Abstract
Objectives:
Conventional anal dilatation for anal fissures has long been abandoned because of the high incidence of anal incontinence. However, less invasive and more precise dilation techniques have been developed that have shown high healing and low incontinence rates. This study aimed to evaluate the efficacy and safety of controlled anal dilatation (CAD) using a standardized maximum anal diameter.
Methods:
This study included 523 patients who underwent CAD for chronic anal fissures between January 2010 and December 2014. CAD was performed under sacral epidural anesthesia. The index fingers of both hands were placed in the anus and dilated evenly in various directions. CAD was completed when the anus was dilated to the sixth scale (35 mm in diameter) using a caliber ruler.
Results:
The mean anal scale size expanded from 3.1 to 5.8 (p<0.001). Non-healing was observed in nine patients (1.7%) at 1 month postoperatively, six of whom underwent additional CAD. The mean maximal anal resting pressure (mmHg) decreased from 90.2 to 79.7 at three months postoperatively (p<0.001). Postoperative complications were observed in 11 (2.1%) patients, of whom three patients with thrombosed hemorrhoids underwent resection. None of the patients complained of anal incontinence during the mean follow-up period of 16.6 months. The cumulative recurrence-free rates at three and five years were 87.9% and 69.2%, respectively.
Conclusions:
CAD is technically simple and safe and can achieve reasonable long-term outcomes. Thus, CAD appears to be the preferred procedure for patients with chronic anal fissures who do not respond to conservative treatments.
Keywords: anal fissure, anal dilatation, anal stretching, anal sphincter, fecal incontinence, lateral internal sphincterotomy
Introduction
Anal fissure is a linear or oval shaped tear in the squamous epithelium of the distal anal canal, mostly in the posterior midline[1,2]. The onset of anal fissures is often associated with trauma due to hard stools or prolonged diarrhea[2]. Anal pain is the most common symptom of anal fissures and may appear only during defecation or last for several hours after defecation[1]. Acute anal fissures are often cured with medical treatments, such as high-fiber diets, sitz baths, analgesics, and topical steroids; however, some patients develop recurrent or chronic anal fissures[3]. Chronic anal fissures are defined by symptoms lasting more than six weeks and the presence of an enlarged proximal papilla, a perianal skin tag, fibrotic edges, or an ulcer with exposed internal sphincter fibers[1].
Although the exact etiology of anal fissures remains unclear, hypertonia of the internal anal sphincter (IAS) and subsequent local ischemia are considered to be the most important factors in the persistence of anal fissures[1]. Therefore, treatment strategies for chronic anal fissures focus on reducing anal pressure using oral or topical medications or surgery[1,2]. Clinical guidelines recommend nonoperative treatments such as topical nitrates, calcium channel blockers, or botulinum toxin as the first-line therapies for chronic anal fissures[2]. If medical management fails, a surgical approach is required.
Lateral internal sphincterotomy (LIS) is the best surgical procedure, with healing rates of 88-100%, and remains the gold standard for chronic anal fissures[2]. In a recent network meta-analysis comparing treatments for anal fissures, LIS had the highest odds of healing compared with botulinum toxin and medical therapy[4]. However, LIS can cause postoperative anal incontinence and wound-related complications[4]. The incontinence rate after LIS in 44 randomized controlled trials (RCTs) varied from 0 to 44.4%[5]. In a meta-analysis based on these RCTs, the incontinence rate was 9.4% at a median follow-up of 2 months after LIS[5]. A meta-analysis of 22 RCTs that reported a follow-up of > 2 years after LIS showed an incontinence rate of 14%[6]. In addition to anal incontinence, severe complications after LIS requiring reoperation, such as bleeding, abscesses, fistulas, and non-healing wounds, have been observed in 3% of patients[7].
Anal dilatation (AD), also known as Lord's procedure, involves inserting the four fingers of both hands into the anus and stretching the anal canal for more than four minutes[8]. AD is easy to perform, does not require significant equipment, and was the recommended operative intervention for anal fissures in the 1960s[9]. Watts et al.[10] reported favorable results in 95% of patients with anal fissures, showing early symptom relief after AD. However, a 17-year follow-up study of 138 patients who received AD revealed that 52% developed varying degrees of anal incontinence[11]. Nielsen et al.[12] performed endoanal ultrasonography in post-AD patients and found IAS defects in 60% of the patients and external anal sphincter (EAS) defects in 10%. A Cochrane review published in 2005 analyzed seven RCTs comparing LIS and AD. The results showed that LIS was superior to AD in both healing rate (OR = 3.35; 95%CI = 1.55-7.26) and postoperative anal incontinence rate (OR = 4.03; 95%CI = 2.04-7.46)[13]. Thus, AD was abandoned because of the unacceptably high risk of postoperative anal incontinence[1,2].
In recent years, accurate, measurable, and reproducible AD techniques have been introduced into clinical practice[9,14-17]. These modified AD techniques use a variety of dilators, including retractors, balloons, and anoscopes, with a target anal diameter of 40-48 mm after dilation[9,14-17]. Such standardized AD is also called controlled AD (CAD), and several reports have shown that the outcomes of CAD are comparable to that of LIS[16,18,19]. In a large series using a CAD-specific dilator kit, the healing rate was 88% and the postoperative anal incontinence rate was only 1%[20]. Renzi et al. performed endoanal ultrasonography in 33 patients after CAD using a pneumatic balloon and found no IAS or EAS defects[15]. Therefore, the latest guidelines list balloon-based CAD as a promising technique[2]. However, they stated that this technique had not been sufficiently investigated for use as a standard procedure[2].
For more than 20 years, we have performed CAD for chronic anal fissures by manually stretching the IAS while strictly measuring the anal diameter using a caliber ruler. We set the maximum anal dilation in CAD to 35 mm in diameter, considering the small physique of Japanese patients. To date, there have been no reports of CAD by manually stretching. This study investigated the long-term outcomes of manual CAD in a large number of patients with chronic anal fissures.
Methods
This study was a retrospective review of medical records and was approved by the institutional review board of our hospital (approval code: K23-001). This study enrolled consecutive patients who underwent manual CAD for chronic anal fissures between January 2010 and December 2014. Written informed consent was obtained from all the patients. Indications for CAD at our institution were patients with normal (≥40 mmHg) or high (≥100 mmHg) maximal resting pressure (MRP) who had failed nonoperative medical therapy such as topical steroids, analgesics, and stool softeners for more than two months. Patients with low MRP (<40 mmHg) or a history of anal incontinence were treated with an anocutaneous advancement flap. Exclusion criteria included anorectal pathologies, such as tumors, abscesses, fistulas, grade ≥3 hemorrhoids, and inflammatory bowel diseases.
Anorectal examinations
Digital rectal examination, proctoscopy, and anal manometry were performed with the patient in the left lateral position, without bowel preparation. Anal manometry was performed using a one-channel microtip transducer mounted on a flexible catheter with a 5-mm in diameter (P-1401; Star Medical Inc., Tokyo, Japan). The MRP was recorded using a rapid pull-through technique and was defined as the highest resting pressure. Next, the maximal squeeze pressure (MSP), defined as the highest pressure above the baseline at any level within the anal canal, was measured.
Surgical technique
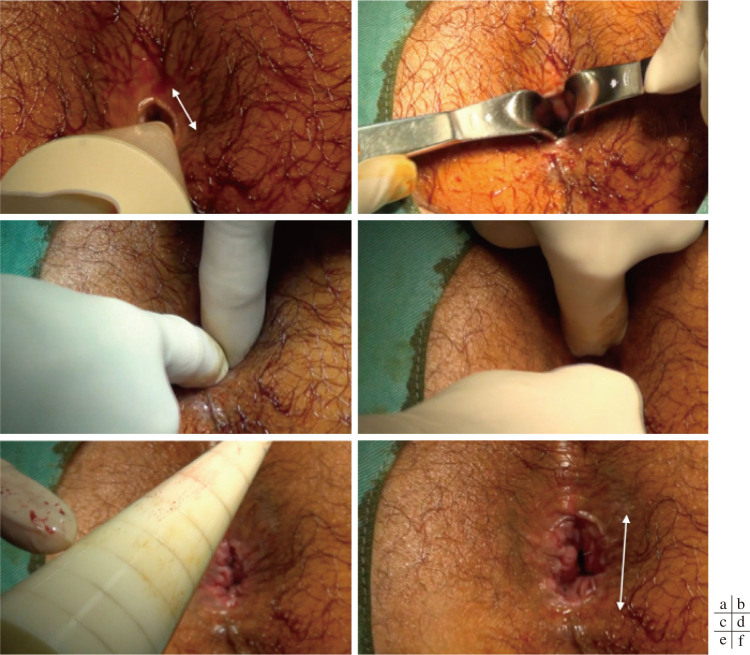
Preoperative preparation consisted of the administration of two suppositories (sodium bicarbonate and anhydrous sodium phosphate) immediately before surgery. CAD was performed with the patient in the jackknife position under sacral epidural anesthesia. After anesthesia, the actual anal diameter was measured using a caliber ruler (Figure 1, 2a). Subsequently, the anus was dilated using handheld surgical retractors to the extent that the index fingers of both hands could be inserted (Figure 2b). The index fingers of both hands were placed in the anus and gradually exerted outward pressure (Figure 2c). The anus was evenly dilated in various directions by hand rotation (Figure 2d). The CAD was completed when the anus was dilated to the sixth scale (35 mm in diameter) on the caliber ruler (Figure 2e). In patients with enlarged anal papillae or sentinel tags, resection was performed at the patient's request. When the IAS was too stiff to be sufficiently dilated using CAD, the procedure was immediately converted to LIS. All patients were hospitalized overnight following CAD.
Figure 1.
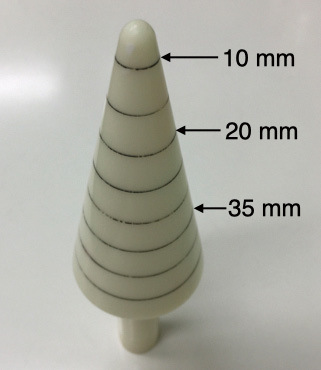
Caliber ruler for measuring anal diameter (custom-made). It has eight scales in 5 mm increments, with the sixth (35 mm) as the target for anal dilatation.
Figure 2.
Procedure image of controlled anal dilation (CAD). The anal size before dilation was the second to third scale (15-20 mm in diameter) on a caliber ruler (a). Next, the anus was dilated using handheld surgical retractors to the extent that the index fingers of both hands could be inserted (b). Place the index fingers of both hands in the anus and gradually exert pressure outward (c). The anus was equally dilated in various directions (d). CAD was completed when the anus was dilated to the sixth scale (35 mm in diameter) (e). The anal diameter was more than doubled by CAD (f).
Outcome measures
The patients were followed up at one, three, and six months, and at one, two, and three years after surgery. Subsequent follow-ups were performed voluntary. Follow-up assessments included interviews regarding anal symptoms (pain, bleeding, difficulty in defecation, and anal incontinence), digital rectal examination, proctoscopy, and anal manometry. The efficacy of the CAD was evaluated at the 1-month postoperative visit. Non-healed cases were defined as those in which the symptoms did not improve, anal fissures remained on anoscopy, and additional surgery was performed or proposed. Changes in MRP and MSP at baseline and at three months postoperatively, and postoperative complications were analyzed. Recurrences were defined as cases in which anal fissures were confirmed by clinical symptoms and proctoscopy after being cured by CAD and reoperation was performed or proposed.
Statistical methods
All statistical analyses were performed using the EZR software (version 1.11; Saitama Medical Center, Jichi Medical University, Saitama, Japan). Categorical variables were reported as frequencies and percentages. Continuous variables were presented as means and standard deviations. Contingency tables were analyzed using chi-square tests. The Wilcoxon signed-rank test was used to compare pre- and postoperative values for anal scale size, MRP, and MSP. The two-sided significance level was set at 5%. The cumulative recurrence-free rate was assessed using the Kaplan-Meier method.
Results
Between January 2010 and December 2014, 559 patients with chronic anal fissures underwent surgery at our hospital. CAD was performed in 547 patients, and an anocutaneous advancement flap was applied in the other 12 patients because of the risk of anal incontinence. Of those who underwent CAD, 24 (4.4%) were converted to LIS due to difficulty in dilatation; therefore, 523 patients were included in this study. The baseline characteristics of the included patients are summarized in Table 1. Posterior anal fissures were more common in men, and anterior anal fissures were more common in women. There was no difference in the mean anal scale size and MRP between men and women (Table 1).
Table 1.
Characteristics of the Study Population at Baseline (n = 523).
| Variable | Men | Women | p-value |
|---|---|---|---|
| Number of patients (%) | 255 (48.8) | 268 (51.2) | – |
| Age (year) | 55.3 (15.6) | 50.5 (16.6) | <0.001 |
| Anal fissure location, n (%) | |||
| Posterior | 152 (71.4) | 130 (57.0) | 0.002 |
| Anterior | 13 (6.1) | 41 (18.0) | <0.001 |
| Posterior and anterior | 45 (21.1) | 51 (22.4) | 0.841 |
| Others | 3 (1.4) | 6 (2.6) | 0.568 |
| Anal scale size | 3.2 (0.9) | 3.1 (0.9) | 0.668 |
| Mean anal pressures (mmHg) | |||
| Maximal resting pressure | 92.8 (23.6) | 88.2 (20.2) | 0.486 |
| Maximal squeeze pressure | 324.1 (106.4) | 208.6 (111.6) | <0.001 |
Values are presented as mean (standard deviation) unless specified otherwise.
None of the patients experienced intraoperative complications. The mean anal scale size was significantly extended from 3.1 ± 0.9 to 5.8 ± 0.5 (p<0.001). Non-healing was observed in nine patients (1.7%) at one month postoperatively, six of whom had additional CAD, two had LIS, and one had an anocutaneous advancement flap. Although manometric data were not available for all patients, the mean MRP decreased from 90.2 ± 21.0 mmHg to 79.7 ± 19.8 mmHg at three months postoperatively (p<0.001). In contrast, the mean MSP did not change significantly three months postoperatively (Table 2).
Table 2.
Change in the Manometric Data before and after Treatment.
| Baseline | 3 months | p-value | |
|---|---|---|---|
| Mean MRP (mmHg) | |||
| Men (n = 160) | 91.2 (21.9) | 77.9 (20.6) | < 0.001 |
| Women (n = 166) | 89.3 (20.0) | 81.4 (18.9) | < 0.001 |
| Mean MSP (mmHg) | |||
| Men (n = 160) | 315.7 (97.4) | 334.0 (248.0) | 0.388 |
| Women (n = 166) | 209.1 (59.8) | 210.4 (63.0) | 0.838 |
MRP, maximal resting pressure; MSP, maximal squeeze pressure
Data are shown mean (standard deviation).
The mean duration of postoperative follow-up was 16.6 months (range, 1-85 months). Postoperative complications were observed in 11 (2.1%) patients, of whom three patients with thrombosed external hemorrhoids underwent resection (Table 3). The remaining eight patients were treated with topical steroids. No patients complained anal incontinence during the follow-up period. Anal fissure recurrence was observed in 41 (7.8%) patients, of whom 32 (78%) underwent re-CAD (Table 4). The cumulative recurrence-free rates (95% confidence interval) at three and five years were 87.9% (82.6-91.6%) and 69.2% (56.7-78.7%), respectively (Figure 3).
Table 3.
Breakdown of Postoperative Complications.
| Number of patients with complications | 11 (2.1) |
|---|---|
| Type of complication | |
| Thrombosed external hemorrhoids | 7 (1.3) |
| Swelling of sentinel tags | 4 (0.8) |
| Anal incontinence | 0 |
Values in parentheses are percentages.
Table 4.
Breakdown of Treatment at Recurrence.
| Number of patients with recurrence | 41 (7.8) |
|---|---|
| Type of treatment at recurrence | |
| Re-controlled anal dilation | 32 (78.0) |
| Anocutaneous advancement flap | 5 (12.2) |
| Lateral internal sphincterotomy | 2 (4.9) |
| Medical treatment | 2 (4.9) |
Values in parentheses are percentage.
Figure 3.
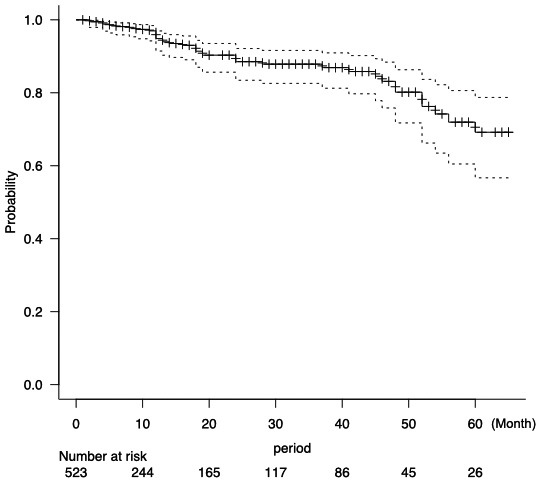
Probability of no recurrence with time for patients who underwent the treatment (n = 523). Cumulative recurrence-free rates at three and five years postoperatively were 87.9% and 69.2%, respectively.
Discussion
In this retrospective study of 523 patients with chronic anal fissures, we demonstrated that manual CAD can achieve high healing rates and low anal incontinence rates comparable to those of balloon- or dilator-based CAD. Although the mean MRP decreased significantly, it was sufficient to maintain anal continence. The long-term recurrence-free rates were also reasonable.
In 1992, Sohn et al.[9] performed the first CAD with a Parks retractor that opened to 48 mm and a 40-mm diameter balloon, and found healing in 93% and 94% of patients, respectively. Based on these results, they developed a dilator kit (Sohn's DilatorsⓇ) with a maximum diameter of 40 mm[20]. Sohn et al.[20] performed CAD using the dilator kit in 292 patients with anal fissures and reported a healing rate of 88% and an anal incontinence rate of 1%. Subsequently, CAD using an anal speculum that opens to 48 mm and a bivalve anoscope that opens to 45 mm have been reported (Table 5)[16,17]. The healing rates in these CAD reports ranged from 90 to 94.5% and the recurrence rates ranged from 0 to 14.8%. Temporary anal incontinence was observed in 0-6.1% of patients, but permanent anal incontinence was not reported (Table 5)[9,14-17].
Table 5.
Comparison of Data from the Literature of Controlled Anal Dilation in Patients with Anal Fissures.
| Authors (years) | Sohn et al.[9] (1992) | Boschetto et al.[14] (2004) | Renzi et al.[15] (2005) | Yucel et al.[16] (2009) | Santander et al.[17] (2010) | Abe et al. (Present study) | |
|---|---|---|---|---|---|---|---|
| Men/women (n) | 58/47 | 39/27 | 65/44 | 15/18 | 10/10 | Total 27 | 234/324 |
| Mean age (years) | NS | 53.3 | 41 | 28.7 | NS | 52.8 | |
| Anesthesia | Local + sedation | Sedation | Local + sedation | General | Local | Sacral epidural | |
| Dilation device | Retractor | Balloon | Balloon | Balloon | Anal speculum | Anoscope | Both hands |
| Dilation diameter (mm) | 48 | 40 | 40 | 40 | 48 | 45 | 35 |
| Mean MRP (mmHg) | |||||||
| At baseline | NS | NS | 91.0 | 89.7 | 122 | 90.2 | |
| After treatment | NS | NS | 70.5 | 76.9 | 91 | 79.7 | |
| Postoperative complications, n (%) | |||||||
| Thrombosed hemorrhoids | 0 | 1 (1.5) | 3 (2.7) | 0 | 0 | 0 | 7 (1.3) |
| Temporary anal incontinence | 2 (1.9) | 0 | 0 | 2 (6.1) | 0 | 1 (3.7) | 0 |
| Permanent anal incontinence | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Non-healing | 7 (6.7) | 4 (6.1) | 6 (5.5) | 2 (6.1) | 2 (10) | 2 (7.4) | 9 (1.7) |
| Anal fissure recurrence | 2 (1.9) | 0 | 0 | 1 (3.0) | 2 (10) | 4 (14.8) | 41 (7.8) |
| Follow up period (month) | NS | 15.5 | 25.7 | NS | 18 | 16.6 | |
MRP, maximal resting pressure; NS, not stated. Values in parentheses are percentage unless specified otherwise.
Previous CAD studies used devices that can expand to 40-48 mm in diameter[9,14-17]. Specifically, the balloons are the same in all studies at 40 mm, but the anoscope or retractors are larger at 45-48 mm[9,14-17]. However, even with a retractor that opens up to 48 mm, it can extend only approximately 25 mm laterally, resulting in a circumference of approximately 120 mm, which translates into a diameter of approximately 38 mm[9]. Considering the Japanese physique, we further limited dilation to a smaller diameter of 35 mm. The results showed a modest MRP decrease of 11.6%, less than the 14.3-25.4% observed in previous studies, and no anal incontinence occurred (Table 5). Conversely, men or large women may be able to dilate to 40 mm or more, which would reduce non-healing and recurrence rates.
The key to the success of the CAD procedure in this study may be the anesthesia method. Strugnell et al.[21] analyzed the results of manual AD performed by a single-skilled surgeon without standardizing the anal diameter and reported a healing rate of 89% and no postoperative anal incontinence. The authors advocated the importance of inhibiting EAS contraction with suxamethonium to prevent sphincter damage during dilation. In previous studies, CAD was performed under general or local anesthesia[9,14-17], whereas we performed CAD under sacral epidural anesthesia to ensure relaxation of the EAS (Table 5). This allows for a more accurate and gentle dilation, as only the hardened IAS can be palpated during the procedure.
Three studies comparing LIS and CAD for chronic anal fissure have been reported[16,18,19]. Renzi et al.[18] conducted an RCT of LIS (n = 25) versus balloon CAD (n = 24) and found no significant differences in healing rates (92% for LIS vs. 83.3% for CAD). However, the incidence of anal incontinence at the 24-month follow-up was significantly higher in the LIS group (16%) than in the CAD group (0%) (p<0.0001)[18]. Yucel et al.[16] performed an RCT on LIS (n = 20) and anal speculum-based CAD (n = 20) and found no significant differences in the healing rates of 85% and 90%, respectively. Although the follow-up period was two months, postoperative anal incontinence was not observed in either group[16]. Walfisch et al.[19] conducted an observational study of LIS (n = 100) and balloon CAD (n = 175) and found no significant differences in healing rates (98% vs. 92%) or anal incontinence rates (2% vs. 0%). However, postoperative perianal abscesses were more common in the LIS group (4%) than in the balloon CAD group (0%) (p=0.018)[19]. These results suggest that CAD may be as effective as LIS in the treatment of chronic anal fissures, and may cause equal or less damage to the anal sphincters.
Thus, CADs have overcome the shortcomings of AD, such as poor reproducibility and a high incidence of anal incontinence, by standardizing dilators or techniques. Similarly, several modifications have been reported to standardize or minimize the extent of sphincterotomy to prevent anal incontinence after LIS[3]. Two RCTs have compared “tailored” LIS, defied as sphincterotomy limited in extent to the fissure apex, to conventional LIS, defined as transecting internal sphincter up to the dentate line[22,23]. The results showed that tailored LIS did not cause persistent or significant anal incontinence, unlike conventional LIS, but the time to anal pain relief was significantly longer than with conventional LIS[22,23]. Menteş et al.[24] performed an RCT of “calibrated” sphincterotomy, which dilates the anal canal to 30 mm in diameter, versus tailored LIS and found significantly less postoperative anal incontinence scores with the calibrated LIS. In a large retrospective study of “minimal” LIS, which divides only the fibrotic band of IAS, the healing rate was 97% and the anal incontinence rate was 0.4%[25]. Thus, these “modified” LISs appear to minimize the deterioration of anal sphincter function. Comparative studies between the modified LIS and CAD have not yet been reported; therefore, future RCTs should be conducted.
The limitations of this study include its retrospective, single-institution, and observational design, and the absence of a control group. Another limitation was that anal pain, bleeding, and quality of life were not evaluated. In addition, the incidence of anal incontinence may have been underestimated because we did not use a validated severity score for anal incontinence but only assessed it through interviews. Furthermore, since office visits after three years were optional, the number of follow-ups steadily decreased. Therefore, the recurrence rate may have been over- or underestimated in the present study. Therefore, the results need to be validated through RCTs.
This is the first report of manual CAD that shows that the anal canal can be safely dilated to a diameter of 35 mm. Although anal incontinence did not occur during the follow-up period in this study, post-CAD patients may have developed anal incontinence decades later. However, unlike LIS, CAD does not involve IAS transection and the risk of age-related anal incontinence is presumed to be low. CAD also carries no risk of wound-related complications that can occur with LIS, such as perianal abscesses or bleeding. Therefore, we consider CAD as the first choice for chronic anal fissures that do not respond to conservative treatment. The cumulative five-year recurrence rate after CAD is > 30%; however, re-CAD can be performed as safely as the initial CAD. However, it should be noted that manual CAD was not possible in all cases. In this study, we observed 4.4% of cases in which the IAS was too stiff to dilate adequately. Forcible dilation may result in the tearing of the entire IAS, and it is safer to convert to LIS.
In conclusion, manual CAD is safe, technically simple, and requires no special dilators. Therefore, it can be implemented in any facility. This study suggests that CAD can dilate the anal canal in patients with chronic anal fissures more safely than LIS. Accordingly, CAD should be, in our opinion, the preferred procedure for patients with chronic anal fissure.
Conflicts of Interest
There are no conflicts of interest.
Author Contributions
Tatsuya Abe contributed to the concept and design and data acquisition and analysis and drafted and revised the manuscript; Masao Kunimoto, Yoshikazu Hachiro, Shigenori Ota, Kei Ohara, Mitsuhiro Inagaki, Yusuke Saitoh, and Masanori Murakami contributed to data acquisition, revised the manuscript, and approved the final version.
Approval by Institutional Review Board (IRB)
This research was approved by the institutional review board of Kunimoto Hospital (approval code: K23-001).
Disclaimer
Tatsuya Abe is one of the Associate Editors of Journal of the Anus, Rectum and Colon and on the journal's Editorial Board. He was not involved in the editorial evaluation or decision to accept this article for publication at all.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
References
- 1.Beaty JS, Shashidharan M. Anal fissure. Clin Colon Rectal Surg. 2016 Mar; 29(1): 30-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davids JS, Hawkins AT, Bhama AR, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Anal Fissures. Dis Colon Rectum. 2023 Feb; 66(2): 190-9. [DOI] [PubMed] [Google Scholar]
- 3.Hwang SH. Trends in treatment for hemorrhoids, fistula, and anal fissure: go along the current trends. J Anus Rectum Colon. 2022 Jul; 6(3): 150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin JZ, Bhat S, Park B, et al. A systematic review and network meta-analysis comparing treatments for anal fissure. Surgery. 2022 Jul; 172(1): 41-52. [DOI] [PubMed] [Google Scholar]
- 5.Ebinger SM, Hardt J, Warschkow R, et al. Operative and medical treatment of chronic anal fissures-a review and network meta-analysis of randomized controlled trials. J Gastroenterol. 2017 Jan; 52(6): 663-76. [DOI] [PubMed] [Google Scholar]
- 6.Garg P, Garg M, Menon GR. Long-term continence disturbance after lateral internal sphincterotomy for chronic anal fissure: a systematic review and meta-analysis. Colorectal Dis. 2013 Mar; 15(3): e104-17. [DOI] [PubMed] [Google Scholar]
- 7.Walker WA, Rothenberger DA, Goldberg SM. Morbidity of internal sphincterotomy for anal fissure and stenosis. Dis Colon Rectum. 1985 Nov; 28(11): 832-5. [DOI] [PubMed] [Google Scholar]
- 8.Lord PH. A new regime for the treatment hemorrhoids. Proc R Soc Med. 1968 Sep; 61(9): 935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sohn N, Eisenberg MM, Weinstein MA, et al. Precise anorectal sphincter dilatation: its role in the therapy of anal fissures. Dis Colon Rectum. 1992 Apr; 35(4): 322-7. [DOI] [PubMed] [Google Scholar]
- 10.Watts JM, Bennett RC, Goligher JC. Stretching of anal sphincters in treatment of fissure-in-ano. BMJ. 1964 Aug; 2(5405): 342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konsten J, Baeten CG. Hemorrhoidectomy vs. Lord's method: 17-year follow-up of a prospective, randomized trial. Dis Colon Rectum. 2000 Apr; 43(4): 503-6. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen MB, Rasmussen OO, Pedersen JF, et al. Risk of sphincter damage and anal incontinence after anal dilatation for fissure-in-ano. An endosonographic study. Dis Colon Rectum. 1993 Jul; 36(7): 677-80. [DOI] [PubMed] [Google Scholar]
- 13.Nelson R. Operative procedures for fissure in ano. Cochrane Database Syst Rev. 2005 Apr; 18(2): CD002199. [DOI] [PubMed] [Google Scholar]
- 14.Boschetto S, Giovannone M, Tosoni M, et al. Hydropneumatic anal dilation in conservative treatment of chronic anal fissure: clinical outcomes and randomized comparison with topical nitroglycerin. Tech Coloproctol. 2004 Aug; 8(2): 89-92. [DOI] [PubMed] [Google Scholar]
- 15.Renzi A, Brusciano L, Pescatori M, et al. Pneumatic balloon dilatation for chronic anal fissure: a prospective, clinical, endosonographic, and manometric study. Dis Colon Rectum. 2005 Jan; 48(1): 121-6. [DOI] [PubMed] [Google Scholar]
- 16.Yucel T, Gonullu D, Oncu M, et al. Comparison of controlled-intermittent anal dilatation and lateral internal sphincterotomy in the treatment of chronic anal fissures: a prospective, randomized study. Int J Surg. 2009 Jun; 7(3): 228-31. [DOI] [PubMed] [Google Scholar]
- 17.Santander C, Gisbert JP, Moreno-Otero R, et al. Usefulness of manometry to select patients with anal fissure for controlled anal dilatation. Rev Esp Enferm Dig. 2010 Dec; 102(12): 691-7. [DOI] [PubMed] [Google Scholar]
- 18.Renzi A, Izzo D, Di Sarno G, et al. Clinical, manometric, and ultrasonographic results of pneumatic balloon dilatation vs. lateral internal sphincterotomy for chronic anal fissure: a prospective, randomized, controlled trial. Dis Colon Rectum. 2008 Jan; 51(1): 121-7. [DOI] [PubMed] [Google Scholar]
- 19.Walfisch S, Silberstein E. Balloon anal dilatation for anal fissure. Tech Coloproctol. 1998 Dec; 2(2): 73-5. [Google Scholar]
- 20.Sohn N, Weinstein MA. Anal Dilatation for Anal Fissures. Seminars in Colon & Rectal Surgery. 1997 Jan; 8(1): 17-23. [Google Scholar]
- 21.Strugnell NA, Cooke SG, Lucarotti ME, et al. Controlled digital anal dilatation under total neuromuscular blockade for chronic anal fissure: a justifiable procedure. Br J Surg. 1999 May; 86(5): 651-5. [DOI] [PubMed] [Google Scholar]
- 22.Menteş BB, Ege B, Leventoglu S, et al. Extent of lateral internal sphincterotomy: up to the dentate line or up to the fissure apex? Dis Colon Rectum. 2005 Feb; 48(2): 365-70. [DOI] [PubMed] [Google Scholar]
- 23.Elsebae MM. A study of fecal incontinence in patients with chronic anal fissure: prospective, randomized, controlled trial of the extent of internal anal sphincter division during lateral sphincterotomy. World J Surg. 2007 Oct; 31(10): 2052-7. [DOI] [PubMed] [Google Scholar]
- 24.Menteş BB, Güner MK, Leventoglu S, et al. Fine-tuning of the extent of lateral internal sphincterotomy: spasm-controlled vs. up to the fissure apex. Dis Colon Rectum. 2008 Jan; 51(1): 128-33. [DOI] [PubMed] [Google Scholar]
- 25.Lee KH, Hyun K, Yoon SG, et al. Minimal lateral internal sphincterotomy (LIS): Is it enough to cut less than the conventional tailored LIS? Ann Coloproctol. 2021; 37(5): 275-80. [DOI] [PMC free article] [PubMed] [Google Scholar]