Abstract
Visfatin is a multifunctional protein which, besides the control of energy homeostasis, seems to be also involved in the regulation of female fertility through the influence on the endocrine hypothalamus-pituitary-gonadal axis, including the pituitary. The aim of this study was to investigate the expression of visfatin mRNA and protein in the anterior (AP) and posterior pituitary lobes of the pig during the oestrous cycle and early pregnancy. In AP, we also examined colocalisation of visfatin with pituitary tropic hormones. Moreover, we aimed to evaluate the in vitro effects of GnRH, FSH, LH, and insulin on visfatin protein concentration and secretion in AP cells during the cycle. The study showed that visfatin is present in all types of porcine pituitary endocrine cells and its expression is reliant on stage of the cycle or pregnancy. GnRH, FSH, LH and insulin stimulated visfatin secretion by AP cells on days 17 to 19 of the cycle, while on days 2 to 3 visfatin release was enhanced only by LH. Summarising, visfatin is locally produced in the pituitary in a way dependent on hormonal milieu typical for reproductive status of pigs. Further research is required to clarify the role of visfatin in the pituitary gland.
Subject terms: Reproductive biology, Homeostasis
Introduction
The adipose tissue, considered as a place of energy storage, is also an endocrine organ. It secretes a group of hormones called adipokines1. One of them, nicotinamide phosphoribosyltransferase (NAMPT), also termed visfatin, was identified in 2005 by Fukuhara et al.2. Visfatin is a 56 kDa multifunctional protein which occurs in two forms: extracellular (eNAMPT), acting as a hormone, and intracellular (iNAMPT), which is involved in the synthesis of nicotinamide dinucleotide adenine3. No visfatin receptor has yet been identified, but it was suggested that visfatin may bind and activate the insulin receptor (for review see Grolla et al.4). Additionally, chemokine receptor CCR55 and toll-like receptor 46 were indicated as potential visfatin receptors. Visfatin plays an important role in the regulation of energy homeostasis, inflammation, cell differentiation7 and angiogenesis8,9. Visfatin, taking part in the control of energy homeostasis, seems to be also involved in the regulation of female fertility. A positive correlation was noted between the hormone concentration in the ovarian follicular fluid of women and the number of oocytes retrieved10. Similarly in mice, visfatin increased the potential of fertility and developmental competence of oocytes11. It is possible that this effect is achieved to some extent by the participation of visfatin in the regulation of ovarian steroidogenesis12–14. The involvement of visfatin in the regulation of uterine contractions, implantation and placentation is also suggested15,16.
It seems that visfatin, apart from its direct effect on the reproductive system, may additionally influence the endocrine hypothalamus-pituitary-gonadal axis (HPG), including the pituitary, what has not been investigated so far. Visfatin is expressed in all structures of the HPG axis: in the hypothalamus of pigs17 and mice18, in the pituitary gland of mice19 and sheep20, as well as in the ovarian follicular cells of humans12, buffaloes14 and pigs21. Its expression is hormonally controlled. In human granulosa cells, visfatin gene (NAMPT) expression was increased in response to hCG and prostaglandin E210. Also in our recent studies on pig luteal cells, we found the effect of luteinising hormone (LH), progesterone (P4), insulin (INS), and prostaglandins E2 and F2α on visfatin protein expression. The visfatin expression in response to the treatments was dependent on the endocrine milieu related to the oestrous cycle22. It seems that also hormonal status related to pregnancy may affect visfatin production, which is strongly suggested by the increase in visfatin plasma concentration with advancing gestational age of woman23.
We hypothesised that the expression of visfatin in the pituitary gland is dependent on the hormonal status of animals. Therefore, the aim of this study was to investigate the visfatin gene expression and protein concentration in the anterior (AP) and posterior pituitary (NP) lobes of the pig during the oestrous cycle (days 2 to 3–the early-luteal phase, 10 to 12–the mid-luteal phase, the phase in which the steroidogenic activity of the corpus luteum is the highest throughout the cycle and similar to its activity observed during pregnancy, 14 to 16–the late-luteal phase and 17 to 19–the follicular phase) and early pregnancy (days 10 to 11–the migration of the embryos within the uterus, 12 to 13–the maternal recognition of pregnancy, 15 to 16–the beginning of implantation and 27 to 28–the end of implantation). In AP lobe, we also examined colocalisation of visfatin with LH, follicle-stimulating hormone (FSH), adrenocorticotrophic hormone (ACTH), thyroid-stimulating hormone (TSH), prolactin (PRL) and growth hormone (GH) on days 10 to 12 of the oestrous cycle. Moreover, we aimed to evaluate the in vitro effects of gonadotrophin-releasing hormone (GnRH) – the main regulator of pituitary gonadotrophs, FSH and LH – hormones produced by these cells, and INS – energy homeostatic signal, on visfatin protein concentration and secretion in AP cells (APc) during the oestrous cycle.
Results
Gene and protein expression of visfatin in the anterior pituitary gland
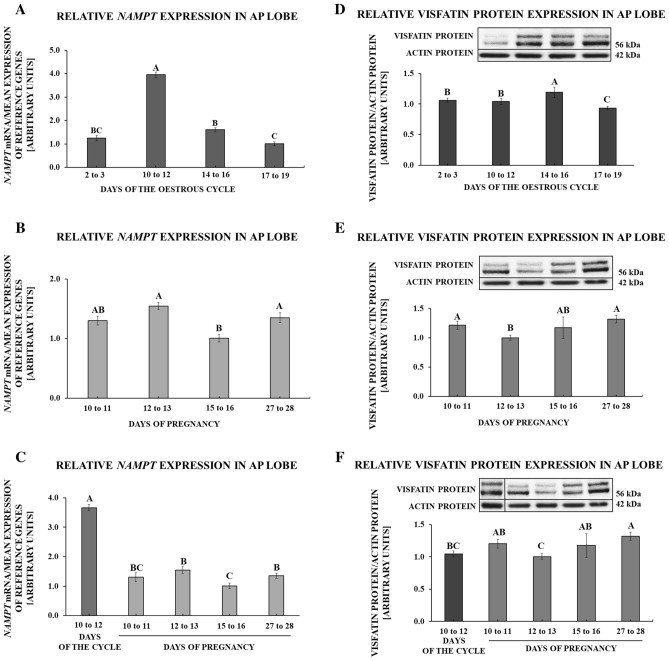
During the oestrous cycle, the greatest expression of visfatin gene was observed on days 10 to 12 (p < 0.001). On days 17 to 19, the expression was decreased relative to days 10 to 12 and 14 to 16 of the cycle (p < 0.001; Fig. 1A). During pregnancy, NAMPT expression was suppressed on days 15 to 16 in comparison to days 12 to 13 and 27 to 28 (p = 0.005; Fig. 1B). Comparing visfatin gene expression throughout the early pregnancy with days 10 to 12 of the oestrous cycle, visfatin mRNA content during all periods of pregnancy was significantly lesser (p < 0.001; Fig. 1C).
Figure 1.
Visfatin gene (NAMPT) and protein expression in the anterior pituitary lobe (AP) of pigs. Relative expression of visfatin mRNA and protein determined using quantitative real-time PCR and Western blot procedures, respectively, in AP tissues collected on days 2 to 3, 10 to 12, 14 to 16, 17 to 19 of the oestrous cycle (A,D), days 10 to 11, 12 to 13, 15 to 16, 27 to 28 of pregnancy (B,E) and days of early pregnancy compared to days 10 to 12 of the oestrous cycle (C,F). Right side of the figure: upper panels contain representative immunoblots (all bands shown in the figure come from the same blot; for an additional comparison at point F, where the concentrations of proteins during chosen periods of the cycle/pregnancy were compared, the artificially arranged bands were separated by a vertical line; uncropped images of visfatin and actin immunoblots are attached in the Supplementary File 1), lower panels demonstrate densitometric analysis of expression of visfatin protein relative to actin protein; results are means ± S.E.M (n = 5). Values associated with bars with different superscripts are different (one way ANOVA followed by Tukey post hoc test), capital letters indicate p < 0.05.
During the oestrous cycle, the greatest expression of visfatin protein was observed on days 14 to 16, whereas the lowest was on days 17 to 19 (p < 0.001; Fig. 1D). During pregnancy, visfatin protein expression was decreased on days 12 to 13 in comparison to days 10 to 11 and 27 to 28 (p = 0.002; Fig. 1E). Comparing visfatin protein abundance throughout the early pregnancy with days 10 to 12 of the oestrous cycle, it was found the increase of the protein concentration on days 27 to 28 of pregnancy (p < 0.001; Fig. 1F).
Gene and protein expression of visfatin in the posterior pituitary gland
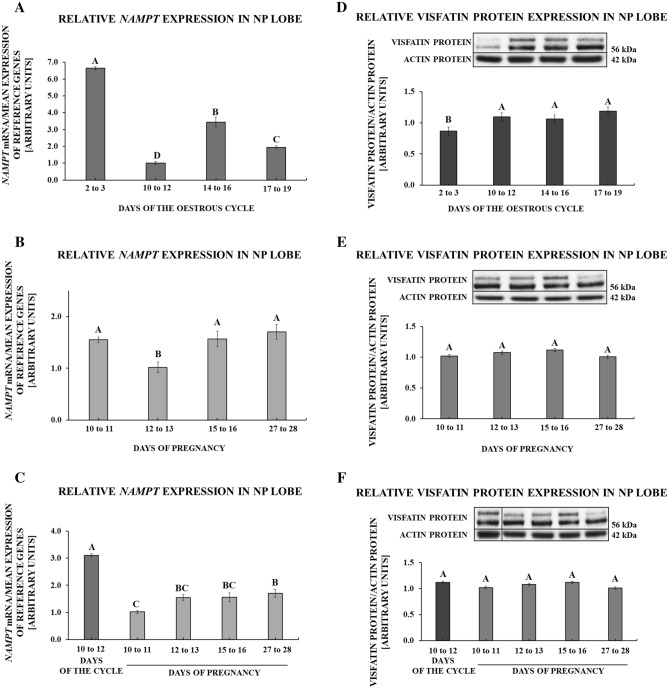
During the oestrous cycle, the greatest expression of visfatin gene was observed on days 2 to 3 (p < 0.001). We observed lesser values on days 14 to 16 and 17 to 19, while the lowest expression of visfatin gene was on days 10 to 12 of the cycle (p < 0.001; Fig. 2A). During pregnancy, NAMPT expression was decreased on days 12 to 13 relative to other studied stages (p = 0.005; Fig. 2B). Comparing NAMPT expression throughout the early pregnancy with days 10 to 12 of the oestrous cycle, visfatin mRNA content during all periods of pregnancy was significantly suppressed (p < 0.001; Fig. 2C).
Figure 2.
Visfatin gene (NAMPT) and protein expression in the posterior pituitary lobe (NP) of pigs. Relative expression of visfatin mRNA and protein determined using quantitative real-time PCR and Western blot procedures, respectively, in NP tissues collected on days 2 to 3, 10 to 12, 14 to 16, 17 to 19 of the oestrous cycle (A,D), days 10 to 11, 12 to 13, 15 to 16, 27 to 28 of pregnancy (B,E) and days of early pregnancy compared to days 10 to 12 of the oestrous cycle (C,F). Right side of the figure: upper panels contain representative immunoblots (all bands shown in the figure come from the same blot; for an additional comparison at point F, where the concentrations of proteins during chosen periods of the cycle/pregnancy were compared, the artificially arranged bands were separated by a vertical line; uncropped images of visfatin and actin immunoblots are attached in the Supplementary File 1), lower panels demonstrate densitometric analysis of expression of visfatin protein relative to actin protein; results are means ± S.E.M (n = 5). Values associated with bars with different superscripts are different (one way ANOVA followed by Tukey post hoc test), capital letters indicate p < 0.05.
During the oestrous cycle, the lowest expression of visfatin protein was observed on days 2 to 3 in comparison to the other days (p < 0.001; Fig. 2D). During pregnancy, visfatin protein expression was constant (p = 0.076; Fig. 2E). We observed no differences in the protein content of visfatin between days 10 to 12 of the cycle and stages of early pregnancy (p = 0.159; Fig. 2F).
The distribution of visfatin in the porcine pituitary cells
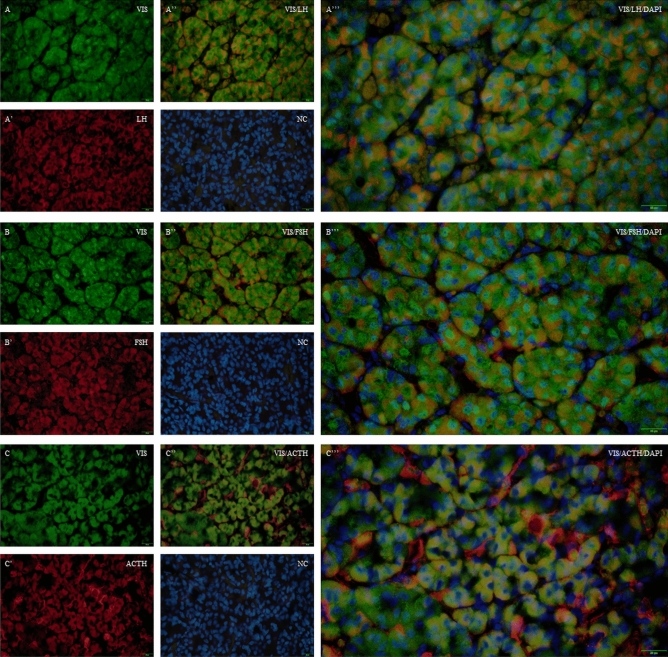
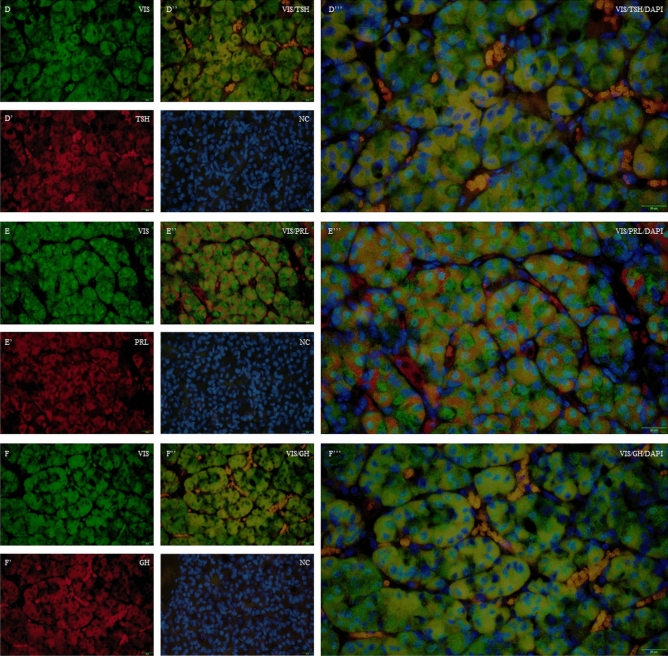
We confirmed the localisation of visfatin in LH-immunoreactive (IR) cells, FSH-IR cells, ACTH-IR cells, TSH-IR cells, PRL-IR cells, GH-IR cells in the porcine pituitaries collected during the oestrous cycle (days 10 to 12; Figs. 3 and 4).
Figure 3.
Immunofluorescence localisation of VIS and LH, FSH or ACTH in the porcine pituitary cells collected on days 10 to 12 of the oestrous cycle. Immunofluorescence labelling of VIS, DAPI (nuclear staining, NC) and LH (A–A’’’), FSH (B–B’’’), and ACTH (C–C’’’) in the porcine pituitary on days 10 to 12 of the oestrous cycle. Magnification of 40x, scale bar 20 µm. VIS visfatin, LH luteinising hormone, FSH follicle-stimulating hormone, ACTH adrenocorticotrophic hormone, NC negative control.
Figure 4.
Immunofluorescence localization of VIS and TSH, PRL or GH in the porcine pituitary cells collected on days 10 to 12 of the oestrous cycle. Immunofluorescence labelling of VIS, DAPI (nuclear staining, NC) and TSH (D–D’’’), PRL (E–E’’’), and GH (F–F’’’) in the porcine pituitary on days 10 to 12 of the oestrous cycle. Magnification of 40x, scale bar 20 µm. VIS visfatin, TSH thyroid-stimulating hormone, PRL prolactin, GH growth hormone, NC negative control.
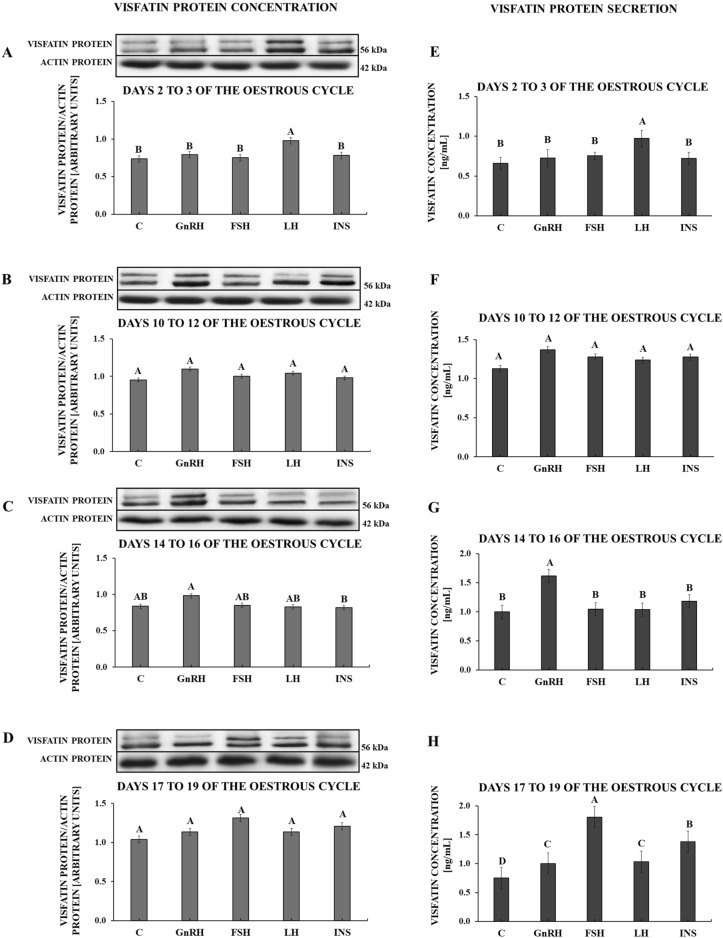
Determination of GnRH, FSH, LH and INS impact on visfatin protein expression and secretion by APc during the oestrous cycle
On days 2 to 3 of the oestrous cycle, only LH stimulated visfatin protein content in APc (p = 0.002; Fig. 5A) as well as visfatin secretion by these cells (p < 0.001; Fig. 5E). On days 10 to 12 of the oestrous cycle, none of the tested hormones affected visfatin protein expression (p = 0.138; Fig. 5B) and visfatin secretion (p = 0.120; Fig. 5F). On days 14 to 16 of the oestrous cycle, none of the tested hormones had any influence on the content of visfatin protein (p = 0.031; Fig. 5C), but only GnRH had the stimulatory effect on visfatin secretion (p < 0.001; Fig. 5G). On days 17 to 19 of the oestrous cycle, no influence of the tested hormones on the content of visfatin protein in APc was observed (p = 0.057; Fig. 5D). However, each of these hormones stimulated visfatin secretion (p < 0.001; Fig. 5H).
Figure 5.
The effect of GnRH (100 ng/mL), FSH (100 ng/mL), LH (100 ng/mL) and INS (10 ng/mL) on visfatin protein expression (A–D) and visfatin secretion by the porcine pituitary cells (E–H) during the oestrous cycle. The visfatin protein expression was analysed by Western blot. Results are shown as representative immunoblots (each of the panels represents one blot; uncropped images of visfatin and actin immunoblots are attached in the Supplementary File 1) and bar graphs with densitometry measurement of relative visfatin protein content normalised with actin protein. Visfatin concentration in culture media was evaluated based on ELISA assay. Results are means ± S.E.M (n = 5). Values associated with bars with different superscripts are different; capital letters indicate p < 0.05. C control, GnRH gonadotrophin-releasing hormone, FSH follicle-stimulating hormone, LH luteinising hormone, INS insulin.
Discussion
The presented research was the first experiment to report the expression of visfatin gene and concentration of visfatin protein in the porcine pituitary during the oestrous cycle and early pregnancy, as well as visfatin cellular localisation in this gland. Moreover, we indicated the impact of GnRH, FSH, LH and INS on visfatin protein expression in the in vitro cultured anterior pituitary cells and visfatin secretion by these cells.
We noted that the highest visfatin mRNA abundance was observed in AP lobe during the mid-luteal phase, while in NP, the highest NAMPT expression was observed during the early-luteal phase of the cycle. Moreover, our study demonstrated the highest concentration of visfatin protein in AP lobe during the late-luteal phase and the lowest during the follicular phase of the oestrous cycle. This, in turn, implies that visfatin expression is tissue-specific and dependent on the phase of the oestrous cycle. Interestingly, visfatin was not detected in NP of the mouse pituitary and dependence between AP visfatin expression and the phase of the cycle was not found in this species19 which suggests that this phenomenon is also species dependent. The porcine pituitary gland, thanks to the presence of both classical oestrogen and progesterone receptors and non-classical membrane receptors, is sensitive to the action of oestradiol (E2) and P424. Approximately 70% of pituitary gonadotrophs, responsible for LH and FSH generation, express oestrogen receptors in different species25–27. Of note, the sensitivity of the gland to steroids changes throughout the cycle. The study by Diekman and Anderson28 indicated that the number of cytoplasmic oestrogen and P4 receptors in the porcine pituitary is many-fold lower on day 18 compared to days 1 to 15 of the cycle. Thus, the observed in our study variations in transcript and protein levels of visfatin in porcine pituitary lobes could result from the hormonal status of animals related to plasma steroids’ concentrations and the gland sensitivity to them.
The visfatin gene and protein expression patterns in AP and NP are different, in particular during the oestrous cycle, which suggests that the regulation of visfatin synthesis and secretion in both pituitary lobes is also different. AP and NP lobes are morphologically and functionally distinct, but it is known that NP affects AP functioning. While AP lobe is populated mainly by six types of cells: somatotrophs, gonadotrophs, lactotrophs, thyrotrophs, corticotrophs, and folliculostellate cells29, the posterior pituitary includes pituicytes that affect AP hormone secretion by releasing cytokines, growth factors and other neuroactive compounds30 and it also consists of oxytocin- and vasopressin-secreting neuroendocrine terminals. The ability of porcine NP to synthesize other adipokines, such as adiponectin31 and chemerin32 is known, but there is no information, apart from the current work, on the possibility of visfatin synthesis. This requires further in-depth research.
During early pregnancy, both visfatin gene and protein expression in AP and NP were relatively constant. It pays attention, however, decreased NAMPT expression in the pituitary lobes from pregnant animals compared to days 10 to 12 of the cycle. It is worth noting that according to Diekman and Anderson28, the number of oestrogen and P4 receptors in the pituitaries of pregnant pigs (days 5 to 15) is essentially lower that during the cycle, indicating lesser sensitivity of the gland to sex steroids. It cannot be ruled out that one of factors controlling the number of pituitary steroid receptors can be also visfatin itself. Such an effect of visfatin on oestrogen receptors α and β (ERα and ERβ) was observed in the mice ovary33.
In addition to the mentioned earlier, presumed effect of visfatin on the pituitary expression of steroid receptors, the adipokine is also able to regulate its own synthesis. It was found that in the process of the circadian rhythm control of some cell functions, NAMPT gene transcription is regulated by a feedback loop involving visfatin itself34,35. The exposure of rat pituitary gland explants to visfatin increased the concentration of NAMPT mRNA36. Thus, it seems that in pituitary cells, the process of autoregulation of visfatin synthesis is active and it is one of elements controlling the final expression of the adipokine.
Relationship between the visfatin expression and the phase of the oestrous cycle was also noted in other structures of the HPG axis and organs of the reproductive system. Changes in the expression of visfatin in the porcine hypothalamus during the oestrous cycle and early pregnancy were reported in our earlier study17. Visfatin gene and protein expression in the hypothalamic structures involved in GnRH synthesis, mediobasal hypothalamus and preoptic area, were dependent on hormonal status related to the phase of the oestrous cycle or early pregnancy and affected by steroid hormones. Analogous observations pertaining to the phase-specific visfatin expression have been made for the ovary of pigs22, mice37 and water buffaloes14. Visfatin protein expression in the porcine corpora lutea was regulated by LH, P4, INS, and prostaglandins E2 and F2α22. Similarly, in the mouse uterus, visfatin expression was steroid dependent with stimulatory effect of E2 and inhibitory of P437.
In this study, we also examined the colocalisation of visfatin protein with LH, FSH, ACTH, TSH, PRL and GH. We observed the presence of visfatin in gonadotrophs, corticotrophs, thyrotrophs, lactotrophs and somatotrophs. Maillard et al.19 confirmed visfatin protein localisation in the anterior and intermediate parts of female mice, mainly in gonadotrophic cells, but not in NP lobe. The presence of visfatin in these cells may suggest its auto/paracrine influence on pituitary cells’ functions.
In the next part of the experiment, we examined the effect of GnRH, FSH, LH and INS on the content and secretion of visfatin protein in AP during the oestrous cycle noting the phase-dependent influence of these hormones. The stimulatory effect of GnRH on visfatin secretion, but no content, was observed on days 14 to 16 and 17 to 19 of the cycle, when P4 plasma level is decreased and E2 enhanced. It is known that P4 is a negative regulator of pituitary GnRH receptors38–41, whereas E2 increased the receptor expression39,42–44. Of note, GnRH receptors have been identified in gonadotrophs, thyrotrophs and somatotrophs45 and the number of gonadotrophs containing ERα, and, consequently, sensitivity to oestrogen action, increases in the follicular phase of the cycle46. It is suggested, however, that the most notable factor inducing GnRH receptors’ expression is GnRH itself (for review see Stamatiades and Kaiser47). Thus, the increased number of pituitary GnRH receptors in the follicular phase in different species, including the pig48, may result mainly from enhanced plasma GnRH concentration and pulse frequency. In addition, insulin can directly promote gonadotrophs’ response to GnRH stimulation49–51. Insulin, apart from its own receptors, is able to bind also insulin-like growth factor 1 (IGF-1) receptors52,53, which number increases in the rat pituitary at prooestrous54. This may justify to some extent the GnRH action seen at the end of the cycle and not in the early- and mid-luteal phase. The effect of GnRH on visfatin secretion seems to be limited to certain types of APc and takes place in the strictly defined physiological status.
In this study, LH stimulated the content and secretion of visfatin protein in the early-luteal phase. Moreover, FSH and LH enhanced the adipokine secretion in the follicular phase, which may be related to the high physiological blood plasma concentration of both gonadotrophins at the turn of two cycles55. The influence of pituitary hormones, including FSH and LH, on visfatin secretion by this gland has been completely unknown until now. It seems however, that there are physiological conditions for such an effect to occur. FSH and LH receptors are localised first of all in gonadal tissues, nevertheless the extragonadal localisation of both receptors in different species was indicated56–60. This also applies to the pituitary gland60–63. It is suggested the direct LH effect on AP through auto feedback action64,65. The ability of LH to down-regulate its own receptors is known66. It therefore seems possible that locally produced pituitary hormones, such as FSH and LH, auto/paracrinaly, in a phase-dependent manner, are able to influence visfatin secretion. The presented research should be an impulse for further studies of the impact of gonadotrophins and other pituitary tropic hormones on the gland secretory activity.
It is known that APc, including gonadotrophs, constitute target cells of INS, which may regulate their secretory functions67 acting as energy homeostatic signal50. Both INS and IGF-1 receptors are present in APc of different species50,51,68,69. The deletion of insulin receptor substrate-2, a component of the insulin/IGF-1 signalling cascade, causes female infertility through, i.a., disturbances in pituitary functions, leading to reduced numbers of gonadotrophs and decreased LH concentration70. As in the case of GnRH, reproductive status influenced the visfatin response to INS. Its effect on the visfatin secretion was observed in this study during the follicular phase. It seems plausible, that the reason for this is mentioned earlier an increase in IGF-1 receptors’ concentration observed in the phase of plasma E2 domination54. Oestrogens can enhance IGF-1 receptors’ concentration in APc of different species, including the pig71. Moreover, feedback mechanism between IGF-1 and E2 is suggested. Oestrogens may sensitise APc to IGF-1 and IGF-1 can up-regulate E2 receptors’ expression52. The insulin influence on visfatin secretion was also noted in other tissues – human adipocytes increased the adipokine release in response to INS72.
It is worth mentioning that the effect of GnRH, gonadotrophins and INS on visfatin protein expression and its secretion was usually different. While the adipokine secretion in the follicular phase was stimulated by these hormones, the protein abundance was unaffected by them. This effect could result from the existence of two forms of visfatin, iNAMPT and eNAMPT29, that could be regulated differently. What is more, the extracellular form is approximately 1% of the total NAMPT (for review see Carbone et al.73). Since the observed effect of the studied factors is more related to the extracellular form of visfatin, it seems that the physiological response of APc to their action is mainly the release of visfatin acting as a hormone, and not a change in the expression of visfatin protein acting as an enzyme.
The role of visfatin in the pituitary is known to a very limited extent. It was found the inhibitory influence of visfatin on LH secretion by the LβT2 gonadotroph cell line. Moreover, in the same study by Maillard et al.19, the increase of LβT2 cells’ proliferation, in response to visfatin, was noted. Other studies using rat corticotrophs have shown that visfatin is able to stimulate ACTH release both directly and indirectly, by the enhancement of interleukin-6 release from folliculostellate cells of the pituitary gland74. These results may explain the observed earlier by the same research team increase of pituitary proopiomelanocortin (ACTH precursor) mRNA concentration under the influence of visfatin36. Generally however, the role of visfatin in the pituitary, especially its extracellular form, is almost completely unexplored and requires further studies.
The study has shown the dependence of visfatin protein expression in the pituitary on the phase of the oestrous cycle or stage of pregnancy. The visfatin secretion by APc was affected by GnRH, FSH, LH and INS. The obtained results suggest that visfatin is locally produced in the porcine pituitary in a way reliant on hormonal milieu typical for the reproductive status of pigs. Further research is required to clarify the role of visfatin in the pituitary gland.
Methods
Experimental animals and tissue collection
Pigs bound for commercial slaughter and meat processing were used in this experiment. Experimental animals were mature cross-breed gilts at the age of 7–8 months and the body weight of 130–150 kg. The diet of the animals was in line with the Polish nutritional standards for domestic pigs. Gilts were monitored daily for oestrus behaviour in the presence of a boar. The phase of the oestrous cycle was also confirmed based on the ovarian morphology characteristic according to Akins and Morrissette75.The day of the onset of the second oestrus was marked as day 0 of the oestrous cycle. Natural insemination was performed on days 1 to 2 of the cycle. The stage of pregnancy (days 15 to 16 and 27 to 28) was additionally confirmed based on the morphology of conceptuses/trophoblasts76. A few minutes after the slaughter, the pituitary glands (separated into AP and NP lobes) were frozen in liquid nitrogen and stored at 80 °C until processing for RNA and protein isolation. The samples assigned to both the quantitative real-time PCR (qPCR) and Western blot were collected from the same animals at the same time. AP glands utilised for in vitro cell culture were collected and placed in the ice-cold Dulbecco’s Phosphate-Buffered Saline with 100 IU/mL penicillin and 100 μg/mL streptomycin, and transported to the laboratory on ice. Additionally, the pituitaries obtained on days 10 to 12 of the cycle, intended for immunofluorescent staining, were placed in 4% buffered paraformaldehyde (pH = 7.4, 4°C).
Isolation of APc and in vitro cultures
APc were isolated according to the method described by Kiezun et al.31 with modifications. In brief, isolation of the cells was performed through the digestion of the pituitary lobes with 0.2% collagenase (Merck, USA) at 37 °C for 30 min, and then the cells were digested with 0.2% collagenase and 0.25% pancreatin (Merck, USA) in cycles of 10 min until the whole tissue was dispersed. The remaining steps of the procedure and cell preincubation were carried out in accordance with the indicated reference. After preincubation, the media were removed and the cells were rinsed with fresh serum-free McCoy’s 5A medium. The cells were incubated for another 24 h (37 °C, 5% CO2 and 95% air) in the presence of the treatments: GnRH (100 ng/mL), FSH (100 ng/mL), LH (100 ng/mL), or INS (10 ng/mL). The cells cultured with medium alone were used as control samples. Insulin and GnRH concentrations were determined based on Kiezun et al.31 and Gavin et al.77, respectively. Concentrations of FSH and LH were chosen based on Gregoraszczuk et al.78. The potential effects of treatments on the cells’ viability were determined using the Alamar Blue test, which revealed that the cultured APc were not affected by the applied treatments. After incubation, media were collected and centrifuged at 800×g, supernatants were collected and stored at -20 °C, and the cells were used to isolate the total protein.
Total RNA isolation, cDNA synthesis and quantitative real-time PCR
RNA isolation, cDNA synthesis and qPCR reactions were performed as described previously17. In the case of qPCR, the conditions and characteristics of primers used in the study are detailed in Table 1. Constitutive expression of reference genes (UBC and 18sRNA) was confirmed statistically. Relative gene expression of visfatin was calculated using the 2−ΔΔCT method according to Livak and Schmittgen79.
Table 1.
Primers specification, reaction mixture composition and reaction conditions used in the study for the quantitative real-time PCR.
| Gene symbol | Primer sequence | Accession number | Reaction mixture composition | Reaction conditions | Reference |
|---|---|---|---|---|---|
| NAMPT |
F: 5′-CCAGTTGCTGATCCCAACAAA-3′ R: 5′-AAATTCCCTCCTGGTGTCCTATG-3′ |
XM_003132281.5 |
Power SYBR Green—12.5 µL cDNA—20 ng Forward primer—300 nM Reverse primer—300 nM H2O—up to a total volume of 20 μL |
Activation and initial denaturation: 95 °C, 10 min 40 cycles of: denaturation: 95 °C, 15 s annealing: 60 °C, 1 min |
80 |
| UBC |
F: 5′-GGAGGAATCTACTGGGGCGG-3′ R: 5′-CAGAAGAAACGCAGGCAAACT-3′ |
XM_003483411.3 |
Power SYBR Green—12.5 µL cDNA—20 ng Forward primer—400 nM Reverse primer—400 nM H2O—up to a total volume of 20 μL |
Activation and initial denaturation: 95 °C, 10 min 40 cycles of: denaturation: 95 °C, 15 s annealing: 60 °C, 1 min elongation: 72 °C, 1 min |
81 |
| 18sRNA |
F: 5′-TCCAATGGATCCTCGCGGAA-3′ R: 5′-GGCTACCACATCCAAGGAAG-3′ |
AY265350.1 |
NAMPT visfatin, UBC ubiquitin C, 18sRNA 18 s ribosomal RNA, F forward primer, R reverse primer.
Western blot
Western blot analysis was conducted similarly as described previously17. Protein isolation was performed using Tissue Protein Extraction Reagent (T-PER; Thermo Fisher Scientific, USA) according to the manufacturer's instructions. Then, samples in the amount of 30 µg of protein (per sample) were used for SDS-PAGE electrophoresis. Protein transfer was performed by semi-dry technique, and blots were blocked with 5% BSA. Specification of antibodies used in the study are detailed in Table 2. The actin protein was used as the reference protein. Constitutive accumulation of actin protein was confirmed statistically. The immunocomplexes were visualised using Immobilon Western Chemiluminescent HRP Western blotting Luminol Reagent (Advansta Inc., USA) and archived using the Chemidoc™ XRS + System (BioRad Laboratories Inc., USA). Specific bands were quantified using the densitometer and ImageJ software (US National Institutes of Health, USA). Data were expressed as the ratio of visfatin protein relative to actin proteins in arbitrary optical density units.
Table 2.
Specifications of antibodies used in the study for the Western blot analysis.
| Antibodies | Host species | Supplier and catalog number | Dilution | |
|---|---|---|---|---|
| Primary | Anti-visfatin | Rabbit | Abcam, UK; cat. no. ab233294 | 1:700 |
| Anti-β-actin | Mouse | Merck, USA; cat. no. A5316 | 1:5000 |
| Antibodies | Type of conjugated enzyme | Supplier and catalog number | Dilution | |
|---|---|---|---|---|
| Secondary | Goat anti-rabbit | HRP | Cell Signaling Technology, USA; cat. no. 7074 | 1:1000 |
| Horse anti-mouse | HRP | Cell Signaling Technology, USA; cat. no. 7076 | 1:1000 |
HRP horseradish peroxidase.
The analysis of visfatin localisation in the porcine pituitary gland using fluorescent immunohistochemistry
Fluorescent immunohistochemistry analysis of colocalisation was conducted on 5 μm thick paraffin pituitary sections (n = 3). To expose the antigen epitopes, sections were boiled with Antigen Retrieval Solution. For the reduction of nonspecific background staining, the sections were incubated with 50 mM NH4CL in phosphate-buffered saline (PBS). Subsequently, sections were permeabilised using 0.1% Triton™ X-100 and the nonspecific sites of antibodies binding were blocked through 1.5 h incubation with Fish Serum Blocking Buffer (cat. no. 37527; Thermo Fisher Scientific, USA). Next, the slides were rinsed with 0.1 M PBS and incubated with proper antibodies (specification of antibodies used in the study are detailed in Table 3). For negative control, the primary antibodies were omitted, and slides were incubated with 0.1 M PBS. The nonspecific background noise was reduced through 20 min. incubation of slides with 0.5% Sudan Black B dissolved in 70% EtOH. The obtained sections were air-dried and covered with histology mounting medium Fluoroshield™ (cat. no. F6057; Merck, USA) with DAPI for nuclear counterstaining. The labelled sections were analysed with the use of Olympus BX51 research microscope (Olympus, Japan) equipped with an EXFO x-CiteSeries 120Q fluorescence illuminator (Excelitas Technologies Corp., USA) using appropriate filters set for DAPI and Alexa Fluor® dyes. Images were acquired with the use of Nikon DS-Qi2 microscope digital camera (Nikon, Japan) and NIS-Elements (v. 5.10) imaging software (Nikon, Japan).
Table 3.
Specifications of antibodies used in the study for the fluorescent immunohistochemistry.
| Antibodies | Host species | Supplier and catalog number | Dilution | |
|---|---|---|---|---|
| Primary | Anti-visfatin | Rabbit | Abcam, UK; cat. no. ab233294 | 1:150 |
| Anti-LH | Mouse | Abcam, UK; cat. no. ab212578 | 1:200 | |
| Anti-FSH | Mouse | Abcam, UK; cat. no. ab233866 | 1:150 | |
| Anti-ACTH | Mouse | Abcam, UK; cat. no. ab212736 | 1:500 | |
| Anti-TSH | Mouse | R&D, USA; cat. no. MAB57941 | 1:200 | |
| Anti-PRL | Mouse | Abcam, UK; cat. no. ab11301 | 1:200 | |
| Anti-GH | Mouse | Abcam, UK; cat. no. ab218405 | 1:200 |
| Antibodies | Type of conjugated fluorophore | Supplier and catalog number | Dilution | |
|---|---|---|---|---|
| Secondary | Goat anti-rabbit | Alexa Fluor® 488 | Jackson ImmunoResearch Labs, UK; cat. no. 111-545-003 | 1:1000 |
| Goat anti-mouse | Alexa Fluor® 594 | Jackson ImmunoResearch Labs, UK; cat. no. 115-585-003 | 1:1000 |
LH luteinising hormone, FSH follicle-stimulating hormone, ACTH adrenocorticotrophic hormone, TSH thyroid-stimulating hormone, PRL prolactin, GH growth hormone.
ELISA assay
The concentration of visfatin in the culture medium was determined using a commercial ELISA kit (cat. no. MBS736963; MyBioSource, USA) as described previously17. The intra- and inter-assay coefficients of variability for performed analysis were 6.03% and 6.17%, respectively.
Statistical analysis
Statistical analyses were performed using Statistica program (StatSoft Inc., USA). All data were tested for the assumption’s normality (Shapiro–Wilk test) and homogeneity of variances (Levene's test), and analysed by one-way ANOVA followed by the Tukey’s honest significance post hoc test. Data were presented as means ± S.E.M. from five independent observations. Values for p < 0.05 were considered statistically significant.
Ethics declarations
The studies were carried out following the Polish Act on the Protection of Animals Used for Scientific or Educational Purposes of the 15th of January 2015 (Journal of Laws Dz. U. 2015 No. item 266) as well as Directive 2010/63/EU of the European Parliament and the Council of the 22nd of September 2010 on the protection of animals used for scientific purposes. Hence, this study did not require the consent of the competent ethics committee for animal experiments.
Supplementary Information
Acknowledgements
This research was financially supported by the National Science Centre, Poland (Project No. 2018/31/B/NZ9/00781). The Society for Reproductive Biology in Poland financed the research stay for Ewa Mlyczynska at the Professor Tadeusz Kaminski laboratory in the Department of Animal Anatomy and Physiology, Faculty of Biology and Biotechnology, University of Warmia and Mazury in Olsztyn in 2020.
Author contributions
Conceptualisation, K.S. and T.K.; methodology, K.S., E.Z., E.M., P.K. and E.R.; formal analysis, K.S., E.Z., K.D. and M.K.; investigation, K.S., E.M., E.Z. and E.R.; resources T.K.; data curation, K.S., E.Z. and T.K.; writing-original draft preparation, K.S.; writing-review and editing, K.S., E.R., B.K., T.K., N.S. and A.R.; visualization, K.S. and E.R.; supervision, T.K.; project administration, T.K. and A.R.; funding acquisition, T.K. and A.R. All authors have read and agreed to the published version of the manuscript.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-45255-4.
References
- 1.Lehr S, Hartwig S, Sell H. Adipokines: A treasure trove for the discovery of biomarkers for metabolic disorders. Proteom. Clin. Appl. 2012;6:91–101. doi: 10.1002/prca.201100052. [DOI] [PubMed] [Google Scholar]
- 2.Fukuhara A, et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 3.Adeghate E. Visfatin: Structure, function and relation to diabetes mellitus and other dysfunctions. Curr. Med. Chem. 2008;15:1851–1862. doi: 10.2174/092986708785133004. [DOI] [PubMed] [Google Scholar]
- 4.Grolla AA, Travelli C, Genazzani AA, Sethi JK. Extracellular nicotinamide phosphoribosyltransferase, a new cancer metabokine. Br. J. Pharmacol. 2016;173:2182–2194. doi: 10.1111/bph.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van den Bergh R, et al. Monocytes contribute to differential immune pressure on R5 versus X4 HIV through the adipocytokine visfatin/NAMPT. PLoS One. 2012;7:e35074. doi: 10.1371/journal.pone.0035074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camp SM, et al. Unique toll-like receptor 4 activation by NAMPT/PBEF induces NFκB signaling and inflammatory lung injury. Sci. Rep. 2015;5:13135. doi: 10.1038/srep13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavlová T, Novák J, Bienertová-Vašků J. The role of visfatin (PBEF/Nampt) in pregnancy complications. J. Reprod. Immunol. 2015;112:102–110. doi: 10.1016/j.jri.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Lovren F, et al. Visfatin activates eNOS via Akt and MAP kinases and improves endothelial cell function and angiogenesis in vitro and in vivo: Translational implications for atherosclerosis. Am. J. Physiol. Endocrinol. Metab. 2009;296:E1440–E1449. doi: 10.1152/ajpendo.90780.2008. [DOI] [PubMed] [Google Scholar]
- 9.Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: Novel insights into visfatin-induced angiogenesis. Cardiovasc. Res. 2008;78:356–365. doi: 10.1093/cvr/cvm111. [DOI] [PubMed] [Google Scholar]
- 10.Shen C-J, et al. The concentrations of visfatin in the follicular fluids of women undergoing controlled ovarian stimulation are correlated to the number of oocytes retrieved. Fertil. Steril. 2010;93:1844–1850. doi: 10.1016/j.fertnstert.2008.12.090. [DOI] [PubMed] [Google Scholar]
- 11.Choi K-H, et al. Administration of visfatin during superovulation improves developmental competency of oocytes and fertility potential in aged female mice. Fertil. Steril. 2012;97:1234–1241.e3. doi: 10.1016/j.fertnstert.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Reverchon M, et al. Visfatin is expressed in human granulosa cells: regulation by metformin through AMPK/SIRT1 pathways and its role in steroidogenesis. Mol. Hum. Reprod. 2013;19:313–326. doi: 10.1093/molehr/gat002. [DOI] [PubMed] [Google Scholar]
- 13.Reverchon M, et al. VISFATIN (NAMPT) improves in vitro IGF1-induced steroidogenesis and IGF1 receptor signaling through SIRT1 in Bovine granulosa cells. Biol. Reprod. 2016 doi: 10.1095/biolreprod.115.134650. [DOI] [PubMed] [Google Scholar]
- 14.Thakre A, et al. Transcriptional and translational abundance of visfatin (NAMPT) in buffalo ovary during estrous cycle and its in vitro effect on steroidogenesis. Domest. Anim. Endocrinol. 2021;75:106583. doi: 10.1016/j.domaniend.2020.106583. [DOI] [PubMed] [Google Scholar]
- 15.Reverchon M, Ramé C, Bertoldo M, Dupont J. Adipokines and the female reproductive tract. Int. J. Endocrinol. 2014;2014:232454. doi: 10.1155/2014/232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazaki-Tovi S, et al. Evidence for differential regulation of the adipokine visfatin in the maternal and fetal compartments in normal spontaneous labor at term. J. Perinat. Med. 2010;38:281–288. doi: 10.1515/JPM.2010.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaminski T, et al. Plasma level and expression of visfatin in the porcine hypothalamus during the estrous cycle and early pregnancy. Sci. Rep. 2021;11:8698. doi: 10.1038/s41598-021-88103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guia RM, et al. Fasting- and ghrelin-induced food intake is regulated by NAMPT in the hypothalamus. Acta Physiol. 2020;228:e13437. doi: 10.1111/apha.13437. [DOI] [PubMed] [Google Scholar]
- 19.Maillard V, et al. Visfatin and resistin in gonadotroph cells: Expression, regulation of LH secretion and signalling pathways. Reprod. Fertil. Dev. 2017;29:2479. doi: 10.1071/RD16301. [DOI] [PubMed] [Google Scholar]
- 20.Dupré SM, et al. Identification of melatonin-regulated genes in the ovine pituitary pars tuberalis, a target site for seasonal hormone control. Endocrinology. 2008;149:5527–5539. doi: 10.1210/en.2008-0834. [DOI] [PubMed] [Google Scholar]
- 21.Mlyczyńska E, et al. Expression of visfatin in the ovarian follicles of prepubertal and mature gilts and in vitro effect of gonadotropins, insulin, steroids, and prostaglandins on visfatin levels. Theriogenology. 2023;211:28–39. doi: 10.1016/j.theriogenology.2023.07.040. [DOI] [PubMed] [Google Scholar]
- 22.Mlyczyńska E, et al. Expression and regulation of visfatin/NAMPT in the porcine corpus luteum during the estrous cycle and early pregnancy. Anim. Reprod. Sci. 2023;250:107212. doi: 10.1016/j.anireprosci.2023.107212. [DOI] [PubMed] [Google Scholar]
- 23.Mastorakos G, et al. The role of adipocytokines in insulin resistance in normal pregnancy: Visfatin concentrations in early pregnancy predict insulin sensitivity. Clin. Chem. 2007;53:1477–1483. doi: 10.1373/clinchem.2006.084731. [DOI] [PubMed] [Google Scholar]
- 24.He J, et al. Zearalenone and alpha-zearalenol inhibit the synthesis and secretion of pig follicle stimulating hormone via the non-classical estrogen membrane receptor GPR30. Mol. Cell. Endocrinol. 2018;461:43–54. doi: 10.1016/j.mce.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Liu Z, Yi S, Cui S. Islet-1 expression and its colocalisation with luteinising hormone, thyroid-stimulating hormone and oestrogen receptor alpha in the developing pituitary gland of the sheep foetus. J. Neuroendocrinol. 2005;17:773–780. doi: 10.1111/j.1365-2826.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- 26.Yuan X, et al. Expression of androgen receptor and its co-localization with estrogen receptor-alpha in the developing pituitary gland of sheep fetus. Histochem. Cell Biol. 2007;127:423–432. doi: 10.1007/s00418-006-0262-6. [DOI] [PubMed] [Google Scholar]
- 27.Sun D, et al. Cell-specific distributions of estrogen receptor alpha (ERα) and androgen receptor (AR) in anterior pituitary glands from adult cockerels as revealed by immunohistochemistry. Cell Tissue Res. 2012;348:551–558. doi: 10.1007/s00441-012-1399-3. [DOI] [PubMed] [Google Scholar]
- 28.Diekman MA, Anderson LL. Quantification of receptors for estradiol-17β and progesterone in the pituitary and hypothalamus of guts during the estrous cycle and early pregnancy. Biol. Reprod. 1983;29:946–952. doi: 10.1095/biolreprod29.4.946. [DOI] [PubMed] [Google Scholar]
- 29.Santiago-Andres Y, Golan M, Fiordelisio T. Functional pituitary networks in vertebrates. Front. Endocrinol. 2021 doi: 10.3389/fendo.2020.619352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boersma CJC, Van Leeuwen FW. Neuron-glia interactions in the release of oxytocin and vasopressin from the rat neural lobe: The role of opioids, other neuropeptides and their receptors. Neuroscience. 1994;62:1003–1020. doi: 10.1016/0306-4522(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 31.Kiezun M, et al. Adiponectin expression in the porcine pituitary during the estrous cycle and its effect on LH and FSH secretion. Am. J. Physiol. Endocrinol. Metab. 2014;307:E1038–E1046. doi: 10.1152/ajpendo.00299.2014. [DOI] [PubMed] [Google Scholar]
- 32.Kisielewska K, et al. Expression of chemerin receptors CMKLR1, GPR1 and CCRL2 in the porcine pituitary during the oestrous cycle and early pregnancy and the effect of chemerin on MAPK/Erk1/2 Akt and AMPK signalling pathways. Theriogenology. 2020;157:181–198. doi: 10.1016/j.theriogenology.2020.07.032. [DOI] [PubMed] [Google Scholar]
- 33.Annie L, Gurusubramanian G, Kumar Roy V. Visfatin protein may be responsible for suppression of proliferation and apoptosis in the infantile mice ovary. Cytokine. 2021;140:155422. doi: 10.1016/j.cyto.2021.155422. [DOI] [PubMed] [Google Scholar]
- 34.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD + salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramsey KM, et al. Circadian clock feedback cycle through NAMPT-mediated NAD + biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Celichowski P, et al. Mitochondrial sirtuins in the rat adrenal gland: Location within the glands of males and females, hormonal and developmental regulation of gene expressions. Folia Histochem. Cytobiol. 2017;55:190–202. doi: 10.5603/FHC.a2017.0020. [DOI] [PubMed] [Google Scholar]
- 37.Annie L, Gurusubramanian G, Roy VK. Estrogen and progesterone dependent expression of visfatin/NAMPT regulates proliferation and apoptosis in mice uterus during estrous cycle. J. Steroid Biochem. Mol. Biol. 2019;185:225–236. doi: 10.1016/j.jsbmb.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Weiss JM, Polack S, Treeck O, Diedrich K, Ortmann O. Regulation of GnRH I receptor gene expression by the GnRH agonist triptorelin, estradiol, and progesterone in the gonadotroph-derived cell line αT3-1. Endocrine. 2006;30:139–144. doi: 10.1385/ENDO:30:1:139. [DOI] [PubMed] [Google Scholar]
- 39.Nett TM, Turzillo AM, Baratta M, Rispoli LA. Pituitary effects of steroid hormones on secretion of follicle-stimulating hormone and luteinizing hormone. Domest. Anim. Endocrinol. 2002;23:33–42. doi: 10.1016/s0739-7240(02)00143-1. [DOI] [PubMed] [Google Scholar]
- 40.Cheon M, et al. Progesterone together with estrogen attenuates homologous upregulation of gonadotropin-releasing hormone receptor mRNA in primary cultured rat pituitary cells. Endocrine. 2000;13:379–384. doi: 10.1385/ENDO:13:3:379. [DOI] [PubMed] [Google Scholar]
- 41.Bauer-Dantoin AC, Weiss J, Jameson JL. Roles of estrogen, progesterone, and gonadotropin-releasing hormone (GnRH) in the control of pituitary GnRH receptor gene expression at the time of the preovulatory gonadotropin surges. Endocrinology. 1995;136:1014–1019. doi: 10.1210/endo.136.3.7867555. [DOI] [PubMed] [Google Scholar]
- 42.Quiñones-Jenab V, et al. Estrogen regulation of gonadotropin-releasing hormone receptor messenger RNA in female rat pituitary tissue. Brain Res. Mol. Brain Res. 1996;38:243–250. doi: 10.1016/0169-328x(95)00322-j. [DOI] [PubMed] [Google Scholar]
- 43.Nett TM, Crowder ME, Wise ME. Role of estradiol in inducing an ovulatory-like surge of luteinizing hormone in sheep. Biol. Reprod. 1984;30:1208–1215. doi: 10.1095/biolreprod30.5.1208. [DOI] [PubMed] [Google Scholar]
- 44.Wu JC, Sealfon SC, Miller WL. Gonadal hormones and gonadotropin-releasing hormone (GnRH) alter messenger ribonucleic acid levels for GnRH receptors in sheep. Endocrinology. 1994;134:1846–1850. doi: 10.1210/endo.134.4.8137751. [DOI] [PubMed] [Google Scholar]
- 45.Sanno N, et al. Gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor messenger ribonucleic acids expression in nontumorous and neoplastic pituitaries 1. J. Clin. Endocrinol. Metab. 1997;82:1974–1982. doi: 10.1210/jcem.82.6.3976. [DOI] [PubMed] [Google Scholar]
- 46.Tobin VA, Pompolo S, Clarke IJ. The percentage of pituitary gonadotropes with immunoreactive oestradiol receptors increases in the follicular phase of the ovine oestrous cycle. J. Neuroendocrinol. 2001;13:846–854. doi: 10.1046/j.1365-2826.2001.00701.x. [DOI] [PubMed] [Google Scholar]
- 47.Stamatiades GA, Kaiser UB. Gonadotropin regulation by pulsatile GnRH: Signaling and gene expression. Mol. Cell. Endocrinol. 2018;463:131–141. doi: 10.1016/j.mce.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clapper J, Taylor A. Components of the porcine anterior pituitary insulin-like growth factor system throughout the estrous cycle. Domest. Anim. Endocrinol. 2011;40:67–76. doi: 10.1016/j.domaniend.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Brothers KJ, et al. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010;12:295–305. doi: 10.1016/j.cmet.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navratil AM, et al. Insulin augments gonadotropin-releasing hormone induction of translation in LβT2 cells. Mol. Cell. Endocrinol. 2009;311:47–54. doi: 10.1016/j.mce.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buggs C, et al. Insulin augments GnRH-stimulated LHβ gene expression by Egr-1. Mol. Cell. Endocrinol. 2006;249:99–106. doi: 10.1016/j.mce.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia Y, Weiss J, Polack S, Diedrich K, Ortmann O. Interactions of insulin-like growth factor-I, insulin and estradiol with GnRH-stimulated luteinizing hormone release from female rat gonadotrophs. Eur. J. Endocrinol. 2001 doi: 10.1530/eje.0.1440073. [DOI] [PubMed] [Google Scholar]
- 53.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis*. Endocr. Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 54.Michels KM, Lee WH, Seltzer A, Saavedra JM, Bondy CA. Up-regulation of pituitary [125I]insulin-like growth factor-I (IGF-I) binding and IGF binding protein-2 and IGF-I gene expression by estrogen. Endocrinology. 1993;132:23–29. doi: 10.1210/endo.132.1.7678216. [DOI] [PubMed] [Google Scholar]
- 55.Van De Wiel DFM, Erkens J, Koops W, Vos E, Van Landeghem AAJ. Periestrous and midluteal time courses of circulating LH, FSH, prolactin, estradiol-17β and progesterone in the domestic pig. Biol. Reprod. 1981;24:223–233. doi: 10.1095/biolreprod24.2.223. [DOI] [PubMed] [Google Scholar]
- 56.Stilley JAW, Segaloff DL. FSH actions and pregnancy: Looking beyond ovarian FSH receptors. Endocrinology. 2018;159:4033–4042. doi: 10.1210/en.2018-00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stilley JAW, et al. FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice1. Biol. Reprod. 2014 doi: 10.1095/biolreprod.114.118562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blair JA, Bhatta S, McGee H, Casadesus G. Luteinizing hormone: Evidence for direct action in the CNS. Horm. Behav. 2015;76:57–62. doi: 10.1016/j.yhbeh.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdallah MA, et al. Human fetal nongonadal tissues contain human chorionic gonadotropin/luteinizing hormone receptors. J. Clin. Endocrinol. Metab. 2004;89:952–956. doi: 10.1210/jc.2003-030917. [DOI] [PubMed] [Google Scholar]
- 60.Apaja PM, Harju KT, Aatsinki JT, Petäjä-Repo UE, Rajaniemi HJ. Identification and structural characterization of the neuronal luteinizing hormone receptor associated with sensory systems. J. Biol. Chem. 2004;279:1899–1906. doi: 10.1074/jbc.M311395200. [DOI] [PubMed] [Google Scholar]
- 61.Yang E-J, Nasipak BT, Kelley DB. Direct action of gonadotropin in brain integrates behavioral and reproductive functions. Proc. Natl. Acad. Sci. U.S.A. 2007;104:2477–2482. doi: 10.1073/pnas.0608391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lei ZM, Rao CV, Kornyei JL, Licht P, Hiatt ES. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology. 1993;132:2262–2270. doi: 10.1210/endo.132.5.8477671. [DOI] [PubMed] [Google Scholar]
- 63.Pawlikowski M, Pisarek H, Kubiak R, Jaranowska M, Stępień H. Immunohistochemical detection of FSH receptors in pituitary adenomas and adrenal tumors. Folia Histochem. Cytobiol. 2012;50:325–330. doi: 10.5603/17850. [DOI] [PubMed] [Google Scholar]
- 64.Patritti-Laborde N, Odell WD. Short-loop feedback of luteinizing hormone: Dose-response relationships and specificity. Fertil. Steril. 1978;30:456–460. [PubMed] [Google Scholar]
- 65.Hirono M, Igarashi M, Matsumoto S. Short-and auto-feedback mechanism of LH. Endocrinol. Jpn. 1971;18:175–178. doi: 10.1507/endocrj1954.18.175. [DOI] [PubMed] [Google Scholar]
- 66.Peegel H, Randolph J, Midgley AR, Menon KM. In situ hybridization of luteinizing hormone/human chorionic gonadotropin receptor messenger ribonucleic acid during hormone-induced down-regulation and the subsequent recovery in rat corpus luteum. Endocrinology. 1994;135:1044–1051. doi: 10.1210/endo.135.3.8070346. [DOI] [PubMed] [Google Scholar]
- 67.Adashi EY, Hsueh AJW, Yen SSC. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology. 1981;108:1441–1449. doi: 10.1210/endo-108-4-1441. [DOI] [PubMed] [Google Scholar]
- 68.Luque RM, Kineman RD. Impact of obesity on the growth hormone axis: Evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology. 2006;147:2754–2763. doi: 10.1210/en.2005-1549. [DOI] [PubMed] [Google Scholar]
- 69.Unger JW, Lange W. Insulin receptors in the pituitary gland: Morphological evidence for influence on opioid peptide-synthesizing cells. Cell Tissue Res. 1997;288:471–483. doi: 10.1007/s004410050833. [DOI] [PubMed] [Google Scholar]
- 70.Burks DJ, et al. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. 2000;407:377–382. doi: 10.1038/35030105. [DOI] [PubMed] [Google Scholar]
- 71.Rempel LA, Clapper JA. Administration of estradiol-17β increases anterior pituitary IGF-I and relative amounts of serum and anterior pituitary IGF-binding proteins in barrows. J. Anim. Sci. 2002;80:214–224. doi: 10.2527/2002.801214x. [DOI] [PubMed] [Google Scholar]
- 72.Haider DG, et al. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia. 2006;49:1909–1914. doi: 10.1007/s00125-006-0303-7. [DOI] [PubMed] [Google Scholar]
- 73.Carbone F, et al. Regulation and function of extracellular nicotinamide phosphoribosyltransferase/visfatin. Compr. Physiol. 2017;7:603–621. doi: 10.1002/cphy.c160029. [DOI] [PubMed] [Google Scholar]
- 74.Celichowski P, et al. Extracellular Nampt (eNampt/Visfatin/PBEF) directly and indirectly stimulates ACTH and CCL2 protein secretion from isolated rat corticotropes. Adv. Clin. Exp. Med. 2021;30:967–980. doi: 10.17219/acem/136172. [DOI] [PubMed] [Google Scholar]
- 75.Akins R, Morrissette J. Gross ovarian changes during estrous cycle of swine. Am. J. Vet. Res. 1968;29:1953–1957. [PubMed] [Google Scholar]
- 76.Anderson LL. Reproductive biology of pigs. Iowa State Univ Anim. Ind. Rep. 2009 doi: 10.31274/ans_air-180814-838. [DOI] [Google Scholar]
- 77.Gavin JR, Roth J, Neville DM, De Meyts P, Buell DN. Insulin-dependent regulation of insulin receptor concentrations: A direct demonstration in cell culture. Proc. Natl. Acad. Sci. U.S.A. 1974;71:84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gregoraszczuk EŁ, Wójtowicz AK, Ptak A, Nowak K. In vitro effect of leptin on steroids’ secretion by FSH- and LH-treated porcine small, medium and large preovulatory follicles. Reprod. Biol. 2003;3:227–239. [PubMed] [Google Scholar]
- 79.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 80.Szeszko K, et al. The influence of adiponectin on the transcriptomic profile of porcine luteal cells. Funct. Integr. Genom. 2016;16:101–114. doi: 10.1007/s10142-015-0470-z. [DOI] [PubMed] [Google Scholar]
- 81.Smolinska N, et al. Expression of chemerin and its receptors in the porcine hypothalamus and plasma chemerin levels during the oestrous cycle and early pregnancy. Int. J. Mol. Sci. 2019;20:3887. doi: 10.3390/ijms20163887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.