Abstract
Early-onset colorectal cancer (CRC), which refers to CRC diagnosed in individuals below the age of 50 years, is a growing health concern that presents unique challenges in diagnosis, treatment, and long-term outcomes. Although approximately 70% of early-onset CRC cases are sporadic, with no apparent family history, approximately 25% have a familial component, and up to 20% may be associated with germline mutations, indicating a higher prevalence compared with the general population. Despite the progress in identifying the environmental, molecular, and genetic risk factors of early-onset CRC, the underlying causes for the global increase in its incidence remain unclear. This comprehensive review aims to provide a thorough analysis of early-onset CRC by examining the trends associated with its incidence, clinical and pathological characteristics, risk factors, molecular and genetic profiles, prognosis and screening strategies. By deepening our understanding of early-onset CRC, significant advances related to improving the outcomes and alleviating the burden of this disease on individuals, families, and healthcare systems can be achieved.
Keywords: colorectal cancer, early-onset colorectal cancer, epidemiology, cancer screening
Introduction
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer-related deaths globally[1]. Recent temporal trends in the incidence and mortality rates of CRC have shown a decline among patients aged ≥50 years mainly in high-income countries[2]. The decline can be explained by the implementation of CRC screening programs, which generally target average-risk populations aged 50-75 years, and the improved treatment strategies that contribute to the decrease in mortality rates[3]. Meanwhile, the incidence and mortality rates in patients with CRCs, especially rectal cancer, have been increasing in patients aged <50 years, which is defined as young-onset CRC or early-onset CRC[4,5]. This trend has attracted significant attention and has prompted extensive research that is focused on early-onset CRC. However, the underlying reasons for the increase in early-onset CRC remain largely unknown. Although the majority of early-onset CRC cases are sporadic and lack a clear genetic predisposition, the exact causes are not identifiable. Although environmental, molecular, and genetic risk factors have been identified in relation to early-onset CRC, they fail to fully account for the observed upward trends. In this review, we explore the epidemiology, clinical and pathological features, risk factors, molecular and genetic features, prognosis, and screening strategies associated with early-onset CRC.
Epidemiology of Early-onset CRC
The increasing incidence of early-onset CRC was initially reported in 2003 based on data from the nine Surveillance, Epidemiology, and End Results (SEER) databases in the United States, covering the period between 1973 and 1999[6]. During this time frame, there was a 17% increase in early-onset colon cancer and a substantial 75% increase in early-onset rectal cancer, whereas late-onset CRC incidence showed a decrease. A subsequent study conducted by the American Cancer Society in 2009, utilizing the 13 SEER databases and examining trends between 1992 and 2005, revealed a continuous rise in the rates of early-onset CRC. The study demonstrated an annual increase in the incidence of early-onset CRC of 1.5% in men and 1.6% in women per 100,000 individuals during this period[7]. Importantly, this recent surge in the incidence of early-onset CRC has been observed globally, predominantly in high-income countries[3,8,9]. A recent study incorporating data from 20 European countries revealed a significant increase in the incidence of early-onset CRC in 14 of those 20 countries and a decrease in Italy only[5]. Additionally, another study revealed that the incidence rates of early-onset CRC were on the rise in nine high-income countries, including Germany, the USA, Australia, Canada, New Zealand, the UK, Denmark, Slovenia, and Sweden. In contrast, the incidence rates of early-onset CRC declined in only three countries, namely, Italy, Austria, and Lithuania[9]. This upward trend in early-onset CRC was also observed in Asian countries, excluding Japan, which already has higher incidence rates of CRC than those observed globally[9]. In Japan, population-based CRC screening has been performed using the fecal immunochemical test (FIT) since 1992. The recommended screening program involves offering an annual two-sample FIT to average-risk individuals aged ≥40 years, and colonoscopy is recommended for individuals with a positive FIT result. The different trend in CRC incidence in Japan, with a decline in individuals aged <50 years, may be influenced by the younger age target for CRC screening. Austria also implemented an earlier initiation age for CRC screening in 2003, starting at 40 years old, using fecal-based testing and colonoscopy[10]. This suggests that early initiation of CRC screening is an effective strategy in addressing the upward trend of incidence of early-onset CRC.
Considering the recent trends, it is estimated that by 2030, the incidence of early-onset colon and rectal cancers will rise by 90% and 124%, respectively[11]. Furthermore, it is projected that approximately 11% of colon cancers and 23% of rectal cancers will occur in individuals aged <50 years in 2030[11]. These projections indicate that the incidence of early-onset CRC will continue to rise in the future globally.
Clinical Features of Early-onset CRC
Clinical features of early-onset CRC differ from those of late-onset CRC, with a higher likelihood of occurrence in the rectum[7,12-14]. A study utilizing the SEER database revealed that early-onset CRC was predominantly diagnosed in the rectum, accounting for 41% and 36% of all male and female patients, respectively. However, as with the increase in age, the proportion of rectal cancer decreased, with rates of 29% in men and 22% in women in the age group of 65-79 years[12]. A recent study conducted in Japan demonstrated similar patterns, indicating a higher prevalence of rectal cancer among patients with early-onset CRC compared with those aged 50-75 and >75 years (50.4%, 43.3%, and 30.4%)[14]. These findings highlight the significance of the anatomical location of early-onset CRC in providing valuable insights into the underlying causative mechanisms. Furthermore, emerging evidence suggests biological differences between right- and left-sided CRC, further emphasizing the importance of considering the anatomical location in understanding the pathophysiology of the disease[15-17].
Patients with early-onset CRC have a higher likelihood of presenting at a more advanced disease stage compared with those with late-onset CRC. Several population-based studies have consistently reported a higher proportion of stage III and IV CRC among individuals with early-onset disease, ranging between 54% and 61.8%[18-20]. Recent research conducted in Japan aligns with this trend, demonstrating that patients with early-onset CRC had a lower proportion of stage I CRC (15.2% vs. 30.3%) and a higher proportion of stage III-IV CRC (64.0% vs. 49.4%) compared with patients aged 50-75 years[14]. These findings emphasize the importance of early detection and improved screening strategies for early-onset CRC to mitigate the potential effect of advanced disease stages on patient outcomes.
It is well established that patients with early-onset CRC are more likely to present with symptoms related to CRC. A study conducted in the USA reported that 86.4% of 1,025 patients with early-onset CRC were symptomatic at the time of diagnosis[21]. Rectal bleeding is the most commonly reported red flag symptom in patients with early-onset CRC, followed by abdominal pain, change in bowel habits, unexplained weight loss, and anemia, which were also frequently reported[22-24]. However, despite the presence of these red flag symptoms, the evaluation and diagnosis of CRC in young individuals may be delayed because of patient- and physician-associated factors. Previous research has indicated that the average delay in presentation, attributed to patient-associated factors, was approximately 6.2 months. Furthermore, 15%-50% of patients experienced a delay in diagnosis due to physician-associated factors[25]. A case-control study focusing on patients with rectal cancer demonstrated a significant difference in the median time to treatment between patients aged <50 years (217 days) and those aged >50 years (29.5 days)[26]. Although some studies have not found an association between delays in diagnosis and worse CRC stage at presentation or 5-year survival[26,27], these findings emphasize the importance of considering CRC as a potential diagnosis in patients aged <50 years. To address these challenges, it is crucial to implement educational initiatives targeting young adults, primary care physicians, and clinicians. By raising awareness and promoting timely diagnosis and intervention, such initiatives can contribute to improving the outcomes for patients with early-onset CRC.
Pathological Features of Early-onset CRC
Patients with early-onset CRC are more likely to present with adverse histopathological features, including poor differentiation and mucinous and signet-ring morphology[28,29]. A comprehensive study analyzing 64,068 patients with early-onset CRC revealed that compared with patients with later-onset CRC, younger patients more frequently exhibited poor differentiation (18% vs. 20.4%) and mucinous and signet-ring morphology (10.8% vs. 12.6%)[18]. These specific histopathological features are known to be associated with microsatellite instability-high (MSI-H) CRC[30]. Given the association between these adverse histopathological features and MSI-H CRC, it is recommended to perform MSI testing in patients displaying such features[31]. This testing can aid in the identification of Lynch syndrome, a hereditary condition associated with a higher risk of developing CRC and other types of cancer. Detecting MSI-H CRC and identifying individuals with Lynch syndrome is crucial for the appropriate management and genetic counseling for patients and their families.
Risk Factors of Early-onset CRC
The majority of patients with CRC have a sporadic onset, accounting for approximately 70% of all cases, whereas only approximately 5% of patients have a germline mutation, and the remaining 25% have a family history of CRC[32,33]. Various risk factors have been identified as potential drivers of early-onset CRC, including diet, lifestyle factors such as alcohol and tobacco use, obesity, and diabetes. Understanding these risk factors is crucial for the effective early detection and management of patients with early-onset CRC.
Consumption of a Western diet, which is characterized by the intake of ultra-processed, high-fat foods, red meat, and low fiber, has been associated with an increased risk of developing early-onset CRC[34-36]. Similarly, both alcohol consumption[37] and tobacco use[38] are well-known risk factors for CRC development, although the exact underlying mechanisms remain largely unknown[39]. Recent studies have highlighted the contribution of these factors to early-onset CRC, particularly when present concurrently[40,41].
Obesity is another established risk factor for CRC or a surrogate marker for other factors such as the increased consumption of red meat or fat-rich diet[34]. A prospective cohort study involving 85,256 women demonstrated that individuals with obesity (body mass index ≥30) had nearly double the risk of developing early-onset CRC compared with women with a normal body mass index[42].
Type 2 diabetes mellitus has also been associated with a significantly increased risk of early-onset CRC. In a Swedish cohort study, patients with type 2 diabetes mellitus diagnosed before the age of 50 years had a 3.5-fold increased risk of early-onset CRC[43]. Another case-control study reported a similar association between type 2 diabetes mellitus and early-onset CRC, with stronger associations observed for uncontrolled and complicated diabetes[44]. The rising prevalence of type 2 diabetes mellitus among younger adults may partially contribute to the increasing incidence of early-onset CRC[45].
By understanding and considering these risk factors, healthcare professionals can enhance their ability to identify individuals at higher risk for early-onset CRC and implement appropriate screening and preventive measures.
Recent research has proposed that gut microbiota plays a role in the pathogenesis of CRC. Studies have demonstrated that the microbiota profiles associated with CRC differ from those found in healthy individuals and are linked to specific mucosal gene expression patterns[46]. Certain microorganisms, including enterotoxigenic Bacteroides fragilis, oral anaerobe Fusobacterium nucleatum, polyketide synthase-expressing Escherichia coli, and enteric fungi Aspergillus rambellii, have been associated with colorectal carcinogenesis[46-48]. The interaction between gut microbes and the host immune system is believed to influence the anticancer immune response[48]. Emerging evidence suggests that the composition of the microbiota affects the development and progression of CRC. Moreover, a recent study demonstrated that classifying CRCs into distinct subgroups based on the oncomicrobial community subtype could provide insights into different clinicomolecular features and outcomes[49]. Further investigations are needed to better understand the relationship between oncomicrobial community subtypes and the behavior of CRC, as well as to explore the potential efficacy of microbiota-targeted interventions. These studies have the potential to uncover novel strategies for preventing and managing CRC by targeting the microbiota and modulating its effect on colorectal carcinogenesis and immune responses.
Molecular and Genetic Features of Early-onset CRC
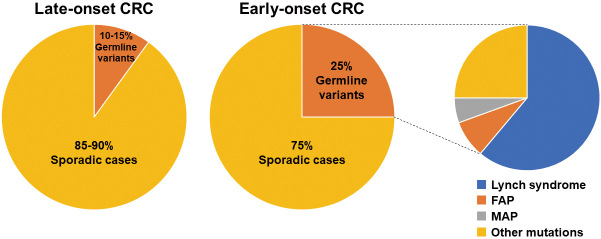
Approximately 25% of patients with early-onset CRC have a family history of CRC in at least one first-degree relative, indicating a potential genetic predisposition[50,51]. The estimated prevalence of germline mutations associated with CRC is approximately 5% of all patients with CRC, including individuals of all age groups[33]. Although there is a lack of population-based studies specifically examining the prevalence of germline variants in patients with early-onset CRC, several studies that focused on resected samples have indicated that the prevalence of germline mutations can be as high as 25% among patients with early-onset CRC, which is higher compared with that of the general population (Figure 1)[50,52,53].
Figure 1.
Prevalence of germline variants in patients with early-onset CRC.
The prevalence of germline variants is shown for early-onset CRC as compared with late-onset CRC.
CRC, colorectal cancer; FAP, familial adenomatous polyposis; MAP, MUTYH-associated polyposis
Lynch syndrome-associated germline variants, including MLH1, MSH2, MSH6, PMS2, and EPCAM, are the most common among hereditary CRC cases, particularly in the context of early-onset CRC[13]. Lynch syndrome, accounting for approximately 10% of early-onset CRC cases, is the most commonly diagnosed hereditary CRC[50,54]. It is characterized by a high lifetime risk of developing CRC (50%-70%) and the onset of CRC before the age of 40 years in 40% of the cases[55]. To ensure early identification and appropriate management, current guidelines recommend universal testing of all newly diagnosed early-onset CRC cases for Lynch syndrome-associated genes[56,57]. This approach has demonstrated high sensitivity (100%) and specificity (93%) in identifying individuals with Lynch syndrome and is considered cost-effective[58]. Germline panel testing using next-generation sequencing should at least include the Lynch syndrome-associated genes.
Heredity polyposis syndromes, although less common than Lynch syndrome, are also associated with an increased risk of early-onset CRC[59]. Familial adenomatous polyposis (FAP), primarily caused by variants in the APC gene, accounts for approximately 1% of all CRC cases. In classic FAP, if left untreated, nearly 100% of individuals develop CRC by the age of 50 years[60]. The offsprings of individuals with FAP have a 50% risk of inheriting the pathogenic variants and associated high risk of early-onset CRC. Indeed, early identification and surveillance are crucial for the prevention and early detection of CRC in patients with FAP. In classic FAP, adenomas can start to form starting from the age of 12 years, with up to 50% of patients developing adenomas by the age of 15 years[61]. Therefore, guidelines recommend 1-3 yearly colonoscopy surveillance starting from 10 to 14 years of age for patients with FAP (Table 1)[62-64].
Table 1.
Colonoscopy Surveillance Recommendations for Individuals with Germline Variants.
| Syndrome | Family history of CRC | Age to begin surveillance (years) | Surveillance interval (years) |
|---|---|---|---|
| No mutation | No | 50 | 10 |
| Yes | 40* | 5-10 | |
| Lynch syndrome | N/A | 20-25 | 1-2 |
| Familial adenomatous polyposis | N/A | 10-15 | 1-3 |
| MUTYH-associated polyposis | N/A | 18-20 | 1-3 |
| Juvenile polyposis | N/A | 12-15 | 1-2 |
| Peutz-Jeghers syndrome | N/A | Baseline: 8 Routine: 18 | 1-3 |
| Cowden syndrome | N/A | 15 | 2 |
*40 years old or 10 years before the age of the youngest first-degree relative diagnosed with CRC.
CRC, colorectal cancer
MUTYH-associated polyposis (MAP), caused by biallelic MUTYH pathogenic variants, is also associated with an increased risk of early-onset CRC. MAP is responsible for <1% of all cases of CRCs[65]. The risk of CRC in patients <60 years with MAP is 48%, and the lifetime risk in nonsurveilled patients is estimated to be 80%-90%[66]. Similar to FAP, scheduled colonoscopy surveillance from 18 to 20 years is recommended in patients with MAP (Table 1)[62-64].
Although germline variants associated with FAP and MAP are more common, other pathogenic variants, such as SMAD4 and BMPR1A (associated with Juvenile polyposis), PTEN (Cowden/multiple hamartoma syndrome), STK11 (Peutz-Jeghers syndrome), POLE, and POLD1 (polymerase proofreading-associated polyposis), are less frequent than FAP and MAP but are also associated with an increased risk of CRC[3,59].
Recent advancements in gene expression-based subtyping have led to the classification of CRC into four consensus molecular subtypes based on distinct molecular characteristics[67]. Among patients with early-onset CRC, CMS1, characterized by hypermutated tumors with MSI and immune activation, is more common. However, CMS3, characterized by metabolic dysregulation, and CMS4, characterized by transforming growth factor-β activation, stromal invasion, and angiogenesis, are less common[3,13]. The role of the immune system in early-onset CRC, particularly within CMS1, is still not fully understood.
The identification of different germline variants associated with CRC has significantly contributed to our understanding of the molecular mechanisms underlying colorectal carcinogenesis. This knowledge will be instrumental in guiding surveillance strategies and personalized management approaches for individuals having a high risk of developing early-onset CRC[68].
Prognosis of Early-onset CRC
The prognosis for patients with early-onset CRC can vary and is influenced by several factors. Although early-onset CRC is often diagnosed at advanced stages, leading to a worse prognosis, younger patients are more likely to receive aggressive treatments, which can improve disease-specific survival[19,69].
Survival data for early-onset CRC are limited and can sometimes yield conflicting outcomes[20,70,71]. However, recent studies have shed some light on the prognosis of early-onset CRC. A large-scale study that included patients with stage II or III CRC treated in clinical trials of adjuvant chemotherapy revealed similar cancer-specific mortality rates between patients with early-onset CRC and those with late-onset CRC. In that study, patients with early-onset CRC had a higher number of metastatic regional lymph nodes but were more likely to complete the planned treatment duration and receive a higher intensity of treatment than patients with late-onset CRC. However, young patients with high risk stage III CRC experienced more frequent disease recurrence despite the higher treatment intensity, suggesting more aggressive disease biology[72]. Another study focusing on stage III colon cancer from 25 randomized studies reported that patients with early-onset colon cancer had better overall survival and survival after recurrence in the analysis without molecular markers[73]. However, when adjusting for molecular markers, such as mismatch repair status, BRAF, and KRAS status, the prognostic value of age at onset was lost. Patients with early-onset colon cancer exhibited more frequent mismatch repair deficiency and were less likely to have BRAF mutation compared with patients with late-onset colon cancer, suggesting a higher rate of Lynch syndrome in the early-onset group than in the late-onset group. That study suggested that tumor biology, including molecular markers, plays a more important role in prognosis than the age of onset among patients with stage III colon cancer. In a large randomized controlled trial involving patients with metastatic CRC, no significant difference in the overall survival and progression-free survival was observed between patients with early-onset CRC and those with late-onset CRC[74]. A recent study from Japan also reported no significant difference in the overall survival between patients with early-onset CRC and those aged 50-75 years[14].
Overall, the stage of CRC at diagnosis and tumor biology, including molecular markers, appear to be more influential prognostic factors than the age of onset. Patients with early-onset CRC may face challenges due to advanced disease at diagnosis, but aggressive treatment approaches can potentially improve their disease-specific survival outcomes.
Screening Strategy for Early-onset CRC
There is strong evidence supporting the effectiveness of cancer screening in reducing the incidence and mortality rates of CRC[75-81]. Multiple screening modalities are available for CRC, including FIT, multitarget stool DNA testing, colonoscopy, sigmoidoscopy, or a combination of these modalities. Many countries have implemented CRC screening programs, typically targeting individuals between the ages of 50 and 75 years[82].
Given the increasing incidence of early-onset CRC, there has been a consideration of lowering the starting age for CRC screening. Microsimulation studies have demonstrated that initiating CRC screening at the age of 45 years provides a favorable balance between screening benefits and burden[83]. In response to this evidence, the American Cancer Society issued a qualified recommendation in 2018 to begin CRC screening at the age of 45 years[84]. Furthermore, the guidelines from the US Preventive Services Task Force were updated in 2021, and the US Multi-Society Task Force on Colorectal Cancer updated their guidelines in 2022, both recommending starting screening at the age of 45 years for individuals with average-risk[85,86]. In contrast, the Asia-Pacific guidelines still maintain the recommended starting age for widespread CRC screening at 50 years[87]. A recent study using Markov modeling analysis suggested that lowering the starting age of population screening for sporadic CRC to 45 years would be cost-effective[88]. Other microsimulation models have also indicated that initiating CRC screening at the age of 45 years could be cost-effective[89,90]. However, it should be noted that lowering the starting age of CRC screening to 45 years would increase the demand for colonoscopy by 3%-14% and could be associated with an additional 55-170 colonoscopies per additional death prevented[89].
Evidence supporting lowering the starting age of CRC screening is primarily based on microsimulation models. Studies confirming the effectiveness of CRC screening in individuals aged 45-49 years are sparse because most countries start CRC screening in individuals from the age of 50 years. However, there are data from Japan where population-based CRC screening has been implemented using annual FIT for individuals aged ≥40 years. A study from Japan revealed that among patients <50 years, screening-detected CRC was associated with a 50% lower risk of mortality compared with nonscreening-detected CRC[14]. The study also reported that cancer-specific survival was worse in the 40-44-year-old group compared with that in the 50-75-year-old group. There was no difference in the cancer-specific survival in the 45-49-year-old group compared with that in the 50-75-year-old group. These findings suggest that CRC screening provides a meaningful survival advantage for individuals aged 45-49 years.
In terms of CRC screening for middle adults aged 40-44 years, the benefits may be less clear compared with those of CRC screening for individuals aged 45-49 years. However, this younger age group could still benefit from earlier screening if the CRC risk is stratified on the basis of factors such as family history, genetic risk, and other lifestyle-related and environmental risks associated with CRC[3,91,92]. Several guidelines recommend initiating CRC screening at 40 years old or 10 years before the age of the youngest first-degree relative diagnosed with CRC, particularly for individuals with a family history of CRC, such as having a first-degree relative diagnosed before the age of 60 years or having two or more first-degree relatives with CRC diagnosed at any age (Table 1)[84-86]. Among patients with early-onset CRC who are aged 40-49 years, 25% meet the family history criteria for early screening, and nearly all the cases who met the criteria could have had CRC diagnosed earlier if earlier screening had been implemented[93]. Obtaining family history data is important in identifying patients at high risk, allowing for the implementation of individualized screening guidelines[94].
Optimizing the screening strategy for early-onset CRC requires the development of precision screening approaches that go beyond age and family history. Incorporating genetic risk factors, lifestyle-related and environmental risk factors, and advancing noninvasive screening methods can help identify high risk individuals and improve early detection and prevention outcomes. Continued research and collaboration among healthcare professionals, researchers, and policymakers are essential to refine the screening guidelines and implement effective strategies for early-onset CRC.
Conclusions
Early-onset CRC is an emerging health concern, with a significant increase in incidence over the past few decades. Although the exact causes for this rise in incidence are not fully understood, it is important to recognize the unique characteristics and risk factors associated with early-onset CRC. Early detection and prevention strategies are crucial in addressing this growing challenge. To optimize the early detection and prevention of early-onset CRC, there is a need to develop precise screening strategies that take into account genetic risk factors, lifestyle-related factors, and other relevant markers. Incorporating these factors into the screening guidelines can help identify high risk individuals who would benefit from earlier and more targeted screening.
Future research should focus on further elucidating the causes and risk factors associated with early-onset CRC, as well as evaluating the real-world effectiveness of lowering the starting age of CRC screening. Continued collaboration among healthcare professionals, researchers, and policymakers is essential to refine the screening guidelines, improve the early detection rates, and reduce the burden of early-onset CRC on individuals and healthcare systems.
By addressing the challenges posed by early-onset CRC through comprehensive screening, risk assessment, and continued research, we can work toward achieving better outcomes and a decreased impact of this disease on individuals and communities.
Conflicts of Interest
There are no conflicts of interest.
Author Contributions
Kazunori Takada wrote the manuscript. Yoshihiro Kishida, Kenichiro Imai, Kinichi Hotta, and Hiroyuki Ono critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Approval by Institutional Review Board (IRB)
N/A
Acknowledgements
We thank Editage for English language editing.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan; 68(1): 7-30. [DOI] [PubMed] [Google Scholar]
- 2.Doubeni CA, Corley DA, Quinn VP, et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut. 2018 Feb; 67(2): 291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology. 2020 Jan; 158(2): 341-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akimoto N, Ugai T, Zhong R, et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol. 2021 Apr; 18(4): 230-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019 Oct; 68(10): 1820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Connell JB, Maggard MA, Liu JH, et al. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003 Oct; 69(10): 866-72. [PubMed] [Google Scholar]
- 7.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009 Jun; 18(6): 1695-8. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017 Aug; 109(8): djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019 Dec; 68(12): 2179-85. [DOI] [PubMed] [Google Scholar]
- 10.Zamora-Ros R, Béraud V, Franceschi S, et al. Consumption of fruits, vegetables and fruit juices and differentiated thyroid carcinoma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Int J Cancer. 2018 Feb; 142(3): 449-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015 Jan; 150(1): 17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017 May; 67(3): 177-93. [DOI] [PubMed] [Google Scholar]
- 13.Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer. 2019 Jun; 125(12): 2002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takada K, Hotta K, Imai K, et al. Favorable survival after screening for young-onset colorectal cancer: benefits of screening in young adults. Dis Colon Rectum. 2022 Aug; 65(8): 996-1004. [DOI] [PubMed] [Google Scholar]
- 15.Suttie SA, Shaikh I, Mullen R, et al. Outcome of right- and left-sided colonic and rectal cancer following surgical resection. Colorectal Dis. 2011 Aug; 13(8): 884-9. [DOI] [PubMed] [Google Scholar]
- 16.Shida D, Inoue M, Tanabe T, et al. Prognostic impact of primary tumor location in Stage III colorectal cancer-right-sided colon versus left-sided colon versus rectum: a nationwide multicenter retrospective study. J Gastroenterol. 2020 Oct; 55(10): 958-68. [DOI] [PubMed] [Google Scholar]
- 17.Kanno H, Miyoshi H, Yoshida N, et al. Differences in the immunosurveillance pattern associated with DNA mismatch repair status between right-sided and left-sided colorectal cancer. Cancer Sci. 2020 Aug; 111(8): 3032-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You YN, Xing Y, Feig BW, et al. Young-onset colorectal cancer: is it time to pay attention? Arch Intern Med. 2012 Feb; 172(3): 287-9. [DOI] [PubMed] [Google Scholar]
- 19.Kneuertz PJ, Chang GJ, Hu CY, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg. 2015 May; 150(5): 402-9. [DOI] [PubMed] [Google Scholar]
- 20.Saraste D, Järås J, Martling A. Population-based analysis of outcomes with early-age colorectal cancer. Br J Surg. 2020 Feb; 107(3): 301-9. [DOI] [PubMed] [Google Scholar]
- 21.Dozois EJ, Boardman LA, Suwanthanma W, et al. Young-onset colorectal cancer in patients with no known genetic predisposition: can we increase early recognition and improve outcome? Medicine (Baltimore). 2008 Sep; 87(5): 259-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olivo R, Ratnayake S. Colorectal cancer in young patients: a retrospective cohort study in a single institution. ANZ J Surg. 2019 Jul; 89(7-8): 905-7. [DOI] [PubMed] [Google Scholar]
- 23.Frostberg E, Rahr HB. Clinical characteristics and a rising incidence of early-onset colorectal cancer in a nationwide cohort of 521 patients aged 18-40 years. Cancer Epidemiol. 2020 Jun; 66: 101704. [DOI] [PubMed] [Google Scholar]
- 24.Dharwadkar P, Greenan G, Singal AG, et al. Is colorectal cancer in patients younger than 50 years of age the same disease as in older patients? Clin Gastroenterol Hepatol. 2021 Jan; 19(1): 192-4.e3. [DOI] [PubMed] [Google Scholar]
- 25.O'Connell JB, Maggard MA, Livingston EH, et al. Colorectal cancer in the young. Am J Surg. 2004 Mar; 187(3): 343-8. [DOI] [PubMed] [Google Scholar]
- 26.Scott RB, Rangel LE, Osler TM, et al. Rectal cancer in patients under the age of 50 years: the delayed diagnosis. Am J Surg. 2016 Jun; 211(6): 1014-8. [DOI] [PubMed] [Google Scholar]
- 27.Chen FW, Sundaram V, Chew TA, et al. Advanced-stage colorectal cancer in persons younger than 50 years not associated with longer duration of symptoms or time to diagnosis. Clin Gastroenterol Hepatol. 2017 May; 15(5): 728-37.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connell JB, Maggard MA, Liu JH, et al. Do young colon cancer patients have worse outcomes? World J Surg. 2004 Jun; 28(6): 558-62. [DOI] [PubMed] [Google Scholar]
- 29.Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012 Aug; 25(8): 1128-39. [DOI] [PubMed] [Google Scholar]
- 30.Toh JWT, Phan K, Reza F, et al. Rate of dissemination and prognosis in early and advanced stage colorectal cancer based on microsatellite instability status: systematic review and meta-analysis. Int J Colorectal Dis. 2021 Aug; 36(8): 1573-96. [DOI] [PubMed] [Google Scholar]
- 31.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004 Feb; 96(4): 261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marmol I, Sanchez-de-Diego C, et al. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci. 2017 Jan; 18(1): 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010 Jun; 138(6): 2044-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta RS, Song M, Nishihara R, et al. Dietary patterns and risk of colorectal cancer: analysis by tumor location and molecular subtypes. Gastroenterology. 2017 Jun; 152(8): 1944-53.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romaguera D, Fernandez-Barres S, Gracia-Lavedan E, et al. Consumption of ultra-processed foods and drinks and colorectal, breast, and prostate cancer. Clin Nutr. 2021 Apr; 40(4): 1537-45. [DOI] [PubMed] [Google Scholar]
- 36.Carroll KL, Fruge AD, Heslin MJ, et al. Diet as a risk factor for early-onset colorectal adenoma and carcinoma: a systematic review. Front Nutr. 2022; 9: 896330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNabb S, Harrison TA, Albanes D, et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int J Cancer. 2020 Feb; 146(3): 861-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008 Dec; 300(23): 2765-78. [DOI] [PubMed] [Google Scholar]
- 39.Johnson CH, Golla JP, Dioletis E, et al. Molecular mechanisms of alcohol-induced colorectal carcinogenesis. Cancers (Basel). 2021 Aug; 13(17): 4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zisman AL, Nickolov A, Brand RE, et al. Associations between the age at diagnosis and location of colorectal cancer and the use of alcohol and tobacco: implications for screening. Arch Intern Med. 2006 Mar; 166(6): 629-34. [DOI] [PubMed] [Google Scholar]
- 41.Rosato V, Bosetti C, Levi F, et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control. 2013 Feb; 24(2): 335-41. [DOI] [PubMed] [Google Scholar]
- 42.Liu PH, Wu K, Ng K, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019 Jan; 5(1): 37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali Khan U, Fallah M, Tian Y, et al. Personal history of diabetes as important as family history of colorectal cancer for risk of colorectal cancer: a nationwide cohort study. Am J Gastroenterol. 2020 Jul; 115(7): 1103-9. [DOI] [PubMed] [Google Scholar]
- 44.Li Z, Chen H, Fritz CDL, et al. Type 2 diabetes and risk of early-onset colorectal cancer. Gastro Hep Advances. 2022; 1(2): 186-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venugopal A, Carethers JM. Epidemiology and biology of early onset colorectal cancer. Excli J. 2022 Jan; 21: 162-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flemer B, Lynch DB, Brown JM, et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017 Apr; 66(4): 633-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin Y, Lau HC, Liu Y, et al. Altered mycobiota signatures and enriched pathogenic Aspergillus rambellii are associated with colorectal cancer based on multicohort fecal metagenomic analyses. Gastroenterology. 2022 Oct; 163(4): 908-21. [DOI] [PubMed] [Google Scholar]
- 48.Garrett WS. The gut microbiota and colon cancer. Science. 2019 Jun; 364(6446): 1133-5. [DOI] [PubMed] [Google Scholar]
- 49.Mouradov D, Greenfield P, Li S, et al. Oncomicrobial community profiling identifies clinicomolecular and prognostic subtypes of colorectal cancer. Gastroenterology. 2023 Mar, online ahead of print. [DOI] [PubMed] [Google Scholar]
- 50.Stoffel EM, Koeppe E, Everett J, et al. Germline genetic features of young individuals with colorectal cancer. Gastroenterology. 2018 Mar; 154(4): 897-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gausman V, Dornblaser D, Anand S, et al. Risk factors associated with early-onset colorectal cancer. Clin Gastroenterol Hepatol. 2020 Nov; 18(12): 2752-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearlman R, Frankel WL, Swanson B, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 2017 Apr; 3(4): 464-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel SG, Karlitz JJ, Yen T, et al. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022 Mar; 7(3): 262-74. [DOI] [PubMed] [Google Scholar]
- 54.You YN, Borras E, Chang K, et al. Detection of pathogenic germline variants among patients with advanced colorectal cancer undergoing tumor genomic profiling for precision medicine. Dis Colon Rectum. 2019 Apr; 62(4): 429-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sehgal R, Sheahan K, O'Connell PR, et al. Lynch syndrome: an updated review. Genes (Basel). 2014 Jun; 5(3): 497-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta S, Provenzale D, Llor X, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, Version 2.2019. J Natl Compr Canc Netw. 2019 Sep; 17(9): 1032-41. [DOI] [PubMed] [Google Scholar]
- 57.Heald B, Hampel H, Church J, et al. Collaborative group of the Americas on inherited gastrointestinal cancer position statement on multigene panel testing for patients with colorectal cancer and/or polyposis. Fam Cancer. 2020 Jul; 19(3): 223-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mvundura M, Grosse SD, Hampel H, et al. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010 Feb; 12(2): 93-104. [DOI] [PubMed] [Google Scholar]
- 59.Valle L, Vilar E, Tavtigian SV, et al. Genetic predisposition to colorectal cancer: syndromes, genes, classification of genetic variants and implications for precision medicine. J Pathol. 2019 Apr; 247(5): 574-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valle L. Genetic predisposition to colorectal cancer: where we stand and future perspectives. World J Gastroenterol. 2014 Aug; 20(29): 9828-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aelvoet AS, Buttitta F, Ricciardiello L, et al. Management of familial adenomatous polyposis and MUTYH-associated polyposis; new insights. Best Pract Res Clin Gastroenterol. 2022 Jun-Aug; 58-59: 101793. [DOI] [PubMed] [Google Scholar]
- 62.van Leerdam ME, Roos VH, van Hooft JE, et al. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2019 Sep; 51(9): 877-95. [DOI] [PubMed] [Google Scholar]
- 63.Monahan KJ, Bradshaw N, Dolwani S, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut. 2020 Mar; 69(3): 411-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang J, Gurudu SR, Koptiuch C, et al. American Society for gastrointestinal endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020 May; 91(5): 963-82.e2. [DOI] [PubMed] [Google Scholar]
- 65.Cleary SP, Cotterchio M, Jenkins MA, et al. Germline MutY human homologue mutations and colorectal cancer: a multisite case-control study. Gastroenterology. 2009 Apr; 136(4): 1251-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Curia MC, Catalano T, Aceto GM. MUTYH: not just polyposis. World J Clin Oncol. 2020 Jul; 11(7): 428-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015 Nov; 21(11): 1350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spaander MCW, Zauber AG, Syngal S, et al. Young-onset colorectal cancer. Nat Rev Dis Primers. 2023 Apr; 9(1): 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdelsattar ZM, Wong SL, Regenbogen SE, et al. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer. 2016 Mar; 122(6): 929-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kolarich A, George TJ, Jr., Hughes SJ, et al. Rectal cancer patients younger than 50 years lack a survival benefit from NCCN guideline-directed treatment for stage II and III disease. Cancer. 2018 Sep; 124(17): 3510-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaborowski AM, Murphy B, Creavin B, et al. Clinicopathological features and oncological outcomes of patients with young-onset rectal cancer. Br J Surg. 2020 Apr; 107(5): 606-12. [DOI] [PubMed] [Google Scholar]
- 72.Fontana E, Meyers J, Sobrero A, et al. Early-onset colorectal adenocarcinoma in the IDEA database: treatment adherence, toxicities, and outcomes with 3 and 6 months of adjuvant fluoropyrimidine and oxaliplatin. J Clin Oncol. 2021 Dec; 39(36): 4009-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin Z, Dixon JG, Fiskum JM, et al. Clinicopathological and molecular characteristics of early-onset stage III colon adenocarcinoma: an analysis of the ACCENT database. J Natl Cancer Inst. 2021 Nov; 113(12): 1693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lipsyc-Sharf M, Zhang S, Ou FS, et al. Survival in young-onset metastatic colorectal cancer: findings from cancer and leukemia group B (Alliance)/SWOG 80405. J Natl Cancer Inst. 2022 Mar; 114(3): 427-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota colon cancer control study. N Engl J Med. 1993 May; 328(19): 1365-71. [DOI] [PubMed] [Google Scholar]
- 76.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996 Nov; 348(9040): 1472-7. [DOI] [PubMed] [Google Scholar]
- 77.Faivre J, Dancourt V, Lejeune C, et al. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004 Jun; 126(7): 1674-80. [DOI] [PubMed] [Google Scholar]
- 78.Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg. 2008 Aug; 95(8): 1029-36. [DOI] [PubMed] [Google Scholar]
- 79.Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012 Feb; 366(8): 697-706. [DOI] [PubMed] [Google Scholar]
- 80.Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016 Jun; 315(23): 2576-94. [DOI] [PubMed] [Google Scholar]
- 81.Shaukat A, Kahi CJ, Burke CA, et al. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol. 2021 Mar; 116(3): 458-79. [DOI] [PubMed] [Google Scholar]
- 82.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015 Jun; 64(10): 1637-49. [DOI] [PubMed] [Google Scholar]
- 83.Peterse EFP, Meester RGS, Siegel RL, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018 Jul; 124(14): 2964-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018 Jul; 68(4): 250-81. [DOI] [PubMed] [Google Scholar]
- 85.US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2021 May; 325(19): 1965-77. [DOI] [PubMed] [Google Scholar]
- 86.Patel SG, May FP, Anderson JC, et al. Updates on age to start and stop colorectal cancer screening: recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2022 Jan; 162(1): 285-99. [DOI] [PubMed] [Google Scholar]
- 87.Sung JJY, Chiu HM, Lieberman D, et al. Third Asia-Pacific consensus recommendations on colorectal cancer screening and postpolypectomy surveillance. Gut. 2022 Nov; 71(11): 2152-66. [DOI] [PubMed] [Google Scholar]
- 88.Ladabaum U, Mannalithara A, Meester RGS, et al. Cost-effectiveness and national effects of initiating colorectal cancer screening for average-risk persons at age 45 years instead of 50 years. Gastroenterology. 2019 Jul; 157(1): 137-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lew JB, St John DJB, Macrae FA, et al. Benefits, harms, and cost-effectiveness of potential age extensions to the National Bowel Cancer Screening Program in Australia. Cancer Epidemiol Biomarkers Prev. 2018 Dec; 27(12): 1450-61. [DOI] [PubMed] [Google Scholar]
- 90.Knudsen AB, Rutter CM, Peterse EFP, et al. Colorectal cancer screening: an updated modeling study for the US preventive services task force. JAMA. 2021 May; 325(19): 1998-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jeon J, Du M, Schoen RE, et al. Determining risk of colorectal cancer and starting age of screening based on lifestyle, environmental, and genetic factors. Gastroenterology. 2018 Feb; 154(8): 2152-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Archambault AN, Su YR, Jeon J, et al. Cumulative burden of colorectal cancer-associated genetic variants is more strongly associated with early-onset vs late-onset cancer. Gastroenterology. 2020 Apr; 158(5): 1274-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gupta S, Bharti B, Ahnen DJ, et al. Potential impact of family history-based screening guidelines on the detection of early-onset colorectal cancer. Cancer. 2020 Jul; 126(13): 3013-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sinicrope FA. Increasing incidence of early-onset colorectal cancer. N Engl J Med. 2022 Apr; 386(16): 1547-58. [DOI] [PubMed] [Google Scholar]