Abstract
The goal of the present study was to evaluate the antileishmanial effects of topically applied lipid-based formulations containing amphotericin B (AmB) in CBA mice as a model for human cutaneous leishmaniasis. Such treatment, if efficacious, is expected to be superior to systemic treatments since, by acting in a localized manner, it will require lower, and therefore less toxic, drug dosages. Three preparations of AmB complexed to polar lipids were tested: Fungizone (mixed micelles composed of AmB and deoxycholate), Amphocil (AmB and cholesteryl sulfate complex), and ABPLC (AmB and phospholipid complex). All these formulations killed parasites in vitro with similar efficacies but were ineffective when they were applied topically. However, Amphocil and ABPLC, but not Fungizone, when dispersed in an aqueous solution containing 5 to 25% ethanol, induced a statistically significant improvement in lesion size from week 2 or 3 onward (a total of 15 mg of AmB per kg of body weight was applied over 3 weeks). AmB biodistribution measurements following topical application of Amphocil, determined by high-pressure liquid chromatography, showed that AmB was detectable in the skin but not in the internal organs. Application of at least 10 times more drug was necessary to obtain detectable levels of AmB in the internal organs. After application of therapeutic doses of ABPLC, very low levels of AmB were detected in the internal organs. These experiments show for the first time that AmB administered topically as a complex either with cholesteryl sulfate or with phospholipids and in the presence of ethanol can penetrate the skin and kill sensitive organisms in a localized manner by using very low total drug concentrations.
Amphotericin B (AmB), a polyene antibiotic, is a potent antifungal and antiparasitic agent with a broad spectrum of activity against organisms that have ergosterol in their membranes. It is commonly used as a systemic agent, administered intravenously, with high levels of activity against leishmania infection. However, because AmB is highly toxic, particularly nephrotoxic, when it is given systemically, it is thus generally used for the treatment of leishmaniasis only after other treatments have failed (13).
Toxic side effects are typically greatly reduced when a drug is administered topically, since much lower doses can generally be used and a very small amount of the drug reaches sensitive internal organs such as the kidneys. The topical use of AmB preparations on mucous membrane surfaces, such as those of the eye, mouth, and respiratory and digestive passages, often in combination with other agents, has been described (1, 5, 18, 22).
The skin, however, is a very effective barrier for substances with molecular masses greater than a few hundred daltons. For example, topical treatment of cutaneous leishmaniasis (CL), a widespread and potentially disfiguring protozoal infection of the skin, with paromomycin, an aminoglycoside antibiotic, has been shown to be effective in some cases (10) but not in others (4, 15, 19). Cutaneous Leishmania major lesions in mice—used as an experimental model for CL—were also unresponsive to a topical preparation of AmB in soft white paraffin containing 12% methylbenzethonium chloride (9), although the drug is effective against leishmaniasis when it is administered systemically. In order to obtain effective drug penetration through the skin, therefore, an efficacious drug-carrier system is required.
The data presented here show that formulations of AmB-cholesteryl sulfate and AmB-phospholipid complex, but not AmB-deoxycholate complex, when dispersed in nonviscous solutions containing 5 to 25% ethanol and then administered topically, induced significant improvements in lesion size with very low drug concentrations.
MATERIALS AND METHODS
Drugs and formulations.
Fungizone (Bristol Myers Squibb, Princeton, N.J.) is composed of AmB and sodium deoxycholate (mixed micelle formulation in a 54:94 [by mole] ratio); Amphocil (SEQUUS Pharmaceuticals, Menlo Park, Calif.) is a colloidal dispersion composed of AmB and cholesteryl sulfate (a 1:1 colloidal complex); ABPLC (AmB-phospholipid complex) is a 1:3 molar complex of AmB with a 7:3 mole mixture of dimyristoyl phosphatidylcholine (DMPC) and dimyristoyl phosphatidylglycerol (DMPG) and was prepared as described previously (11). For more details on the formulations, see the report by Barenholz and Cohen (3).
Quantification of AmB.
The amount of AmB was quantified by high-pressure liquid chromatography (HPLC; Pump 420, Autosampler 460, Detector 430, and Data system 450; Kontron, Zürich, Switzerland). Quantification was obtained by using an Econosphere C18 (3-μm particle size) plus guard column (100 by 4.6 mm; Alltech, Deerfield, Ill.) with a mobile phase of acetonitrile and 0.02 M EDTA (pH 4.5) in H2O in a ratio of 110 to 90 by volume. The injection volume was 80 μl, and the flow rate was 1 ml/min. The quantification was based on the absorbances at 405 and 386 nm recorded with the Kontron 430 detector. Spectral snapshots in the range of 300 to 500 nm were taken to verify the identity of the analyte as AmB. Mean size and size distribution were measured by photon correlation spectroscopy by Coulter submicroparticle analysis (Coulter N4SD submicroparticle analyzer; Coulter Electronics Ltd., Luton, England). In some formulations the particles were too large to be measured in this way. Therefore, these preparations were measured with a multisizer (Multisizer II; Coulter), which measures particle sizes from 0.8 to 30 μm. The levels of AmB in body tissues after topical application or intravenous (i.v.) injection of various formulations were measured by HPLC. Levels in skin, spleen, liver, and kidney were measured 1 day after administration was terminated; the level in the skin (at the site of application) was in some cases also measured 1 h after administration was terminated.
Tissue extractions.
The animals were killed 1 or 24 h after the last drug application or injection, and the organs were removed, dried with filter paper, weighed, and kept frozen in a “wet” freezer until extraction. For skin, enzymatic digestion was performed with tissue cut into small pieces, before tissue extraction, by using 0.25% trypsin, 25 U of collagenase, and 0.1 mg of DNase I (Sigma Chemical Co.) per ml of phosphate-buffered saline with overnight digestion at 37°C.
For AmB extraction of the organs, the tissue was homogenized with methanol (3 ml/g of tissue) by using a Polytron CH-6010 (Kinematika GmbH, Luzern, Switzerland) high-speed homogenizer. The homogenate was incubated for 1 h at room temperature; the sample was then vortexed, transferred to polypropylene Eppendorf centrifuge tubes, and centrifuged for 10 min. Eighty microliters of the supernatant was injected into the chromatograph; it was found that the sensitivity based on this volume was 40 ng/ml, i.e., 3 ng of AmB per analysis.
Assay validation.
Assay validation was performed in order to assess the degree and reproducibility of the extraction recovery from the tissues. This was accomplished by spiking experiments in which an aliquot of AmB from each of the formulations was injected into homogenates of various organs, followed by the addition of methanol and extraction as described above. The value of the aliquot injected into water was used as a measurement of 100% extraction efficiency. The results showed >95% reproducible extraction over a broad range of concentrations.
Parasites.
Leishmania major WR1045 parasites were isolated from BALB/c mice (Harlan Laboratories, Jerusalem, Israel) and were maintained in RPMI 1640 culture medium supplemented with 20% fetal calf serum (Biological Industries, Beth Haemek, Israel) at 28°C for no more than 2 months.
In vivo experiments.
Female CBA mice (age, 6 to 10 weeks; Harlan Laboratories, Indianapolis, Ind.) were injected subcutaneously in the base of the tail with 3 × 106 stationary-phase promastigotes (9, 24); eight mice were included in each experimental group. From 24 h after injection onward and for the next 3 weeks, 10 μl of each preparation (concentration, 2 mg/ml) was applied daily to the base of the tail. Each mouse received a total of 15 drug applications, representing approximately 300 μg of AmB. Two investigators independently measured the lesion sizes weekly. To determine the diameter, the average was taken between the longest distance across the lesion and the length of the line bisecting this distance at a 90° angle. Mice were kept in a sterile pathogen-free animal facility throughout the experiments.
Healthy mice were given 1 mg of Fungizone, Amphocil, or ABPLC per kg of body weight by i.v. injection for 5 consecutive days.
In vitro experiments.
L. major promastigotes (5 × 106/ml) were incubated at 28°C in RPMI 1640 medium. AmB formulations were added, and the number of viable (moving) parasites was counted with a Neubauer hemocytometer after 24 h. The results obtained were expressed as percent death of parasites in treated cultures [100 − (number of viable parasites in treated cultures divided by number of viable parasites in untreated cultures)].
Statistical methods.
The paired Student t test was used for statistical analysis.
RESULTS
Characterization of AmB-cholesteryl sulfate formulations.
Various formulations that were based on Amphocil, a complex of amphotericin B and cholesteryl sulfate which is available as a colloidal dispersion, were prepared. This preparation, which is normally administered by injection, contains small (approximately 120 nm) diskoid particles (3) composed of AmB and cholesteryl sulfate at a 1:1 mole ratio. Amphocil reconstituted with water to 5 mg/ml contains 5% glucose. Dispersion of Amphocil was prepared in various media that are commonly used to enhance percutaneous absorption. Amphocil dispersed easily in aqueous solutions of 5% glucose or 5 to 25% ethanol. Particle size distribution, measured by photon correlation spectroscopy (Table 1), demonstrates that the size of Amphocil particles increases somewhat with an increase in the ethanol concentration, from 122 nm without ethanol to 259 nm in the presence of 25% ethanol. To obtain a dispersion in propylene glycol or glycerol, strong vortexing and ultrasonic irradiation were necessary. Particle size, which was much larger (4 to 15 μm), was measured with a Coulter multisizer.
TABLE 1.
Particle size distribution of Amphocil in various media measured by photon correlation spectroscopy
| Formulationa | Amt (%) | Mean size (nm) | SD size (nm) |
|---|---|---|---|
| DDW | 100 | 122 | 24 |
| 5% EtOH in DDW | 2 | 18 | 3 |
| 90 | 167 | 53 | |
| 8 | 822 | 96 | |
| 10% EtOH in DDW | 80 | 205 | 83 |
| 20 | 1,270 | 150 | |
| 25% EtOH in DDW | 90 | 259 | 81 |
| 10 | 1,260 | 150 | |
| 5% glucose in DDW | 97 | 7.3 | 1 |
| 3 | 104 | 23 |
The reconstituted Amphocil dispersion contained 5 mg of AmB per ml in 5% glucose. EtOH, ethanol.
Effect of AmB formulations on the course of disease.
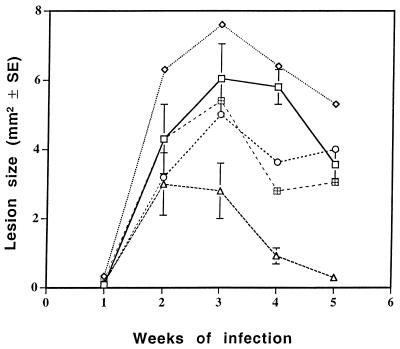
Initially, dispersions of Amphocil in glycerol, propylene glycol, 10% ethanol in double-distilled water (DDW), and 5% glucose in DDW were topically applied for 3 weeks on the site of L. major injection. Lesion size was measured weekly. The effects of the formulations on disease outcome are summarized in Fig. 1, which shows that only topical administration of AmB complexed with cholesteryl sulfate in 10% ethanol caused a marked reduction in lesion size. The effect was statistically significant from week 3 onward (P < 0.003 at week 3 and P < 0.0001 at weeks 4 and 5). The 5% glucose group had statistically smaller lesions than the controls at only one time point (week 4), and little or no effect was found for the other groups. No relapse was observed after the discontinuation of therapy (in any of the experiments performed).
FIG. 1.
Effect of Amphocil in various solvents on the course of disease. Dispersions of Amphocil were prepared in various solvents and topically applied to the base of the tail during the first 3 weeks of an L. major infection. The effect on lesion size was determined weekly. Each group included eight mice. □, untreated; ◊, glycerol; ○, polypropylene glycol; ▵, 10% ethanol; ⊞, glucose.
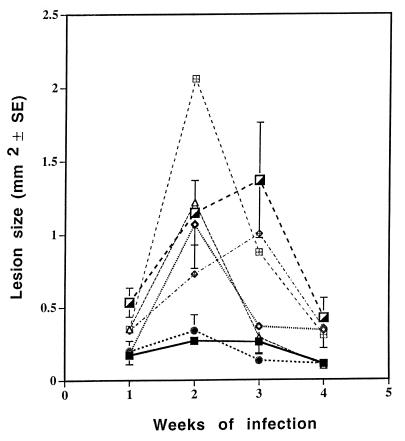
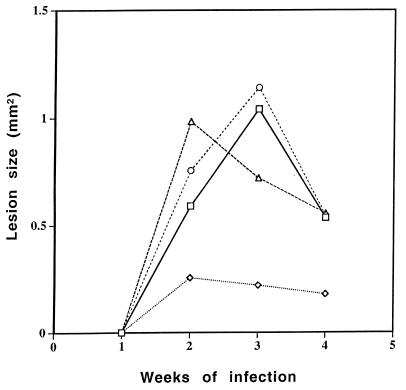
Additional experiments showed that dispersions of AmB and cholesteryl sulfate in 5, 10, and 25% ethanol had approximately equivalent effects on lesion size. Aqueous ethanol alone, applied at 5, 10, and 25% to three experimental groups, did not affect lesion size (Fig. 2), nor did a suspension of AmB and cholesteryl sulfate in water (Fig. 3). An ethanolic formulation containing Fungizone (AmB-sodium deoxycholate) was much less effective topically than those formulations described above (Fig. 3).
FIG. 2.
Effect of Amphocil dissolved in different ethanol concentrations on the course of disease. Amphocil in 5, 10, and 25% ethanol in aqueous solution or ethanol without Amphocil was topically applied to the base of the tail during the first 3 weeks of an L. major infection. The effect on lesion size was determined weekly. Each group included eight mice. ■, AmB and 5% ethanol; ◊, AmB and 10% ethanol; •, AmB and 25% ethanol; ▵, 5% ethanol; ⊞, 10% ethanol;  , 25% ethanol, ┌, untreated.
, 25% ethanol, ┌, untreated.
FIG. 3.
Comparison of the effect of the addition of 5% ethanol to Amphocil and to Fungizone. The effect of Amphocil in the presence of 5% ethanol was compared to the effect of Fungizone in the presence of 5% ethanol and to the effect of Amphocil without ethanol when the formulations were topically applied during the first 3 weeks of an L. major infection. The effect on lesion size was determined weekly. Each experiment included eight mice. □, untreated; ◊, AmB in ethanol; ○, AmB in DDW; ▵, Fungizone in ethanol.
AmB was also tested in a form of AmB-phospholipid (DMPC:DMPG, 7:3) complex (ABPLC). A topical formulation of ABPLC in 0.9% NaCl–5% ethanol was found to be as effective as the AmB-cholesteryl sulfate formulations in reducing lesion size, despite the large particle size (approximately 2.75 μm) in this formulation (data not shown). Therefore, it seems that no apparent correlation exists between particle size and the therapeutic efficacy of a given dispersion.
Effects of AmB formulations on L. major promastigotes in vitro.
The effects of Amphocil formulations in aqueous ethanol or DDW on the viability of L. major promastigotes were determined. The results from two independent experiments, summarized in Table 2,, indicate that Fungizone, although highly effective in killing parasites in vitro, was ineffective in vivo, even when it was applied topically in 5% ethanol (Fig. 3). The results further show that ethanol is not required for the effectiveness of AmB in vitro since identical parasite killing was obtained with AmB-cholesteryl sulfate prepared in DDW or in aqueous ethanolic solutions, whereas Fungizone, in the presence or absence of ethanol, was the most efficacious in vitro but lacked therapeutic efficacy in vivo.
TABLE 2.
Effects of AmB formulations on promastigotes in vitroa
| Formulation and AmB concn (μM) | % Parasite death
|
|||
|---|---|---|---|---|
| 5% EtOHb | 10% EtOH | 25% EtOH | DDW | |
| Amphocil | ||||
| 1 | 99 ± 2 | 98 ± 2 | 97 ± 4 | 99 ± 1 |
| 0.3 | 78 ± 13 | 62 ± 25 | 65 ± 31 | 81 ± 5 |
| 0.1 | 25 ± 25 | 33 ± 13 | 35 ± 11 | 21 ± 15 |
| 0.03 | 11 ± 11 | 0 | 6 ± 6 | 3 ± 3 |
| Fungizone | ||||
| 1 | 100 ± 0 | |||
| 0.1 | 96 ± 4 | |||
| 0.01 | 85 | |||
| 0.001 | 6 | |||
L. major promastigotes were grown at 28°C in the presence of ethanolic Amphocil or Fungizone. The results are presented as percent death of parasites (average ± standard error) determined after 24 h.
EtOH, ethanol.
Comparison of organ biodistribution of AmB formulations after topical and systemic administration.
The levels of AmB in body tissues after topical administration of various levels of Amphocil in 10% ethanol were determined and were compared to those obtained after i.v. administration. The level of AmB in the kidneys, liver, and spleen after the application of a topical total dose of 0.9 mg of AmB was below the detection limit. After the application of a total dose of 8.1 mg, 2 to 5.5 μg/g of tissue was detected in the internal organs. In comparison, after i.v. administration of 0.1 mg, 0.5, 14, and 50 μg/g of tissue were detected in the kidneys, spleen, and liver, respectively. In a similar experiment with topical ethanolic aqueous ABPLC, 3.5 to 4.5 μg/g of tissue was detected in the internal organs after the application of a total topical dose of 0.9 mg of AmB.
The data indicate that even for much smaller cumulative doses (40 to 400 μg versus 1 to several milligrams for topical application), comparable or sometimes greater levels of drug were detected in the internal organs. For Amphocil, in particular, large amounts of the drug were found in the liver and the spleen.
DISCUSSION
Cutaneous leishmaniasis is a potentially disfiguring disease that occurs in more than 60 countries, with approximately 200 million people at risk and 300,000 cases occurring annually (2). Three lipid formulations of AmB are now marketed for clinical use in many countries worldwide (see reference 14 and the references listed therein). These formulations are successful treatments for visceral leishmaniasis both in mice (12, 21) and in humans (8) because the drug is administered i.v. and thus is easily delivered to the affected organs. However, studies with these formulations for the treatment of CL have shown that they have little or no effect against experimental CL (20) or clinical mucocutaneous leishmaniasis (17). A more recent study has shown that one formulation, AmBisome, when injected i.v., is effective against experimental CL, albeit at doses approximately five times higher than those required for the treatment of visceral disease (24). These results support the notion that a lack of effective treatment for CL is due to a lack of proper delivery rather than a lack of drug efficacy. The efficacious in vitro killing of L. major promastigotes by Fungizone, Amphocil, and Ambisome (24; this study) support this notion.
The results obtained in this study suggest that the therapeutic efficacies of AmB-lipid formulations in ethanolic aqueous carriers are related to the presence of low concentrations of ethanol in the carrier. The addition of ethanol does not affect the parasites directly (Table 2) and does not improve the efficacy of AmB against the parasite in vitro. The effect of ethanol at the percent used (5 to 25%) should therefore be related to the effects on the skin or on skin-carrier interactions. Several factors may be involved in the ethanol-induced topical therapeutic effects of Amphocil and ABPLC: (i) reduction of skin-water surface tension and/or reduction of viscosity of the drug dispersion. However, because the presence of ethanol did not improve the performance of the AmB-deoxycholate mixed micelles (Fungizone), the effect of ethanol on surface tension and viscosity is either not important or not sufficient, and ethanol at 5 to 25% is not an effective enhancer of skin permeability. (ii) We must therefore assume that the effects of ethanol on the physical properties of Amphocil and ABPLC are the dominant factors. In this respect sodium deoxycholate-AmB mixed micelles are very different from Amphocil and ABPLC. Although the last two formulations differ from each other, they share a much higher association constant of AmB to their lipids (3, 7). Also, in Amphocil discoids (16) and in ABPLC large particles, the curvature is much smaller than that in Fungizone. The increase in the size of the Amphocil particles with increasing ethanol concentration from 120 nm without ethanol to 167 nm in 5% (0.8 M) ethanol to 259 nm in 25% (∼4 M) ethanol (Table 1) supports our hypothesis that the ethanol which partitions into the Amphocil particles affects its properties. This size increase resembles an earlier observation for dipalmitoylphosphatidylcholine (DPPC) large unilamellar vesicles (LUV; 100 nm) (23). As in the case of DPPC LUV, the Amphocil particles remain intact in the range of 0.8 to 4 M ethanol used in the present study. The similarities in the effects of ethanol on particle size increases for Amphocil and DPPC LUV suggest that the partition of Amphocil into the interface region of the discoid particles increases the lateral as well as the transverse repulsion between the lipid molecules, which leads to increases in the area per molecule and head group solvation. The increase in the ratio of the polar region cross section/hydrophobic region cross section induces interdigitation, thereby causing the less flexible AmB to protrude from the assembly and become more available to the skin. For ABPLC we assume that the effect of ethanol will be similar, although it is technically not feasible to measure size changes, because the size distribution is very heterogeneous and most particles are in the micron size range.
As demonstrated by the biodistribution studies, the doses of the ethanolic formulations of AmB applied topically result in much lower levels of the drug in internal organs than those obtained with lower doses administered systemically (especially for Amphocil). Accordingly, higher doses may be used topically with greatly reduced toxic side effects.
The profile of the organ biodistribution of AmB delivered topically as Amphocil or ABPLC is very different from the biodistribution when these formulations are given i.v. When given topically, the skin (site of application) is the major tissue of accumulation. For both Amphocil and ABPLC the order of tissue concentration is skin > kidney > spleen > liver, while after i.v. delivery the liver is by far the major organ of accumulation, especially for Amphocil (liver > spleen > kidney) (3, 14). This suggests that, when applied topically, AmB is dissociated from the lipid carrier molecule and there is no transdermal delivery of the intact AmB-lipid assembly.
ACKNOWLEDGMENTS
This work was supported by the Leslie Nicholas Fund, Hadasit Medical Research Services and Development, the Horowitz Foundation, and the Chief Scientist’s Office, Ministry of Health, Israel.
REFERENCES
- 1.Aerdts S J, van-Dalen R, Clasener H A, Festen J, van-Lier H J, Vollaard E J. Antibiotic prophylaxis of respiratory tract infection in mechanically ventilated patients. A prospective, blinded, randomized trial of the effect of a novel regimen. Chest. 1991;100:783–791. doi: 10.1378/chest.100.3.783. [DOI] [PubMed] [Google Scholar]
- 2.Ashford R W, Desjeux P, deRaadt P. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol Today. 1992;8:104–105. doi: 10.1016/0169-4758(92)90249-2. [DOI] [PubMed] [Google Scholar]
- 3.Barenholz Y, Cohen R. Rational design of amphiphile-based drug carriers and sterically stabilized carriers. J Liposome Res. 1995;5:905–932. [Google Scholar]
- 4.Bensalah A, Zakraoui H, Zaatour A, Ftaiti A, Zaafouri B, Garraoui A, Olliaro P L, Dellagi K, Benismail R. A randomized, placebo-controlled trial in Tunisia treating cutaneous leishmaniasis with paromomycin ointment. Am J Trop Med Hyg. 1995;53:162–166. doi: 10.4269/ajtmh.1995.53.162. [DOI] [PubMed] [Google Scholar]
- 5.Bent J P, III, Kuhn F A. Antifungal activity against allergic fungal sinusitis organisms. Laryngoscope. 1996;106:1331–1334. doi: 10.1097/00005537-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Brajtburg J, Bolard J. Carrier effects on biological activity of amphotericin B. J Clin Microbiol Rev. 1996;9:512–531. doi: 10.1128/cmr.9.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen R, Polacheck I, Benita S, Levi M, Barenholz Y. Program and abstracts of the Conference on Liposome Advances; Progress in Drug Delivery and Vaccine Delivery. London, United Kingdom: Centre for Drug Delivery Research, School of Pharmacy, University of London; 1996. Comparative evaluation of five types of amphiphile-based assemblies containing amphotericin B. [Google Scholar]
- 8.Dimartino L, Davidson R N, Giacchino R, Scotti S, Raimondi F, Casstagnola E, Tasso L, Cascio A, Gradoni L, Gramiccia M, Pettoellomantovani M, Bryceson A D M. Treatment of visceral leishmaniasis in children with liposomal amphotericin B. J Pediatr. 1997;131:271–277. doi: 10.1016/s0022-3476(97)70165-3. [DOI] [PubMed] [Google Scholar]
- 9.El-On J, Geoffrey J P, Witztum E, Greenblatt C L. Development of topical treatment for cutaneous leishmaniasis caused by Leishmania major in experimental animals. Antimicrob Agents Chemother. 1984;26:745–751. doi: 10.1128/aac.26.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-On J, Weinrauch L, Livshin R, Even Paz Z, Jacobs G P. Topical treatment of recurrent cutaneous leishmaniasis with ointment containing paromomycin and methyl-benzethonium chloride. Br Med J. 1985;291:704. doi: 10.1136/bmj.291.6497.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelhard D, Eldor A, Polacheck I, Hardan I, Ben-Yehuda D, Amselem S, Salkin I F, Lopez-Berestein G, Sacks T, Rachmilewitz E A, Barenholz Y. Disseminated fusariosis treated with amphotericin B-phospholipid complex. Leuk Lymphoma. 1993;9:385–392. doi: 10.3109/10428199309148539. [DOI] [PubMed] [Google Scholar]
- 12.Gangneux J P, Sulahian A, Garin Y J F, Derouin F. Lipid formulations of amphotericin B in the treatment of experimental visceral leishmaniasis due to Leishmania infantum. Trans R Soc Trop Med Hyg. 1996;90:574–577. doi: 10.1016/s0035-9203(96)90330-2. [DOI] [PubMed] [Google Scholar]
- 13.Giri O P. Amphotericin B therapy in kala-azar J. Indian Med Assoc. 1993;91:91–93. [PubMed] [Google Scholar]
- 14.Hiemenz J W, Walsh T J. Lipid formulations of amphotericin B: recent progress and future directions. Clin Infect Dis. 1996;2:S133–S144. doi: 10.1093/clinids/22.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 15.Klaus S, Axelrod O, Jonas F, Frankenburg S. Changing patterns of cutaneous leishmaniasis in Israel. Trans R Soc Trop Med Hyg. 1994;88:649–650. doi: 10.1016/0035-9203(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 16.Lasic D D. Mixed micelles in drug delivery. Nature. 1992;355:279–280. doi: 10.1038/355279a0. [DOI] [PubMed] [Google Scholar]
- 17.Llanos-Cuentas, A., J. Echevarria, J. Chang, P. Campos, J. Cieza, D. Lentnek, D. Gonzalez, M. Robbins, and R. Williams. 1991. Randomized trial of amphotericin B complex (ABLC) versus Fungizone in patients with mucocutaneous leishmaniasis. Mem. Inst. Oswaldo Cruz 86(Suppl.):238.
- 18.Lotery A J, Kerr J R, Page B A. Fungal keratitis caused by Scopulariopsis brevicaulis: successful treatment with topical amphotericin B and chloramphenicol without the need for surgical debridement. Br J Ophthalmol. 1994;78:730. doi: 10.1136/bjo.78.9.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neva F A, Ponce C, Ponce E, Kreutzer R, Modabber F, Olliaro P. Non ulcerative cutaneous leishmaniasis in Honduras fails to respond to topical paromomycin. Trans R Soc Trop Med Hyg. 1997;91:473–475. doi: 10.1016/s0035-9203(97)90290-x. [DOI] [PubMed] [Google Scholar]
- 20.Panosian C B, Barza M, Szoka F, Wyler D J. Treatment of experimental cutaneous leishmaniasis with liposome-intercalated amphotericin B. Antimicrob Agents Chemother. 1984;25:655–656. doi: 10.1128/aac.25.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul M, Durand R, Fessi H, Rivollet D, Houin R, Astier A, Deniau M. Activity of a new liposomal formulation of amphotericin B against two strains of leishmania infantum in a murine model. Antimicrob Agents Chemother. 1997;41:1731–1734. doi: 10.1128/aac.41.8.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pleyer U, Legmann A, Mondino B J, Lee D A. Use of collagen shields containing amphotericin B in the treatment of experimental Candida albicans-induced keratomycosis in rabbits. Am J Ophthalmol. 1992;113:303–308. doi: 10.1016/s0002-9394(14)71583-1. [DOI] [PubMed] [Google Scholar]
- 23.Vierl U, Lobbecke L, Nagel N, Cevc G. Solute effects on the colloidal and phase behavior of lipid bilayer membranes: ethanol-dipalmitoylphosphatidylcholine mixtures. Biophys J. 1994;67:1067–1079. doi: 10.1016/S0006-3495(94)80572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yardley V, Croft S. Activity of liposomal amphotericin B against experimental cutaneous leishmaniasis. Antimicrob Agents Chemother. 1997;41:752–756. doi: 10.1128/aac.41.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]