Abstract
There are currently no universally accepted guidelines for the management of digoxin toxicity. In the absence of clinical practice guidelines, a set of consensus recommendations for management of digoxin toxicity in the clinical setting were developed through a modified Delphi approach. The recommendations highlight the importance of early recognition of signs of potentially life-threatening toxicity that requires immediate treatment with digoxin-specific antibodies. The consensus identifies a straightforward approach to dosing immune antibody fragments according to the presence or absence of signs of life-threatening toxicity. Supportive measures and management of specific signs of toxicity are also covered.
Keywords: antidote, atrial fibrillation, digitalis, digoxin, Fab fragments, heart failure, poisoning, toxicity
Background
Cardiac glycosides are some of the oldest medications that remain in contemporary therapeutic use. For common cardiac disorders such as atrial fibrillation and heart failure, digoxin does not have a significant impact on mortality but has been shown in randomised controlled trials to reduce hospital admissions and improve patient well-being [1,2].
Although digoxin has a narrow therapeutic window, toxicity is an uncommon occurrence particularly now that low-dose regimens are standard in clinical practice. However, careful consideration and monitoring is needed in all patients to avoid rare, potentially life-threatening events. Healthcare professionals need to be able to identify patients at risk of developing digoxin toxicity, recognise toxicity quickly if it develops, and act promptly with appropriate interventions.
Using clinical experience and the limited evidence available in the literature, a consensus on the management of digoxin toxicity in adults was reached.
Methods
A group of physicians with experience in the management of digoxin toxicity was convened (see Table, Supplemental digital content 1, http://links.lww.com/EJEM/A394). Consensus statements were developed using a variation of the Delphi method (estimate-talk-estimate) in which participants were allowed to interact between iterations of the statements. Comments and scoring were not anonymized so that multi-speciality input was visible at each stage. Based on a review of the literature, a preliminary set of statements was prepared and sent to the panel as an online questionnaire. For each statement, participants were asked to indicate whether the statement should be retained or deleted and to provide their qualitative feedback.
The list of statements was revised and re-circulated via an online questionnaire. In the second round of review, participants were asked to provide quantitative feedback, rating their agreement with each statement on a scale of 1 (strongly disagree) to 10 (strongly agree). There was also the opportunity to include comments alongside the quantitative ratings.
The feedback from the second round of review was incorporated into a revised set of statements, which were then discussed in a videoconference attended by all of the authors. Based on the discussions, a final set of statements was prepared and circulated to the group for review and approval.
Results
The final set of statements on which the panel reached consensus is presented in Table 1 (Diagnosis and investigation) and Table 2 (Management).
Table 1.
Consensus statements on diagnosis and investigation of digoxin toxicity
| Presentation and diagnosis |
|---|
|
| Assessments and investigations |
|
Table 2.
Consensus statements on management of digoxin toxicity
| Use of digoxin immune antibody fragments |
|---|
|
| Other treatment options |
|
| Other management considerations |
|
Diagnosis of digoxin toxicity
Diagnosis is based primarily on clinical suspicion and clinical features including electrocardiographic (ECG) changes suggestive of digoxin intoxication. Most cases involve chronic over-ingestion and develop insidiously [3,4].
Symptoms of digoxin toxicity are mostly non-specific in nature and include gastrointestinal and neurological disturbances. Visual disturbances are more characteristic of digoxin toxicity.
Digoxin toxicity can result in a broad range of arrhythmias including bradycardia and tachycardia (none of which are specific to digoxin toxicity) [5–7]. Signs of life-threatening digoxin toxicity include:
Ventricular tachycardia or fibrillation;
Asystole, symptomatic high-degree atrioventricular block or atropine-resistant bradycardia;
Severe hyperkalaemia (serum potassium >6.5 mmol/L);
Hypotension associated with end-organ dysfunction.
Serum digoxin levels do not reliably correlate with toxicity, which can occur even at levels normally regarded as within the therapeutic range (0.8–2.0 ng/ml, with a narrower range of 0.5–0.8 ng/ml increasingly favoured in heart failure) [8].
Risk factors for digoxin toxicity
Various factors are associated with an increased risk of toxicity occurring during chronic digoxin treatment (Table 3) [9]. Most cases of digoxin toxicity occur in older individuals receiving chronic digoxin therapy and against a background of deteriorating renal function. Interactions between digoxin and other drugs are possible.
Table 3.
Factors associated with an increased risk of digoxin toxicity
| • Advanced age |
| • Hypokalaemia |
| • Hypomagnesaemia |
| • Hypercalcaemia |
| • Renal insufficiency |
| • Dehydration |
| • Hypoxaemia |
| • Myocardial ischaemia |
Assessments and investigations
General health status
General and cardiac health status as well as concomitant therapy and recent intercurrent illnesses should be assessed for all patients who present with suspected digoxin toxicity.
Serum digoxin measurement
Complete distribution of digoxin to tissues takes several hours and serum digoxin levels in the first few hours after dosing are not reflective of either therapeutic activity or potential toxicity. Consequently, at least 6 h and ideally 8–12 h should be allowed between the last dose of digoxin and measurement of serum digoxin levels to indicate the severity of intoxication. Nevertheless, an initial measurement of digoxin levels should be carried out at the time when overdose is suspected. Treatment of life-threatening digoxin toxicity should not be delayed while waiting for the results of serum digoxin measurements.
Most digitalis immunoassays do not distinguish between free and bound digoxin. Hence digoxin levels can be misleading in patients treated with digoxin immune Fab during the time it takes for this to be cleared completely from the body (variable, but can be up to 3 weeks) [10].
Serum digoxin measurements can be of value in assessing potential toxicity in cases where digoxin intoxication is suspected but clinical or ECG signs of toxicity are absent. For the reasons described above, at least 6 h should have elapsed after the last dose of digoxin before measuring serum digoxin.
Renal function and electrolytes
Digoxin is eliminated primarily in the urine and many cases of chronic digoxin toxicity occur in the context of declining renal function. Renal function should be assessed and monitored in all patients presenting with suspected digoxin toxicity.
Hyperkalaemia is an indicator of disease severity, particularly in cases of acute digoxin toxicity. At the same time, acute or chronic hypokalaemia [11], hypomagnesaemia [12] and hypercalcaemia [13] can increase the potential for toxicity. Therefore, serum electrolytes should be monitored regularly.
Cardiac monitoring
Electrocardiogram monitoring is essential for all patients with suspected toxicity. In addition to the arrhythmias considered above, changes on the ECG typical of digoxin toxicity can include down-sloping ST segment depression (‘scooped out’ or ‘reverse tick’ appearance), flattened/inverted T waves and increased U wave amplitude. However, all these changes can also be seen in patients receiving digoxin without toxicity. Continuous cardiac monitoring should be offered, where available, particularly for patients with ECG disturbances.
Management of digoxin toxicity
Use of digoxin immune Fab for life-threatening toxicity
Treatment should be initiated immediately by the first medical contact, which may be an emergency or acute medicine physician, intensive care physician or cardiologist depending on the setting. Subsequent care may require input from an interdisciplinary team with expertise in toxicology and intensive care. Withdrawing digoxin can have an impact on the underlying management of the patient’s atrial fibrillation and/or heart failure and so early involvement of cardiologists is advisable.
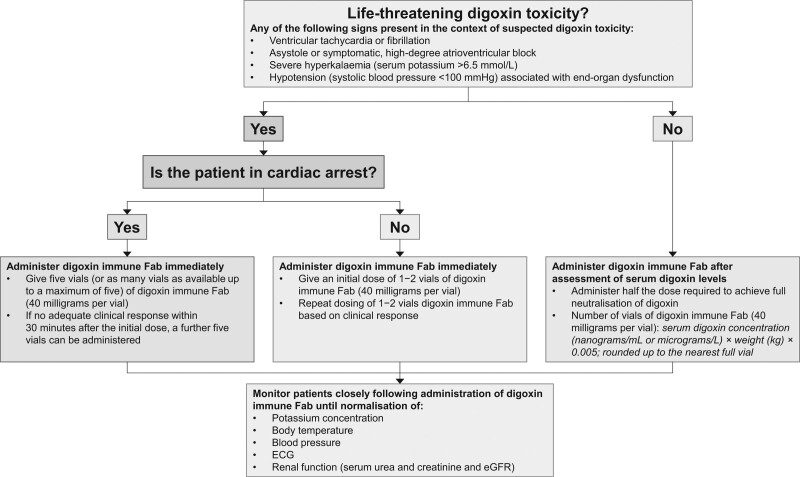
Digoxin immune antibody fragments (digoxin immune Fab, DigiFab, Protherics Medicines Developments Ltd) should be initiated immediately in patients with signs of life-threatening toxicity (Fig. 1). Response rates in the range of 50–90% have been reported following digoxin immune Fab treatment in prospective [14,15] and observational studies [16–18], with rapid resolution of toxicity [15,19–21]. Non-significant trends to reduced in-hospital mortality and early rehospitalisation were observed in a recent retrospective analysis [22].
Fig. 1.
Guidelines for treatment of digoxin toxicity with digoxin immune Fab.
The consensus expert opinion was that for patients with digoxin toxicity in cardiac arrest (loss of cardiac output due to asystole, pulseless electrical activity or tachyarrhythmias), five vials of digoxin immune Fab (40 milligrams per vial) are administered immediately. If five vials are not immediately accessible, as many vials as available up to the maximum of five should be given. If there is not an adequate clinical response after the initial dose of digoxin immune Fab, a further five vials can be administered. For all other cases of life-threatening digoxin toxicity (i.e. patients not in cardiac arrest), an initial dose of 1–2 vials should be sufficient for neutralisation of digoxin toxicity in most cases, and can be followed with incremental doses of 1–2 vials, until resolution of toxicity. Expert advice should be sought for patients who have ingested very large amounts of digoxin and in cases of ongoing toxicity after administration of digoxin immune Fab.
Potential use of digoxin immune Fab in absence of life-threatening signs
Administration of digoxin immune Fab is also sometimes appropriate for selected patients without life-threatening signs. This includes both asymptomatic patients and patients with non-life-threatening signs of toxicity and with very high serum digoxin (>12 nanograms/ml). Use of digoxin immune Fab is also appropriate following serum digoxin measurement for patients with less elevated serum digoxin levels when associated with troublesome toxicity (e.g. moderate to severe gastrointestinal symptoms or bradycardia without high-degree atrioventricular block). In such cases, the number of vials of digoxin immune Fab given should be based on the measured serum digoxin level using the formula provided in Table 1 and Fig. 1 (calculated as half that required to achieve full neutralisation of digoxin). Half the full neutralisation dose is recommended in these patients as full neutralisation may not be required to achieve adequate symptom relief and could also adversely affect patients who are dependent on the therapeutic actions of digoxin.
Monitoring and adverse effects
Hypokalaemia associated with digoxin immune Fab administration generally resolves spontaneously within several hours, but potassium levels should be assessed regularly, particularly during the first 6 h following treatment. The reduction in free digoxin following treatment with digoxin immune Fab may lead not only to a reduction in digoxin-induced toxicity but also of therapeutic effects. Frequent ECG measurement is therefore recommended to monitor both cardiac disturbances due to digoxin toxicity and potential worsening of pre-existing cardiac disorders. Renal function should also be monitored, including serum urea and creatinine as well as estimated glomerular filtration rate. Monitoring of potassium, ECG and renal function should be continued until the patient has regained their pre-intoxication status, including achievement of ‘normal’ clinical status, resolution of ECG changes and potassium disturbances, and restoration of baseline renal function.
After toxicity has resolved, digoxin may need to be reinitiated or alternative therapy introduced. The timing of reinitiation and the dose given requires careful consideration with sufficient time allowed for full clearance of digoxin immune Fab.
Adverse events associated with digoxin immune Fab treatment may occur up to 14 days after the administration of digoxin immune Fab [16,23]. Infusion-related and hypersensitivity reactions including serum sickness are possible but rarely occur.
Other treatment options
Cases of chronic digoxin toxicity with mild symptoms but without ECG changes or arrhythmias, or conduction disturbances, can generally be managed by stopping digoxin or reducing the digoxin dose. Patients should be carefully monitored for worsening of underlying heart failure or arrhythmia associated with reduced therapeutic action of digoxin.
Treatment with activated charcoal is not a substitute for other interventions, including use of digoxin immune Fab, but can be considered within the first 2 h following acute digoxin ingestion [24–26]. Caution is required in patients with confusion or a decreased consciousness level due to the risk of charcoal aspiration and consequent pneumonitis [27]. Extracorporeal treatments directed at enhancing the elimination of digoxin, such as dialysis and haemoperfusion, are of limited effectiveness [28] and are not generally recommended.
Severe hypokalaemia can be managed by intravenous administration of additional potassium. Potassium levels should be carefully monitored to avoid rebound hyperkalaemia. Mild-to-moderate hyperkalaemia should resolve following digoxin immune Fab treatment without the need for additional measures.
Alongside digoxin immune Fab as first-choice treatment, ventricular arrhythmias can be treated with lidocaine or magnesium sulfate, and bradyarrhythmias with atropine [29–32]. Adrenaline and isoprenaline can trigger ventricular fibrillation [33–35], and so should be avoided where possible. Cardiac pacing requires extreme caution [36] and, while necessary and beneficial for some patients [37], should only be undertaken if digoxin immune Fab is not immediately available and by clinicians with the requisite expertise and within a coronary care or cardiac intensive care setting.
Discussion
The consensus process produced a series of recommendations to help physicians to recognise and treat digoxin toxicity so as to improve outcomes for patients and enhance the efficiency of healthcare facilities in managing this challenging toxicity. One area of repeated discussion was the advice for dosing of digoxin immune Fab. The statements reflect the consensus that the presence or absence of cardiac arrest is the critical consideration. The pattern of toxicity (chronic, acute-on-chronic and acute) is less important in the emergency setting. The intention was to identify a simple and practical approach that can be rapidly implemented without the need for time-consuming calculations. Most patients with chronic toxicity have digoxin levels <5 ng/ml, and so 1–2 vials of digoxin immune Fab should usually be sufficient for full neutralisation [38–40]. The recommendation for an initial dose of five vials of digoxin immune Fab for patients in cardiac arrest is directed at balancing the clinical urgency of achieving neutralisation with the often limited supplies of digoxin Fab immediately available. Five vials (200 mg) of digoxin immune Fab will achieve neutralisation of 3 mg digoxin and is likely to be sufficient in most cases. However, other approaches to dosing have been recommended and used. The posology section in the labelling for DigiFab [15] is based on pharmacological considerations [41] and describes calculating the dose required to achieve half or full neutralisation of circulating digoxin, and taking into account the type of poisoning, body weight and whether the amount of digoxin ingested or the serum concentration of digoxin is known. Some authors have proposed initial dosing with half of the dose calculated as specified in the label, with further doses administered if required according to response [41].
A number of limitations should be noted. First, there is little robust evidence as to the effectiveness and safety of interventions for the management of digoxin toxicity. Therefore, the consensus process drew on personal experience and interpretation to a greater extent than is ideal. Second, all participants were based in Western Europe and, as such, the consensus is based on experience that may not be wholly representative of, or applicable to, management of digoxin toxicity in other parts of the world.
Conclusion
Digoxin toxicity presents a complex and challenging medical emergency that requires immediate management to achieve optimal outcomes. The consensus statements developed by a group of specialists in intensive care medicine, toxicology and cardiology provide practical and pragmatic recommendations for the management of digoxin toxicity in routine clinical care.
Acknowledgements
Protherics Medicines Development Ltd funded the services of a medical writer (Ian Faulkner, Aspire Scientific, UK) who provided support in searching the literature, drafting and revising consensus statements, managing the consensus process and virtual meetings, and drafting and revising the publication. All supporting activities were at the direction of the expert group and independent of Protherics Medicines Development Ltd, who made no contribution and gave no direction to the content of either the consensus statements or their publication.
Authors are listed alphabetically; all authors contributed equally to development of the consensus statements and preparation of the manuscript.
Conflicts of interest
Prof. Kotecha reports grants from the National Institute for Health Research (NIHR CDF-2015-08-074 RATE-AF; NIHR130280 DaRe2THINK; NIHR132974 D2T-NeuroVascular; NIHR203326 Biomedical Research Centre); British Heart Foundation (PG/17/55/33087, AA/18/2/34218 and FS/CDRF/21/21032); EU/EFPIA Innovative Medicines Initiative (BigData@Heart 116074) EU Horizon (HYPERMARKER 101095480); UK National Health Service -Data for R&D- Subnational Secure Data Environment programme; UK Dept. for Business, Energy & Industrial Strategy Regulators Pioneer Fund; the Cook & Wolstenholme Charitable Trust; European Society of Cardiology supported by educational grants from Boehringer Ingelheim/BMS-Pfizer Alliance/Bayer/Daiichi Sankyo/Boston Scientific, the NIHR/University of Oxford Biomedical Research Centre and British Heart Foundation/University of Birmingham Accelerator Award (STEEER-AF NCT04396418); Bayer; Amomed Pharma; Protherics Medicines Development; and IRCCS San Raffaele and Menarini (Beta-blockers in Heart Failure Collaborative Group NCT0083244); all outside the submitted work. Frédéric Lapostolle reports grants from AstraZeneca, Boehringer Ingelheim, Medtronic, Mundipharma, Nova Biomedical, Novartis, Serb and Teleflex. For the remaining authors, there are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.euro-emergencymed.com).
References
- 1.Kotecha D, Bunting KV, Gill SK, Mehta S, Stanbury M, Jones JC, et al.; Rate Control Therapy Evaluation in Permanent Atrial Fibrillation (RATE-AF) Team. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA 2020; 324:2497–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziff OJ, Lane DA, Samra M, Griffith M, Kirchhof P, Lip GY, et al. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ 2015; 351:h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapostolle F, Borron SW, Verdier C, Arnaud F, Couvreur J, Mégarbane B, et al. Assessment of digoxin antibody use in patients with elevated serum digoxin following chronic or acute exposure. Intensive Care Med 2008; 34:1448–1453. [DOI] [PubMed] [Google Scholar]
- 4.Supervía Caparrós A, Salgado García E, Calpe Perarnau X, Galicia Paredes M, García Gibert L, Córdoba Ruiz F, et al. Immediate and 30 days mortality in digoxin poisoning cases attended in the Hospital Emergency Services of Catalonia, Spain. Emergencias 2019; 31:39–42. [PubMed] [Google Scholar]
- 5.Arbabian H, Lee HM, Graudins A. Elderly patients with suspected chronic digoxin toxicity: a comparison of clinical characteristics of patients receiving and not receiving digoxin-Fab. Emerg Med Australas 2018; 30:242–248. [DOI] [PubMed] [Google Scholar]
- 6.See I, Shehab N, Kegler SR, Laskar SR, Budnitz DS. Emergency department visits and hospitalizations for digoxin toxicity: United States, 2005 to 2010. Circ Heart Fail 2014; 7:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wofford JL, Hickey AR, Ettinger WH, Furberg CD. Lack of age-related differences in the clinical presentation of digoxin toxicity. Arch Intern Med 1992; 152:2261–2264. [PubMed] [Google Scholar]
- 8.Goldberger ZD, Goldberger AL. Therapeutic ranges of serum digoxin concentrations in patients with heart failure. Am J Cardiol 2012; 109:1818–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings ED, Swoboda HD, Digoxin toxicity. In StatPearls. Treasure Island (FL): StatPearls Publishing LLC; 2023. https://www.ncbi.nlm.nih.gov/books/NBK470568/. [PubMed] [Google Scholar]
- 10.Ujhelyi MR, Robert S. Pharmacokinetic aspects of digoxin-specific Fab therapy in the management of digitalis toxicity. Clin Pharmacokinet 1995; 28:483–493. [DOI] [PubMed] [Google Scholar]
- 11.Hall RJ, Gelbart A, Billingham M, Snidow G, Goldman RH. Effect of chronic potassium depletion on digitalis-induced inotropy and arrhythmias. Cardiovasc Res 1981; 15:98–107. [DOI] [PubMed] [Google Scholar]
- 12.Young IS, Goh EM, McKillop UH, Stanford CF, Nicholls DP, Trimble ER. Magnesium status and digoxin toxicity. Br J Clin Pharmacol 1991; 32:717–721. [PMC free article] [PubMed] [Google Scholar]
- 13.Arispe N, Diaz JC, Simakova O, Pollard HB. Heart failure drug digitoxin induces calcium uptake into cells by forming transmembrane calcium channels. Proc Natl Acad Sci U S A 2008; 105:2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith TW. Review of clinical experience with digoxin immune Fab (ovine). Am J Emerg Med 1991; 9(2 Suppl 1):1–6; discussion 33–34. [DOI] [PubMed] [Google Scholar]
- 15.Protherics UK Limited DigiFab®. Summary of product characteristics. 2016. https://digifab.health/getmedia/6f38d7ed-1832-4b7d-863a-9c71905e4d02/SmPC_DigiFab_Dec17.pdf.
- 16.Chan BS, Buckley NA. Digoxin-specific antibody fragments in the treatment of digoxin toxicity. Clin Toxicol (Phila) 2014; 52:824–836. [DOI] [PubMed] [Google Scholar]
- 17.Schaeffer TH, Mlynarchek SL, Stanford CF, Delgado J, Holstege CP, Olsen D, et al. Treatment of chronically digoxin-poisoned patients with a newer digoxin immune fab: a retrospective study. J Am Osteopath Assoc 2010; 110:587–592. [PubMed] [Google Scholar]
- 18.Thomas E, Tomlinson S, Thomas S, Ward S, Daugherty C, Gallardo E, et al. Treatment of life-threatening digoxin toxicity with digoxin-specific antibody fragments: results from a prospective, non-interventional observational UK patient registry study. Eur J Hosp Pharm 2022. doi: 10.1136/ejhpharm-2022-003416. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.Husby P, Farstad M, Brock-Utne JG, Koller ME, Segadal L, Lund T, et al. Immediate control of life-threatening digoxin intoxication in a child by use of digoxin-specific antibody fragments (Fab). Paediatr Anaesth 2003; 13:541–549. [DOI] [PubMed] [Google Scholar]
- 20.Zucker AR, Lacina SJ, DasGupta DS, Fozzard HA, Mehlman D, Butler VP, Jr, et al. Fab fragments of digoxin-specific antibodies used to reverse ventricular fibrillation induced by digoxin ingestion in a child. Pediatrics 1982; 70:468–471. [PubMed] [Google Scholar]
- 21.Antman EM, Wenger TL, Butler VP, Jr, Haber E, Smith TW. Treatment of 150 cases of life-threatening digitalis intoxication with digoxin-specific Fab antibody fragments. Final report of a multicenter study. Circulation 1990; 81:1744–1752. [DOI] [PubMed] [Google Scholar]
- 22.Peters AE, Chiswell K, Hofmann P, Ambrosy A, Fudim M. Characteristics and outcomes of suspected digoxin toxicity and immune Fab treatment over the past two decades - 2000-2020. Am J Cardiol 2022; 183:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickey AR, Wenger TL, Carpenter VP, Tilson HH, Hlatky MA, Furberg CD, et al. Digoxin Immune Fab therapy in the management of digitalis intoxication: safety and efficacy results of an observational surveillance study. J Am Coll Cardiol 1991; 17:590–598. [DOI] [PubMed] [Google Scholar]
- 24.Lalonde RL, Deshpande R, Hamilton PP, McLean WM, Greenway DC. Acceleration of digoxin clearance by activated charcoal. Clin Pharmacol Ther 1985; 37:367–371. [DOI] [PubMed] [Google Scholar]
- 25.Park GD, Goldberg MJ, Spector R, Johnson GF, Feldman RD, Quee CK, et al. The effects of activated charcoal on digoxin and digitoxin clearance. Drug Intell Clin Pharm 1985; 19:937–941. [DOI] [PubMed] [Google Scholar]
- 26.Hoegberg LCG, Shepherd G, Wood DM, Johnson J, Hoffman RS, Caravati EM, et al. Systematic review on the use of activated charcoal for gastrointestinal decontamination following acute oral overdose. Clin Toxicol (Phila) 2021; 59:1196–1227. [DOI] [PubMed] [Google Scholar]
- 27.Liisanantti J, Kaukoranta P, Martikainen M, Ala-Kokko T. Aspiration pneumonia following severe self-poisoning. Resuscitation 2003; 56:49–53. [DOI] [PubMed] [Google Scholar]
- 28.Mowry JB, Burdmann EA, Anseeuw K, Ayoub P, Ghannoum M, Hoffman RS, et al.; EXTRIP Workgroup. Extracorporeal treatment for digoxin poisoning: systematic review and recommendations from the EXTRIP Workgroup. Clin Toxicol (Phila) 2016; 54:103–114. [DOI] [PubMed] [Google Scholar]
- 29.Pincus M. Management of digoxin toxicity. Aust Prescr 2016; 39:18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navab F, Honey M. Self-poisoning with digoxin: successful treatment with atropine. Br Med J 1967; 3:660–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiffel JA, Bigger JT, Jr, Cramer M. Effects of digoxin on sinus nodal function before and after vagal blockade in patients with sinus nodal dysfunction: a clue to the mechanisms of the action of digitalis on the sinus node. Am J Cardiol 1979; 43:983–989. [DOI] [PubMed] [Google Scholar]
- 32.Cohen L, Kitzes R. Magnesium sulfate and digitalis-toxic arrhythmias. JAMA 1983; 249:2808–2810. [PubMed] [Google Scholar]
- 33.Freedman RA, Swerdlow CD, Echt DS, Winkle RA, Soderholm-Difatte V, Mason JW. Facilitation of ventricular tachyarrhythmia induction by isoproterenol. Am J Cardiol 1984; 54:765–770. [DOI] [PubMed] [Google Scholar]
- 34.Holmén J, Hollenberg J, Claesson A, Herrera MJ, Azeli Y, Herlitz J, et al. Survival in ventricular fibrillation with emphasis on the number of defibrillations in relation to other factors at resuscitation. Resuscitation 2017; 113:33–38. [DOI] [PubMed] [Google Scholar]
- 35.Straznitskas AD, Wong S, Kupchik N, Carlbom D. Secondary ventricular fibrillation or pulseless ventricular tachycardia during cardiac arrest and epinephrine dosing. Am J Crit Care 2015; 24:e22–e27. [DOI] [PubMed] [Google Scholar]
- 36.Taboulet P, Baud FJ, Bismuth C, Vicaut E. Acute digitalis intoxication: is pacing still appropriate? J Toxicol Clin Toxicol 1993; 31:261–273. [DOI] [PubMed] [Google Scholar]
- 37.Bismuth C, Motte G, Conso F, Chauvin M, Gaultier M. Acute digitoxin intoxication treated by intracardiac pacemaker: experience in sixty-eight patients. Clin Toxicol 1977; 10:443–456. [DOI] [PubMed] [Google Scholar]
- 38.Chan BS, Isbister GK, O’Leary M, Chiew A, Buckley NA. Efficacy and effectiveness of anti-digoxin antibodies in chronic digoxin poisonings from the DORA study (ATOM-1). Clin Toxicol (Phila) 2016; 54:488–494. [DOI] [PubMed] [Google Scholar]
- 39.Chan BS, Isbister GK, Page CB, Isoardi KZ, Chiew AL, Kirby KA, et al. Clinical outcomes from early use of digoxin-specific antibodies versus observation in chronic digoxin poisoning (ATOM-4). Clin Toxicol (Phila) 2019; 57:638–643. [DOI] [PubMed] [Google Scholar]
- 40.Bilbault P, Oubaassine R, Rahmani H, Lavaux T, Castelain V, Sauder P, et al. Emergency step-by-step specific immunotherapy in severe digoxin poisoning: an observational cohort study. Eur J Emerg Med 2009; 16:145–149. [DOI] [PubMed] [Google Scholar]
- 41.Bateman DN. Digoxin-specific antibody fragments: how much and when? Toxicol Rev 2004; 23:135–143. [DOI] [PubMed] [Google Scholar]