Abstract
Objectives
Although biomarkers predicting therapy response in first‐line metastatic renal carcinoma (mRCC) therapy remain to be defined, C‐reactive protein (CRP) kinetics have recently been associated with immunotherapy (IO) response. Here, we aimed to assess the predictive and prognostic power of two contemporary CRP kinetics definitions in a large, real‐world first‐line mRCC cohort.
Methods
Metastatic renal carcinoma patients treated with IO‐based first‐line therapy within 5 years were retrospectively included in this multicentre study. According to Fukuda et al., patients were defined as ‘CRP flare‐responder’, ‘CRP responder’ and ‘non‐CRP responder’; according to Ishihara et al., patients were defined as ‘normal’, ‘normalised’ and ‘non‐normalised’ based on their early CRP kinetics. Patient and tumor characteristics were compared, and treatment outcome was measured by overall (OS) and progression‐free survival (PFS), including multivariable Cox regression analyses.
Results
Out of 316 mRCC patients, 227 (72%) were assigned to CRP groups according to Fukuda. Both CRP flare‐ (HR [Hazard ratio]: 0.59) and CRP responders (HR: 0.52) had a longer PFS, but not OS, than non‐CRP responders. According to Ishihara, 276 (87%) patients were assigned to the respective groups, and both normal and normalised patients had a significantly longer PFS and OS, compared with non‐normalised group.
Conclusion
Different early CRP kinetics may predict therapy response in first‐line mRCC therapy in a large real‐world cohort. However, further research regarding the optimal timing and frequency of measurement is needed.
Keywords: biomarker, checkpoint inhibition, C‐reactive protein, CRP flare‐response, first‐line therapy, metastatic renal cell carcinoma
We investigated the predictive potential of early serum C‐reactive protein (CRP) kinetics during first‐line immunotherapy in metastatic renal cell carcinoma. Out of 316 patients, more than 70% of patients were assigned to respective CRP kinetics groups according to different definitions [Fukuda et al. (a), Ishihara et al. (b)], which correctly identified patients who will most likely respond to therapy and live longer.
Introduction
In recent years, the first‐line treatment for metastatic renal cell carcinoma (mRCC) has undergone significant changes because of the introduction of immune checkpoint inhibition (IO)‐based therapy regimens. Two different types of first‐line combination therapies are currently applied for the treatment of mRCC, namely an intensified immune checkpoint inhibition (IO + IO) and a combination of immune checkpoint inhibition plus anti‐angiogenic therapy (IO + TKI). 1 , 2 , 3 However, only a subset of patients responds to these first‐line IO combination therapies. 1 Therefore, predictive biomarkers are needed to identify early therapy failure and avoid severe unnecessary side effects.
Several studies have linked the occurrence and kinetics of systemic inflammatory response reflected by serum C‐reactive protein (CRP) with clinical outcome and treatment response in various cancers, including urothelial cancer, non‐small‐cell lung cancer and mRCC. 4 , 5 , 6 , 7 , 8 , 9 Previous research has explored CRP levels at the initial diagnosis or baseline before therapy initiation and associated increased systemic inflammation with a poor prognosis. As cancers can also trigger chronic inflammation, the kinetics of CRP levels during treatment may have predictive value for successful immunotherapy. 10 Here, a multitude of differing CRP kinetic models have been described for mRCC in various treatment lines including IO regimens. Klümper et al. and Fukuda et al., for example, investigated the predictive value of the CRP ‘flare‐response’ phenomenon in mRCC cohorts receiving first‐line standard of care therapy with either IO+IO or IO+TKI or second‐line therapy with nivolumab. 11 , 12 Early CRP changes after IO treatment initiation were found to reliably predict therapy success and identify patients who are unlikely to benefit from IO combination therapy. However, both studies were limited by low patient numbers. Ishihara et al., on the contrary, examined the association between baseline and early on‐treatment CRP levels and clinical outcomes in mRCC patients treated with nivolumab second line. 13 The study found that elevated baseline CRP levels were associated with poor overall survival (OS), progression‐free survival (PFS) and objective response rate in mRCC patients. These and other studies highlight the potential predictive value of longitudinal CRP measurements and baseline CRP levels as a potential prognostic biomarker. 5 , 14 , 15 The aim of this study was to examine two CRP kinetic regimens in an updated, multicentre mRCC cohort of 316 patients receiving systemic first‐line mRCC treatment.
Results
Descriptive characteristics of the study population
The study cohort comprised 316 mRCC patients treated with first‐line IO + IO/IO + TKI. Among those, 227 (72%) and 276 (87%) mRCC patients could be assigned to the respective CRP classifications by Fukuda et al. as well as Ishihara et al. (Supplementary figure 1). The patient and tumor characteristics did not significantly differ between patients that could be assigned to the respective groups compared with the remaining ones (Supplementary tables 1 and 2). The median follow‐up in the whole cohort was 13 (Interquartile range (IQR): 6–23) months.
CRP dynamics according to Fukuda et al.
Among 227 mRCC patients, 80 (35%) vs. 44 (19%) vs. 103 (45%) were non‐CRP responder vs. CRP flare‐responder vs. CRP responder. In general, patient and tumor characteristics did not significantly differ between the groups (Table 1). Only the proportion of patients receiving radiotherapy was higher in CRP flare‐responders vs. CRP responder and non‐CRP responder (57% vs. 49%, vs. 32%, P = 0.02). The systemic treatment regimen did not differ between the groups. The median time from diagnosis to treatment initiation was significantly longer in the non‐CRP responder group (12 months) than in the CRP flare‐responder (9 months) and CRP responder group (3 months), respectively (P = 0.005). At first staging, median maximum target lesion change was 7 (IQR: −25; 50), 1 (IQR: −28; 27) and −20% (IQR: −41; −5) for non‐CRP responder, CRP flare‐responder and CRP responder (P = 0.002).
Table 1.
Patient and tumor characteristics of 227 metastatic renal cell carcinoma patients treated with first‐line IO + IO/IO + TKI and C‐reactive protein data available to be stratified according CRP kinetics defined by Fukuda et al.
| N | Overall, N = 227 | Non‐CRP responder, N = 80 (35%) | CRP flare‐responder, N = 44 (19%) | CRP responder, N = 103 (45%) | P‐value | |
|---|---|---|---|---|---|---|
| Age at treatment initiation [years], Median (IQR) | 227 | 66 (60, 73) | 66 (58, 73) | 67 (58, 72) | 67 (60, 73) | 0.7 |
| Male gender, n (%) | 157 (69%) | 57 (71%) | 31 (70%) | 69 (67%) | ||
| ECOG, n (%) | 220 | 0.9 | ||||
| 0 | 91 (41%) | 31 (41%) | 14 (33%) | 46 (46%) | ||
| 1 | 107 (49%) | 37 (49%) | 25 (58%) | 45 (45%) | ||
| 2 | 15 (6.8%) | 5 (6.6%) | 3 (7.0%) | 7 (6.9%) | ||
| 3 | 6 (2.7%) | 3 (3.9%) | 1 (2.3%) | 2 (2.0%) | ||
| 4 | 1 (0.5%) | 0 (0%) | 0 (0%) | 1 (1.0%) | ||
| IMDC, n (%) | 226 | 0.2 | ||||
| Favorable | 47 (21%) | 20 (25%) | 10 (23%) | 17 (17%) | ||
| Intermediate | 128 (57%) | 45 (57%) | 27 (61%) | 56 (54%) | ||
| Poor | 51 (23%) | 14 (18%) | 7 (16%) | 30 (29%) | ||
| Combined T‐stage, n (%) | 195 | 0.9 | ||||
| T1 | 49 (25%) | 17 (23%) | 8 (21%) | 24 (29%) | ||
| T2 | 18 (9.2%) | 6 (8.2%) | 4 (10%) | 8 (9.6%) | ||
| T3 | 90 (46%) | 35 (48%) | 21 (54%) | 34 (41%) | ||
| T4 | 19 (9.7%) | 8 (11%) | 4 (10%) | 7 (8.4%) | ||
| Tx | 19 (9.7%) | 7 (9.6%) | 2 (5.1%) | 10 (12%) | ||
| Combined N‐Stage, n (%) | 185 | 0.7 | ||||
| N0 | 82 (44%) | 29 (43%) | 19 (50%) | 34 (43%) | ||
| N1 | 60 (32%) | 20 (29%) | 11 (29%) | 29 (37%) | ||
| Nx | 43 (23%) | 19 (28%) | 8 (21%) | 16 (20%) | ||
| M‐Stage, n (%) | 223 | 0.068 | ||||
| M0 | 70 (31%) | 31 (40%) | 17 (40%) | 22 (22%) | ||
| M1 | 131 (59%) | 40 (51%) | 23 (53%) | 68 (67%) | ||
| Mx | 22 (9.9%) | 7 (9.0%) | 3 (7.0%) | 12 (12%) | ||
| Nephrectomy, n (%) | 217 | 135 (62%) | 50 (68%) | 30 (70%) | 55 (55%) | 0.12 |
| Radiotherapy, n (%) | 188 | 84 (45%) | 22 (32%) | 21 (57%) | 41 (49%) | 0.029 |
| Clear‐cell histology, n (%) | 177 (82%) | 66 (84%) | 33 (79%) | 78 (81%) | ||
| Grade histology, n (%) | 174 | > 0.9 | ||||
| 1 | 9 (5.2%) | 3 (4.7%) | 2 (5.6%) | 4 (5.4%) | ||
| 2 | 69 (40%) | 29 (45%) | 12 (33%) | 28 (38%) | ||
| 3 | 71 (41%) | 24 (38%) | 17 (47%) | 30 (41%) | ||
| 4 | 25 (14%) | 8 (12%) | 5 (14%) | 12 (16%) | ||
| Systemic treatment combination, n (%) | 224 | 0.4 | ||||
| IO‐IO | 86 (38%) | 34 (43%) | 18 (42%) | 34 (33%) | ||
| IO‐TKI | 138 (62%) | 45 (57%) | 25 (58%) | 68 (67%) | ||
| Baseline CRP [mg L−1] Median (IQR) | 227 | 9 (2, 38) | 3 (1, 15) | 7 (2, 17) | 29 (6, 61) | < 0.001 |
| Duration for 1 month CRP [days] Median (IQR) | 227 | 21 (13, 22) | 21 (12, 22) | 21 (14, 22) | 21 (14, 22) | 0.8 |
| Time to therapy [months] Median (IQR) | 227 | 5 (1, 34) | 12 (2, 56) | 9 (1, 31) | 3 (0, 24) | 0.005 |
| Maximum target lesion at 1st staging [%], Median (IQR) | 99 | −8 (−33, 27) | 7 (−25, 50) | 1 (−28, 27) | −20 (−41, −5) | 0.002 |
All values are median (Interquartile range) or frequencies (%).
ECOG, Eastern Cooperative Oncology Group; IMDC, International metastatic RCC database consortium; IO, Immunotherapy; IQR, Interquartile range; TKI, Tyrosine kinase inhibitor.
The median PFS was 8 (95% CI [95% confidence interval] 4; 15), 14 (95% CI 11; 30) and 18 months (95% CI 12; n.r. [not reached]) for non‐CRP responder, CRP flare‐responder and CRP responder (Figure 1a) and differed significantly (P = 0.012; Figure 2a). In univariate Cox regression analyses, CRP response (Hazard ratio [HR]: 0.55; 95% CI 0.37; 0.83), but not CRP flare‐response, was associated with prolonged PFS. It is of note that following adjustment for covariables, both CRP responder (HR: 0.59; 95% CI 0.35; 0.99) and CRP flare‐responder (HR: 0.52; 95% CI 0.33; 0.82) gained statistically significant predictors for PFS (Supplementary table 3). The median OS was 32 (95% CI 23; n.r.), not reached (95% CI 18; n.r.) and 33 months (95% CI 31; n.r.) for non‐CRP responder, CRP flare‐responder and CRP responder and did not significantly differ. Moreover, CRP stratification was not predictive for OS in both univariable and multivariable Cox regression analyses (Figure 1b, Supplementary table 3).
Figure 1.
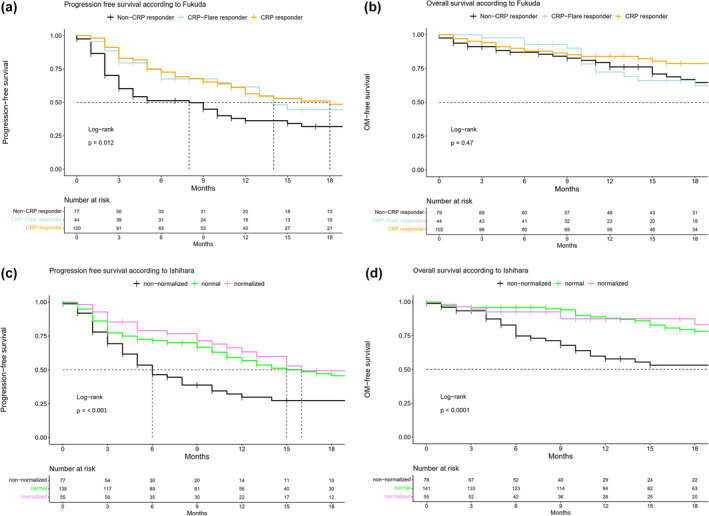
Kaplan–Meier plots depicting (a, c) progression‐free survival and (b, d) overall survival for mRCC patients stratified according to C‐reactive protein kinetics definition by Fukuda (a, b) or Ishihara et al. (c, d).
Figure 2.

Waterfall plots depicting target lesion change at first line, stratified by the C‐reactive protein kinetics definition according to Fukuda et al. (a) and Ishihara et al. (b).
CRP dynamics according to Ishihara et al.
Out of 276 mRCC patients, 79 (29%) vs. 141 (51%) vs. 56 (20%) were assigned to the non‐normalised vs. normal vs. normalised groups according to Ishihara et al. In general, patient characteristics did not statistically significantly differ between the groups (Table 2). However, more patients in the normal CRP group were IMDC favorable risk and less poor risk than the other groups (P < 0.001). Moreover, the proportion of M1 patients was higher in CRP non‐normalised patients (P = 0.002). Median baseline CRP values differed between the groups with 55 (IQR: 26;101) vs. 2 (IQR: 1; 5) vs. 33 (IQR: 20; 56) mg L−1 for the non‐normalised vs. normal vs. normalised groups, respectively (Table 2). At first staging, median maximum target lesion change was −19 (IQR: −34; 11), −11 (IQR: −36; 20) and −32% (IQR: −48; −8) for the non‐normalised vs. normal vs. normalised groups, and did not significantly differ.
Table 2.
Patient and tumor characteristics of 276 metastatic renal cell carcinoma patients being treated with first‐line IO + IO/IO + TKI and C‐reactive protein data available to be stratified according to CRP kinetics defined by Ishihara et al.
| N | Overall, N = 276 | Non‐normalised, N = 79 (29%) | Normal, N = 141 (51%) | Normalised, N = 56 (20%) | P‐value | |
|---|---|---|---|---|---|---|
| Age at treatment initiation [years], Median (IQR) | 276 | 67 (60, 73) | 66 (60, 72) | 67 (60, 73) | 66 (58, 74) | > 0.9 |
| Male gender, n (%) | 192 (70%) | 54 (68%) | 100 (71%) | 38 (68%) | ||
| ECOG, n (%) | 266 | 0.3 | ||||
| 0 | 111 (42%) | 34 (45%) | 56 (41%) | 21 (39%) | ||
| 1 | 130 (49%) | 35 (46%) | 70 (51%) | 25 (46%) | ||
| 2 | 18 (6.8%) | 4 (5.3%) | 7 (5.1%) | 7 (13%) | ||
| 3 | 6 (2.3%) | 3 (3.9%) | 3 (2.2%) | 0 (0%) | ||
| 4 | 1 (0.4%) | 0 (0%) | 0 (0%) | 1 (1.9%) | ||
| IMDC, n (%) | 275 | < 0.001 | ||||
| Favorable | 54 (20%) | 8 (10%) | 42 (30%) | 4 (7.1%) | ||
| Intermediate | 160 (58%) | 45 (57%) | 83 (59%) | 32 (57%) | ||
| Poor | 61 (22%) | 26 (33%) | 15 (11%) | 20 (36%) | ||
| Combined* T‐stage, n (%) | 240 | 0.14 | ||||
| T1 | 60 (25%) | 10 (15%) | 38 (30%) | 12 (26%) | ||
| T2 | 26 (11%) | 7 (10%) | 17 (13%) | 2 (4.3%) | ||
| T3 | 108 (45%) | 28 (42%) | 57 (45%) | 23 (49%) | ||
| T4 | 26 (11%) | 14 (21%) | 7 (5.6%) | 5 (11%) | ||
| Tx | 20 (8.3%) | 8 (12%) | 7 (5.6%) | 5 (11%) | ||
| Combined* N‐Stage, n (%) | 230 | 0.8 | ||||
| N0 | 105 (46%) | 28 (45%) | 59 (47%) | 18 (42%) | ||
| N1 | 69 (30%) | 19 (31%) | 34 (27%) | 16 (37%) | ||
| Nx | 56 (24%) | 15 (24%) | 32 (26%) | 9 (21%) | ||
| M‐Stage, n (%) | 271 | 0.002 | ||||
| M0 | 85 (31%) | 16 (21%) | 51 (36%) | 18 (33%) | ||
| M1 | 164 (61%) | 58 (76%) | 71 (51%) | 35 (64%) | ||
| Mx | 22 (8.1%) | 2 (2.6%) | 18 (13%) | 2 (3.6%) | ||
| Nephrectomy, n (%) | 254 | 152 (60%) | 33 (50%) | 91 (68%) | 28 (51%) | 0.013 |
| Radiotherapy, n (%) | 236 | 104 (44%) | 31 (46%) | 52 (41%) | 21 (50%) | 0.6 |
| Clear‐cell histology, n (%) | 220 (83%) | 58 (76%) | 124 (91%) | 38 (72%) | 0.03 | |
| Grade histology, n (%) | 210 | 0.063 | ||||
| 1 | 14 (6.7%) | 4 (8.7%) | 9 (7.3%) | 1 (2.5%) | ||
| 2 | 89 (42%) | 17 (37%) | 58 (47%) | 14 (35%) | ||
| 3 | 79 (38%) | 16 (35%) | 48 (39%) | 15 (38%) | ||
| 4 | 28 (13%) | 9 (20%) | 9 (7.3%) | 10 (25%) | ||
| Systemic treatment combination, n (%) | 272 | 0.13 | ||||
| IO‐IO | 108 (40%) | 37 (48%) | 54 (39%) | 17 (31%) | ||
| IO‐TKI | 164 (60%) | 40 (52%) | 86 (61%) | 38 (69%) | ||
| Baseline CRP [mg L−1], Median (IQR) | 276 | 9 (2, 41) | 55 (26, 101) | 2 (1, 5) | 33 (20, 56) | < 0.001 |
| Duration for 1 month CRP [days], Median (IQR) | 238 | 21 (14, 23) | 20 (7, 25) | 21 (17, 22) | 21 (14, 24) | 0.3 |
| Time to therapy [months], Median (IQR) | 276 | 5 (1, 33) | 2 (0, 10) | 11 (2, 53) | 3 (0, 17) | < 0.001 |
| Maximum target lesion at 1st staging [%], Median (IQR) | 123 | −16 (−38, 15) | −19 (−34, 11) | −11 (−36, 20) | −32 (−48, −8) | 0.2 |
All values are median (Interquartile range) or frequencies (%).
ECOG, Eastern Cooperative Oncology Group; IMDC, International metastatic RCC database consortium; IO, Immunotherapy; IQR, Interquartile range; TKI, Tyrosine kinase inhibitor.
The median PFS was 6 (95% CI 5; 10), 15 (95% CI 12; 23) and 16 months (95% CI 13; n.r.) for the non‐normalised vs. normal vs. normalised groups (Figure 1c) and differed significantly (P < 0.001). In univariable Cox regression analyses for PFS, both the normal (HR: 0.54, 95% CI 0.37; 0.83) and the normalised groups (HR: 0.40, 95% CI 0.24; 0.66) were associated with prolonged PFS (Supplementary table 4). It is of note that following adjustment for other covariables, both normal and normalised CRP dynamics remained statistically significant predictors for PFS in multivariable Cox regression analyses (Supplementary table 4).
The median OS was 23 (95% CI 11; n.r.), 39 months (95% CI 32; n.r.) and not reached (95% CI 33; n.r.) for the non‐normalised vs. normal vs. normalised group (Figure 2b) and differed significantly (P < 0.001, Figure 1d). Moreover, normal (HR: 0.36, 95% CI 0.22; 0.59) and normalised (HR: 0.32, 95% CI 0.16; 0.65) CRP dynamics were associated with prolonged OS in univariable Cox regression analyses (both P < 0.01). Its predictor status remained unchanged following the adjustment for other variables in multivariable Cox regression analyses (Supplementary table 4).
Comparison of both CRP kinetics definitions
When stratifying the CRP kinetics groups by clinical response at first staging, the proportion of clinical responders (complete response [CR] + partial response [PR] + stable disease [SD]) was higher in CRP flare‐responders than in CRP nonresponders (83.2% vs. 55.6%) and normalised patients according to Ishihara than non‐normalised patients (89.7% vs. 67.7%; Figure 3a and b). In direct comparison, the proportion of patients with progressive disease was slightly higher in CRP flare‐responders than in normalised patients (16.7% vs. 10.3%). When comparing both CRP kinetics definitions, the overlap of patients belonging to the corresponding groups was low (Figure 4).
Figure 3.
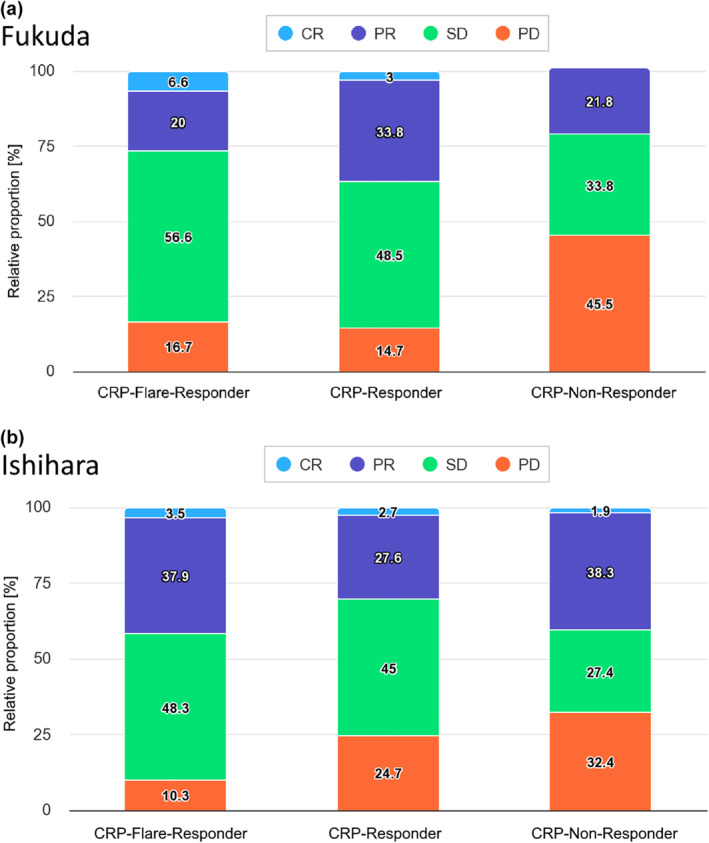
Stacked bar plots stratifying patients by C‐reactive protein kinetic group according to Fukuda (a) or Ishihara et al. (b) by the respective target lesion change at first staging 3 months after therapy initiation. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 4.
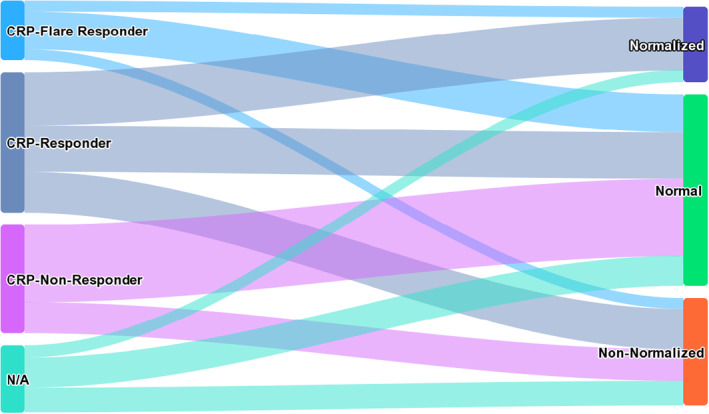
Sankey diagram illustrating the patient distribution compared between the two diverging C‐reactive protein kinetics definitions by Fukuda et al. and Ishihara et al.
Discussion
Against the background of increasing treatment options in first‐line mRCC therapy, offering clinical benefit to a significant number of patients on the one hand, but on the other hand subjecting nonresponders to considerable side effects, reliable predictive biomarkers represent an unmet need. 16 Although the PD‐L1 expression on tumor cells has been linked with a negative prognostic role in mRCC, 17 its predictive value could ultimately not be confirmed. 18 , 19 Moreover, it remains questionable from a biological point of view, whether the primarily metastasis‐directed systemic therapy should be guided by the analysis of the primary tumor. 20 Hence, the ideal biomarker should rather provide a snapshot of all tumor cells, and potentially its dynamic changes during therapy. Liquid biomarkers may combine all these requirements, and especially the inflammatory marker CRP is not only easy to measure and cost‐effective, but also nearly ubiquitously available. 6 , 12
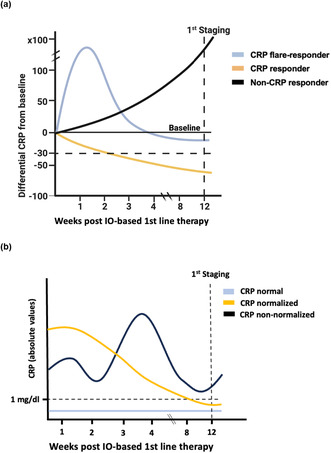
The concept of quantifying the immunological activation status of the patient by measuring CRP values before treatment initiation is not new, and its prognostic power in RCC had already been hypothesized by Iimura et al. in 2009. 21 However, in order to predict therapy response, the dynamic measurement of biomarkers might exert a significantly higher predictive power. 22 Ishihara et al. recently proposed their definition of CRP kinetics, differentiating three patient groups: those whose pretreatment levels were < 10 mg L−1 before therapy (‘normal’), those with decreasing CRP levels from ≥ 10 mg L−1 before treatment initiation to nadir levels below < 10 mg L−1 within 3 months (‘normalised’) and ‘non‐normalised’ patients with CRP levels ≥ 10 mg L−1 before and under therapy. ‘Normal’ and ‘normalised’ patients had a significantly longer OS and PFS under second‐line nivolumab therapy in mRCC. 23 On the contrary, Fukuda et al. proposed to differentiate ‘CRP flare‐responders’ (at least doubling of CRP levels within 1 month after initiation and the decrease to lower baseline within 3 months), ‘CRP responders’ (decrease ≥ 30% from baseline within 3 months w/o flare) and ‘non‐CRP‐responders’ (all other remaining CRP dynamics). 11 The predictive power of the CRP flare‐up phenomenon as an on‐treatment biomarker has been confirmed by different groups in different tumor entities, such as urothelial or lung carcinoma. 12 , 24 , 25 Recently, Guer et al. investigated CRP kinetics – combined with early‐on treatment related adverse events – as an predictor for treatment success in mRCC. 9 Even though that the authors reported noteworthy findings, the results need to be interpreted under the light of a small, single‐centre study cohort (n = 57) and limited follow‐up. As a consequence, above‐mentioned diverging definitions of the CRP kinetics have not been compared in a large, contemporary real‐world first‐line mRCC cohort, hence, we performed the present analysis comprising more than 300 mRCC patients. Ultimately, the application of both definitions enabled to correctly identify many therapy (non)responders, as CRP (flare) response and normal/normalised CRP values were significant independent predictors of OS and PFS.
However, in our German real‐world clinical experience, early CRP measurements within the first weeks after initiation of first‐line therapy in mRCC are no standard, as portrayed by 89 (28.2%) missing early measurements to successfully classify our cohort according to CRP kinetics defined by Fukuda. Of note, especially the CRP flare‐response phenomenon appears to occur within the first 2 weeks after therapy initiation. 24 Widespread indication of such measurements obviously could have the power to identify patients undergoing progression before first staging with the potential not to delay further interventions or therapy lines. On the contrary, easy‐to‐apply CRP algorithms pre‐/post‐therapy initiation as introduced by Ishihara et al. are also available and applicable in the majority of treating centres – although 40 (12.7%) patients could neither be classified according to the definition by Ishihara. It is of note that this definition also possesses a significant predictive power as illustrated in our analysis and can potentially lead to better patient care in the sense of treatment oversight and planning.
Interestingly, both examined CRP kinetic definitions in the cohort presented here vastly differ: CRP kinetics defined by Fukuda rely on relative changes in CRP values independently from the magnitude of initial CRP values, whereas Ishihara's definition is based on absolute CRP thresholds. Moreover, the overlap of patients belonging to comparable CRP kinetics group was low; however, this did not affect the predictive power of both definitions. In our opinion, further investigation is warranted to potentially identify patients profiting exceptionally from therapy (or not). Ideal questions to be asked here would aim at the significance of initial CRP values and their subsequent kinetic, be it relative proportions or absolute drop. For instance, Klümper et al. recently further distinguished CRP flare‐responders into short‐ and long‐flare‐responders, 24 or integrated also albumin levels within the ‘modified Glasgow prognostic score’ (mGPS). 26 However, these approaches were beyond our study objective.
Limitations
Even though the majority of patient and tumor characteristics did not differ between patients with sufficient CRP information to be classified according to Fukuda or Ishihara compared to those without sufficient CRP information, same characteristics differed statistically significantly in sensitivity analyses. As a consequence, a potential selection bias could not be formally ruled out; yet, its magnitude and clinical relevance for interpreting the current results is of limited nature.
Conclusions
In summary, both examined CRP kinetics presented here reliably predicted mRCC patient outcomes in first‐line mRCC therapy, highlighting further potential improvements of patient care. Interestingly, there was no significant group overlap of both examined models suggesting that further improvement through CRP kinetic model modification might be possible.
Methods
Study population
This multi‐institutional retrospective study included mRCC patients treated with either IO+IO or IO+TKI 1st line between 11/2017 and 12/2022 in eight tertiary referral centres. Patients with sufficient CRP data to be stratified according to the CRP kinetics classifications previously introduced by Fukuda et al. (‘Fukuda’) and Ishihara et al. (‘Ishihara’) were identified and further analysed (Supplementary figure 2a, b). Regarding ‘Fukuda’, minimum CRP data consisted of (a) CRP prior (< 6 weeks) to treatment initiation, (b) CRP within 1 month of treatment initiation and (c) at least one further CRP prior to first staging. Regarding ‘Ishihara’, minimum CRP data consisted of (a) CRP prior to treatment initiation (< 30 days) and at least one further CRP prior to first staging. Relying on the ‘Fukuda’ definition, mRCC patients were classified as ‘CRP flare‐responder’, ‘CRP responder’ and ‘non‐CRP responder’ following CRP dynamics, whereas following the ‘Ishihara’ definition, mRCC patients were classified as ‘normal’, ‘normalised’ and ‘non‐normalised’, respectively. 11 , 13 Patient demographics and baseline parameters, including IMDC (International Metastatic RCC Database Consortium) risk criteria, were obtained. Tumor response was graded according to response evaluation criteria in solid tumors (RECIST v1.1). 27 Serum CRP concentration was measured in accredited routine laboratories in each participating centre and is given in mg L−1 (clinical reference < 5 mg L−1). For each patient, a recent follow‐up was conducted. This study was conducted according to the Declaration of Helsinki and approved by the local responsible ethical review boards (reference #20201211‐01).
Statistical analyses
Metastatic renal carcinoma patients with sufficient CRP data to be classifiable according to ‘Fukuda’ definition, patient and tumor characteristics were tabulated according to CRP dynamics. Descriptive statistics included frequencies and proportions for categorical variables. Medians and IQRs were reported for continuously coded variables. Maximum target lesion change for the three different CRP dynamic groups at first staging was depicted relying on waterfall plot illustration. Subsequently, separate Kaplan–Meier plots and Cox regression analyses were used to test for (a) PFS and (b) OS differences according to CRP dynamics. Progression was defined according to the RECIST v1.1 criteria including death from any cause. In Cox regression analyses, IMDC criteria (favorable vs intermediate vs poor), age at treatment initiation (per year), ECOG (Eastern Cooperative Oncology Group) performance status (continuously) and combined tumor stage (T1/2 vs T3/4 vs Tx) were defined as adjustment variables. In order to test for a potential underlying selection bias, sensitivity analyses were conducted to compare the tumor and patient characteristics between ‘Fukuda’‐eligible mRCC patients and patients for whom CRP data were missing. The statistical approach was repeated for mRCC patients who had enough CRP data to be classified according to the ‘Ishihara’ definition (‘normal’, ‘normalised’, ‘non‐normalised’). All tests were two‐sided with a level of significance set at P < 0.05 and R software environment for statistical computing and graphics (version 4.1.1) was used for all analyses. 28
Author contributions
Benedikt Hoeh: Conceptualization; data curation; formal analysis; writing – original draft. Cristina Cano Garcia: Data curation; software. Severine Banek: Data curation; supervision. Niklas Klümper: Data curation; investigation; writing – review and editing. Alexander Cox: Data curation; writing – review and editing. Jörg Ellinger: Supervision; writing – review and editing. Philipp Schmucker: Data curation; writing – review and editing. Oliver Hahn: Conceptualization; data curation; writing – review and editing. Angelika Mattigk: Data curation; investigation; visualization; writing – review and editing. Friedemann Zengerling: Resources; writing – review and editing. Philippe Becker: Conceptualization; data curation; writing – review and editing. Kati Erdmann: Data curation; methodology; writing – review and editing. Bjoern Thorben Buerk: Data curation; supervision; writing – review and editing. Luka Flegar: Data curation; writing – review and editing. Johannes Huber: Supervision; writing – review and editing. Charis Kalogirou: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; supervision; writing – original draft; writing – review and editing. Philip Zeuschner: Conceptualization; data curation; formal analysis; methodology; project administration; software; visualization; writing – original draft; writing – review and editing.
Conflict of interest
The authors declare that there is no conflict of interests.
Ethics consent statement
The study was conducted according to the Declaration of Helsinki and approved by the responsible ethical review board (reference #20201211–01).
Funding statement
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Supplementary figure 1
Supplementary figure 2
Supplementary table 1
Supplementary table 2
Supplementary table 3
Supplementary table 4
Acknowledgments
CCG was awarded a scholarship by the STIFTUNG GIERSCH. Open Access funding enabled and organized by Projekt DEAL.
Data availability
All data sets generated for this study are included in the manuscript.
References
- 1. Grunwald V, Bergmann L, Brehmer B et al. Systemic therapy in metastatic renal cell carcinoma (mRCC): An evidence‐based recommendation of the german interdisciplinary RCC guidelines group. World J Urol 2022; 40: 2381–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ljungberg B, Albiges L, Bedke J et al. European Association of Urology guidelines on renal cell carcinoma: The 2022 update. Eur Urol 2022; 82: 399–410. [DOI] [PubMed] [Google Scholar]
- 3. Hoeh B, Schmucker P, Klumper N et al. Comparison of first‐line anti‐PD‐1‐based combination therapies in metastatic renal‐cell carcinoma: Real‐world experiences from a retrospective, multi‐institutional cohort. Urol Int 2022; 106: 1150–1157. [DOI] [PubMed] [Google Scholar]
- 4. Saito K, Tatokoro M, Fujii Y et al. Impact of C‐reactive protein kinetics on survival of patients with metastatic renal cell carcinoma. Eur Urol 2009; 55: 1145–1153. [DOI] [PubMed] [Google Scholar]
- 5. Saito K, Kihara K. C‐reactive protein as a biomarker for urological cancers. Nat Rev Urol 2011; 8: 659–666. [DOI] [PubMed] [Google Scholar]
- 6. Kalogirou C, Mulfinger P, Sokolakis I et al. Preoperative C‐reactive protein values as a potential component in outcome prediction models of metastasized renal cell carcinoma patients receiving cytoreductive nephrectomy. Urol Int 2017; 99: 297–307. [DOI] [PubMed] [Google Scholar]
- 7. Riedl JM, Barth DA, Brueckl WM et al. C‐reactive protein (CRP) levels in immune checkpoint inhibitor response and progression in advanced non‐small cell lung cancer: A bi‐centre study. Cancers (Basel) 2020; 12: 2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kijima T, Yamamoto H, Saito K et al. Early C‐reactive protein kinetics predict survival of patients with advanced urothelial cancer treated with pembrolizumab. Cancer Immunol Immunother 2021; 70: 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guer M, Janitzky A, Schostak M. On‐treatment risk model for predicting treatment response in advanced renal cell carcinoma. World J Urol 2023; e‐pub ahead of print. 10.1007/s00345-023-04545-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ozawa Y, Amano Y, Kanata K et al. Impact of early inflammatory cytokine elevation after commencement of PD‐1 inhibitors to predict efficacy in patients with non‐small cell lung cancer. Med Oncol 2019; 36: 33. [DOI] [PubMed] [Google Scholar]
- 11. Fukuda S, Saito K, Yasuda Y et al. Impact of C‐reactive protein flare‐response on oncological outcomes in patients with metastatic renal cell carcinoma treated with nivolumab. J Immunother Cancer 2021; 9: e001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klumper N, Schmucker P, Hahn O et al. C‐reactive protein flare‐response predicts long‐term efficacy to first‐line anti‐PD‐1‐based combination therapy in metastatic renal cell carcinoma. Clin Transl Immunology 2021; 10: e1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishihara H, Takagi T, Kondo T et al. Predictive impact of an early change in serum C‐reactive protein levels in nivolumab therapy for metastatic renal cell carcinoma. Urol Oncol 2020; 38: 526–532. [DOI] [PubMed] [Google Scholar]
- 14. Tomita Y, Larkin J, Venugopal B et al. Association of C‐reactive protein (crp) with efficacy of avelumab + axitinib (a + ax) in advanced renal cell carcinoma (arcc): Long‐term follow‐up results from JAVELIN Renal 101. J Clin Oncol 2021; 39: 4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saal J, Bald T, Holzel M et al. In the phase III IMmotion151 trial of metastatic renal cell carcinoma the easy‐to‐implement modified Glasgow prognostic score predicts outcome more accurately than the IMDC score. Ann Oncol 2022; 33: 982–984. [DOI] [PubMed] [Google Scholar]
- 16. Bosma NA, Warkentin MT, Gan CL et al. Efficacy and safety of first‐line systemic therapy for metastatic renal cell carcinoma: A systematic review and network meta‐analysis. Eur Urol Open Sci 2022; 37: 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ueda K, Suekane S, Kurose H et al. Prognostic value of PD‐1 and PD‐l1 expression in patients with metastatic clear cell renal cell carcinoma. Urol Oncol 2018; 36: 499.e9–499.e16. [DOI] [PubMed] [Google Scholar]
- 18. Rini BI, Powles T, Atkins MB et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open‐label, phase 3, randomised controlled trial. Lancet 2019; 393: 2404–2415. [DOI] [PubMed] [Google Scholar]
- 19. Motzer RJ, Robbins PB, Powles T et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: Biomarker analysis of the phase 3 JAVELIN renal 101 trial. Nat Med 2020; 26: 1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zeuschner P, Junker K. Optimal selection of patients with genitourinary cancers for anti‐PD1/PD‐l1 treatment with a focus on urothelial and renal cell carcinoma. Eur Urol Focus 2022; 8: 907–909. [DOI] [PubMed] [Google Scholar]
- 21. Iimura Y, Saito K, Fujii Y et al. Development and external validation of a new outcome prediction model for patients with clear cell renal cell carcinoma treated with nephrectomy based on preoperative serum C‐reactive protein and TNM classification: The TNM‐C score. J Urol 2009; 181: 1004–1012; discussion 1012. [DOI] [PubMed] [Google Scholar]
- 22. Lesterhuis WJ, Bosco A, Millward MJ, Small M, Nowak AK, Lake RA. Dynamic versus static biomarkers in cancer immune checkpoint blockade: Unravelling complexity. Nat Rev Drug Discov 2017; 16: 264–272. [DOI] [PubMed] [Google Scholar]
- 23. Ishihara H, Tachibana H, Takagi T et al. Predictive impact of peripheral blood markers and C‐reactive protein in nivolumab therapy for metastatic renal cell carcinoma. Target Oncol 2019; 14: 453–463. [DOI] [PubMed] [Google Scholar]
- 24. Klumper N, Sikic D, Saal J et al. C‐reactive protein flare predicts response to anti‐pd‐(l)1 immune checkpoint blockade in metastatic urothelial carcinoma. Eur J Cancer 2022; 167: 13–22. [DOI] [PubMed] [Google Scholar]
- 25. Saal J, Bald T, Eckstein M et al. Early C‐reactive protein kinetics predicts immunotherapy response in non‐small cell lung cancer in the phase III OAK trial. JNCI Cancer Spectrum 2023; 7: pkad027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saal J, Bald T, Eckstein M et al. Integrating on‐treatment modified Glasgow prognostic score and imaging to predict response and outcomes in metastatic renal cell carcinoma. JAMA Oncol 2023; 9: 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 28. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. [cited 2023 24.05.2023]. Available from: https://www.R‐project.org/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1
Supplementary figure 2
Supplementary table 1
Supplementary table 2
Supplementary table 3
Supplementary table 4
Data Availability Statement
All data sets generated for this study are included in the manuscript.


