Abstract
The presence and genetic content of integrons were investigated for 37 epidemiologically unrelated multiple-drug-resistant strains of Salmonella enterica serotype Typhimurium from humans. All isolates were resistant to ampicillin, chloramphenicol, kanamycin, streptomycin, sulfonamides, and trimethoprim, as well as to tetracycline and/or nalidixic acid; 20% of them were also resistant to gentamicin and amikacin. Three different class 1 integrons (In-t1, In-t2, and In-t3) were identified by Southern blot hybridization, PCR, and DNA sequencing, and these integrons were found to carry the aadB, catB3, oxa1, aadA1a, aacA4, and aacC1 gene cassettes. Integrons In-t1 (aadB and catB3) and In-t2 (oxa1 and aadA1a) were both located on a conjugative IncFI plasmid of 140 kb. In-t3 (aacA4, aacC1, and aadAIa) was located on an IncL/M plasmid of 100 kb which was present, in association with the IncFI plasmid, in gentamicin- and amikacin-resistant isolates. Despite the extensive similarity at the level of the antibiotic resistance phenotype, integrons were not found on the prototypic IncFI plasmids carried by epidemic Salmonella strains isolated during the late 1970s. The recent appearance and the coexistence of multiple integrons on two conjugative plasmids in the same Salmonella isolate are examples of how mobile gene cassettes may contribute to the acquisition and dissemination of antibiotic resistance.
Bacterial resistance to antimicrobial agents is a serious problem worldwide, and understanding of the molecular basis of how resistance genes are acquired and transmitted may contribute to the creation of new antimicrobial strategies (47). One efficient mechanism for the acquisition and dissemination of resistance determinants is their transmission through mobile genetic elements. It has been proposed that promiscuous plasmids, conjugative transposons, and transposons carried by conjugative plasmids are responsible for the horizontal spread of resistance genes throughout bacteria (9). Recently, naturally occurring gene expression elements called “integrons” have been described as vehicles for the acquisition of resistance genes carried by mobile elements (14, 26, 46). These structures have also been found to be involved in the genetic reassortment of resistance determinants frequently observed in multiple-antibiotic-resistant bacterial pathogens (5).
Three classes of integrons have been identified. Class 1 integrons are prevalent among clinical isolates and are composed of two conserved regions, the 5′CS and the 3′CS regions, surrounding an interposed variable region (14, 16). The variable region contains gene cassettes for antibiotic resistance integrated, all in the same orientation, at the attI site. Coordinate expression of the gene cassettes is driven by the tandemly arranged Pant and P2 promoters (39). The 5′CS region of class 1 integrons contains the intI1 gene, which encodes the type 1 integrase protein, which is responsible for site-specific insertion and excision of gene cassettes (5). As a consequence of this activity, integrons exist in a large variety of forms with respect to the number, type, and order of the inserted genes. Each gene cassette includes an open reading frame and a recombination site known as the “59-base element” located downstream of each coding sequence (15). The 3′CS contains the sul1 and the qacEΔ1 genes, which confer resistance to sulfonamides and to quaternary ammonium compounds, respectively (33, 36). Gene cassette arrays similar to those of the class 1 integrons were observed for the transposon Tn7 and its close relatives, forming a second class of integrons. A putative defective integrase gene (intI2), whose product is 40% identical to that of intI1, is located in the distal portion of Tn7 (11). Recently, a third class of integron has been identified, and the putative integrase (intI3), located at the 5′ of the blaIMP cassette, is 61% identical to the intI1 integrase (31).
The occurrence of integrons among clinical bacterial isolates is under investigation, and recent studies report a widespread distribution of these elements among multiple-antibiotic-resistant nosocomial bacteria (18, 20, 34).
Salmonella enterica serotype Typhimurium is one of the more frequent agents of bacterial enteritis worldwide (29). This serotype has often been described as being resistant to multiple drugs, most commonly showing resistance to ampicillin (Ap), chloramphenicol (Cm), streptomycin (Sm), sulfonamides (Su), and tetracycline (Te). The R types R-ApCmSmSuTe and R-ApSmSuTe have been associated with the phage types DT104 and DT193, respectively (17, 23, 48).
In the study described in this report, we investigated the genetic determinants for antibiotic resistance of S. enterica serotype Typhimurium strains isolated from epidemiologically unrelated pediatric patients with gastroenteritis. Our results indicate that the multiple-drug resistance phenotype is determined by integrons carrying an unusually wide repertoire of resistance gene cassettes. Up to three types of integrons located on two conjugative plasmids were identified. The quality, number, and organization of the resistance genes were then determined.
MATERIALS AND METHODS
Bacterial strains.
Thirty-seven S. enterica serotype Typhimurium strains were isolated from the stools of children hospitalized for acute gastroenteritis at the Pediatric Clinic of the University of Tirana (Tirana, Albania) from June 1996 to February 1997 and were sent to the Laboratory of Bacteriology and Medical Mycology of the Istituto Superiore di Sanità in Rome, Italy, for characterization. All patients originated from different districts of Albania.
The serotype of the isolates was determined with anti-O and anti-H antisera obtained from Behringwerke AG (Marburg, Germany). None of the isolates was phage typeable as described by Callow (3), nor did their lytic patterns resemble those of multi-drug-resistant S. enterica serotype Typhimurium phage types DT104 or DT193 (17, 23, 48).
Antimicrobial susceptibility and transfer of resistance.
S. enterica serotype Typhimurium isolates were preliminarily tested for antibiotic resistance by the disk diffusion assay on Mueller-Hinton agar with commercial antimicrobial susceptibility disks (Oxoid, Basingstoke, United Kingdom, and Becton Dickinson Microbiological System, Cockeysville, Md.) according to the recommendations of the National Committee for Clinical Laboratory Standards (30).
The rifampin-resistant mutant strain Escherichia coli K-12 CSH26-R was used as the recipient in conjugation experiments (24). Exconjugants were selected on Luria-Bertani agar containing 100 μg of rifampin per ml plus 20 μg of amikacin (Ak) per ml or 30 μg of chloramphenicol per ml and were tested on Simmons citrate-agar plates (Biogenetics, Padova, Italy) to distinguish exconjugants from spontaneous rifampin-resistant donor mutants.
Intraspecific matings were performed with the nalidixic acid (Na)-resistant strain E. coli CSH26-N as the recipient.
Characterization of plasmids and identification of integrons by DNA hybridization.
Plasmid DNA was extracted by the procedure of Kado and Liu (21), electrophoresed in a 0.8% agarose gel at 5 V/cm, and stained with ethidium bromide.
Extraction of genomic DNA and colony hybridizations were performed as described elsewhere (10, 41).
Plasmid DNA, digested genomic DNA, and bacterial colonies were transferred onto a positively charged nylon membrane (Boehringer Mannheim, Mannheim, Germany) by standard methods (41) and were hybridized under high-stringency conditions with a specific probe for integrase genes intI1 and intI2. The intI1 probe was obtained by restriction and agarose elution of the 596-bp PvuII-RsaI fragment from the transposon-carrying plasmid pACYC184::Tn21 (7). The intI2 probe was obtained by PCR amplification on R483::Tn7 (45) with primers intI2A and intI2B (Table 1) internal to the released intI2 sequence. DNA fragments were labelled with [α-32P]dCTP with a random labelling kit (Bethesda Research Laboratories, Inc., Gaithersburg, Md.).
TABLE 1.
Primers used for integron detection by PCR
| Primer | DNA sequence | Position in submitted sequence | Accession no. |
|---|---|---|---|
| 5′CS | 5′-GGCATCCAAGCAGCAAG-3′ | 1190–1206 | M73819 |
| 3′CS | 5′-AAGCAGACTTGACCTGA-3′ | 1342–1326 | M73819 |
| intI2A | 5′-ATGTCTAACAGTCCATTTTTAAATTCTA-3′ | 1914–1886 | AJ002782 |
| intI2B | 5′-AAATCTTTAACCCGCAAACGC-3′ | 1475–1495 | AJ002782 |
| intI3A | 5′-GTGGCGCAGGGTGTGGAC-3′ | 194–211 | D50438 |
| intI3B | 5′-ACAGACCGAGAAGGCTTATG-3′ | 959–938 | D50438 |
Plasmids were also probed with each clone of the inc rep plasmid bank generously provided by Werner K. Maas, as described by Couturier et al. (8).
PCR amplification, cloning, and sequencing of integrons.
Amplification reactions were carried out by PCR with 10 μl of boiled bacterial suspensions, with 200 μM deoxynucleoside triphosphate, 1 μM the primer pairs listed in Table 1, Taq Plus buffer (Promega), and 5 U of Taq Plus polymerase (Stratagene). The PCR was run at 94°C for 30 s, 59°C for 30 s, and 72°C for 3.5 min for a total of 35 cycles. Amplicons were blunt-end ligated at the SmaI site of the pGEM 3z(f+) vector (Promega) and were used to transform E. coli DH5α cells (41). Sequencing reactions were performed with double-stranded plasmid preparations by the dideoxy chain termination method with universal primers (43) and a commercial kit purchased from Pharmacia Biotech and products were analyzed with a Pharmacia Biotech ALFexpress automated DNA sequencing apparatus. The presence of the sul-1 gene was investigated by PCR amplification with the primers described by Sandvang et al. (42).
Nucleotide sequence accession numbers.
The nucleotide sequences of In-t1, In-t2, and In-t3 have been assigned EMBL accession nos. AJ009818, AJ009819, and AJ009820, respectively.
RESULTS
Antibiotic resistance profiles and characterization of plasmids of S. enterica serotype Typhimurium isolates.
Thirty-seven S. enterica serotype Typhimurium isolates of human origin were tested for antimicrobial susceptibility. All strains were resistant to ampicillin, chloramphenicol, kanamycin (Km), streptomycin, sulfonamides, and trimethoprim (Tp). Additionally, 75% of the isolates were also resistant also to tetracycline, 38% were also resistant to nalidixic acid, and 20% were also resistant to amikacin and gentamicin (Gm).
Plasmids from six Salmonella strains were examined (Table 2). The approximate sizes of the Salmonella plasmids were estimated by direct comparison with three reference plasmids harbored by the multi-drug-resistant strain S. enterica Wien WZM6 (R-ApCmKmSmSuTe) (24). This strain carries an IncFI plasmid of 140 kb (pZM61; R-ApCmKmTe) and two smaller plasmids of 13 kb (pZM62; R-ApSmSu) and 2.1 kb (pZM63 [a cryptic plasmid]; Fig. 1A, lane 1). S. enterica serotype Typhimurium strains 44, 516, 252 (Fig. 1A, lanes 2, 3, and 4), 298, 202 (Fig. 1A, lanes 5 and 6), and 341 (Fig. 1A, lane 7) all carried a large plasmid similar in size to pZM61. Strains 202 and 298, representative of the R-ApCmKmSmSuTpTeGmAk type, carried an additional large plasmid of approximately 100 kb. Other smaller heterogeneous plasmids were present in all strains (27).
TABLE 2.
Antibiotic resistance phenotypes of S. enterica serotype Typhimurium donor strains and E. coli exconjugants, transferred plasmids, and type of integrons found on plasmids
| Salmonella R type | No. of strains | Donor strain | Exconjugant R type | Frequency of transfer | Transferred plasmid | Integron(s) |
|---|---|---|---|---|---|---|
| ApCmKmSmSuTpTeNa | 5 | 366 | ApCmKmSmSuTpTe | 5 × 10−5 | 140-kb IncFI | In-t1,In-t2 |
| ApCmKmSmSuTpNa | 9 | 341 | ApCmKmSmSuTp | 7 × 10−5 | 140-kb IncFI | In-t1,In-t2 |
| ApCmKmSmSuTpTe | 15 | 252 | ApCmKmSmSuTe | 6 × 10−5 | 140-kb IncFI | In-t1,In-t2 |
| ApCmKmSmSuTpTeGmAk | 8 | 202 | ApKmSuTeGmAk | 1 ×10−6 | 100-kb IncL/M | In-t3 |
| 202 | ApCmKmSmSuTpTe | 7 × 10−5 | 140-kb IncFI | In-t1,In-t2 | ||
| 298 | ApKmSuGmAk | 7 × 10−7 | 100-kb IncL/M | In-t3 | ||
| 298 | ApCmKmSmSuTpTe | 5 × 10−5 | 140-kb IncFI | In-t1,In-t2 |
FIG. 1.
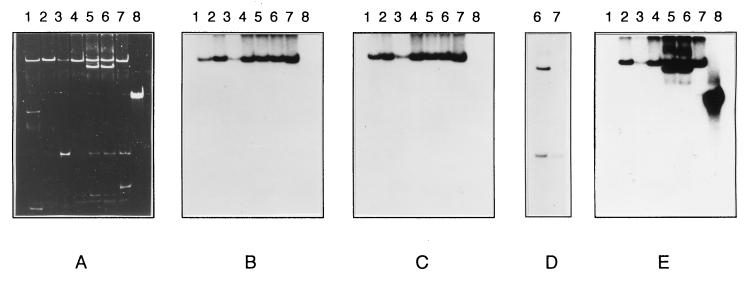
Plasmids in S. enterica serotype Typhimurium strains. (A) Plasmid DNA in S. enterica serotype Wien WZM6 (lane 1) and in six prototypic isolates of S. enterica serotype Typhimurium, strains 44 (lane 2), 516 (lane 3), 252 (lane 4), 298 (lane 5), 202 (lane 6), and 341 (lane 7). Plasmids were separated by agarose gel electrophoresis and stained with ethidium bromide. Replicas of the electropherograms shown in panel A were blotted onto nylon membrane filters and hybridized with the following probes: repFIA (B), repFIB (C), repL/M (D), and an integrase gene (intI1) (E). Plasmid pACYC184::Tn21 (lane 8) was used as positive control for the presence of the intI1 gene.
We further characterized the 140-kb plasmid, which was present in all isolates, and the 100-kb plasmid, which was present in strains 202 and 298. Plasmid DNA was hybridized with the complete repertoire of inc and rep probes specific for the major incompatibility groups (8). The 140-kb replicon was assigned to the IncFI group because it reacted with the two FI-specific probes, repFIA and repFIB (Fig. 1B and C, lanes 2 to 7), as did the prototypic FI plasmid pZM61 (Fig. 1B and C, lanes 1). The 100-kb plasmid belongs to the IncL/M group, since it hybridized with the specific repL/M probe (Fig. 1D, lane 6).
Transfer of resistance.
Prototypic Salmonella strains 366, 341, 252, 202, and 298 were conjugated with E. coli CSH26-R. Two types of exconjugants were obtained from strains 202 and 298 upon selection on rifampin-amikacin and rifampin-chloramphenicol plates: the Ak-type exconjugants of the R-ApKmSuGmAk type and the Cm-type exconjugants of the R-ApCmKmSmSuTmTe type. Cm-type exconjugants were also obtained from strains 366, 341, and 252 (Table 2).
Transconjugants were examined for their plasmid profiles. We found that all Cm-type transconjugants harbored the IncFI plasmid, while the Ak-type transconjugants carried the IncL/M replicon. Figure 2A illustrates the independent transmission of the two plasmids from S. enterica serotype Typhimurium 202 to E. coli. Both Cm and Ak types of transconjugants were able to transfer their complete R patterns in intraspecific matings with E. coli CSH26-N as the recipient strain, showing transfer frequencies comparable to those observed in interspecific matings (data not shown).
FIG. 2.
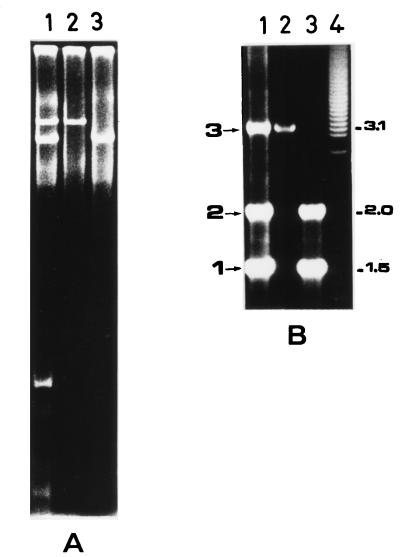
Integrons found on plasmids carried by S. enterica serotype Typhimurium. (A) Plasmids were separated by agarose gel electrophoresis and were visualized by ethidium bromide staining. Lane 1, S. enterica serotype Typhimurium 202 donor strain; lane 2, Cm-type E. coli exconjugant carrying the IncFI replicon; lane 3, Ak-type E. coli exconjugant carrying the IncL/M replicon. (B) PCR analysis of integron-borne gene cassettes amplified with the 5′CS and 3′CS primers (Table 1). DNA templates were from the S. enterica serotype Typhimurium 202 (lane 1) and from Ak-type and Cm-type exconjugants (lanes 2 and 3, respectively). In-t1, In-t2, and In-t3 integrons are indicated as 1, 2, and 3, respectively, on the left of the figure. Molecular size standards (lane 4; in kilobases) are indicated on the right.
These results led us to conclude that all of the S. enterica serotype Typhimurium prototypic strains carry the IncFI plasmid which accounts for the R-ApCmKmSmSuTpTe phenotype; the R-ApCmKmSmSuTpTeGmAk strains harbor an additional self-transferable IncL/M plasmid which confers resistance to ampicillin, kanamycin, sulfonamides, gentamicin, and amikacin.
Integrons in S. enterica serotype Typhimurium plasmids.
The presence of the sulfonamide resistance determinant is strongly suggestive of the presence of class 1 integrons when it is carried by conjugative plasmids (36). Therefore, we analyzed the IncFI and IncL/M plasmids harbored by S. enterica serotype Typhimurium for the presence of these genetic elements. We initially searched for the presence of the class 1 integrase gene, and Southern hybridization experiments were carried out with plasmid DNA by using the intI1 probe. Plasmid pACYC184::Tn21 carrying the Tn21 integron was used as the positive control (Fig. 1A, lane 8). Figure 1E (lanes 2 to 7) shows that the 140-kb IncFI plasmid of all representative strains and the 100-kb IncL/M plasmid carried by strains 202 and 298 hybridize with the integrase gene (intI1) probe. Interestingly, none of the S. enterica serotype Wien WZM6 plasmids were found to be positive when they were probed for the presence of the class 1 integrase gene (Fig. 1E, lane 1).
Of note, three additional multi-drug-resistant epidemic S. enterica serotype Wien strains (W537, W811, and W569; R-ApCmKmTe, R-ApCmKmSmTe, and R-ApCmSuTpGmNa, respectively) isolated in Italy at the Istituto Superiore di Sanità during the period from 1983 to 1989 were also negative by colony hybridization for the intI1 gene. Conversely, the presence of class 1 integrons was demonstrated by colony hybridization with the intI1 probe in all 37 multi-drug-resistant S. enterica serotype Typhimurium isolates (data not shown).
To better characterize the integronic structures associated with S. enterica serotype Typhimurium conjugative plasmids, we performed a PCR analysis with the 5′CS and 3′CS primers (22) with DNA extracted from the strain 202 Cm-type and strain 202 Ak-type transconjugants, as well as from the donor S. enterica serotype Typhimurium 202 isolate. The results showed the presence in S. enterica serotype Typhimurium 202 of three types of integrons, which we designated In-t1, In-t2, and In-t3, respectively; they carried variable regions of 1.5, 2.1, and 3.2 kb, respectively (Fig. 2B, lane 1). These three integrons were conjugatively transferable by the two IncFI and IncL/M plasmids. In fact, the Cm-type transconjugant, which had acquired the IncFI plasmid, generated both In-t1 and In-t2 amplicons (Fig. 2B, lane 3). Moreover, the 3.2-kb DNA band, corresponding to In-t3, was generated by the Ak-type exconjugant which had received the IncL/M plasmid only (Fig. 2B, lane 2). The recipient strain E. coli CSH26-R was negative by PCR for the class 1 integrons (data not shown).
Southern blot analysis of BamHI- and PvuII-digested total DNA from prototypic multi-drug-resistant S. enterica serotype Typhimurium isolates confirmed the existence of all three types of integrons carrying the variable regions revealed by PCR and allowed us to rule out the possibility that class 1 integrons other than those identified on the IncFI and IncL/M plasmids were present in these isolates (data not shown). In addition, the presence of class 2 and 3 integrons in these isolates was ruled out by Southern blot analysis and amplification reactions performed with intI3-specific primers intI3A and intI3B (Table 1).
Integron-borne gene cassettes.
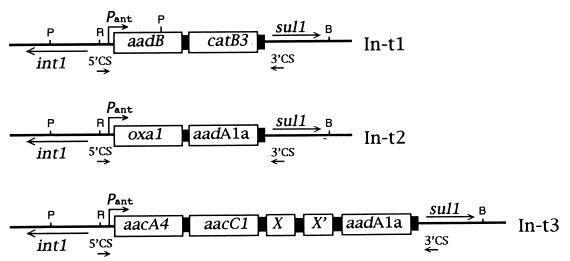
The In-t1, In-t2, and In-t3 PCR products were cloned in pGem 3Zf(+) and were entirely sequenced (Fig. 3). The nucleotide sequence of In-t1 showed the presence of two gene cassettes, the aadB gene [also named ant(2")-Ia] and the catB3 gene. The aadB gene cassette is 596 bp long and encodes the enzyme aminoglycoside adenyltransferase AAD(2"). This enzyme has been shown to confer resistance to kanamycin and to low levels of gentamicin (44). The catB3 gene corresponds to an open reading frame of 633 bp which encodes the enzyme chloramphenicol acetyltransferase (CatB3) (2). On the basis of a sequence comparison, the putative Pant promoter was found to consist of a −35 signal (TGGAGA) separated by 17 bases from a −10 signal (TAAGCT). The secondary promoter P2 was also identified ca. 80 nucleotides downstream of Pant and was found to consist of the sequence −35TTGTTA-[N17]-−10TACAGT (6).
FIG. 3.
Structural organization of gene cassettes in S. enterica serotype Typhimurium integrons. Integron-borne gene cassettes are represented as open boxes; black boxes are the 59-base elements (not in scale). The positions of an integrase gene (intI1), Pant promoter, a sulfonamides resistance gene (sul-1), and the 5′CS and 3′CS primers are indicated by arrows. Restriction sites for BamHI (B), PvuII (P), and Rsa I (R) are indicated.
The In-t2 integron was found to contain two gene cassettes: oxa-1 and aadA1a [also named ant(3")-Ia]. The oxa-1 gene is 1,039 bp long, and its product is a beta-lactamase, which accounts for the ampicillin resistance (32). The 862 bp aadA1a gene cassette encodes the aminoglycoside adenyltransferase AAD(3")-Ia enzyme, which confers streptomycin resistance (39). The Pant and P2 sequences of In-t2 were identical to the Pant and P2 sequences of In-t1.
In-t3 was found to contain three antibiotic resistance gene cassettes: aacA4, aacC1, and aadA1a. Between the aacC1 and aadA1a genes two open reading frames (X and X′) were present; their products are still uncharacterized, and the sequences of the products do not show any significant similarity with published sequences at either the nucleotide or the deduced protein level. The aacA4 gene cassette encodes the 6′-N-aminoglycoside acetyltransferase [AAC(6′)-Ib] described in Pseudomonas aeruginosa BM 2656. This enzyme confers resistance to amikacin (13). The aacC1 gene encodes the enzyme aminoglycoside acetyltransferase [AAC(3)-Ia] which confers resistance to gentamicin (44). The aadA1a gene found on In-t3 had the same sequence as the aadA1a gene found on In-t2. Divergence among In-t2 and In-t3 aadA1a gene cassettes starts immediately before the GTTAAAC sequence at the 5′ end, which represents the integrase-specific recombination site (38, 39). In the Ak-type transconjugants, the aadA1a-encoded streptomycin resistance was not expressed (Table 2). The Pant sequence of this integron consists of the −35TTGACA-[N17]-−10TAAACT element. The P2 promoter was identical in sequence and location to those found in In-t1 and In-t2.
DISCUSSION
In this report we describe the presence of multiple class 1 integrons conferring resistance to a very broad spectrum of antibiotics in S. enterica serotype Typhimurium strains isolated from infants with acute gastroenteritis. These strains were resistant to β-lactams, chloramphenicol, co-trimoxazole, and the most commonly used aminoglycosides. The antibiotic resistance determinants of the S. enterica serotype Typhimurium isolates were all transferable, being carried by conjugative plasmids belonging to the IncFI (R-ApCmKmSmSuTpTe) and IncL/M (R-ApKmSuGmAk) incompatibility groups.
We identified three different class 1 integrons which account for almost the entire resistance phenotype observed in Salmonella isolates; tetracycline and trimethoprim resistance is the only plasmid-encoded resistance not located within integrons. In-t1 (R-CmKmSu) and In-t2 (R-ApSmSu) were located on the IncFI plasmid in all isolates tested, while In-t3 (R-KmSuGmAk) was located on the IncL/M plasmid, which was carried by ca. 20% of the isolates. All these isolates were negative for class 2 and 3 integrons.
The presence in the same isolate of three class 1 integrons located on two different plasmids has, to our knowledge, never been observed. In addition, the repertoire of antibiotic resistance carried by In-t1, In-t2, and In-t3 represents one of the most extensive examples of gene cassette arrays thus far described among multi-drug-resistant isolates.
The role of integrons in the acquisition and spread of antibiotic resistance has not yet been fully investigated. Several studies have reported the presence of class 1 integrons in gram-negative bacteria from patients with hospital-acquired infections (i.e., Klebsiella pneumoniae, P. aeruginosa, E. coli, and Citrobacter freundii [20]) or in Klebsiella oxytoca strains responsible for nosocomial outbreaks (34). The integron-borne gene cassettes found in the S. enterica serotype Typhimurium isolates have previously been identified within class 1 integrons. The oxa-1 gene was found to be associated with aadA1a in transposon Tn2603 (32), and the aacA4-aacC1 association was reported for a K. oxytoca integron (34). The catB3 and aadB gene cassettes are located in the same class 1 integron in plasmid pBWH301 (2). Moreover, the aadA1a gene cassette, which we found in both In-t2 and In-t3, occurs at a high frequency in Tn21 derivatives (40) and has recently been described in multi-drug-resistant S. enterica serotype Typhimurium DT104 strains isolated from pig herds in Denmark (42).
Our data indicated that the integron-borne aadA1a gene cassette is silent in In-t3, while it is expressed when it is located in In-t2. This is likely due to the polarity effects observed for promoter-proximal and promoter-distal gene cassettes (39). In fact, the sequence determined for the Pant promoter of In-t3 has previously been reported to be 20-fold more active than those of the variants found in In-t1 and In-t2 (6).
The presence of In-t1 and In-t2 on the Salmonella IncFI plasmid leads to other interesting considerations. In fact, this plasmid has been implicated in the wide diffusion of multi-drug-resistant Salmonella strains. From 1969 to 1980, considerable clinical and epidemiological evidence indicated that the emergence and prevalence of some epidemic strains of human Salmonella spp. were correlated with the acquisition of conjugative or defective conjugative IncFI plasmids conferring resistance to multiple drugs and ranging in size from 100 to 180 kb. These plasmids were very common in different serotypes of Salmonella when the R type was R-ApCmKmSmSuTe or R-ApCmSmSuTe (1, 4, 37). The IncFI plasmid that we found in the recent isolates of S. enterica serotype Typhimurium has been compared with the prototypic pZM61 IncFI plasmid (R-ApCmKmTe) harbored by the epidemic strain S. enterica serotype Wien WZM6 isolated in Italy in 1974 (24). It is not known whether some ancestral IncFI plasmids harbored integrons, but our data clearly demonstrate that strain WZM6 does not contain integrons. Our observations suggest that the IncFI plasmids of Salmonella spp. could have evolved through the acquisition of integrons as genetic vehicles of the resistance genes. Several pieces of evidence indicate that the acquisition of integrons by R plasmids may have originated from the spread of Tn5090-like transposons (35). However, multiple site-specific recombination events occurring between the primary site attI and secondary sites can also lead to the fusion of the conserved integron sequences to plasmids and could represent a further mechanism for the acquisition of integrons (19). It will be interesting to determine whether the integrase site-specific recombination sites recently found in the E. coli F plasmid (12) are also present in the homologous Salmonella IncFI plasmid (28) and if these secondary sites have been involved in the acquisition of In-t1 and/or In-t2.
The presence of an additional integron, In-t3, in a limited number of isolates derives from the acquisition of a 100-kb IncL/M plasmid and results in an extended spectrum of resistance (resistance to gentamicin and amikacin). The presence in In-t3 of two putative open reading frames (X and X′) endowed with no apparent function suggests that coding DNA modules other than antibiotic resistance genes can be packaged within integrons, providing a natural mechanism for the engineering of bacterial genomes (39).
Molecular models for the generation of new integron gene cassette arrays require the simultaneous presence of different integrons in the same cell (12, 14, 15, 26). The coexistence of two plasmids carrying integrons with different gene cassettes is expected to result in the transfer of cassettes from one plasmid to the other through the intI1-dependent integrative and excisive model (25, 38). Furthermore, the coexistence of multiple integrons on the same plasmid can frequently result in deletions of these elements (15). Although both In-t1 and In-t2 appear to be very stable on the IncFI plasmid, since they were identical in all prototypic strains tested, future analysis of more recent Salmonella isolates might demonstrate gene cassette exchanges among In-t1, In-t2, and In-t3. It should be pointed out that the IncL/M plasmid, which contains In-t3, confers resistance to β-lactams through a still unidentified beta-lactamase gene which was not found among integron-borne gene cassettes. The copy number of this plasmid is higher than that of the IncFI plasmid, and this could result in an enhancement of the antibiotic resistance levels due to an increased gene dose. Under selective antibiotic pressure, these last two features might have contributed to the stable maintenance of the entire IncL/M plasmid rather than to the acquisition of a third integron by the resident IncFI plasmid or the in trans integration of additional gene cassettes within In-t1 and/or In-t2.
Although our findings strongly support the hypothesis that integron exchange represents a very efficient strategy for the acquisition of new antibiotic resistance genes, a prospective long-term observation of the natural evolution of the S. enterica serotype Typhimurium IncFI plasmids could provide further insight into the natural forces behind the acquisition and spread of integrons.
ACKNOWLEDGMENTS
We are very grateful to Asmi Dibra of the Institute of Public Health of Tirana, Tirana, Albania, for his enthusiastic collaboration and for kindly providing the Salmonella strains. We thank Werner K. Maas for generously providing the inc and rep plamid bank. We also thank Antonio Cassone, Mauro Nicoletti, Annalisa Pantosti, and Alfredo Caprioli for critical review of the manuscript and Susanna Mariotti, Sergio Arena, Ildo Benedetti, and Teodoro Squatriti for excellent technical assistance.
This work was partially supported by the UNICEF project Control of Acute Diarrheal Diseases Including Cholera in Albania.
REFERENCES
- 1.Anderson E S, Threlfall E J, Carr J M, McConnell M M, Smith H R. Clonal distribution of resistance plasmid-carrying Salmonella typhimurium mainly in the Middle East. J Hyg Camb. 1977;79:425–448. doi: 10.1017/s0022172400053286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunny K L, Hall R M, Stokes H W. New mobile gene cassettes containing an aminoglycoside resistance gene, accA7, and a chloramphenicol resistance gene, catB3, in an integron in pBWH301. Antimicrob Agents Chemother. 1995;39:686–693. doi: 10.1128/AAC.39.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callow B. A new phage typing scheme for Salmonella typhimurium. J Hyg Camb. 1959;57:346–359. doi: 10.1017/s0022172400020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casalino M, Comanducci A, Nicoletti M, Maimone F. Stability of plasmid content in Salmonella wien in late phases of the epidemic history. Antimicrob Agents Chemother. 1984;25:499–501. doi: 10.1128/aac.25.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collis C M, Hall R M. Site-specific deletion and rearrangement of integron inserted genes catalyzed by the integron DNA integrase. J Bacteriol. 1992;174:1574–1585. doi: 10.1128/jb.174.5.1574-1585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collis C M, Hall R M. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob Agents Chemother. 1995;39:155–162. doi: 10.1128/aac.39.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colonna B, Bernardini M, Micheli G, Maimone F, Nicoletti M, Casalino M. The Salmonella wien virulence plasmid pZM3 carries Tn1935, a multiresistance transposon containing a composite IS1936-kanamycin resistance element. Plasmid. 1988;20:221–231. doi: 10.1016/0147-619x(88)90028-5. [DOI] [PubMed] [Google Scholar]
- 8.Couturier M, Bex F, Bergquist P L, Maas W K. Identification and classification of bacterial plasmids. Microbiol Rev. 1988;52:375–395. doi: 10.1128/mr.52.3.375-395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264:375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 10.Ezaki T, Takeuchi N, Liu S L, Kai A, Yamamoto H, Yabuuchi E. Small-scale DNA preparation for rapid genetic identification of Campylobacter species without radioisotope. Microbiol Immunol. 1988;32:141–150. doi: 10.1111/j.1348-0421.1988.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 11.Flores C, Qadri M I, Lichtenstein C. DNA sequence analysis of five genes; tnsA, B, C, D and E, required for Tn7 transposition. Nucleic Acids Res. 1990;18:901–911. doi: 10.1093/nar/18.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francia M V, Avila P, de la Cruz F, Garcia Lobo J M. A hot spot in plasmid F for site-specific recombination mediated by Tn21 integron integrase. J Bacteriol. 1997;179:4419–4425. doi: 10.1128/jb.179.13.4419-4425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galimand M, Lambert T, Gerbaud G, Courvalin P. Characterization of the aac(6′)-Ib gene encoding an aminoglycoside 6′-N-acetyltransferase in Pseudomonas aeruginosa BM 2656. Antimicrob Agents Chemother. 1993;37:1456–1462. doi: 10.1128/aac.37.7.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall R M, Collis C M. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol. 1995;15:593–600. doi: 10.1111/j.1365-2958.1995.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 15.Hall R M, Brookes D E, Stokes H W. Site-specific insertion of genes into integrons: role of the 59-base element and determination of the recombination of cross-over point. Mol Microbiol. 1991;5:1941–1958. doi: 10.1111/j.1365-2958.1991.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 16.Hall R M, Brown H J, Brookes D E, Stokes H V. Integrons found in different locations have identical 5′ ends but variable 3′ ends. J Bacteriol. 1994;176:6286–6294. doi: 10.1128/jb.176.20.6286-6294.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hampton M D, Threlfall E J, Frost J A, Ward L R, Rowe B. Salmonella typhimurium DT193: differentiation of an epidemic phage type by antibiogram, plasmid profile, plasmid fingerprint and Salmonella plasmid virulence (spv) gene probe. J Appl Bacteriol. 1995;78:402–408. doi: 10.1111/j.1365-2672.1995.tb03425.x. [DOI] [PubMed] [Google Scholar]
- 18.Hannecart-Pokorni E, Depuydt F, de Wit L, van Bossuyt E, Content J, Vanhoof R. Characterization of the 6′-N-aminoglycoside acetyltransferase gene aac (6′)-Il associated with a sulI-type integron. Antimicrob Agents Chemother. 1997;41:314–318. doi: 10.1128/aac.41.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson K, Skold O, Sundstrom L. Non-palindromic attI sites of integrons are capable of site-specific recombination with one another and with secondary targets. Mol Microbiol. 1997;26:441–453. doi: 10.1046/j.1365-2958.1997.5401964.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones M E, Peters E, Weersink A M, Fluit A, Verhoef J. Widespread occurrence of integrons causing multiple antibiotic resistance in bacteria. Lancet. 1997;349:1742. doi: 10.1016/S0140-6736(05)62954-6. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 21.Kado C I, Liu S. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levesque C, Pichè L, Larose C, Roy P. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother. 1995;39:185–191. doi: 10.1128/aac.39.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Low J C, Angus M, Hopkins G, Munro D, Rankin S C. Antimicrobial resistance of Salmonella enterica Typhimurium DT104 isolates and investigation of strains with transferable apramycin resistance. Epidemiol Infect. 1997;118:97–103. doi: 10.1017/s0950268896007339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maimone F, Colonna B, Bazzicalupo P, Oliva B, Nicoletti M, Casalino M. Plasmids and transposable elements in Salmonella wien. J Bacteriol. 1979;193:369–375. doi: 10.1128/jb.139.2.369-375.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez E, de la Cruz F. Transposon Tn21 encodes RecA-independent site-specific integration system. Mol Gen Genet. 1988;211:320–325. doi: 10.1007/BF00330610. [DOI] [PubMed] [Google Scholar]
- 26.Martinez E, de la Cruz F. Genetic elements involved in Tn21 site-specific integration, a novel mechanism for the dissemination of antibiotic resistance genes. EMBO J. 1990;9:1275–1281. doi: 10.1002/j.1460-2075.1990.tb08236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer L W. Use of plasmid profiles in epidemiologic surveillance of disease outbreaks and in tracing the transmission of antibiotic resistance. Clin Microbiol Rev. 1988;1:228–243. doi: 10.1128/cmr.1.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McConnell M M, Smith H R, Leonardopoulos J, Anderson E S. The value of plasmid studies in the epidemiology of infections due to drug-resistant Salmonella wien. J Infect Dis. 1979;139:178–190. doi: 10.1093/infdis/139.2.178. [DOI] [PubMed] [Google Scholar]
- 29.Moss P J, McKendrick M W. Bacterial gastroenteritis. Curr Opin Infect Dis. 1997;10:402–407. [Google Scholar]
- 30.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disc susceptibility tests. M2A2. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 31.Osano E, Arakawa Y, Wacharotayankum R, Ohta M, Horii T, Ito H, Yoshimura F, Nato N. Molecular characterization of an enterobacterial metallo-β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouellette M, Roy P H. Homology of ORFs from Tn2603 and from R46 to site specific recombinases. Nucleic Acids Res. 1987;15:10055. doi: 10.1093/nar/15.23.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulsen I T, Littlejohn T G, Radstrom P, Sundstrom L, Skold O, Swedberg G, Skurray R A. The 3′ conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob Agents Chemother. 1993;37:761–768. doi: 10.1128/aac.37.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preston K E, Kacica M A, Limberger R J, Archinal W A, Venezia R A. The resistance and integrase genes of pACM1, a conjugative multiple-resistance plasmid, from Klebsiella oxytoca. Plasmid. 1997;37:105–118. doi: 10.1006/plas.1997.1284. [DOI] [PubMed] [Google Scholar]
- 35.Radstrom P, Skold O, Swedberg G, Flensburg J, Roy P H, Sundstrom L. Transposon Tn5090 of plasmid R751 which carries an integron, is related to Tn7, Mu, and retroelements. J Bacteriol. 1994;176:3257–3268. doi: 10.1128/jb.176.11.3257-3268.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radstrom P, Swedberg G, Skold O. Genetic analysis of sulfonamide resistance and its dissemination in gram-negative bacteria illustrate new aspects of R plasmid evolution. Antimicrob Agents Chemother. 1991;35:1840–1848. doi: 10.1128/aac.35.9.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rangnekar V M, Banker D D, Jhala H I. Antimicrobial resistance and incompatibility groups of R plasmids in Salmonella typhimurium isolated from human sources in Bombay from 1978 to 1980. Antimicrob Agents Chemother. 1983;23:54–58. doi: 10.1128/aac.23.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Recchia G D, Stokes H W, Hall R M. Characterisation of specific and secondary recombination sites recognised by the integron DNA integrase. Nucleic Acids Res. 1994;22:2071–2078. doi: 10.1093/nar/22.11.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Recchia G D, Hall R M. Gene cassettes: a new class of mobile element. Microbiology. 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 40.Sallen B, Rajoharison A, Desvarenne S, Malibat C. Molecular epidemiology of integron-associated antibiotic resistance genes in clinical isolates of Enterobacteriaceae. Microb Drug Resist. 1995;1:195–202. doi: 10.1089/mdr.1995.1.195. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook J E, Fritsch F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Sandvang D, Aerestrup F M, Jensen L B. Characterization of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica Typhimurium DT104. FEMS Microbiol Lett. 1997;157:177–181. doi: 10.1111/j.1574-6968.1997.tb12770.x. [DOI] [PubMed] [Google Scholar]
- 43.Sanger F, Nicklen S, Coulson A R, Roc B A. DNA sequencing with chain terminating inhibition. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw K J, Rather P N, Hare R S, Miller G H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simonsen C, Ey C, Levinson A. Identification of the types of trimethoprim-resistant dihydrofolate reductase specified by Escherichia coli R-plasmid R483: comparison with procaryotic and eucaryotic dihydrofolate reductase. J Bacteriol. 1983;155:1001–1008. doi: 10.1128/jb.155.3.1001-1008.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stokes H V, Hall R M. A novel family of potential DNA elements encoding site-specific gene-integration function: integrons. Mol Microbiol. 1989;3:1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 47.Swartz M N. Use of antimicrobial agents and drug resistance. N Engl J Med. 1997;337:491–492. doi: 10.1056/NEJM199708143370709. [DOI] [PubMed] [Google Scholar]
- 48.Threlfall E J, Frost J A, Ward L R, Rowe B. Increasing spectrum of resistance in multi-resistant Salmonella typhimurium. Lancet. 1996;347:1053–1054. doi: 10.1016/s0140-6736(96)90199-3. . (Letter.) [DOI] [PubMed] [Google Scholar]