Abstract
Background
The aim of this study was to analyze the effectiveness and safety of H101 in Chinese patients with malignant pleural effusion and ascites (MPE/MA) in the real world.
Methods
This multicenter, observational, real‐world study recruited patients with MPE/MA caused by malignant tumor receiving H101‐containing treatment between January 2020 and June 2022. Effectiveness was evaluated by overall remission rate (ORR), and safety was evaluated based on adverse events (AEs). Subgroup analysis was performed on patients grouped according to tumor type, the volume of MPE and MA, and dosage of H101.
Results
A total of 643 eligible patients were enrolled, and 467 received H101 monotherapy and 176 received H101 combined with chemotherapy. The ORR of total patients was60.3% with 388 case of PR. In the H101 monotherapy group, the decrease of MPE or MA was achieved in 282 (60.4%, PR) patients, 176 (37.7%, NC) patients showed no change in volume of MPE or MA, and nine (1.9%, PD) patients showed an increase, yielding an ORR of 60.4% (282/467). The ORR for the combination therapy group was 60.2% (106/176), with 106 cases of PR, 69 cases of NC and one case of PD. Subgroup analyses based on tumor type, volume of MPE and MA, and dosage of H101 all showed high ORR, approximately 60%. The main AEs associated with H101‐containing regimens were fever, nausea and vomiting. No serious AEs occurred in both groups.
Conclusion
Encouraging clinical benefits and manageable toxicity of H101 against MPE/MA were preliminarily observed in the real‐world clinical setting, indicating that the H101‐containing regimen is reliable, safe, and feasible, providing a novel and effective option for the treatment of this disease.
Keywords: malignant serous effusion, malignant pleural effusion, malignant ascites, human type 5 recombinant adenovirus, real‐world
A diagram summarizing the flow of participants through the study is presented. Before drug administration, the patient's PE and ascites should be drained. Then, the patients received intrapleural or intraperitoneal administration of H101 monotherapy or a combination of conventional chemotherapy drugs. According to the condition of malignant serous effusion, the remission rate of patients post‐treatment was evaluated.

INTRODUCTION
Malignant pleural effusion (MPE) and malignant ascites (MA) are pathological manifestations of fluid accumulation in the serous cavity and are the most common serious complications of late‐stage malignancies, with an incidence of about 50%. 1 , 2 Accumulated evidence shows that tumor burden, pleural and peritoneal effusion quantity, and tumor growth rate are closely related to the life expectancy of patients. 3 Malignant serous effusion (MSE) has a poor prognosis and a high recurrence rate. If it is not properly controlled, it will seriously affect the quality of life of patients and is often life‐threatening. 4 Thus, the management of these conditions is a challenging but important issue.
Currently, there are no standard treatment models or reference guideline for MSE, which makes treatment a daunting task. The main method of clinical treatment is to actively remove PE and ascites to prevent further deterioration of the disease. 5 Intracavitary chemotherapy is one of the main treatment methods for MPE and MA. However, standard chemotherapy is less specific to cancer cells and is not concentrated in tumor tissue, resulting in low response rates and side effects. 6 Therefore, a new and effective treatment strategy is needed to relieve the symptoms of patients with MSE.
Oncolytic viruses (OVs) are an emerging class of therapeutic drugs that can preferentially infect and replicate in cancer cells, killing infected cells directly without damaging normal cells. 7 The application of OVs in various malignancies has become a promising therapeutic strategy. 8 , 9 , 10 Recombinant human adenovirus type 5 (H101) is a genetically modified oncolytic adenovirus that can selectively replicate in p53 mutated tumors and induce p53 accumulation during replication to produce direct and selective cytotoxic effects on tumor cells. 11 As p53 is the most frequently mutated gene in human cancers, H101 has exhibited anticancer properties in a variety of cancers, such as head and neck carcinoma, gastric cancer, and hepatocellular carcinoma. 12 , 13 , 14 Although extensive clinical data for OVs in treating solid tumors have been reported, there is limited research on the therapeutic efficacy of H101 in the treatment of MPE/MA, especially the lack of real‐world evidence. The dense stroma and hypoxic microenvironment within a solid tumor mass limit the effectiveness of viral infection and intratumoral penetration of OVs. 15 , 16 In contrast, the microenvironment within MPE and MA may create favorable conditions for OVs infection and OVs‐induced immune activation, indicating the feasibility of OVs in treating MSE.
With OVs still representing a relatively novel treatment option in the management of MSE, there is a demand to use the experience gained through using H101 in a real‐life, clinical setting, to further evaluate its safety and utility. Hence, we conducted a multicenter, observational, real‐world study in order to investigate the effectiveness and safety of H101 in the treatment of MPE/MA in a real‐world setting, and seek a more efficient therapeutic approach for the clinical management of these cancer‐related diseases.
METHODS
Study design and patients
This multicenter, observational, real‐world study recruited patients with MPE or MA caused by malignant tumor receiving H101‐containing treatment between January 2020 and June 2022 across five hospitals, including the First Affiliated Hospital of Xi'an Jiaotong University, Sun Yat‐sen University Cancer Center, Shaanxi Provincial Cancer Hospital, the Second Hospital of Tianjin Medical University and the 960th Hospital of the People's Liberation Army. This study was approved by the Ethics Committee of all the above‐mentioned hospitals, and informed consent was exempted owing to its retrospective nature.
The inclusion criteria were: (1) Histologically or cytologically diagnosed solid tumor malignancy. (2) Patients diagnosed with MPE or MA by ultrasonography or CT. (3) Patients who received H101‐based regimen by pleural or abdominal perfusion during treatment. (4) Patients who underwent one ultrasound or CT examination of PE and ascites by the same method before and after treatment; The exclusion criteria were: (1) Patients who received H101 treatment by nonintrapleural or intraperitoneal infusion. (2) During the continuous observation period after H101 administration, the patients’ imaging records were less than 4 weeks.
The patients were divided into two groups according to treatment methods, H101 alone and H101 combined with chemotherapy. Subgroup analysis was performed based on tumor type, the volume of MPE and MA, and dosage of H101.
Treatment protocol
The patients underwent chest and abdominal ultrasonography or CT examination for localization of PE and ascites, followed by therapeutic paracentesis (Figure 1). Overall, before administration, the patients’ PE and ascites were drained. Then, they received intrapleural or intraperitoneal administration of H101 monotherapy or a combination of conventional chemotherapy drugs, including cisplatin, paclitaxel, gemcitabine and docetaxel. Each vial of H101 (Shanghai Sunway Biotech) contained 0.5 mL sterile viral solution with 5.0 × 1011 viral particles (vp), and titered at a median tissue culture infective dose (TCID50) <1:60. Then, 0.5–2 mL of H101 diluted in 20 mL 0.9% sodium chloride solution was intrapleurally or intraperitoneally injected through the drainage catheter. The dosage and course for the use of H101 was adjusted according to the patient's tolerance and the severity of the disease. Chemotherapy was performed according to the recommended standard treatment dose and cycles. After bed rest, patients were asked to turn over every 15 min in order to encourage full access of the delivered drugs to the coelom. No drainage of PE and ascites was performed after H101 administration and during subsequent CT or ultrasound.
FIGURE 1.
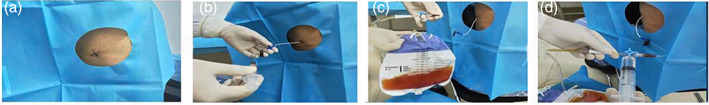
Administration process of H101. (a) The patients underwent chest and abdominal ultrasound/computed tomography (CT) examination for localization of pleural effusion (PE) and ascites. (b) Puncture catheter insertion. (c) Continue draining until there is no fluid in the chest or abdomen. (d) Then 0.5–2 mL of H101 diluted in 20 mL 0.9% sodium chloride solution was intrapleurally or intraperitoneally injected through the drainage catheter. PE, pleural effusion.
Clinical evaluation
Patient history, pathology, and treatment‐related information were collected separately, both by an oncologist and a pharmacist, whereas imaging evaluation was conducted separately by two doctors, from which any objection was determined by the imaging director.
Remission rate was determined according to previous studies. 1 , 17 Complete remission (CR) was considered when the accumulated fluid had disappeared and remained stable for at least 4 weeks; partial remission (PR) was considered when >50% of the accumulated fluid had disappeared, symptoms had improved, and the remaining fluid had failed to increase for at least 4 weeks; remission not obvious (NC) was considered when <50% of the accumulated fluid had disappeared or there was no noticeable change in symptoms; progression (PD) was considered when the accumulated fluid had increased with worsening of symptoms. The overall remission rate (ORR) was the proportion of the total number of CR + PR patients treated compared to the total number of cases. Adverse reactions were evaluated by the Common Toxicity Evaluation Criteria (CTC) according to the National Cancer Institute (NCI).
Statistical analysis
SPSS version 26.0 software (SPSS Inc.) was used to analyze the data. In descriptive analysis, continuous variables are expressed as median and range and categorical variables as a percentage.
RESULTS
Baseline characteristics
A total of 643 eligible patients were enrolled in this study, among whom 467 received H101 monotherapy and 176 received H101 combined with chemotherapy (Figure 2). Their age ranged from 55.0 to 66.0 years, with a median age of 60.0 and 61.5, respectively. The primary tumor types in the two groups were lung cancer, breast cancer, and gastrointestinal carcinoma. According to the fluid volume at baseline, 407 (87.2%) patients in the H101 group had a massive volume of PE and ascites, and 60 (12.8%) patients had a moderate volume of PE and ascites. The proportions of massive and moderate volume of PE and ascites in the combination group were 141 (80.1%) and 35 (19.9%), respectively. In these two groups, the administration of H101 varied from 0.5 mL to 2 mL, and the number of patients was balanced among different doses. The detailed patient characteristics are listed in Table 1.
FIGURE 2.
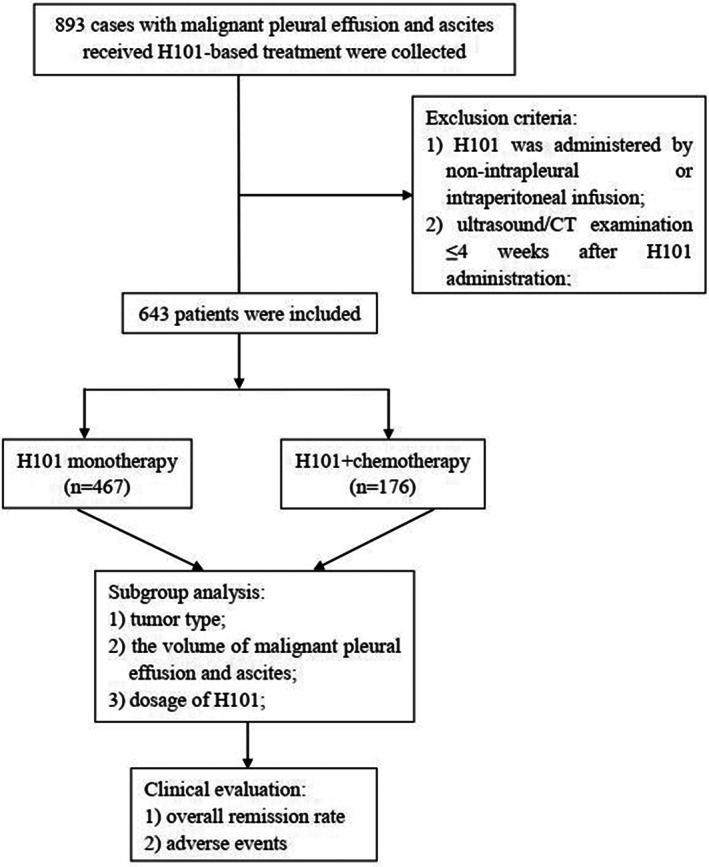
Flow diagram of this study.
TABLE 1.
Baseline characteristics of the patients.
| Variables | H101 (n = 467) | H101 + chemotherapy (n = 176) |
|---|---|---|
| Age (years), median (IQR) | 60.0 (55.0, 66.0) | 61.5 (55.0, 66.0) |
| Gender, n (%) | ||
| Male | 165 (35.3) | 64 (36.4) |
| Female | 302 (64.7) | 112 (63.6) |
| Bodyweight (kg), median (IQR) | 58.6 (54.3, 64.1) | 59.0 (55.0, 65.0) |
| Pathological type, n (%) | ||
| Lung cancer | 237 (50.7) | 100 (56.8) |
| Breast cancer | 117 (25.1) | 52 (29.5) |
| Gastrointestinal carcinoma | 39 (8.4) | 15 (8.5) |
| Others | 74 (15.8) | 9 (5.1) |
| Volume of MPE and MA, n (%) | ||
| Massive | 407 (87.2) | 141 (80.1) |
| Moderate | 60 (12.8) | 35 (19.9) |
| Dosage of H101, n (%) | ||
| 0.5 mL | 80 (17.1) | 35 (19.9) |
| 1.0 mL | 155 (33.2) | 51 (29.0) |
| 1.5 mL | 116 (24.8) | 45 (25.6) |
| 2.0 mL | 116 (24.8) | 45 (25.6) |
Abbreviations: IQR, interquartile range; MA, malignant ascites; MPE, malignant pleural effusion.
Efficacy analysis
The ORR of total patients was 60.3% with 388 cases of PR. Of the 467 patients with MPE or MA who received H101 monotherapy, the decrease of MPE or MA was achieved in 282 (60.4%, PR) patients, 176 (37.7%, NC) patients showed no change in volume of MPE or MA, and nine (1.9%, PD) patients showed an increase, yielding an ORR of 60.4% (282/467). The ORR for the combination therapy group was 60.2% (106/176), with 106 cases of PR, 69 cases of NC and one case of PD.
Subgroup analysis was performed based on tumor type, the volume of MPE and MA, and dosage of H101. In subgroup analysis of different tumor types, efficacy analysis mainly focused on lung cancer, breast cancer, and gastrointestinal carcinoma. As shown in Table 2, the ORR of H101 monotherapy for lung cancer with MSE, breast cancer with MSE, and gastrointestinal carcinoma with MSE were 59.5% (141/237), 59.0% (69/117), and 61.5% (24/39), respectively, while the ORR of the combination treatment group were 57.0% (57/100), 59.6% (31/52), and 73.3% (11/15), respectively. In patients with different volume of MPE/MA, a large proportion of decreased effusion was observed in both the H101 monotherapy group and the combination group. Specifically, for patients with a massive volume of MPE/MA, the ORR of the two groups was 59.5% (242/407) and 61.7% (87/114), and 66.7% (40/60) and 54.3% (19/35) of the patients with moderate MPE/MA achieved remission, respectively (Table 3). Of the 657 patients who were treated with the H101‐based regimen, both monotherapy and combination therapy showed considerable curative effect. In the monotherapy group, 66.2% (53/80), 54.8% (85/155), 62.9% (73/116) and 61.2% (71/116) of the patients achieved remission after administration of 0.5, 1.0, 1.5 and 2 mL H101, respectively. Correspondingly, 57.1% (20/35), 56.9% (29/51), 64.4% (29/45), and 62.2% (28/45) of the patients in the combination group achieved remission. Higher remission rates, greater than 60%, were observed with high‐dose administration of H101 (1.5 mL and 2 mL) in in both groups (Table 4).
TABLE 2.
Subgroup analysis of different tumor types.
| H101 (n = 467) | H101+ chemotherapy (n = 176) | |||||||
|---|---|---|---|---|---|---|---|---|
| Response (n, %) | Lung cancer (n = 237) | Breast cancer (n = 117) | Gastrointestinal carcinoma (n = 39) | Other (n = 74) | Lung cancer (n = 100) | Breast cancer (n = 52) | Gastrointestinal carcinoma (n = 15) | Other (n = 9) |
| PR | 141 (59.5) | 69 (59.0) | 24 (61.5) | 48 (64.9) | 57 (57.0) | 31 (59.6) | 11 (73.3) | 7 (77.8) |
| NC | 92 (38.8) | 44 (37.6) | 14 (35.9) | 26 (35.1) | 43 (43.0) | 20 (38.5) | 4 (26.7) | 2 (22.2) |
| PD | 4 (1.69) | 4 (3.4) | 1 (2.6) | 0 (0) | 0 (0) | 1 (1.9) | 0 (0) | 0 (0.) |
| ORR | 141 (59.5) | 69 (59.0) | 24 (61.5) | 48 (64.9) | 57 (57.0) | 31 (59.6) | 11.0 (73.3) | 7 (77.8) |
Abbreviations: NC, remission not obvious; ORR, overall remission rate; PD, progression; PR, partial remission.
TABLE 3.
Subgroup analysis of different volume of fluid.
| H101 (n = 467) | H101+ chemotherapy (n = 176) | |||
|---|---|---|---|---|
| Response (n, %) | Massive volume (n = 407) | Moderate volume (n = 60) | Massive volume (n = 141) | Moderate volume (n = 35) |
| PR | 242 (59.5) | 40 (66.7) | 87 (61.7) | 19 (54.3) |
| NC | 158 (38.8) | 18 (30.0) | 53 (37.6) | 16 (45.7) |
| PD | 7 (1.7) | 2 (3.3) | 1 (0.7) | 0 (0) |
| ORR | 242 (59.5) | 40 (66.7) | 87 (61.7) | 19 (54.3) |
Abbreviations: NC, remission not obvious; ORR, overall remission rate; PD, progression; PR, partial remission.
TABLE 4.
Subgroup analysis of different doses of H101.
| H101 (n = 467) | H101+ chemotherapy (n = 176) | |||||||
|---|---|---|---|---|---|---|---|---|
| Response (n, %) | 0.5 mL (n = 80) | 1.0 mL (n = 155) | 1.5 mL (n = 116) | 2.0 mL (n = 116) | 0.5 mL (n = 35) | 1.0 mL (n = 51) | 1.5 mL (n = 45) | 2.0 mL (n = 45) |
| PR | 53 (66.2) | 85 (54.8) | 73 (62.9) | 71 (61.2) | 20 (57.1) | 29 (56.9) | 29 (64.4) | 28 (62.2) |
| NC | 24 (30.0) | 69 (44.5) | 40 (34.5) | 43 (37.1) | 15 (42.9) | 22 (43.1) | 16 (35.6) | 16 (35.6) |
| PD | 3 (3.8) | 1 (0.7) | 3 (2.6) | 2 (1.7) | 0 (0) | 0 (0) | 0 (0) | 1 (2.2) |
| ORR | 53 (66.2) | 85 (54.8) | 73 (62.9) | 71 (61.2) | 20 (57.1) | 29 (56.9) | 29 (64.4) | 2 (62.2) |
Abbreviations: NC, remission not obvious; ORR, overall remission rate; PD, progression; PR, partial remission.
Safety analysis
The treatment‐related AEs are listed in Table 5. Of all these events, the most common AEs were fever, followed by nausea and vomiting. The major AEs associated with H101 monotherapy included fever, and nausea and vomiting, while the main AEs in the combination group were fever, nausea and vomiting and hypertension. Apart from the incidence of fever in the H101 monotherapy group being higher than that in the combination group, the incidence of other AEs was higher in the H101 combined chemotherapy group than in the H101 monotherapy group. Notably, although the proportion of fever treated with H101 monotherapy was high, the symptoms of patients were mild and most of them recovered to normal within 48 h, which did not affect subsequent treatment. No serious AEs occurred in both groups.
TABLE 5.
Treatment‐related adverse events.
| Complications | H101 (n = 37) | H101 + chemotherapy (n = 16) |
|---|---|---|
| Fever | 19 (51.4) | 3 (18.8) |
| Nausea and vomiting | 6 (16.2) | 7 (43.8) |
| Increased cholesterol | 2 (5.4) | 1 (6.3) |
| Chill | 1 (2.7) | 1 (6.3) |
| Rash | 1 (2.7) | 1 (6.3) |
| Hypertension | 0 (0) | 2 (12.5) |
| Pruritus | 0 (0) | 1 (6.3) |
| Constipation | 0 (0) | 1 (6.3) |
DISCUSSION
MPE and MA are leading causes of death among patients with late‐stage malignant tumors, and their formation mechanism is related to factors such as tumor invasion of serous membrane, blockage of lymphatic vessels and mural serosa vessels, enhancement of permeability of thoracic and abdominal capillaries caused by inflammation, blockage of reflux caused by lymph node metastasis. 2 Cough, chest tightness, dyspnea and abdominal distension are the main clinical manifestations. MPE and MA have a very serious negative impact on the quality of life of patients, and further accelerate the disease progression of advanced malignant tumors. 18 As an emerging candidate for the treatment of MSE, H101 has been reported to show a promising curative effect, but the evidence is limited and most of the focus has been on small sample sizes, in single center studies. Thus, confirmatory studies are required.
This is the first multicenter, real‐world study to evaluate the effectiveness and safety of H101 in the treatment of MPE and MA. With this novel study design, we hoped to develop a more comprehensive understanding of its real‐world effectiveness from a variety of aspects. In this study, of our 643 patients, 388 patients achieved PR, reaching an ORR of 60.3%. In addition, H101 alone or in combination both achieved a high response rate of 60.4% and 60.2%, respectively, suggesting that the selective direct cytotoxicity of H101 to tumor cells may be sufficient to eliminate metastatic tumor cells on the surface of the thorax and peritoneum and lead to reduced effusion. These positive outcomes were consistent with published reports which showed that that effusion could be markedly reduced by treatment with H101‐based therapy in patients with MSE. 7 , 19 Notably, our study is superior because it was conducted in a real‐world setting with a larger sample size, whereas previous studies only assessed a limited number of study subjects. This study did not compare H101 monotherapy with the combination group, as this is the first large exploratory study focused on evaluating real‐world effectiveness of H101, but available data indicates that the ORR in both groups are similar. H101 was reported effective not only by introducing wild human tumor suppressor gene fragments into target cells and replicating defective adenovirus, but also by killing tumor cells through bystander effects, increased chemotherapy sensitivity, and obstruction of tumor angiogenesis. 20 The slight difference in response rate between that study and ours was possibly due to different research populations, treatment regimens, different diagnosis and treatment environment, diverse health status of patients, and variety of sample size. The possible causes need further analysis.
MPE/MA mostly occurs in common tumors, including lung cancer, breast cancer, and gastrointestinal cancer. 4 Until now, a large number of scholars have sought related drugs in order to gain clinical benefits in MSE treatment. Zhou et al. explored the efficacy of bevacizumab in lung cancer patients with PE and ascites, and confirmed that bevacizumab significantly reduced the volume of effusion. 2 Similarly, Zhao and colleagues reported that Endostar successfully controlled the formation of MPE and MA in patients including gastric and colon cancer. 17 Here, the H101 regimen directed high ORR in lung, breast, and gastrointestinal cancers, all of which were with an ORR of about 60%, suggesting that all patients with these tumors can benefit from H101 treatment. In addition, different from previous literature, this study not only performed the total efficacy analysis for all tumor types included, but also separately analyzed the efficacy for different tumor types, which is the advantage of this study. Moreover, we included more other tumor types, which also showed promising therapeutic effects. However, due to the small sample size, the results are not fully displayed, but it is a new tumor exploration.
Clinically, the primary reason for clinical symptoms of MSE is a large accumulation of exudates, and different amounts of fluid reflect the severity of the patient. 21 In this study, H101 showed considerable effectiveness in treating both massive volume and moderate volume of MPE and MA. In the study reported by Zhang et al. evaluating the efficacy of H101 on ascites, they administered the drug according to the ascites volume of patients, which may explain why we stratified by volume of fluid. 7 With H101 still representing a relatively novel treatment option in the management of MSE, there are no guidelines for H101 dosing. This study explored the efficacy of different doses of H101 on MPE/MA, and found that all can effectively control fluid accumulation, and patients who received higher doses of H101 benefited more. Our finding provides a possible explanation why the H101 dose in the currently published studies are concentrated in 2 mL. 19 , 22 More clinical investigations are still required regarding the description of the required dosage of the drugs used with this aim.
Our study provides evidence for an H101‐containing treatment strategy on MSE caused by malignant tumors. Either monotherapy or combination therapy can readily diminish effusion, with a positive health outcome for the patients. During this study, we did not find any serious side effects, indicating that this line of therapy is well tolerated. A total of 37 patients in the H101 treatment group showed elevated body temperature, but it only appeared within 24 h after injection of H101, which was relieved after symptomatic treatment, and most of them returned to normal within 48 h. Other AEs reported mainly included nausea and vomiting, and improved spontaneously without special treatment. The AEs which occurred in this study are consistent with those previously reported to be caused by H101. 7 , 19 Although H101 has side‐effects such as nausea and vomiting, there is no evidence that H101 combined with chemotherapy significantly increases side effects, as nausea and vomiting are typical side effects of chemotherapy, whether or not H101 is introduced. 23 Overall, H101 has good safety in the treatment of MPE and MA.
There were some limitations in this study. First, owing to its retrospective nature, some information might not have been recorded and lost. However, the advantage of this study was that a subgroup analysis of the enrolled patients was also performed. In addition, the current analysis did not include an assessment of patients' quality of life, which has been shown to correlate with MPE and MA. Second, due to the primary study purpose, a “chemotherapy alone” group was not set up and there was no comparison of different treatment methods. Third, the follow‐up was too short to measure OS and entire PFS. We will continue to conduct prospective, more complete grouping, longer follow‐up studies, and continue to expand the sample size for each subgroup.
In conclusion, our study preliminarily demonstrated the effectiveness and safety of H101‐based therapy in the real‐world clinical setting and may provide useful information to physicians treating patients with MPE and MA in clinical practice.
AUTHOR CONTRIBUTIONS
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
Wang B, Zhong C, Liao Z, Wang H, Cai X, Zhang Y, et al. Effectiveness and safety of human type 5 recombinant adenovirus (H101) in malignant tumor with malignant pleural effusion and ascites: A multicenter, observational, real‐world study. Thorac Cancer. 2023;14(30):3051–3057. 10.1111/1759-7714.15101
DATA AVAILABILITY STATEMENT
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Jiang L, Li P, Gong Z, Hu B, Ma J, Wang J, et al. Effective treatment for malignant pleural effusion and ascites with combined therapy of bevacizumab and cisplatin. Anticancer Res. 2016;36(3):1313–1318. [PubMed] [Google Scholar]
- 2. Zhou Z, Li H, Hu D, Xie L. Clinical efficacy of bevacizumab combined with cisplatin in the treatment of malignant pleural effusion and ascites caused by lung cancer: a randomized trial. Ann Palliat Med. 2021;10(10):10575–10583. [DOI] [PubMed] [Google Scholar]
- 3. Hou W, Sanyal AJ. Ascites: diagnosis and management. Med Clin North Am. 2009;93(4):801–817. vii. [DOI] [PubMed] [Google Scholar]
- 4. Li JX, Shi YM, An LY, Yang JX, Qi YX, Yang T, et al. Quality assessment of the guidelines for the management of malignant pleural effusions and ascites. World J Surg Oncol. 2020;18(1):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen D, Song X, Zhang Y, Kong L, Wang H, Yu J. Optimizing intrapleural bevacizumab dosing in non‐small‐cell lung cancer‐mediated malignant pleural effusion: less is more. Future Oncol. 2018;14(21):2131–2138. [DOI] [PubMed] [Google Scholar]
- 6. Chu H, Du F, Gong Z, Lian P, Wang Z, Li P, et al. Better clinical efficiency of TILs for malignant pleural effusion and ascites than cisplatin through Intrapleural and intraperitoneal infusion. Anticancer Res. 2017;37(8):4587–4591. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Y, Qian L, Chen K, Gu S, Wang J, Meng Z, et al. Intraperitoneal oncolytic virotherapy for patients with malignant ascites: characterization of clinical efficacy and antitumor immune response. Mol Ther Oncolytics. 2022;25:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti‐PD‐1 immunotherapy. Cell. 2017;170(6):1109–19.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. [DOI] [PubMed] [Google Scholar]
- 10. Zhang Y, Li Y, Chen K, Qian L, Wang P. Oncolytic virotherapy reverses the immunosuppressive tumor microenvironment and its potential in combination with immunotherapy. Cancer Cell Int. 2021;21(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang J, Zhang Q, Liu Z, Wang J, Shi F, Su J, et al. Efficacy and safety of recombinant human adenovirus type 5 (H101) in persistent, recurrent, or metastatic gynecologic malignancies: a retrospective study. Front Oncol. 2022;12:877155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mell LK, Brumund KT, Daniels GA, Advani SJ, Zakeri K, Wright ME, et al. Phase I trial of intravenous oncolytic vaccinia virus (GL‐ONC1) with cisplatin and radiotherapy in patients with Locoregionally advanced head and neck carcinoma. Clin Cancer Res. 2017;23(19):5696–5702. [DOI] [PubMed] [Google Scholar]
- 13. Zhang R, Cui Y, Guan X, Jiang X. A recombinant human adenovirus type 5 (H101) combined with chemotherapy for advanced gastric carcinoma: a retrospective cohort study. Front. Front Oncol. 2021;11:752504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin XJ, Li QJ, Lao XM, Yang H, Li SP. Transarterial injection of recombinant human type‐5 adenovirus H101 in combination with transarterial chemoembolization (TACE) improves overall and progressive‐free survival in unresectable hepatocellular carcinoma (HCC). BMC Cancer. 2015;15:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng M, Huang J, Tong A, Yang H. Oncolytic viruses for cancer therapy: barriers and recent advances. Mol Ther Oncolytics. 2019;15:234–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pipiya T, Sauthoff H, Huang YQ, Chang B, Cheng J, Heitner S, et al. Hypoxia reduces adenoviral replication in cancer cells by downregulation of viral protein expression. Gene Ther. 2005;12(11):911–917. [DOI] [PubMed] [Google Scholar]
- 17. Zhao WY, Chen DY, Chen JH, Ji ZN. Effects of intracavitary administration of Endostar combined with cisplatin in malignant pleural effusion and ascites. Cell Biochem Biophys. 2014;70(1):623–628. [DOI] [PubMed] [Google Scholar]
- 18. Prado‐Garcia H, Romero‐Garcia S, Rumbo‐Nava U, Lopez‐Gonzalez JS. Predominance of Th17 over regulatory T‐cells in pleural effusions of patients with lung cancer implicates a proinflammatory profile. Anticancer Res. 2015;35(3):1529–1535. [PubMed] [Google Scholar]
- 19. Yang F, Lu B, Hu C, Li X, Liu Z, Zhou L, et al. Effect of intrathoracic injection of recombinant human adenovirus type 5 on malignant pleural effusion of advanced lung cancer. Journal of Practical Medicine. 2013;29(17):2885–2886. [Google Scholar]
- 20. Heise C, Lemmon M, Kirn D. Efficacy with a replication‐selective adenovirus plus cisplatin‐based chemotherapy: dependence on sequencing but not p53 functional status or route of administration. Clin Cancer Res. 2000;6(12):4908–4914. [PubMed] [Google Scholar]
- 21. Yin T, Wang G, He S, Shen G, Su C, Zhang Y, et al. Malignant pleural effusion and ascites induce epithelial‐mesenchymal transition and cancer stem‐like cell properties via the vascular endothelial growth factor (VEGF)/phosphatidylinositol 3‐kinase (PI3K)/Akt/mechanistic target of rapamycin (mTOR) pathway. J Biol Chem. 2016;291(52):26750–26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhan Z. Effect of intrathoracic injection of recombinant human adenovirus type 5 on malignant pleural effusion of advanced lung cancer. J Front Med. 2015;5(36):3. [Google Scholar]
- 23. Du N, Li X, Li F, Zhao H, Fan Z, Ma J, et al. Intrapleural combination therapy with bevacizumab and cisplatin for non‐small cell lung cancer‐mediated malignant pleural effusion. Oncol Rep. 2013;29(6):2332–2340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.


