Abstract
Abnormal brain–gut interaction is considered the core pathological mechanism behind the disorders of gut–brain interaction (DGBI), in which the intestinal microbiota plays an important role. Microglia are the “sentinels” of the central nervous system (CNS), which participate in tissue damage caused by traumatic brain injury, resist central infection and participate in neurogenesis, and are involved in the occurrence of various neurological diseases. With in-depth research on DGBI, we could find an interaction between the intestinal microbiota and microglia and that they are jointly involved in the occurrence of DGBI, especially in individuals with comorbidities of mental disorders, such as irritable bowel syndrome (IBS). This bidirectional regulation of microbiota and microglia provides a new direction for the treatment of DGBI. In this review, we focus on the role and underlying mechanism of the interaction between gut microbiota and microglia in DGBI, especially IBS, and the corresponding clinical application prospects and highlight its potential to treat DGBI in individuals with psychiatric comorbidities.
Keywords: gut microbiota, microglia, disorders of gut–brain interaction, irritable bowel syndrome
Graphical Abstract
Graphical Abstract.
Introduction
Disorders of gut–brain interactions (DGBIs), formerly known as functional gastrointestinal disorders (FGIDs), is a general term for a series of gastrointestinal (GI) symptoms without underlying structural abnormalities (Black et al., 2020a; Sperber et al., 2021). The three representative DGBIs are irritable bowel syndrome (IBS), functional dyspepsia (FD), and functional constipation (FC). A large-scale multinational study has shown that > 40% of people worldwide suffer from DGBIs (Sperber et al., 2021). Several clinical epidemiological studies have also found that patients with DGBIs have a high proportion of psychiatric comorbidities, such as anxiety and depression (Fond et al., 2014). These comorbidities lead to difficulties in the treatment of DGBIs and affect the quality-of-life and social functioning of patients (Sperber et al., 2021).
The pathophysiology of DGBIs is complex and diverse (Drossman and Hasler, 2016). Visceral hypersensitivity, abnormal GI motility, and psychological disorders are thought to be the main mechanisms that lead to DGBIs. Later, low-grade intestinal inflammation, increased intestinal mucosal permeability, altered immune function, and disturbances of the intestinal microbiota were also discovered to be involved in DGBIs. Based on this mechanism, the microbiota–gut–brain axis was proposed.
The gut microbiota of patients with DGBIs, particularly IBS, has been extensively studied (Simrén et al., 2013; Jalanka-Tuovinen et al., 2014; Lembo et al., 2016; Duan et al., 2019; Saffouri et al., 2019; Liu et al., 2022). Changes in the structure of the gut microbiota are associated with low-grade inflammation, such as mast cell activity in the colonic mucosa and elevated levels of colonic interleukin (IL)-12 in patients with IBS. In addition to low-grade intestinal inflammation, systemic inflammation was observed in these patients, manifested by elevated IL-12 levels and a decreased IL-10/IL-12 ratio in peripheral blood (Liu et al., 2022). Systemic inflammation can also lead to central nervous system (CNS) inflammation. Further studies using functional magnetic resonance imaging (fMRI) have found that patients with DGBIs have different degrees of activation of brain regions (Wilder-Smith, 2011), such as the medial prefrontal cortex, amygdala, and hippocampus. In addition, there was a significant correlation between IBS and the incidence of Alzheimer’s disease (AD) (Fu et al., 2020), Parkinson’s disease (PD) (Lu et al., 2022) and dementia (Chen et al., 2016). Mendelian randomization studies also found a significant genetic correlation between IBS and AD (Adewuyi et al., 2022). The gut microbiota is an important factor that affects the gut–brain axis. The symptoms of IBS can be improved through interventions targeting the gut microbiota, including probiotics (Zhang et al., 2022a), prebiotics (Ford et al., 2014; Huaman et al., 2018), low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diets (low-FODMAP diets) (Staudacher et al., 2014; Huaman et al., 2018), non-absorbable antibiotic rifaximin (Pimentel et al., 2011) and fecal microbiota transplantation (FMT) (Johnsen et al., 2018; El-Salhy et al., 2020).
Microglia are the resident immune cells of the brain that play key roles in a variety of neurodevelopmental processes required for proper brain maturation and function, such as neurogenesis, synapse shaping, and defense against infection. Changes in the structure and function of microglia are associated with various psychiatric disorders, such as depression (Torres-Platas et al., 2014; Tay et al., 2017) and Alzheimer’s disease (AD) (Moonen et al., 2023). In addition to the role of microglia in the CNS, microglia are involved in GI function and diseases. For example, visceral pain is associated with microglial activation in an IBS-like rat model (Yuan et al., 2020). Efferent nerves of the brain regulate GI motility, secretions, and permeability. Owing to the important physiological and pathological roles of microglia in CNS diseases, microglia are indispensable for the regulation of the gut–brain axis.
The gut microbiota can influence the CNS through neuronal, endocrine, and immune pathways (Margolis et al., 2021). Meanwhile, the CNS may also affect the gut microbiota structure. Gut microbiota and CNS have potential interactions and may mainly act on microglia. This relationship may be an important mechanism for DGBIs and psychiatric comorbidities, and has potential to be a future therapeutic target. Here, combined with our previous studies and recent research progress, we review and discuss whether the crosstalk between the gut microbiota and microglia participates in the development of DGBIs, especially IBS, from both gut-to-brain and brain-to-gut perspectives.
Abnormal microbiota–gut–brain interaction in patients with IBS
IBS is the most typical DGBI. Approximately 40%–60% of patients with IBS have symptoms of anxiety and depression. Approximately 50% of these patients have a history of trauma (Fond et al., 2014). Greater insular activation and reduced inactivation of the pregenual anterior cingulate cortex have been observed in patients with IBS during noxious rectal distension. During anticipation of rectal distension, non-hypersensitive patients with IBS had more activation in the right hippocampus than the controls (Larsson et al., 2012). Notably, patients with IBS have increased levels of systemic cytokines, which is manifested by increased expression of IL-10 and IL-12 in peripheral blood (Zhang et al., 2019a). Patients with IBS with psychiatric comorbidities are often the most difficult to treat. Tricyclic antidepressants are most effective in alleviating abdominal pain in patients with IBS (Black et al., 2020b).
Previous cross-sectional and longitudinal studies have demonstrated an altered gut microbiota structure and function in patients with IBS (Mars et al., 2020; Mujagic et al., 2022). However, the results of various studies are highly heterogeneous. Most studies have mainly focused on the structure of the microbiota, and it is difficult to summarizing their microbial characteristics. However, the mechanism by which the intestinal microbiota causes IBS symptoms has not yet been elucidated. A recent study used metatranscriptomics and metabolomics to conduct a multi-omic assessment of the intestinal microbiota function in patients with IBS and found that after a series of parameter adjustments, IBS was associated with a differential abundance of bacterial taxa, such as Bacteroides dorei. The transcript abundance of some bacteria was increased in IBS, such as Eggerthella lenta and Blautia hydrogenotrophica; however, it decreased in Bilophila wadsworthia, Roseburia inulinivorans, and Bifidobacterium animals (Jacobs et al., 2023). Particularly, the gut microbiota structure is similar at the phylum level between patients with IBS and depression (Liu et al., 2016). This can be understood to a certain extent, as the pathogenesis of IBS is associated with comorbidities of mental disorders.
A study by Bercik provided new evidence regarding a species of gut microbiota that contributes to abdominal symptoms in IBS. The aforementioned low-FODMAP diet can relieve the symptoms of patients with DGBIs, which may be related to the lower histamine concentration in the urine of patients with IBS (McIntosh et al., 2017). Mast cells in the intestinal mucosa contain large amounts of histamine and are thought to be involved in the development of visceral hypersensitivity (Wouters et al., 2016; Balemans et al., 2019; Perna et al., 2021). In addition to mast cells, certain strains of gut microbiota are important sources of histamine. A recent study had detected high enrichment of Klebsiella aerogenes, a bacterium carrying a histidine decarboxylase gene variant, in fecal samples from patients with IBS. This revealed that Klebsiella aerogenes is an important source of intestinal lumen histamine, which partly explains why enterobacteria cause IBS abdominal pain (De Palma et al., 2022).
Intervention of the gut microbiota is effective in improving abdominal symptoms in patients with IBS and relieving psychiatric symptoms and central inflammation. Intervention with mixed probiotics (Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis) has been shown to significantly relieve IBS symptom severity scale (IBS-SSS) scores in patients with IBS and diarrhea (IBS-D), relieve abdominal pain symptoms, and reduce plasma monocyte chemoattractant protein-1 (MCP-1) and IL-1β levels (Zhang et al., 2019a). Furthermore, depression and coexisting GI symptoms can be significantly improved by probiotic intervention (Tian et al., 2022). In one such study, Bifidobacterium longum NCC3001 could increase the quality-of-life score and reduce the depression score in patients with IBS. Moreover, fMRI analysis showed that, compared with placebo, the Bifidobacterium longum NCC3001 intervention group reduced response of brain regions to negative emotional stimuli, including the amygdala and fronto-limbic regions. In other words, Bifidobacterium longum NCC3001 reduces the tendency of depression in patients with IBS (Pinto-Sanchez et al., 2017).
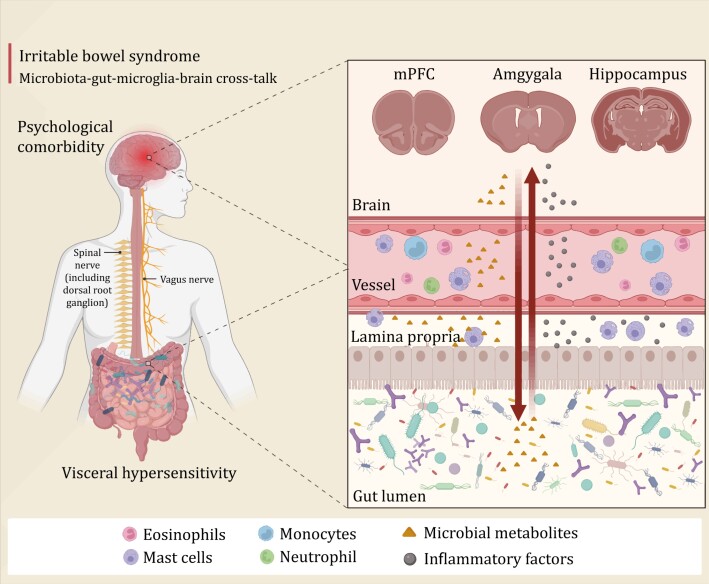
In summary, abnormal brain–gut interactions are widely present in patients with DGBIs, which is the basis for the comorbidity of DGBIs and mental disorders. By regulating the intestinal microbiota, the “gut and brain co-treatment” can be partially realized, which is worthy of further discussion (Fig. 1).
Figure 1.
Gut–brain interaction in irritable bowel syndrome. Gut microbiota and its metabolites affect central nervous system function through neural, endocrine, and immune pathways. Disruption of the microbiota can lead to neuroinflammation that results in abnormal activation of multiple brain regions. Conversely, the activation of brain regions and neuroinflammation will release a variety of pro-inflammatory cytokines, which affect intestinal function and change the structure of intestinal microbiota through neural, immune, and endocrine pathways. Abnormalities in this gut–brain interaction collectively contribute to the comorbidity of psychiatric disorders in patients with IBS. mPFC, medial prefrontal cortex; IBS, irritable bowel syndrome.
Microglia play an important role in gut–brain interactions
Microglial properties and functions
Microglia are macrophages located in the brain parenchyma and spinal cord, and accounts for approximately 10% of all total CNS cells and 10%–15% of all glial cells. Unlike meninges and perivascular macrophages, microglia originate from the yolk sac and are derived from bone marrow-derived hematopoietic cells (Aguzzi et al., 2013). Microglial development can be divided into three phases based on gene expression: progenitor cell, embryonic, and mature. During the progenitor phase, microglia highly expresses genes involved in cell proliferation and DNA replication. The embryonic phase expresses genes related to nervous system development, intercellular signaling, and cell motility. The adult stage is characterized by involvement in cell development and immune activation (Matcovitch-Natan et al., 2016; Thion et al., 2018).
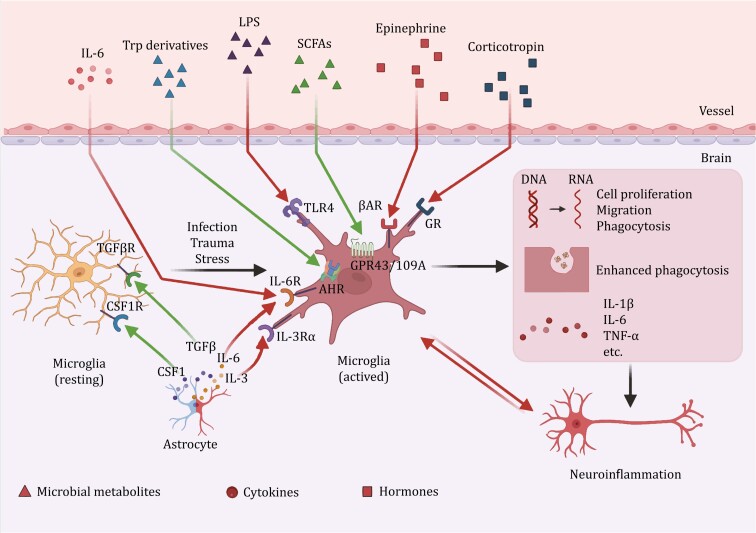
Microglia are considered a part of the innate immune system in the brain and are known to exhibit a variety of morphologies. Resting microglia have small cell bodies and ramified processes that perform normal physiological functions. These microglia support the development and survival of neurons by releasing neurotrophic factors, removing dead cells from the CNS, preventing dysfunction in the CNS environment, regulating synaptic density and synaptic activity, and participating in the development of synaptic plasticity (Nayak et al., 2014). Amebic microglia appear in response to infection, trauma, mental stress, and central inflammation. Its cell body became larger with almost no processes, and the expression of cell proliferation, migration, and phagocytosis genes increased. Amebic microglia secrete high levels of inflammatory cytokines and mediate pathological pro-inflammatory, phagocytic, and immune-clearance functions (Wolf et al., 2017) (Fig. 2 and Table 1).
Figure 2.
Homeostasis and activation of microglia. Resting microglia maintain homeostasis under the regulation of cytokines secreted by astrocytes and other cells. However, under conditions such as infection, trauma and stress, microglia are activated by various microbial metabolites, cytokines and hormones, leading to pro-inflammatory manifestations, causing neuroinflammation in the CNS and PNS. SCFAs and tryptophan derivatives play roles in inhibiting microglial activation. The red arrows represent pro-inflammatory pathways, and the green arrows represent mediators that inhibit inflammation or maintain normal growth and development of microglia. AHR, aryl hydrocarbon receptor; βAR, beta adrenergic receptor; CSF1, colony-stimulating factor 1; CSF1R, CSF1 receptor; GPR43/109A, G protein-coupled receptor 43/109A; GR, glucocorticoid receptor; IL, interleukin; IL-3Rα, IL-3 receptor alpha; IL-6R, IL-6 receptor; LPS, lipopolysaccharide; SCFAs, short-chain fatty acids; TGFβ: transforming growth factor beta; TGFβR, TGFβ receptor; TLR, toll-like receptor; TNF-α, tumor necrosis factor alpha; Trp, tryptophan.
Table 1.
Properties and functions of microglia.
| Function of microglia | State | Action | Pathways and molecules | References |
|---|---|---|---|---|
| Neuronal support | Developing | Promote the development, and maintain the function of neurons | CX3CR1, IGF1, P2Y13 | Araujo and Cotman (1992), Yamagata et al. (1995), Nakajima et al. (2001), Trang et al. (2011) and Ribeiro Xavier et al. (2015) |
| Resting | Maintain the development and survival of neurons | BDNF, NGF, bFGF, EGF, HGF | Ziv et al. (2006), Ueno et al. (2013) and Stefani et al. (2018) | |
| Resting or activating | Monitor neural environment and response to damage | ATP, UTP | Davalos et al. (2005), Nimmerjahn et al. (2005) and Castellano et al. (2016) | |
| Stroke | Includes reduction of excitotoxic injury | NA | Szalay et al. (2016) | |
| Neuronal damage | Developing | Promote neuronal apoptosis during development | NGF, CD11b, DAP12 | Wakselman et al. (2008) |
| Depression | Impair neurogenesis | IL-10, CX3CR1 | Bassett et al. (2021) and Yang et al. (2021) | |
| AD | Cause damage or loss of neurons | CSF1R | Spangenberg et al. (2016) and Sosna et al. (2018) | |
| PD | Cause degeneration and depletion of dopaminergic neurons | CD200-CD200R, CX3CR1 | Shan et al. (2011) and Zhang et al. (2011) | |
| Bacterial infection | Impair neurogenesis | TLR2, NO | Lehnardt et al. (2006) and Hoffmann et al. (2007a) | |
| Virus infection | Induce neuronal apoptosis | IFN-γ | Garber et al. (2019) | |
| Phagocytosis | Developing | Regulate phagocytosis and shape CNS function | Endocannabinoid | Cunningham et al. (2013) and VanRyzin et al. (2019) |
| Resting | Promote the clearance of apoptotic neurons | TREM2, DAP12, Mertk | Takahashi et al. (2005) and Damisah et al. (2020) | |
| AD | Mediate the phagocytosis of Aβ | CD47, TREM2, | Li et al. (2012), Zhao et al. (2018) and Dionisio-Santos et al. (2019) | |
| Bacterial infection | Increase phagocytosis activity against pathogens | TLR2, TLR4, TLR9 | Ribes et al. (2010) | |
| Synaptic pruning | Developing | Eliminate synapses to ensure proper brain connectivity | CX3CR1, complement, TREM2 | Paolicelli et al. (2011), Filipello et al. (2018) and Gunner et al. (2019) |
| Resting | Modify synaptic morphology | P2Y12, TWEAK | Tremblay et al (2010), Sipe et al. (2016) and Cheadle et al. (2020) | |
| Resting | Regulate synaptic function | ATP, THIK-1 | Wake et al. (2009) and Badimon et al. (2020) | |
| Depression | Prune synapses in excess | complement | Li et al. (2023c) and Wang et al. (2023b) | |
| AD | Prune synapses in excess | CD47 | Ding et al. (2021) | |
| Virus infection | Eliminate synapse Pathologically | IFN-γ | Garber et al. (2019) | |
| Immune response | Depression | Secret IL-1β, IL-2, IL-6, TNF-α, and IFN-γ | NLRP3, PGE2 | Jia et al. (2021) |
| PD | Release TNF-α, NO, ROS | MMPs, LRRK2 | Lee et al. (2010) and Moehle et al. (2012) | |
| Bacterial infection | Secrete pro-inflammatory cytokines, and chemokine | TLR1, TLR2, TLR4, TLR6 | Prinz et al. (1999), Kielian (2004), Domínguez-Punaro et al. (2007) and Hoffmann et al. (2007b) | |
| Bacterial infection | Play the role of antigen presentation | MHC II, CD40, CD80, CD86 | (Kielian T. 2004) | |
| Virus infection | Present the antigen (Moseman et al., 2020), and activate the T cells | MHC I, CSF1R | Wheeler et al. (2018) and Funk and Klein (2019) |
ATP, adenosinetriphosphate; BDNF, brain-derived neurotrophic factor; bFGF, basic fibroblast growth factor; CX3CR1, C-X3-C motif chemokine receptor 1; DAP12, DNAX activation protein of 12 kDa; EGF, epidermal growth factor; HGF, hepatocyte growth factor; IFN, interferon; IGF1, insulin like growth factor 1; IL, interleukin; LRRK2, leucine-rich repeat kinase 2; MHC, major histocompatability complex; MMPs, matrix metalloproteinases; NGF, nerve gowth factor; NLRP3, pyrin-domain-containing 3; NO, nitric oxide; PGE2, prostaglandin E2; THIK-1, TWIK-related halothane-inhibited K channel; TLR, toll-like receptor; TREM2, triggering receptor expressed on myeloid cells-2; TWEAK, TNF-associated weak inducer of apoptosis; UTP, uridine triphosphate.
Gut microbiota affects microglia
Gut microbiota influences microglia development, maturation, and function
Recently, the role of the gut microbiota and their metabolites in microglial function and central and peripheral nervous system dysfunction has received extensive attention. The most direct evidence of this is the abnormal development and function of microglia in germ-free (GF) animals. A previous study has shown that GF mice display global defects in microglia, with altered cell proportions (Erny et al., 2015; Thion et al., 2018) and an immature phenotype, leading to impaired innate immune responses (Erny et al., 2015). This suggests that the intestinal microbiota play a substantial role in maintaining the normal development of microglia. Further studies have shown that microglial colonization in GF mice is altered in a time- and sex-specific manner. Specifically, GF mice showed increased microglial density and hyper-differentiation at the embryonic stage, and the microglial density in male brains was significantly higher than that in females; however, in adult females, it increased relative to males (Thion et al., 2018). Gut microbiota also influences the development and aging of microglia. Microglia from GF mice exhibit reduced expression of genes associated with inflammatory and defense responses (Matcovitch-Natan et al., 2016). Microglia in aged specific pathogen-free (SPF) mice showed markedly altered morphology compared to young SPF mice. However, no significant difference was observed in microglia between aged and young GF mice (Mossad et al., 2022). Similarly, a key feature of microglial aging is increased oxidative stress, manifested by elevated levels of intracellular reactive oxygen species (ROS) (Streit, 2006). Microglial oxidative stress and ROS expression significantly increased in aged SPF mice; however, this increase was less pronounced in GF mice (Mossad et al., 2022).
Depletion of the gut microbiota and reduced diversity of the microbiota also affect microglial maturation and function. In addition to their antibacterial effects, antibiotics cause “side effects” that disturb the intestinal microbiota. Antibiotic (ABX) treatment promotes the downregulation of homeostasis-related genes P2ry12, P2ry13, Selplg, Gpr165, and Cst3 in the spinal microglial cells of superoxide dismutase 1 (SOD1) mice (an amyotrophic lateral sclerosis animal model), whereas neurodegenerative disease characteristic-related genes include the upregulation of Apoe, Lgals3 bp, and Cst7. Antibiotics induce the transition of microglia from a homeostatic to a pathogenic state in SOD1 mice (Cox et al., 2022). Additionally, microglial overactivation due to antibiotic depletion of the gut microbiota exacerbates multiple diseases. For example, antibiotic treatment can worsen herpes simplex virus type 1 (HSV-1) infection, causing herpes simplex encephalitis. HSV-1 infection causes mitochondrial dysfunction, and the downregulation of PINK1-PRKN inhibits mitophagy and activates microglia to promote inflammation. Consequently, the nicotinamide oxidation product of neomycin-susceptible bacteria can limit microglial activation, promote mitophagy, and inhibit inflammation (Li et al., 2022a, 2023a). Furthermore, an FMT from non-ABX-treated mice to ABX-treated male mice reduced cecal enlargement and restored microglial structure and morphology in ABX mice (Dodiya et al., 2019). More importantly, the use of antibiotics will affect microglia and have a profound impact on future generations. Antibiotic-induced maternal microbiota dysbiosis (MMD) led to dysfunction of the intestinal microbiota and their metabolites in pregnant mice, increased expression of microglial senescence genes Trp53 and Il1β, and caused abnormal social behavior in offspring mice. Maternal supplementation with Lactobacillus murinus HU-1 alleviated MMD-induced abnormalities in microglia and social behavior in offspring (Lebovitz et al., 2019). This result has been confirmed in clinical trials showing that prenatal antibiotic exposure during fetal delivery may increase the risk of various postpartum infection incidents (Nakitanda et al., 2023). Therefore, whether it is a GF or an antibiotic-treated animal, the morphology and function of microglia will be disturbed, which fully illustrates the regulation of the intestinal microbiota in microglia. However, how such a large number of intestinal microbiota affect and regulate microglia still requires elucidation.
Indeed, the composition of the microbiota structure in certain disease states may be an important factor in disease development. AD-associated gut microbiota (characterized by enrichment in Bacteroides spp.) can upregulate pro-inflammatory polyunsaturated fatty acid metabolism through activation of the C/EBPβ/AEP pathway, enhancing microglial activation and aggravating neuroinflammation, thereby promoting AD pathology and cognitive impairment (Chen et al., 2022a). Similarly, when sleep-deprived mouse microbiota were transplanted into normal mice, the normal mice showed excessive activation of microglia, apoptosis of neurons in the hippocampus, and cognitive decline (Wang et al., 2023a).
However, in this state of disease, what role does the microbiota play in regulating microglia? The commensal microbiota can promote the antigen presentation of microglia by activating the Toll-like receptor (TLR) 4 signaling pathway to help the host resist viral infection (Brown et al., 2019). Intervention with heat-killed Mycobacterium vaccae reduced the basal levels of genes involved in microglial priming (Nlrp3 and Nfkbia), blocked microglial priming in response to immune stress, and attenuated stress-induced anxiety-like behavior (Frank et al., 2018). Prebiotic intervention can inhibit related pro-inflammatory and neurotoxic signaling pathways in α-synuclein-overexpressing mice and upregulate the neuroprotective phenotype of microglia (Abdel-Haq et al., 2022).
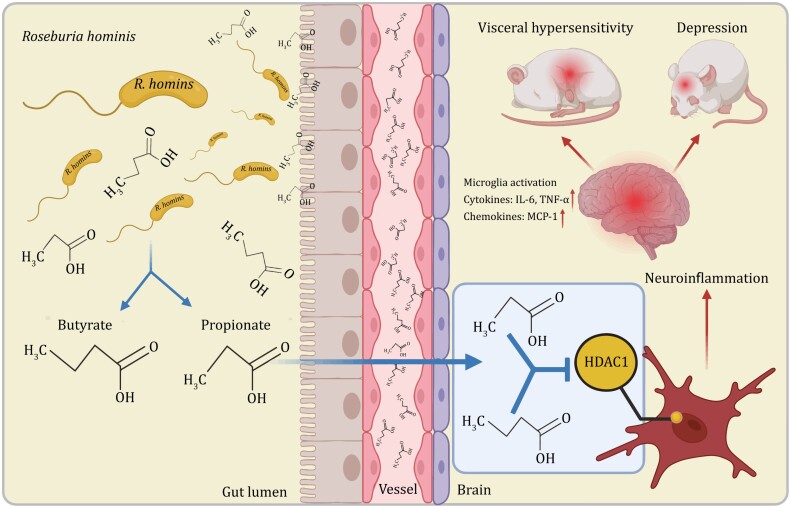
It is a promising method for regulating microglia by regulating the intestinal microbiota to prevent and treat diseases. Therefore, understanding the effects of specific strains, such as the positive effects of known probiotics is urgently required. Various substances produced during the metabolism of the intestinal microbiota, such as short-chain fatty acids (SCFAs), are important factors that affect microglia (Silva et al., 2020). First, SCFA receptor G protein-coupled receptor (GPR43) (encoded by Ffar2)-deficient mice exhibit microglial defects consistent with those in GF mice (Erny et al., 2015). This suggests that SCFAs are important for microglial development and function. When we focused on one of specific SCFA, we found that microbiota-derived acetic acid can be taken up by microglia, and defects in gene expression, cell morphology, metabolic characteristics, and mitochondria of microglia in GF mice can be restored by supplementing acetic acid (Erny et al., 2021). Clostridium butyricum intervention improves motor function, inhibits microglial activation, and improves synaptic dysfunction in mice with Parkinson’s disease (PD) (Sun et al., 2021). Butyrate supplementation ameliorates chronic alcoholic CNS injury by inhibiting microglia-mediated neuroinflammation via the GPR109A/PPAR-γ/TLR4-NF-κB signaling pathway (Wei et al., 2023). Our study has shown that gavage with propionate and butyrate can improve abnormal microglial morphology in GF rats by inhibiting histone deacetylase-1 (HDAC1) expression and histone H3 acetylation K9 levels and reduce the expression of various inflammatory factors in the brain, such as those of IL-6, IFN-γ, and MCP-1 (Song et al., 2022) (Fig. 3). However, SCFAs released by microbiota also negatively affect the regulation of microglia. In a mouse model of AD, acetic acid inhibited the phagocytosis of amyloid-beta (Aβ) by microglia and increased Aβ plaques (Erny et al., 2021).
Figure 3.
Roseburia hominis alleviates neuroinflammation in rat. Roseburia hominis alleviates neuroinflammation by producing propionate and butyrate, which serve as HDAC inhibitors. This provides a potential psychoprobiotic to reduce neuroinflammation. HDAC1, histone deacetylase-1; IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha.
In addition to SCFAs, plant foods are metabolized by intestinal microorganisms to produce tryptophan derivatives, which activate the aryl hydrocarbon receptor (AHR) on microglial cells, directly promote transforming growth factor (TGF)-α, and inhibit the transcription of VEGF-β, thereby inhibiting the expression of the astrocyte inflammatory response and suppressing CNS inflammation (Rothhammer et al., 2018). Bile acid levels are also regulated by the gut microbiome. For microglia, activation of primary bile acid receptors such as sphingosine-1-phosphate receptor 2 can lead to activation of microglia and increased expression of chemokine ligand 2 release from neurons (McMillin et al., 2017; Agus et al., 2021). In contrast, secondary bile acids such as hyodeoxycholic acid and tauroursodeoxycholic acid may ameliorate CNS and microglial inflammation via the Takeda G protein-coupled receptor 5 (TGR5) and farnesoid X receptor (FXR) pathways (Bhargava et al., 2020; Zhu et al., 2022). Isoamylamine (IAA), a small-molecule metabolite produced by Ruminococcus, promotes microglial cell death and cognitive decline in aged mice by inducing S100A8 expression (Teng et al., 2022). Senescence of microglia manifests as ROS accumulation. Accumulation of ROS in the aging brain is associated with mitochondrial damage and mitochondrial dysfunction (Stefanatos and Sanz, 2018). Moreover, the intestinal microbiota can interact with intestinal epithelial mitochondria, affect mitochondrial function, and participate in the regulation of body homeostasis (Zhang et al., 2022b). Metabolomic analysis revealed that N6-carboxymethyllysine (CML), produced by bacterial metabolism, accumulated in the blood and brain of aged non-GF mice, and CML also increased with aging in human serum and brain. CML increases ROS levels and inhibits mitochondrial activity and adenosinetriphosphate (ATP) accumulation in microglia (Mossad et al., 2022). Branched-chain amino acids (BCAAs) are a group of essential amino acids, and the gut microbiota plays a role in regulating their levels (Agus et al., 2021). The accumulation of BCAAs caused by intestinal microbiota disturbance activates the AKT/STAT3/NF-κB pathway and exacerbates microglia-mediated neuroinflammation (Shen et al., 2023). The gut microbiome produces the metabolite trimethylamine, which is converted to trimethylamine N-oxide (TMAO) in the liver. High levels of TMAO increase the number of activated microglia and CNS inflammation (Li et al., 2022b). This process may be mediated by ROS production and the downregulation of methionine sulfoxide reductase A76 (Meng et al. 2019).
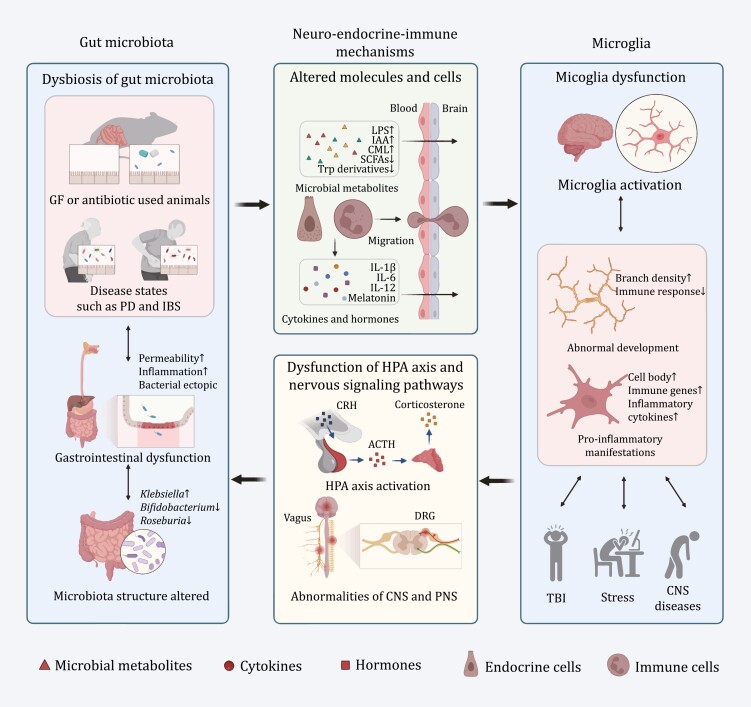
We briefly summarized and exemplified some possible mechanisms by which the gut microbiota regulates microglia (Figs. 2 and 4).
Figure 4.
Potential mechanisms of microbiota–microglia interaction. In the absence or disturbance of the gut microbiota, the composition and abundance of microbial metabolites are altered. These metabolites, microbiota-derived inflammatory cytokines and hormones can enter the circulation and pass through the blood‒brain barrier, simultaneously with the migration of immune cells, leading to immaturity, abnormal activation and increased pro-inflammatory manifestations in microglia. Microglia are activated and participate in central inflammation and nervous signaling pathway dysfunction in brain injury, stress and a variety of psychiatric and neurodegenerative diseases, therefore causing HPA axis activation and abnormalities of the CNS and PNS. Then, GI dysfunction appears and further alters the dysbiosis of the gut microbiota. ACTH, adrenocorticotropic hormone; CML, N6-carboxymethyllysine; CNS, central nervous system; CRH, corticotropin-releasing hormone; DRG, dorsal root ganglion; GF, germ-free; IAA, isoamylamine; HPA, hypothalamic‒pituitary‒adrenal; IBS, irritable bowel syndrome; LPS, lipopolysaccharide; PD, Parkinson’s disease; PNS, peripheral nervous system; SCFAs, short-chain fatty acids; Trp, tryptophan.
Influence of gut microbiota on microglia in IBS
Although there is a lack of evidence for microglial activation in population cohort studies, various animal models of visceral hypersensitivity in IBS support this hypothesis. In animal models of IBS visceral hypersensitivity, the number of microglial cells in the dorsal horn of the spinal cord and brain increases, and is substantially activated (Zhang et al., 2016; Lucarini et al., 2020; Yuan et al., 2020; Atmani et al., 2022; Ji et al., 2022). The number of microglia in the dorsal horn of the L6-S1 spinal cord increased in a mouse model of visceral hypersensitivity, induced by intravesical perfusion of acetic acid (Atmani et al., 2022). The number of M1 pro-inflammatory microglia and M1 pro-inflammatory microglia-derived cytokines IL-6 and tumor necrosis alpha (TNF-α) increased in the anterior lateral bed nucleus of the stria terminalis of neonatal colorectal distension-induced chronic visceral hyperalgesia model rats (Ji et al., 2022).
Additionally, disturbances in gut microbiota have been reported in different IBS models. As previously mentioned, gut microbiota has three pathways affecting the CNS (Margolis et al., 2021).
For immune pathways, gut microbiota plays essential role in immune development and function (Belkaid and Harrison, 2017). For example, segmented filamentous bacteria (SFB) can adhere to the ileum and induce differentiation of T helper 17 cells (Ivanov et al., 2009). In addition, the gut microbiota can release a variety of metabolites and components such as lipopolysaccharide (LPS) and peptidoglycan into the circulating blood, thereby affecting the functions of the central and peripheral nervous systems (Agirman et al., 2021). The entry of gut-derived inflammatory factors (such as IL-1β, IL-6, and TNF-α) into the circulation may alter the integrity of the blood–brain barrier (BBB) and affect brain development (Erny et al., 2015; Thion et al., 2018; Parker et al., 2020). These metabolites and cytokines cause the activation of immune cells in the brain through the BBB, and can also activate immune cells in the intestinal tract. Some immune cells can even migrate to the CNS to promote or inhibit neuroinflammation and peripheral inflammation. Our previous studies have shown that transplantation of fecal microbiota from patients with IBS-D into GF rats can lead to visceral hypersensitivity and intestinal inflammation (as shown by an increased number of mast cells). Simultaneously, the spinal cord microglial cells of GF rats transplanted with feces from patients with IBS-D were significantly activated, and the branch area, length, number of branch points, number of segments, and number of terminal points were significantly lower than those in the control group (Zhang et al., 2021). Roseburia hominis gavage significantly reduces visceral hypersensitivity and improves microglial activation in the cingulate gyrus, dorsal hippocampus, and spinal cord of GF rats (Song et al., 2022).
For endocrine pathway, inflammation-induced activation of the hypothalamic–pituitary–adrenal (HPA) axis triggers the release of glucocorticoids, altering gut function (Cryan et al., 2019). The function of the HPA axis is regulated by the gut microbiota. Corticosterone levels and HPA axis activity were increased in GF mice, suggesting a central role for gut microbiota in HPA axis development and neuromodulation. Moreover, probiotics may affect neuroendocrine physiological homeostasis through immune regulation and reducing intestinal microbiota translocation (Cohen et al., 2015).
Melatonin is the main hormone in the pineal gland and is primarily synthesized by enterochromaffin cells in the GI tract. Melatonin regulates immunity, inflammation, and mood, relieves anxiety and depression, promotes sleep, and regulating GI motility (Ma et al., 2020). Patients with IBS-D have elevated melatonin levels and increased numbers of mast cells in the colonic mucosa. Melatonin may participate in the regulation of visceral hypersensitivity in IBS-D by inhibiting the activation of colonic mucosal mast cells. When GF rats received FMT from patients with IBS-D, the colonic mucosal melatonin levels increased. Further analysis revealed that Roseburia and Lachnospira species may promote colonic mucosal melatonin production through their metabolite butyrate (Wang et al., 2020). When GF rats were administered Roseburia hominis, melatonin levels in the ileum and colonic mucosa increased. Propionate and butyrate levels also increased. Furthermore, after gavage with propionic acid and butyric acid in GF rats, melatonin levels in the peripheral blood, ileum, and colonic mucosa also increased (Song et al., 2021).
For neuronal pathways, the most typical is the two-way regulation of gut microbiota and vagus nerve. Campylobacter jejuni can induce anxiety-related behaviors by enhancing vagal signaling to the nucleus tractus solitarius (NTS) (Goehler et al., 2005). Ameliorative effects of Lactobacillus rhamnosus JB1 on anxiety-related and depression-like behaviors can be blocked by vagotomy (Bravo et al., 2011). In turn the stimulation of afferent vagal and dorsal root ganglion (DRG) neurons that sense inflammation triggers CNS circuits involved in HPA axis activation, disease behavior [such as anorexia (Rao et al., 2017)], and visceral pain perception. Efferent nerves send signals to immune cells, inhibit the invasion of pathogenic bacteria, and induce the enrichment of protective microbiota by inhibiting pro-inflammatory macrophages, regulating M cell function (Lai et al., 2020), and inhibiting intestinal inflammation. A rat model of water avoidance stress (WAS) exhibited elevated visceral sensitivity, increased fecal particle counts, and activation of microglial cells in the dorsal horn of the spinal cord (Zhang et al., 2019b, 2021). Similar results have been observed in a mouse model of visceral hypersensitivity induced by chronic WAS (Yuan et al., 2020). Additional supplementation of Roseburia hominis can improve the abnormalities caused by WAS stress in rats, such as the decline of corticotropin-releasing hormone (CRH), reduction in visceral sensitivity, and decreasing of the fecal pellet number (Zhang et al., 2019b).
Correction or intervention of intestinal microbiota can inhibit microglial activation, relieve central inflammation, and improve IBS symptoms. The relative abundances of Ruminococcus and Spirillum increased when rifaximin was administered to animals with chronic and unpredictable mild stress (CUMS). In addition, rifaximin, a non-absorbable antibiotic, prevents and ameliorates CUMS-induced central functional abnormalities and elevates butyrate levels in the brain (Li et al., 2021). Some traditional Chinese medicine has been shown to have certain beneficial effects. Shugan granules are patented Chinese medicines. Shugan granule treatment in rats with chronic restraint stress can regulate intestinal microbiota, increase the abundance of Bacteroides, and improve the activation of microglia and intestinal barrier repair in model animals (Li et al., 2022c). Ginsenosides are the main active ingredients in ginseng and have a wide range of pharmacological effects. Ginsenoside Rh4 alleviates intestinal microbiota disturbances in depression-like mice, increases the content of SCFAs, inhibits excessive activation of microglia and astrocytes, and improves depressive behavior. Correlation analysis revealed that the Rh4-inhibited LPS/NLRP3/caspase-1/IL-1β signaling pathway plays a key role in improving depression (Shao et al., 2023). Berberine (BBR) is a natural alkaloid that exerts various physiological functions in the body by regulating the intestinal microbiota. BBR can significantly alleviate visceral hypersensitivity induced by chronic WAS and reduce the activation of colonic mast cells and spinal cord microglia by enriching SCFA-producing bacteria (Zhang et al., 2021). In addition, the psychbiotic Roseburia hominis inhibits the activation of microglial cells, reducing the level of inflammation in the brain, relieving neuroinflammation, and reducing depression-like behavior (Song et al., 2022) (Fig. 3).
Microglia affect gut microbiota
Effects of the CNS on intestinal microbiota
Many studies have found that the CNS, especially microglia, can regulate the structure and function of intestinal microbiota. Traumatic brain injury (TBI) is an excellent candidate model for studying the brain–gut–microbiota axis as the earliest pathological change that occurs in the brain. TBI is accompanied by abnormal changes in microglia. In addition to central manifestations, patients with TBI have abnormal GI function with decreased GI smooth muscle contractility, delayed transit time, epithelial cell shedding, intestinal villus rupture, focal ulceration, and decreased expression of intestinal tight junction proteins such as occludin and zonula occludens-1. In TBI animal models, an imbalance in the intestinal microbiota of mice has also been observed. Specifically, the severity of TBI was related to the abundance of Bacteroides, Porphyromonas, Firmicutes, and Proteobacteria (Sundman et al., 2017). In TBI, these changes in the gut microbiota are directly caused by damage to the CNS and abnormal changes in microglia.
Central stress also induces changes in the gut microbiota and is involved in the activation of microglia through β-adrenergic signaling and the glucocorticoid receptor (GR) on microglia (Johnson et al., 2005; Blandino et al., 2006; Picard et al., 2021). In clinical studies, stress has been shown to disrupt the gut microbiota. Under stress, the permeability of the intestinal mucosa increases, α-diversity of the intestinal microbiota increases, and relative abundance of approximately half of the species changes, including the increase in the abundance of the weaker dominant groups, while the more dominant groups, such as Bacteroides and lactic acid bacteria, decrease in abundance (Knowles et al., 2008; Karl et al., 2017). In stress-induced animal models, the abundance of the families Prevotellaceae and Coriobacteriaceae and the genus Prevotella_1 increased significantly, whereas those of Peptococcaceae and Veillonellaceae were significantly reduced (Zhang et al., 2019b). Central stress can alter the transcription of intestinal epithelial genes, such as Reg3b, Duox2, Nos2, and other inflammation-related genes, accompanied by changes in the intestinal microbiota and translocation of bacteria (Allen et al., 2022). Piglets stressed by maternal separation have altered gut microbiota and are more prone to diarrhea, weight loss, and death. Alkaline mineral water can inhibit the HPA axis, thereby reshaping the intestinal microbiota, maintaining the stability of the intestinal epithelium through the brain–microbiota–gut axis, and improving digestive system symptoms (Chen et al., 2022b).
As stated above, antibiotic-induced changes in the maternal microbiota can affect CNS function in offspring, especially that of microglia. Conversely, the central stress of the mother affects the composition of the intestinal microbiota of the offspring while affecting microglia. Clinical data have shown that infants born to mothers with high levels of anxiety, depression, and stress have reduced alpha diversity and a relative abundance of Bifidobacterium dentalis in their gut microbiota (Galley et al., 2023). In animal studies, dams exposed to prenatal stress had altered gut microbiota in their female offspring and not in their male offspring and exhibited more severe colonic damage in a model of neonatal necrotizing enterocolitis-like injury. Tissue damage is due to higher microbiota IgA binding in female offspring than in male offspring (Brawner et al., 2020).
Microglia, as important immune cells of the CNS, affect the gut microbiota in several ways. In Ang II-induced hypertension model mice, the number and activation of microglia increased, α-diversity of the intestinal microbiota decreased, and evident clustering appeared (Sharma et al., 2019). Considering the broad physiological functions of microglia in brain development and maintenance of homeostasis, they are thought to be involved in the pathogenesis of various psychiatric and neurodegenerative diseases, which consequently, may affect the gut microbiota. For example, in patients with autism spectrum disorder (ASD), the density of sensitized microglia is increased and the expression of pro-inflammatory genes in microglia is upregulated (Gupta et al., 2014; Lee et al., 2017). Altered gut microbiota with increased Clostridia and Lactobacilli and decreased Bacteroides and Bifidobacteria were found in stool samples from children with ASD (Abdel-Haq et al., 2019). AD is another disease in which microglia play an important role. Microglia act as phagocytes in the brain and are responsible for clearing amyloid Aβ (Abdel-Haq et al., 2019). Chronic neuroinflammation and the release of pro-inflammatory factors caused by the activation of microglia are also important causes of AD (Li et al., 2018). AD also disturbs the intestinal microbiota. Increased proportions of Bacteroidetes, Firmicutes, Lachnospiraceae, Ruminococcaceae, and Bacteroidaceae have also been observed in patients with AD. In aged AD mouse models, reduced abundance of Firmicutes, Verrucomicrobia, Proteobacteria, and Actinobacteria have been observed (Zhou et al., 2022). Microglia are also involved in the pathogenesis of PD. Studies have shown that abnormally activated microglia affect the intercellular transfer of α-synuclein and promote the progression of PD (George et al., 2019). The composition of the gut microbiota is significantly altered in patients with PD. A clinical study found that the gut microbiota diversity of patients with PD was lower than that in healthy controls. The abundance of Verrucomicrobia, Prevotella, Porphyromonas, Lactobacillus, and Parabacteroides increased, and dysbiosis of the microbiota correlated with intestinal inflammation (Lin et al., 2019). In addition, SCFAs were significantly reduced among the intestinal microbiota metabolites in patients with PD (Unger et al., 2016).
This chapter focuses on correlation studies and lacks the exploration of causality. Diseases and changes in the intestinal microbiota are the result of the joint action of several factors and are not necessarily caused solely by microglial regulation. As there is a two-way connection between the CNS and the GI tract, the role of microglia in shaping the intestinal microbiota is inevitable; however, more research is needed to confirm this. Therefore, we speculated a possible mechanism by which microglia affect the gut microbiota from the perspective of CNS regulatory mechanisms (Fig. 4).
The potential mechanism by which the CNS affects the gut microbiota
Activation of the HPA axis under stress is an important pathway by which the CNS and microglia affect the gut microbiota. Stress can lead to intestinal barrier dysfunction, abnormal activation of intestinal inflammation, and ectopic intestinal bacteria, leading to changes in the gut microbiota (Shaler et al., 2021). Mechanistically, the activation of the HPA axis is accompanied by increased levels of circulating CRH and corticosterone. The corticotropin-releasing hormone receptor 1 (CRHR1) mediates colonic inflammation and mucosal damage (Li et al., 2017). Elevated corticosterone levels reduce intestinal permeability, increase systemic inflammation, and change the structure of intestinal microbiota, which is dependent on the existence of GR (Zheng et al., 2013).
Another possible mechanism involves afferent and efferent neural pathways. Cholinergic vagal efferent fibers can stimulate enteric neurons and subsequently inhibit macrophages to release inflammatory cytokines such as IL-1β, IL-6, IL-18, and TNF-α (Agirman et al., 2021). The DRG is a terminal region that originates from the transmission of peripheral sensations (including general somatic and visceral senses) to the brain. The intestinal nociceptor Nav1.8 and transient receptor potential vanilloid 1+ (TRPV1+) neurons derived from the DRG can mediate the body’s defense against Salmonella by regulating the Peyer’s patch microfold cells to change the ileal microbiota structure, abundance, and levels of segmented filamentous bacteria (Lai et al., 2020). The sympathetic nervous system is widely distributed throughout the digestive tract and regulated by the CNS. Postganglionic neurons of the sympathetic nerve can release neurotransmitters, such as norepinephrine and dopamine into the gut lumen. After receiving these neurotransmitters, the commensal and pathogenic microbiota in the digestive tract express virulence genes, accelerate growth, and cause changes in the structure of the microbiota (Mayer et al., 2022) (Fig. 4).
Influence of central microglia on the microbiota in IBS
As mentioned above, some brain regions in patients with IBS are activated and the gut microbiota is significantly altered. The microglia and gut microbiota of IBS-like model animals are also disturbed. Visceral hypersensitivity is the most important pathophysiological mechanism of IBS, and some studies have suggested that the pro-inflammatory cytokine granulocyte-colony-stimulating factor released by microglia is a key mediator of visceral sensitization (Basso et al., 2017). Increased visceral sensitivity is markedly attenuated by the inhibition of microglial activation (Zhang et al., 2016; Yuan et al., 2020; Atmani et al., 2022; Ji et al., 2022). Furthermore, directly targeting the P2RY12 purinergic receptor on microglia for blockade can inhibit chronic visceral pain mediated by TRPV1+ visceral afferent nerves (Defaye et al., 2022).
Patients with IBS commonly have psychiatric comorbidities. The amelioration of depression-like behaviors in multiple animal models can also be achieved by targeting microglia (Li et al., 2023b; Yang et al., 2023). Minocycline is a microglia-specific inhibitor. Orally administered minocycline in rats with spinal cord injury was found to alleviate cognitive and affective impairments in injured rats and cause changes in the gut microbiota, implying a regulatory function of the gut microbiota of microglia (Schmidt et al., 2021). Minocycline reduces the number of prefrontal microglial cells in a rat model of depression and improves the cecal microbiome by increasing the number of butyric acid-producing bacteria, such as Lachnospiraceae (Schmidtner et al., 2019). However, minocycline is an antibiotic, and whether this change in the gut microbiota is involved remains unknown. Chemically modified tetracycline-3 (CMT-3) is a tetracycline derivative that lacks antibacterial properties, while retaining the inhibitory activity of minocycline on microglia. Intrathecal injection of CMT-3 inhibited microglial activation and it was found to induce a significant antihypertensive effect. This effect is likely mediated by unique changes in the gut microbiota (Sharma et al., 2019).
Although there is no clinical trial evidence at present, we can reasonably speculate that if the microglia of patients with IBS can be inhibited in some way, it is likely to improve the crosstalk between CNS inflammation and the gut microbiota, thereby alleviating their peripheral and central conditions (Fig. 4).
Prospects for the regulating the interaction between microbiota and microglia in the treatment of DGBIs
The treatment of DGBIs remains a challenge for clinicians, especially for DGBIs with comorbid psychiatric disorders. At present, the main method of treatment is to manage symptoms while considering the psychosocial factors of the patients and treating psychological comorbidities. Tricyclic antidepressants (TcAs) and selective serotonin reuptake inhibitors (SSRIs) are recommended for the treatment of the psychological comorbidities of DGBIs; however, there exist a series of side effects associated with the use of these drugs. Based on the content of this review, interventions targeting the intestinal microbiota to regulate microglia could achieve co-treatment of gut and brain, which is very promising and is a field worthy of further exploration. Limited by the lack of current technological development, there are few studies on the changes of human microglia in vivo (Suzuki et al., 2013), and relatively more studies on microglia have chosen to use animal experiments.
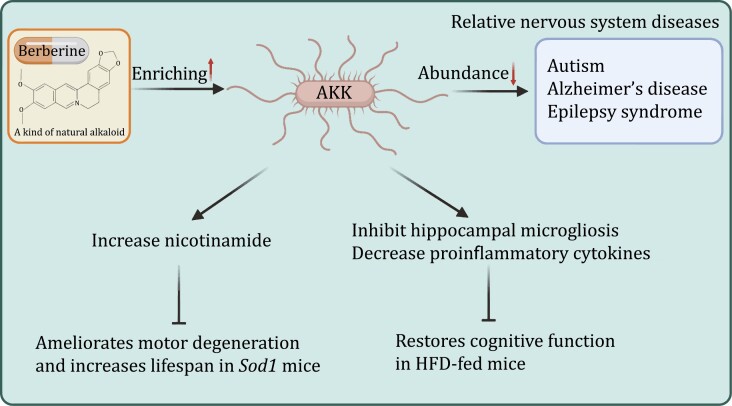
Currently, there are many interventions targeting intestinal microbiota. Roseburia hominis, a kind of typical probiotics, could inhibit abnormal activation of microglia in GF rats, relieve increased visceral sensitivity, and improve depression-like behavior (Song et al., 2022). Early intervention with a high-fiber diet (rich in prebiotics) reduces PD-like symptoms and brain lesions in α-synuclein-overexpressing ASO mice and upregulates a neuroprotective phenotype of microglia (Abdel-Haq et al., 2022). Similarly, as a typical representative of postbiotics, propionic acid and butyric acid produced by the metabolism of the gut microbiota can also inhibit the activation of microglia (Song et al., 2022). But most of the current research focuses on IBS. Although this has an evident effect, the shortcomings of obvious individual variations have also emerged. In addition, the research and development of some agents is important, similar to the ginsenosides mentioned in this review. BBR, a natural alkaloid, is a highly promising drug. Although clinical studies are lacking, multiple animal experiments provide some evidence. BBR could modulate the intestinal microbiota to alleviate visceral hypersensitivity in WAS-induced IBS mice model. In the IBS mouse model constructed by FMT of IBS patients, BBR also exerted a similar effect, alleviating the increased visceral sensitivity (Zhang et al., 2021). Notably, while improving abdominal symptoms, BBR’s treatment of central inflammation and mental disorders, such as anxiety (Fang et al., 2021) and depression (Huang et al., 2023), has also been partially verified in animal experiments. Our previous studies have shown that BBR intervention can affect the structure and function of the intestinal microbiota, especially the enrichment of Akkermansia muciniphila (AKK) and Bacteroidaceae. Recent studies have shown that AKK is related to the occurrence of diseases, such as autism and multiple sclerosis, and can be used as a potential psychobiotic for the next generation (Fig. 5 and Table 2). Further exploration of the potential mechanisms and implementation of clinical trials would be of great significance.
Figure 5.
Potential role of Akkermansia muciniphila in the treatment of neurological diseases. Berberine could significantly enhance the accumulation of AKK in the gut. Altered abundance of AKK in the gut is associated with various neurological diseases. Animal studies have shown that AKK can increase the level of nicotinamide in the brain and alleviate amyotrophic lateral sclerosis. AKK also attenuates HFD-induced cognitive impairment and increases microglia in the hippocampus. AKK, Akkermansia muciniphila; HFD, high-fat diet.
Table 2.
Potential therapeutic progress of regulating of metabolites or microglia in DGBIs or mental disorders.
| Treatment | Subject | Diseases and disorders | Results | References |
|---|---|---|---|---|
| Probiotics | ||||
| Bacillus coagulans | Human | IBS | Improving IBS symptom relief rate, as well as global symptom, abdominal pain, bloating, and straining scores. | Zhang et al. (2022a) |
| Mixed probiotics (Bifidobacterium longum, Lactobacillus acidophilus and Enterococcus faecalis) | Human | IBS | Relief IBS-SSS scale scores in IBS-D patients, relieve abdominal pain symptoms, and reduce plasma MCP-1 and IL-1β levels | Zhang et al. (2019a) |
| Mixed probiotics (Bifidobacterium breve CCFM1025, Bifidobacterium longum CCFM687, and Pediococcus acidilactici CCFM6432) | Human | Depression | Reduced depression scores (Hamilton Depression Rating, Montgomery-Asberg Depression Rating, and Brief Psychiatric Rating Scales), improved the patients’ gastrointestinal functions (Gastrointestinal Symptom Rating Scale) | Tian et al. (2022) |
| Bifidobacterium longum NCC3001 | Human | Depression comorbidity in IBS | Increasing the quality-of-life score and reduce the depression score in IBS patients | Pinto-Sanchez et al. (2017) |
| Heat-killed Mycobacterium vaccae | Mice | Anxiety | Reducing basal levels of genes (Nlrp3 and Nfkbia) involved in microglial priming | Frank et al. (2018) |
| Clostridium butyricum | Mice | PD | Improved motor deficits, loss of dopaminergic neurons, synaptic dysfunction, and microglial activation | Sun et al. (2021) |
| Roseburia hominis | Rat | Depression | Producing SCFAs and inhibits microglia activation | Song et al. (2022) |
| FMT | Human | IBS | Relief of global IBS symptoms | Johnsen et al. (2018) and El-Salhy et al. (2020) |
| Prebiotics | ||||
| Low-FODMAP | Mice | PD | Inhibiting related pro-inflammatory and neurotoxic signaling pathways, upregulates the neuroprotective phenotype of microglia | Abdel-Haq et al. (2022) |
| Low-FODMAP | Human | IBS | Altering the composition of intestinal microbiota reduces the concentration of histamine in urine | Staudacher et al. (2014), McIntosh et al. (2017) and Huaman et al. (2018) |
| Postbiotics | ||||
| Propionic acid and butyric acid | Rat | Depression | Inhibiting HDAC1 expression and reduce the expression of various inflammatory factors in the brain | Song et al. (2022) |
| Butyrate | Mice | Chronic alcoholic central nervous system injury | Improve locomotor hypoactivity, anxiety and depression behaviour, impairs learning, spatial recognition memory, and effectively reduce chronic alcoholic central nervous system damage and correct microglial cell polarization (M1/M2) imbalance | Wei et al. (2023) |
| NAMO | Mice | HSE | Restoring NAD+-dependent mitophagy, inhibiting microglial inflammatory response, and slowing down the progression of HSE |
Li et al. (2022a, 2023a) |
| Drugs | ||||
| Rifaximin | Human | IBS | Relief of global IBS symptoms | Lembo et al. (2016) |
| Shugan granule | Rat | Depression | Regulating the intestinal microbiota, inhibits the activation of microglia and improves intestinal barrier repair | Li et al. (2022c) |
| Ginsenoside Rh4 | Mice | Depression | Alleviating the intestinal microbiota disturbance, inhibiting the excessive activation of microglia and astrocytes, and improve depressive behaviour | Shao et al. (2023) |
| Berberine | Rat | IBS | Modulating the gut microbiota, alleviates visceral hypersensitivity and reduce the activation of colonic mast cells and spinal cord microglia | Zhang et al. (2021) |
| Berberine | Rat | Anxiety | Regulating gut microbiota | Fang et al. (2021) |
| Minocycline | Rat | Depression | Reducing the number of prefrontal microglial cells and improves the cecal microbiome by increasing butyric acid-producing bacteria | Schmidtner et al. (2019) |
FMT, fecal microbiota transplantation; FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols; HDAC1, histone deacetylase-1; HSE, Herpes Simplex Encephalitis; IBS, irritable bowel syndrome.; IBS-D, IBS with diarrhea; IBS-SSS, IBS symptom severity scale; IL-1β, interleukin-1 beta; MCP-1, monocyte chemoattractant protein-1; NAD, nicotinamide adenine dinucleotide; NAMO, nicotinamide n-oxide; PD, Parkinson’s disease; SCFAs, short-chain fatty acids.
Summary
Microglia play an important role in gut–brain regulation in DGBIs. The gut microbiota is an important regulator of homeostasis, and the regulation and mechanism of the gut–brain axis have received increasing attention. As an important therapeutic target, the regulation of intestinal microbiota may achieve gut–brain co-treatment of DGBI. Given the complexity and inter-individual variability of the gut microbiota, the development of probiotics, prebiotics, synbiotics, and plant drugs that can regulate the intestinal microbiota and potentially affect central microglia, is an important research direction in the future. Restricted by techniques, studies on intestinal microbiota and microglia crosstalk are mainly based on animal models. With the vigorous development of microglia labeling technology and brain functional imaging technology, more studies on brain–gut–microbiota interactions in humans are worth expecting. Furthermore, the mechanism by which microglia affect the gut microbiota is far from being elucidated. It will greatly benefit the understanding and treatment of diseases if future work can uncover more brain–gut mechanisms.
Acknowledgements
All figures were created with BioRender.com. We would like to thank Editage for English language editing.
Glossary
Abbreviations
- Aβ
amyloid-beta
- ABX
antibiotic
- ACTH
adrenocorticotropic hormone
- AD
Alzheimer’s disease
- AHR
aryl hydrocarbon receptor
- AKK
Akkermansia muciniphila
- ASD
autism spectrum disorder
- ATP
adenosinetriphosphate
- βAR
beta adrenergic receptor
- BBB
blood–brain barrier
- BCAAs
branched-chain amino acids
- BDNF
brain-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- CML
N6-carboxymethyllysine
- CMT-3
chemically modified tetracycline-3
- CNS
central nervous system
- CRH
corticotropin-releasing hormone
- CRHR1
corticotropin-releasing hormone receptor 1
- CSF1
colony-stimulating factor 1
- CSF1R
CSF1 receptor
- CUMS
chronic and unpredictable mild stress
- CX3CR1
C-X3-C motif chemokine receptor 1
- DAP12
DNAX activation protein of 12 kDa
- DGBIs
disorders of gut–brain interaction
- EGF
epidermal growth factor
- FC
functional constipation
- FD
functional dyspepsia
- FGIDs
functional gastrointestinal disorders
- fMRI
functional magnetic resonance imaging
- FMT
fecal microbiota transplantation
- FXR
farnesoid X receptor
- GF
germ-free
- GI
gastrointestinal
- GPR43/109A
G protein-coupled receptor 43/109A
- GR
glucocorticoid receptor
- HDAC1
histone histone deacetylase-1
- HFD
high-fat diet
- HGF
hepatocyte growth factor
- HPA
hypothalamic‒pituitary‒adrenal
- HSV-1
herpes simplex virus type 1
- IAA
isoamylamine
- IBS
irritable bowel syndrome
- IBS-D
IBS with diarrhea
- IBS-SSS
IBS symptom severity scale
- IFN
interferon
- IGF1
insulin like growth factor 1
- IL
interleukin
- IL-3Rα
IL-3 receptor alpha
- IL-6R
IL-6 receptor
- low-FODMAP diets
low fermentable oligosaccharides, disaccharides, monosaccharides and polyols diets
- LPS
lipopolysaccharide
- LRRK2
leucine-rich repeat kinase 2
- MCP-1
monocyte chemoattractant protein-1
- MHC
major histocompatability complex
- MMD
maternal microbiota dysbiosis
- MMPs
matrix metalloproteinases
- mPFC
medial prefrontal cortex
- NGF
nerve gowth factor
- NLRP3
pyrin-domain-containing 3
- PD
Parkinson’s disease
- PGE2
prostaglandin E2
- PNS
peripheral nervous system
- ROS
reactive oxygen species
- SCFAs
short-chain fatty acids
- SOD1
superoxide dismutase 1
- SPF
specific pathogen-free
- SSRIs
selective serotonin reuptake inhibitors
- TBI
traumatic brain injury
- TcAs
tricyclic antidepressants
- TGF
transforming growth factor
- TGFβR
TGFβ receptor
- TGR5
Takeda G protein-coupled receptor 5
- TLR
toll-like receptor
- TMAO
trimethylamine N-oxide
- TNF-α
tumor necrosis factor alpha
- TREM2
triggering receptor expressed on myeloid cells-2
- Trp
tryptophan
- TRPV1+
transient receptor potential vanilloid 1+
- THIK-1
TWIK-related halothane-inhibited K channel
- TWEAK
TNF-associated weak inducer of apoptosis
- UTP
uridine triphosphate
- WAS
water avoidance stress
Contributor Information
Haonan Zheng, Department of Gastroenterology, Peking University Third Hospital, Beijing 100191, China; Beijing Key Laboratory for Helicobacter Pylori Infection and Upper Gastrointestinal Diseases, Beijing 100191, China.
Cunzheng Zhang, Department of Gastroenterology, Peking University Third Hospital, Beijing 100191, China; Beijing Key Laboratory for Helicobacter Pylori Infection and Upper Gastrointestinal Diseases, Beijing 100191, China.
Jindong Zhang, Department of Gastroenterology, Peking University Third Hospital, Beijing 100191, China; Beijing Key Laboratory for Helicobacter Pylori Infection and Upper Gastrointestinal Diseases, Beijing 100191, China.
Liping Duan, Department of Gastroenterology, Peking University Third Hospital, Beijing 100191, China; Beijing Key Laboratory for Helicobacter Pylori Infection and Upper Gastrointestinal Diseases, Beijing 100191, China.
Funding
This work was supported by the National Key R&D Program of China (No. 2021YFA1301300, 2019YFA0905600) and the National Natural Science Foundation of China (No. 82170557, 81670491, 82000510).
Conflict of interest
The authors declare that they have no conflicts of interest.
Author contributions
HZ and CZ designed the contents. LD obtained funding. HZ, CZ and JZ wrote the draft of the manuscript. LD performed critical revisions of the manuscript.
References
- Abdel-Haq R, Schlachetzki JCM, Glass CKet al. Microbiome-microglia connections via the gut–brain axis. J Exp Med 2019;216:41–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Haq R, Schlachetzki JCM, Boktor JCet al. A prebiotic diet modulates microglial states and motor deficits in α-synuclein overexpressing mice. Elife 2022;11:e81453. doi: 10.7554/eLife.81453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adewuyi EO, O’Brien EK, Porter Tet al. Relationship of cognition and Alzheimer’s Disease with gastrointestinal tract disorders: a large-scale genetic overlap and mendelian randomisation analysis. Int J Mol Sci 2022;23(24):16199. doi: 10.3390/ijms232416199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agirman G, Yu KB, Hsiao EY.. Signaling inflammation across the gut–brain axis. Science 2021;374:1087–1092. [DOI] [PubMed] [Google Scholar]
- Agus A, Clément K, Sokol H.. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021;70:1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A, Barres BA, Bennett ML.. Microglia: scapegoat, saboteur, or something else? Science 2013;339:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JM, Mackos AR, Jaggers RMet al. Psychological stress disrupts intestinal epithelial cell function and mucosal integrity through microbe and host-directed processes. Gut Microbes 2022;14:2035661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo DM, Cotman CW.. Basic FGF in astroglial, microglial, and neuronal cultures: characterization of binding sites and modulation of release by lymphokines and trophic factors. J Neurosci 1992;12:1668–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmani K, Wuestenberghs F, Baron Met al. Bladder-colon chronic cross-sensitization involves neuro-glial pathways in male mice. World J Gastroenterol 2022;28:6935–6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badimon A, Strasburger HJ, Ayata Pet al. Negative feedback control of neuronal activity by microglia. Nature 2020;586:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balemans D, Aguilera-Lizarraga J, Florens MVet al. Histamine-mediated potentiation of transient receptor potential (TRP) ankyrin 1 and TRP vanilloid 4 signaling in submucosal neurons in patients with irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2019;316:G338–G349. [DOI] [PubMed] [Google Scholar]
- Bassett B, Subramaniyam S, Fan Yet al. Minocycline alleviates depression-like symptoms by rescuing decrease in neurogenesis in dorsal hippocampus via blocking microglia activation/phagocytosis. Brain Behav Immun 2021;91:519–530. [DOI] [PubMed] [Google Scholar]
- Basso L, Lapointe TK, Iftinca Met al. Granulocyte-colony-stimulating factor (G-CSF) signaling in spinal microglia drives visceral sensitization following colitis. Proc Natl Acad Sci USA 2017;114:11235–11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Harrison OJ.. Homeostatic immunity and the microbiota. Immunity 2017;46:562–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava P, Smith MD, Mische Let al. Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J Clin Invest 2020;130:3467–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black CJ, Drossman DA, Talley NJet al. Functional gastrointestinal disorders: advances in understanding and management. Lancet 2020a;396:1664–1674. [DOI] [PubMed] [Google Scholar]
- Black CJ, Yuan Y, Selinger CPet al. Efficacy of soluble fibre, antispasmodic drugs, and gut–brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2020b;5:117–131. [DOI] [PubMed] [Google Scholar]
- Blandino P Jr, Barnum CJ, Deak T.. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol 2006;173:87–95. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MVet al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 2011;108:16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner KM, Yeramilli VA, Kennedy BAet al. Prenatal stress increases IgA coating of offspring microbiota and exacerbates necrotizing enterocolitis-like injury in a sex-dependent manner. Brain Behav Immun 2020;89:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DG, Soto R, Yandamuri Set al. The microbiota protects from viral-induced neurologic damage through microglia-intrinsic TLR signaling. Elife 2019;8:e47117. doi: 10.7554/eLife.47117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano B, Bosch-Queralt M, Almolda Bet al. Purine signaling and microglial wrapping. Adv Exp Med Biol 2016;949:147–165. [DOI] [PubMed] [Google Scholar]
- Cheadle L, Rivera SA, Phelps JSet al. Sensory experience engages microglia to shape neural connectivity through a non-phagocytic mechanism. Neuron 2020;108:451–468.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Lin CL, Kao CH.. Irritable bowel syndrome is associated with an increased risk of dementia: a Nationwide Population-Based Study. PLoS One 2016;11:e0144589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liao J, Xia Yet al. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022a;71:2233–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhao BC, Dai XYet al. Drinking alkaline mineral water confers diarrhea resistance in maternally separated piglets by maintaining intestinal epithelial regeneration via the brain–microbe–gut axis. J Adv Res 2022b;S2090-1232(22)00287-9. doi: 10.1016/j.jare.2022.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Tsien RW, Goff DCet al. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res 2015;167:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Calcagno N, Gauthier Cet al. The microbiota restrains neurodegenerative microglia in a model of amyotrophic lateral sclerosis. Microbiome 2022;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, O’Riordan KJ, Cowan CSMet al. The microbiota–gut–brain axis. Physiol Rev 2019;99:1877–2013. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Martínez-Cerdeño V, Noctor SC.. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 2013;33:4216–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damisah EC, Hill RA, Rai Aet al. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci Adv 2020;6:eaba3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang Get al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005;8:752–758. [DOI] [PubMed] [Google Scholar]
- Defaye M, Abdullah NS, Iftinca Met al. Gut-innervating TRPV1+ neurons drive chronic visceral pain via microglial P2Y12 receptor. Cell Mol Gastroenterol Hepatol 2022;13:977–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma G, Shimbori C, Reed DEet al. Histamine production by the gut microbiota induces visceral hyperalgesia through histamine 4 receptor signaling in mice. Sci Transl Med 2022;14:eabj1895. [DOI] [PubMed] [Google Scholar]
- Ding X, Wang J, Huang Met al. Loss of microglial SIRPα promotes synaptic pruning in preclinical models of neurodegeneration. Nat Commun 2021;12:2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio-Santos DA, Olschowka JA, O’Banion MK.. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J Neuroinflammation 2019;16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodiya HB, Kuntz T, Shaik SMet al. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J Exp Med 2019;216:1542–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Punaro MC, Segura M, Plante MMet al. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J Immunol 2007;179:1842–1854. [DOI] [PubMed] [Google Scholar]
- Drossman DA, Hasler WL.. Rome IV-functional GI disorders: disorders of gut–brain interaction. Gastroenterology 2016;150:1257–1261. [DOI] [PubMed] [Google Scholar]
- Duan R, Zhu S, Wang Bet al. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing: a systematic review. Clin Transl Gastroenterol 2019;10:e00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Salhy M, Hatlebakk JG, Gilja OHet al. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020;69:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Hrabě de Angelis AL, Jaitin Det al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18:965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Dokalis N, Mezö Cet al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab 2021;33:2260–2276.e7. [DOI] [PubMed] [Google Scholar]
- Fang Y, Zhang J, Zhu Set al. Berberine ameliorates ovariectomy-induced anxiety-like behaviors by enrichment in equol generating gut microbiota. Pharmacol Res 2021;165:105439. [DOI] [PubMed] [Google Scholar]
- Filipello F, Morini R, Corradini Iet al. The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity 2018;48:979–991.e8. [DOI] [PubMed] [Google Scholar]
- Fond G, Loundou A, Hamdani Net al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 2014;264:651–660. [DOI] [PubMed] [Google Scholar]
- Ford AC, Quigley EM, Lacy BEet al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol 2014;109:1547–61; quiz 1546, 1562. [DOI] [PubMed] [Google Scholar]
- Frank MG, Fonken LK, Dolzani SDet al. Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior. Brain Behav Immun 2018;73:352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P, Gao M, Yung KKL.. Association of intestinal disorders with Parkinson’s disease and Alzheimer’s disease: a systematic review and meta-analysis. ACS Chem Neurosci 2020;11:395–405. [DOI] [PubMed] [Google Scholar]
- Funk KE, Klein RS.. CSF1R antagonism limits local restimulation of antiviral CD8(+) T cells during viral encephalitis. J Neuroinflamm 2019;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley JD, Mashburn-Warren L, Blalock LCet al. Maternal anxiety, depression and stress affects offspring gut microbiome diversity and bifidobacterial abundances. Brain Behav Immun 2023;107:253–264. [DOI] [PubMed] [Google Scholar]
- Garber C, Soung A, Vollmer LLet al. T cells promote microglia-mediated synaptic elimination and cognitive dysfunction during recovery from neuropathogenic flaviviruses. Nat Neurosci 2019;22:1276–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Rey NL, Tyson Tet al. Microglia affect α-synuclein cell-to-cell transfer in a mouse model of Parkinson’s disease. Mol Neurodegener 2019;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Opitz Net al. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun 2005;19:334–344. [DOI] [PubMed] [Google Scholar]
- Gunner G, Cheadle L, Johnson KMet al. Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nat Neurosci 2019;22:1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Ellis SE, Ashar FNet al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat Commun 2014;5:5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann O, Mahrhofer C, Rueter Net al. Pneumococcal cell wall-induced meningitis impairs adult hippocampal neurogenesis. Infect Immun 2007a;75:4289–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann O, Braun JS, Becker Det al. TLR2 mediates neuroinflammation and neuronal damage. J Immunol 2007b;178:6476–6481. [DOI] [PubMed] [Google Scholar]
- Huaman JW, Mego M, Manichanh Cet al. Effects of prebiotics vs a diet low in FODMAPs in patients with functional gut disorders. Gastroenterology 2018;155:1004–1007. [DOI] [PubMed] [Google Scholar]
- Huang M, He Y, Tian Let al. Gut microbiota–SCFAs–brain axis associated with the antidepressant activity of berberine in CUMS rats. J Affect Disord 2023;325:141–150. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel Net al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009;139:485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JP, Lagishetty V, Hauer MCet al. Multi-omics profiles of the intestinal microbiome in irritable bowel syndrome and its bowel habit subtypes. Microbiome 2023;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalanka-Tuovinen J, Salojärvi J, Salonen Aet al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014;63:1737–1745. [DOI] [PubMed] [Google Scholar]
- Ji NN, Meng QX, Wang Yet al. Microglia-derived TNF-α inhibiting GABAergic neurons in the anterior lateral bed nucleus of the stria terminalis precipitates visceral hypersensitivity induced by colorectal distension in rats. Neurobiol Stress 2022;18:100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Gao Z, Hu H.. Microglia in depression: current perspectives. Sci China Life Sci 2021;64:911–925. [DOI] [PubMed] [Google Scholar]
- Johnsen PH, Hilpüsch F, Cavanagh JPet al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol 2018;3:17–24. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CMet al. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 2005;135:1295–1307. [DOI] [PubMed] [Google Scholar]
- Karl JP, Margolis LM, Madslien EHet al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol Gastrointest Liver Physiol 2017;312:G559–G571. [DOI] [PubMed] [Google Scholar]
- Kielian T. Immunopathogenesis of brain abscess. J Neuroinflamm 2004;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles SR, Nelson EA, Palombo EA.. Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: a possible mechanism underlying susceptibility to illness. Biol Psychol 2008;77:132–137. [DOI] [PubMed] [Google Scholar]
- Lai NY, Musser MA, Pinho-Ribeiro FAet al. Gut-innervating nociceptor neurons regulate Peyer’s patch microfold cells and SFB levels to mediate Salmonella host defense. Cell 2020;180:33–49.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MB, Tillisch K, Craig ADet al. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology 2012;142:463–472.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz Y, Kowalski EA, Wang Xet al. Lactobacillus rescues postnatal neurobehavioral and microglial dysfunction in a model of maternal microbiome dysbiosis. Brain Behav Immun 2019;81:617–629. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Woo MS, Moon PGet al. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol 2010;185:615–623. [DOI] [PubMed] [Google Scholar]
- Lee AS, Azmitia EC, Whitaker-Azmitia PM.. Developmental microglial priming in postmortem autism spectrum disorder temporal cortex. Brain Behav Immun 2017;62:193–202. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Henneke P, Lien Eet al. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol 2006;177:583–592. [DOI] [PubMed] [Google Scholar]
- Lembo A, Pimentel M, Rao SSet al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology 2016;151:1113–1121. [DOI] [PubMed] [Google Scholar]
- Li E, Noda M, Doi Yet al. The neuroprotective effects of milk fat globule-EGF factor 8 against oligomeric amyloid β toxicity. J Neuroinflamm 2012;9:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Lee C, Filler Tet al. Inhibition of corticotropin-releasing hormone receptor 1 and activation of receptor 2 protect against colonic injury and promote epithelium repair. Sci Rep 2017;7:46616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Chen L, Liu Xet al. Pterostilbene inhibits amyloid-β-induced neuroinflammation in a microglia cell line by inactivating the NLRP3/caspase-1 inflammasome pathway. J Cell Biochem 2018;119:7053–7062. [DOI] [PubMed] [Google Scholar]
- Li H, Xiang Y, Zhu Zet al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J Neuroinflamm 2021;18:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang Y, Song Xet al. The intestinal microbial metabolite nicotinamide n-oxide prevents herpes simplex encephalitis via activating mitophagy in microglia. Gut Microbes 2022a;14:2096989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhu L, Dai Yet al. Diet-induced high serum levels of trimethylamine-N-oxide enhance the cellular inflammatory response without exacerbating acute intracerebral hemorrhage injury in mice. Oxid Med Cell Longev 2022b;2022:1599747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li Y, Duan Wet al. Shugan granule contributes to the improvement of depression-like behaviors in chronic restraint stress-stimulated rats by altering gut microbiota. CNS Neurosci Ther 2022c;28:1409–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang Y, Zheng K.. Microglial mitophagy integrates the microbiota–gut–brain axis to restrain neuroinflammation during neurotropic herpesvirus infection. Autophagy 2023a;19:734–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chen K, Shao Qet al. Nanoparticulate MgH(2) ameliorates anxiety/depression-like behaviors in a mouse model of multiple sclerosis by regulating microglial polarization and oxidative stress. J Neuroinflamm 2023b;20:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Liu B, Xu Jet al. Phloretin decreases microglia-mediated synaptic engulfment to prevent chronic mild stress-induced depression-like behaviors in the mPFC. Theranostics 2023c;13:955–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Chen CC, Chiang HLet al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J Neuroinflamm 2019;16:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang L, Wang Xet al. Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin Gastroenterol Hepatol 2016;14:1602–1611.e5. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhu S, He Met al. Patients with breath test positive are necessary to be identified from irritable bowel syndrome: a clinical trial based on microbiomics and rifaximin sensitivity. Chin Med J (Engl) 2022;135:1716–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Jiang HY, Shi YD.. Association between irritable bowel syndrome and Parkinson’s disease: a systematic review and meta-analysis. Acta Neurol Scand 2022;145:442–448. [DOI] [PubMed] [Google Scholar]
- Lucarini E, Parisio C, Branca JJVet al. Deepening the mechanisms of visceral pain persistence: an evaluation of the gut–spinal cord relationship. Cells 2020;9:1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Zhang J, Reiter RJet al. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: a therapeutic target to reduce intestinal inflammation. Med Res Rev 2020;40:606–632. [DOI] [PubMed] [Google Scholar]
- Margolis KG, Cryan JF, Mayer EA.. The microbiota–gut–brain axis: from motility to mood. Gastroenterology 2021;160:1486–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RAT, Yang Y, Ward Tet al. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell 2020;182:1460–1473.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcovitch-Natan O, Winter DR, Giladi Aet al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016;353:aad8670. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Nance K, Chen S.. The gut–brain axis. Annu Rev Med 2022;73:439–453. [DOI] [PubMed] [Google Scholar]
- McIntosh K, Reed DE, Schneider Tet al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut 2017;66:1241–1251. [DOI] [PubMed] [Google Scholar]
- McMillin M, Frampton G, Grant Set al. Bile acid-mediated Sphingosine-1-Phosphate receptor 2 signaling promotes neuroinflammation during hepatic encephalopathy in mice. Front Cell Neurosci 2017;11:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Li N, Li Det al. The presence of elevated circulating trimethylamine N-oxide exaggerates postoperative cognitive dysfunction in aged rats. Behav Brain Res 2019;368:111902. [DOI] [PubMed] [Google Scholar]
- Moehle MS, Webber PJ, Tse Tet al. LRRK2 inhibition attenuates microglial inflammatory responses. J Neurosci 2012;32:1602–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonen S, Koper MJ, Van Schoor Eet al. Pyroptosis in Alzheimer’s disease: cell type-specific activation in microglia, astrocytes and neurons. Acta Neuropathol 2023;145:175–195. [DOI] [PubMed] [Google Scholar]
- Moseman EA, Blanchard AC, Nayak Det al. T cell engagement of cross-presenting microglia protects the brain from a nasal virus infection. Sci Immunol 2020;5(48):eabb1817. doi: 10.1126/sciimmunol.abb1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossad O, Batut B, Yilmaz Bet al. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N(6)-carboxymethyllysine. Nat Neurosci 2022;25:295–305. [DOI] [PubMed] [Google Scholar]
- Mujagic Z, Kasapi M, Jonkers DMet al. Integrated fecal microbiome-metabolome signatures reflect stress and serotonin metabolism in irritable bowel syndrome. Gut Microbes 2022;14:2063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Honda S, Tohyama Yet al. Neurotrophin secretion from cultured microglia. J Neurosci Res 2001;65:322–331. [DOI] [PubMed] [Google Scholar]
- Nakitanda AO, Kieler H, Odsbu Iet al. In-utero antibiotic exposure and subsequent infections in infancy: A register-based cohort study with si,bling analysis. Am J Obstet Gynecol MFM 2023;5(4):100860. doi: 10.1016/j.ajogmf.2023.100860 [DOI] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB.. Microglia development and function. Annu Rev Immunol 2014;32:367–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F.. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005;308:1314–1318. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani Fet al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011;333:1456–1458. [DOI] [PubMed] [Google Scholar]
- Parker A, Fonseca S, Carding SR.. Gut microbes and metabolites as modulators of blood–brain barrier integrity and brain health. Gut Microbes 2020;11:135–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna E, Aguilera-Lizarraga J, Florens MVet al. Effect of resolvins on sensitisation of TRPV1 and visceral hypersensitivity in IBS. Gut 2021;70:1275–1286. [DOI] [PubMed] [Google Scholar]
- Picard K, Bisht K, Poggini Set al. Microglial-glucocorticoid receptor depletion alters the response of hippocampal microglia and neurons in a chronic unpredictable mild stress paradigm in female mice. Brain Behav Immun 2021;97:423–439. [DOI] [PubMed] [Google Scholar]
- Pimentel M, Lembo A, Chey WDet al. ; TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011;364:22–32. [DOI] [PubMed] [Google Scholar]
- Pinto-Sanchez MI, Hall GB, Ghajar Ket al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a Pilot Study in patients with irritable bowel syndrome. Gastroenterology 2017;153:448–459.e8. [DOI] [PubMed] [Google Scholar]
- Prinz M, Kann O, Draheim HJet al. Microglial activation by components of Gram-positive and -negative bacteria: distinct and common routes to the induction of ion channels and cytokines. J Neuropathol Exp Neurol 1999;58:1078–1089. [DOI] [PubMed] [Google Scholar]
- Rao S, Schieber AMP, O’Connor CPet al. Pathogen-mediated inhibition of anorexia promotes host survival and transmission. Cell 2017;168:503–516.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Xavier AL, Kress BT, Goldman SAet al. A distinct population of microglia supports adult neurogenesis in the subventricular zone. J Neurosci 2015;35:11848–11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes S, Ebert S, Regen Tet al. Toll-like receptor stimulation enhances phagocytosis and intracellular killing of nonencapsulated and encapsulated Streptococcus pneumoniae by murine microglia. Infect Immun 2010;78:865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Borucki DM, Tjon ECet al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018;557:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffouri GB, Shields-Cutler RR, Chen Jet al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun 2019;10:2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EKA, Raposo PJF, Torres-Espin Aet al. Beyond the lesion site: minocycline augments inflammation and anxiety-like behavior following SCI in rats through action on the gut microbiota. J Neuroinflamm 2021;18:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtner AK, Slattery DA, Gläsner Jet al. Minocycline alters behavior, microglia and the gut microbiome in a trait-anxiety-dependent manner. Transl Psychiatry 2019;9:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaler CR, Parco AA, Elhenawy Wet al. Psychological stress impairs IL22-driven protective gut mucosal immunity against colonising pathobionts. Nat Commun 2021;12:6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan S, Hong-Min T, Yi Fet al. New evidences for fractalkine/CX3CL1 involved in substantia nigral microglial activation and behavioral changes in a rat model of Parkinson’s disease. Neurobiol Aging 2011;32:443–458. [DOI] [PubMed] [Google Scholar]
- Shao J, Ma X, Qu Let al. Ginsenoside Rh4 remodels the periphery microenvironment by targeting the brain–gut axis to alleviate depression-like behaviors. Food Chem 2023;404:134639. [DOI] [PubMed] [Google Scholar]
- Sharma RK, Yang T, Oliveira ACet al. Microglial cells impact gut microbiota and gut pathology in angiotensin II-induced hypertension. Circ Res 2019;124:727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Guo H, Liu Set al. Aberrant branched-chain amino acid accumulation along the microbiota–gut–brain axis: crucial targets affecting the occurrence and treatment of ischaemic stroke. Br J Pharmacol 2023;180:347–368. [DOI] [PubMed] [Google Scholar]
- Silva YP, Bernardi A, Frozza RL.. The role of short-chain fatty acids from gut microbiota in gut–brain communication. Front Endocrinol (Lausanne) 2020;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simrén M, Barbara G, Flint HJet al. ; Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 2013;62:159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe GO, Lowery RL, Tremblay Met al. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat Commun 2016;7:10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, He M, Sun Qet al. Roseburia hominis increases intestinal melatonin level by activating p-CREB-AANAT pathway. Nutrients 2021;14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Sun Q, Zheng Het al. Roseburia hominis alleviates neuroinflammation via short-chain fatty acids through histone deacetylase inhibition. Mol Nutr Food Res 2022;66:e2200164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosna J, Philipp S, Albay R 3rdet al. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol Neurodegener 2018;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenberg EE, Lee RJ, Najafi ARet al. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-β pathology. Brain 2016;139:1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber AD, Bangdiwala SI, Drossman DAet al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology 2021;160:99–114.e3. [DOI] [PubMed] [Google Scholar]
- Staudacher HM, Irving PM, Lomer MCet al. Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat Rev Gastroenterol Hepatol 2014;11:256–266. [DOI] [PubMed] [Google Scholar]
- Stefanatos R, Sanz A.. The role of mitochondrial ROS in the aging brain. FEBS Lett 2018;592:743–758. [DOI] [PubMed] [Google Scholar]
- Stefani J, Tschesnokowa O, Parrilla Met al. Disruption of the microglial ADP receptor P2Y(13) enhances adult hippocampal neurogenesis. Front Cell Neurosci 2018;12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ. Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci 2006;29:506–510. [DOI] [PubMed] [Google Scholar]
- Sun J, Li H, Jin Yet al. Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson’s disease via gut microbiota-GLP-1 pathway. Brain Behav Immun 2021;91:703–715. [DOI] [PubMed] [Google Scholar]
- Sundman MH, Chen NK, Subbian Vet al. The bidirectional gut–brain–microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun 2017;66:31–44. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sugihara G, Ouchi Yet al. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry 2013;70:49–58. [DOI] [PubMed] [Google Scholar]
- Szalay G, Martinecz B, Lénárt Net al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun 2016;7:11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H.. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med 2005;201:647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay TL, Béchade C, D’Andrea Iet al. Microglia gone rogue: impacts on psychiatric disorders across the lifespan. Front Mol Neurosci 2017;10:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Mu J, Xu Fet al. Gut bacterial isoamylamine promotes age-related cognitive dysfunction by promoting microglial cell death. Cell Host Microbe 2022;30:944–960.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion MS, Low D, Silvin Aet al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 2018;172:500–516.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P, Zou R, Wang Let al. Multi-probiotics ameliorate major depressive disorder and accompanying gastrointestinal syndromes via serotonergic system regulation. J Adv Res 2022;45:117–125. doi: 10.1016/j.jare.2022.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GGet al. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 2014;42:50–59. [DOI] [PubMed] [Google Scholar]
- Trang T, Beggs S, Salter MW.. Brain-derived neurotrophic factor from microglia: a molecular substrate for neuropathic pain. Neuron Glia Biol 2011;7:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M, Lowery RL, Majewska AK.. Microglial interactions with synapses are modulated by visual experience. PLoS Biol 2010;8:e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka Tet al. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci 2013;16:543–551. [DOI] [PubMed] [Google Scholar]
- Unger MM, Spiegel J, Dillmann KUet al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 2016;32:66–72. [DOI] [PubMed] [Google Scholar]
- VanRyzin JW, Marquardt AE, Argue KJet al. Microglial phagocytosis of newborn cells is induced by endocannabinoids and sculpts sex differences in juvenile rat social play. Neuron 2019;102:435–449.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno Set al. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 2009;29:3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S, Béchade C, Roumier Aet al. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci 2008;28:8138–8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhu S, Liu Zet al. Increased expression of colonic mucosal melatonin in patients with irritable bowel syndrome correlated with gut dysbiosis. Genomics Proteom Bioinform 2020;18:708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Z, Cao Jet al. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 2023a;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen HS, Li HHet al. Microglia-dependent excessive synaptic pruning leads to cortical underconnectivity and behavioral abnormality following chronic social defeat stress in mice. Brain Behav Immun 2023b;109:23–36. [DOI] [PubMed] [Google Scholar]
- Wei H, Yu C, Zhang Cet al. Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome–gut–brain axis. Biomed Pharmacother 2023;160:114308. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Sariol A, Meyerholz DKet al. Microglia are required for protection against lethal coronavirus encephalitis in mice. J Clin Invest 2018;128:931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith CH. The balancing act: endogenous modulation of pain in functional gastrointestinal disorders. Gut 2011;60:1589–1599. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Boddeke HW, Kettenmann H.. Microglia in physiology and disease. Annu Rev Physiol 2017;79:619–643. [DOI] [PubMed] [Google Scholar]
- Wouters MM, Balemans D, Van Wanrooy Set al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 2016;150:875–87.e9. [DOI] [PubMed] [Google Scholar]
- Yamagata T, Muroya K, Mukasa Tet al. Hepatocyte growth factor specifically expressed in microglia activated Ras in the neurons, similar to the action of neurotrophic factors. Biochem Biophys Res Commun 1995;210:231–237. [DOI] [PubMed] [Google Scholar]
- Yang L, Liu C, Li Wet al. Depression-like behavior associated with E/I imbalance of mPFC and amygdala without TRPC channels in mice of knockout IL-10 from microglia. Brain Behav Immun 2021;97:68–78. [DOI] [PubMed] [Google Scholar]
- Yang Y, Huang T, Zhang Het al. Formononetin improves cardiac function and depressive behaviours in myocardial infarction with depression by targeting GSK-3β to regulate macrophage/microglial polarization. Phytomedicine 2023;109:154602. [DOI] [PubMed] [Google Scholar]
- Yuan T, Manohar K, Latorre Ret al. Inhibition of microglial activation in the Amygdala reverses stress-induced abdominal pain in the male rat. Cell Mol Gastroenterol Hepatol 2020;10:527–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang XJ, Tian LPet al. CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. J Neuroinflammation 2011;8:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Yu L, Chen ZYet al. Activation of corticotropin-releasing factor neurons and microglia in paraventricular nucleus precipitates visceral hypersensitivity induced by colorectal distension in rats. Brain Behav Immun 2016;55:93–104. [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu YX, Wang Zet al. Clinical characteristic and fecal microbiota responses to probiotic or antidepressant in patients with diarrhea-predominant irritable bowel syndrome with depression comorbidity: a pilot study. Chin Med J (Engl) 2019a;132:346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Song L, Wang Yet al. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J Gastroenterol Hepatol 2019b;34:1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JD, Liu J, Zhu SWet al. Berberine alleviates visceral hypersensitivity in rats by altering gut microbiome and suppressing spinal microglial activation. Acta Pharmacol Sin 2021;42:1821–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhang C, Zhang Jet al. Efficacy of probiotics for irritable bowel syndrome: a systematic review and network meta-analysis. Front Cell Infect Microbiol 2022a;12:859967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Duan L.. The role of microbiota-mitochondria crosstalk in pathogenesis and therapy of intestinal diseases. Pharmacol Res 2022b;186:106530. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wu X, Li Xet al. TREM2 Is a Receptor for β-amyloid that mediates microglial function. Neuron 2018;97:1023–1031.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Wu SP, Hu Yet al. Corticosterone mediates stress-related increased intestinal permeability in a region-specific manner. Neurogastroenterol Motil 2013;25:e127–e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Qian S, Cho WCSet al. Microbiota-microglia connections in age-related cognition decline. Aging Cell 2022;21:e13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Bai Y, Wang Get al. Hyodeoxycholic acid inhibits lipopolysaccharide-induced microglia inflammatory responses through regulating TGR5/AKT/NF-κB signaling pathway. J Psychopharmacol 2022;36:849–859. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Avidan H, Pluchino Set al. Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. Proc Natl Acad Sci USA 2006;103:13174–13179. [DOI] [PMC free article] [PubMed] [Google Scholar]