Abstract
Severe asthma in children and adolescents exerts a substantial health, financial, and societal burden. Severe asthma is a heterogeneous condition with multiple clinical phenotypes and underlying inflammatory patterns that might be different in individual patients. Various add-on treatments have been developed to treat severe asthma, including monoclonal antibodies (biologics) targeting inflammatory mediators. Biologics that are currently approved to treat children (≥ 6 years of age) or adolescents (≥ 12 years of age) with severe asthma include: anti-immunoglobulin E (omalizumab), anti-interleukin (IL)-5 (mepolizumab), anti-IL5 receptor (benralizumab), anti-IL4/IL13 receptor (dupilumab), and antithymic stromal lymphopoietin (TSLP) (tezepelumab). However, access to these targeted treatments varies across countries and relies on few and crude indicators. There is a need for better treatment stratification to guide which children might benefit from these treatments. In this narrative review we will assess the most recent developments in the treatment of severe pediatric asthma, as well as potential biomarkers to assess treatment efficacy for this patient population.
Key Points
| Childhood asthma is a heterogeneous inflammatory condition. Immunological pathways driving the chronic inflammation differ per individual patient. |
| The introduction of biologics as add-on therapy for severe asthma has revolutionized severe asthma management and paved the way for precision medicine in children and adolescents. |
| Five biologics are currently licensed to treat severe asthma in children and/or adolescents: omalizumab (anti-IgE), mepolizumab (anti-IL5), benralizumab (anti-IL5Rα), dupilumab (anti-IL4Rα), and tezepelumab (anti-TSLP). |
| Despite marketing approval access to these biologics differ per country, and indicators to guide choice of the biologic and monitor treatment success are scarce. |
| Especially for the most recently approved biologics, such as dupilumab and tezepelumab, efficacy and safety data in the pediatric population is scarce. |
Introduction
Severe asthma in children has a large impact on quality of life and well-being of patients and their families [1, 2]. Asthma is one of the leading causes of school absenteeism and the level of absences correlates with asthma severity [2]. Especially during adolescence, building relationships with peers is essential. However, uncontrolled asthma symptoms negatively influence participation in everyday activities and societal participation in this important life phase [3]. Furthermore, studies indicate that quality of life impairment and internalizing behavioral problems are significantly more prevalent in children with severe asthma compared with children with moderate asthma [4].
The introduction of inhaled corticosteroids (ICS) in the 1970s to treat underlying airway inflammation was an enormous step forward in controlling the disease in pediatric patients with asthma [5]. Currently, it is the mainstream therapeutic maintenance option for persistent asthma in children and most children respond well. However, for a small group of children and adolescents, the disease remains insufficiently controlled and requires additional treatment. Treatment options consist of increasing the ICS doses and adding additional medication such as long-acting beta-agonists (LABAs), leukotriene receptor antagonists (LTRA), or long-acting muscarinic antagonists (LAMAs). Even though treatment with ICS is generally considered safe in children, daily use of these drugs over a long period of time, especially with higher dosages, has been associated with growth reduction and other adverse effects, such as adrenal suppression, fatigue, anemia, coughing, and oropharyngeal candidiasis [6, 7]. Evidence suggests that the effect of ICS on growth may not only depend on medication dose and duration of use, but also on the type of steroid and delivery device, which physicians should take into account when determining the optimal therapy [7]. Furthermore, physicians should take other factors into account, such as therapy adherence, the patient’s personal preference, and comorbidities [7, 8]. In addition, oral corticosteroids (OCS) are commonly used to treat severe asthma exacerbations in children. However, systematic steroid-related adverse effects are frequent among patients with severe asthma and recurrent exposure to OCS is associated with an increased risk of infections, weight gain, growth retardation, and Cushingoid features in children [9, 10].
It has been estimated that approximately 5% of children with asthma suffer from severe asthma [11]. However, exact estimations are difficult due to the lack of a uniform definition for severe asthma [12]. Different guidelines and professional societies apply different definitions. A joint task force of the European Respiratory Society (ERS) and American Thoracic Society (ATS) defined severe asthma in 2014 as “When a diagnosis of asthma is confirmed and comorbidities addressed, severe asthma is defined as ‘asthma that requires treatment with high dose inhaled corticosteroids […] plus a second controller (and/or systemic corticosteroids) to prevent it from becoming ‘uncontrolled’ or which remains ‘uncontrolled’ despite this therapy’.” The World Health Organization (WHO) holds a slightly different definition and defines severe asthma as “uncontrolled asthma which can result in risk of frequent severe exacerbations (or death) and/or adverse reactions to medications and/or chronic morbidity (including impaired lung function or reduced lung growth in children).” According to the WHO, patients with severe disease can be subclassified into three different categories [11, 13]: (I) untreated severe asthma, (II) difficult-to-treat severe asthma, and (III) severe therapy-resistant asthma (STRA). In both definitions intensive multidisciplinary evaluation is required to assess whether children with severe asthma are truly STRA and might have a biological profile that predisposes them to have a poor response to treatment, or whether comorbidities, (psycho)social, environmental, and/or behavioral factors drive the observed lack of response to treatment [11, 14]. Most children with severe symptoms will improve upon structured evaluation and management of comorbidities, inhalation technique, and adherence [15]. If this is not the case, these children might be a candidate for add-on treatment with biologics (Table 1). Biologics are monoclonal antibodies that target specific components of the immune pathways (including cytokines and/or cell surface markers).
Table 1.
Biologics approved to treat severe asthma in children and/or adolescents
| Biologic | Trade name | Target | Indication |
|---|---|---|---|
| Omalizumab | Xolair | IgE | ≥ 6 years with moderate to severe persistent asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and whose symptoms are inadequately controlled with ICS |
| Mepolizumab | Nucala | IL5 | ≥ 6 years with severe eosinophilic asthma |
| Dupilumab | Dupixent | IL4/IL13 receptor alpha | ≥ 6 years moderate-to-severe asthma characterized by an eosinophilic phenotype or with oral corticosteroid dependent asthma |
| Benralizumab | Fasenra | IL5 receptor alpha |
FDA: ≥ 12 years with severe asthma and an eosinophilic phenotype EMA: ≥ 18 years with eosinophilic asthma |
| Tezepelumab | Tezspire | TSLP | ≥ 12 years with severe asthma who are inadequately controlled despite high dose inhaled corticosteroids + another medicinal product for maintenance treatment |
ICS, inhaled corticosteroids; IL, interleukin; TSLP, thymic stromal lymphopoietin
The approval of the first asthma biologic, a monoclonal antibody (mAb) targeted at immunoglobin E (IgE) in 2003 [16], has revolutionized asthma management once more. It indicated the introduction of biologic therapies that target key inflammatory mediators [17]. These drugs improve outcomes in a substantial subset of patients with severe asthma, including decreased exacerbation rates and improved symptom control, while reducing the need for systemic steroids [18]. Biologics pave the way for precision medicine in the asthma clinic; however, there is still a lack of clear indicators for treatment choice and treatment efficacy, especially in children [19, 20].
In this narrative review we will assess the most recent developments in the treatment of severe pediatric asthma, building upon the previously published reviews in this journal by Licari et al. in 2019 [21] and 2020 [22]. We will mainly focus on the studies that have led to the approval of the newest kids on the block: dupilumab and tezepelumab. Furthermore, we will review potential biomarkers of treatment efficacy for this severe pediatric patient population.
The Concept of Asthma Endotypes
Severe asthma is a heterogeneous inflammatory condition with multiple clinical phenotypes and distinct inflammatory patterns that might be different in individual patients and dynamic over time [23]. To better recognize shared molecular pathways, the concept of distinct asthma “endotypes” was proposed in 2008 [24, 25].
Currently, the best-defined asthma endotype is type 2-high asthma [26]. Type 2 (T2) high inflammation is defined by increased type 2 cytokines, such as IL5, IL13, and IL4. Particularly in asthma, an intrinsic downregulation of the expression of tight junction and adhesion proteins in airway epithelium, including claudins and E-cadherins, are directly interconnected with the reduced structural integrity of the epithelial cell barrier, as well as the enhanced permeability and responsiveness to exogenous stimuli [27–29]. Activated airway epithelial cells display an increased capacity to produce and release alarmins (i.e., IL25, IL33, and TSLP) that create a local tissue inflammatory microenvironment that promotes recruitment, activation and inflammatory function of other more specialized immune cells residing in the local tissue or upon recruitment from the peripheral blood. Epithelial cell-derived alarmins are crucial in the development and regulation of type 2 innate lymphoid cells (ILC2), as well as the activation and proliferation of dendritic cells (DCs) and type 2 T-helper cells (Th2) [30, 31]. Both the adaptive and the innate immune processes contribute to the ultimate pool of T2 cytokines. These inflammatory mediators are predominately produced by ILC2s, Th2 cells, and eosinophils that are present in increased numbers in the inflamed tissue as well as in the peripheral blood. Recently, a novel subtype of inflammatory ILC2 characterized by the surface expression of CD45RO was identified in the blood of patients with severe asthma and uncontrolled T2-high asthma [32]. These inflammatory ILC2s produced increased levels of IL5 and lL13 as compared with their resting/conventional CD45+ ILC2 equivalents and displayed reduced sensitivity to glucocorticosteroids [32]. Currently approved biological agents target specific components of T2 inflammation, including inflammatory molecules or surface cell receptors. In a clinical setting, a T2-high asthma phenotype is often defined on the basis of increased blood eosinophil counts or an increased level of fractional exhaled nitric oxide (FeNO). This non-invasive exhaled biomarker is considered a surrogate marker of T2 inflammation [33]. T2-high asthma is associated with a better response to antiinflammatory treatment, such as ICS [34].
The T2-high endotype is often further differentiated in eosinophilic allergic inflammation and eosinophilic non-allergic inflammation. In children, the most common asthma phenotype is allergic and eosinophilic [35]. The allergic subset of T2-high asthma usually presents at a young age and is often referred to as the early-onset asthma. The characteristics of this asthma subtype include positive allergy skin tests and increased serum total and specific IgE, as well as clinical symptoms upon allergen provocation and/or exposure. Little is known about the immunological mechanisms driving the pathology of early-onset asthma. IL2-mediated pathways were shown to drive and control the lung homing and retention of allergen-specific memory Th2 that drive the pathological mechanisms in asthma [36].
Type 2 low (also known as non-type 2 or non-eosinophilic asthma) encompasses neutrophilic asthma and paucigranulocytic asthma. Although T2-high inflammation is most common in childhood-onset asthma, characteristics of both endotypes have been reported in children with severe asthma [37–39].
Neutrophilic asthma is mainly defined by the high prevalence of neutrophils in the sputum and the inflammation is the results of a mixed T helper 1 (Th1) and T helper 17 (Th17) activation, triggered by infections and/or inhaled pollutants [40]. Th1 subsequently produce and release IFN-γ, TNF-α, and IL8, while Th17 cells produce IL17, and IL22. The role of neutrophils in childhood asthma is still unclear. From a clinical perspective, however, neutrophilic asthma has been associated with severe asthma, poor asthma control, reduced pulmonary function, smoking, obesity, steroid-resistance, and bacterial airway colonization [41–43]. Studies have suggested that neutrophilic asthma is in part caused by treatment with corticosteroids as steroids are known to induce apoptosis in eosinophils, but neutrophils are less sensitive to these drugs [44, 45]. Nevertheless, this effect cannot explain the increased frequencies of neutrophils in the bronchi and the fact that neutrophilic asthma has been observed in steroid-naïve patients [46]. Another factor attributed to the limited response to antiinflammatory medication in neutrophilic asthma is the increased prevalence of bacteria found in the airways of adults, but reduced microbial diversity [40, 47].
Paucigranulocytic asthma is characterized by persistent symptoms and evidence of airway hyperresponsiveness; however, without evidence of increased eosinophils or neutrophils in sputum or blood. For this reason, in these patients, the anti-inflammatory therapies are ineffective at controlling symptoms. The pathogenesis is poorly understood, but several mechanisms have been proposed such as structural abnormalities involving airway smooth muscle and abnormal response to neuronal activation [48]. Other studies have proposed a role of oxidative stress and oxidative phosphorylation in the pathogenesis of paucigranuocytic asthma [49].
Another subgroup is obesity-related asthma. The interaction between obesity and asthma is complex and multifactorial [50]. A growing amount of evidence shows that obesity is associated with low-grade systemic inflammation, which may interact with asthma inflammation. Obesity can therefore make it more challenging to achieve good asthma control in T2-high asthma. However asthma can also be the consequence of obesity, which is associated with the T2-low endotype [50]. Considering the increase in obesity worldwide, further studies focusing on elucidating the biological mechanisms are necessary, as this may offer more therapeutic targets in pediatric obese asthma [51].
Overall, the mechanistic basis of severe pediatric asthma remains largely unknown and there is evidence that inflammatory endotypes in children are not stable [23]. Research on the mechanistic basis in children is challenging due to ethical concerns of performing bronchoscopies in healthy controls, and repeat bronchoscopies in children with asthma to assess the airway response to an intervention. However, it has become clear that results from studies with adults cannot be automatically extrapolated to the pediatric population [52].
Stepwise Asthma Management
Asthma treatment in children uses a stepwise approach, focusing on gaining and maintaining symptom control, and halting the ongoing inflammation. The first step in children 6–12 years, according to the recommendations of the Global Initiative for Asthma 2022, consists of short-acting beta 2 agonists (SABA) as rescue therapy combined with a low dose of ICS whenever the SABA is taken [8]. In step 2, the frequency of ICS use is increased to a daily low dose, or a daily leukotriene receptor antagonist is started. In step 3, either low-dose ICS is combined with a daily leukotriene receptor antagonist, a long-acting beta 2 agonist is added as an additional controller, ICS is increased to a medium dose, or a very low dose ICS–formoterol maintenance and reliever (MART) is prescribed. In step 4, a medium dose ICS and long-acting beta 2 agonist are prescribed or low dose MART. Step 5 recommends referring the patient with uncontrolled asthma despite high treatment dosages for further assessment, increasing the ICS–LABA dose or starting an add-on therapy including treatment with biologics.
Add-on Therapy with Biologics
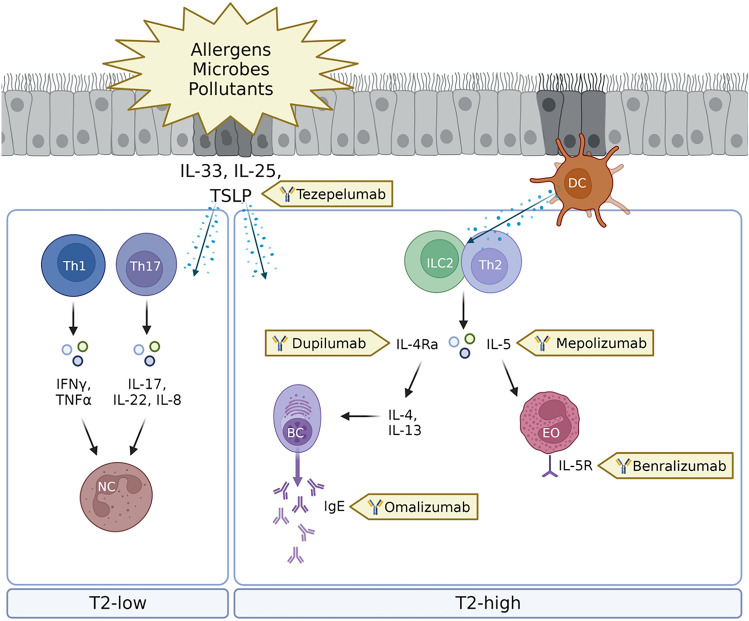
Currently, five biologics are licensed to treat severe asthma in children and adolescents by the US Food and Drug Administration (FDA) and four by the European Medicines Agency (EMA). A mAb targeting IgE (omalizumab) was the first biologic to receive approval for treating severe pediatric asthma. Already in 2003, it was approved by the US FDA and in 2005 by the EMA to treat adolescents (12 years and older) with severe allergic (IgE-mediated) asthma. In 2009, the approval of omalizumab was expanded to children with severe allergic asthma ≥ 6 years of age. Omalizumab blocks free serum IgE and limits its binding to the FcεRI receptor that prevents the release of proinflammatory mediators after an encounter with an allergen. In addition, omalizumab reduces cell-bound IgE and downregulates IgE receptors [53, 54]. A large amount of data from clinical trials and observational studies showed that omalizumab is generally well tolerated with a favorable safety profile [55]. The second biologic that entered the stage to treat severe asthma in children was mepolizumab. It was first approved in 2015 by the FDA to treat adolescents of 12 years and older. It has been available in Europe since 2018 and 2019 in the USA to treat children 6 years and above with severe eosinophil-mediated asthma. Mepolizumab binds to and neutralizes IL5, which prevents IL5 from binding to the IL5 receptor complex on the eosinophil. IL5 is a cytokine involved in the proliferation, differentiation, mobilization, activation, recruitment, and survival of eosinophils [56, 57]. As a result it selectively inhibits eosinophilic inflammation and reduces the number of eosinophils in both sputum and blood [58]. In placebo-controlled trials, the most commonly reported adverse events were injection-site reactions, respiratory infections, worsening of asthma and headaches [59]. In 2017, benralizumab was approved for patients with severe eosinophilic asthma (12 year and older) in the USA, but not in Europe. Benralizumab binds to the IL5 receptor expressed on eosinophils and basophils, resulting in drug induced apoptosis of these cells through antibody-dependent cell-mediated cytotoxicity [60]. Long-term follow-up showed that benralizumab was safe and well tolerated. The most commonly reported adverse events after administration were nasopharyngitis, worsening asthma, headaches, and respiratory tract infections [61]. Two years later, dupilumab (targeting the IL4/IL13 receptor) was approved to treat adolescents (12 years and older) with severe type 2 inflammation, and in 2021 the FDA expanded the approval to include children ≥ 6 years and older. The approval for this age group in Europe followed in 2022. In 2021, tezepelumab-ekko (antithymic stromal lymphopoietin: TSLP) was approved by the FDA for the treatment of adolescents (12 years and older) with severe asthma with no phenotype or biomarker limitations. The approval for use in Europe followed in 2022. A visual representation of the biologics used in severe pediatric asthma and their immunological targets is shown in Fig. 1.
Fig. 1.
Visual representation of the biologics used in severe pediatric asthma and their immunological targets. External triggers (e.g., allergens, microbes, and pollutants) trigger the inflammatory cascade in children with asthma. Biologics target specific inflammatory compounds to reduce airway inflammation. BC, B cell; DC, dendritic cell; EO, eosinophil; IgE, Immunoglobulin E; IL, interleukin; ILC2, innate lymphoid cell type 2; NC, neutrophil; Th, T helper cell; TSLP, thymic stromal lymphopoietin. Created with BioRender.com
Despite regulatory approval, clinical practice shows that the access to these biologics and criteria used to prescribe these drugs largely vary across countries [62, 63]. A survey among severe pediatric asthma experts from 25 European countries showed a wide variation in the biologics available in the different countries, the healthcare providers allowed to prescribe these drugs and the indicators to assess treatment success [63]. To gain more insight into the differences in clinical practice, prescription of biologics, and subsequent response to treatment, National and International Pediatric Registries of severe pediatric asthma are of importance [64, 65].
Dupilumab: IL4/IL13R mAb
Mechanism of Action
In contrast to the previously available asthma biologics, dupilumab targets a more upstream mediator of type 2 inflammation. The drug is a human IgG4 mAb directed against a shared component for IL4 and IL13 receptor complexes; IL4Rα. There are two types of IL4R: (1) type I consisting of IL4Rα and a gamma (c) component, and (2) type II consisting of IL4Rα and IL13α1 [66]. Dupilumab blocks the shared IL4Rα component, thereby inhibiting both IL4 and IL13-mediated pathways. IL4 and IL13 are mainly produced by CD4+ Th2 cells and ILC2, but a wide range of immune cells, such as eosinophils, basophils, mast cells, CD8+ cells, and natural killer cells, can also produce them [22]. Binding of IL4 and IL13 to their respective receptors activates signal transducer and activator of transcription (STAT) 6-mediated signaling, as well as other signaling pathways involved in the allergic response [66]. A study that used human Th2 cells, performed a kinetic analysis and found that 80% of IL4 regulated genes were dependent on STAT6 and included CRTH2, IL24, LTB, and SOCS1 [67]. Blocking of the receptor leads to downregulation of T2 inflammation, but there is little known on the exact mechanism of action of dupilumab [66].
Efficacy in Adolescents and Children
The initial approval of dupilumab for adults and adolescents (≥ 12 years of age) with moderate-to-severe asthma was based on three randomized clinical trials (RCTs): a phase 2b study in 769 adults [68], a phase 3 trial of 24 weeks with 210 patients (including 3 adolescents) (LIBERTY ASTHMA VENTURE) [69], and a phase 3 trial of 52 weeks with 1902 patients (including 107 adolescents ≥ 12 years of age) (LIBERTY ASTHMA QUEST) [70]. Overall, dupilumab treatment was associated with lower rates of asthma exacerbations, better lung function, and symptom control in these studies, with the greatest treatment effects observed in patients with a type 2 inflammation phenotype (based on baseline blood eosinophil counts or increased levels of FeNO [69, 71]. An overview of clinical trials of dupilumab including children and/or adolescents, is provided in Table 2.
Table 2.
Completed clinical trials of dupilumab including children or adolescents with severe asthma
| Reference | Study name | population | Intervention | Treatment duration | Primary end point | Effect |
|---|---|---|---|---|---|---|
| Bacharier et al. 2021 [73] | VOYAGE [NCT02948959] | 408 children (6–11 years); 350 had a type 2 inflammatory phenotype (defined as a blood eosinophil count of ≥150 cells per cubic millimeter or a FeNO of ≥ 20 ppb at baseline) | add-on sc dupilumab every 2 weeks (100 mg for ≤ 30 kg, 200 mg for > 30 kg) | 52 weeks | Efficacy: annualized rate of severe exacerbations | Dupilumab reduced risk of severe exacerbations. RR of severe exacerbations (treatment versus placebo) in total population: 0.46 (95% CI 0.31–0.67), in patients with type 2 inflammatory phenotype: 0.41 (95% CI: 0.27–0.61) |
| Wechsler et al. 2021 [129] | LIBERTY ASTHMA TRAVERSE [NCT02134028] | 2193 adults and 89 adolescents (aged 12–17 years) with moderate-to-severe or oral-corticosteroid-dependent severe asthma who had completed a previous dupilumab asthma study | add-on sc dupilumab every 2 weeks, 300 mg | Up to 148 weeks | Long term safety: number and percentage of patients with any treatment-emergent AE | Treatment-emergent AE were similar to shorter clinical trials and ranged from 76.3–94.7% |
| Rabe et al. 2018 [69] | LIBERTY ASTHMA VENTURE [NCT02528214] | 207 adult and 3 adolescent patients (12–17 years) patients with OCS-treated asthma | Add-on sc dupilumab every 2 weeks, 300 mg | 24 weeks | Efficacy: reduction in OCS dose from baseline | Dupilumab reduced OCS use: % change in OCS dose −70.1% in the dupilumab arm versus −41.9% on the placebo arm |
| Castro et al. 2018 [70]; Maspero et al. 2021 [72] (post hoc analysis) | LIBERTY ASTHMA QUEST [NCT02414854] | 1795 adult and 107 adolescent patients | Add-on sc dupilumab every 2 weeks, 200 mg or 300 mg | 52 weeks | Efficacy: annualized rate of severe asthma exacerbations; lung function improvement at week 12 [change in the forced expiratory volume in 1 second (FEV1)] |
Total study population: patients in the dupilumab arm had lower rates of exacerbations and better lung function. For 200 mg dosage: RR for severe exacerbations 0.52 (95% CI: 0.41–0.66), in subgroup with high eosinophil counts (≥ 300 cells/mm3) RR: 0.34 (95% CI: 0.24–0.48) Dupilumab improved lung function by week 12 by 0.47 L versus 0.22 L (placebo) Post hoc analysis in the adolescent population (n = 107): dupilumab (200 mg) decreased risk of exacerbations with 46%, while higher dose (300 mg) was associated with increased risk (13%) of exacerbations Both dupilumab dosages were associated with improved lung function by week 12 |
Only completed trials with published results are included in the table. AER, annualized rate of asthma exacerbations; FeNO, fraction of exhaled nitric oxide; OCS, oral corticosteroids; ppb, parts per billion; RR, relative risk/risk ratio; sc, subcutaneous; 95% CI, 95% confidence interval
A post hoc analysis on the efficacy of dupilumab in the adolescent population in the LIBERTY ASTHMA QUEST trial was published separately [72]. This analysis included 34 adolescents treated with 200 mg dupilumab, 34 adolescents treated with 300 mg dupilumab every 2 weeks, and 39 patients treated with a placebo. Remarkably, dupilumab (200 mg every 2 weeks) led to a reduced rate of severe exacerbations (adjusted annualized rate of severe exacerbations on 52 weeks of treatment: 0.19 [0.08–0.44] versus 0.36 [0.17–0.75] in the placebo group), while an opposite effect was seen in the group treated with the higher dosage of dupilumab (300 mg every 2 weeks). In this group, there was a 13% increased risk of exacerbations (adjusted annualized rate of severe exacerbations: 0.37 [0.19–0.72] versus 0.33 [0.14–0.78] in the respective placebo arm). This increased risk was also visible when the analyses were restricted to adolescents with a marked T2 inflammatory phenotype [based on blood eosinophils ≥ 150 cells/µl or FeNO ≥ 20 parts per billion (ppb)]. The investigators hypothesized that an imbalance in the number of severe exacerbations in the preceding year between the 300 mg group and the matched placebo (mean: 1.53 versus 2.22) caused this effect. Both dupilumab dosages (200 mg and 300 mg) were associated with improved lung function upon 12 weeks of treatment; however, there was no statistically significant improvement in symptom scores [72].
The expanded approval of dupilumab to children ≥ 6 years of age was based on the results of the VOYAGE trial [73]. This phase 3 RCT assessed the efficacy of add-on dupilumab among 408 children (6–11 years) with uncontrolled moderate-to-severe asthma [73]. The majority of children had a T2 inflammatory phenotype (n = 350) based on increased blood eosinophil levels (≥ 150 cells/mm3) or increased fraction of exhaled nitric oxide (FeNO ≥ 20 ppb). Participants received a subcutaneous injection of dupilumab or matched placebo every 2 weeks and were followed for 52 weeks. The primary outcome was the annualized rate of severe exacerbations; defined as asthma-related OCS use for at least 3 days, hospitalization, or emergency department (ED) visit leading to OCS use. Dupilumab treatment reduced the risk of severe exacerbations in the total study population [rate ratio (RR): dupilumab versus placebo: 0.46, 95% confidence interval (CI): 0.31–0.67]. As expected, based on the mechanism of action of dupilumab, the effect size was slightly higher in the subgroup of patients with T2 inflammation (RR: 0.41, 95% CI: 0.27–0.61) and highest in the subgroup of patients with blood eosinophil counts ≥ 300 cells/mm3 (RR: 0.35, 95% CI: 0.22–0.56). Overall, 63% of the trial participants had eosinophil levels ≥ 300 cells/mm3. Secondary end points included lung function improvement upon 12 weeks of treatment and change in asthma control score upon 24 weeks of treatment. Dupilumab treatment significantly improved lung function, with least squares (LS) mean difference versus placebo in change in percent predicted forced expiratory volume in 1 second (ppFEV1) from baseline being 4.7 (1.9–7.5) in the total population, and symptoms scores, with LS mean difference in change from baseline asthma control questionnaire 7 (ACQ-7) score being −0.28 (−0.44 to −0.12) in the total study population. Also, for the secondary outcomes, effect estimates were slightly higher for the children with a T2 inflammatory phenotype [73].
In the VOYAGE trial, the effects of dupilumab on median levels of T2 biomarkers was also assessed over the 52-week treatment period: serum total IgE (IU/mL), serum thymus and activation-regulated chemokine (TARC) (ng/L), blood eosinophil count (cells/μL), and FeNO (ppb). At week 52, reductions in baseline were seen in al T2 biomarkers [74].
A RCT that aims to assess the effect of add-on dupilumab for 12 months on asthma exacerbations in urban children and adolescents 6–17 years with T2-high exacerbation-prone asthma [NCT05347771] is currently ongoing [75].
Safety
The post hoc analysis of the LIBERTY ASTHMA QUEST showed a similar safety profile in adolescents and adults [72]. The most common side effects of dupilumab are injection site reactions such as pain, swelling, itching and erythema, (allergic) conjunctivitis, oral herpes, eosinophilia, and arthralgia. The VOYAGE trial did not find significant differences in the frequency of serious adverse events in children in the intervention arm compared with the children in the placebo arm. However, eosinophilia was more frequently observed in the dupilumab study arm (5.9% versus 0.7%) [73].
Tezepelumab: TSLP mAb
Mechanism of Action
Tezepelumab-ekko (tezepelumab) is a human IgG2 mAb that targets thymic stromal lymphopoietin (TSLP) [76]. TSLP is an upstream regulator of airway inflammation. The airway epithelium acts as a first line of defense against potentially immunogenic triggers such as allergens, viruses, and pollutants. TSLP is an alarmin cytokine produced in copious amounts by airway epithelium [77]. Upon exposure to environmental triggers, epithelial cells and stromal cells in the respiratory tract can release TSLP. However, other immune cells, e.g., mast cells, dendritic cells, and basophils, are also sources of TSLP [78]. Upon binding to the TSLP receptor, multiple signaling cascades are triggered that induce a Th2 response. TSLP induces JAK1 and JAK2 activation, essential for the signaling of type I and type II cytokines [79]. In the end, dendritic cells are activated driving the polarization of naïve T cells toward Th2, and activated ILC2s produce IL4, IL5, and IL13 [80, 81]. Moreover, TSLP has also been implicated in Th2-low inflammation; however, this process is less well understood [82]. It has been proposed that TSLP activates dendritic cells resulting in the polarization of naïve T cells toward a Th17 phenotype [83]. The subsequent release of IL17, which induces neutrophilic chemokines, is then responsible for recruiting neutrophils to the airway, resulting in neutrophilic inflammation [84].
Efficacy in Adolescents
Tezepelumab is the most recently approved biologic and the first asthma biologic to have been approved without an asthma phenotype limitation [85]. In contrast to the other approved asthma biologics, this drug is also available to treat patients with a T2-low phenotype. The market approval in the USA was based on two large RCTs: a phase 2 study including 550 adult patients (PATHWAY; NCT02054130) [86] and a phase 3 study (NAVIGATOR; NCT03347279) including 1061 patients (of which 82 were adolescent patients) [87]. An overview of clinical trials of tezepelumab, including adolescents, is reported in Table 3.
Table 3.
Completed clinical trials of tezepelumab including children or adolescents with severe asthma
| Reference | Study name | Population | Intervention | Treatment duration | Primary end point | Effect |
|---|---|---|---|---|---|---|
| Menzies-Gow et al., 2021 [87] | NAVIGATOR (NCT03706079) | 979 adults and 82 adolescents (12–17 years) with severe uncontrolled asthma | Sc tezepelumab (210mg) every 4 weeks | 52 weeks | Efficacy: annualized rate of asthma exacerbations (AER) | Tezepelumab treatment reduced risk of asthma exacerbations: AER in tezepelumab group 0.93 (95% CI: 0.80–1.07) versus 2.10 (95%CI: 1.84–2.39) [placebo]; RR: 0.44 (95% CI: 0.37–0.53). In patients with high eosinophil counts (≥ 300 cells/mm3) RR: 0.30 (95% CI: 0.22–0.40) |
| Shinkai et al., 2023 [90] | NOZOMI (NCT04048343) | 65 Japanese patients with inadequately controlled severe asthma (including 1 patient ≤ 18 years of age) | Sc tezepelumab (210mg) every 4 weeks | 52 weeks | Safety: (serious) adverse events | 4 out of 65 participants experienced SAE, 39/65 experienced AE |
| Menzies-Gow et al., 2020 [130] | DESTINATION (NCT03706079) | 951 patients with severe, uncontrolled asthma, including 82 adolescents who participated previously in the NAVIGATOR trial | Sc tezepelumab (210 mg) every 4 weeks | 104 weeks | Safety: (serious) adverse events | Incidence rate of SAE in the tezepelumab arm (of participants originally in NAVIGATOR trial): 7.9 versus 12.5 in the placebo arm |
| Alpizar et al., 2021 [94] | PATH–HOME (NCT03968978) | 216 patients with severe, uncontrolled asthma (including 24 adolescents) | Sc tezepelumab (210 mg) every 4 weeks via accessorized prefilled syringe (APFS) or via autoinjector (AI) | 24 weeks | Performance of autoinjector device/accessorized prefilled syringe for at-home administration | APFS and AI performed equally well at home and in the clinic |
Only completed trials with published results are included in the table. AE, adverse effects; AER, annualized rate of asthma exacerbations; AI, autoinjector; APFS, accessorized prefilled syringe; RR, relative risk/risk ratio; Sc, subcutaneous; 95% CI, 95% confidence interval
Overall, data on the efficacy of tezepelumab in adolescents are scarce and have not been published separately. The NAVIGATOR study recruited patients with asthma from 12 to 80 years of age with severe uncontrolled asthma. In total, 82 out of the 1061 participants were ≤ 18 years old. Patients in the intervention arm received add-on tezepelumab (210 mg) subcutaneously every 4 weeks for 52 weeks. The primary outcome was asthma exacerbations. Secondary outcomes included lung function, asthma control, and health-related quality of life. In the total study population, tezepelumab treatment was associated with a decreased rate of asthma exacerbations (annualized rate of asthma exacerbations was 0.93, 95% CI: 0.80–1.07 versus 2.10, 95% CI: 1.84–2.39 in the placebo group). More than half of the participants had baseline blood eosinophil counts < 300 cells/μl, and over a quarter of the study population had baseline blood eosinophil counts < 150 cells/μl. In these patient groups, the beneficial effect of tezepelumab treatment on exacerbation rates was observed; RR in patients with blood eosinophil counts < 300 cells/μl was 0.59 (95% CI: 0.46–0.75), RR in patients with blood eosinophil counts < 150 cells/μl was 0.61 (95% CI: 0.42–0.88). This illustrates that the efficacy of tezepelumab increases with increasing concentrations of T2 biomarkers [88]. Furthermore, restricted to the adolescent participants in the trial no significant reduction in exacerbation frequency was found, with a RR of 0.7 (95% CI: 0.34–1.46). This could be due to a lack of power. Nonetheless, tezepelumab was registered for adolescents. More research is needed to assess the efficacy of tezepelumab in adolescents [87].
A safety extension study to assess the safety and tolerability of tezepelumab (DESTINATION, NCT03706079) (up to 2 years of treatment) has recently been completed [89]. Patients from the NAVIGATOR or SOURCE trial (the latter did not include patients < 18 years of age) were followed for 104 weeks. A secondary outcome included annualized exacerbation rate upon 2 years of treatment. In the overall study population originally included in the NAVIGATOR trial, long-term tezepelumab had a beneficial effect on exacerbations with RR of 0.42 (95% CI: 035–0.51).
Safety
The DESTINATION trial primarily investigated long-term safety and tolerability and reported a decreased incidence rate of serious adverse events (SAE) in the tezepelumab arm (of participants originally in NAVIGATOR trial): 7.9 versus 12.5 in the placebo arm. Results for the adolescent population have not been reported separately. Another clinical trial (NOZOMI; NCT04048343) assessed safety upon 52 weeks of tezepelumab in Japanese patients (including one patient ≤ 18 years of age) [90]. In this single-arm clinical trial, 4 out of 65 participants experienced SAE (atrial fibrillation, viral gastroenteritis, lung abscess, tonsillitis), 39/65 experienced adverse events (AE), with the most common being nasopharyngitis (20%).
From Hospital to Home Administration of Biologics
Previously, biologic treatment required regular (2–4 weekly) clinical visits to administrate the drugs and monitor potential adverse reactions. However, self-injection pens and prefilled syringes have recently become available, and all biologics used for treating severe asthma have now gained approval for patient/caregiver administration. Home administration is a big step forward in the treatment of severe childhood asthma, as it brings health care toward the patient, saves travel time and decreases hospital-related school absences. The coronavirus disease 2019 (COVID-19) pandemic has accelerated the transition from hospital to home administrations of biologics for patients with severe asthma [91].
In 2019, mepolizumab was the first biologic to receive approval for self-administration in the USA and EU for patients 12 years and older. The approval was based on positive patient experience data from two open-label single-arm phase 3a studies that also included a small proportion of adolescents. Bel et al., performed a single-arm trial in which mepolizumab was administrated via a safety syringe [92]. The study included 56 patients, including 2 adolescents. Upon training, a first drug dose was provided in the clinic, the second was self-administered at home, and a final drug dose was self-administered at the clinic under observation. All participants successfully administered mepolizumab. Second, Bernstein et al., assessed the usability of a single-use prefilled autoinjector (AI) for mepolizumab self-administration in 159 patients, including 11 adolescent patients [93]. The drug was administered with an AI every 4 weeks for a total treatment duration of 12 weeks. Almost all patients (> 95%) successfully self-administered the drug in the clinic or at home using AI. In 2022, the approval was extended for at-home administration in patients 6–11 years old with severe eosinophilic asthma.
The PATH–HOME study assessed the functionality of an AI device or accessorized prefilled syringe for tezepelumab treatment in 216 patients with asthma, including 24 adolescents [94]. Patients received 6 dosages of tezepelumab over a period of 24 weeks. The first 3 dosages were provided in the clinic, followed by 2 dosages at home and a final dose in the clinic. Patients or caregivers were trained to administer the study drug. Results showed that tezepelumab could be administrated successfully at home in the adolescent population by themselves or by a caregiver.
Real-world experiences from a severe asthma clinic in the UK showed that home administration of omalizumab and mepolizumab in children (6–18 years) was feasible and did not decrease the quality of care [95]. Sixteen out of the 23 families with children who received biologic treatment were willing to administer the drug at home. Administration by patients or caregivers was supported by video calls. During 2 out of 75 occasions, the biologic was administered inaccurately; however, due to the video supervision, these issues were resolved and there were no adverse effects.
An international qualitative study among 75 adult patients and 12 healthcare providers found that both groups agree that the benefits of home administration of biologics outweigh the disadvantages, but the transition to home administration should be closely monitored [91]. Adult patients indicate the importance of clear instructions, training of administration in the clinic, accessible contact options with the health care providers, and close monitoring of the patients.
Furthermore, at home administrations might not be suitable for all children or adolescents with severe asthma and/or their caregivers since administration of biologics is currently by injection and not by nebulizers or orally. Children for instance, are more likely to fear receiving an intramuscular injection and caregivers could find administering intramuscular injections difficult and burdensome. There is a need for studies assessing perceptions of pediatric and adolescent patients and their caregivers regarding home administration since these might differ from adult patients.
Biomarkers to Guide Treatment Efficacy
With the introduction of biologics for severe asthma, there is an increased need to improve patient selection, predict treatment response and monitor the efficacy of these costly and, for children, sometimes burdensome therapies. Biomarkers can provide information on the underlying mechanisms driving the disease process and help identify the optimal treatment for each patient and monitor treatment response. The most studied biomarkers for severe asthma are related to T2 inflammation and include eosinophils, FeNO, IgE, and periostin [26]. These biomarkers largely overlap for the various biologics available since most target T2 inflammation, resulting in patients meeting the criteria for more than one biologic. Furthermore, with the biomarkers that are currently used, levels fluctuate over time due to the clinical condition and the recent or current use of OCS. In addition, applied cut-off values of these biomarker levels are predominantly based on adult data.
Eosinophils
Several studies have shown a relation between a high eosinophil count and response to biologics that target eosinophilic inflammation, such as anti-IL5 (mepolizumab), anti-IL5Rα (benralizumab), and anti-IL4Rα (dupilumab). For example, a secondary meta-analysis of the DREAM and MENSA study with a total of 1192 participants, including 26 adolescents, showed that the reduction in exacerbation rate with mepolizumab versus placebo increased progressively with increasing blood eosinophil count [96]. Similar results have been reported in adults for dupilumab and benralizumab [97]. For omalizumab, a meta-analysis of two pediatric studies evaluating efficacy predictors also found that higher blood eosinophils was a valuable marker for selecting patients who may benefit most [98]. Studies in tezepelumab, however, found the treatment response to be irrespective of baseline high and low type 2 inflammatory status [86, 87].
FeNO
FeNO is a marker of airway inflammation in the respiratory tract that can be used as an additional biomarker of T2 inflammation, primarily reflecting IL13 activity. In a post hoc analysis of the LIBERTY ASTHMA QUEST study, increased baseline FeNO was associated with greater clinical effects from dupilumab compared with placebo [72]. This association was independent of eosinophil levels and other clinical characteristics [99]. In response to these findings, a retrospective review of 229 adult patients with severe eosinophilic asthma treated with mepolizumab or benralizumab was performed to compare treatment responses across patients grouped by baseline FeNO level. Here FeNO did not seem to predict response [100]. Lastly, for omalizumab baseline FeNO seems to differ significantly between responders and non-responders in adults [weighted mean difference (WMD): 19.2 ppb, 95% CI: 14.82–23.56 ppb; p < 0.001] [98]. However, in a subgroup analysis for 643 pediatric patients, only a trend of lower FeNO without statistical significance was observed [98]. More studies in adults and children are necessary to establish which biologic is optimal for FeNO high patients with a high eosinophil count. Furthermore, reference values for FeNO levels are dependent on age in children, making its interpretation more challenging [101].
IgE
Other important biomarkers of T2 inflammation are total and allergen-specific IgE levels. However, for omalizumab, a recent meta-analysis in pediatric patients showed a trend but no statistical significance towards better therapeutic response with higher IgE baseline levels (WMD 50.30, 95% CI: − 5.91 to − 106.51) [98], therefore making the role for IgE as a biomarker for prediction of treatment response uncertain.
Periostin
Periostin is a protein that, besides being involved in the development of various tissues, such as bone and cartilage, also has a role as an inflammatory regulator. Some studies found elevated expression in adults with T2-high asthma, and it has therefore been proposed as a biomarker for severe asthma and to monitor biologic treatment in adults [102]. Periostin differs from other biomarkers related to T2 inflammation, as it seems to have an association with subepithelial fibrosis and fixed airflow limitation [103], and thus may contribute to airway remodeling in asthma. In a RCT of adults with steroid-naïve asthma before and after treatment with an ICS, serum periostin concentrations were correlated with airway wall thickness and inversely correlated with airflow limitation [104]. However, in children it cannot be used as reliable biomarker since it is produced in bone and its production is influenced by growth and age [105].
Non-type 2 biomarkers
T2-low asthma is currently defined by the absence of T2-high biomarkers, and as a result no validated biomarkers are used in clinical practice [106]. However, in vitro and in vivo studies have identified pathways and possible related biomarkers. The biomarker most frequently discussed is neutrophil levels in sputum. However, sputum analysis might not always be feasible to perform in children and is often limited to research services and specialized centers. Although there is no consensus on the percentage, a value above 40–76% has been recognized as a patient having neutrophilic asthma [41, 107]. In addition to sputum leukocyte count, multiple cytokines are under investigation as potential T2-low biomarkers. In adult patients with severe asthma correlations between sputum neutrophil levels and levels of IL17 in circulation and induced sputum have been found [106, 108]. Furthermore, IL8 levels in the sputum correlated with the sputum neutrophil count in adult severe asthmatics [109]. Other potential biomarkers of neutrophilic asthma are myeloperoxidase (MPO) and neutrophilic elastase [106, 110]. There are presently no biomarkers for patients with paucigranulocytic asthma.
Future perspectives
In addition to the biologics currently available, multiple new biologics targeting the immunological pathway are being developed and researched. Especially for patients with a T2-low asthma phenotype, more treatment options must become available. Phase II clinical trials are now running to test ecleralimab, the first inhaled anti-TSLP therapy for treating severe asthma [111]. Ecleralimab is a neutralizing antibody fragment directed against human TSLP and formulated as a powder for delivery to the lungs through an inhaler device. Administration of a mAb via the lungs may have advantages compared with systemic drug delivery; potentially, lower doses can be administered, leading to fewer side effects. Furthermore, many asthmatic patients are already accustomed to dry powder inhaler devices, and administration might be perceived as less burdensome compared with intravenous or subcutaneous administration.
Astegolimab is another biologic in development, potentially targeting T2-high and T2-low asthma. Astegolimab is a human mAb that blocks IL33 signaling by targeting ST2, the IL33 receptor [112]. IL33 is predominantly produced by airway epithelial cells and is released in response to tissue injury as an alarmin and can activate Th1 and Th2 cells, as well as ILC2s. A phase II study (ZENTYATTA) evaluated the efficacy and safety of subcutaneous administration of astegolimab and found reduced asthma exacerbation rates versus placebo after 54 weeks of treatment, irrespective of the patients’ asthma phenotype (T2-high or T2-low).
In addition to developing new medication, it is important to collect real-life data of children currently prescribed biologics. Previous results indicate that in adults, only 25–35% of patients who are prescribed biologics would have been identified as eligible candidates to participate in a clinical trial due to the strict inclusion criteria, demonstrating a discrepancy between the trial and real-life population [113]. In pediatric patients treated with omalizumab a real-life study has been performed, confirming the positive results found in clinical trials [114]. The MUPPITS-2 study also provided more insight into the effect of biologics in an under-represented population in clinical trials [115]. This RCT assessed the efficacy and safety of phenotype-directed therapy with mepolizumab in an urban pediatric population in the USA, with a high number of Black and Hispanic individuals, and found that mepolizumab significantly reduced the number of asthma exacerbations. They did not find an improvement in other asthma outcomes, such as Composite Asthma Severity Index (CASI) scores, physician–patient global assessment of response to therapy, and lung function. A European Respiratory Society (ERS) initiative to learn more about the clinical characteristics of pediatric patients with severe asthma in secondary/tertiary care centers across Europe is the Severe Pediatric Asthma Collaborative (SPACE) [64]. SPACE aims to provide real-life data by setting up a prospective web-based database, incorporating baseline and annual follow-up data. Gaining more insight into the real-life setting of the prescription and response to biologic treatment will help to guide treatment in the future. Furthermore, when assessing treatment response, it is important to focus on patient-centered definitions and to understand the views of children and adolescents with severe asthma and their caregivers [116]. Over the last decades, a third of the RCTs on treatments of severe asthma (37%) did not include health-related quality of life (QoL) as the clinical trial end point [117]. However, most patients consider improved QoL as the most important outcome to assess response to biological therapy [118]. Other important outcomes for patients when assessing response were social interaction, fatigue, ability to participate in everyday life, and impact on mental health [119, 120].
Another real-life research gap that studies could contribute to is information on the long-term consequences of biologic use in children and its effects on a developing immune system. Since eosinophils have an important role in homeostasis and immunity, it remains unclear whether there are health related risks concerning long-term eosinophil depletion in a developing immune system. In addition, long-term follow-up is crucial to answer uncertainties regarding optimal treatment duration, approach to discontinuation and to explore if there is a prolonged effectiveness of these immunomodulatory drugs [121]. Uncontrolled asthma in children has been associated with an increased risk of irreversible lung damage and life-long complications. The Preventing Asthma in high-Risk Kids study (PARK, NCT02570984) is currently ongoing and is the first to evaluate the disease modifying effect of biologics by exploring whether a 2-year treatment of preschool children at high risk for asthma with omalizumab will prevent the progression to childhood asthma.
Moreover, a substantial proportion of asthmatic children receiving treatment with biologics are considered to be non-responders. For example, omalizumab has an estimated response rate of only 60% in severe asthmatic children ≥ 12 years [114]. Hence, to optimize the treatment and management of children with severe asthma, there is a need for a head-to-head comparison. Yet this is complicated due to the differences in indications of the various biologics (Table 1). The Treating Severe Pediatric Asthma (TREAT) trial, is a randomized controlled non-inferiority trial that is currently being performed to compare mepolizumab versus omalizumab [122]. In addition, there is a need for new trial designs addressing biologic switching in case of non-response, as performed in adult patients with severe asthma [123]. Furthermore, in responders to biologics, various questions remain to be answered, such as: can the time between subsequent dose administration be extended? When and in which patients can biologics treatment be discontinued without an increase in symptoms? [124, 125] Which biomarkers can aid with these decisions?
Consensus on a set of core outcome measures is of utmost importance to compare the effectiveness of biologic therapies for asthma. Recently, the Core Outcome Measures sets for pediatric and adult Severe Asthma (COMSA) working group of the 3TR consortium published a statement on core outcome measures [126]. For pediatric asthma, this included the Pediatric Asthma Quality of Life Questionnaire, Asthma Control Test (ACT) or Childhood-ACT, FEV1 as z-scores, annual frequency of severe exacerbations, and maintenance OCS use. However, there is still no consensus on the definition of response to biologic therapy, which would facilitate better comparison of treatment efficacy.
Lastly, as the predictive value of currently available biomarkers is still relatively limited. There is an urgent need for a better understanding of underlying molecular mechanisms and identification of novel (clinically applicable) biomarkers to assess treatment efficacy [19]. Rapid advances in omics technology might provide novel candidates, such as non-invasive markers in exhaled breath or microbiome, epigenomic, transcriptomic, or metabolomics patterns reflecting distinct underlying inflammatory patterns [127, 128]. However, the road from preclinical biomarker discovery to clinical implementation is long and costly. It requires discipline overarching collaborations to successfully pave the way for precision medicine in pediatric asthma.
Conclusion
Biologics to treat severe pediatric asthma have provided the opportunity for targeted therapy to the immunological pathway driving the disease. Recently two more biologics were licensed to treat severe asthma in children and adolescents: dupilumab (anti-IL4/IL13R) and tezepelumab (anti-TSLP). However, efficacy and safety data in the pediatric population still remains scarce, and more research is needed to look at predictive biomarkers to improve treatment selection for each individual patient and predict and monitor response. There is a continuous need to develop new medication for T2-low asthma, for real-life studies in populations currently underrepresented in clinical trials and to assess patient-centered outcomes when assessing response. Furthermore, at-home administration might lessen the burden of drug administration for children, as it brings health care toward the patient, saves travel time, and decreases hospital-related school absences.
Declarations
Funding
S.J.H.V. is PI of the PERMEABLE consortium. The PERMEABLE consortium is supported by ZonMW (project number: 456008004), the Swedish Research Council (project number 2018-05619), the Ministry of Education, Science and Sport of the Republic of Slovenia (contract number C3330-19-252012), and the German Ministry of Education and Research (BMBF) (project number FKZ01KU1909A), under the frame of the ERA PerMed JTC 2018 Call. Furthermore, she is PI of the PANDA study (supported by Lung Foundation Netherlands, YI grant: 6.2.18.244JO).
Conflict of interest
N.R. has received a fee for participating in advisory boards for Sanofi (2021) and GSK (2018). A.H.M.v.d.Z. has been reimbursed for visiting the ATS by Chiesi, received a fee for participating in advisory boards for Boehringer lngelheim and AstraZeneca, and received an unrestricted research grant from GSK. Y.E.v.D., G.K, S.H., S.H. and S.J.H.V. have nothing to disclose.
Availability of data and material
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
YD: conceptualization, writing—original draft preparation, NR: conceptualization, writing—reviewing and editing, KG: writing—reviewing and editing, HS: writing—reviewing and editing, SH: writing—reviewing and editing, A-HM-Z: writing—reviewing and editing, SV: conceptualization, writing—original draft preparation, supervision.
References
- 1.Fleming L, Murray C, Bansal AT, Hashimoto S, Bisgaard H, Bush A, et al. The burden of severe asthma in childhood and adolescence: results from the paediatric U-BIOPRED cohorts. Eur Respir J. 2015;46(5):1322–1333. doi: 10.1183/13993003.00780-2015. [DOI] [PubMed] [Google Scholar]
- 2.Moonie SA, Sterling DA, Figgs L, Castro M. Asthma status and severity affects missed school days. J Sch Health. 2006;76(1):18–24. doi: 10.1111/j.1746-1561.2006.00062.x. [DOI] [PubMed] [Google Scholar]
- 3.van den Bemt L, Kooijman S, Linssen V, Lucassen P, Muris J, Slabbers G, et al. How does asthma influence the daily life of children? Results of focus group interviews. Health Qual Life Outcomes. 2010;8:5. doi: 10.1186/1477-7525-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montalbano L, Ferrante G, Montella S, Cilluffo G, Di Marco A, Bozzetto S, et al. Relationship between quality of life and behavioural disorders in children with persistent asthma: a Multiple Indicators Multiple Causes (MIMIC) model. Sci Rep. 2020;10(1):6957. doi: 10.1038/s41598-020-62264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown HM, Storey G. Beclomethasone dipropionate steriod aerosol in treatment of perennial allergic asthma in children. Br Med J. 1973;3(5872):161–164. doi: 10.1136/bmj.3.5872.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kachroo P, Stewart ID, Kelly RS, Stav M, Mendez K, Dahlin A, et al. Metabolomic profiling reveals extensive adrenal suppression due to inhaled corticosteroid therapy in asthma. Nat Med. 2022;28(4):814–822. doi: 10.1038/s41591-022-01714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Axelsson I, Naumburg E, Prietsch SO, Zhang L. Inhaled corticosteroids in children with persistent asthma: effects of different drugs and delivery devices on growth. Cochrane Database Syst Rev. 2019;6(6):Cd010126. [DOI] [PMC free article] [PubMed]
- 8.GINA Report. Global Strategy for Asthma Management and Prevention. Available at: http://www.ginasthma.org. 2022. Accessed August 2023.
- 9.Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71(4):339–346. doi: 10.1136/thoraxjnl-2015-207630. [DOI] [PubMed] [Google Scholar]
- 10.Aljebab F, Choonara I, Conroy S. Systematic review of the toxicity of long-course oral corticosteroids in children. PLoS ONE. 2017;12(1):e0170259. doi: 10.1371/journal.pone.0170259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush A, Fitzpatrick AM, Saglani S, Anderson WC, Szefler SJ. Difficult-to-treat asthma management in school-age children. J Allergy Clin Immunol Pract. 2022;10(2):359–375. doi: 10.1016/j.jaip.2021.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Bagnasco D, Paggiaro P, Latorre M, Folli C, Testino E, Bassi A, et al. Severe asthma: One disease and multiple definitions. World Allergy Organ J. 2021;14(11):100606. doi: 10.1016/j.waojou.2021.100606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bousquet J, Mantzouranis E, Cruz AA, Aït-Khaled N, Baena-Cagnani CE, Bleecker ER, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126(5):926–938. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Bossley CJ, Fleming L, Ullmann N, Gupta A, Adams A, Nagakumar P, et al. Assessment of corticosteroid response in pediatric patients with severe asthma by using a multidomain approach. J Allergy Clin Immunol. 2016;138(2):413–20.e6. doi: 10.1016/j.jaci.2015.12.1347. [DOI] [PubMed] [Google Scholar]
- 15.Cook J, Beresford F, Fainardi V, Hall P, Housley G, Jamalzadeh A, et al. Managing the pediatric patient with refractory asthma: a multidisciplinary approach. J Asthma Allergy. 2017;10:123–130. doi: 10.2147/JAA.S129159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Food & Drug Administration. Drugs@FDA: FDA-Approved Drugs - Xolair. Available at: https://www.accessdata.fda.gov/SCRIPTS/CDER/DAF/index.cfm?event=overview.process&ApplNo=103976. Accessed August 2023.
- 17.Alizadeh Bahmani AH, Abdel-Aziz MI, Maitland-van der Zee AH, Vijverberg SJH. Recent advances in the treatment of childhood asthma: a clinical pharmacology perspective. Expert Rev Clin Pharmacol. 2022;15(10):1165–76. [DOI] [PubMed]
- 18.Principe S, Vijverberg SJH, Abdel-Aziz MI, Scichilone N, Maitland-van der Zee AH. Precision medicine in asthma therapy. Handb Exp Pharmacol. 2022. [DOI] [PubMed]
- 19.Golebski K, Dankelman LHM, Björkander S, Bønnelykke K, Brinkman P, Deschildre A, et al. Expert meeting report: towards a joint European roadmap to address the unmet needs and priorities of paediatric asthma patients on biologic therapy. ERJ Open Res. 2021;7(4). [DOI] [PMC free article] [PubMed]
- 20.Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Resp J. 2020;55(1). [DOI] [PubMed]
- 21.Licari A, Manti S, Castagnoli R, Parisi GF, Salpietro C, Leonardi S, et al. Targeted therapy for severe asthma in children and adolescents: current and future perspectives. Paediatr Drugs. 2019;21(4):215–237. doi: 10.1007/s40272-019-00345-7. [DOI] [PubMed] [Google Scholar]
- 22.Licari A, Castagnoli R, Marseglia A, Olivero F, Votto M, Ciprandi G, et al. Dupilumab to treat type 2 inflammatory diseases in children and adolescents. Paediatr Drugs. 2020;22(3):295–310. doi: 10.1007/s40272-020-00387-2. [DOI] [PubMed] [Google Scholar]
- 23.Fleming L, Tsartsali L, Wilson N, Regamey N, Bush A. Sputum inflammatory phenotypes are not stable in children with asthma. Thorax. 2012;67(8):675–681. doi: 10.1136/thoraxjnl-2011-201064. [DOI] [PubMed] [Google Scholar]
- 24.Pijnenburg MW, Frey U, De Jongste JC, Saglani S. Childhood asthma: pathogenesis and phenotypes. Eur Resp J. 2022;59(6). [DOI] [PubMed]
- 25.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372(9643):1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 26.Diamant Z, Vijverberg S, Alving K, Bakirtas A, Bjermer L, Custovic A, et al. Towards clinically applicable biomarkers for asthma—an EAACI position paper. Allergy. 2019. [DOI] [PubMed]
- 27.Heijink IH, Kies PM, Kauffman HF, Postma DS, van Oosterhout AJ, Vellenga E. Down-regulation of E-cadherin in human bronchial epithelial cells leads to epidermal growth factor receptor-dependent Th2 cell-promoting activity. J Immunol. 2007;178(12):7678–7685. doi: 10.4049/jimmunol.178.12.7678. [DOI] [PubMed] [Google Scholar]
- 28.Wawrzyniak P, Wawrzyniak M, Wanke K, Sokolowska M, Bendelja K, Rückert B, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017;139(1):93–103. doi: 10.1016/j.jaci.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 29.Striz I, Golebski K, Strizova Z, Loukides S, Bakakos P, Hanania NA, et al. New insights into the pathophysiology and therapeutic targets of asthma and comorbid chronic rhinosinusitis with or without nasal polyposis. Clin Sci (Lond) 2023;137(9):727–753. doi: 10.1042/CS20190281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golebski K, Röschmann KI, Toppila-Salmi S, Hammad H, Lambrecht BN, Renkonen R, et al. The multi-faceted role of allergen exposure to the local airway mucosa. Allergy. 2013;68(2):152–160. doi: 10.1111/all.12080. [DOI] [PubMed] [Google Scholar]
- 31.Golebski K, Layhadi JA, Sahiner U, Steveling-Klein EH, Lenormand MM, Li RCY, et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity. 2021;54(2):291–307.e7. doi: 10.1016/j.immuni.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 32.van der Ploeg EK, Golebski K, van Nimwegen M, Fergusson JR, Heesters BA, Martinez-Gonzalez I, et al. Steroid-resistant human inflammatory ILC2s are marked by CD45RO and elevated in type 2 respiratory diseases. Sci Immunol. 2021;6(55). [DOI] [PubMed]
- 33.Marcos MC, Cisneros SC. What is the added value of FeNO as T2 biomarker? Front Allergy. 2022;3:957106. doi: 10.3389/falgy.2022.957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109(3):410–418. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 35.Comberiati P, Di Cicco ME, D'Elios S, Peroni DG. How much asthma is atopic in children? Front Pediatr. 2017;5:122. doi: 10.3389/fped.2017.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hondowicz BD, An D, Schenkel JM, Kim KS, Steach HR, Krishnamurty AT, et al. Interleukin-2-dependent allergen-specific tissue-resident memory cells drive asthma. Immunity. 2016;44(1):155–166. doi: 10.1016/j.immuni.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bossley CJ, Fleming L, Gupta A, Regamey N, Frith J, Oates T, et al. Pediatric severe asthma is characterized by eosinophilia and remodeling without T(H)2 cytokines. J Allergy Clin Immunol. 2012;129(4):974–82.e13. doi: 10.1016/j.jaci.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su MW, Lin WC, Tsai CH, Chiang BL, Yang YH, Lin YT, et al. Childhood asthma clusters reveal neutrophil-predominant phenotype with distinct gene expression. Allergy. 2018;73(10):2024–2032. doi: 10.1111/all.13439. [DOI] [PubMed] [Google Scholar]
- 39.Wisniewski JA, Muehling LM, Eccles JD, Capaldo BJ, Agrawal R, Shirley DA, et al. TH1 signatures are present in the lower airways of children with severe asthma, regardless of allergic status. J Allergy Clin Immunol. 2018;141(6):2048–60.e13. doi: 10.1016/j.jaci.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crisford H, Sapey E, Rogers GB, Taylor S, Nagakumar P, Lokwani R, et al. Neutrophils in asthma: the good, the bad and the bacteria. Thorax. 2021;76(8):835–844. doi: 10.1136/thoraxjnl-2020-215986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133(6):1557–63.e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanović R. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161(1):9–16. doi: 10.1164/ajrccm.161.1.9802048. [DOI] [PubMed] [Google Scholar]
- 43.Shaw DE, Berry MA, Hargadon B, McKenna S, Shelley MJ, Green RH, et al. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest. 2007;132(6):1871–1875. doi: 10.1378/chest.07-1047. [DOI] [PubMed] [Google Scholar]
- 44.Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax. 2010;65(5):384–390. doi: 10.1136/thx.2009.126722. [DOI] [PubMed] [Google Scholar]
- 45.Saffar AS, Ashdown H, Gounni AS. The molecular mechanisms of glucocorticoids-mediated neutrophil survival. Curr Drug Targets. 2011;12(4):556–562. doi: 10.2174/138945011794751555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimoda T, Obase Y, Nagasaka Y, Nakano H, Kishikawa R, Iwanaga T. Airway inflammation phenotype prediction in asthma patients using lung sound analysis with fractional exhaled nitric oxide. Allergol Int. 2017;66(4):581–585. doi: 10.1016/j.alit.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 47.Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L, et al. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS ONE. 2014;9(6):e100645. doi: 10.1371/journal.pone.0100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sze E, Bhalla A, Nair P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy. 2020;75(2):311–325. doi: 10.1111/all.13985. [DOI] [PubMed] [Google Scholar]
- 49.Kuipers I, Louis R, Manise M, Dentener MA, Irvin CG, Janssen-Heininger YM, et al. Increased glutaredoxin-1 and decreased protein S-glutathionylation in sputum of asthmatics. Eur Resp J. 2013;41(2):469–472. doi: 10.1183/09031936.00115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Cicco M, Ghezzi M, Kantar A, Song WJ, Bush A, Peroni D, et al. Pediatric obesity and severe asthma: targeting pathways driving inflammation. Pharmacol Res. 2023;188:106658. doi: 10.1016/j.phrs.2023.106658. [DOI] [PubMed] [Google Scholar]
- 51.Reyes-Angel J, Kaviany P, Rastogi D, Forno E. Obesity-related asthma in children and adolescents. Lancet Child Adolesc Health. 2022;6(10):713–724. doi: 10.1016/S2352-4642(22)00185-7. [DOI] [PubMed] [Google Scholar]
- 52.Fleming L, Heaney L. Severe asthma-perspectives from adult and pediatric pulmonology. Front Pediatr. 2019;7:389. doi: 10.3389/fped.2019.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Platts-Mills TA. The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med. 2001;164(8 Pt 2):S1–5. doi: 10.1164/ajrccm.164.supplement_1.2103024. [DOI] [PubMed] [Google Scholar]
- 54.Oliver JM, Tarleton CA, Gilmartin L, Archibeque T, Qualls CR, Diehl L, et al. Reduced FcepsilonRI-mediated release of asthma-promoting cytokines and chemokines from human basophils during omalizumab therapy. Int Arch Allergy Immunol. 2010;151(4):275–284. doi: 10.1159/000250436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu Z, Xu Y, Cai C. Efficacy and safety of omalizumab in children with moderate-to-severe asthma: a meta-analysis. J Asthma. 2021;58(10):1350–1358. doi: 10.1080/02770903.2020.1789875. [DOI] [PubMed] [Google Scholar]
- 56.Akuthota P, Weller PF. Eosinophils and disease pathogenesis. Semin Hematol. 2012;49(2):113–119. doi: 10.1053/j.seminhematol.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavord ID, Bel EH, Bourdin A, Chan R, Han JK, Keene ON, et al. From DREAM to REALITI-A and beyond: mepolizumab for the treatment of eosinophil-driven diseases. Allergy. 2022;77(3):778–797. doi: 10.1111/all.15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Investig. 2003;112(7):1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khatri S, Moore W, Gibson PG, Leigh R, Bourdin A, Maspero J, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019;143(5):1742–51.e7. doi: 10.1016/j.jaci.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 60.Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–53.e2. doi: 10.1016/j.jaci.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Korn S, Bourdin A, Chupp G, Cosio BG, Arbetter D, Shah M, et al. Integrated safety and efficacy among patients receiving benralizumab for up to 5 years. J Allergy Clin Immunol Pract. 2021;9(12):4381–92.e4. doi: 10.1016/j.jaip.2021.07.058. [DOI] [PubMed] [Google Scholar]
- 62.Porsbjerg CM, Menzies-Gow AN, Tran TN, Murray RB, Unni B, Audrey Ang SL, et al. Global variability in administrative approval prescription criteria for biologic therapy in severe asthma. J Allergy Clin Immunol Pract. 2022;10(5):1202–16.e23. doi: 10.1016/j.jaip.2021.12.027. [DOI] [PubMed] [Google Scholar]
- 63.Santos-Valente E, Buntrock-Döpke H, Abou Taam R, Arasi S, Bakirtas A, Lozano Blasco J, et al. Biologicals in childhood severe asthma: the European PERMEABLE survey on the. ERJ Open Res. 2021;7(3). [DOI] [PMC free article] [PubMed]
- 64.Rusconi F, Fernandes RM, Pijnenburg MWH, Grigg J. The Severe Paediatric Asthma Collaborative in Europe (SPACE) ERS Clinical Research Collaboration: enhancing participation of children with asthma in therapeutic trials of new biologics and receptor blockers. Eur Resp J. 2018;52(4). [DOI] [PubMed]
- 65.Mathioudakis AG, Miligkos M, Boccabella C, Alimani GS, Custovic A, Deschildre A, et al. Management of asthma in childhood: study protocol of a systematic evidence update by the Paediatric Asthma in Real Life (PeARL) Think Tank. BMJ Open. 2021;11(7):e048338. doi: 10.1136/bmjopen-2020-048338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harb H, Chatila TA. Mechanisms of Dupilumab. Clin Exp Allergy. 2020;50(1):5–14. doi: 10.1111/cea.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elo LL, Järvenpää H, Tuomela S, Raghav S, Ahlfors H, Laurila K, et al. Genome-wide profiling of interleukin-4 and STAT6 transcription factor regulation of human Th2 cell programming. Immunity. 2010;32(6):852–862. doi: 10.1016/j.immuni.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 68.Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 69.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi: 10.1056/NEJMoa1804093. [DOI] [PubMed] [Google Scholar]
- 70.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
- 71.Blaiss MS, Castro M, Chipps BE, Zitt M, Panettieri RA, Jr, Foggs MB. Guiding principles for use of newer biologics and bronchial thermoplasty for patients with severe asthma. Ann Allergy Asthma Immunol. 2017;119(6):533–540. doi: 10.1016/j.anai.2017.09.058. [DOI] [PubMed] [Google Scholar]
- 72.Maspero JF, FitzGerald JM, Pavord ID, Rice MS, Maroni J, Rowe PJ, et al. Dupilumab efficacy in adolescents with uncontrolled, moderate-to-severe asthma: LIBERTY ASTHMA QUEST. Allergy. 2021;76(8):2621–2624. doi: 10.1111/all.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bacharier LB, Maspero JF, Katelaris CH, Fiocchi AG, Gagnon R, de Mir I, et al. Dupilumab in children with uncontrolled moderate-to-severe asthma. N Engl J Med. 2021;385(24):2230–2240. doi: 10.1056/NEJMoa2106567. [DOI] [PubMed] [Google Scholar]
- 74.Jackson DJ, Bacharier LB, Phipatanakul W, Sher L, Domingo C, Papadopoulos N, et al. Dupilumab pharmacokinetics and effect on type 2 biomarkers in children with moderate-to-severe asthma. Annals Allergy Asthma Immunol. 2023. [DOI] [PubMed]
- 75.NCT05347771 CgI. Prevention of asthma exacerbations using dupilumab in urban children and adolescents (PANDA).
- 76.Hoy SM. Tezepelumab: first approval. Drugs. 2022;82(4):461–468. doi: 10.1007/s40265-022-01679-2. [DOI] [PubMed] [Google Scholar]
- 77.Golebski K, van Tongeren J, van Egmond D, de Groot EJ, Fokkens WJ, van Drunen CM. Specific induction of TSLP by the viral RNA analogue Poly(I:C) in primary epithelial cells derived from nasal polyps. PLoS ONE. 2016;11(4):e0152808. doi: 10.1371/journal.pone.0152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Furci F, Murdaca G, Pelaia C, Imbalzano E, Pelaia G, Caminati M, et al. TSLP and HMGB1: inflammatory targets and potential biomarkers for precision medicine in asthma and COPD. Biomedicines. 2023;11(2). [DOI] [PMC free article] [PubMed]
- 79.Ebina-Shibuya R, Leonard WJ. Role of thymic stromal lymphopoietin in allergy and beyond. Nat Rev Immunol. 2023;23(1):24–37. doi: 10.1038/s41577-022-00735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krabbendam L, Bal SM, Spits H, Golebski K. New insights into the function, development, and plasticity of type 2 innate lymphoid cells. Immunol Rev. 2018;286(1):74–85. doi: 10.1111/imr.12708. [DOI] [PubMed] [Google Scholar]
- 81.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202(9):1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gauvreau GM, Sehmi R, Ambrose CS, Griffiths JM. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets. 2020;24(8):777–792. doi: 10.1080/14728222.2020.1783242. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka J, Watanabe N, Kido M, Saga K, Akamatsu T, Nishio A, et al. Human TSLP and TLR3 ligands promote differentiation of Th17 cells with a central memory phenotype under Th2-polarizing conditions. Clin Exp Allergy. 2009;39(1):89–100. doi: 10.1111/j.1365-2222.2008.03151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao H, Ying S, Dai Y. Pathological roles of neutrophil-mediated inflammation in asthma and its potential for therapy as a target. J Immunol Res. 2017;2017:3743048. doi: 10.1155/2017/3743048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.US Food & Drug Administration. FDA approves maintenance treatment for severe asthma. 2021. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-maintenance-treatment-severe-asthma.
- 86.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–946. doi: 10.1056/NEJMoa1704064. [DOI] [PubMed] [Google Scholar]
- 87.Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–1809. doi: 10.1056/NEJMoa2034975. [DOI] [PubMed] [Google Scholar]
- 88.Brusselle G, Riemann S. Is efficacy of tezepelumab independent of severe asthma phenotype? Am J Respir Crit Care Med. 2023. [DOI] [PMC free article] [PubMed]
- 89.Trail.GOV C. Extension Study to Evaluate the Safety and Tolerability of Tezepelumab in Adults and Adolescents With Severe, Uncontrolled Asthma (DESTINATION) [Available from: Extension Study to Evaluate the Safety and Tolerability of Tezepelumab in Adults and Adolescents With Severe, Uncontrolled Asthma—Study Results—ClinicalTrials.gov.
- 90.Shinkai M, Ebisawa M, Fukushima Y, Takeuchi S, Okada H, Tokiyo T, Hayashi N, Takikawa M, Colice G, Almqvist G. One-year safety and tolerability of tezepelumab in Japanese patients with severe uncontrolled asthma: results of the NOZOMI study. J Asthma. 2023;60(3):616–24. 10.1080/02770903.2022.2082309 [DOI] [PubMed]
- 91.Flokstra-de Blok B, Kocks J, Wouters H, Arling C, Chatelier J, Douglass J, et al. Perceptions on home-administration of biologics in the context of severe asthma: an international qualitative study. J Allergy Clin Immunol Pract. 2022;10(9):2312–23.e2. doi: 10.1016/j.jaip.2022.04.015. [DOI] [PubMed] [Google Scholar]
- 92.Bel EH, I Bernstein D, Bjermer L, Follows R, Bentley JH, Pouliquen I, et al. Usability of mepolizumab single-use prefilled syringe for patient self-administration. J Asthma. 2020;57(7):755–64. [DOI] [PubMed]
- 93.Bernstein D, Pavord ID, Chapman KR, Follows R, Bentley JH, Pouliquen I, et al. Usability of mepolizumab single-use prefilled autoinjector for patient self-administration. J Asthma. 2020;57(9):987–998. doi: 10.1080/02770903.2019.1630641. [DOI] [PubMed] [Google Scholar]
- 94.Alpizar S, Megally A, Chen C, Raj A, Downie J, Colice G. Functionality and performance of an accessorized pre-filled syringe and an autoinjector for at-home administration of tezepelumab in patients with severe, uncontrolled asthma. J Asthma Allergy. 2021;14:381–392. doi: 10.2147/JAA.S305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Makhecha S, Jamalzadeh A, Irving S, Hall P, Sonnappa S, Saglani S, et al. Paediatric severe asthma biologics service: from hospital to home. Arch Dis Child. 2021;106(9):900–902. doi: 10.1136/archdischild-2020-320626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Albers FC, Licskai C, Chanez P, Bratton DJ, Bradford ES, Yancey SW, et al. Baseline blood eosinophil count as a predictor of treatment response to the licensed dose of mepolizumab in severe eosinophilic asthma. Respir Med. 2019;159:105806. doi: 10.1016/j.rmed.2019.105806. [DOI] [PubMed] [Google Scholar]
- 97.Akenroye A, Lassiter G, Jackson JW, Keet C, Segal J, Alexander GC, et al. Comparative efficacy of mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: A Bayesian network meta-analysis. J Allergy Clin Immunol. 2022;150(5):1097–105.e12. doi: 10.1016/j.jaci.2022.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y, Li X, Zhang B, Yu Q, Lu Y. Predictive biomarkers for response to omalizumab in patients with severe allergic asthma: a meta-analysis. Expert Rev Respir Med. 2022;16(9):1023–1033. doi: 10.1080/17476348.2022.2092100. [DOI] [PubMed] [Google Scholar]
- 99.Pavord ID, Deniz Y, Corren J, Casale TB, FitzGerald JM, Izuhara K, et al. Baseline FeNO independently predicts the dupilumab response in patients with moderate-to-severe asthma. J Allergy Clin Immunol Pract. 2022. [DOI] [PubMed]
- 100.Hearn AP, Kavanagh J, d'Ancona G, Roxas C, Green L, Thomson L, et al. The relationship between Feno and effectiveness of mepolizumab and benralizumab in severe eosinophilic asthma. J Allergy Clin Immunol Pract. 2021;9(5):2093–6.e1. doi: 10.1016/j.jaip.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 101.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sonnenberg-Riethmacher E, Miehe M, Riethmacher D. Periostin in allergy and inflammation. Front Immunol. 2021;12:722170. doi: 10.3389/fimmu.2021.722170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takahashi K, Meguro K, Kawashima H, Kashiwakuma D, Kagami SI, Ohta S, et al. Serum periostin levels serve as a biomarker for both eosinophilic airway inflammation and fixed airflow limitation in well-controlled asthmatics. J Asthma. 2019;56(3):236–243. doi: 10.1080/02770903.2018.1455855. [DOI] [PubMed] [Google Scholar]
- 104.Mansur AH, Srivastava S, Sahal A. Disconnect of type 2 biomarkers in severe asthma; dominated by FeNO as a predictor of exacerbations and periostin as predictor of reduced lung function. Respir Med. 2018;143:31–38. doi: 10.1016/j.rmed.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 105.Kashima TG, Nishiyama T, Shimazu K, Shimazaki M, Kii I, Grigoriadis AE, et al. Periostin, a novel marker of intramembranous ossification, is expressed in fibrous dysplasia and in c-Fos-overexpressing bone lesions. Hum Pathol. 2009;40(2):226–237. doi: 10.1016/j.humpath.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 106.Schleich F, Demarche S, Louis R. Biomarkers in the management of difficult asthma. Curr Top Med Chem. 2016;16(14):1561–1573. doi: 10.2174/1568026616666151015093406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Demarche S, Schleich F, Henket M, Paulus V, Van Hees T, Louis R. Detailed analysis of sputum and systemic inflammation in asthma phenotypes: are paucigranulocytic asthmatics really non-inflammatory? BMC Pulm Med. 2016;16:46. doi: 10.1186/s12890-016-0208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med. 2010;104(8):1131–1137. doi: 10.1016/j.rmed.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 109.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma : evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119(5):1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 110.Wood LG, Baines KJ, Fu J, Scott HA, Gibson PG. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest. 2012;142(1):86–93. doi: 10.1378/chest.11-1838. [DOI] [PubMed] [Google Scholar]
- 111.O'Byrne PM, Panettieri RA, Jr, Taube C, Brindicci C, Fleming M, Altman P. Development of an inhaled anti-TSLP therapy for asthma. Pulm Pharmacol Ther. 2023;78:102184. doi: 10.1016/j.pupt.2022.102184. [DOI] [PubMed] [Google Scholar]
- 112.Kelsen SG, Agache IO, Soong W, Israel E, Chupp GL, Cheung DS, et al. Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: a randomized clinical trial. J Allergy Clin Immunol. 2021;148(3):790–8. 10.1016/j.jaci.2021.03.044. [DOI] [PubMed]
- 113.Richards LB, van Bragt J, Aarab R, Longo C, Neerincx AH, Sont JK, et al. Treatment eligibility of real-life mepolizumab-treated severe asthma patients. J Allergy Clin Immunol Pract. 2020;8(9):2999–3008.e1. doi: 10.1016/j.jaip.2020.04.029. [DOI] [PubMed] [Google Scholar]
- 114.Nieto García A, Garriga-Baraut T, Plaza Martín AM, Nieto Cid M, Torres Borrego J, FolquéGiménez MDM, et al. Omalizumab outcomes for up to 6 years in pediatric patients with severe persistent allergic asthma. Pediatr Allergy Immunol. 2021;32(5):980–991. doi: 10.1111/pai.13484. [DOI] [PubMed] [Google Scholar]
- 115.Jackson DJ, Bacharier LB, Gergen PJ, Gagalis L, Calatroni A, Wellford S, et al. Mepolizumab for urban children with exacerbation-prone eosinophilic asthma in the USA (MUPPITS-2): a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet. 2022;400(10351):502–511. doi: 10.1016/S0140-6736(22)01198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coleman C, Khaleva E, Rattu A, Frankemölle B, Nielsen H, Roberts G, et al. Narrative review to capture patients' perceptions and opinions about non-response and response to biological therapy for severe asthma. Eur Resp J. 2023;61(1). [DOI] [PMC free article] [PubMed]
- 117.Lanario JW, Burns L. Use of health related quality of life in clinical trials for severe asthma: a systematic review. J Asthma Allergy. 2021;14:999–1010. doi: 10.2147/JAA.S320817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Clark VL, Gibson PG, McDonald VM. What matters to people with severe asthma? Exploring add-on asthma medication and outcomes of importance. ERJ Open Res. 2021;7(1). [DOI] [PMC free article] [PubMed]
- 119.Clark VL, Gibson PG, McDonald VM. The patients' experience of severe asthma add-on pharmacotherapies: a qualitative descriptive study. J Asthma Allergy. 2021;14:245–258. doi: 10.2147/JAA.S296147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.de Graaff MB, Bendien SA, van de Bovenkamp HM. 'Like a fish on dry land': an explorative qualitative study into severe asthma and the impact of biologicals on patients' everyday life. J Asthma. 2022;59(5):980–988. doi: 10.1080/02770903.2021.1888976. [DOI] [PubMed] [Google Scholar]
- 121.Licari A, Manti S, Castagnoli R, Marseglia A, Foiadelli T, Brambilla I, et al. Immunomodulation in pediatric asthma. Front Pediatr. 2019;7:289. doi: 10.3389/fped.2019.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saglani S, Bush A, Carroll W, Cunningham S, Fleming L, Gaillard E, et al. Biologics for paediatric severe asthma: trick or TREAT? Lancet Respir Med. 2019;7(4):294–296. doi: 10.1016/S2213-2600(19)30045-1. [DOI] [PubMed] [Google Scholar]
- 123.Scioscia G, Nolasco S, Campisi R, Quarato CMI, Caruso C, Pelaia C, et al. Switching biological therapies in severe asthma. Int J Mol Sci. 2023;24(11). [DOI] [PMC free article] [PubMed]
- 124.Deschildre A, Roussel J, Drumez E, Abou-Taam R, Rames C, Le Roux P, et al. Omalizumab discontinuation in children with severe allergic asthma: an observational real-life study. Allergy. 2019;74(5):999–1003. doi: 10.1111/all.13678. [DOI] [PubMed] [Google Scholar]
- 125.Jeffery MM, Inselman JW, Maddux JT, Lam RW, Shah ND, Rank MA. Asthma patients who stop asthma biologics have a similar risk of asthma exacerbations as those who continue asthma biologics. J Allergy Clin Immunol Pract. 2021;9(7):2742–50.e1. doi: 10.1016/j.jaip.2021.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khaleva E, Rattu A, Brightling C, Bush A, Bossios A, Bourdin A, et al. Development of Core Outcome Measures sets for paediatric and adult Severe Asthma (COMSA). Eur Resp J. 2022. [DOI] [PMC free article] [PubMed]
- 127.Golebski K, Kabesch M, Melén E, Potočnik U, van Drunen CM, Reinarts S, et al. Childhood asthma in the new omics era: challenges and perspectives. Curr Opin Allergy Clin Immunol. 2020;20(2):155–161. doi: 10.1097/ACI.0000000000000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carraro S, di Palmo E, Licari A, Barni S, Caldarelli V, De Castro G, et al. Metabolomics to identify omalizumab responders among children with severe asthma: a prospective study. Allergy. 2022;77(9):2852–2856. doi: 10.1111/all.15385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wechsler ME, Ford LB, Maspero JF, Pavord ID, Papi A, Bourdin A, et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med. 2022;10(1):11–25. doi: 10.1016/S2213-2600(21)00322-2. [DOI] [PubMed] [Google Scholar]
- 130.Menzies-Gow A, Ponnarambil S, Downie J, Bowen K, Hellqvist Å, Colice G. DESTINATION: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the long-term safety and tolerability of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res. 2020;21(1):279. doi: 10.1186/s12931-020-01541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]