Abstract
In the present study, a novel glass system containing Neodymium(III) oxide with BaO, Al2O3, and B2O3 were created via a popular melt-quenching technique. Nd2O3 were added, in different concentrations, instead of B2O3 to study its impact on the crystallization, and electro-magnetic behaviors of the prepared poly-crystalline materials. Thermal characteristics via DTA, XRD and SEM techniques were involved to explore the crystallization and structural properties. The magnetic parameters of the prepared glass–ceramics were studied by VSM measurements. As well the electric properties were also explored. BaB2O4 and Al(BO3) phases were firstly crystallized then Ba3Nd(BO3)3 phase was incompletely precipitated instead of BaB2O4 phase owing to Nd2O3 additions. As well, the internal structure was modified from coarse crystals to fine grain microstructure. The crystallization study proved that the addition of neodymium improved the crystallization process of the BaO–Al2O3–B2O3 glass system. The VSM and conductivity analysis for the crystalline materials proved that the Nd2O3/B2O3 substitutions led to an increase in the electrical and magnetic parameters of the investigated materials. The data obtained from the prepared crystalline specimen showed that these materials are with a distinct and promising ferro-electrical property for use in diverse modern applications.
Subject terms: Chemistry, Materials science
Introduction
Nowadays, many world scientists are attracted to developing modern materials to keep pace with the rapid development of our life requirements. A glass and glass–ceramics are one of these gifted materials. Glass–ceramics have caught the interest of academics due to their excellent combination of properties and numerous commercially available products for consumers and specific markets1. Glass–ceramics are now defined as nonmetallic, inorganic materials that are created by controlling the crystallization of glass systems using a variety of processing methods2. They are composed of at least one functional crystal stage imbedded in a glassy matrix. Among these, borate glasses and their glass–ceramic derivatives have consistently drawn attention and been the topic of numerous studies and publications3. They are suggested as potential hosts for radioactive waste4, candidates for fast ionization5, or materials with electro-optic, 2nd harmonic generation, and nonlinear optical activity. Borate-based glasses have a blend of planar B3O6 boroxol rings with triangular [BO3] units. The concentration of boroxol rings decreases when some alkali or alkaline-earth oxide ions are introduced to the matrix of the borate glass because three-coordinated boron is changed to four-coordinated boron, and the concentration of boroxol rings falls, which increases network connectivity in the structure of the created glasses6.
Barium oxide (BaO) is one of the alkaline earth oxides that has a significant impact on the properties when introduced in the borate-based glasses, including phase formation and thermal stability7. In barium-borate glasses, BaO can react with B2O3, leading to the conversion of some [BO3] to [BO4] in the glass structure. This is a special characteristic of frequently manufactured borate glasses, which exhibit two coordination states in their network structure. These materials possess fascinating, beneficial physical characteristics such as dielectric properties and second harmonic generation8.
The crystallization characteristics of barium borate as a binary the glass system in different concentrations have been investigated by several researchers9–11. Some studies8,12–14 confirmed the effect of TiO2 doped in Ba-borate glasses as nucleating agent. Środa et al.15 examined the impact of CeO2 doping on the structure, thermal stability, and luminescence features of barium borate glasses and glass–ceramics. Glass–ceramics in the barium aluminium borate system were studied by Russel and others16–18. Recently, the structural role of Nd2O3/B2O3 replacement in the borate glasses and glass ceramics based on the 46B2O3–27CaO–24.4Na2O–2.6P2O5 (mol%) system has been studied19. The amorphous specimens were synthesized through the melt quenching technique. Increasing the Nd2O3 content at the expense of B2O3 led to a growth in the glass transition temperature (Tg) and the measured density, which increased the glass hardness. However, the calculated molar volume decreases. The change in the glass batches by an Nd+3 large cation rather than a B+3 led to a rise in the toughness of the glass network by increasing the bridging bonds in the glass network as a result of decreasing both the molar volume and free spaces of the investigated compositions. The state of crystallization in the high-Nd2O3-containing glass (4 mol%) is more affected by the glass compositions than by the applied thermal treatment schedule.
The presence of rare-earth oxide (REO) in the glass structure led to increased permittivity, reduced dielectric loss, raised resistivity and a high elastic modulus20 and other beneficial electrical and magnetic properties21–23. Neodymium oxide (Nd2O3) is a REO that gives glass unique features such as thermal, mechanical, chemical, optical, and electrical properties21,22. Due to these outstanding characteristics, Nd2O3-containing glasses have already been used in numerous modern applications23–25. Neodymium oxide has an astonishingly high melting point of 2300 °C26. These characteristics could influence the progress of hermetic materials for sealing purposes at high temperatures or refractory materials with great corrosion resistance. Despite the fact that Nd2O3 acts as a network modifier, Kohli and Shelby21 found that increasing the Nd2O3 content in aluminosilicate glasses increased the Tg and Td slightly. This behavior is attributed to the main effect of Nd2O3 as high field strength rather than a glass modifier.
The aim of our research is to examine the impact of the Nd2O3/B2O3 replacements on crystallization behavior. The electro-magnetic properties of the new novel poly-crystallized glasses containing Nd2O3 were prepared via fabricated glasses based on the BaO–B2O3(Nd2O3)–Al2O3 system (mol%), followed by heat treatment according to DTA data. The structural characteristics of these unique glass–ceramics were investigated by the XRD technique and SEM images using five different concentrations. The impact of Nd2O3/B2O3 replacements on the density, magnetic parameters, and electric properties was explored to develop new promising electro-magnetic glass–ceramics for future applications.
Experimental techniques
Glass and glass–ceramic synthesis
The 20 BaO–(70 − x) B2O3–xNd2O3–10 Al2O3 glass system was carefully synthesized by the melt-quenching method, (where x = 0.5, 1, 2, and 3 mol%). The glass batches of this work are shown in Table 1. High purity grade (˃ 99%) raw materials of BaCO3, H3BO3, Al2O3, were purchased from Fluka chemei AG, Switzerland, and Nd2O3 was purchased from BDH chemicals, England were used. The suitable amounts of these compositions were combined and placed in a platinum crucible after being accurately weighted using an electronic balance that had an accuracy of ± 0.0002 g. The mixture was then melted at 1250 °C for 90 min in an electrical furnace. Molds made of hot stainless steel were used to form the desired shapes from the molten glass for experimental measurements. These glass species were then transferred to an electric furnace and annealed at 450 °C for two hours to prevent cracking. Then, the furnace was turned off after the definite amount of time (120 min) of annealing, and the glass specimens were kept inside until the temperature reaches to ambient temperature. Lastly, the solid glass samples were heat treated at specific temperatures per time through double-stage schedules according to DTA data, as presented in Table 2, to prepare glass–ceramic samples.
Table 1.
The studied glass compositions (mol%).
| Composition (mol%) | ||||
|---|---|---|---|---|
| Sample ID | BaO | B2O3 | Al2O3 | Nd2O3 |
| GNd0 | 20.00 | 70.0 | 10 | – |
| GNd0.5 | 20.00 | 69.5 | 10 | 0.5 |
| GNd1 | 20.00 | 69.0 | 10 | 1 |
| GNd2 | 20.00 | 68.0 | 10 | 2 |
| GNd3 | 20.00 | 67.0 | 10 | 3 |
Table 2.
The thermal study and glass–ceramics characterization.
| Sample ID | Heat-treatment (°C/h) | ∆T = Tc − Tg [Refs.59,60] | Crystalline phases | Density (g/cm3) | Magnetic properties | ||
|---|---|---|---|---|---|---|---|
| Ms (emu/g) | Hc (G) | Mr (emu/g) | |||||
| GNd0 | 530/2–755/1 | 225 | BaB2O4, AlBO3 | 2.92 | 0.104 | 35 | 3.07 |
| GNd0.5 | 548/2–779/1 | 222 | BaB2O4, AlBO3 | 2.94 | – | – | – |
| GNd1 | 616/2–786/1 | 170 | BaB2O4, AlBO3, Ba3Nd(BO3)3 | 2.99 | 0.132 | 65 | 4.03 |
| GNd2 | 636/2–789/1 | 153 | Ba3Nd(BO3)3, BaB2O4, AlBO3 | 3.06 | – | – | – |
| GNd3 | 652/2–799/1 | 147 | Ba3Nd(BO3)3, BaB2O4, AlBO3 | 3.11 | 0.203 | 91 | 5.95 |
Material characterizations
The powdered glass samples (about 10 mg) will subjected to a differential scanning Calorimetry, (DTA-Netzsch-STA449C, Germany) with a temperature region of 24–800 °C with heating rate of 5 °C per minute using alumina crucible. From the results obtained, it is possible to know the controlled heat-treatment regime at which crystallization occurs, such as the glass transition temperature (Tg) and the crystallization temperature (Tc).
XRD (X-Ray Diffraction) analysis of the heat-treated glasses will performed to determine the crystalline phases precipitated. X-ray diffractometer (XRD) was performed to examine the crystalline phases of each samples using (Philips X-ray diffractometer PW 1730). The Cu-Kα X-ray radiation (λ = 1.5406 A) powered was at 40 kV and 40 mA in the 2θ range of 10°–70° in steps of (2θ) = 0.01.The microstructure morphology of selected crystallized glass-ceramic specimens was studied by Scanning Electron Microscopy (SEM, Quanta 250 FEG-FEI Company, Netherlands). The specimens was detected on fresh fracture surfaces after etched by soaking in 2% HF acid for 45 s.
The Archimedes method will be used to calculate the densities of bulk crystalline specimens with distilled water (density of water, ρw = 1 g/cm3) as the immersion liquid. Each sample contained five unique pieces. Using an electrical digital balance with an accuracy of ± 0.02 mg, weight the poly-crystalline specimens in air (Wair) and distilled water (Ww) to determine their respective weights (g). Density was calculated according to the following equation the sample density value, ρ sample, (g/cm3):
| 1 |
The room-temperature magnetic parameters of the powder glass–ceramic specimens were examined using (VSM, Lake Shore Model 7410, USA). The used magnetic field is 20 kOe. The obtained measurements were used to decide the saturation magnetization (Ms), remanence magnetization (Mr), and coercive field (Hc) of the crystalline samples. The conductivity behavior of the studied crystalline samples was determined by Hitester Impedance Analyzer (HIOKI 3522-50 LCR) with 0.100 V voltage depending on the frequency. In a fully dry state, an alternating electrical current (AC) was used with the 0.1 Hz–2 MHz as frequency range at room temperature. The fine powders of the examined glass–ceramic sample were pressed at room temperature, by using a mass of 5 tons, to prepare the desired disks for the measurements, which then solidified in an electric furnace at 200 °C for 3 h. The surface of the glass–ceramic discs was covered with silver.
Ethics approval
The study was approved by the Ethics Committee of National Research Centre.
Consent to participate
A written informed consent was taken from all participants.
Results and discussion
Crystallization behavior of the glasses
The crystallization process involves the production of glass–ceramic from glass through a vital step called heat treatment, in which the glass transforms into a polycrystalline material with a fine microstructure and beneficial properties. These materials are called glass ceramics. To do this, the glass must be subjected to the best possible heat treatment conditions for a desired period of time to form a large number of nuclei and then complete the growth of crystals. To determine the ideal condition for heat treating the prepared glass samples, two techniques can be combined or used separately: the conventional method of trial and error, which involves systematic heat treatment of the samples at various conditions to determine the optimal condition, and differential thermal analysis (DTA).
Thermal analysis (DTA studies)
The DTA technique was used on a fine powder of the studied glass samples to investigate the thermal behavior and determine temperatures of crystallization parameters such as the glass transformation temperature (Tg) and the crystallization temperature (Tc), the values of which are shown in Table 2, and the recorded data is shown graphically in Fig. 1. The measurements were done in temperature ranges up to 900 °C at a rate of 10 °C per minute. The DTA patterns of the synthesized glass samples based on the BaO, Al2O3, B2O3, and Nd2O3 compositions have an interesting feature. The reported data (Table 2, Fig. 1) involved a markedly increased Tg with increasing Nd2O3 contents in the examined glasses, from 530 °C (for sample GNd0, free of Nd2O3) to 652 °C (for sample GNd3, with high Nd2O3 content). As well, the Tc temperatures were slightly increased from 755 to 799 °C for the samples GNd0 and GNd3, respectively.
Figure 1.
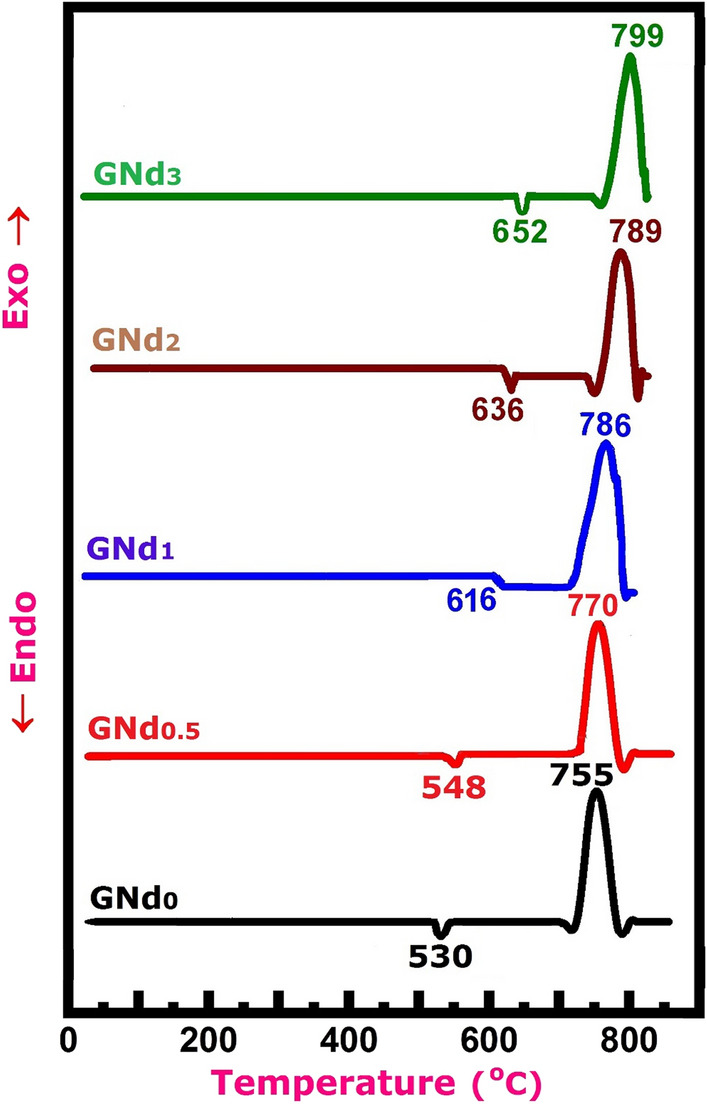
DTA thermographs for the synthesized glass samples.
It is known that Nd2O3 is a modifier oxide27, so this behavior is unexpected. It was anticipated to disorder the glass network, causing a decrease in both the Tg and Tc. The opposite was observed; despite this, given the high field strength of Nd+3 ions27, this anomaly may be acceptable. Our DTA results are similar to those reported by Kohli and Shelby21, which support the suggestion that the effect of Nd2O3 in the aluminosilicate glasses is in terms of its field strength rather than as a modifier oxide. Additionally, as seen in Table 2, a falling trend of ΔT is found for the Nd2O3-containing sample, which appears to be related to the rapid crystallization and the distinction in the heat capacities between the base glass, free of Neodymium oxide (GNd0 sample), and the other modified glass samples with different Nd2O3 content (GNd0.5–GNd3 samples). According to the important principles of the thermal analysis, Gabbott stated that the formed exothermic peaks are related to the thermal impact of crystallization behavior28. As a result, the sharp exothermic peaks shown in the DTA curves (Fig. 1) are an indication of the rapid crystallization process taking place over a small temperature interval (Table 2).
Crystalline phases formed (XRD study)
The type and amounts of the crystals precipitated during the crystallization of the glasses in any system depend mainly on the effects of composition, nucleating agents, and the applied heat treatment on both glass formation and. The XRD patterns for the developed crystalline phases as a result of treating glass samples at different temperatures according to the obtained DTA data are presented in Fig. 2, whereas their corresponding phases are recorded in Table 2. It is clear that the diffraction peaks of three different types of crystals formed exactly match their normal diffraction PDF cards. The XRD spectra of the un-doped base glass sample (GNd0) revealed that the control crystallization of the base glass at 530 °C for 2 h and 755 °C for 1 h led to the formation of the BaB2O4 phase (JCPDS Card No. 44-0584) as a main crystalline phase in addition to the Al(BO3) phase (JCPDS Card No. 26-0007) as a minor crystalline phase (Fig. 2, Table 2). The two crystalline phases may be precipitated in the glassy matrix by the use of oxides as in the following Eqs. (2) and (3):
| 2 |
| 3 |
Figure 2.

XRD patterns of the studied crystalline samples.
For the glass in the BaO–Al2O3–B2O3 system, BaB2O4 nanocrystals were developed as the major phase during the heat treatment of the glass samples29. Additionally, the presence of Al increases the surface tension of the BaB2O4 melt and extends the glass-forming range30. The structure of BaB2O4 crystals can be seen as a layer-step type of lattice that built up via Ba2+ and (B3O6)3− rings instead31.
Doping the studied glass composition (20 BaO, 10 Al2O3, and 70 B2O3) with a low content of Nd2O3 (0.5 mol%) at the expense of B2O3 (i.e., GNd0.5, heated at 548 °C for 2 h and 770 °C for 1 h), does not add any change in the type or amount of the phases that crystallized in the treated base glass, free of Nd2O3. This may be attributed to the low content of neodymium oxide (0.5 mol%), which adds to the glass, precipitates in the glass matrix, and is not enough to develop any Nd2O3-containing phases but the crystallization behavior was improved. These suggestions were confirmed by the obtained XRD results (Fig. 2). Partial increasing of the Nd2O3 content from 0.5 to 1 mol% (i.e., GNd1) instead of B2O3 led to the development of the Nd2O3-containing phase in the form of the Ba3Nd(BO3)3 phase (JCPDS Card No. 51-0425), at the expense of the BaB2O4 phase, together with the Al(BO3) phase. The crystallization of the barium neodymium borate crystalline phase [Ba3Nd(BO3)3] may be formed in a rich media of boric and barium oxide through the reaction described as follows:
| 4 |
Further increasing the Nd2O3 content up to 2 and 3 mol% (i.e., GNd2 and GNd3, respectively), the diffraction peak intensity of the Ba3Nd(BO3)3 phase was increased and became the main crystal phase together with the appearance of BaB2O4 and Al(BO3) as minor phases (Fig. 2 and Table 2). The introduction of Nd2O3 with high content (2 mol% and 3 mol%) in the studied glasses improved the crystallization behavior, but the kinds of crystal phases remained unchanged as shown in XRD patterns (Fig. 2). Furthermore, the growth of Nd-containing crystals adds a further boost to the value of glass ceramics as promising candidates to contain radioactive waste32.
Microstructural evolution (SEM study)
Microstructure is a powerful factor controlling the properties of glass–ceramic, and it can decrease or increase the features of key phases33. The design of an appropriate composition of the glass and the application of a suitable heat treatment control the microstructure developed for the crystalline phases in terms of size and morphology34. Figure 3 shows the morphologies of the distribution phases in the selected crystalline samples that were produced by melting and then subjecting them to heat treatment at two different temperatures per specific time as presented in Table 2. The fracture surfaces of the examined samples (GNd0, GNd1, and GNd3) were etched by 2% HF acid for 45 s to reduce the glassy matrix layer. The SEM image of the base crystallized sample, free of Nd2O3 (GNd0), shows a volume crystallization of dendritic microstructure with micro pores developed inside the formed crystals (Fig. 3a). A denser microstructure of fine prismatic-like crystals was developed, with fine pores formed in between these crystals and distributed in the image of the crystalline GNd1 sample (Fig. 3b). As shown in Fig. 3c, for the sample GNd3, an ultra-fine microstructure was formed with a new generation of fine lath-like growths of Nd-containing crystals that grow as a function of increasing Nd2O3 content in the glass composition, and this suggestion is evidenced by the XRD patterns for the GNd3 sample (Fig. 2). The observed SEM images show a denser morphology of fine grain microstructure in the three examined samples. However, the average grain size of the crystals decreased with an increase in the Nd2O3 content (Fig. 3). This observation is in agreement with a previous study by Cheng et al.35, who found that the SEM images of the waste doped with Nd2O3 (0, 10, 19, and 30 wt%) show the formation of very small crystals of micron size and pores formed directly next to these crystals.
Figure 3.
SEM images and micrographs for some crystalline samples.
Materials characterization
Figure 4 shows the density values of the prepared crystalline materials. The data presented that the density values of the crystallized samples were in the variety from 2.92 to 3.11 g/cm3. It is clear from the data that with the increase the addition Nd2O3 instead of B2O3, the bulk density displays a continuous increase. For the base crystalline specimen (GNd0) the bulk density value is 2.92 g/cm3. While, the addition of neodymium oxide up to 3.0 mol% at the expense of boric oxide (GNd3) the bulk density increase to 3.11 g/cm3. This may be due to formation of high atomic mass crystalline barium neodymium borate phase Ba3Nd(BO3)3 instead of relatively less dense BaB2O4 phase. The density of controlled crystallized glasses is affected by a combination of factors, the most significant of which is the density of the crystalline phases formed36–38. Another factor may be affecting the density of the synthesized glass–ceramic is formation of fine microstructure with increasing the Nd2O3/B2O3 replacement. The density of the crystallized glasses is mainly affected by the granular texture formed39–41. Higher bulk density means a condensed microstructure of the glass–ceramic materials39–41.
Figure 4.
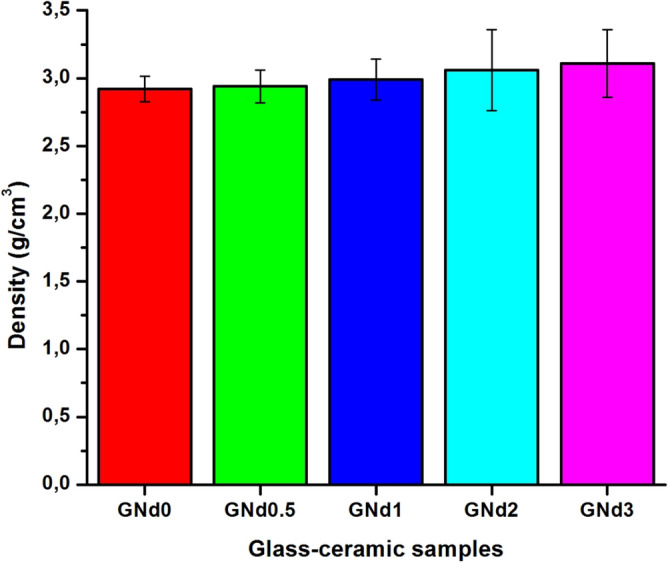
Density of the synthesized glass–ceramic specimens.
Recently, interest has increased in the synthesis of new materials that have distinct magnetic and electrical properties42–44. This is due to its new and distinct uses in many fields such as electronics, electrolytes, and electrochemical devices42–44. Ceramic materials are among the commonly used materials in these fields, especially glass–ceramics, because it has distinct properties that enable it to be used strongly in these fields. The ionic conductivity of the prepared glass–ceramic materials is shown in Fig. 5. The base crystalline sample GNd0 containing BaB2O4 and Al(BO3) as main and secondary crystalline phases respectively showed the lowest conductivity value. It is well known that BaB2O4 based materials are significant attributed to their amazing nonlinear optical, and piezoelectric properties for technical applications45,46. The obtained data indicate that the partial substitution of B2O3 with Nd2O3 in the glass specimens led to increase the conductivity values of the corresponding crystalline specimens. The crystalline sample GNd3 with 3 mol% Nd2O3 showed the highest conductivity value. This may be due to the crystallization of high conducting Ba3Nd(BO3)3 phase on account of the relatively low conducting BaB2O4 phase. The conductivity of crystallized glasses counts on mainly the type of crystallized phases47. The electrical conductivity of barium borate glass doped with Nd2O3 was studied48. They reported that the conductivity of materials increase with increasing neodymium content48.
Figure 5.

Variation of electrical conductivity with frequency for some crystalline samples.
The VSM (Vibrating Sample Magnetometry) hysteresis loops of the synthesized barium aluminum borate glass–ceramics containing different amounts of neodymium oxide is shown in Fig. 6, and Table 2. The magnetic parameters of the crystalline samples were detected at ambient atmosphere under a magnetic field of 20 kOe. In our current study, the focus was on the effect of neodymium content on the magnetic parameters measured of prepared glass–ceramics. The results presented that the measured glass–ceramic samples displayed a soft ferromagnetic attitude. The effects of microstructure as well as crystalline phases designed in the crystallized glasses on the different magnetic parameters, such as saturation magnetization (Ms), remanence magnetization (Mr) and coercivity (Hci), were studied as presented in Tables 2. The (Hci), (Ms), and (Mr) of the crystalline specimens are 35, 87, 91 G, 0.104, 0.132, 0.2039 (emu/g) and 3.07, 4.03 and 5.95 emu/g for GNd0, GNd1, and GNd3 respectively. The estimated magnetic parameters show that the crystalline specimens that Nd2O3/B2O3 replacements led to enhance the saturation magnetization (Ms) particularly the sample with high Nd2O3/B2O3 replacement ratio i.e., GNd3. This may be due to crystallization of barium neodymium borate Ba3Nd(BO3)3 instead of barium borate phase BaB2O4 as detected from the XRD patterns (Fig. 2). The change of saturation magnetization in glass–ceramic materials is largely depending on type and amount of crystallized phases49–53. Addition of Nd2O3 led to increase of magnetic properties of barium heksaferit (BaFe12O19) phase54. According the characterization results show that the addition of 0.5%wt.Nd2O3, the magnetic properties can increase about 40%55. On the other side, there is a clear increase in the coercivity and remanence magnetization parameters with addition of Nd2O3 instead of B2O3 as shown in Table 2. This may be due to the formation of fine-grained microstructure in the crystalline specimens with increasing Nd2O3 content, as shown in microstructure images for the crystallized samples in Fig. 3. The formed microstructure and the applied magnetic field are the main and effective factors influencing the values of coercivity and remanence magnetization56. When the grain microstructure becomes fine led to increase the coercivity and remanence magnetization parameters57,58.
Figure 6.

VSM hysteresis loops of the glass–ceramic specimens.
Conclusion
The formation of novel glasses based on the BaO–B2O3–Nd2O3–Al2O3 system was studied. The principal focus, however, will be on the controlled crystallization of glasses in the studied system. The effect of composition, modifying oxide, and applied heat treatment on both glass formation and crystallization was investigated. The DTA, XRD, SEM, and VSM techniques were used to explore the glass–ceramic materials. As well, density, electric, and magnetic properties are discussed in detail. The obtained Tg and Tc temperatures were increased as a result of the high field strength of Nd2O3 rather than a glass modifier oxide. The XRD and SEM studies revealed that BaB2O4, Al(BO3), and Ba3Nd(BO3)3 crystalline phases with different morphologies were obtained by modifying the composition with Nd2O3 dopants. The VSM and conductivity analysis for the crystalline materials proved that the Nd2O3/B2O3 substitutions led to an increase in the electrical and magnetic parameters of the investigated materials. The results showed a great potential of the prepared glass ceramics as promising soft ferro-electrical materials that can be used in different modern applications. The obtained data led to further improving and developing the abilities of these Nd-containing glass–ceramic materials with desirable properties for radioactive waste applications in the future.
Acknowledgements
The authors acknowledge National Research Centre for providing the necessary facilities for carrying out the research work.
Author contributions
H.A.-M.: Conceptualization, Methodology, Writing-Original draft preparation, Writing-Reviewing and editing, Replied to reviewers’ comments and revised the final version. E.M.: Methodology, Writing-Original draft preparation, Writing-Reviewing and Editing, Software, replied to reviewers’ comments and revised the final version. M.A.A. Data curation, Methodology, Software, Writing-Reviewing and Editing, Software. All authors approved the version of the manuscript to be published.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
Data and materials are all available and prepared, authors will be pleased to provide it if requested during the publication process. The corresponding author (M.A. Azooz) has a permission from other authors to reply if someone wants any data from this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mhareb M, Alajerami Y, Sayyed M, Mahmoud K, Ghrib T, Hamad MK, et al. Morphological, optical, structural, mechanical, and radiation-shielding properties of borosilicate glass–ceramic system. Ceram. Int. 2022;48:35227–35236. [Google Scholar]
- 2.Davis MJ, Zanotto ED. Glass-ceramics and realization of the unobtainable: Property combinations that push the envelope. MRS Bull. 2017;42:195–199. [Google Scholar]
- 3.Wright AC, Feller SA, Hannon AC. Borate Glasses, Crystals & Melts: Society of Glass Technology. Springer; 1997. [Google Scholar]
- 4.Martinez AL, Lebullenger R, Feitosa C, Hernandes AC. Semi-transparent barium borate surface crystallization for second harmonic generation. J. Non-Cryst. Solids. 2005;351:1372–1376. [Google Scholar]
- 5.Abdelghany A, Ouis M, Azooz M, ElBatal H, El-Bassyouni G. Role of SrO on the bioactivity behavior of some ternary borate glasses and their glass ceramic derivatives. Spectrochim. Acta Part A. 2016;152:126–133. doi: 10.1016/j.saa.2015.07.072. [DOI] [PubMed] [Google Scholar]
- 6.Ouis M, ElBatal H. Comparative studies of IR spectra, optical and thermal properties of binary CdO–B2O3, SrO–B2O3, and BaO–B2O3. Silicon. 2017;9:703–710. [Google Scholar]
- 7.Zhu H, Fu R, Agathopoulos S, Fang J, Li G, He Q. Crystallization behaviour and properties of BaO–CaO–B2O3–SiO2 glasses and glass-ceramics for LTCC applications. Ceram. Int. 2018;44:10147–10153. [Google Scholar]
- 8.Marzouk M, ElBatal F, ElBatal H. Effect of TiO2 on the optical, structural and crystallization behavior of barium borate glasses. Opt. Mater. 2016;57:14–22. [Google Scholar]
- 9.Rüssel C, Tauch D, Garkova R, Woltz S, Völksch G. Phase separation and crystallisation in borate glasses. Phys. Chem. Glass. Eur. J. Glass Sci. Technol. B. 2006;47:397–404. [Google Scholar]
- 10.Hovhannisyan RM. Binary alkaline earth borates: Phase diagram correction and low thermal expansion of crystallised stoichiometric glass compositions. Phys. Chem. Glass. Eur. J. Glass Sci. Technol. B. 2006;47:460–464. [Google Scholar]
- 11.Pevzner B, Klyuev V, Polyakova I, Borodzyulya V. Peculiar properties of barium diborate polycrystals. Phys. Chem. Glass. Eur. J. Glass Sci. Technol. B. 2006;47:534–537. [Google Scholar]
- 12.Kasuga T. Bioactive calcium pyrophosphate glasses and glass-ceramics. Acta Biomater. 2005;1:55–64. doi: 10.1016/j.actbio.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Monem AS, ElBatal HA, Khalil EM, Azooz MA, Hamdy YM. In vivo behavior of bioactive phosphate glass-ceramics from the system P2O5–Na2O-CaO containing TiO2. J. Mater. Sci. Mater. Med. 2008;19:1097–1108. doi: 10.1007/s10856-007-3044-3. [DOI] [PubMed] [Google Scholar]
- 14.Rajyasree C, Rao DK. Spectroscopic properties of RBiBO4 (R = Ca, Sr) glasses doped with TiO2. J. Mol. Struct. 2012;1007:168–174. [Google Scholar]
- 15.Środa M, Świontek S, Gieszczyk W, Bilski P. The effect of CeO2 on the thermal stability, structure and thermoluminescence and optically stimulated luminescence properties of barium borate glass. J. Non-Cryst. Solids. 2019;517:61–69. [Google Scholar]
- 16.Baumann TF, Worsley MA, Han TY-J, Satcher JH. High surface area carbon aerogel monoliths with hierarchical porosity. J. Non-Cryst. Solids. 2008;354:3513–3515. [Google Scholar]
- 17.Tauch D, Rüssel C. Glass-ceramics in the system BaO/TiO2 (ZrO2)/Al2O3/B2O3 and their thermal expansion. J. Non-Cryst. Solids. 2007;353:2109–2114. [Google Scholar]
- 18.Wisniewski W, Schröter B, Zscheckel T, Rüssel C. A global glassy layer on BaAl2B2O7 crystals formed during surface crystallization of BaO· Al2O3· B2O3 glass. Cryst. Growth Des. 2012;12:1586–1592. [Google Scholar]
- 19.Shaalan M, El-Damrawi G, Hassan A, Misbah MH. Structural role of Nd2O3 as a dopant material in modified borate glasses and glass ceramics. J. Mater. Sci. Mater. Electron. 2021;32:12348–12357. [Google Scholar]
- 20.Volf MB. Chemical approach to glass. Glass Sci. Technol. 1984;7:465–469. [Google Scholar]
- 21.Kohli J, Shelby J. Formation and properties of rate earth aluminosilicate glasses. Phys. Chem. Glass. 1991;32:67–71. [Google Scholar]
- 22.Sebaï M, Penot C, Goursat P, Liddell K, Thompson D, Nestor E, et al. Oxidation resistance of Nd–Si–Al–ON glasses and glass-ceramics. J. Eur. Ceram. Soc. 1998;18:169–182. [Google Scholar]
- 23.Tanabe S, Hirao K, Soga N. Elastic properties and molar volume of rare-earth aluminosilicate glasses. J. Am. Ceram. Soc. 1992;75:503–506. [Google Scholar]
- 24.Ramesh R, Nestor E, Pomeroy M, Hampshire S, Liddell K, Thompson D. Potential of Nd–Si–Al–ON glasses for crystallisation to glass-ceramics. J. Non-Cryst. Solids. 1996;196:320–325. [Google Scholar]
- 25.Snitzer E. Lasers and glass technology. Am. Ceram. Soc. Bull. 1973;52:516–525. [Google Scholar]
- 26.Thaddeus BM. Binary Alloy Phase Diagrams. 2. Springer; 1990. pp. 2705–2708. [Google Scholar]
- 27.Mitang W, Cheng J, Mei L. Effect of rare earths on viscosity and thermal expansion of soda-lime-silicate glass. J. Rare Earths. 2010;28:308–311. [Google Scholar]
- 28.Gabbott P. Principles and Applications of Thermal Analysis. Wiley; 2008. [Google Scholar]
- 29.Pernice P, Esposito S, Aronne A, Sigaev V. Structure and crystallization behavior of glasses in the BaO–B2O3–Al2O3 system. J. Non-Cryst. Solids. 1999;258:1–10. [Google Scholar]
- 30.Kimura H, Sato M, Shimamura K, Fukuda T. Viscosity and surface tension change in BaB2O4 melt by substitution of Al or Ga for B. J. Mater. Sci. Lett. 1997;16:911–913. [Google Scholar]
- 31.Lu, S.-F., He, M.-Y., Huang, J.-L. Crystal structure of the low temperature form of barium barate Ba3(B3O6)2 (2005).
- 32.Jantzen C, Neurgaonkar R. Solid state reactions in the system Al2O3 Nd2O3 CaO: A system pertinent to radioactive waste disposal. Mater. Res. Bull. 1981;16:519–524. [Google Scholar]
- 33.Holand W, Beall G. Glass-Ceramic Technology. Wiley; 2020. [Google Scholar]
- 34.Sigaev V, Lopatina E, Sarkisov P, Stefanovich SY, Molev V. Grain-oriented surface crystallization of lanthanum borosilicate and lanthanum borogermanate glasses. Mater. Sci. Eng. B. 1997;48:254–260. [Google Scholar]
- 35.Cheng Y, Shu X, Wen M, Lu Y, Tan P, Lu X, et al. Immobilize Nd2O3 as simulated nuclear waste in silicate-apatite glass-ceramics. Process. Saf. Environ. Protect. 2023;171:783–793. [Google Scholar]
- 36.Gao H, Liu X, Chen J, Qi J, Wang Y, Ai Z. Preparation of glass-ceramics with low density and high strength using blast furnace slag, glass fiber and water glass. Ceram. Int. 2018;44:6044–6053. [Google Scholar]
- 37.Zhang H, Liu J, Shi F, Zhang H, Yuan X, Li Y, et al. Controlling the microstructure and properties of lithium disilicate glass-ceramics by adjusting the content of MgO. Ceram. Int. 2023;49:216–225. [Google Scholar]
- 38.Huang X, Zhao W, Guo H, Yan B, Li P, Li C. Crystallization enhancement and microstructure evolution characteristics of Ti-bearing blast furnace slag glass-ceramics with the introduction of ferrochromium slag. Ceram. Int. 2023;49:9708–9718. [Google Scholar]
- 39.Ma X, Li Q, Xie L, Chang C, Li H. Effect of Ni addition on the properties of CoMoPB bulk metallic glasses. J. Non-Cryst. Solids. 2022;587:121573. [Google Scholar]
- 40.Li Z, Ma G, Zheng D, Zhang X, Muvunyi RA. Effect of ZnO on the crystallization behavior and properties of SiO2–CaO–Al2O3–Fe2O3 glass-ceramics prepared from simulated secondary slag after reduction of copper slag. Ceram. Int. 2022;48:21245–21257. [Google Scholar]
- 41.Abo-Mosallam H, Mahdy EA. Effect of strontium on crystallization characteristics and properties of ZnO-Fe2O3-B2O3-P2O5 glass-ceramics for biomedical applications. J. Non-Cryst. Solids. 2022;583:121467. [Google Scholar]
- 42.Gautam S, Gupta DC. Analysis of structural, electro-magnetic, mechanical, thermodynamic, and thermoelectric properties of potassium based perovskites. J. Magn. Magn. Mater. 2023;572:170593. [Google Scholar]
- 43.Das A, Sahu S, Mohapatra M, Verma S, Bhattacharyya AJ, Basu S. Lithium-ion conductive glass-ceramic electrolytes enable safe and practical Li batteries. Mater. Today Energy. 2022;29:101118. [Google Scholar]
- 44.Grava A-M, Marian M, Grava C, Curilă S, Trip N-D. Bond-graph analysis and modelling of a metal detector as an example of electro-magnetic system. Ain Shams Eng. J. 2023;14:102204. [Google Scholar]
- 45.Maia LJ, Bernardi MI, Zanatta AR, Hernandes AC, Mastelaro VR. β-BaB2O4 nanometric powder obtained from the ternary BaO–B2O3–TiO2 system using the polymeric precursor method. Mater. Sci. Eng. B. 2004;107:33–38. [Google Scholar]
- 46.Ferreira LH, Dantelle G, Ibanez A, Maia LJ. Thermal sensitivity of the Nd3+/Yb3+ co-doped β-BaB2O4 nanoparticles for luminescence nanothermometry. Physica B. 2022;644:414193. [Google Scholar]
- 47.Abo-Mosallam H, Salama S, Salman S. Synthesis and characterization of some molybdenum-containing glass-ceramics. J. Austral. Ceram. Soc. 2021;57:1291–1299. [Google Scholar]
- 48.Ali A, Shaaban M. Electrical properties of LiBBaTe glass doped with Nd2O3. Solid State Sci. 2010;12:2148–2154. [Google Scholar]
- 49.Mortazavi SR, Karimzadeh F, Emadi R, Ahmadvand H. Synthesis and evaluation of a glass-ceramic system containing zinc ferrite nanocrystals. J. Non-Cryst. Solids. 2021;559:120704. [Google Scholar]
- 50.Bartek N, Shvartsman VV, Salamon S, Wende H, Lupascu DC. Influence of calcination and sintering temperatures on dielectric and magnetic properties of Pb (Fe0.5Nb0.5)O3 ceramics synthesized by the solid state method. Ceram. Int. 2021;47:23396–23403. [Google Scholar]
- 51.Feng H, Huang J, Wang X, Li J, Yin X, Xu Z, et al. Microstructure and enhanced electromagnetic wave absorbing performance of Zn0.6Ni0.3Cu0.1Fe2O4 ferrite glass-ceramic. Ceram. Int. 2022;48:9090–9098. [Google Scholar]
- 52.Kaur J, Kaur P, Mudahar I, Singh K. Effect of Sm2O3 on the physical, structural and optical properties of 40 SiO2–40 B2O3–10 V2O5-(10–x) Fe2O3 glasses. Ceram. Int. 2022;49:13610–13617. [Google Scholar]
- 53.Abo-Mosallam H, Farag M. The impact of NiO on crystallization and thermo-magnetic properties of Li2O–NiO–P2O5 glasses as new magnetic materials. J. Mater. Sci. Mater. Electron. 2023;34:602. [Google Scholar]
- 54.Sembiring T, Sasniati P, Muljadi SP, Sebayang K. Study of physical and magnetic properties of barium hexaferrite substituted by Nd2O3. AIP Conf. Proc. 2020;2020:110025. [Google Scholar]
- 55.Sardjono P, Sembiring T. Synthesis and characterization of Ba-Ferrite with variation of Nd2O3 additive by powder metallurgy method. J. Phys. Conf. Ser. 2019;1:012016. [Google Scholar]
- 56.Cullity BD, Graham CD. Introduction to Magnetic Materials. Wiley; 2011. [Google Scholar]
- 57.Bretcanu O, Spriano S, Verné E, Cöisson M, Tiberto P, Allia P. The influence of crystallised Fe3O4 on the magnetic properties of coprecipitation-derived ferrimagnetic glass–ceramics. Acta Biomater. 2005;1:421–429. doi: 10.1016/j.actbio.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 58.Salman S, Salama S, Abo-Mosallam H. The effect of aluminum and germanium oxides on the crystallization process and magnetic properties of Li2O–Fe2O3–SiO2 glass system. Ceram. Int. 2015;41:1521–1529. [Google Scholar]
- 59.Mahdy EA, Khattari ZY, Salem WM, Ibrahim S. Study the structural, physical, and optical properties of CaO–MgO–SiO2–CaF2 bioactive glasses with Na2O and P2O5 dopants. Mater. Chem. Phys. 2022;286:126231. [Google Scholar]
- 60.Muiva CM, Sathiaraj ST, Mwabora JM. Crystallisation kinetics, glass forming ability and thermal stability in glassy Se100-xInx chalcogenide alloys. J. Non-Cryst. Solids. 2011;357:3726–3733. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials are all available and prepared, authors will be pleased to provide it if requested during the publication process. The corresponding author (M.A. Azooz) has a permission from other authors to reply if someone wants any data from this study.



