titer (GMT) was 880 (9.8 log2) at baseline and increased to 1316 (10.4 log2) at 2 weeks and 1312 (10.4 log2) at 4 weeks after vaccination (P < 0.05), reflecting a geometric mean fold rise (GMFR) of 1.5. RSV nAb seroresponses (≥1.5-fold rise) were identified in 7 of 14 (50%) participants. In group 2, the nAb GMT was 850 (9.7 log2) at baseline, similar to that in group 1, and increased to 1103 (10.1 log2) at 2 weeks (P < 0.05) and 1372 (10.4 log2) at 4 weeks after vaccination (P < 0.05), reflecting GMFRs of 1.3 and 1.5, respectively. RSV nAb seroresponses were identified in 6 of 15 (40%) participants in this group (Table 3) (individual nAb titer changes were shown in fig. S1A). Notably, RSV nAb seroresponses were identified in all five participants positive for vaccine virus shedding, suggesting a potential correlation between replication and systemic Ab responses.
Fig. 2. Serum RSV-specific Ab titers before and after vaccination.
(A) RSV nAbs by microneutralization assay and (B) F-specific RSV IgA Abs and (C) F-specific IgG Abs by enzyme-linked immunosorbent assay (ELISA). Individual log2 titers and mean log2 titers with the respective SDs (error bars) at days 1, 15, and 29 by age group are shown in the graphs in the left column, and GMFRs from baseline on days 15 and 29 by age group are shown in the graphs in the right column. *P < 0.05, **P < 0.01, and ***P < 0.001 by Wilcoxon test.
Table 3. Summary of RSV Ab and CMI response rates.
A positive seroresponse was defined as ≥1.5-fold rise from baseline for RSV F IgG and IgG. A positive nasal IgA Ab response was defined as ≥2-fold rise over baseline. CMI response was defined as >0.1% rise of sum of individual T cell secreting >1 cytokine after subtraction of baseline (before vaccination). CTL response was defined by the change in IFN-γ–producing CD8+ cells. CMI, cell-mediated immunity; CTL, cytotoxic T lymphocyte; PBMC, peripheral blood mononuclear cell; PIV5, parainfluenza virus type 5; RSV, respiratory syncytial virus.
| Sample | Immune parameter | Group 1 responders | Group 2 responders |
|---|---|---|---|
| n/N (% responder) | n/N (% responder) | ||
| Serum | RSV nAb | 7/14 (50%) | 6/15 (40%) |
| RSV F IgA | 6/14 (43%) | 5/15 (36%) | |
| RSV F IgG | 3/14 (21%) | 1/15 (7%) | |
| PIV5 nAb | 7/14 (50%) | 10/15 (67%) | |
| PIV5 IgG | 12/14 (86%) | 13/15 (87%) | |
| Nasal swab | Nasal RSV F IgA | 9/14 (64%) | 5/15 (33%) |
| PBMC | Total CMI | 13/14 (93%) | 15/15 (100%) |
| CD8 CTL | 12/14 (86%) | 13/15 (87%) |
All participants were also seropositive for RSV F–specific serum IgA and IgG Abs at baseline (Fig. 2). In froup 1, F-specific serum IgA GMT increased from 530 (9.0 log2) at baseline to 821 (9.7 log2) and 756 (9.6 log2) at 2 and 4 weeks after vaccination, respectively. F-specific serum IgG GMT increased from 1640 (10.7 log2) at baseline to 2049 (11.0 log2) at 2 weeks and 2055 (11.0 log2) at 4 weeks after vaccination. F-specific serum IgA and IgG seroresponses (≥1.5-fold) were identified in 6 of 14 (43%) and 3 of 14 (21%) participants, respectively (Table 3). In group 2, F-specific serum IgA GMT increased from 445 (8.8 log2) at baseline to 582 (9.2 log2) and 641 (9.3 log2) at 2 and 4 weeks after vaccination, respectively. F-specific serum IgG GMT increased from 1088 (10.1 log2) at baseline to 1198 (10.2 log2) and 1325 (10.4 log2) at 2 and 4 weeks after vaccination. F-specific IgA and IgG seroresponses were identified in 5 of 15 (33%) and 1 of 15 (7%) participants, respectively (Table 3).
Overall, the results suggested that BLB201 boosted RSV-specific serum Ab levels in young adults (33 to 59 years old) and elderly (61 to 75 years old), although with greater magnitude in adults versus elderly. The fold increases in RSV-specific Ab titers inversely correlated with baseline titers. It is possible that the higher baseline titers were close to maximum levels.
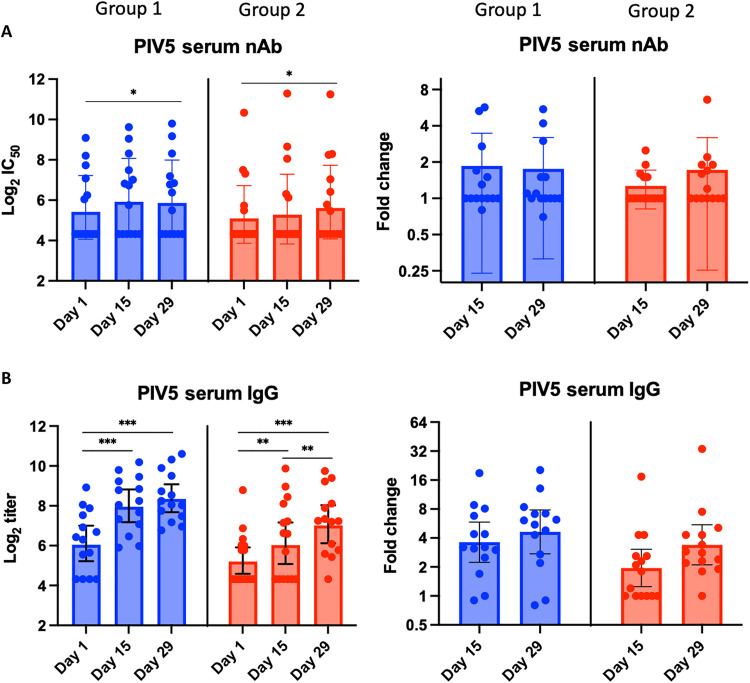
We next examined vector-specific immune responses. For PIV5 nAbs at baseline, 15 of 29 (52%) participants were seropositive as defined by a titer of 1:10 (Fig. 3A). This level of background seropositivity likely reflects passive exposure to PIV5 through exposure to dogs receiving the kennel cough vaccine. In group 1, the PIV5 nAb GMT increased from 26 (4.7 log2) at baseline to 48 (5.6 log2) and 51 (5.7 log2) at 2 and 4 weeks after vaccination, respectively, reflecting a GMFRs of 1.8 and 2.0, respectively. PIV5 nAb seroresponses (≥1.5-fold rise) were identified in 7 of 14 (50%) participants (Table 3). In group 2, the PIV5 nAb GMT increased from 19 (4.2 log2) at baseline to 34 (5.1 log2) and 44 (5.5 log2) at 2 and 4 weeks after vaccination, respectively, reflecting a GMFRs of 1.8 and 2.2, respectively. PIV5 nAb seroresponses (≥1.5-fold rise) were identified in 10 of 15 (67%) participants. Similar kinetics were observed with PIV5-specific IgG titers (Fig. 3B) but with greater GMFRs (≤4.6-fold in group 1 and ≤3.4-fold in group 2) and seroresponses (86% in group 1 and 87% in group 2). By comparing participants with low PIV5 nAb titers with those with high PIV5 nAb titers, baseline PIV5 nAb titers had no clear effect on PIV5 nAb seroresponse rate and appeared to not inhibit RSV nAb seroresponse rate (fig. S2).
Fig. 3. Serum PIV5 Ab titers before and after vaccination.
(A) PIV5 nAbs by microneutralization assay and (B) PIV5-specific RSV IgG Abs by ELISA. Individual log2 titers, and mean log2 titers with the respective SDs (error bars) at days 1, 15, and 29 by age group are shown in the graphs in the left column, and GMFRs from baseline on days 15 and 29 by age group are shown in the graphs in the right column. *P < 0.05, **P < 0.01, and ***P < 0.001 by Wilcoxon test.
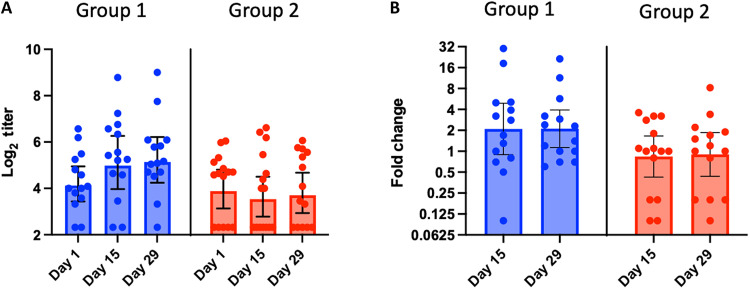
RSV F–specific IgA Ab titers in nasal swabs
At baseline, RSV F–specific IgA Ab titers in nasal swabs were variable ranging from below the LOD to 514 (9.0 log2) (Fig. 4A). In group 1, the F-specific nasal IgA GMT was 19 (4.2 log2) at baseline and increased to 40 (5.3 log2) at 2 and 4 weeks after vaccination (P < 0.05), reflecting a GMFR of 2.1 (Fig. 4B). F-specific nasal IgA responses (≥2-fold rise) were identified in 9 of 14 (64%) participants (Table 3). In group 2, the baseline F-specific nasal IgA GMT of 18 (4.2 log2) was similar to that in group 1, but GMTs were not boosted (P > 0.05) 2 and 4 weeks after vaccination (Fig. 4, A and B). However, F-specific nasal IgA responses were identified in 5 of 15 (33%) participants in group 2 (individual’s nasal IgA Ab changes were shown in fig. S1B). In groups 1 and 2, F-specific nasal IgA responses were identified in four of five participants positive for vaccine virus shedding.
Fig. 4. Nasal IgA Ab titers before and after vaccination.
F-specific RSV IgA Abs by ELISA. Individual log2 titers, and mean log2 titers with the respective SDs (error bars) at days 1, 15, and 29 by age group are shown in the graphs in (A), and GMFRs from baseline on days 15 and 29 by age group are shown in the graphs in (B).
Overall, the results suggested that BLB201 boosted RSV-specific nasal IgA levels in adults and elderly but to a lesser degree in elderly. Similar to results seen with systemic Ab responses, the fold increases in F-specific nasal IgA titers in both groups inversely correlated with baseline titers (fig. S2).
Cell mediated immunity
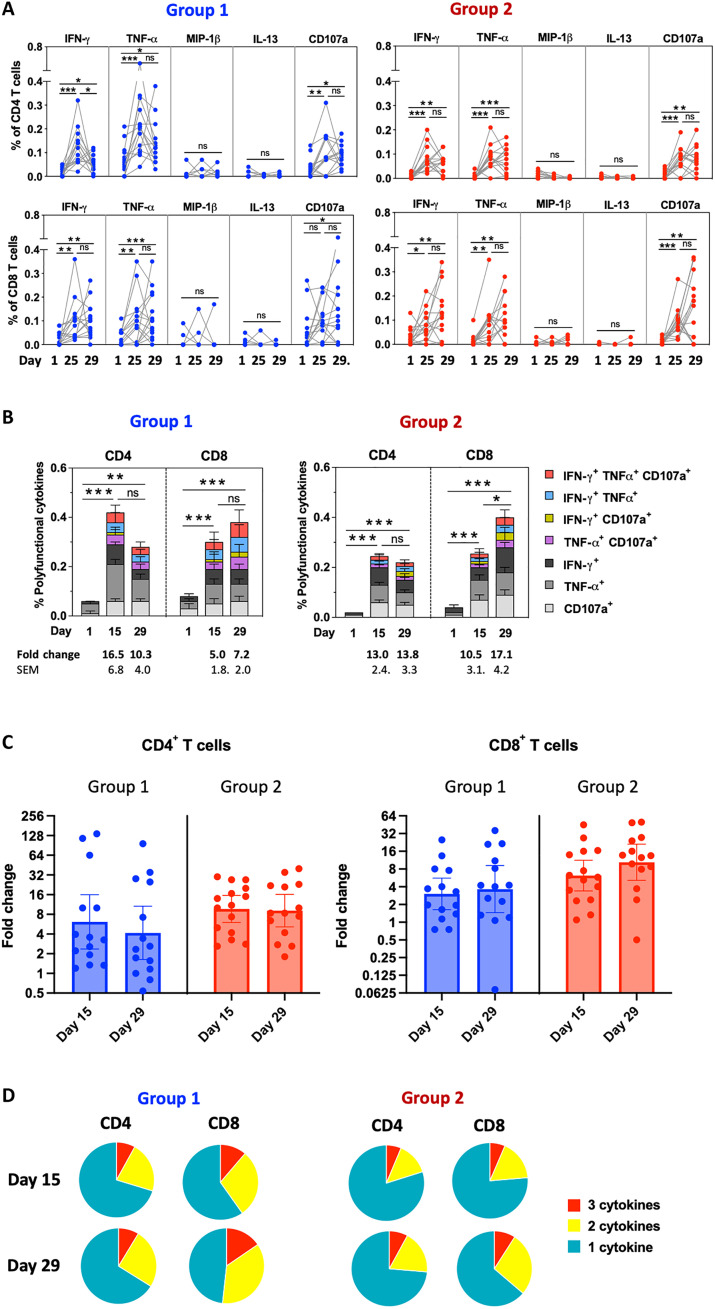
At baseline, RSV F–specific CD4+ and CD8+ T cells were detected on the basis of the expression of T helper 1 (TH1) or cytotoxic T cell cytokines/markers interferon-γ (IFN-γ), tumor necrosis factor–α (TNF-α), macrophage inflammatory protein–1β (MIP-1β), and CD107a and on the expression of TH2 cytokine interleukin-13 (IL-13) (Fig. 5A). In group 1, the mean percentages of F-specific CD4+ T cells expressing at least one of the TH1/cytotoxic markers increased from 0.06% at baseline to a maximum of 0.42% 2 weeks after vaccination (P < 0.001; Fig. 5B), and the mean percentages of the same phenotypes of CD8+ T cells increased from 0.08% at baseline to a maximum of 0.38% 4 weeks after vaccination (P < 0.001). In group 2, the mean percentages of the same phenotypes of CD4+ T cells increased from 0.02 to a maximum of 0.26% 2 weeks after vaccination (P < 0.001), and CD8+ T cells increased from 0.04 to a maximum of 0.40% 4 weeks after vaccination (P < 0.001). In groups 1 and 2, F-specific CMI responses to BLB201 (defined as an increase from baseline of >0.1% of T cells expressing at least two TH1/cytotoxic biomarkers) were identified in 13 of 14 (93%) and 15 of 15 (100%) participants, respectively (Table 3). Also, F-specific cytotoxic T lymphocyte (CTL) responses to BLB201 (defined as an increase from baseline of >0.1% of CD8+ T cells expressing IFN-γ) were identified in 12 of 14 (86%) and 13 of 15 (87%) participants, respectively. This suggested that BLB201 enhanced the production of F-specific TH1 (CD4+) and cytotoxic (CD8+) T cells in nearly all participants. Although numbers of CD4+ and CD8+ T cells were similar after immunization in both groups, the geometric mean of CD4+ T cells rose to 6.1- and 9.1-fold by day 15 for groups 1 and 2, respectively, and the geometric mean of CD8+ T cells rose to 3.6- and 10-fold by day 29 for groups 1 and 2, respectively (Fig. 5C), reflecting lower baseline for group 2 than for group 1. This result suggests that, in contrast to nasal IgA response, similar levels of CMI in adults and elderly was induced by BLB201.
Fig. 5. RSV F–specific CD4+ and CD8+ T cell frequencies per million PBMCs (by percentage) before and after vaccination.
(A) Percentages of CD4+ T cells (top graphs) and CD8+ T cells (bottom graphs) by age group on days 1, 15, and 29 for each individual (represented by symbols and contiguous line) by expression of the indicated immune marker. (B) Mean percentages of CD4+ T cells (left graphs) and CD8+ T cells (right graphs) by age group on days 1, 15, and 29 by expression of the indicated TH1/cytotoxic marker combinations (IFN-γ, TNF-α, and CD107a) represented in stacked histograms. (C) GMFRs from baseline on days 15 and 29 by age group for CD4+ and CD8+ T cells. (D) Pie chart representations of the proportions of F-specific CD4+ and CD8+ T cells expressing one, two, or three TH1/cytotoxic markers (IFN-γ, TNF-α, and CD107a) at days 15 and 29 in groups 1 and 2. *P < 0.05, **P < 0.01, and ***P < 0.001. ns, not significant.
In both groups 1 and 2, T cells expressing a single TH1/cytotoxic biomarker were more frequent than those expressing at least two TH1/cytotoxic markers (Fig. 5D). However, T cells coexpressing at least two TH1/cytotoxic biomarkers tended to be more frequent in group 1 versus group 2, suggesting that BLB201 may induce better quality of antigen-specific effector/memory T cells in the younger age group based on polyfunctional TH1/cytotoxic responses. In contrast to TH1 and cytotoxic phenotypes, the percentages of F-specific CD4+ T cells and CD8+ T cells expressing IL-13 did not increase after vaccination in either group 1 or group 2 (Fig. 5A), indicating that there was no TH2-biased response to BLB201, which has been identified as a potential risk factor for vaccine-associated enhanced RSV disease (33, 34).
DISCUSSION
This clinical study of the PIV5-vectored RSV vaccine candidate BLB201 was the first in-human study for this vaccine and for the replicating PIV5 vector. The study showed that BLB201 administered as a single intranasal dose had an acceptable safety profile. Solicited local and systemic AEs were generally mild and only reported by approximately half of the vaccine recipients. No SAE related to vaccination was reported. This result is consistent with the long history of PIV5 vector’s use in dogs. The vector has been used in dogs for 50 years, and yet no human disease has ever been reported from human exposure to PIV5-immunized animals. In this phase 1 study, approximately half of the enrolled participants were PIV5 seropositive based on nAb titer >1:10 at baseline, suggesting that asymptomatic exposures occur in a high percentage of the U.S. population. The safety profile of BLB201 is comparable to other intranasal live virus–based vaccines including live-attenuated RSV vaccines, MEDI-534, and PanAd3-RSV in adults (18, 20, 21). The acceptable safety profile of PIV5 vector in this first in-human trial will enable the development of other vaccines for humans using PIV5 vector platform.
Although RSV preF protein–based vaccines have been approved for reducing symptoms in lower respiratory tract of RSV-infected elderly, an RSV vaccine that prevents symptomatic infection in lower respiratory tract and even symptomatic infection in upper respiratory tract is highly desirable. While it is thought that serum Ab, nasal IgA, and CMI all contribute to protection against symptomatic RSV infection, RSV-specific nasal IgA is thought to be the most critical in preventing RSV infection (35, 36). While high levels of serum nAb against RSV can reduce severity of RSV diseases, data for demonstrating that serum nAb can prevent RSV infection has been inconsistent. It was reported that higher RSV-specific nasal IgA level correlates with lower level of RSV infection-associated clinical symptoms in human challenge studies with wild-type virus (37). Furthermore, lower level of RSV-specific nasal IgA is a risk factor for RSV infection (17). Thus, generating RSV-specific nasal IgA is a viable approach to develop an RSV vaccine that can prevent RSV infection. CMI plays a critical role in virus clearance. However, the study of CMI in RSV immunity is limited. BLB201 was immunogenic in seropositive adults, even in those with pre-existing antivector immunity. BLB201 induced a range of immune responses, from the enhanced production of RSV F–specific Ab in the nasal mucosa to enhanced RSV nAbs and cytotoxic T cells in the peripheral blood.
There are several intranasal RSV vaccines advanced to human clinical trials. Most are live-attenuated RSV vaccines. Because of the existing anti-RSV antibodies, these live-attenuated RSV vaccines did not generate detectable immune responses in RSV-seropositive people (38). Viral vectored RSV vaccines such as MEDI-534 (b/hPIV3-based RSV), Sendai virus–vectored RSV vaccine and ChAd-RSV did not generate measurable immune responses in adults after a single dose of intranasal immunization (18, 20, 21). Unlike these intranasal RSV vaccines that failed to generate detectable vaccine-mediated immune responses in RSV-seropositive adults, an increase in both serum RSV nAbs and nasal F-specific IgA Abs has been observed for BLB201 intranasal vaccine in adults. BLB201 induced nasal IgA in both age groups, albeit at a higher frequency in the younger age group. We posit that the more robust immune responses generated by BLB201 is likely due to the fact that BLB201 replication was detected in human adults in this clinical trial. The mucosal immunity elicited by BLB201 has the potential to limit virus replication by reducing RSV infection at the portal of viral entry, an important protection mechanism as a prophylactic vaccine. To the best of our knowledge, BLB201 generates the most robust RSV F–specific nasal IgA response among all RSV vaccine candidates.
The induction of cytotoxic CD8+ T cells by BLB201 was observed in the majority of participants in this study. Notably, cytotoxic CD8+ T cell induction is often minimally induced by parenteral RSV subunit vaccine (21, 39). Cytotoxic CD8+ T cells are considered essential effectors that eliminate infected cells, enabling viral clearance (40). Polyfunctional T cells that have been associated with protection against disease (41) were also induced by BLB201, while no TH2-biased T cell responses to RSV were induced in this study; TH2 type of responses are considered a risk factor for vaccine-enhanced RSV disease (33, 34). The lack of TH2-biased responses from BLB201 immunization further supports the BLB201 safety profile. While the precise role of CD8+ CTL in preventing RSV infection and disease is not well defined, it is probable that high levels of CD8+ CTL induced by BLB201 can play a positive role in immunity against RSV disease. Expectedly, base levels of CD8+ in group 1 (33 to 59) with younger participants (0.08%) is a little higher than group 2 (61 to 75) with elderly (0.04%). Both groups reached similar levels of CD8+ at 0.38 and 0.40% after BLB201 immunization, indicating that BLB201 can induce robust CD8+ CTL in elderly (group 2).
The rate of the induction of nasal F-specific IgA Ab response was higher in the younger age group than the elderly group, indicating that, similar to many other vaccines, there was an age-dependent difference in magnitude for responses to BLB201 in nasal IgA Ab. This may be attributed to age-related immunosenescence (42), suggesting that a higher dosage or a second dose may be required in the older population. Because BLB201 generated similar CMI but different levels of nasal IgA in elderly, investigating the efficacy of BLB201 in elderly may elucidate the roles of CD8+ CTL in preventing RSV-associated diseases in elderly.
In this study, approximately 50% (group 1) and 40% (group 2) of participants were seropositive at baseline for PIV5 nAbs. Seropositivity to PIV5 in this study was a little higher than previously reported at about 30% (43), potentially due to prior exposure to dogs vaccinated with PIV5-containing live kennel cough vaccine. In this study, pre-existing PIV5-specific Ab titers did not appear to negatively affect RSV-specific immune responses to BLB201, suggesting that immune experience to PIV5 had no or limited impact on the ability to elicit RSV-specific responses to BLB201. This result is consistent with our previous report that PIV5-vectored influenza vaccine generated similar immune responses in PIV5 immune naïve dogs and in kennel cough vaccinated dogs, which might be due to its ability to infect multiple mucosal cell types in the presence of serum PIV5 nAb (43).
The shedding of vaccine virus in nasal swabs after vaccination was transient, lasting less than 4 weeks, suggesting that BLB201 has the capacity to replicate in the nasal cavities of RSV-experienced adults. This self-limiting infection in the respiratory mucosa by BLB201 may help in the development of RSV-specific immunity. Although vaccine virus shedding was only identified in five participants, it is plausible that it had occurred in other participants but at earlier time points than were sampled here, i.e., within 1 week after vaccination. The result that over 93% participants had robust anti-RSV F cellular immune responses is consistent with replication of BLB201 in humans: BLB201 likely entered human cells and expressed RSV F that was presented by major histocompatibility complex I in infected cells.
The genetic stability of the viral vaccine vector is important for its use in humans. MEDI-534 was not stable in infants, resulting in the termination of its clinical development (19, 44). Extensive testing of BLB201 genetic stability in vitro, in animals, and in this study has indicated that BLB201 is genetically stable in RSV-seropositive adults (31).
In conclusion, BLB201 is a PIV5-based intranasal RSV candidate vaccine that demonstrated safety and immunogenicity in this phase 1 study. The ability of BLB201 to boost pre-existing cellular, humoral, and mucosal responses in adults supports further clinical evaluation of this approach in infants and adult populations.
MATERIALS AND METHODS
Study design and vaccination
The phase 1 clinical trial of PIV5-RSV vaccine (BLB201; ClinicalTrials.gov, NCT05281263) was approved by the Advarra central Institutional Review Board (IRB) and conducted at two study sites in the United States. Participants were recruited to two study cohorts, healthy young adults (group 1, 33 to 59 years old) and healthy older adults (group 2, 61 to 75 years old). Participants of childbearing potential were required to practice contraceptive measurement to prevent pregnancy. Exclusion criteria included any live vaccine within the 30 days before trial vaccine; any prior receipt of any investigational RSV vaccine or any PIV5-based vaccine (CVXGA1) that was actively enrolling during the trial period; and known infection with human immunodeficiency virus, hepatitis B virus, or hepatitis C virus. A full list of inclusion and exclusion criteria can be found at https://clinicaltrials.gov. Participants were not prescreened for their RSV serum Ab levels. Eligible participants were administered on day 1, a single dose at a concentration of 107.5 PFU of BLB201 and a 0.25-ml spray to each nostril (total volume 0.5 ml) using a MAD Nasal Intranasal Mucosal Atomization Device (Teleflex MAD300), and observed for 30 min immediately after dosing. In addition, participants were asked to maintain a memory aid for solicited systemic AEs and local reactions during the week after vaccination. Four sentinel participants were dosed first in each group, and their safety data were reviewed by the safety monitory committee before the remainder of the participants in the group were enrolled.
Primary outcome measures included (i) solicited AEs (days 1 to 8) and (ii) unsolicited AEs (days 1 to 29). Secondary outcome measures included (i) serum IgG titers to RSV protein (days 15 and 29), (ii) SAEs (days 1 to 181), and (iii) AEs of special interest (AESIs) including new onset chronic medical conditions and medically attended AEs (days 1 to181).
Safety assessments
The safety and tolerability of PIV5-RSV intranasal vaccine were assessed over the period of 6 months after vaccination. Participants recorded the information of local and systemic AEs for 7 days after vaccination (solicited local and systematic events) and unsolicited AEs for 28 days using a memory aid. SAEs and AESIs were collected at scheduled study follow-up visits or unscheduled visits for the full duration of the study (6 months). AEs were graded according to the U.S. Food and Drug Administration Center for Biologics Evaluation and Research Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials guidelines. All unsolicited AEs were categorized by the investigator for possible causative association with vaccination (i.e., relatedness to vaccination).
Vaccine shedding and genetic stability
Vaccine-virus shedding was analyzed by RT-qPCR of nasal swabs collected on days 1 (before vaccination) and days 8, 15, and 29 after vaccination. A regular sized flocked nylon swab was used to collect nasal secretion from one nostril, placed in 1-ml universal transport medium (VTM, Copan), and stored at −80°C until sample testing. RNA was extracted from 140 μl (group 2) or 560 μl (group 1) of nasal swab by the QIAmp Viral RNA Mini Kit (QIAGEN) and eluted in 40 μl of buffer. The primers targeted the trailer region of PIV5 genome to quantify PIV5-RSV vaccine virus genomic RNA. The PCR reaction mix included Luna Universal probe One-Step RT-qPCR (New England BioLabs), PIV5 trailer region primers/probe (sequence information is available upon request), and 8 μl of RNA in a QuantStudio 3 instrument (Thermo Fisher Scientific). The assay had a lower LOD of 4.6 log10 copies/ml (~2.5 log10 PFU/ml) and a linearity from 4.6 to 9.0 log10 copies/ml.
Vaccine virus shedding samples with RNA genome levels greater than 5.0 log10 PFU/ml were subjected to sequence analysis of the inserted RSV F gene and its flanking sequences to evaluate stability of the vaccine insert. Viral RNA extracted from nasal swabs was amplified by RT-PCR (OneTaq One-Step RT-PCR, New England BioLabs) and cDNA sequenced by the Sanger method (Azenta).
Immunogenicity assessment
Blood and nasal samples were collected at baseline (day 1, before vaccination), day 15, and day 29 before vaccination. To reduce sample variation, samples from the same participant were run on the same plate in a blinded fashion in all the assays. Serum RSV nAb levels were determined by a qualified RSV A2 microneutralization (MN) assay based on RSV-A2-rLuc reporter virus (45). Briefly, serially twofold diluted serum samples in quadruplicate from a starting dilution of 1:100 were incubated with 175 ± 75 PFU RSV-rLuc for 1 hour and infected Vero cells on 96-well, white-walled, clear bottom plates. After 20 to 24 hours incubation, the cells were lysed using Renilla-Glo Luciferase assay (Promega) and luciferase signals were read on a SpectraMax iD3 multimode microplate reader (Molecular Devices). RSV nAb titer was defined as reciprocal dilution that inhibited at least 50% signal of the virus control as determined by 5PL curve fitting using Prism (version 9.5.1 for macOS, GraphPad Software). The RSV nAb titer was converted to international units based on the standard serum (16/284) obtained from The National Institute for Biological Standard and Control (NIBSC) (London, UK).
RSV F–specific serum IgG and IgA Ab and nasal IgA Ab levels were determined by enzyme-linked immunosorbent assay (ELISA) assay using purified RSV F protein (25 ng per well; SinoBiological, catalog no. 11049-V08B) and twofold serial dilutions in blocking buffer (5% milk/0.5% bovine serum albumin in 1× KPL wash buffer (SeraCare) in duplicate. End-point titer was calculated by 4PL curve fitting using Prism and reported as reciprocal dilution. PIV5-specific IgG and nAb titers were determined by ELISA assay using PIV5 virus-coated plates and by PIV5-rLuc–based MN assay in Vero cells, respectively. The PIV5 IgG end-point titer was calculated by 4PL curve fitting and reported as reciprocal dilution. The PIV5 MN assay was performed similarly to the RSV-rLuc–based MN assay, except in duplicate instead of quadruplicate. Analysis of PIV5 MN was identical to the RSV-rLuc–based MN assay, and the nAb titer was defined as the reciprocal dilution that inhibited at least 50% signal of the virus control.
Antigen-specific T cell frequencies were evaluated by intracellular cytokine staining assay using cryopreserved peripheral blood mononuclear cells (PBMCs) isolated from whole blood on day 1 (pre-vaccine dosing), day 15, and day 29. One million cryopreserved PBMCs were thawed in complete 10% fetal bovine serum (FBS) RPMI medium and rested overnight at 37°C before being incubated with an RSV F peptide pool from GenScript at a final concentration of 1 μg/ml in the presence of anti-CD28 ECD (1 μg/ml; Beckman Coulter, clone CD28.2), anti-CD107a fluorescein isothiocyanate (BD Biosciences, clone H4A3), and anti-CD49d (BD Biosciences, clone 9F10). In addition, PBMCs were stimulated with 1 μl of complete medium with 0.5% dimethyl sulfoxide (DMSO; negative control corresponding to the DMSO concentration of the RSV F peptide pool) or 1 μl of phorbol 12-myristate 13-acetate (PMA)/ionomycin [PMA (25 ng/ml) and ionomycin (1 μg/ml)] for negative and positive controls, respectively. After 2-hour incubation at 37°C, brefeldin A (10 μg/ml; BD Biosciences) were added and the cells were incubated for another 4 hours. The cells were washed with phosphate-buffered saline (PBS) and incubated with aqua viability dye (Invitrogen) at room temperature for 15 min. The cells were washed with PBS supplemented with 2% FBS and surface-stained at 4°C for 30 min with anti-CD3 Alexa 700 (BD Biosciences, clone SP34-2), anti-CD4 BV605 (BD Biosciences, clone L200), anti-CD8 BV450 (BD Biosciences, clone RPA-T8), and anti-CD95 phycoerythrin (PE)–Cy5 (BD Biosciences, clone DX2). The cells were washed with PBS with 2% FBS, fixed with Cytofix/Cytoperm (BD Biosciences), permeabilized with 1× Perm/Wash (BD Biosciences), and incubated with anti–IFN-γ PE-Cy7 (BD Biosciences, clone B27), anti–TNF-α allophycocyanin (APC)–Cy7 (BioLegend, clone Mab11), anti–IL-13 PE (Miltenyi Biotec, clone JES10-5A2.2), and anti–MIP-1β APC (eBioscience, clone FL34Z3L) antibodies at 4°C for 30 min. The cells were washed with 1× Perm/Wash and PBS with 2% FBS then resuspended in PBS/2% formaldehyde for acquisition on a BD FACSAria Fusion cell sorter. CD3+ cells were gated for CD4+ and CD8+ T cells and separated into memory and naïve cells with CD28 and CD95. The net percentage of cytokine-secreting cells was determined by subtraction of the values obtained with DMSO-stimulated samples (negative control). T cell frequencies were considered positive if the detected frequency of cytokine-positive CD4+ or CD8+ T cells was >0.1% after subtraction of the baseline. Data were analyzed using FlowJo software (version 10). A Boolean combination and SPICE software were used to determine polyfunctional responses of CD4+ and CD8+ T cells producing two or more cytokines.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (Version 9). Given the small sample size, statistical analysis was mostly descriptive and summative. P values were used to show difference of potential significance at a 0.05 significance level. Two-group comparisons of RSV-specific CMI responses and Ab responses were evaluated by the Wilcoxon matched-pairs signed rank test (day 15 or day 29 versus day 1 or day 15 versus day 29). Antibody titer rises of ≥1.5-fold after vaccination versus baseline was considered significant because assay characteristics showed a change of 1.2- to 1.3-fold (95% confidence) to be a significant change when samples from a single participant were tested on the same plate.
Acknowledgments
We thank the participants of the clinical trial, the clinical site investigators, and their staff for conducting the trial; the Emmes Corporation for managing the trial; and M. Teng for providing rRSV-Luc. The central IRB of Advarra approved the study, and participants provided informed consent. MG Science Communications (Belgium) provided scientific writing services.
Funding: Blue Lake Biotechnology Inc funded this study.
Author contributions: P.S., H.J., F.J.V., and B.H. contributed to study concept, methodology, and writing. P.X., K.K., M.C.G., B.F., Z.W., M.M., S.P.J., K.S., and J.K. for data generation and analysis. H.R., S.W., and M.T. for clinical protocol, regulatory submission, and manuscript review.
Competing interests: H.J., K.K., M.C.G., H.R., S.W., M.T., M.M., B.F., Z.W., and B.H. are employees of CyanVac LLC and have equity in its affiliated Blue Lake Biotechnology, who funded the study. P.S. has received contracts from CyanVac LLC and from Blue Lake Biotechnology for the performance of clinical trials (awarded to CCHMC). F.J.V. received contract from Blue Lake Biotechnology for CMI sample testing. Other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The BLB201 can be provided by Blue Lake Biotechnology Inc. pending scientific review and a completed material transfer agreement. Requests for the BLB201 should be submitted to B.H.
Supplementary Materials
This PDF file includes:
Figs. S1 and S2
REFERENCES AND NOTES
- 1.B. S. Graham, L. J. Anderson, Challenges and opportunities for respiratory syncytial virus vaccines. Curr. Top. Microbiol. Immunol. 372, 391–404 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D. K. Shay, R. C. Holman, G. E. Roosevelt, M. J. Clarke, L. J. Anderson, Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979-1997. J. Infect Dis. 183, 16–22 (2001). [DOI] [PubMed] [Google Scholar]
- 3.C. B. Hall, G. A. Weinberg, A. K. Blumkin, K. M. Edwards, M. A. Staat, A. F. Schultz, K. A. Poehling, P. G. Szilagyi, M. R. Griffin, J. V. Williams, Y. Zhu, C. G. Grijalva, M. M. Prill, M. K. Iwane, Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 132, e341–e348 (2013). [DOI] [PubMed] [Google Scholar]
- 4.T. S. D. A. McAllister, K. L. O'Brien, E. A. F. Simoes, S. A. Madhi, B. D. Gessner, F. P. Polack, E. Balsells, S. Acacio, C. Aguayo, I. Alassani, A. Ali, M. Antonio, S. Awasthi, J. O. Awori, E. Azziz-Baumgartner, H. C. Baggett, V. L. Baillie, A. Balmaseda, A. Barahona, S. Basnet, Q. Bassat, W. Basualdo, G. Bigogo, L. Bont, R. F. Breiman, W. A. Brooks, S. Broor, N. Bruce, D. Bruden, P. Buchy, S. Campbell, P. Carosone-Link, M. Chadha, J. Chipeta, M. Chou, W. Clara, C. Cohen, E. de Cuellar, D. A. Dang, B. Dash-Yandag, M. Deloria-Knoll, M. Dherani, T. Eap, B. E. Ebruke, M. Echavarria, C. C. de Freitas Lázaro Emediato, R. A. Fasce, D. R. Feikin, L. Feng, A. Gentile, A. Gordon, D. Goswami, S. Goyet, M. Groome, N. Halasa, S. Hirve, N. Homaira, S. R. C. Howie, J. Jara, I. Jroundi, C. B. Kartasasmita, N. Khuri-Bulos, K. L. Kotloff, A. Krishnan, R. Libster, O. Lopez, M. G. Lucero, F. Lucion, S. P. Lupisan, D. N. Marcone, J. P. McCracken, M. Mejia, J. C. Moisi, J. M. Montgomery, D. P. Moore, C. Moraleda, J. Moyes, P. Munywoki, K. Mutyara, M. P. Nicol, D. J. Nokes, P. Nymadawa, M. T. da Costa Oliveira, H. Oshitani, N. Pandey, G. Paranhos-Baccalà, L. N. Phillips, V. S. Picot, M. Rahman, M. Rakoto-Andrianarivelo, Z. A. Rasmussen, B. A. Rath, A. Robinson, C. Romero, G. Russomando, V. Salimi, P. Sawatwong, N. Scheltema, B. Schweiger, J. A. G. Scott, P. Seidenberg, K. Shen, R. Singleton, V. Sotomayor, T. A. Strand, A. Sutanto, M. Sylla, M. D. Tapia, S. Thamthitiwat, E. D. Thomas, R. Tokarz, C. Turner, M. Venter, S. Waicharoen, J. Wang, W. Watthanaworawit, L. M. Yoshida, H. Yu, H. J. Zar, H. Campbell, H. Nair; RSV Global Epidemiology Network , Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 390, 946–958 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.A. R. Falsey, P. A. Hennessey, M. A. Formica, C. Cox, E. E. Walsh, Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352, 1749–1759 (2005). [DOI] [PubMed] [Google Scholar]
- 6.E. E. Walsh, D. R. Peterson, A. R. Falsey, Risk factors for severe respiratory syncytial virus infection in elderly persons. J. Infect. Dis. 189, 233–238 (2004). [DOI] [PubMed] [Google Scholar]
- 7.W. W. Thompson, D. K. Shay, E. Weintraub, L. Brammer, N. Cox, L. J. Anderson, K. Fukuda, Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289, 179–186 (2003). [DOI] [PubMed] [Google Scholar]
- 8.S. W. Hanson, C. Abbafati, J. G. Aerts, Z. Al-Aly, C. Ashbaugh, T. Ballouz, O. Blyuss, P. Bobkova, G. Bonsel, S. Borzakova, D. Buonsenso, D. Butnaru, A. Carter, H. Chu, C. De Rose, M. M. Diab, E. Ekbom, M. El Tantawi, V. Fomin, R. Frithiof, A. Gamirova, P. V. Glybochko, J. A. Haagsma, S. H. Javanmard, E. B. Hamilton, G. Harris, M. H. Heijenbrok-Kal, R. Helbok, M. E. Hellemons, D. Hillus, S. M. Huijts, M. Hultström, W. Jassat, F. Kurth, I. M. Larsson, M. Lipcsey, C. Liu, C. D. Loflin, A. Malinovschi, W. Mao, L. Mazankova, D. McCulloch, D. Menges, N. Mohammadifard, D. Munblit, N. A. Nekliudov, O. Ogbuoji, I. M. Osmanov, J. L. Peñalvo, M. S. Petersen, M. A. Puhan, M. Rahman, V. Rass, N. Reinig, G. M. Ribbers, A. Ricchiuto, S. Rubertsson, E. Samitova, N. Sarrafzadegan, A. Shikhaleva, K. E. Simpson, D. Sinatti, J. B. Soriano, E. Spiridonova, F. Steinbeis, A. A. Svistunov, P. Valentini, B. J. van de Water, R. van den Berg-Emons, E. Wallin, M. Witzenrath, Y. Wu, H. Xu, T. Zoller, C. Adolph, J. Albright, J. O. Amlag, A. Y. Aravkin, B. L. Bang-Jensen, C. Bisignano, R. Castellano, E. Castro, S. Chakrabarti, J. K. Collins, X. Dai, F. Daoud, C. Dapper, A. Deen, B. B. Duncan, M. Erickson, S. B. Ewald, A. J. Ferrari, A. D. Flaxman, N. Fullman, A. Gamkrelidze, J. R. Giles, G. Guo, S. I. Hay, J. He, M. Helak, E. N. Hulland, M. Kereselidze, K. J. Krohn, A. Lazzar-Atwood, A. Lindstrom, R. Lozano, D. C. Malta, J. Månsson, A. M. M. Herrera, A. H. Mokdad, L. Monasta, S. Nomura, M. Pasovic, D. M. Pigott, R. C. Reiner Jr., G. Reinke, A. L. P. Ribeiro, D. F. Santomauro, A. Sholokhov, E. E. Spurlock, R. Walcott, A. Walker, C. S. Wiysonge, P. Zheng, J. P. Bettger, C. J. L. Murray, T. Vos, Global burden of disease long COVID collaborators, estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 328, 1604–1615 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.E. E. Walsh, G. P. Marc, A. M. Zareba, A. R. Falsey, Q. Jiang, M. Patton, F. P. Polack, C. Llapur, P. A. Doreski, K. Ilangovan, M. Ramet, Y. Fukushima, N. Hussen, L. J. Bont, J. Cardona, E. DeHaan, G. C. Villa, M. Ingilizova, D. Eiras, T. Mikati, R. N. Shah, K. Schneider, D. Cooper, K. Koury, M. M. Lino, A. S. Anderson, K. U. Jansen, K. A. Swanson, A. Gurtman, W. C. Gruber, B. Schmoele-Thoma; RENOIR Clinical Trial Group , Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N. Engl. J. Med. 388, 1465–1477 (2023). [DOI] [PubMed] [Google Scholar]
- 10.A. R. Falsey, K. Williams, E. Gymnopoulou, S. Bart, J. Ervin, A. R. Bastian, J. Menten, E. De Paepe, S. Vandenberghe, E. K. H. Chan, J. Sadoff, M. Douoguih, B. Callendret, C. A. Comeaux, E. Heijnen; CYPRESS Investigators , Efficacy and safety of an Ad26.RSV.preF-RSV preF protein vaccine in older adults. N. Engl. J. Med. 388, 609–620 (2023). [DOI] [PubMed] [Google Scholar]
- 11.A. Papi, M. G. Ison, J. M. Langley, D. G. Lee, I. Leroux-Roels, F. Martinon-Torres, T. F. Schwarz, R. N. van Zyl-Smit, L. Campora, N. Dezutter, N. de Schrevel, L. Fissette, M. P. David, M. Van der Wielen, L. Kostanyan, V. Hulstrom; AReSVi-006 Study Group , Respiratory syncytial virus prefusion F protein vaccine in older adults. N. Engl. J. Med. 388, 595–608 (2023). [DOI] [PubMed] [Google Scholar]
- 12.FDA, FDA approves first respiratory syncytial virus (RSV) vaccine: Arexvy approved for individuals 60 years of age and older (FDA, 2023).
- 13.J. Shan, P. N. Britton, C. L. King, R. Booy, The immunogenicity and safety of respiratory syncytial virus vaccines in development: A systematic review. Influenza Other Respi. Viruses 15, 539–551 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A. R. Falsey, E. E. Walsh, Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 13, 371–384 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R. A. Karron, J. E. Atwell, E. J. McFarland, C. K. Cunningham, P. Muresan, C. Perlowski, J. Libous, S. A. Spector, R. Yogev, M. Aziz, S. Woods, K. Wanionek, P. L. Collins, U. J. Buchholz, Live-attenuated vaccines prevent respiratory syncytial virus-associated illness in young children. Am. J. Respir. Crit. Care Med. 203, 594–603 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.E. Jordan, S. J. Lawrence, T. P. H. Meyer, D. Schmidt, S. Schultz, J. Mueller, D. Stroukova, B. Koenen, R. Gruenert, G. Silbernagl, S. Vidojkovic, L. M. Chen, H. Weidenthaler, N. Samy, P. Chaplin, Broad antibody and cellular immune response from a phase 2 clinical trial with a novel multivalent poxvirus-based respiratory syncytial virus vaccine. J. Infect. Dis. 223, 1062–1072 (2021). [DOI] [PubMed] [Google Scholar]
- 17.M. S. Habibi, A. Jozwik, S. Makris, J. Dunning, A. Paras, J. P. DeVincenzo, C. A. de Haan, J. Wrammert, P. J. Openshaw, C. Chi, Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 191, 1040–1049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R. S. Tang, R. R. Spaete, M. W. Thompson, M. MacPhail, J. M. Guzzetta, P. C. Ryan, K. Reisinger, P. Chandler, M. Hilty, R. E. Walker, M. M. Gomez, G. A. Losonsky, Development of a PIV-vectored RSV vaccine: Preclinical evaluation of safety, toxicity, and enhanced disease and initial clinical testing in healthy adults. Vaccine 26, 6373–6382 (2008). [DOI] [PubMed] [Google Scholar]
- 19.C. F. Yang, C. K. Wang, E. Malkin, J. H. Schickli, C. Shambaugh, F. Zuo, M. S. Galinski, F. Dubovsky, G. Study, R. S. Tang, Implication of respiratory syncytial virus (RSV) F transgene sequence heterogeneity observed in Phase 1 evaluation of MEDI-534, a live attenuated parainfluenza type 3 vectored RSV vaccine. Vaccine 31, 2822–2827 (2013). [DOI] [PubMed] [Google Scholar]
- 20.C. A. Green, C. J. Sande, E. Scarselli, S. Capone, A. Vitelli, A. Nicosia, L. Silva-Reyes, A. J. Thompson, C. M. de Lara, K. S. Taylor, K. Haworth, C. L. Hutchings, T. Cargill, B. Angus, P. Klenerman, A. J. Pollard, Novel genetically-modified chimpanzee adenovirus and MVA-vectored respiratory syncytial virus vaccine safely boosts humoral and cellular immunity in healthy older adults. J. Infect. 78, 382–392 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.C. A. Green, E. Scarselli, C. J. Sande, A. J. Thompson, C. M. de Lara, K. S. Taylor, K. Haworth, M. Del Sorbo, B. Angus, L. Siani, S. Di Marco, C. Traboni, A. Folgori, S. Colloca, S. Capone, A. Vitelli, R. Cortese, P. Klenerman, A. Nicosia, A. J. Pollard, Chimpanzee adenovirus- and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. Sci. Transl. Med. 7, 300ra126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Z. Chen, Parainfluenza virus 5-vectored vaccines against human and animal infectious diseases. Rev. Med. Virol. 28, e1965 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D. An, K. Li, D. K. Rowe, M. C. H. Diaz, E. F. Griffin, A. C. Beavis, S. K. Johnson, I. Padykula, C. A. Jones, K. Briggs, G. Li, Y. Lin, J. Huang, J. Mousa, M. Brindley, K. Sakamoto, D. K. Meyerholz, P. B. McCray Jr., S. M. Tompkins, B. He, Protection of K18-hACE2 mice and ferrets against SARS-CoV-2 challenge by a single-dose mucosal immunization with a parainfluenza virus 5-based COVID-19 vaccine. Sci. Adv. 7, eabi5246 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A. J. Mooney, Z. Li, J. D. Gabbard, B. He, S. M. Tompkins, Recombinant parainfluenza virus 5 vaccine encoding the influenza virus hemagglutinin protects against H5N1 highly pathogenic avian influenza virus infection following intranasal or intramuscular vaccination of BALB/c mice. J. Virol. 87, 363–371 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.A. J. Mooney, J. D. Gabbard, Z. Li, D. A. Dlugolenski, S. K. Johnson, R. A. Tripp, B. He, S. M. Tompkins, Vaccination with recombinant parainfluenza virus 5 expressing neuraminidase protects against homologous and heterologous influenza virus challenge. J. Virol. 91, e01579-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.S. M. Tompkins, Y. Lin, G. P. Leser, K. A. Kramer, D. L. Haas, E. W. Howerth, J. Xu, M. J. Kennett, R. K. Durbin, J. E. Durbin, R. Tripp, R. A. Lamb, B. He, Recombinant parainfluenza virus 5 (PIV5) expressing the influenza A virus hemagglutinin provides immunity in mice to influenza A virus challenge. Virology 362, 139–150 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Z. Li, A. J. Mooney, J. D. Gabbard, X. Gao, P. Xu, R. J. Place, R. J. Hogan, S. M. Tompkins, B. He, Recombinant parainfluenza virus 5 expressing hemagglutinin of influenza A virus H5N1 protected mice against lethal highly pathogenic avian influenza virus H5N1 challenge. J. Virol. 87, 354–362 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Z. Li, J. D. Gabbard, S. Johnson, D. Dlugolenski, S. Phan, S. M. Tompkins, B. He, Efficacy of a parainfluenza virus 5 (PIV5)-based H7N9 vaccine in mice and guinea pigs: Antibody titer towards HA was not a good indicator for protection. PLOS ONE 10, e0120355 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.K. Li, Z. Li, C. Wohlford-Lenane, D. K. Meyerholz, R. Channappanavar, D. An, S. Perlman, P. B. McCray Jr., B. He Single-Dose, Intranasal immunization with recombinant parainfluenza virus 5 expressing Middle East Respiratory Syndrome Coronavirus (MERS-CoV) spike protein protects mice from fatal MERS-CoV infection. mBio 11, e00554-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D. Wang, S. Phan, D. J. DiStefano, M. P. Citron, C. L. Callahan, L. Indrawati, S. A. Dubey, G. J. Heidecker, D. Govindarajan, X. Liang, B. He, A. S. Espeseth, A single-dose recombinant parainfluenza virus 5-vectored vaccine expressing Respiratory Syncytial Virus (RSV) F or G protein protected cotton rats and african green monkeys from RSV challenge. J. Virol. 91, e00066-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.S. I. Phan, J. R. Zengel, H. Wei, Z. Li, D. Wang, B. He, Parainfluenza virus 5 expressing wild-type or prefusion Respiratory Syncytial Virus (RSV) fusion protein protects mice and cotton rats from RSV challenge. J. Virol. 91, e00560-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.S. I. Phan, Z. Chen, P. Xu, Z. Li, X. Gao, S. L. Foster, M. N. Teng, R. A. Tripp, K. Sakamoto, B. He, A respiratory syncytial virus (RSV) vaccine based on parainfluenza virus 5 (PIV5). Vaccine 32, 3050–3057 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.P. L. Acosta, M. T. Caballero, F. P. Polack, Brief history and characterization of enhanced respiratory syncytial virus disease. Clin. Vaccine Immunol. 23, 189–195 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.F. P. Polack, M. N. Teng, P. L. Collins, G. A. Prince, M. Exner, H. Regele, D. D. Lirman, R. Rabold, S. J. Hoffman, C. L. Karp, S. R. Kleeberger, M. Wills-Karp, R. A. Karron, A role for immune complexes in enhanced respiratory syncytial virus disease. J. Exp. Med. 196, 859–865 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.B. Bagga, J. E. Cehelsky, A. Vaishnaw, T. Wilkinson, R. Meyers, L. M. Harrison, P. L. Roddam, E. E. Walsh, J. P. DeVincenzo, Effect of preexisting serum and mucosal antibody on experimental Respiratory Syncytial Virus (RSV) challenge and infection of adults. J. Infect. Dis. 212, 1719–1725 (2015). [DOI] [PubMed] [Google Scholar]
- 36.E. E. Walsh, A. R. Falsey, Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J. Infect. Dis. 190, 373–378 (2004). [DOI] [PubMed] [Google Scholar]
- 37.P. J. Watt, B. S. Robinson, C. R. Pringle, D. A. Tyrrell, Determinants of susceptibility to challenge and the antibody response of adult volunteers given experimental respiratory syncytial virus vaccines. Vaccine 8, 231–236 (1990). [DOI] [PubMed] [Google Scholar]
- 38.R. A. Karron, C. Luongo, J. S. Mateo, K. Wanionek, P. L. Collins, U. J. Buchholz, Safety and immunogenicity of the respiratory syncytial virus vaccine RSV/ΔNS2/Δ1313/I1314L in RSV-seronegative children. J. Infect. Dis. 222, 82–91 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.J. Baber, M. Arya, Y. Moodley, A. Jaques, Q. Jiang, K. A. Swanson, D. Cooper, M. S. Maddur, J. Loschko, A. Gurtman, K. U. Jansen, W. C. Gruber, P. R. Dormitzer, B. Schmoele-Thoma, A phase 1/2 study of a respiratory syncytial virus prefusion F vaccine with and without adjuvant in healthy older adults. J. Infect. Dis. 226, 2054–2063 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.A. Jozwik, M. S. Habibi, A. Paras, J. Zhu, A. Guvenel, J. Dhariwal, M. Almond, E. H. C. Wong, A. Sykes, M. Maybeno, J. Del Rosario, M. B. Trujillo-Torralbo, P. Mallia, J. Sidney, B. Peters, O. M. Kon, A. Sette, S. L. Johnston, P. J. Openshaw, C. Chiu, RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun. 6, 10224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.B. N. Blunck, L. S. Angelo, D. Henke, V. Avadhanula, M. Cusick, L. Ferlic-Stark, L. Zechiedrich, B. E. Gilbert, P. A. Piedra, Adult memory T cell responses to the respiratory syncytial virus fusion protein during a single RSV season (2018-2019). Front. Immunol. 13, 823652 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.A. Aiello, F. Farzaneh, G. Candore, C. Caruso, S. Davinelli, C. M. Gambino, M. E. Ligotti, N. Zareian, G. Accardi, Immunosenescence and its hallmarks: How to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 10, 2247 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Z. Chen, P. Xu, G. W. Salyards, S. B. Harvey, B. Rada, Z. F. Fu, B. He, Evaluating a parainfluenza virus 5-based vaccine in a host with pre-existing immunity against parainfluenza virus 5. PLOS ONE 7, e50144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.C. L. Nelson, R. S. Tang, E. A. Stillman, Genetic stability of RSV-F expression and the restricted growth phenotype of a live attenuated PIV3 vectored RSV vaccine candidate (MEDI-534) following restrictive growth in human lung cells. Vaccine 31, 3756–3762 (2013). [DOI] [PubMed] [Google Scholar]
- 45.S. Fuentes, R. L. Crim, J. Beeler, M. N. Teng, H. Golding, S. Khurana, Development of a simple, rapid, sensitive, high-throughput luciferase reporter based microneutralization test for measurement of virus neutralizing antibodies following respiratory syncytial virus vaccination and infection. Vaccine 31, 3987–3994 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 and S2