Abstract
INTRODUCTION
Diabetes and overweight/obesity are described as accelerating aging processes, yet many individuals with these conditions maintain high levels of cognitive and physical function and independence late into life. The Look AHEAD Aging study is designed to identify 20‐year trajectories of behaviors, risk factors, and medical history associated with resilience against geriatric syndromes and aging‐related cognitive and physical functional deficits among individuals with these conditions.
METHODS
Look AHEAD Aging extends follow‐up of the cohort of the former 10‐year Look AHEAD trial. The original cohort (N = 5145) was enrolled in 2001 to 2004 when participants were aged 45 to 76 years and randomly assigned to a multidomain intensive lifestyle intervention (ILI) or a diabetes support and education (DSE) condition. The trial interventions ceased in 2012. Clinic‐based follow‐up continued through 2020. In 2021, the cohort was invited to enroll in Look AHEAD Aging, an additional 4‐year telephone‐based follow‐up (every 6 months) enhanced with Medicare linkage. Standardized protocols assess multimorbidity, physical and cognitive function, health care utilization, and health‐related quality of life.
RESULTS
Of the original N = 5145 Look AHEAD participants, N = 1552 active survivors agreed to participate in Look AHEAD Aging. At consent, the cohort's mean age was 76 (range 63 to 94) years and participants had been followed for a mean of 20 years. Of the original Look AHEAD enrollees, those who were younger, female, or with no history of cardiovascular disease were more likely to be represented in the Look AHEAD Aging cohort. Intervention groups were comparable with respect to age, diabetes duration, body mass index, insulin use, hypertension, cardiovascular disease, and cognitive function. ILI participants had significantly lower deficit accumulation index scores.
DISCUSSION
By continuing the long‐term follow‐up of an extensively characterized cohort of older individuals with type 2 diabetes, Look AHEAD Aging is well positioned to identify factors associated with resilience against aging‐related conditions.
Keywords: cognitive function, geriatric syndromes, lifestyle, obesity, type 2 diabetes mellitus
1. INTRODUCTION
Type 2 diabetes mellitus and overweight/obesity are often described as accelerators of aging processes, for example, as increasing the rate in which geriatric syndromes and age‐related chronic diseases develop, due to associations with decreased lifespan, increased disability risk, and reduced health‐related quality of life. 1 , 2 Yet, not all individuals with these conditions face the same prognosis in later life, and some continue to maintain relatively high levels of cognitive and physical function and independence. This suggests that strategies could be identified to promote healthier later‐life experiences in these individuals. It is natural to hypothesize that behavioral interventions to promote and maintain weight loss may mitigate against the consequences of aging. Many short term (≤18 months) trials of behavioral interventions have been conducted in older adults with obesity and some with diabetes, 3 , 4 , 5 , 6 , 7 , 8 , 9 but longer‐term trials are necessary for age‐related outcomes.
The Action for Health in Diabetes (Look AHEAD) randomized controlled clinical trial was designed to assess whether a multidomain intensive lifestyle intervention (ILI) targeting weight loss (including counseling on caloric restriction, increased physical activity, improved diet, and metabolic risk factor monitoring) versus a control condition (diabetes support and education [DSE]) would, a reduce risks for major cardiovascular disease events (primary outcome) and benefit many other aging‐related outcomes. 10 , 11 From the initial enrollment (in 2001 to 2004), of individuals aged 45 to 76 with established type 2 diabetes and overweight or obesity, through the end of its 10‐year intervention (in 2012), and as observed through extended follow‐up (through 2020), its intervention was shown to have successfully induced and maintained long‐term weight loss and to have provided benefits for many age‐related health conditions (eg, chronic kidney disease, depression, mobility, frailty indices, disability‐free life years). 12
It is unknown whether or not these benefits are maintained and translate to better later‐life experiences and long‐term associations with cognitive function and geriatric syndromes. Individuals in the cohort are now reaching ages when risks for age‐related health issues markedly increase. The Action for Health in Diabetes Aging (Look AHEAD Aging) observational study initiated 4 years of additional follow‐up of the cohort, beginning in 2022. It provides an unprecedented opportunity to characterize the long‐term aging processes in a well‐characterized cohort that represents an understudied but growing segment of the population, that is, older individuals with type 2 diabetes and obesity. The Look AHEAD Aging study features a change from the clinic‐based assessment used throughout prior study follow‐up to assessment based on telephone interviews and Medicare linkage. This manuscript is organized to (1) provide a rationale for the Look AHEAD Aging study; and (2) describe its protocol, recruitment approach, enrollment rates, and characteristics of its participants.
2. BACKGROUND
The Look AHEAD trial enrolled 5145 adults, aged 45 to 76 years, with established type 2 diabetes. 11 All participants met the following criteria: 45 to 76 years of age, body mass index (BMI) > 25 kg/m2 (>27 kg/m2 if on insulin), glycated hemoglobin (HbA1c) < 97 mmol/mol (11%), systolic/diastolic blood pressure < 160/<100 mmHg, triglycerides < 600 mg/dl, and successful passing of a maximum graded exercise test. The trial contrasted an ILI to induce and maintain ≥7% weight loss with an intervention featuring DSE. 13 , 14 The Look AHEAD trial's primary measure of effectiveness was achieved weight loss. Additional measures were changes in fitness based on a graded exercise test and intervention session attendance. ILI participants achieved a mean weight reduction of 8.6% at year 1 and sustained relative lower weights compared with DSE participants throughout follow‐up. 11 , 15 After the intervention phase of the trial ended in 2012, participants were enrolled in an observational follow‐up ending in 2020.
The Look AHEAD Aging study has as its primary objective the identification of long‐term effects of a multidomain ILI compared to a DSE in five areas: (1) multimorbidity and geriatric syndromes, (2) physical function, (3) cognitive function, (4) health care utilization and costs, and (5) health‐related quality of life. Shorter‐term benefits of ILI have been reported for multimorbidity, 16 physical function, 17 , 18 health care costs and medication use, 19 , 20 and some aspects of health‐related quality of life. 21
Cognitive outcomes are an important component of the Look AHEAD Aging study because the impact of ILI on cognitive function may be variable depending on an individual's obesity status and medical history, with potential benefits for those without obesity or cardiovascular disease and potential harm for others. 22 , 23 In the Look AHEAD cohort, cognitive function appeared to be relatively preserved among individuals maintaining relatively better diabetes control and those without severe obesity. 24 , 25 There have been inconsistent reports of cognitive benefits from other major clinical trials of lifestyle interventions, 26 , 27 but none have fostered the substantial long‐term weight losses that were achieved in the Look AHEAD program.
The secondary objectives of the Look AHEAD Aging study include evaluating whether any long‐term differences in its primary outcomes between intervention groups might be attributable to differences in the nearly two decades of longitudinal trajectories in clinical measures (eg, HbA1c, blood pressure, depressive symptoms, weight), medication use (eg, metformin, insulin, statins), and behaviors (eg, physical activity, weight control practices). They also include evaluating whether intervention group differences vary among baseline subgroups defined by sex, race/ethnicity, age, obesity, and health status. Other objectives include continuing to assess aging‐related measures of function and abilities (ie, gait speed, balance, strength, activities of daily living, instrumental activities of daily living, independent living) over time and examining the interplay between stressors (diabetes, obesity/overweight, and chronological aging) and resilience against the development of physical and cognitive deficits, geriatric syndromes, and expanding health care needs adopting the paradigm described by Ferrucci, et al. 28 The study also will maintain its cohort as a source of discovery by promoting ancillary studies, dissemination, and distribution of well‐documented public use data, and through scientific interest groups.
The overarching goal of Look AHEAD Aging is to provide information that is vital for improving the care of older individuals with type 2 diabetes who are overweight or obese and to identify strategies that, if applied in mid‐life, yield better later life experiences.
3. ENROLLMENT IN THE Look AHEAD AGING STUDY
All individuals who participated in the Look AHEAD program and who had given permission for further contact by a central unit (Wake Forest University School of Medicine) were eligible for inclusion in the Look AHEAD Aging study. Of the original 5145 Look AHEAD participants, 641 had dropped from the study, 1279 had died, and 495 of the remaining had not provided permission (this included 174 participants for whom their tribal leadership had prohibited further engagement). Excluding the 32 participants of those remaining who had previously been classified with dementia, this resulted in a pool of 2698 potential participants. Look AHEAD Aging adopted the following inclusion criteria:
Hearing ability adequate to complete a telephone interview.
Adequate comprehension of the consent process, based on a consent comprehension tool.
Fluency in English or Spanish.
RESEARCH IN CONTEXT
Systematic Review: The Look AHEAD Aging study builds on the 20 years of deep phenotyping of a large cohort of adults with diabetes and overweight or obesity, the many research findings from the study group, and an extensive review of geriatric literature related to diabetes and obesity.
Interpretation: Type 2 diabetes and overweight/obesity are often described as accelerators of aging due to associations with cognitive impairment, decreased lifespan, increased disability risk, and reduced health‐related quality of life. More information is required to understand the characteristics and behaviors that are associated with better health and functional profiles in later life for individuals with these conditions.
Future Directions: The Look AHEAD Aging study will identify factors associated with resilience against the development of functional deficits in cognition and physical function, geriatric syndromes, and the expanding health care utilization that are associated with aging. We view diabetes, obesity/overweight, and chronological age as stressors challenging resilience.
Look AHEAD Aging recruitment was based on mailings followed by scheduled telephone interviews to obtain consent and to ascertain outcomes. Figure 1 diagrams the recruitment of participants. Of the 2698 potential enrollees, 1606 (60%) eventually provided informed consent, and of these 54 withdrew or died prior to being contacted for assessments, yielding an evaluable cohort of 1552. Of these evaluable participants, baseline cognitive testing was completed on 1437 (93%).
FIGURE 1.
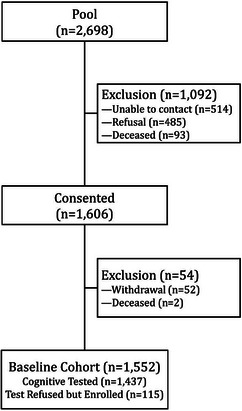
Look AHEAD Aging enrollment.
4. CONSENT STATEMENT
All participants provided informed consent prior to enrollment.
5. COHORT CHARCTERISTICS
The Look AHEAD Aging cohort has been altered from the original Look AHEAD study group by attrition, mortality, the reconsenting process, and the above inclusion criteria. Table 1 compares the distribution of the original cohort with respect to characteristics at the Look AHEAD baseline in 2001 to 2004 with those of the Look AHEAD Aging cohort in 2022 to 2023, both overall and by intervention assignment. Those enrolling in Look AHEAD Aging tended to be younger, women, more highly educated, and to not have had a history of cardiovascular disease. As noted above, the clinical sites that contributed the majority of Native American participants declined to participate in Look AHEAD Aging, leading to very few of these individuals being enrolled and resulting in overall differences in the racial/ethnic distribution. African‐American and Hispanic/Latino participants continued to be well represented in the Look AHEAD Aging cohort. Importantly, enrollment rates were consistent across intervention groups, that is, prior intervention assignment appeared to be unrelated to participants’ enrollment in Look AHEAD Aging, both overall and among important subgroups.
TABLE 1.
Comparison of ILI and DSE sociodemographic characteristics among the Look AHEAD cohort at baseline with the characteristics of the Look AHEAD Aging Cohort at Look AHEAD baseline: N and (percent).
| Look AHEAD baseline, N = 5145, enrolled 2001–2004 | Look AHEAD Aging, N = 1552, enrolled 2022–2023 | Comparisons (p‐values) | ||||
|---|---|---|---|---|---|---|
| Characteristics of Look AHEAD at baseline | DSE N = 2575 | ILI N = 2570 | DSE N = 739 | ILI N = 813 | Look AHEAD versus look AHEAD Aging | Consistency of look AHEAD Aging enrollment across intervention groups |
| Age at Look AHEAD Enrollment | ||||||
| 45–54 | 600 (23%) | 642 (25%) | 216 (29%) | 272 (33%) | p < 0.001 | p = 0.18 |
| 55–64 | 1422 (55%) | 1428 (56%) | 457 (62%) | 467 (57%) | ||
| 65–76 | 553 (22%) | 500 (19%) | 66 (9%) | 74 (9%) | ||
| Gender | ||||||
| Female | 1537 (60%) | 1526 (59%) | 452 (61%) | 517 (64%) | p = 0.005 | p = 0.17 |
| Male | 1038 (40%) | 1044 (41%) | 287 (39%) | 296 (36%) | ||
| Education | ||||||
| HS or less | 1482 (59%) | 1454 (58%) | 374 (52%) | 388 (49%) | p < 0.001 | p = 0.22 |
| College | 542 (22%) | 576 (23%) | 168 (23%) | 221 (28%) | ||
| Post college | 485 (19%) | 491 (19%) | 179 (25%) | 190 (24%) | ||
| Race and ethnicity | ||||||
| African American | 404 (16%) | 400 (16%) | 124 (17%) | 137 (17%) | p < 0.001 | p = 0.17 |
| Native American | 128 (5%) | 130 (5%) | 2 (0%) | 4 (0%) | ||
| Asian/Pacific Islander | 21 (1%) | 29 (1%) | 7 (1%) | 13 (2%) | ||
| Hispanic/Latino | 340 (13%) | 340 (13%) | 79 (11%) | 112 (14%) | ||
| Non‐Hispanic White | 1631 (63%) | 1621 (63%) | 513 (69%) | 526 (65%) | ||
| Other/Multiple | 51 (2%) | 50 (2%) | 15 (2%) | 21 (3%) | ||
| BMI, kg/m2 | ||||||
| 25–29 | 362 (14%) | 403 (16%) | 106 (14%) | 135 (17%) | p = 0.70 | p = 0.68 |
| 30–39 | 1639 (64%) | 1592 (62%) | 475 (64%) | 494 (61%) | ||
| >40 | 574 (22%) | 575 (22%) | 158 (21%) | 184 (23%) | ||
| CVD history | ||||||
| No | 2228 (87%) | 2205 (86%) | 682 (92%) | 749 (92%) | p < 0.001 | p = 0.72 |
| Yes | 347 (13%) | 365 (14%) | 57 (8%) | 64 (8%) | ||
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; DSE, diabetes support and education; HS, high school; ILI, intensive lifestyle intervention; Look AHEAD, Action for Health in Diabetes.
When the interventions were terminated on September 14, 2012, there were differences between treatment arms for key measures among the subset of participants who were to later enroll in Look AHEAD Aging. The most recent mean (SE) percent changes in BMI from baseline for ILI participants was −5.45 (0.33)% and for DSE participants it was −3.79 (0.38)%, p < 0.001. There were differences in 400 m gait speed: 1.015 (0.007) m/s for ILI versus 0.982 (0.008) m/s for DSE, p = 0.002. There were also differences for increases in multimorbidity from baseline: 0.79 (0.04) for ILI versus 0.90 (0.04) for DSE, p = 0.049. However, there were no differences in composite cognitive function: 0.125 (0.024) for ILI versus 0.115 (0.023) for DSE, p = 0.78.
6. Look AHEAD AGING PROTOCOL
Data collection for the Look AHEAD Aging study is conducted via standardized telephone interviews approximately every 6 months (calls of <1h), as well as Medicare linkage. The transition from the prior clinic‐based assessments conducted in the Look AHEAD program to remote assessments was necessitated by the closing of the study's clinical sites. We drew from past experience in transitioning from clinic‐ to telephone‐based assessments and used rigorous central training of interviewers designed to enhance rigor and reliability. All interviews start with a standardized brief hearing assessment. The study team has extensive experience in telephone‐based data collection, including measures of cognition and physical function.
Telephone‐based interviews consist of one panel (A) that is administered annually (4 times) and two panels (M1 and M2) that are administered at alternate mid‐years (2 times each) in a random order (see Figure 2 for details). Participants are asked to weigh themselves at home the morning of the call, or for those without home scales to provide their weight (and date) from their last physician visit. Participants confirm (or provide for the first time) their Medicare Beneficiary Number for assessment of health care utilization and cost. Look AHEAD Aging is designed to address the following hypotheses.
FIGURE 2.
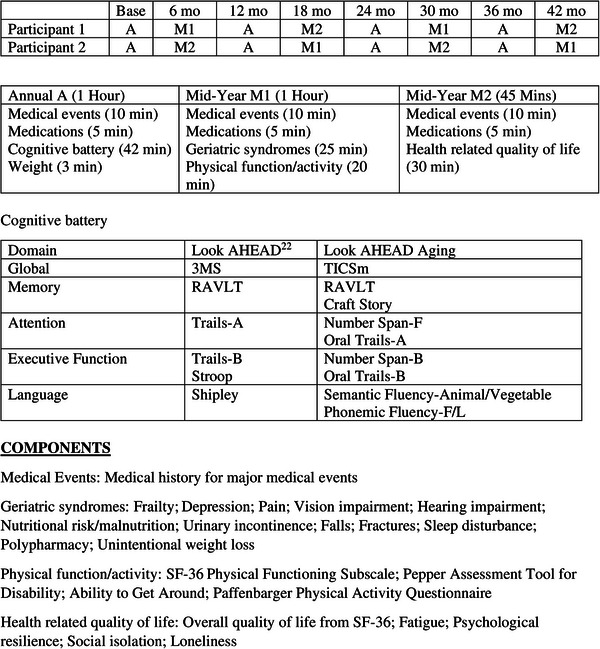
Assessment schedule for Look AHEAD Aging: example for two potential participants. 3MS, Modified Mini‐Mental State Examination; Look AHEAD, Action for Health in Diabetes; RALT, Rey Auditory Verbal Learning Test; SF‐36, 36‐Item Short Form Survey; TICSm, Modified Telephone Interview for Cognitive Status.
Multimorbidity. The primary hypothesis related to multimorbidity is that random assignment to 10 years of ILI versus DSE results in a mean difference in a 9‐component multimorbidity index—comprising cancer, cardiac arrhythmia, chronic kidney disease, congestive heart failure, coronary artery disease, depression, dyslipidemia, hypertension, and stroke—over Look AHEAD Aging follow‐up. A secondary hypothesis is that the mean rate of change in the multimorbidity index over the follow‐up since the original randomization will vary between ILI and DSE. Supporting analyses will compare the prevalence, severity, and change in the following geriatric syndromes: depression, pain, vision impairment, hearing impairment, nutritional risk, urinary incontinence, falls, fractures, sleep, polypharmacy, and weight and unintentional weight loss. Look AHEAD Aging will also describe differences between ILI and DSE in a frailty index based on deficit accumulation, modeled after the indices previously developed in Look AHEAD. 29 , 30
Physical function. The primary hypothesis related to physical function is that random assignment to 10 years of ILI versus DSE results in a mean difference in the 36‐Item Short Form Survey (SF‐36) physical functioning subscale over Look AHEAD Aging follow‐up. A secondary hypothesis is that the mean rate of change in this scale over the full Look AHEAD follow‐up varies between participants who had been assigned to ILI versus DSE. Supporting analyses will compare the prevalence, severity, and change in self‐reported mobility, activities of daily living, abilities, and physical activity.
Cognitive function. The primary hypothesis related to cognitive function is that random assignment to 10 years of ILI versus DSE results in mean differences in a composite score of cognitive function over Look AHEAD Aging follow‐up. A secondary hypothesis is that the mean rate of change in this score over the full Look AHEAD follow‐up varies between ILI and DSE. Supporting analyses will compare scores for the cognitive domains of memory, executive function, and global cognitive function and in subjective memory complaints.
The cognitive battery has been harmonized with the National Alzheimer Coordinating Center (NACC) Uniform Dataset Version 3 (UDS v3) 31 and assesses global cognitive function, memory, attention, executive function, and language. It is modeled after the validated telephone‐based Women's Health Initiative Memory Study that has been administered successfully since 2008. 32 The battery was slightly modified for Look AHEAD Aging. It includes the Modified Telephone Interview for Cognitive Status (TICSm) 33 , 34 instead of the Montreal Cognitive Assessment (MoCA) 35 and the Rey Verbal Learning Test (RAVLT). 36 Telephone‐administered versions of other tests have been used. 37 , 38 Proxy questionnaires that were previously administered in Look AHEAD have been continued: the Functional Activity Questionnaire (FAQ) 39 and Dementia Questionnaire (DQ). 40 , 41 The battery provides anchors for harmonization with the prior Look AHEAD cognitive protocol to allow the assessment of cognitive decline over time. An ancillary study provides standardized adjudication of mild cognitive impairment and dementia.
Assessing depressive symptoms is important for supporting the interpretation of cognitive function data. Depression was previously assessed in Look AHEAD with the Patient Health Questionnaire (PHQ‐9), a 9‐item self‐report measure of depressive symptoms in adults that corresponds directly with Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria for a major depressive episode, which will continue to be administered by telephone. 42 , 43
Health care utilization and costs. The primary hypothesis related to health care utilization and costs is that random assignment to 10 years of ILI versus DSE results in a mean difference in total costs of health care over Look AHEAD Aging follow‐up. A secondary hypothesis is that the mean rate of change in these costs over the full Look AHEAD follow‐up varies between ILI and DSE. Supporting analyses will compare associated costs and rates of hospitalizations, outpatient care, home care medications, and procedures.
Health‐related quality of life. Each of the above outcomes contribute to overall health‐related quality of life. Look AHEAD Aging will additionally describe differences between ILI and DSE in mean levels and changes in measures of SF‐36 component scores, fatigue, psychological resilience, social isolation, and loneliness. 44 , 45 , 46
Look AHEAD Aging will also investigate the associations that clinical measures, medication use, and behaviors have with long‐term outcomes. Look AHEAD has collected extensive (for 18 years) data on clinical measures (HbA1c, blood pressure, lipids, depressive symptoms, and health‐related deficits in abilities and function). There has been considerable variation in modifiable risk factors such as weight, waist circumference, fitness, and body composition in the participants. 47 There is a vast literature suggesting that medications commonly used among participants may affect the rate (positively or negatively) of aging and accrual of age‐related chronic diseases. Examples include metformin, insulin, statins, antihypertensive medications, antidepressants, anti‐inflammatories, and many more. From its annual inventories, Look AHEAD provides a rich platform to explore associations that exposures to drugs and drug combinations have with later‐life outcomes. It has extensive data on weight control practices, alcohol intake, smoking, physical activity, sleep, and self‐care. Also included are prior measures of psychological resilience and loneliness, 45 , 46 which are extended to Look AHEAD Aging. These resources allow us to retrospectively examine trajectories related to the preservation of function, independence, and better overall health later in life. Look AHEAD Aging will also describe incidence and trajectories of outcomes across the 20 to 24 year span of combined Look AHEAD and Look AHEAD Aging follow‐up. It will share these data resources for other investigators to mine as we continue to publish public use databases.
7. COHORT CHARACTERISTICS AT Look AHEAD AGING ENROLLMENT
Table 2 describes the characteristics of participants at the time when they were enrolled in the Look AHEAD Aging study based on current self‐report or the most recent Look AHEAD clinic‐based measure. Eight percent of participants were aged 85 years or older and nearly half had durations of diabetes of at least 25 years. Fifteen percent had BMI values of 40 kg/m2 or more. The two intervention groups had similar distributions with respect to age, diabetes duration, BMI, and prevalence of hypertension and cardiovascular disease. The most marked difference between groups was with respect to deficit accumulation frailty, 29 which averaged about 14% lower among former ILI versus DSE participants.
TABLE 2.
Characteristics of the Look AHEAD Aging cohort by ILI and DSE at last assessment: current age, body mass index, diabetes duration, insulin use, hypertension, and cardiovascular disease history (updated throughout Look AHEAD), N (percent) or mean (standard deviation).
|
DSE N = 739 |
ILI N = 813 |
p‐value | |
|---|---|---|---|
| Age | |||
| 65–74 | 248 (34%) | 288 (35%) | 0.76 |
| 75–84 | 431 (58%) | 461 (57%) | |
| ≥85 | 60 (8%) | 64 (8%) | |
| Diabetes duration, years | |||
| 18–24 | 419 (57%) | 462 (57%) | 0.40 |
| 25–29 | 207 (28%) | 211 (26%) | |
| 30–34 | 65 (9%) | 91 (11%) | |
| ≥35 | 43 (6%) | 45 (6%) | |
| BMI, kg/m2 a | |||
| <25 | 24 (3%) | 39 (5%) | 0.36 |
| 25–29 | 182 (25%) | 208 (26%) | |
| 30–39 | 413 (56%) | 449 (55%) | |
| ≥40 | 119 (16%) | 117 (14%) | |
| Insulin use a | |||
| No | 380 (51%) | 463 (57%) | 0.03 |
| Yes | 359 (49%) | 350 (43%) | |
| Hypertension a | |||
| No | 55 (7%) | 74 (9%) | 0.23 |
| Yes | 684 (93%) | 739 (91%) | |
| Cardiovascular disease a | |||
| No | 587 (79%) | 664 (82%) | 0.27 |
| Yes | 152 (21%) | 149 (18%) | |
| Deficit accumulation FI a | 0.14 (0.06) | 0.12 (0.06) | <0.001 |
| Multimorbidity Index a | 1.77 (1.02) | 1.68 (1.04) | 0.09 |
| Modified Mini‐Mental State Score | 90.5 (7.4) | 90.6 (7.4) | 0.68 |
Abbreviations: BMI, body mass index; DSE, diabetes support and education; FI, frailty index; ILI, intensive lifestyle intervention; Look AHEAD, Action for Health in Diabetes.
Last assessed during years 16 to 18.
Table 3 lists mean cognitive test scores by intervention group. There was no evidence of overall differences in cognitive function between groups, with perhaps slightly higher phonemic fluency scores among ILI compared with DSE participants.
TABLE 3.
Cognitive test scores at Look AHEAD Aging entry: mean (SD) and results from t‐tests.
| Cognitive test | DSE N = 781 | ILI N = 820 | p‐value |
|---|---|---|---|
| TICSm | 34.5 (4.9) | 34.4 (4.7) | 0.87 |
| Craft Story | |||
| Immediate | 21.0 (6.4) | 21.2 (6.3) | 0.90 |
| Delayed | 17.7 (6.7) | 17.9 (6.5) | 0.61 |
| RAVLT Delayed | 7.01 (3.96) | 6.94 (3.96) | 0.71 |
| Number Span–F | 7.15 (2.33) | 7.08 (2.33) | 0.59 |
| Number Span–B | 6.33 (2.32) | 6.32 (2.33) | 0.91 |
| Oral Trails–A | 13.8 (4.9) | 13.8 (5.5) | 0.79 a |
| Oral Trails–B | 84.4 (88.3) | 83.6 (88.6) | 0.54 a |
| Semantic Fluency–Animal | 18.1 (5.3) | 18.3 (5.4) | 0.72 |
| Semantic Fluency–Vegetable | 13.0 (4.1) | 13.0 (4.0) | 0.79 |
| Phonemic Fluency–L/F | 20.7 (8.1) | 21.5 (8.2) | 0.08 |
Abbreviations: DSE, diabetes support and education; ILI, intensive lifestyle intervention; Look AHEAD, Action for Health in Diabetes; RALT, Rey Auditory Verbal Learning Test; TICSm, Modified Telephone Interview for Cognitive Status.
Based on log‐transformed data.
8. MEASURES AND STATISTICAL CONSIDERATIONS
The primary aims of Look AHEAD Aging are to identify the long‐term effects of ILI compared to DSE in five domains of outcomes. Look AHEAD Aging will also identify factors and behaviors (eg, exposure to obesity, risk factor control, and physical activity) that are associated with better late‐life health‐related profiles. In general, analyses will use data collected during Look AHEAD Aging follow‐up. All data available since randomization will be used to examine changes over time as supporting analysis.
Multimorbidity. A generalized Poisson or negative binomial distribution model will be used to address the overdispersion that is likely present when summing over several different Poisson processes contributing to multimorbidity. A similar approach will be used when analyzing claims‐based measures of multimorbidity. One challenge is that there are incomplete records due to death or lost follow‐up. Additionally, multimorbidity can influence death and dropout, which subsequently censor the observation of multimorbidity. Therefore, a joint modeling of the longitudinal and survival processes will be used to account for potential informative censoring and allow valid statistical inferences for multimorbidity. 48 A general linear model for the count data and a proportional hazards model for survival data will be fitted with subject‐specific random effects to link the two submodels.
Physical function. The physical functioning score from SF‐36 is a simple, effective measure of mobility disability in older people 44 and has been collected regularly since the beginning of Look AHEAD. For these continuous outcomes, linear mixed effects models will be used to estimate the intervention effect. The basic model will include intervention assignment, time, and their interaction term. Contrasts will be used to estimate the mean difference between ILI and DSE across Look AHEAD Aging follow‐up. Similar to the analysis of multimorbidity index, analyses will account for death by adopting a joint modeling approach that links the linear mixed models and the survival model with a latent bivariate normal process. 49 , 50
Cognitive function. Cognitive assessment includes global cognitive function, attention, memory, language, and executive function. For participants who score below a validated cutoff on global cognitive function, a questionnaire will be administered to a proxy informant with questions about the participant's memory and function. Cognitive status will be adjudicated by an expert consensus panel and classifications of normal, mild cognitive impairment, and probable dementia will be assigned. Z‐scores will be created for individual cognitive tests, aggregated to create domain‐specific z‐scores, and averaged to get a measure of composite cognitive function, similar to what we have done for the earlier Look AHEAD cognitive data. Linear mixed models will be used to assess differences between ILI and DSE with respect to means (primary) and slopes (secondary). Supporting analyses will describe differences for each individual domain.
Total health care utilization and costs. These data will be collected with Medicare claims linkage. The distribution of annual health care use and costs is often positively skewed. General linear models that account for skewness in two ways will be used, allowing specification of (1) a “link” function (specifically the functional form for the outcome variable that best matches a linear combination of covariates), and (2) the appropriate distributional family of the outcome variable (eg, gamma, Poisson, inverse Gaussian). 51 The link function will be determined using a Box‐Cox test and the appropriate distribution family by employing modified Park tests. 52 Logistic models will be used for binary outcomes. Independent variables include intervention (the coefficient provides a measure of the difference in cost or use associated with the intervention), original clinic site, and baseline participant characteristics.
Health‐related quality of life. Differences between intervention groups will be described for the levels and changes in measures of fatigue, psychological resilience, social isolation, loneliness, and the SF‐36 scales using linear mixed models.
Supporting analyses will use longitudinal trajectories in measures collected during earlier follow‐up to describe relationships with longer‐term health outcomes and assess whether any intervention effects on outcomes differ among pre‐specified subgroups formed at baseline (race/ethnicity, age, obesity, health status, and sex as a biological variable). We are interested in understanding whether the long‐term exposure to lower weight over the course of Look AHEAD follow‐up is associated with health‐related benefits. Exploratory analyses will mine the rich data resources to describe long‐term intervention effects on other outcomes, to characterize aging trajectories, and to identify factors related to preserved health and function. A broad range of analytical techniques will be used: survival analysis, generalized linear models (ie, logistic regression, Poisson regression, negative binomial regression), generalized estimating equations models for binary data, and mixed‐model analysis of variance and covariance.
9. STATISTICAL POWER
Our power projections are based on the 1552 participants enrolled in Look AHEAD Aging, with the assumption that attrition will accrue at 5%/year over its 4 years of follow‐up. We adopt a two‐sided Type 1 error for each of the primary outcomes.
Multimorbidity. In Look AHEAD participants aged ≥65 years, the mean difference in rate of multimorbidity expansion between intervention groups was 0.02/year. 15 For N = 1552 and the standard deviation seen in Look AHEAD, this projects to 90% power to detect a mean difference of 0.022/year.
Physical function. The SF‐36 will be collected annually in Look AHEAD Aging. The estimated variance and correlation among repeated measures for the SF‐36 physical function score is 130 and 0.64, respectively, using current Look AHEAD data. For N = 1552 we will have 90% power to detect a difference of 1.4 units in the SF‐36 physical function scores.
Cognitive function. Longitudinal sequences of composite cognitive scores from the Women's Health Initiative Memory Study of the Epidemiology of Cognitive Health Outcomes (WHIMS‐ECHO) annual telephone assessments were used to calculate the longitudinal covariance of measures for a simulation analysis. 53 With loss‐to‐follow‐up at 5%/year, N = 1437 is projected to provide >90% power to detect a mean difference of 0.13 standard deviation.
Total health care costs. We anticipate collecting Medicare claims data on approximately 1395 participants. The standard deviation of Log(total health care costs) from the Look AHEAD cohort has been 1.10. Based on this, we project 90% power to detect a 21% difference in total health care costs from cross‐sectional data. Repeated data over years of follow‐up will increase power.
10. CLINIC VERSUS TELEPHONE‐BASED ASSESSMENTS
Many studies have transitioned from clinic‐based assessments to telephone‐based assessments 53 and this transition has recently become more frequent with the social distancing triggered by the COVD‐19 pandemic. 54 Changes in assessment modes can threaten the validity of longitudinal analyses spanning transitions. An analytical goal of Look AHEAD Aging is to bridge differences in ascertainment modes and domains from the current assessments to previous assessments collected by the Look AHEAD program.
11. DISCUSSION
The original Look AHEAD cohort was shaped by recruitment practices (eg, mass mailing, registries), eligibility criteria (eg, graded exercise testing, diabetes control, overweight/obesity), and recruitment targets (eg, >35% persons of color, <25% insulin users). 10 The influence of these design characteristics has likely faded over time as the forces of mortality and attrition have shaped the current Look AHEAD Aging cohort. Thus, while the original cohort was developed to reflect moderately well controlled, middle‐aged individuals with type 2 diabetes who were good candidates for a multidomain lifestyle intervention, the current cohort is thought to more adequately reflect older individuals with long‐term exposure to diabetes and overweight or obesity. The characteristics of this cohort that are described in this manuscript frame an opportunity to study aging in representatives of an important and understudied population.
Look AHEAD Aging lacks direct objective geroscience‐based measures of function, a limitation related to its remote data collection. There has been recent progress in identifying blood‐based biomarkers of aging 55 ; this raises the possibility of expanding the Look AHEAD Aging breadth through the development of ancillary studies involving a (mail‐based) collection of biospecimens.
Look AHEAD Aging also provides the opportunity to study the legacy effects of a 10‐year multidomain lifestyle intervention on later‐life trajectories of health, function, health care, and quality of life. It is designed to address important research questions. How are different trajectories of weight, physical activity, risk factors, and health care associated with the health‐related experiences of adults with diabetes and obesity later in life? Should mid‐life behavioral changes be recommended as a cost‐effective means to improve the health and well‐being of adults with diabetes and obesity as they age? What accounts for the remarkable range of function and health‐related quality of life among older individuals with diabetes? These questions can only be answered with continued observation as the Look AHEAD cohort enters this critical phase of the life course.
12. SUMMARY
Type 2 diabetes and both overweight and obesity are known to accelerate aging processes, increasing the rate at which geriatric syndromes and age‐related chronic diseases occur; however, there remains much to be learned about how this unfolds in later life. Look AHEAD Aging is designed to draw from the long‐term follow‐up and extensive characterization of the Look AHEAD cohort to describe trajectories of aging‐related outcomes and how these may be related to changes in lifestyle and risk factors over time. The study will identify factors related to resilience against the development of geriatric syndromes, which preserves independence and quality of life in later life.
CONFLICT OF INTEREST STATEMENT
M.A.E. and T.B. receive research support from the Alzheimer's Association. The authors collectively have no other relationships related to the content of the manuscript to disclose. Author disclosures are available in the supporting information.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors have nothing to report. The Action for Health in Diabetes (Look AHEAD) Extended Follow‐up (Look AHEAD Aging) is funded from the National Institute on Aging U01AG073697 and a diversity supplement U01AG073697‐S1. Additional support is provided by the Look AHEAD Sleep ancillary study: AG074562‐01. The Action for Health in Diabetes was supported through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. Additional support was provided by Look AHEAD Mind (AG058571) and the Wake Forest Alzheimer's Disease Core Center (P30AG049638‐01A1).
APPENDIX 1.
APPENDIX 1.1. Look AHEAD Aging study group
Wake Forest University School of Medicine
Lynne E. Wagenknecht, mPI; Mark A. Espeland, mPI; Judy L. Bahnson; Denise K. Houston, Kathleen M. Hayden; Stephen R. Rapp; Bonnie Sachs; Debbie D. Pleasants; Debbie Booth; Darrin Harris; Haiying Chen; Jerry M. Barnes, MA; Tara D. Beckner; Katie Garcia; Rebecca H. Neiberg; Michael P. Walkup; Anthony Alvarado; Cheryl Summerville; Mia Yang; Heather Dailey‐Farley; Ashley Lentz; Allison Lang; Doris Clark; Nancy Kessler; Anne Harris; Jennette Blanco; Jorgelis Quijano; Stephen Dennis; Nikita Makwana; Ann Furstenberg; Mary Barr University of Minnesota
Peter J. Huckfeldt; Mark Woodhouse
University of Southern California
Dana P. Goldman; Ann S. M. Harada; Ian Davis
Columbia University
Jose A. Luchsinger
External Advisors
Rena R. Wing (Miriam Hospital); Jeanne C. Clark (The Johns Hopkins School of Medicine); Helen P. Hazuda (The University of Texas Health Science Center at San Antonio); Karen C. Johnson (The University of Tennessee Health Science Center)
Espeland MA, Houston DK, Hayden KM, et al. Rationale, design, and cohort characteristics of the Action for Health in Diabetes Aging study. Alzheimer's Dement. 2023;9:e12430. 10.1002/trc2.12430
Mark A. Espeland and Lynne E. Wagenknecht contributed equally to this work.
REFERENCES
- 1. Morley JE. Diabetes and aging: epidemiologic overview. Clin Geriatr Med. 2008;24(3):395‐405. [DOI] [PubMed] [Google Scholar]
- 2. Monickaraj F, Aravind S, Gokulakrishnan K, et al. Accelerated aging as evidenced by increased telomere shortening and mitochondrial DNA depletion in patients with type 2 diabetes. Mol Cell Biochem. 2012;365(1‐2):343‐350. [DOI] [PubMed] [Google Scholar]
- 3. Batsis JA, Gill LE, Masutani RK, et al. Weight loss interventions in older adults with obesity: a systematic review of randomized controlled trials since 2005. J Am Geriatr Soc. 2017;65(2);257‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the arthritis, diet, and activity promotion trial. Arthritis Rheumat. 2004;50(5):1501‐1510. [DOI] [PubMed] [Google Scholar]
- 5. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12);1263‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nicklas BJ, Brinkley TE, Houston DK, et al. Effects of caloric restriction on cardiorespiratory fitness, fatigue, and disability responses to aerobic exercise in older adults with obesity: a randomized controlled trial. J Gerontol Series A Biol Sci Med Sci. 2019;74;1084‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutrit. 2015;101(5);991‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. New Eng J Med. 2017;376(20):1943‐1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. New Eng J Med. 2011;364(13):1218‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Look AHEAD Research Group . Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610‐628. [DOI] [PubMed] [Google Scholar]
- 11. The Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New Eng J Med. 2013;369:145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wing RR. Does lifestyle intervention improve health of adults with overweight/obesity and Type 2 diabetes? Findings from the Look AHEAD randomized trial. Obesity (Silver Spring). 2021;29:1246‐1258. [DOI] [PubMed] [Google Scholar]
- 13. The Look AHEAD Research Group . The Look AHEAD Study: a description of the lifestyle intervention and the evidence supporting it. obes. 2006;14:737‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The Look AHEAD Research Group . The development and description of the diabetes support and education (comparison group) intervention for the Action for Health in Diabetes (Look AHEAD) Trial. Clin Trials. 2011;8:320‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wing RR, Neiberg RH, Bahnson JL, et al. Weight change during the postintervention follow‐up of Look AHEAD. Diabetes Care. 2022;45(6):1306‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Espeland MA, Gaussoin SA, Bahnson J, et al. Impact of an 8‐year intensive lifestyle intervention on an index of multimorbidity. J Am Geriatr Soc. 2020;68:2249‐2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Houston DK, Leng X, Bray GA, et al. A long‐term intensive lifestyle intervention and physical function: the look AHEAD Movement and Memory Study. Obesity (Silver Spring). 2015;23(1):77‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Houston DK, Neiberg RH, Miller ME, et al. Physical function following a long‐term lifestyle intervention among middle aged and older adults with Type 2 diabetes: the Look AHEAD Study. J Gerontol A Biol Sci Med Sci. 2018;73(11):1552‐1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Espeland MA, Glick HA, Bertoni A, et al. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014;37(9):2548‐2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huckfeldt PJ, Frenier C, Pajewski NM, et al. Associations of intensive lifestyle intervention in type 2 diabetes with health care use, spending, and disability: an ancillary study of the Look AHEAD Study. JAMA Netw Open. 2020;3(11):e2025488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rubin RR, Wadden TA, Bahnson JL, et al. Impact of intensive lifestyle intervention on depression and health‐related quality of life in type 2 diabetes: the Look AHEAD Trial. Diabetes Care. 2014;37(6):1544‐1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rapp SR, Luchsinger JA, Baker LD, et al. Effect of a long‐term intensive lifestyle intervention on cognitive function: action for Health in Diabetes Study. J Am Geriatr Soc. 2017;9(10);14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Espeland MA, Carmichael O, Hayden K, et al. Long‐term impact of weight loss intervention on changes in cognitive function: exploratory analyses from the Action for Health in Diabetes Randomized Controlled Clinical Trial. J Gerontol A Biol Sci Med Sci. 2018;73(4):484‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carmichael OT, Neiberg RH, Dutton GR, et al. Long‐term change in physiological markers and cognitive performance in type 2 diabetes: the look AHEAD study. J Clin Endocrinol Metab. 2020;105(12):e4778‐e4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bancks MP, Lovato J, Balasubramanyam A, et al. Association of type 2 diabetes subgroups with cognitive status without modification from lifestyle intervention. J Clin Endocrinol Metab. 2023;108:e334‐e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653‐666. [DOI] [PubMed] [Google Scholar]
- 27. Barnes LL, Dhana K, Liu X, et al. Trial of the MIND Diet for prevention of cognitive decline in older persons. N Engl J Med. 2023;389(7):602–611. 10.1056/NEJMoa2302368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrucci L, Levine ME, Kuo P‐L, Simonsick EM. Time and the metrics of aging. Circ Res. 2018;123:740‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simpson FR, Pajewski NM, Nicklas B, et al. Impact of multidomain lifestyle intervention on frailty through the lens of deficit accumulation in adults with type 2 diabetes mellitus. J Gerontol Series A Biol Sci Med Sci. 2020;75,1921‐1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Evans JK, Usoh CO, Simpson F. Long‐term Impact of a 10‐year intensive lifestyle intervention on a deficit accumulation frailty index: for the Action for Health In Diabetes (Look AHEAD) study group. J Gerontol A Biol Sci Med Sci. 2023:glad088. 10.1093/gerona/glad088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weintraub S, Besser L, Dodge HH, et al. Version 3 of the Alzheimer Disease Centers’ neuropsychological test battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord. 2018;32(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rapp SR, Legault C, Espeland MA, et al. Validation of a cognitive assessment battery administered over the telephone. J Am Geriatr Soc. 2012;60(9):1616‐1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brandt J, Spencer M, Folstein MF. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111‐117. [Google Scholar]
- 34. Welsh KA, Breitner J, Magruder‐Habib KM. Detection of dementia in the elderly using the telephone interview for cognitive status. Neuropsychiatr Neuropsychol Behav Neurol. 1993;6:103110. [Google Scholar]
- 35. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695‐699. [DOI] [PubMed] [Google Scholar]
- 36. Estévez‐González A, Kulisevsky J, Boltes A, Otermín P, García‐Sánchez, C . Rey verbal learning test is a useful tool for differential diagnosis in the preclinical phase of Alzheimer's disease: comparison with mild cognitive impairment and normal aging. Int. J Geriat Psychiatry. 2003;18:1021‐1028. [DOI] [PubMed] [Google Scholar]
- 37. Johnson RM. A validation study of the oral trail makng test with a geriatric population. ProQeust Information & Learning; 2001. [Google Scholar]
- 38. Ricker JH, Axelrod BN, Houtler BD. Clinical validation of the oral trail making test. Neuropsychiatry Neuropsychol Behav Neurol. 1996;9(1):50‐53. [Google Scholar]
- 39. Pfeffer RI, Kurosaki TT, Harrah CH Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323‐329. [DOI] [PubMed] [Google Scholar]
- 40. Ellis RJ, Jan K, Kawas C, et al. Diagnostic validity of the dementia questionnaire for Alzheimer disease. Arch Neurol. 1998;55(3):360‐365. [DOI] [PubMed] [Google Scholar]
- 41. Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51(9):901‐906. [DOI] [PubMed] [Google Scholar]
- 42. Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory‐II (BDI‐II), Center for Epidemiologic Studies Depression Scale (CES‐D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire‐9 (PHQ‐9). Arthritis Care Res (Hoboken). 2011;63 Suppl 11(S11):S454‐S466. [DOI] [PubMed] [Google Scholar]
- 43. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self‐report version of PRIME‐MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737‐1744. [DOI] [PubMed] [Google Scholar]
- 44. Ware J, Sherbourne CD The MOS 36‐item short‐form health survey (SF‐36): I. Conceptual Framework and Item Selection. Med Care. 30:473‐483, 1992. [PubMed] [Google Scholar]
- 45. Olson KL, Howard M, McCaffery JM, et al. Psychological resilience in older adults with type 2 diabetes from the Look AHEAD Trial. J Am Geriatr Soc. 2023;71(1):206‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McCaffery JM, Anderson A, Coday M, et al. Loneliness relates to functional mobility in older adults with type 2 diabetes: the look AHEAD study. J Aging Res. 2020;2020:7543702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wadden TA, Neiberg RH, Wing RR, et al. Four‐year weight losses in the look ahead study: factors associated with long‐term success. obes. 2011;19:1987‐1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Piulachs X, Alemany R, Guillen M, Rizopoulos D. Joint models for longitudinal counts and left‐truncated time‐to‐event data with applications to health insurance. SORT. 2017;41:347‐372. [Google Scholar]
- 49. Guo X. Separate and joint modeling of longitudinal and event time data using standard computer packages. Am Statist. 2004;58(16):24. [Google Scholar]
- 50. Rizopoulos DJ. An r package for the joint modelling of longitudinal and time‐to‐event data. J Statist Software. 2010;35(9):1‐33. [Google Scholar]
- 51. Deb P, Norton EC. Modeling health care expenditures and use. Ann Rev Pub Health. 2018;39:489‐505. [DOI] [PubMed] [Google Scholar]
- 52. Park RE. Estimation with heteroscedastic error terms. Econometrica. 1966;34(4):888. [Google Scholar]
- 53. Espeland MA, Rapp SR, Manson JE, et al. Long term effects on cognitive trajectories of postmenopausal hormone therapy in two age groups. J Gerontol A Biol Sci Med Sci. 2017;72:838‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Röhr S, Arai H, Mangialasche F, et al. Impact of COVID‐19 pandemic on statistical design and analysis plans for multidomain intervention clinical trials: Experience from World‐Wide FINGERS. Alz Dem Trans Res Clin Intervent. 2021;7(1):e12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Justice JN, Ferrucc, Newman AB, et al. A framework for selection of blood‐based biomarkers for geroscience‐guided clinical trials: report from the TAME Biomarkers Workgroup. Geroscience. 2018;40(5‐6):419‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


