Abstract
Introduction:
Chronic lymphocytic leukemia (CLL) commonly affects older adults. However, few studies have examined the relationship between baseline geriatric domains and clinical outcomes in this population. Here, we aim to evaluate the use of a comprehensive geriatric assessment in older (>65 years) untreated patients with CLL to predict outcomes.
Materials and Methods:
We conducted a planned analysis of 369 patients with CLL age 65 or older treated in a phase 3 randomized trial of bendamustine plus rituximab versus ibrutinib plus rituximab versus ibrutinib alone (A041202). Patients underwent evaluations of geriatric domains including functional status, psychological status, social activity, cognition, social support, and nutritional status. We examined associations among baseline geriatric domains with grade 3+ adverse events using multivariable logistic regression and overall survival (OS) and progression-free survival (PFS) using multivariable Cox regression models.
Results:
In this study, the median age was 71 years (range: 65–87). In the combined multivariable model, the following geriatric domains were significantly associated with PFS: Medical Outcomes Study (MOS) - social activities survey score (hazard ratio [HR] [95% confidence interval (CI)] 0.974(0.961, 0.988), p=0.0002) and nutritional status (≥5% weight loss in the preceding six months: (HR [95% CI] 2.717[1.696, 4.354], p<0.001). MOS - social activities score [HR (95% CI) 0.978(0.958, 0.999), p=0.038] was associated with OS. No geriatric domains were significantly associated with toxicity. There were no statistically significant interactions between geriatric domains and treatment.
Discussion:
Geriatric domains of social activity and nutritional status were associated with OS and/or PFS in older adults with CLL. These findings highlight the importance of assessing geriatric domains to identify high-risk patients with CLL who may benefit from additional support during treatment.
Keywords: CLL, geriatric assessment, treatment outcome, functional status, nutritional status
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults.1 CLL predominantly affects older adults, and the advent of novel targeted therapies has transformed the treatment landscape for this population.2,3 Alliance for Clinical Trials in Oncology A041202 was a phase 3 clinical trial which established the superiority of the Bruton’s Tyrosine Kinase (BTK) inhibitor ibrutinib, alone or in combination with rituximab, over standard chemoimmunotherapy for older adults with previously untreated CLL.4 With the introduction of novel therapies like ibrutinib, genomic assessment evaluating p53 alterations and immunoglobulin heavy chain variable region (IGHV) hypermutation have largely replaced prior risk stratification tools.5,6 However, risk stratification systems in CLL have not focused on geriatric domains, such as subjective and objective measures of function as well as cognition.1 Older adults with cancer frequently present with a complex constellation of medical and psychosocial issues that impact their ability to tolerate treatment and contribute to negative outcomes and increased morbidity.7,8
A comprehensive geriatric assessment (CGA) can help to robustly characterize health status and represents a better measures of older patients’ health than simply assessing performance status or considering chronologic age alone. Studies primarily in solid tumors have focused on utilizing the CGA to identify older patients at risk for poor clinical outcomes, including toxicity and mortality.9–11 The CGA entails a formal evaluation of multiple geriatric domains: functional status, comorbid conditions, medications, psychological state, social support, cognition, and nutritional status. Few studies have focused on the use of CGA in hematologic malignancies, with existing results suggesting utility in prediction of chemotherapy associated toxicities as well as survival, including in studies of acute myeloid leukemia12 and multiple myeloma.13 However, no studies have examined the use of CGA in CLL.1,14 Thus, for older adults with CLL, we need a better understanding of how geriatric domains contribute to clinical outcomes, particularly in the era of targeted therapies.
The CGA can require considerable time and expertise to complete, often representing a barrier to the use of CGA in routine clinical practice. To improve access to pertinent domains of the CGA, the Cancer in Aging Research Group (CARG) developed the CARG chemo-toxicity calculator. This calculator tool is based on a multidimensional assessment of geriatric domains, laboratory testing (hemoglobin and glomerular filtration rate), sociodemographic information, and treatment intensity.9 The CARG model was established in 500 patients with solid tumors aged 65–91 years with this scoring system stratifying patients into low, intermediate, or high risk for chemotherapy toxicities. The CARG chemo-toxicity calculator may have limitations in patients with CLL for several reasons, including the use of anemia as a risk factor and unknown utility in patients receiving continuous therapy. Given the lack of validation of CARG chemo-toxicity calculator in CLL, this study used the CGA as the primary measure of functional status, and correlation of outcomes with CARG chemo-toxicity calculator is a post-hoc exploratory analysis.
In the present study, we sought to evaluate the association of baseline geriatric domains with clinical outcomes in CLL, including adverse events, therapy discontinuation, overall survival (OS), and progression-free survival (PFS). We also sought to explore how CGA domains changed over time during therapy. We hypothesized that geriatric domains of functional status, nutritional status, social activity, social support, psychological status, and cognition would be associated with toxicity, OS, and PFS. We further sought to investigate the association between the CARG score and toxicity in this cohort.
Methods
Patients and Measures
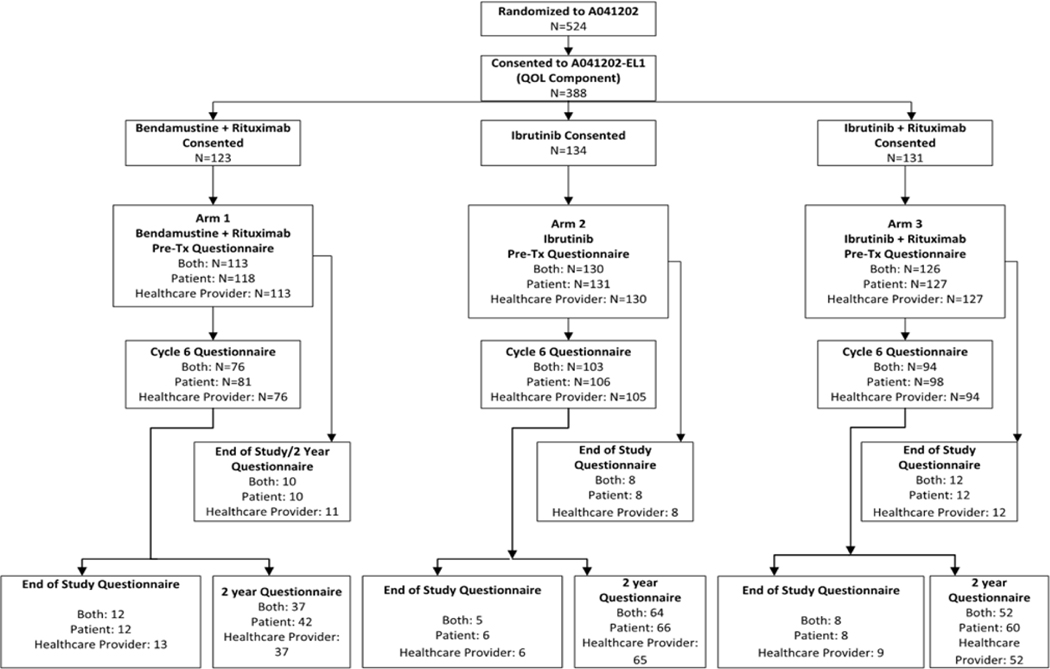
Alliance for Clinical Trials in Oncology A041202 (NCT01886872) was a phase 3 clinical trial comparing standard bendamustine plus rituximab to ibrutinib given alone or in combination with rituximab for untreated patients ≥65 years with CLL. 15 Each participant signed an IRB-approved, protocol-specific, informed consent document in accordance with federal and institutional guidelines. As a planned correlative study optional to participants, we performed geriatric assessments prior to therapy, after six months of treatment, and after two years (or at disease progression)(Figure 1). The geriatric domains included validated measures of functional status, psychological status, social activity, cognition, social support, and nutritional status.16–22 Geriatric domains were scored based on established procedures.16,17 For pertinent characteristics, we dichotomized patients into “impaired” versus “not impaired” based upon prior studies. 23 For the models, if any cell of the dichotomized variable by arm had a sample size less than 10 in any cell, the non-dichotomized score was used.
Figure 1:
CONSORT diagram showing timing of assessments and number of patients who completed each assessment
Clinical Outcomes
The outcomes analyzed in this analysis included toxicity, OS, and PFS, and were measured as in the primary manuscript.4 Toxicity was defined as a grade 3 or higher adverse event (AE) as defined by CTCAE version 4.0 while on study treatment. OS was defined as the time from the date of randomization until the date of death from any cause. Data from patients who were alive were censored on the date of the last assessment. We defined PFS as the time from the date of randomization until the earliest date on which disease progression (as defined by IWCLL criteria)24 or death from any cause was recorded. Data from patients who were alive and had not experienced disease progression were censored on the date of the last assessment. Data from patients who started a therapy for CLL that was not specified in the protocol or withdrew consent for further follow-up were also censored on the date of the last assessment.4
Statistical Analysis
Patients’ sociodemographic and clinical characteristics, as well as geriatric domains, and changes in geriatric domains were summarized by treatment arm using mean (standard deviation) and/or median (range) for continuous variables and using frequency (percentage) for categorical variables. The proportion of patients that reported grade 3+ AEs was summarized by arm and the association between grade 3+ AEs and arm was evaluated using the Chi-squared test. The proportion of patients that reported grade 3+ AEs was summarized and the association between CARG risk category and grade 3+ AEs was evaluated using the Chi-squared test.
Multivariable logistic regression models were used to evaluate the association between components of CGA domains and grade 3+ AEs. First, separate multivariable analyses were conducted for each individual component of the CGA, examining the relationship of the individual CGA domain with toxicity, adjusting for baseline characteristics and treatment arm. Components found to be associated with toxicity (p< 0.20) were then included in separate multivariable logistic regression models to test for the interaction between arm and components of CGA domains, adjusting for the following covariates that were a priori defined based on our review of the literature and prior studies: age, sex, Rai stage, Eastern Cooperative Oncology Group (ECOG) performance status, Zap70 methylation status, presence or absence of TP53 mutation, and/or deletion of 17p13 on FISH25,26. Zap70 methylation status is used in this study as a surrogate for IGHV mutational status, where methylated Zap70 is a surrogate for mutated IGHV. The final model for our multivariable analyses included all components of CGA domains associated with toxicity, adjusted for arm, any statistically significant interactions (p<0.05), and the previously specified covariates. Similar methods were used to investigate the relationship between various components of CGA domains with clinical outcomes (OS and PFS) using Cox proportional hazards regression analyses. In the final model, the geriatric domains that reached the 0.05 significance level were considered statistically significant. Due to the exploratory nature of this analysis, no adjustment was made for multiple comparisons. We performed statistical analyses using Stata version 14.2. on a dataset lock on October 4, 2018. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Management Center. Data quality was ensured by review of data by the Alliance Statistics and Data Management Center and by the study chairperson following Alliance policies.
Results
Patient Characteristics
Overall, 388 patients consented to the geriatric assessments (74% of 524 patients enrolled on A041202), and 369 completed all pre-treatment assessments and were included in analyses. Figure 1 outlines the assessments completed. Table 1 describes the clinical characteristics of these patients. Baseline characteristics of this patient subset were similar to the overall cohort of patients enrolled to A041202. Briefly, median age was 71 years (range: 65–87), most patients were male (70%), and approximately half (56%) had Rai stage III or IV disease. Overall, 54% of patients had Zap70 unmethylated disease, and 26% had either del(17p13) or del(11q22) on baseline FISH. These clinical characteristics were balanced among the three arms. The number of patients considered to have a geriatric impairment in emotional/informational support (MOS Social Support Survey: Emotional/Informational) was significantly different across the three treatment arms, with 50%, 45%, and 62% considered impaired in Bendamustine + Rituximab, Ibrutinib, and Ibrutinib + Rituximab, respectively (p=0.02). There were no other significant differences across the arms in terms of baseline geriatric assessment data or percent of patients who were considered impaired (Supplemental Table 2). Only 8% of patients and 5% of physicians reported Karnofsky performance status (KPS) as impaired. The median score on the activities of daily living (ADL) scale was 14 (range: 9–14), with 21% having an impairment. The median on the Timed “Up and Go” test was 10.3, with 25% of patients having a time of greater than 13.5 seconds. Overall, 13% of patients had one or more falls in the last six months, and the median number of comorbidities was two (range: 0–14). Only 4% of patients had cognitive impairment based upon the Blessed Orientation Memory Concentration (BOMC) Score. Approximately one-fifth (21%) of patients experienced at least 5% weight loss in the preceding six months, and 28% had either a very low or high body mass index (BMI).
Table 1:
Patient Characteristics
| Bendamustine + Rituximab (N=113) | Ibrutinib (N=130) | Ibrutinib + Rituximab (N=126) | Total (N=369) | p value | |
|---|---|---|---|---|---|
| Age | 0.671 | ||||
| Median | 71 | 71 | 71 | 71 | |
| Range | (65–86) | (65–87) | (65–86) | (65–87) | |
| Sex | 0.512 | ||||
| Female | 39 (35%) | 37 (28%) | 36 (29%) | 112 (30%) | |
| Male | 74 (65%) | 93 (72%) | 90 (71%) | 257 (70%) | |
| Race | 0.502 | ||||
| White | 103 (91%) | 121 (93%) | 122 (97%) | 346 (94%) | |
| Black or African American | 5 (4%) | 5 (4%) | 2 (2%) | 12 (3%) | |
| Asian | 3 (3%) | 1 (1%) | 0 (0%) | 4 (1%) | |
| American Indian or Alaska Native | 0 (0%) | 0 (0%) | 1 (1%) | 1 (0%) | |
| Not reported: patient refused or not available | 1 (1%) | 2 (2%) | 1 (1%) | 4 (1%) | |
| Unknown: Patient unsure | 1 (1%) | 1 (1%) | 0 (0%) | 2 (1%) | |
| Ethnicity | 0.592 | ||||
| Hispanic or Latino | 2 (2%) | 1 (1%) | 1 (1%) | 4 (1%) | |
| Not Hispanic or Latino | 107 (95%) | 122 (94%) | 122 (97%) | 351 (95%) | |
| Not Reported | 3 (3%) | 6 (5%) | 1 (1%) | 10 (3%) | |
| Unknown | 1 (1%) | 1 (1%) | 2 (2%) | 4 (1%) | |
| Rai Stage | 0.642 | ||||
| Intermediate (Stage I/II) | 49 (43%) | 62 (48%) | 53 (42%) | 164 (44%) | |
| High (Stage III/IV) | 64 (57%) | 68 (52%) | 73 (58%) | 205 (56%) | |
| ECOG Performance Status | 0.162 | ||||
| 0 | 64 (57%) | 65 (50%) | 64 (51%) | 193 (52%) | |
| 1 | 45 (40%) | 63 (48%) | 62 (49%) | 170 (46%) | |
| 2 | 4 (4%) | 2 (2%) | 0 (0%) | 6 (2%) | |
| Zap70 Methylation Status, Central Results | 0.702 | ||||
| <20% | 63 (56%) | 67 (52%) | 71 (56%) | 201 (54%) | |
| >= 20% | 50 (44%) | 63 (48%) | 55 (44%) | 168 (46%) | |
| Presence of del(11q22) or del(17p13), Local Result | 0.782 | ||||
| Yes | 31 (27%) | 35 (27%) | 30 (24%) | 96 (26%) | |
| No | 82 (73%) | 95 (73%) | 96 (76%) | 273 (74%) | |
| Education | 0.802 | ||||
| High school or less or GED | 39 (35%) | 42 (33%) | 44 (35%) | 125 (34%) | |
| Some college | 50 (44%) | 54 (42%) | 47 (38%) | 151 (41%) | |
| Advanced degree | 24 (21%) | 32 (25%) | 34 (27%) | 90 (25%) | |
| Missing | 0 | 2 | 1 | 3 | |
| Marital Status | 0.692 | ||||
| Married/Partnership | 87 (77%) | 101 (78%) | 93 (74%) | 281 (76%) | |
| Other | 26 (23%) | 28 (22%) | 33 (26%) | 87 (24%) | |
| Missing | 0 | 1 | 0 | 1 | |
| Employment Status | 0.842 | ||||
| Employed | 25 (22%) | 28 (22%) | 23 (18%) | 76 (21%) | |
| Retired | 76 (67%) | 87 (68%) | 92 (74%) | 255 (70%) | |
| Unemployed/Other | 12 (11%) | 13 (10%) | 10 (8%) | 35 (10% | |
| Missing | 0 | 2 | 1 | 3 | |
| Time since Diagnosis (in years) | 0.791 | ||||
| Median | 2.57 | 1.99 | 2.60 | 2.42 | |
| Mean | 3.46 | 3.21 | 3.93 | 3.53 | |
| Range | (0.01 – 18.45) | (0.02 – 17.06) | (0.02 – 19.82) | (0.01 – 19.82) | |
| Missing | 6 | 7 | 9 | 22 |
Kruskal Wallis
Chi-Square
ECOG=Eastern Cooperative Oncology Group; GED=General Education Development
Note: Variables where complete data is not available have the frequency of patients with missing data provided. Variables with complete data are provided without the missing category.
Baseline Geriatric Domains and Risk of High-Grade Adverse Events (Grade 3–5 toxicities)
Using the CARG toxicity calculator,9 54% of patients were at low risk, 40% were at medium risk, and 7% were at high risk for grade 3 or higher toxicities. In this population, the CARG chemo-tox calculator did not reliably predict grade 3 or higher toxicities (Supplemental Table 3; p=0.81). Prevalence of grade 3 or higher AE was not different across treatment arms overall (p=0.92) nor when analyzed only within the first year of therapy (p=0.08; Supplemental Table 4).
In separate multivariable logistic regression models adjusting for baseline characteristics and treatment arm, lower tangible social support (MOS Social Support Survey; odds ratio [OR] = 0.62; 95% confidence interval [CI] 0.35–1.11; p=0.11) and a higher number of comorbidities (OR 1–2 comorbidities = 1.29; 95% CI 0.56–2.94; OR 3+ comorbidities = 2.42; 95% CI 0.99–5.92; overall p=0.08) were associated with higher toxicity, though not statistically significant. After including both social support and the number of comorbidities in a multivariable model, adjusting for baseline characteristics and treatment arm, neither baseline geriatric assessment items (MOS Social Support Survey: Tangible subscale - OR = 0.579; 95% CI 0.32–1.04; p=0.07; number of Comorbidities: OR 1–2 Comorbidities = 1.28; 95% CI 0.56–2.95; OR 3+ comorbidities = 2.57; 95% CI 1.04–6.33; overall p=0.06) were significantly associated with a higher risk for toxicity. Among the baseline characteristics included in the model, high Rai stage (OR = 1.95; 95% CI 1.08–3.50; p=0.03) was significantly associated with a higher risk for grade 3+ adverse events (Table 2). The interactions between each geriatric assessment item and treatment arm were not statistically significant.
Table 2:
Multivariable Logistic Regression Model for Grade 3+ Adverse Events with All Significant Geriatric Assessments
| Factor | Level | N | OR (95% CI) | P-Value |
|---|---|---|---|---|
| Age Group | 65–69 | 144 | 1 (Reference) | 0.2696* |
| 70–74 | 119 | 1.166 (0.588, 2.309) | 0.6412 | |
| 75+ | 104 | 1.849 (0.875, 3.906) | 0.1314 | |
| Presence of del(17p13) | Normal | 335 | 1 (Reference) | |
| Abnormal | 32 | 1.382 (0.445, 4.287) | 0.5756 | |
| ECOG Performance Status | 0 | 192 | 1 (Reference) | |
| 1–2 | 175 | 0.653 (0.363, 1.173) | 0.1542 | |
| Treatment Arm | Bendamustine + Rituximab | 112 | 1 (Reference) | 0.9157* |
| Ibrutinib | 130 | 1.138 (0.557, 2.322) | 0.8513 | |
| Ibrutinib + Rituximab | 125 | 1.153 (0.553, 2.405) | 0.8049 | |
| Number of Comorbidities | 0 | 45 | 1 (Reference) | 0.0567* |
| 1–2 | 168 | 1.281 (0.557, 2.945) | 0.4660 | |
| 3+ | 154 | 2.567 (1.041, 6.331) | 0.0171 | |
| RAI Stage | Intermediate (Stage I/II) | 164 | 1 (Reference) | |
| High (Stage III/IV) | 203 | 1.946 (1.081, 3.504) | 0.0265 | |
| Sex | Male | 256 | 1 (Reference) | |
| Female | 111 | 1.749 (0.884, 3.463) | 0.1085 | |
| Social Support Survey: Tangible subscale | =100 (Not Impaired) | 194 | 1 (Reference) | |
| <100 (Impaired) | 173 | 0.579 (0.322, 1.041) | 0.0677 | |
| Zap70 Methylation Status | >=20% | 167 | 1 (Reference) | |
| <20% | 200 | 1.411 (0.785, 2.535) | 0.2494 |
overall p-value; ECOG=Eastern Cooperative Oncology Group
We also evaluated whether baseline geriatric assessment domains could predict whether patients would discontinue therapy for adverse events. No specific subjective or objective measures of function were associated with discontinuation of therapy for toxicity. However, in models looking at each CGA item individually, after adjusting for patient characteristics, lower levels of depression and anxiety (HR 0.97, 95% CI 0.95–1; p=0.018), higher levels of social activities scores (HR 0.98, 95% CI 0.97–1; p=0.03), and lower levels of weight loss over the previous six months (HR 2.83, 95% CI 1.5–5.36; p=0.001) were all protective against therapy discontinuation for toxicity.
Baseline Geriatric Assessment and Clinical Outcomes
In the Cox regression models with individual components of the CGA, adjusting for baseline characteristics and treatment arm, better functional status (instrumental activities of daily living [IADL] score: HR 2.26, 95% CI: 1.4–3.66, p=0.001), better performance status (patient-reported KPS: HR 0.96, 95% CI: 0.94–0.98, p<0.001), and higher social activity score (HR 0.97, 95% CI: 0.96–0.98, p<0.001) were associated with better PFS. Conversely, worse nutritional status (>5% weight loss in last six months: HR 2.81, 95% CI: 1.77–4.44, p<0.001) was associated with worse PFS. Worse social support (MOS Social Support Survey: Emotional/Informational subscale) was also associated with worse PFS, but this was not statistically significant (HR 1.37, 95% CI: 0.9–2.1, p=0.144). Timed “Up and Go”, number of falls in last six months, number of comorbidities, psychological status, social support, cognition, and low BMI were not associated with PFS. After including all individually associated components of CGA domains in a multivariable model and adjusting for baseline characteristics and treatment arm, higher social activity score (HR 0.97, 95% CI: 0.96–0.99, p=0.002) remained associated with better PFS. Similarly, worse nutritional status (>5% weight loss in last six months: HR 2.72, 95% CI: 1.70–4.35, p<0.001) was associated with worse PFS. Social support, functional status, and performance status were not associated with PFS (Table 3). The interactions between each geriatric assessment item and treatment arm were not statistically significant.
Table 3:
Multivariable Cox Regression Model for Progression Free Survival with all Significant Geriatric Assessments
| Factor | Level | N | HR (95% CI) | P-Value |
|---|---|---|---|---|
| Age Group | 65–69 | 143 | 1 (Reference) | 0.0899* |
| 70–74 | 116 | 1.652 (0.984, 2.773) | 0.0577 | |
| 75+ | 98 | 1.730 (0.977, 3.063) | 0.0602 | |
| Presence of del(17p13) | Normal | 325 | 1 (Reference) | |
| Abnormal | 32 | 2.492 (1.332, 4.661) | 0.0043 | |
| ECOG Performance Status | 0 | 187 | 1 (Reference) | |
| 1–2 | 170 | 0.767 (0.470, 1.250) | 0.2868 | |
| MOS - Social Support Survey: Emotional/Information | =100 (Not Impaired) | 167 | 1 (Reference) | |
| <100 (Impaired) | 190 | 1.257 (0.811, 1.949) | 0.3066 | |
| IADL - Activities of Daily Living subscale | =14 (Not Impaired) | 283 | 1 (Reference) | |
| <14 (Impaired) | 74 | 1.364 (0.804, 2.315) | 0.2496 | |
| Patient Reported Karnofsky Performance Status (Score 30–100) | 357 | 0.988 (0.964, 1.012) | 0.3220 | |
| Treatment Arm | Bendamustine + Rituximab | 110 | 1 (Reference) | <.0001* |
| Ibrutinib | 124 | 0.262 (0.153, 0.451) | <.0001 | |
| Ibrutinib + Rituximab | 123 | 0.289 (0.171, 0.490) | <.0001 | |
| Rai Stage | Intermediate (Stage I/II) | 161 | 1 (Reference) | |
| High (Stage III/IV) | 196 | 0.689 (0.440, 1.079) | 0.1037 | |
| MOS - Social Activities Score | 357 | 0.974 (0.961, 0.988) | 0.0002 | |
| Sex | Male | 248 | 1 (Reference) | |
| Female | 109 | 0.785 (0.483, 1.277) | 0.3302 | |
| Percent Unintentional Weight Loss in last 6 months | < 5% (Not Impaired) | 282 | 1 (Reference) | |
| >= 5% (Impaired) | 75 | 2.717 (1.696, 4.354) | <.0001 | |
| Zap70 Methylation Status | >=20% | 164 | 1 (Reference) | |
| <20% | 193 | 1.831 (1.154, 2.907) | 0.0103 |
overall p-value; ECOG=Eastern Cooperative Oncology Group, MOS=Medical Outcomes Study; IADL=Instrumental Activities of Daily Living
9 Patients were excluded in this final model due to missing values for one or more of the covariates.
In individual multivariable Cox regression models, higher performance status (patient-reported KPS: HR 0.96, 95% CI: 0.93–1, p=0.03; physician-reported KPS: HR 0.95, 95% CI: 0.91–0.99, p=0.02) and higher social activity score (HR 0.97, 95% CI: 0.95–0.99, p<0.001) were associated with better OS. In contrast, worse functional status (IADL score <14: HR 3.73, 95% CI: 1.85–7.53, p<0.001) and worse nutritional status (>5% weight loss in the preceding six months: HR 2.36, 95% CI: 1.17–4.76, p=0.02) was associated with worse OS. Timed “Up and Go”, psychological status, social support, cognition, and low BMI were not significantly associated with OS. The number of falls in last six months was not included in the model due to the low number of patients with no falls that experienced a death event (n=1). After including all individually associated components of CGA domains in a multivariable model, adjusting for baseline characteristics and treatment arm, higher social activity score (HR 0.98, 95% CI: 0.96–1.00, p=0.04) was associated with better OS (Table 4). The interactions between each CGA component and treatment arm were not statistically significant.
Table 4:
Multivariable Cox Regression of Overall Survival with all Significant Geriatric Assessments
| Factor | Level | N | HR (95% CI) | P-Value |
|---|---|---|---|---|
| Age Group (years) | 65–69 | 142 | 1 (Reference) | 0.0293* |
| 70–74 | 115 | 1.629 (0.661, 4.015) | 0.2888 | |
| 75+ | 98 | 3.229 (1.337, 7.795) | 0.0092 | |
| Presence of del(17p13) | Normal | 324 | 1 (Reference) | |
| Abnormal | 31 | 3.527 (1.430, 8.698) | 0.0062 | |
| ECOG Performance Status | 0 | 187 | 1 (Reference) | |
| 1–2 | 168 | 0.369 (0.167, 0.814) | 0.0135 | |
| IADL - Activities of Daily Living subscale | =14 (Not Impaired) | 282 | 1 (Reference) | |
| <14 (Impaired) | 73 | 2.041 (0.911, 4.575) | 0.0831 | |
| Physician Reported Karnofsky Performance Status (Score 0–100) | 355 | 0.980 (0.932, 1.030) | 0.4218 | |
| Patient Reported Karnofsky Performance Status (Score 30–100) | 355 | 0.999 (0.960, 1.039) | 0.9524 | |
| Treatment Arm | Bendamustine + Rituximab | 110 | 1 (Reference) | 0.9319* |
| Ibrutinib | 122 | 1.151 (0.493, 2.684) | 0.7455 | |
| Ibrutinib + Rituximab | 123 | 1.160 (0.490, 2.747) | 0.7363 | |
| Rai Stage | Intermediate (Stage I/II) | 160 | 1 (Reference) | |
| High (Stage III/IV) | 195 | 0.822 (0.414, 1.630) | 0.5741 | |
| MOS - Social Activities Score | 355 | 0.978 (0.958, 0.999) | 0.0377 | |
| Sex | Male | 246 | 1 (Reference) | |
| Female | 109 | 0.704 (0.319, 1.557) | 0.3864 | |
| Percent Unintentional Weight Loss in last 6 months | < 5% (Not Impaired) | 281 | 1 (Reference) | |
| >= 5% (Impaired) | 74 | 1.795 (0.846, 3.807) | 0.1271 | |
| Zap70 Methylation Status | >=20% | 163 | 1 (Reference) | |
| <20% | 192 | 1.291 (0.636, 2.620) | 0.4798 |
overall p-value; ECOG=Eastern Cooperative Oncology Group, MOS=Medical Outcomes Study; IADL=Instrumental Activities of Daily Living
17 Patients were excluded from the final model due to missing values for one or more of the covariates
Change in Comprehensive Geriatric Assessment Domains over Time
Overall, 74% of patients who had completed baseline CGA also had completed assessments at six months (Figure 1). While patients were evaluated within treatment arm, there were no significant changes among treatment arms relative to the change in geriatric assessment domains over time. Overall, many domains of the geriatric assessment improved with the initiation of therapy, with commonalities of improvements in performance status and weight, with a reduction in anxiety (Table 5). In patients receiving bendamustine plus rituximab, we observed improvements in physician-reported KPS (p=0.008), increases in weight (p=0.003), increase in social activity total score (p=0.040) and a reduction in patient-reported anxiety (p<0.001) from baseline to six months. Within the ibrutinib arm, we observed increases in patient- and physician-reported KPS (p=0.004 and <0.001 respectively); increase in weight (p<0.001), social activity total score (p=0.017), and physical functioning score (p=0.003); and reduction in patient reported anxiety (p<0.001) from baseline to six months. Within the ibrutinib plus rituximab arm, we observed an improvement in physician-reported KPS (p=0.034), an increase in weight (p<0.001), and a reduction in patient anxiety (p=0.003) from baseline to six months. However, the number of falls increased slightly from baseline to six months in this group (mean 0.3 to 0.4; p=0.018).
Table 5:
Change in Geriatric Assessment Domains Over Time
| Bendamustine + Rituximab (N=113) | Ibrutinib (N=130) | Ibrutinib + Rituximab (N=126) | Total (N=369) | |||||
|---|---|---|---|---|---|---|---|---|
| GA | Difference (6 months-Baseline) | p-value | Difference (6 months-Baseline) | p-value | Difference (6 months-Baseline) | p-value | Difference (6 months-Baseline) | p-value |
| Physician-reported KPS Mean (SD) | 2.7 (8.5) | 0.008 | 4.4 (7.1) | <0.001 | 2.2 (9.7) | 0.034 | 3.2 (8.5) | 0.067 |
| Patient-reported KPS Mean (SD) | 1.6 (9.3) | 0.124 | 3.6 (12.6) | 0.004 | 2.0 (10.4) | 0.056 | 2.5 (11.0) | 0.457 |
| Number of Falls | −0.1 (1.1) | 0.695 | 0.1 (0.9) | 0.197 | 0.2 (0.8) | 0.018 | 0.1 (1.0) | 0.393 |
| Timed Up and Go | −1.6 (16.1) | 0.384 | −0.4 (14.1) | 0.786 | −0.6 (8.7) | 0.516 | −0.8 (13.2) | 0.161 |
| IADLs | −0.1 (0.7) | 0.530 | 0.1 (0.8) | 0.146 | 0.0 (0.9) | 0.641 | 0.0 (0.8) | 0.348 |
| MOS (Physical Functioning Score) | 1.6 (13.1) | 0.280 | 4.7 (15.8) | 0.003 | 0.5 (17.7) | 0.793 | 2.4 (15.8) | 0.371 |
| Number of Comorbidities | −0.1 (1.4) | 0.424 | 0.1 (2.0) | 0.771 | 0.4 (2.2) | 0.081 | 0.1 (1.9) | 0.478 |
| BMI | −0.1 (2.6) | 0.855 | 0.5 (2.1) | 0.013 | 0.5 (1.3) | <0.001 | 0.4 (2.1) | 0.033 |
| Percent Weight Change | 2.8 (7.8) | 0.003 | 4.2 (7.2) | <0.001 | 3.0 (5.5) | <0.001 | 3.4 (6.8) | 0.181 |
| Mood Total Score | 0.8 (8.2) | 0.368 | 2.0 (9.1) | 0.024 | −0.3 (11.4) | 0.796 | 0.9 (9.7) | 0.079 |
| Social Activity Total Score | −4.1 (17.7) | 0.040 | 4.4 (18.8) | 0.017 | 2.3 (21.4) | 0.289 | 1.3 (19.7) | 0.064 |
| Social Support Total Score | −0.9 (17.7) | 0.655 | −0.1 (19.1) | 0.950 | 0.6 (13.8) | 0.675 | −0.1 (17.0) | 0.627 |
| Anxiety Scale | −10.4 (22.2) | <0.001 | −9.6 (21.1) | <0.001 | −6.6 (21.8) | 0.003 | −8.8 (21.7) | 0.655 |
| BOMC | −1.0 (3.3) | 0.013 | 0.1 (4.3) | 0.868 | −1.1 (4.0) | 0.014 | −0.6 (4.0) | 0.227 |
KPS=Karnofsky Performance Status; IADL=Instrumental Activities of Daily Living; MOS=Medical Outcomes Study; BOMC=Blessed Orientation-Memory Concentration
When looking specifically at patients defined as impaired at baseline, within all arms from baseline to six months we observed improvement in every domain studied, with a trend toward greater gains in the two ibrutinib arms.
Discussion
In this correlative analysis of the pivotal phase 3 A041202 study of older patients with CLL, we found that specific geriatric assessment domains, rather than a CGA, predict drug discontinuation, PFS, and OS in this setting. We found that the specific geriatric domains of social activities and nutritional status assessed prior to therapy initiation were associated with OS and PFS. Notably, these associations were statistically significant regardless of treatment received and despite controlling for known important patient and clinical factors such as age, Rai stage, ECOG performance status, and TP53 status. Importantly, we also found that the widely used CARG chemotoxicity calculator does not accurately predict high grade toxicity in this setting. Collectively, these findings demonstrate the importance of assessing geriatric domains to identify patients with CLL at high risk for worse clinical outcomes, but also highlight the need for further work to identify pertinent geriatric domains among older adults with CLL to better predict toxicity.
This study highlights the fact that all cancers and treatment strategies are not equal. Unexpectedly, the validated CARG chemotherapy-toxicity calculator did not reliably predict which patients would develop high-grade toxicity or discontinue therapy due to toxicity. This is not due to low frequency of toxicity, as >80% of patients experience some type of high-grade toxicity, regardless of treatment arm, and approximately 15% of patients discontinued therapy due to toxicity during this analysis period. Our findings suggest that different domains may be important for patients undergoing treatment with novel therapies, and further research is needed to determine specific pre-treatment factors that accurately predict toxicity and therapy discontinuation. The measures found to be significant from this study should be prospectively evaluated in future trials, including phase 3 studies.
Our findings underscore the importance of geriatric domains in the context of clinical outcomes of patients with CLL in the era of novel targeted therapies. In a prior study of 75 patients with CLL receiving chemotherapy enrolled in the CLL9 trial, the Timed “Up and Go” and cognitive function were associated with survival. Another small study of older adults with CLL also demonstrated an associated between functional status and survival.27,28 We did not find the “Up and Go” test to significantly predict survival and did not detect a relationship in our cohort between cognition and clinical outcomes, potentially due to different treatment regimens. Prior studies in patients with other hematologic malignancies treated with chemotherapy have also found an association between extent of geriatric impairments in older adults with OS.12–14,29,30 However, these studies did not include patients receiving targeted therapy and immunotherapy, the current mainstay treatments for older adults with CLL. Many oncologists consider targeted therapies and immunotherapies less toxic, thus making them more inclined to prescribe these therapies to older adults.31 Our findings highlight the prognostic importance of multiple geriatric domains in older adults with CLL regardless of the type of systemic therapy received. In fact, the International Society of Geriatric Oncology (SIOG) has recommended the routine use of the geriatric assessment in all older adults with CLL.32 Our results suggest that future studies should continue to assess geriatric domains in patients with CLL treated with novel therapies to identify those at risk for worse clinical outcomes and to further risk-stratify patients in clinical practice. Specifically, we identified nutritional status and social activity scales as especially important, and future work will be needed to validate these in this context. It may be the case that other measures (e.g., Timed “Up and Go” and cognition testing) may not be as important to measure in patients undergoing BTK inhibitor treatment for CLL, however, it would be helpful to have these findings validated in other large studies, potentially with more impaired patients, to make firm recommendations.
The exact mechanism(s) by which various geriatric domains impact OS and PFS is likely multifactorial. Nearly half of older adults with a good performance status, as assessed by clinician-reported ECOG performance status, have IADL or physical capacity impairments when assessed with validated tools,33 reflecting the ability of geriatric domains to identify impairments that are difficult to ascertain otherwise. Moreover, research in patients with solid tumors has demonstrated that impairments in functional status and social activities are associated with treatment toxicity and chemotherapy interruptions.9 Notably, pre-rehabilitation programs and geriatric assessment-driven interventions have shown promise as a strategy for improving functional status in older adults with solid tumors.34–36 Future supportive care interventions utilizing pre-rehabilitation programs and/or geriatric assessment-driven interventions tailored to the needs of patients with CLL merit testing and have the potential to improve clinical outcomes in this population.
This study has several limitations that are important to consider. First, despite inclusive entry criteria to try to make the study population representative of a broad CLL patient population, the study enrolled primarily fit patients with relatively low levels of impairment, and thus our findings may not be easily generalizable. In addition, patients enrolled to A041202 had the option to enroll in the GA substudy and thus our findings may not be easily generalizable to the broad CLL population or to patients enrolled in A041202. Some assessments of geriatric domains, such as cognitive impairment, were underrepresented, and therefore our data may not have fully captured the impact of these geriatric domains on clinical outcomes. Moreover, though this is a large study for a CLL population, due to the sample size we were limited in the number of predictors we could analyze in multivariate analyses; thus, our model may not fully account for all possible confounders. In addition, no adjustments were made for multiple comparisons in this exploratory analysis and further validation of the models is needed to confirm the association between these geriatric domains and clinical outcomes.
In conclusion, we demonstrated that baseline assessment of geriatric domains of social activity and nutritional status are associated with OS and PFS in older adults with CLL receiving treatment with chemoimmunotherapy and targeted therapy. Our findings highlight that pre-treatment geriatric assessment provides clinicians and investigators with important prognostic information to identify older adults with CLL at higher risk of poor clinical outcomes.
Supplementary Material
Acknowledgements of Research Support
Support:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821,U10CA180882 and UG1 CA189823 (to the Alliance for Clinical Trials in Oncology), UG1CA233178, UG1CA233180, UG1CA233193, UG1CA233331, UG1CA233339; U10CA180863 and Canadian Cancer Society grant # 707213 (CCTG), U10CA180820 and UG1CA232760 (ECOG-ACRIN), U10CA180888 and UG1CA233178 (SWOG). https://acknowledgments.alliancefound.org. Also supported in part by Pharmacyclics, Inc. JAW is a Clinical Scholar of the Leukemia and Lymphoma Society. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
ClinicalTrials.gov Identifier: NCT01886872
Conflict of Interest Disclosures: PCJ has consulted for AstraZeneca, ADC Therapeutics, and Seagen. JAW has consulted for Abbvie, AstraZeneca, Beigene, Genentech, Janssen, Pharmacyclics, Loxo/Lilly, Merck, and Newave. RAN received equity stock payment from TimeDoc. CO has consulted for Janssen, Beigene, AbbVie, Astrazeneca, Merck and Roche. WD has consulted for Merck, BeiGene, MEI pharama, Alexion and Octapharma. DMS has consulted for Pharmacyclics/Janssen, Karyopharm Therapeutics, Beigene, Innate, AstraZeneca, Abbvie, CSL Behring, Celegene, TG Therapeutics, and Innate Pharma. ASR served on a DSMC for Telios and is an employee of Eli Lilly, although employment began after this work was completed. RS has consulted for GlaxoSmithKline, Hermavant, Takeda, Abbvie, Amgen, AvenCell, BerGenBio, Cellularity, CTI pharma, Jazz, Kura Onc, Rigel, Syros, Novartis, Actinium, Arog, BMS, Boston Pharmaceuticals, and Janssen
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woyach JA. What is the optimal management of older CLL patients? Best Pract Res Clin Haematol. Mar 2018;31(1):83–89. doi: 10.1016/j.beha.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 2.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. The New England journal of medicine. Dec 17 2015;373(25):2425–37. doi: 10.1056/NEJMoa1509388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. The New England journal of medicine. Jun 6 2019;380(23):2225–2236. doi: 10.1056/NEJMoa1815281 [DOI] [PubMed] [Google Scholar]
- 4.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. The New England journal of medicine. Dec 27 2018;379(26):25172528. doi: 10.1056/NEJMoa1812836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International CLLIPIwg. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol. Jun 2016;17(6):779–790. doi: 10.1016/S1470-2045(16)30029-8 [DOI] [PubMed] [Google Scholar]
- 6.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. The New England journal of medicine. Dec 28 2000;343(26):1910–6. doi: 10.1056/NEJM200012283432602 [DOI] [PubMed] [Google Scholar]
- 7.Ommundsen N, Wyller TB, Nesbakken A, et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist. Dec 2014;19(12):1268–75. doi: 10.1634/theoncologist.2014-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamaker ME, Vos AG, Smorenburg CH, de Rooij SE, van Munster BC. The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. Oncologist. 2012;17(11):1439–49. doi: 10.1634/theoncologist.2012-0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. Sep 01 2011;29(25):3457–65. doi: 10.1200/JCO.2011.34.7625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giantin V, Valentini E, Iasevoli M, et al. Does the Multidimensional Prognostic Index (MPI), based on a Comprehensive Geriatric Assessment (CGA), predict mortality in cancer patients? Results of a prospective observational trial. J Geriatr Oncol. Jul 2013;4(3):208–17. doi: 10.1016/j.jgo.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 11.Corre R, Greillier L, Le Caer H, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08–02 Study. J Clin Oncol. May 1 2016;34(13):1476–83. doi: 10.1200/JCO.2015.63.5839 [DOI] [PubMed] [Google Scholar]
- 12.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. May 23 2013;121(21):4287–94. doi: 10.1182/blood-2012-12-471680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. Mar 26 2015;125(13):2068–74. doi: 10.1182/blood-2014-12-615187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheepers ERM, Vondeling AM, Thielen N, van der Griend R, Stauder R, Hamaker ME. Geriatric assessment in older patients with a hematologic malignancy: a systematic review. Haematologica. Jun 2020;105(6):1484–1493. doi: 10.3324/haematol.2019.245803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woyach JA, Ruppert AS, Guinn D, et al. BTKC481S-Mediated Resistance to Ibrutinib in Chronic Lymphocytic Leukemia. Journal of Clinical Oncology. 0(0):JCO.2016.70.2282. doi: 10.1200/jco.2016.70.2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. Apr 1 2011;29(10):1290–6. doi: 10.1200/JCO.2010.30.6985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. Nov 1 2005;104(9):1998–2005. doi: 10.1002/cncr.21422 [DOI] [PubMed] [Google Scholar]
- 18.George LK, Fillenbaum GG. OARS methodology. A decade of experience in geriatric assessment. Journal of the American Geriatrics Society. Sep 1985;33(9):607–15. [DOI] [PubMed] [Google Scholar]
- 19.Veit CT, Ware JE Jr., The structure of psychological distress and well-being in general populations. J Consult Clin Psychol. Oct 1983;51(5):730–42. doi: 10.1037//0022-006x.51.5.730 [DOI] [PubMed] [Google Scholar]
- 20.Sherbourne CD, Stewart AL. The MOS social support survey. Social science & medicine. 1991;32(6):705–14. [DOI] [PubMed] [Google Scholar]
- 21.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society. Feb 1991;39(2):142–8. [DOI] [PubMed] [Google Scholar]
- 22.Kawas C, Karagiozis H, Resau L, Corrada M, Brookmeyer R. Reliability of the Blessed Telephone Information-Memory-Concentration Test. Journal of geriatric psychiatry and neurology. Oct 1995;8(4):238–42. doi: 10.1177/089198879500800408 [DOI] [PubMed] [Google Scholar]
- 23.Nawas MT, Andreadis C, Martin TG, et al. Limitation in Patient-Reported Function Is Associated with Inferior Survival in Older Adults Undergoing Autologous Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. Jun 2019;25(6):1218–1224. doi: 10.1016/j.bbmt.2019.01.028 [DOI] [PubMed] [Google Scholar]
- 24.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. Jun 15 2008;111(12):5446–56. doi: 10.1182/blood-2007-06-093906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn IE, Farber CM, Davids MS, et al. Early progression of disease as a predictor of survival in chronic lymphocytic leukemia. Blood Adv. Nov 28 2017;1(25):2433–2443. doi: 10.1182/bloodadvances.2017011262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aarup K, Rotbain EC, Enggaard L, et al. Real-world outcomes for 205 patients with chronic lymphocytic leukemia treated with ibrutinib. Eur J Haematol. Jul 31 2020;doi: 10.1111/ejh.13499 [DOI] [PubMed] [Google Scholar]
- 27.Goede V, Bahlo J, Chataline V, et al. Evaluation of geriatric assessment in patients with chronic lymphocytic leukemia: Results of the CLL9 trial of the German CLL study group. Leuk Lymphoma. 2016;57(4):789–96. doi: 10.3109/10428194.2015.1091933 [DOI] [PubMed] [Google Scholar]
- 28.Molica S, Giannarelli D, Levato L, et al. A simple score based on geriatric assessment predicts survival in elderly newly diagnosed chronic lymphocytic leukemia patients. Leuk Lymphoma. Mar 2019;60(3):845–847. doi: 10.1080/10428194.2018.1508674 [DOI] [PubMed] [Google Scholar]
- 29.Huang LW, Sheng Y, Andreadis C, et al. Functional Status as Measured by Geriatric Assessment Predicts Inferior Survival in Older Allogeneic Hematopoietic Cell Transplantation Recipients. Biol Blood Marrow Transplant. Jan 2020;26(1):189–196. doi: 10.1016/j.bbmt.2019.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu MA, DuMontier C, Murillo A, et al. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood. Jul 25 2019;134(4):374–382. doi: 10.1182/blood.2019000758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parry JL, Hall PS, Young J. New horizons in systemic anti-cancer therapy in older people. Age Ageing. May 1 2018;47(3):340–348. doi: 10.1093/ageing/afy024 [DOI] [PubMed] [Google Scholar]
- 32.Stauder R, Eichhorst B, Hamaker ME, et al. Management of chronic lymphocytic leukemia (CLL) in the elderly: a position paper from an international Society of Geriatric Oncology (SIOG) Task Force. Ann Oncol. Feb 1 2017;28(2):218–227. doi: 10.1093/annonc/mdw547 [DOI] [PubMed] [Google Scholar]
- 33.Buckstein R, Wells RA, Zhu N, et al. Patient-related factors independently impact overall survival in patients with myelodysplastic syndromes: an MDS-CAN prospective study. Br J Haematol. Jul 2016;174(1):88–101. doi: 10.1111/bjh.14033 [DOI] [PubMed] [Google Scholar]
- 34.Driessen EJ, Peeters ME, Bongers BC, et al. Effects of prehabilitation and rehabilitation including a home-based component on physical fitness, adherence, treatment tolerance, and recovery in patients with non-small cell lung cancer: A systematic review. Crit Rev Oncol Hematol. Jun 2017;114:63–76. doi: 10.1016/j.critrevonc.2017.03.031 [DOI] [PubMed] [Google Scholar]
- 35.Cheville AL, Moynihan T, Herrin J, Loprinzi C, Kroenke K. Effect of Collaborative Telerehabilitation on Functional Impairment and Pain Among Patients With Advanced-Stage Cancer: A Randomized Clinical Trial. JAMA oncology. May 1 2019;5(5):644–652. doi: 10.1001/jamaoncol.2019.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez-Velilla N, Casas-Herrero A, Zambom-Ferraresi F, et al. Effect of Exercise Intervention on Functional Decline in Very Elderly Patients During Acute Hospitalization: A Randomized Clinical Trial. JAMA Intern Med. Jan 1 2019;179(1):28–36. doi: 10.1001/jamainternmed.2018.4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.