Abstract
Antibiotics with different mechanisms of action may vary with respect to their effects on the release and immunostimulatory activities of cell wall fragments from gram-positive bacteria. Therefore, after Staphylococcus aureus was cultured for 4 h in the absence of antibiotics (control) and in the presence of β-lactam antibiotics (imipenem, flucloxacillin, or cefamandole) and protein synthesis-inhibiting antibiotics (erythromycin, clindamycin, or gentamicin), the lipoteichoic acid (LTA) and peptidoglycan (PG) levels in the bacterial supernatants were measured. β-Lactam antibiotics greatly enhanced the release of LTA and PG (4- to 9-fold and 60- to 85-fold, respectively), whereas protein synthesis inhibitors did not affect PG release and even inhibited the release of LTA compared to the amount of LTA released in control cultures. The capacity of β-lactam supernatants to stimulate the production of tumor necrosis factor alpha and interleukin-10 in human whole blood was significantly higher than that of protein synthesis inhibitor or control supernatants; the amounts of these cytokines released were directly proportional to the concentrations of PG and LTA in the supernatants. Enzymatic degradation of PG in the supernatants indicated that PG was mainly responsible for the observed biological reactivity.
Recent research on septic shock has increased the awareness that this syndrome is caused by a host response to bacterial cell wall components. In the case of gram-negative bacteria, lipopolysaccharide (LPS)—a part of the outer membrane—has been identified as the major immunostimulatory component. LPS is released during bacterial growth as well as after lysis of the bacterial cells, for instance, by the actions of antibiotics, although antibiotics may differ in their capacity to cause lysis (5, 6, 13, 32). Analogous to the LPS in gram-negative bacteria, two cell wall components of the gram-positive microorganism Staphylococcus aureus, i.e., peptidoglycan (PG) and lipoteichoic acid (LTA), are able to induce the production of proinflammatory cytokines by monocytes in vitro (8, 15, 17, 21, 26). When these components were injected intravenously into animals, several of the characteristic features of septic shock, such as leukocytopenia, thrombocytopenia, renal failure, and hypotension, were observed (4, 24).
LTA and PG are released spontaneously into the culture medium during growth of gram-positive bacteria (19, 23). Moreover, incubation with antibiotics was found to enhance the release of LTA and PG (2, 12, 22, 30, 35). In many of these studies, however, the release of bacterial cell wall components was measured indirectly and their biological reactivities often were not examined.
The aim of the present study was to investigate whether antibiotics with different mechanisms of action vary with respect to the release of bacterial cell wall components. Therefore, two assays for direct quantification of LTA and PG were developed, and subsequently, the amounts of these components released from S. aureus during incubation with three β-lactam antibiotics (imipenem, flucloxacillin, or cefamandole) and three protein synthesis-inhibiting antibiotics (erythromycin, clindamycin, or gentamicin) were measured. In addition, the immunostimulatory activities of the bacterial supernatants were determined by measuring cytokine production in human whole blood.
MATERIALS AND METHODS
Bacteria.
S. aureus ATCC 25923 was cultured aerobically in nutrient broth (NB) at 37°C. Stock solutions were prepared in NB supplemented with 10% glycerol and were frozen in small aliquots at −20°C.
Antibiotics.
Three representatives of β-lactam antibiotic subclasses were used in this study, i.e., imipenem (a carbapenem), flucloxacillin (a penicillin), and cefamandole (a cephalosporin). These antibiotics were purchased from Merck Sharp & Dohme B. V. (Haarlem, The Netherlands), SmithKline Beecham (Herdfordshire, United Kingdom), and Eli Lilly (Nieuwegein, The Netherlands), respectively. In addition, three different protein synthesis inhibitors were studied, i.e., erythromycin, clindamycin, and gentamicin. These antibiotics were purchased from Abbott B. V. (Amstelveen, The Netherlands), Pharmacia-Upjohn (Puurs, Belgium), and Sigma-Aldrich Chemie B. V. (Zwijndrecht, The Netherlands), respectively. The MICs for S. aureus ATCC 25923 were 0.016 μg/ml for imipenem, 0.125 μg/ml for clindamycin, cefamandole, and gentamicin, and 0.25 μg/ml for flucloxacillin and erythromycin, as determined by standard microdilution techniques (1). In the experiments, antibiotics were added to the bacterial cultures to final concentrations of 1, 2.5, 5, 10, and 20× the MIC.
Radioactive labeling of the cell wall of S. aureus.
Bacteria were cultured overnight in NB in the presence of 2 μCi of [3H]N-acetylglucosamine (Amersham Life Science, Buckinghamshire, United Kingdom) per ml in order to label the cell wall. Unincorporated label was removed by washing the bacteria four times (10 min at 6,000 × g) in warm (37°C) medium. In total, 71.1% ± 4.3% (mean ± standard error of the mean [SEM]; n = 18) of the label added to the culture was incorporated. Localization of the incorporated label in S. aureus via ultrasonification and centrifugation indicated that the cell wall fraction contained 92.9% of the incorporated label, whereas only 7.1% of the label was present in the cytosol fraction, confirming that [3H]N-acetylglucosamine is incorporated preferentially into the cell wall of S. aureus (34). Others have shown that approximately 56% of this label is found in the PG fraction; the remainder is covalently bound to teichoic acid molecules (34).
Bacterial killing assay.
In two parallel experiments S. aureus ATCC 25923 was cultured overnight in the presence or absence of [3H]N-acetylglucosamine. After washing of both cultures, an inoculum of 8.68 × 106 ± 1.95 × 106 bacteria per ml (with a log10 of 6.89 ± 0.11) was prepared in NB. To obtain logarithmic growth the bacteria were cultured for 2 h at 37°C. The antibiotics were then added, and aliquots of both cultures were collected after 1, 2, and 4 h. The number of viable bacteria was determined microbiologically by plating serial 10-fold dilutions of the unlabeled samples on blood agar plates. The amount of viable bacteria was expressed as the numbers of CFU per milliliter. The remainder of the unlabeled sample was filtered (pore size, 0.45 μm), and the supernatants were stored at −20°C for measurement of LTA and PG release.
The radioactive samples were used to determine the release of cross-linked cell wall material caused by the antibiotics. For this purpose, the samples were divided into two aliquots of equal volumes; one aliquot was used to measure the total amount of label in the sample, and the second aliquot was filtered and the amount of radioactivity in the supernatant was determined as a measure of the release of cell wall fragments into the incubation medium. The release of label into the supernatants was expressed as a fraction of the total amount of radioactivity in the sample.
Finally, to determine the biological reactivities of the bacterial fragments, it was necessary to grow the bacteria in a tissue culture medium which does not stimulate the production of cytokines in whole blood, in contrast to the NB medium. Thus, similar experiments were performed with unlabeled bacteria cultured in M199 medium (Gibco BRL Life Technologies, Grand Island, N.Y.) which was enriched with 1% d-(+)-glucose and 2 mM glutamine, a medium that supports logarithmic bacterial growth.
LTA ELISA.
An LTA enzyme-linked immunosorbent assay (ELISA) was developed with a mouse immunoglobulin G3 (IgG3) monoclonal antibody directed against the glycerol phosphate moiety of the LTA molecule (B. J. Appelmelk). The specificity and immunoreactivity of this monoclonal antibody have been reported elsewhere (9). A standard curve was made for purified LTA of S. aureus (Sigma-Aldrich Chemie) at concentrations of 0 to 500 ng per ml of NB. The standard samples and different dilutions of the bacterial supernatants in NB were incubated overnight at room temperature on a 96-well Nunc Polysorb Immunoplate (Nunc A/S, Roskilde, Denmark). After washing, the plate was blocked for 1 h with phosphate-buffered saline containing 0.5% bovine serum albumin and 0.05% Tween 20 to prevent aspecific binding. For detection, 1.2 μg of mouse IgG3 anti-LTA per ml was added for 1 h at 37°C. The plate was washed and incubated with 2 μg of Goat-α-Mouse IgG (Fcγ-specific)-peroxidase conjugate (Jackson Immunoresearch Laboratories Inc., West Grove, Pa.) per ml. A color reaction was obtained with a substrate of 1 mg of 3,3′,5,5′-tetramethylbenzidine per ml in 0.1 M sodium acetate buffer (pH 6.0) containing 0.006% H2O2. The reaction was stopped after 5 min by the addition of 4 N H2SO4, and the optical density at 450 nm was measured. The LTA concentration in the bacterial supernatants was calculated by using the LTA standard curve. The detection limit of the LTA ELISA was 30 ng/ml. The LTA ELISA did not cross-react with other bacterial cell wall components, such as insoluble PG isolated from the cell wall of S. aureus (50 μg/ml; a kind gift of A. C. Fluit, Department of Medical Microbiology, University Hospital, Utrecht, The Netherlands) and LPS of Escherichia coli O111:B4 (100 ng/ml). Furthermore, the LTA ELISA was not influenced by any of the antibiotics used in this study.
PG measurement.
The amount of PG in the bacterial supernatants was determined by means of a silkworm larva plasma (SLP) test (Wako Pure Chemical Industries Ltd., Osaka, Japan), as described previously (28). Plasma from the silkworm Bombyx mori contains multiple serine proteases which can be activated by bacterial PG or (1→3)-β-d-glucan, a cell wall component of yeast and fungi. This activation starts a cascade of reactions, leading eventually to the formation of melanin, a substance that can be measured optically. In short, serial 10-fold dilutions of the bacterial supernatants were prepared in distilled water. As a standard, PG of Micrococcus luteus (Wako) was used in a range of 0 to 10 ng/ml (in twofold dilutions) in distilled water. The different dilutions were transferred to a 96-well plate, the SLP reagent was reconstituted in dilution buffer, and an equal volume was added to the dilutions in the wells. After 30 minutes of incubation at 30°C the optical density was measured at 690 nm. The amount of PG in the samples was calculated by using the standard curve; the sensitivity of the test was 0.3 ng/ml. In addition, insoluble PG of S. aureus (gift of A. C. Fluit) was assessed to compare the effectiveness of the SLP test for the measurement of PG from this microorganism. The results showed that PG of S. aureus is also detectable with the SLP kit and that the sensitivity is comparable to that for detection of PG of M. luteus. No cross-reactivity with LTA from S. aureus (Sigma-Aldrich Chemie) was observed.
Enzymatic degradation of PG.
To determine which cell wall component is responsible for the immunostimulatory activity, the PG molecules in the bacterial supernatants were degraded with enzymes, a method which has been shown to reduce PG-induced cytokine production in vitro (10, 26). For this purpose, the bacterial supernatants were treated for 3 h at 37°C with a mixture of the enzymes lysostaphin (20 U/ml; Sigma) and N-acetylmuramyl-l-alanine amidase (NAMLAA; 0.4 μg/ml) (a kind gift of M. P. Hazenberg, Department of Immunology, Erasmus University, Rotterdam, The Netherlands). After enzymatic degradation the biological reactivities of the bacterial supernatants were tested in whole blood. Control experiments indicated that cleavage of PG in the supernatants was complete because the enzymes totally eradicated the biological reactivity of insoluble PG from S. aureus at a concentration similar to that found in our supernatants.
Whole-blood stimulation and cytokine measurements.
Heparinized whole blood from healthy donors was diluted four times in warm M199 medium and added to a 24-well cell culture plate (Costar Corporation, Cambridge, Mass.). The different supernatants of S. aureus in enriched M199 medium, either complete or degraded enzymatically, were added to obtain a final 10-fold dilution. The blood was incubated at 37°C for 24 h; after centrifugation (10 min at 500 × g) the supernatants were collected and stored at −70°C. The production of human tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in whole blood was measured by specific sandwich ELISAs (BPRC, Rijswijk, The Netherlands, and CLB, Amsterdam, The Netherlands, respectively) according to the instructions of the manufacturers, as described earlier (31). The antibiotics or enzymes, at the concentrations used in this study, did not influence the LPS-induced cytokine production in whole blood or the cytokine ELISAs.
Exotoxin ELISAs.
The bacterial supernatants were screened for the presence of staphylococcal enterotoxins A, B, and C and toxic shock syndrome toxin type 1 by means of specific ELISAs (a kind gift of J. B. Dufrenne, National Institute of Public Health and Environmental Protection, Bilthoven, The Netherlands), as described elsewhere (18).
Statistical analysis.
The release of LTA and PG in NB and in enriched M199 medium, the release of radioactively labeled S. aureus cell wall material, and the induction of cytokines were analyzed by means of the Mann-Whitney U test. The Pearson correlation coefficient was used to analyze the correlation between the release of bacterial components and the induction of cytokines in whole blood. Data from three to four different experiments were expressed as means ± SEMs; P values of ≤0.05 were considered significant.
RESULTS
Killing or growth inhibition of S. aureus by antibiotics.
During logarithmic growth of S. aureus in NB in the absence of an antibiotic, the log number of viable bacteria increased from 7.18 ± 0.04 at the start of the experiment to 7.42 ± 0.03 at 1 h, 7.73 ± 0.05 at 2 h, and 8.28 ± 0.02 after 4 h of culture. Incubation of the bacteria with gentamicin, imipenem, or flucloxacillin resulted in a concentration-dependent decrease in the number of viable microorganisms. At 20× the MIC the maximum decreases in the log10 numbers of bacteria relative to the outgrowth in the control culture at 4 h were 3.19 ± 0.62, 2.84 ± 0.89, and 1.74 ± 0.27 log for gentamicin, imipenem, and flucloxacillin, respectively. After incubation of S. aureus with erythromycin or clindamycin bacterial growth stopped rapidly; a bacteriostatic effect was observed during the 4-h culture period for the range of concentrations of from 1 to 20× the MIC.
In enriched M199 medium the growth of S. aureus ATCC 25923 was similar to that in NB. The log number of viable bacteria in the control cultures increased from 7.08 ± 0.06 to 8.55 ± 0.14 over the 4-h period of the experiment. Moreover, the effects of the antibiotics studied on bacterial growth were similar to the effects observed in NB.
Release of LTA.
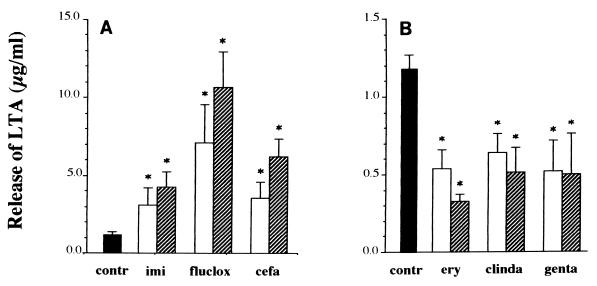
During logarithmic growth of S. aureus in NB, the amount of LTA that was released spontaneously into the supernatant increased from 0.17 ± 0.01 μg/ml at the start of the experiment to 0.21 ± 0.02 μg/ml at 1 h, 0.54 ± 0.09 μg/ml at 2 h, and 1.18 ± 0.10 μg/ml after 4 h of culture. The three β-lactam antibiotics markedly enhanced the release of LTA compared to the level of release of LTA by the control culture (Fig. 1). Added at a concentration of 1× the MIC, imipenem, flucloxacillin, and cefamandole significantly increased the amount of LTA released (2.6-, 6.0-, and 3.0-fold, respectively). At an antibiotic concentration of 20× the MIC, the amount of LTA released increased 3.6-fold for cultures treated with imipenem, 9.1-fold for cultures treated with flucloxacillin, and 5.3-fold for cultures treated with cefamandole.
FIG. 1.
Release of LTA from S. aureus ATCC 25923 in the absence (■) or presence of different antibiotics at a concentration of 1× the MIC (□) or 20× the MIC ( ). Four hours after the addition of medium or antibiotics, the bacterial supernatants were collected by centrifugation and filtration of the cultures, and the release of LTA was measured by means of a specific ELISA. Antibiotics studied were the β-lactam antibiotics imipenem (imi), flucloxacillin (fluclox), and cefamandole (cefa) (A) and the protein synthesis inhibitors erythromycin (ery), clindamycin (clinda), and gentamicin (genta) (B). Note the 10-fold difference in the y axes. Data are expressed as means ± SEMs for three separate experiments, and the asterisks indicate a significant difference (P ≤ 0.05) between the amount of LTA released during incubation with antibiotic and that found to be released by the control (contr) culture.
). Four hours after the addition of medium or antibiotics, the bacterial supernatants were collected by centrifugation and filtration of the cultures, and the release of LTA was measured by means of a specific ELISA. Antibiotics studied were the β-lactam antibiotics imipenem (imi), flucloxacillin (fluclox), and cefamandole (cefa) (A) and the protein synthesis inhibitors erythromycin (ery), clindamycin (clinda), and gentamicin (genta) (B). Note the 10-fold difference in the y axes. Data are expressed as means ± SEMs for three separate experiments, and the asterisks indicate a significant difference (P ≤ 0.05) between the amount of LTA released during incubation with antibiotic and that found to be released by the control (contr) culture.
In contrast, incubation for 4 h with erythromycin, clindamycin, or gentamicin at a concentration as low as 1× the MIC significantly decreased the amount of LTA released to approximately half the amount that was released in the absence of an antibiotic (Fig. 1); similar results were obtained when a concentration of 20× the MIC was used.
In enriched M199 medium the pattern of the spontaneous release of LTA and the release caused by antibiotics was very similar to that found for NB. After 4 h the amount of LTA released from control cultures was 2.1 ± 0.48 μg/ml. Incubation with imipenem, flucloxacillin, and cefamandole (at 20× the MIC) enhanced the amount of LTA released 2- to 2.5-fold, whereas incubation with erythromycin, clindamycin, and gentamicin reduced the amount released to approximately half this amount (data not shown).
Release of PG.
During logarithmic growth in NB, the amount of PG released from the control cultures was 0.59 ± 0.13 μg/ml after 4 h (Fig. 2). After the addition of the β-lactam antibiotics this release was significantly enhanced to 36.0 ± 6.3 μg/ml for cultures treated with imipenem, 37.5 ± 11.5 μg/ml for cultures treated with flucloxacillin, and 51.4 ± 4.3 μg/ml for cultures treated with cefamandole. In contrast, incubation with erythromycin, clindamycin, or gentamicin at a concentration of 20× the MIC did not significantly change the amount of PG released into the culture medium.
FIG. 2.
Release of PG from S. aureus in the absence (■) or presence of different antibiotics (β-lactam antibiotics [A] and protein synthesis inhibitors [B]) at a concentration of 20× the MIC ( ; for abbreviations see the legend to Fig. 1). The bacterial supernatants were collected 4 h after the addition of medium or antibiotics, and the amount of PG was measured by means of an SLP test. Note the 10-fold difference in the y axes. Data are expressed as means ± SEMs for three separate experiments. The asterisks indicate significant differences (∗, P ≤ 0.05; ∗∗, P ≤ 0.005) in the amount of PG released between cultures incubated with an antibiotic and the control culture.
; for abbreviations see the legend to Fig. 1). The bacterial supernatants were collected 4 h after the addition of medium or antibiotics, and the amount of PG was measured by means of an SLP test. Note the 10-fold difference in the y axes. Data are expressed as means ± SEMs for three separate experiments. The asterisks indicate significant differences (∗, P ≤ 0.05; ∗∗, P ≤ 0.005) in the amount of PG released between cultures incubated with an antibiotic and the control culture.
In enriched M199 medium the pattern of PG release was again similar to that found for NB. In control cultures the amount of PG released was 2.1 ± 1.1 μg/ml after 4 h. Incubation with imipenem, flucloxacillin, and cefamandole (at 20× the MICs) enhanced the amount released 10- to 13-fold, whereas incubation with erythromycin, clindamycin, and gentamicin did not cause a change in this level (data not shown).
Release of radioactively labeled cell wall material by antibiotics.
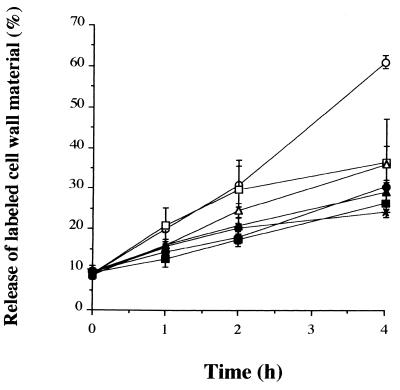
To determine whether the PG released into the supernatants after incubation of the bacteria with antibiotics is derived from the cross-linked cell wall, S. aureus was labeled overnight with [3H]N-acetylglucosamine. The spontaneous release of labeled cell wall material during logarithmic growth of S. aureus increased from 8.9% ± 0.4% at the start of the experiment (after preincubation for 2 h to obtain logarithmic growth) to 21.5% ± 2.0% at 1 h, 37.5% ± 2.2% at 2 h, and 61.3% ± 2.1% at 4 h. When S. aureus was incubated with the β-lactam antibiotics imipenem, flucloxacillin, or cefamandole concentration-dependent (data not shown) and time-dependent inhibitions of the release of labeled cell wall material were observed (Fig. 3), indicating that the PG released by exposure of the bacteria to antibiotics may not be derived from radioactively labeled cross-linked cell wall material. The inhibitory effects of the β-lactams were slightly more pronounced and occurred earlier than those found for the protein synthesis inhibitors. Inhibition had already started 1 h after the addition of the antibiotic and was significant at 2 h (P ≤ 0.01) and 4 h (P ≤ 0.001) of incubation with any of the three antibiotics.
FIG. 3.
Release of [3H]N-acetylglucosamine from the cell wall of S. aureus during incubation in the absence (○) or presence of different antibiotics at a concentration of 20× the MIC (for abbreviations see the legend to Fig. 1). At various time points samples were collected from the bacterial cultures and the release of labeled cell wall was measured by scintillation counting. The release is depicted as a percentage of the total amount of label present in each sample. Data are expressed as means ± SEMs for three experiments. ○, control; ■, imipenem; ▴, flucloxacillin; •, cefamandole; □, erythromycin; ▵, clindamycin; ∗, gentamicin.
Incubation of the bacteria with protein synthesis-inhibiting antibiotics also caused concentration-dependent (data not shown) and time-dependent inhibitions of the release of labeled cell wall material (Fig. 3). No significant differences in the inhibition of the release of labeled cell wall were seen between the β-lactam antibiotics and the protein synthesis inhibitors.
Biological reactivities of the supernatants of S. aureus.
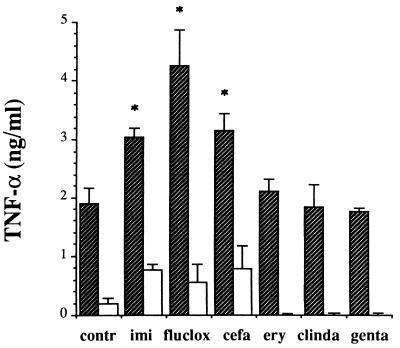
The cytokine-inducing capacities of the different antibiotic supernatants were tested in a whole-blood stimulation assay. At 24 h, the basal production of TNF-α in unstimulated whole blood amounted to 0.03 ± 0.01 ng/ml. Incubation with supernatants of control cultures induced the secretion of 1.89 ± 0.27 ng of TNF-α per ml (Fig. 4). When whole blood was incubated with β-lactam supernatants of S. aureus the level of production of TNF-α was significantly higher than those found for supernatants of control cultures and protein synthesis inhibitors. The levels of TNF-α production did not differ significantly for the separate β-lactam supernatants. For the anti-inflammatory cytokine IL-10 a pattern similar to that found for TNF-α was observed (data not shown). Incubation with supernatants of control cultures induced the secretion of 0.72 ± 0.19 ng of IL-10 per ml. Incubation with β-lactam supernatants increased the level of IL-10 production approximately twofold, whereas supernatants of protein synthesis inhibitors slightly reduced the level of IL-10 production compared to the levels induced by control supernatants. The amounts of LTA and PG in the bacterial supernatants were significantly associated with the amounts of TNF-α (r = 0.691 and 0.726; P values for both are ≤0.01) and IL-10 (r = 0.597 and 0.852; P values for both are ≤0.025). This stimulatory effect could not be due to contamination of the supernatants with LPS because the concentration of LPS was below the detection range of the Limulus amoebocyte lysate assay (i.e., 2 pg/ml) and the addition of polymyxin B to the bacterial supernatants did not affect their stimulatory activities (data not shown). Furthermore, cytokine secretion due to the production of bacterial exotoxins (7) was excluded by determining the amounts of staphylococcal enterotoxins A, B, and C and toxic shock syndrome toxin type 1 in the bacterial supernatants by specific ELISAs. In addition, experiments with LPS-stimulated whole blood showed that none of the antibiotics influenced cytokine production in this assay.
FIG. 4.
Production of TNF-α in human whole blood that was stimulated with supernatants of S. aureus cultured in enriched M199 medium. Bacterial supernatants were collected after 4 h of incubation with or without the indicated antibiotic. These supernatants were either untreated ( ) or pretreated for 3 h at 37°C with two PG-degrading enzymes (□) (see Fig. 5), and 24 h after the addition of these supernatants to whole blood, the cytokine concentrations were measured by means of a specific sandwich ELISA. Data are expressed as the means ± SEMs for four experiments; an asterisk indicates a significant difference (P ≤ 0.05) in cytokine levels between whole blood incubated with antibiotic-induced supernatants and control supernatant. See the legend to Fig. 1 for definitions of abbreviations.
) or pretreated for 3 h at 37°C with two PG-degrading enzymes (□) (see Fig. 5), and 24 h after the addition of these supernatants to whole blood, the cytokine concentrations were measured by means of a specific sandwich ELISA. Data are expressed as the means ± SEMs for four experiments; an asterisk indicates a significant difference (P ≤ 0.05) in cytokine levels between whole blood incubated with antibiotic-induced supernatants and control supernatant. See the legend to Fig. 1 for definitions of abbreviations.
Enzymatic degradation of PG in the bacterial supernatants.
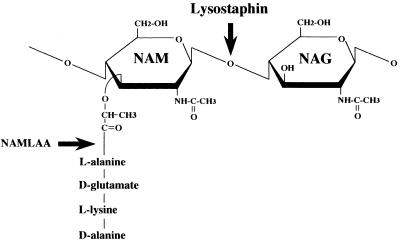
To determine which of the bacterial cell wall components exerted the observed immunostimulatory activity, the PG molecules in the bacterial supernatants were degraded enzymatically with lysostaphin and NAMLAA. The first enzyme cleaves the bond between N-acetyl-muramic acid and N-acetylglucosamine molecules, i.e., the carbohydrate molecules that form the PG backbone (Fig. 5). NAMLAA cleaves the amide bond between N-acetyl-muramic acid and l-alanine in the peptide side chain of PG, thus removing the peptide cross bridges between the PG strands (11). The enzymatic treatment reduced the TNFα-inducing capacity of the β-lactam supernatants by approximately 79% (range, 74.6 to 87.1%), whereas for protein synthesis inhibitors no residual TNF-α production was observed (Fig. 4). This indicates that the biological reactivity in whole blood exerted by supernatants of S. aureus is primarily effected by intact PG molecules.
FIG. 5.
Schematic representation of the minimal repetitive unit of peptidoglycan with the cleavage sites for the enzymes lysostaphin and NAMLAA. Lysostaphin cuts between N-acetyl-muramic acid (NAM) and N-acetylglucosamine (NAG) molecules, whereas NAMLAA cleaves the amide bond between N-acetyl-muramic acid and l-alanine.
DISCUSSION
The main finding of the present study is that β-lactam antibiotics differ strikingly from protein synthesis-inhibiting antibiotics in the amount of immunostimulatory cell wall components that they cause to be released from S. aureus. β-Lactam antibiotics greatly enhanced the release of PG and LTA, whereas the protein synthesis inhibitors did not affect PG release and even inhibited the release of LTA compared to the release of LTA from control cultures. Furthermore, the capacities of the β-lactam supernatants to stimulate the production of TNF-α and IL-10 in human whole blood were significantly higher than those of supernatants of protein synthesis inhibitors or control supernatants, and the amounts of these cytokines released were directly proportional to the concentrations of LTA and PG in the supernatants. Experiments in which PG in the supernatants was enzymatically degraded suggested that intact PG molecules were especially responsible for the observed biological reactivities of the bacterial supernatants.
In this study two groups of antibiotics with different mechanisms of action, i.e., β-lactam antibiotics, which inhibit cell wall synthesis via binding to penicillin-binding proteins, and protein synthesis-inhibiting antibiotics, which exert their effect via reversible or irreversible binding to the bacterial ribosome, were compared. In each of the two groups, the effects of the three different antibiotics on the release of LTA and PG from S. aureus were similar, despite variations in target specificity and bacterial killing.
An important issue to consider is whether the release of cell wall components is related to the antibacterial activities of the separate antibiotics. In our experimental setup it is unclear whether the cell wall components are released from viable or killed bacteria. The finding that bacterial killing induced by β-lactam antibiotics coincided with the release of large amounts of cell wall components could suggest that LTA and PG are predominantly released from killed bacteria. However, gentamicin had potent bactericidal activity, yet it induced the release of only small amounts of LTA and PG. This suggests that the release of cell wall components is influenced substantially by the specific mechanism of action of the antibiotic that leads to bacterial killing.
The exact cause of the release of LTA after incubation of microorganisms with antibiotics is still unknown. With respect to the β-lactam antibiotics it has been hypothesized that binding of the antibiotic to penicillin-binding proteins and subsequent inhibition of de novo synthesis of the cell wall lead to accumulation of cell wall precursors in the cell, which in turn could reduce the stability of LTA in the cell membrane (27). Because LTA molecules bind and inhibit the autolytic enzymes present in the cell wall of the gram-positive bacteria (25), an enhanced release of LTA from the bacterial cell membrane could reduce the inhibition of autolytic enzymes, resulting in penicillin-induced lysis of the bacteria. How protein synthesis inhibitors cause the observed inhibition of the release of LTA is unknown, but at least a diminished synthesis of autolysins does not appear to be responsible (27).
As far as the mechanism of PG release is concerned, we have shown that β-lactam antibiotics greatly enhanced the release of PG, whereas at the same time these antibiotics inhibited the release of incorporated labeled cell wall material, which was shown to consist of approximately 60% PG (34). This apparent discrepancy can be explained by the fact that in our experimental design PG molecules synthesized after removal of the free label will not contain incorporated label and therefore will not be measured, indicating that the large amount of PG released by exposure of the bacteria to β-lactam antibiotics, as measured in the SLP assay, may not be derived from cross-linked radioactively labeled cell wall material but instead consists of newly synthesized PG molecules. This finding is in accordance with those of other studies which have shown that benzylpenicillin caused the release of a low-molecular-weight soluble form of PG that was not incorporated into the cell wall (2, 35).
When investigating the immunostimulatory capacities, the biochemical structures of bacterial cell wall components are important factors to be considered because they appear to determine their biological reactivities. Various biochemical alterations, such as deacylation (3), sonication, enzymatic degradation (26), and purification (14), can reduce the cytokine-inducing capacities of these cell wall components. More importantly, antibiotics can influence the form in which the LTA (19) and PG (2, 35) molecules are released, which in turn could affect their biological reactivities. For instance, when human monocytes were incubated with purified penicillin-induced, low-molecular-weight soluble PG of S. aureus, little TNF-α was secreted into the incubation medium, which is in sharp contrast to the high levels of cytokine production induced by insoluble, high-molecular-weight PG isolated from S. aureus cell walls in the absence of penicillin (21). The latter finding raises doubts about studies in which purified bacterial components isolated from the cell wall are used to investigate their immunostimulatory capacities in vitro, because this is probably not the relevant biochemical form in which these components are released during normal growth and while under the influence of antibiotics. Therefore, in the present study the immunostimulatory capacities of the released bacterial components were examined without purification or biochemical alterations. We investigated cytokine induction in human whole blood, a model that has already been studied in detail (31, 33). The cytokine pattern found in our study after stimulation of whole blood with antibiotic supernatants corresponded closely with that produced by isolated monocytes incubated with different S. epidermidis supernatants (16).
As to the question of which bacterial component was responsible for the observed biological reactivity in whole blood, experiments with enzymatic degradation showed that the TNF-α production was primarily due to the presence of intact PG molecules in the bacterial supernatants. The fact that LTA has been shown to stimulate cytokine production in vitro (8, 15) and the fact that the LTA concentration in our supernatants closely correlates with the residual biological reactivity after enzymatic degradation seem to point in the direction indicating that LTA is another stimulator of proinflammatory cytokine secretion. Our findings, however, do not rule out the existence of other stimulatory cell wall components, e.g., teichoic acid (29).
In conclusion, we have shown that β-lactam antibiotics greatly enhanced the release of LTA and PG from S. aureus compared to the amounts released by control cultures and that this release correlated with increases in the levels of in vitro secretion of the proinflammatory cytokine TNF-α and its counterpart IL-10 in human whole blood. The protein synthesis-inhibiting antibiotics decreased the level of secretion of LTA but did not affect the amount of PG that was released. Subsequently, incubation of whole blood with these antibiotic supernatants led to the secretion of smaller amounts of cytokines.
It is uncertain whether the release of bacterial cell wall fragments during antibiotic treatment of patients plays an important role in the systemic reaction to infection. After the start of antibiotic treatment for infections caused by gram-negative bacteria, an increase in plasma or urine endotoxin levels in some patients has been described (5, 20), but the interpretation in relation to morbidity is uncertain. In the case of infections caused by gram-positive bacteria, neither the release of bacterial components nor their effect on the clinical outcome has yet been studied. When such studies confirm our in vitro findings, possibly, a beneficial effect of the rapid killing of bacteria by the β-lactam antibiotic should be weighed against an enhanced release of immunostimulatory bacterial components.
ACKNOWLEDGMENTS
This study was supported financially by the Praeventiefonds (project 28-2293) and an educational grant from Glaxo Wellcome B.V. (Zeist, The Netherlands).
REFERENCES
- 1.Acar J F, Goldstein F W. Dilution in agar. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 28–32. [Google Scholar]
- 2.Barrett J F, Shockmann G D. Isolation and characterization of soluble peptidoglycan from several strains of Streptococcus faecium. J Bacteriol. 1984;159:511–519. doi: 10.1128/jb.159.2.511-519.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakdi S, Klonisch T, Nuber P, Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991;59:4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Kimpe S J, Thiemermann C, Vane J R. Role for intracellular platelet-activating factor in the circulatory failure in a model for gram-positive shock. Br J Pharmacol. 1995;116:3191–3198. doi: 10.1111/j.1476-5381.1995.tb15123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dofferhoff A S M, Nijland J H, de Vries-Hospers H G, Mulder P O M, Weits J, Bom V J J. Effects of different types and combinations of antimicrobial agents on endotoxin release from gram-negative bacteria: an in-vitro and in-vivo study. Scand J Infect Dis. 1991;23:745–754. doi: 10.3109/00365549109024303. [DOI] [PubMed] [Google Scholar]
- 6.Evans M E, Pollack M. Effects of antibiotic class and concentration on the release of lipopolysaccharide from Escherichia coli. J Infect Dis. 1993;167:1336–1343. doi: 10.1093/infdis/167.6.1336. [DOI] [PubMed] [Google Scholar]
- 7.Henderson B, Wilson M. Cytokine induction by bacteria: beyond lipopolysaccharide. Cytokine. 1996;8:269–282. doi: 10.1006/cyto.1996.0036. [DOI] [PubMed] [Google Scholar]
- 8.Heumann D, Barras C, Severin A, Glauser M P, Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogg S D, Whiley R A, De Soet J J. Occurrence of lipoteichoic acid in oral streptococci. Int J Syst Bacteriol. 1997;47:62–66. doi: 10.1099/00207713-47-1-62. [DOI] [PubMed] [Google Scholar]
- 10.Hoijer M A, Melief M-J, Debets R, Hazenberg M P. Inflammatory properties of peptidoglycan are decreased after degradation by human N-acetylmuramyl-l-alanine amidase. Eur Cytokine Network. 1997;8:375–382. [PubMed] [Google Scholar]
- 11.Hoijer M A, Melief M-J, Keck W, Hazenberg M P. Purification and characterization of N-acetylmuramyl-l-alanine amidase from human plasma using monoclonal antibodies. Biochim Biophys Acta. 1996;1298:57–64. doi: 10.1016/0304-4165(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 12.Horne D, Tomasz A. Release of lipoteichoic acid from Streptococcus sanguis: stimulation of release during penicillin treatment. J Bacteriol. 1979;137:1180–1184. doi: 10.1128/jb.137.3.1180-1184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson J J, Kropp H. β-Lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP)2-specific imipenem and PBP3-specific ceftazidime. J Infect Dis. 1992;165:1033–1041. doi: 10.1093/infdis/165.6.1033. [DOI] [PubMed] [Google Scholar]
- 14.Keller R, Fischer W, Keist R, Bassetti S. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect Immun. 1992;60:3664–3672. doi: 10.1128/iai.60.9.3664-3672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindemann R A, Economou J S, Rothermel H. Production of interleukin-1 and tumor necrosis factor by human peripheral monocytes activated by periodontal bacteria and extracted lipopolysaccharides. J Dent Res. 1988;67:1131–1135. doi: 10.1177/00220345880670081401. [DOI] [PubMed] [Google Scholar]
- 16.Mattsson E, van Dijk H, Verhoef J, Norrby R, Rollof J. Supernatants from Staphylococcus epidermidis grown in the presence of different antibiotics induce differential release of tumor necrosis factor alpha from human monocytes. Infect Immun. 1996;64:4351–4355. doi: 10.1128/iai.64.10.4351-4355.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattsson E, Verhage R, Rollof J, Fleer A, Verhoef J, van Dijk H. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-α, interleukin-1β, and interleukin-6. FEMS Immunol Med Microbiol. 1993;7:281–288. doi: 10.1111/j.1574-695X.1993.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 18.Notermans S, Boot R, Tips P D, Nooy M P. Extraction of staphylococcal enterotoxins (SE) from minced meat and subsequent detections of SE with ELISA. J Food Prot. 1983;46:238–241. doi: 10.4315/0362-028X-46.3.238. [DOI] [PubMed] [Google Scholar]
- 19.Pollack J H, Ntamere A S, Neuhaus F C. d-Alanyl-lipoteichoic acid in Lactobaccilus casei: secretion of vesicles in response to benzylpenicillin. J Gen Microbiol. 1992;138:849–859. doi: 10.1099/00221287-138-5-849. [DOI] [PubMed] [Google Scholar]
- 20.Prins J M, van Agtmael M A, Kuijper E J, van Deventer S J H, Speelman P. Antibiotic-induced endotoxin release in patients with gram-negative urosepsis: a double-blind study comparing imipenem and ceftazidime. J Infect Dis. 1995;172:886–891. doi: 10.1093/infdis/172.3.886. [DOI] [PubMed] [Google Scholar]
- 21.Rabin R L, Bieber M M, Teng N N H. Lipopolysaccharide and peptidoglycan share binding sites on human peripheral blood monocytes. J Infect Dis. 1979;168:135–142. doi: 10.1093/infdis/168.1.135. [DOI] [PubMed] [Google Scholar]
- 22.Raynor R H, Scott D F, Best G K. Oxacillin-induced lysis of Staphylococcus aureus. Antimicrob Agents Chemother. 1979;16:134–140. doi: 10.1128/aac.16.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soto A, Evans T J, Cohen J. Proinflammatory cytokine production by human peripheral blood mononuclear cells stimulated with cell-free supernatants of viridans streptococci. Cytokine. 1996;8:300–304. doi: 10.1006/cyto.1996.0040. [DOI] [PubMed] [Google Scholar]
- 24.Spika J S, Peterson P K, Wilkinson B J, Hammerschmidt D E, Verbrug H A, Verhoef J, Quie P G. Role of peptidoglycan from Staphylococcus aureus in leukopenia, thrombocytopenia, and complement activation associated with bacteraemia. J Infect Dis. 1982;146:227–233. doi: 10.1093/infdis/146.2.227. [DOI] [PubMed] [Google Scholar]
- 25.Suginaka H, Shimatani M, Ogawa M, Kotani S. Prevention of penicillin-induced lysis of Staphylococcus aureus by cellular lipoteichoic acid. J Antibiot. 1979;32:73–77. doi: 10.7164/antibiotics.32.73. [DOI] [PubMed] [Google Scholar]
- 26.Timmerman C P, Mattsson E, Martinez-Martinez L, de Graaf L, van Strijp J A G, Verbrugh H A, Fleer A. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993;61:4167–4172. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomasz A, Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci USA. 1975;72:4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuchiya M, Asahi N, Suzuoki F, Ashida M, Matsuura S. Detection of peptidoglycan and β-glucan with silkworm larvae plasma test. FEMS Immunol Med Microbiol. 1996;15:129–134. doi: 10.1111/j.1574-695X.1996.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 29.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985;151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 30.Utsui Y, Ohya S, Takenouchi Y, Tajima M, Sugawara S, Deguchi K, Suginaka H. Release of lipoteichoic acid from Staphylococcus aureus by treatment with cefmetazole and other beta-lactam antibiotics. J Antibiot. 1983;36:1380–1386. doi: 10.7164/antibiotics.36.1380. [DOI] [PubMed] [Google Scholar]
- 31.van Furth A M, Seijmonsbergen E M, Langermans J A M, van der Meide P H, van Furth R. Effect of xanthine derivates and dexamethasone on Streptococcus pneumoniae-stimulated production of tumor necrosis factor alpha, interleukin-1β (IL-1β), and IL-10 by human leukocytes. Clin Diagn Lab Immunol. 1995;2:689–692. doi: 10.1128/cdli.2.6.689-692.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Langevelde P, Kwappenberg K M C, Groeneveld P H P, Mattie H, van Dissel J. Antibiotic-induced lipopolysaccharide (LPS) release from Salmonella typhi: delay between killing by ceftazidime and imipenem and release of LPS. Antimicrob Agents Chemother. 1998;42:739–743. doi: 10.1128/aac.42.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westendorp R G J, Langermans J A M, Huizinga T W J, et al. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–173. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- 34.Wong W, Young F E, Chatterjee A N. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974;120:837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeiger A R, Wong W, Chatterjee A N, Young F E, Tuazon C U. Evidence for the secretion of soluble peptidoglycans by clinical isolates of Staphylococcus aureus. Infect Immun. 1982;37:1112–1118. doi: 10.1128/iai.37.3.1112-1118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]