Abstract
The mechanism(s) of activity of pentavalent antimony [Sb(V)] is poorly understood. In a recent study, we have shown that potassium antimonyl tartrate, a trivalent antimonial [Sb(III)], was substantially more potent than Sb(V) against both promastigotes and axenically grown amastigotes of three Leishmania species, supporting the idea of an in vivo metabolic conversion of Sb(V) into Sb(III). We report that amastigotes of Leishmania infantum cultured under axenic conditions were poorly susceptible to meglumine [Glucantime; an Sb(V)], unlike those growing inside THP-1 cells (50% inhibitory concentrations [IC50s], about 1.8 mg/ml and 22 μg/ml, respectively). In order to define more precisely the mode of action of Sb(V) agents in vivo, we first induced in vitro Sb(III) resistance by direct drug pressure on axenically grown amastigotes of L. infantum. Then we determined the susceptibilities of both extracellular and intracellular chemoresistant amastigotes to the Sb(V)-containing drugs meglumine and sodium stibogluconate plus m-chlorocresol (Pentostam). The chemoresistant amastigotes LdiR2, LdiR10, and LdiR20 were 14, 26, and 32 times more resistant to Sb(III), respectively, than the wild-type one (LdiWT). In accordance with the hypothesis described above, we found that intracellular chemoresistant amastigotes were resistant to meglumine [Sb(V)] in proportion to the initial level of Sb(III)-induced resistance. By contrast, Sb(III)-resistant cells were very susceptible to sodium stibogluconate. This lack of cross-resistance is probably due to the presence in this reagent of m-chlorocresol, which we found to be more toxic than Sb(III) to L. infantum amastigotes (IC50s, of 0.54 and 1.32 μg/ml, respectively). Collectively, these results were consistent with the hypothesis of an intramacrophagic metabolic conversion of Sb(V) into trivalent compounds, which in turn became readily toxic to the Leishmania amastigote stage.
Leishmaniasis is a significant cause of morbidity and mortality in several countries of the world. A vertebrate host is infected with flagellated extracellular promastigote forms via the bite of a sandfly. Promastigotes are rapidly transformed into nonflagellated amastigotes, which actively divide within the mononuclear phagocytes of the vertebrate host. The basic treatment consists of the administration of sodium stibogluconate (Pentostam), meglumine (Glucantime), pentamidine, or amphotericin B. Treatment failure, especially in patients with kala-azar, mucosal leishmaniasis, and diffuse cutaneous leishmaniasis, is becoming a common problem in many areas of endemicity. Immunological, physiological, or pharmacological deficiencies in the host are possible explanations for variations in clinical response (25). There is evidence, however, that an inherent lack of susceptibility and/or the development of resistance can also contribute to parasite unresponsiveness to drugs (12, 16, 24, 37, 38).
The mode of action of pentavalent antimony [Sb(V)] also remains poorly understood (4–6). Moreover, the mechanisms that contribute to Sb(V) toxicity and resistance are still unknown. An in vivo metabolic conversion of Sb(V) into trivalent antimony [Sb(III)] was suggested more than 50 years ago by Goodwin (14) and Goodwin and Page (15). This hypothesis was supported by the high level of toxicity of Sb(III) against both parasite stages of different Leishmania species (23, 31, 32). Recently, we pointed out that axenically grown amastigotes of Leishmania represented a powerful model that can be used to investigate the activities of drugs on the active and dividing populations of amastigote forms (32). Using this model, we have previously shown that potassium antimonyl tartrate trihydrate [Sb(III)] was generally more toxic than Sb(V) to both parasite stages of different Leishmania species. We have also demonstrated that the extracellular amastigotes of Leishmania infantum were the Leishmania species that were most susceptible to Sb(III) (32). These results are in agreement with data reported by Goodwin (14) and Goodwin and Page (15) and favor an in vivo reduction of Sb(V) to active Sb(III). To better understand the effect of Sb(V) in vivo and to define more precisely its mode of action, we decided to select, under axenic conditions, Sb(III)-resistant amastigotes of L. infantum by direct drug pressure at Sb(III) levels as high as those potentially generated inside macrophages during human therapy, and we evaluated their cross-resistance to Sb(V) extracellularly and inside THP-1 monocytes. Our results strongly support the hypothesis of an in vivo reduction of sodium stibogluconate to active Sb(III) derivatives.
MATERIALS AND METHODS
Materials.
Sodium stibogluconate (Pentostam; liquid form containing 100 mg of sodium stibogluconate per ml and 0.1% m-chlorocresol) was a generous gift of H. Amini, University of Teheran, Teheran, Iran. Meglumine (Glucantime; batch 331-2), which does not contain m-chlorocresol as a preservative, was supplied by Rhône Poulenc Specia. 4-Chloro-3-methylphenol (chlorocresol) and potassium antimonyl tartrate trihydrate were from Sigma.
Parasites and cultures.
A cloned line of L. infantum (MHOM/MA/67/ITMAP-263) was used in all experiments. Axenically grown amastigote forms of L. infantum were maintained at 37°C with 5% CO2 by weekly subpassages in a cell-free medium called MAA/20 (medium for axenically grown amastigotes) in 25-cm2 flasks as described previously (19, 20, 32). From a starting inoculum of 5 × 105 amastigote forms/ml, a cell density of about 5 × 107 parasites/ml was obtained on day 7. MAA/20 consisted of modified medium 199 (Gibco BRL) with Hanks’ salts supplemented with 0.5% soy trypto-casein (Pasteur Diagnostics, Marne la Coquette, France), 0.01 mM bathocuproine disulfonic acid, 3 mM l-cysteine, 15 mM d-glucose, 5 mM l-glutamine, 4 mM NaHCO3, 0.023 mM bovine hemin, and 25 mM HEPES to a final pH of 6.5 and supplemented with 20% pretested fetal calf serum (FCS). The population of axenically grown amastigote forms appeared homogeneous, round to ovoid, aflagellate, and immobile. We and other investigators have previously shown that axenically grown amastigotes of different Leishmania species clearly resembled intracellular amastigotes with regard to their ultrastructural, biological, biochemical, and immunological properties (1–3, 20). Moreover, the characterized extracellular amastigotes, like intracellular ones, differed from promastigotes in a variety of biochemical characteristics, including proteinase, RNase, adenine deaminase, and peroxidase activities; glucose catabolism; nucleic acid synthesis; dehydrogenase activities; and nitric oxide and drug susceptibilities (8, 10, 17, 19, 20, 27, 32).
Selection of Sb(III)-resistant amastigote forms.
Cloned wild-type amastigote forms of L. infantum (designated LdiWT) were adapted to survive in medium containing about 0.5 μg of potassium antimony tartrate, an Sb(III), per ml. The cultures were stabilized for several subcultures before increasing the drug level (1, 2, 4, 6, 10, and 20 μg/ml). Axenically grown amastigotes were subjected to stepwise increasing drug pressure until cell lines resistant to 2, 10, and 20 μg of potassium antimonyl tartrate trihydrate per ml (designated LdiR2, LdiR10, and LdiR20, respectively) were established. The cell lines selected for further studies were then cultured in MAA/20 containing 2, 10, and 20, μg of potassium antimonyl tartrate trihydrate per ml, respectively. All amastigote populations [wild-type and Sb(III)-resistant clones] were subjected to similar in vitro culture subpassages.
Viability test.
To estimate the 50% inhibitory concentrations (IC50s) and the viability of THP-1 cells, the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) micromethod described previously (32, 34, 35) was mainly used throughout the experiments. The results were analyzed with the mathematical model described previously (18, 32). Indeed, the MTT-based assay may not be accurate due to the interaction of meglumine with tetrazolium (32). Therefore, the leishmanicidal activities of increasing concentrations of meglumine on axenically grown amastigotes of L. infantum were ascertained by cell-counting experiments. The leishmanicidal activities of all the other compounds against axenic amastigotes were determined by the MTT micromethod.
Drug efficacy assay in THP-1.
The in vitro sensitivity of L. infantum in human leukemia monocyte cell line (THP-1 cells) was evaluated by the method described by Gebre-Hiwot et al. (13), with modifications. Briefly, THP-1 cells were cultured in RPMI 1640 medium supplemented with 10% FCS. THP-1 cells in the logarithmic phase of growth were differentiated by incubation for 2 days in medium containing 20 ng of phorbol myristate acetate (PMA; Sigma) per ml, which induced differentiation and caused the cells to become adherent (36). THP-1 cells treated with PMA were washed and then infected with stationary-phase extracellular amastigotes in eight-chamber Lab Teck tissue culture slides (Nunc) at a host cell:parasite ratio of 1:5 at 37°C with 5% CO2. After 2 h of incubation, noninternalized parasites were removed. Serial dilutions of each drug were made in RPMI 1640 medium supplemented with 10% FCS and were dispensed into the wells. Medium containing the drug was changed after 3 days. After 5 days of drug exposure, wells containing adherent, differentiated THP-1 cells were washed. The cells were fixed with methanol and stained with Giemsa stain. Measures of both the number of infected host cells and the number of intracellular amastigotes/100 infected host cells are required to establish drug efficacy. Therefore, drug activity was assessed by determining (i) the percentage of infected macrophages and (ii) the percentage of growth inhibition, which was calculated as 100 − (number of amastigotes/100 infected macrophages in treated wells)/(number of amastigotes/100 infected macrophages in untreated wells).
RESULTS
Drug toxicity toward THP-1 cells.
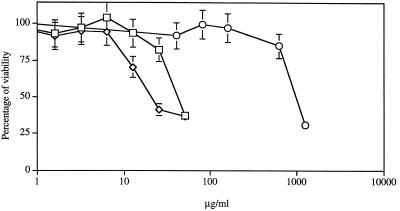
Before assessing the antileishmanial activities of the tested drugs against amastigotes of L. infantum growing in THP-1 cells, we have evaluated the toxicities of sodium stibogluconate, m-chlorocresol, and potassium antimonyl tartrate on THP-1 cells (Fig. 1). THP-1 cells were highly susceptible to m-chlorocresol (IC50, about 41 μg/ml; IC10, about 16 μg/ml). Potassium antimonyl tartrate, an Sb(III), also had highly potent activity against THP-1 cells (IC50, 31.5 μg/ml; IC10, less than 8 μg/ml), while sodium stibogluconate was less toxic to these cell lines, with an IC50 of about 998 μg/ml and an IC10 of less than 430 μg/ml. Drug concentrations below the evaluated IC10 were used in the experiments examining the susceptibilities of amastigotes growing within THP-1. The meglumine concentrations used in our study were below the IC10 evaluated previously (13).
FIG. 1.
Susceptibilities of THP-1 cells to drugs. The in vitro susceptibilities of THP-1 cells to sodium stibogluconate (Pentostam) (○), m-chlorocresol (□), and potassium antimonyl tartrate (◊) were ascertained by the MTT-based in vitro micromethod. Results are expressed as means ± standard deviations of triplicate experiments.
Susceptibilities of extracellular and intracellular wild-type amastigotes of L. infantum to Sb(V).
Under our experimental conditions, we showed that meglumine was poorly toxic to the axenically grown amastigote forms of L. infantum at concentrations as high as 1.28 mg/ml (Table 1) and had no effect at concentrations achieved in vivo in antimonial agent-treated individuals (about 20 μg/ml) (28, 39). Sodium stibogluconate, the second antimonial agent in clinical use, was 20-fold more toxic to these amastigotes than meglumine. As shown in Table 1, the IC50 of sodium stibogluconate for the wild-type extracellular amastigotes was about 104 ± 23 μg of Sb(V) per ml, whereas meglumine produced 50% growth inhibition at a concentration of as high as 1.28 mg/ml (Table 1). Because data suggest that the toxicity of sodium stibogluconate (Pentostam) to Leishmania panamensis promastigotes and Leishmania donovani amastigotes is due to the preservative agent (m-chlorocresol) and not to sodium stibogluconate (11, 29), we determined the susceptibilities of wild-type extracellular amastigotes of L. infantum to m-chlorocresol. Wild-type amastigotes were highly susceptible to m-chlorocresol, with an IC50 of 0.54 μg/ml, which was in the range of that found (1.24 μg/ml) by other investigators for axenically cultured amastigotes of L. donovani (5). The high level of toxicity of sodium stibogluconate compared to the toxicity of meglumine is probably due to the preservative agent m-chlorocresol, which is present only in sodium stibogluconate (Pentostam).
TABLE 1.
IC50s of potassium antimonyl tartrate, sodium stibogluconate (Pentostam), m-chlorocresol, and meglumine for wild-type and Sb(III)-resistant L. infantum amastigotes grown axenically
| Drug | Mean ± SD IC50 (μg/ml)
(n = 3)
|
Relative drug resistance
(IR)a
|
||||||
|---|---|---|---|---|---|---|---|---|
| LdiWT | LdiR2 | LdiR10 | LdiR20 | LdiWT | LdiR2 | LdiR10 | LdiR20 | |
| Potassium antimonyl tartrate [Sb(III)] | 1.32 ± 0.06 | 18.45 ± 1.33 | 34.96 ± 1.53 | 42.86 ± 6.11 | 1 | 14 | 26 | 32 |
| Sodium stibogluconate [Pentostam; Sb(V)] | 104 ± 23 | 69 ± 32 | 54 ± 16 | 64b | 1 | 0.6 | 0.5 | 0.6 |
| m-Chlorocresol | 0.54 ± 0.11 | 0.54 ± 0.10 | 0.36 ± 0.04 | 0.25b | 1 | 1 | 0.6 | 0.5 |
| Meglumine [Sb(V)]c | 1,280 ± 150 | 1,800 | >1,800 | >1,800 | 1 | >1 | >1 | >1 |
IR, index of resistance.
Value of one experiment performed in triplicate.
Due to a substantial interaction of meglumine with tetrazolium, the susceptibilities of wild-type and chemoresistant amastigotes to this drug were ascertained by a counting method. Briefly, axenically grown amastigotes of L. infantum were seeded at 106 amastigotes/ml in 25-cm2 flasks, and drug was added at the appropriate concentration. After 3 days of incubation, cell concentrations were determined by microscopic observation in a Thoma chamber at ×400 magnification after adequate dilution in 0.01 M phosphate-buffered saline (pH 7.2).
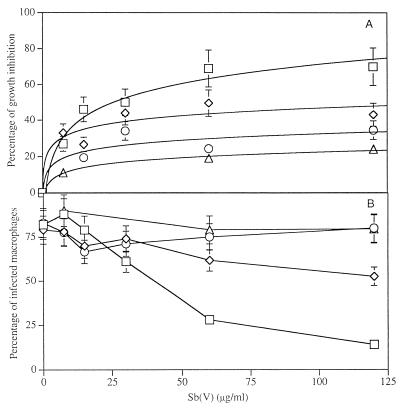
By contrast, wild-type amastigotes of L. infantum growing in THP-1 cells were highly susceptible to meglumine (IC50, about 22 μg/ml) (Fig. 2). They were more susceptible to sodium stibogluconate (IC50, less than 10 μg/ml) (data not shown). Both Sb(V)-containing drugs were readily more toxic to intracellular amastigotes than to the axenically cultured ones used to infect THP-1 cells. We have also assessed the leishmanicidal activity of m-chlorocresol against intracellular amastigotes. This molecule was highly toxic, with IC50s ranging between 0.1 and 1 μg/ml (Fig. 3), showing that the preservative m-chlorocresol was also responsible for the cytotoxicity of sodium stibogluconate (Pentostam) to intracellular amastigotes of L. infantum. Since meglumine does not contain chlorocresol, its activity against amastigotes growing in THP-1 cells could not be attributed to this preservative.
FIG. 2.
Toxicity of meglumine to L. infantum wild-type and chemoresistant amastigotes in THP-1 cells. Host cells were infected with chemoresistant (LdiR2 [◊], LdiR10 [○], and LdiR20 [▵]) or wild-type (LdiWT [□]) amastigotes at a cell-parasite ratio of 1:5 in medium supplemented with 10% FCS after differentiation with PMA. Infected cells were exposed to drugs for 5 days. The percentage of growth (A) and the percentage of infected macrophages (B) were evaluated. The results are the means of duplicate experiments.
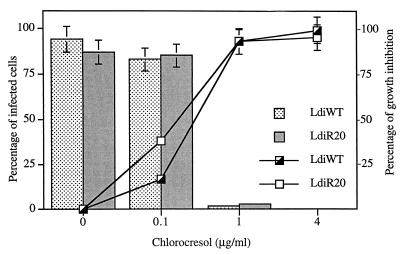
FIG. 3.
Toxicity of m-chlorocresol to L.
infantum amastigotes in THP-1 cells. Human cell lines were
infected with wild-type (LdiWT) or chemoresistant (LdiR20) amastigotes
at a cell-parasite ratio of 1:5 in medium supplemented with 10% FCS
after differentiation with PMA. Infected cells were exposed to the
drugs for 5 days. The percentage of growth inhibition of wild-type
(┌) and the LdiR20 chemoresistant variant (□) (A) and the
percentage of macrophages infected by the wild-type (
) and the
chemoresistant LdiR20 parasites
( ) (B)
were evaluated. The results are the means of duplicate experiments.
) (B)
were evaluated. The results are the means of duplicate experiments.
Characterization of the Sb(III)-resistant amastigotes.
The induction of Sb(III) resistance by direct exposure of axenically grown amastigotes of L. infantum to potassium antimonyl tartrate trihydrate results in the selection of extracellular amastigotes, with indexes of resistance (IRs) of 14 for LdiR2, 26 for LdiR10, and 32 for LdiR20 (Table 1). The time required to induce antimony resistance in vitro varied with the degree of resistance induced (approximately less than 8 weeks for LdiR2, about 19 weeks for LdiR10, and approximately 21 weeks for LdiR20). During drug resistance acquisition chemoresistant amastigote populations grew more slowly than the wild-type clone. However, after Sb(III) resistance stabilization, both resistant and sensitive amastigotes exhibited similar growth doubling times.
Sensitivity of the Sb(III)-resistant amastigotes to Sb(V).
Interestingly, in a macrophage model, chemoresistant variants were cross-resistant to meglumine. After 5 days of exposure to 120 μg of Sb(V) per ml, LdiR2, LdiR10, and LdiR20 amastigotes presented growth inhibitions of 43, 34, and 24%, respectively, whereas the level of growth inhibition of the wild-type clone reached 70% (Fig. 2A). Moreover, less than 15% of the macrophages could be seen to be infected with the wild-type amastigote, whereas 50, 80, and 79.5% of the macrophages remained infected with LdiR2, LdiR10, and LdiR20, respectively (Fig. 2B). Collectively, these results demonstrate the cross-resistance of the Sb(III)-resistant amastigotes to meglumine.
By contrast, chemoresistant variants, like the wild-type parasites, were highly susceptible to sodium stibogluconate (IC50s, less than 10 μg/ml) (data not shown). As shown in Fig. 3, after 5 days of incubation, an m-chlorocresol concentration of 1 μg/ml inhibited more than 90% of the intracellular growth of the wild-type clone as well as that of the chemoresistant variant. At this drug concentration, only 8% of the macrophage population was still infected by at least one wild-type or LdiR20 parasites (Fig. 3). The IC50 calculated by the mathematical model described previously (18) was about 0.4 μg/ml for both LdiWT and LdiR20 variants (Fig. 3). These data demonstrate that LdiR20 mutants are not cross-resistant to m-chlorocresol.
DISCUSSION
We have previously shown that axenically grown amastigotes could be a useful tool for the investigation of antileishmanial agents (32) and thus represented a powerful model that could be used to investigate the activities of drugs against active and dividing populations of amastigote forms. It presents numerous advantages, and in particular, coupled with in vitro models with macrophages, the influence of the latter on drug activity could be analyzed. We have shown that the extracellular amastigote forms of different Leishmania species clearly resemble the intracellular amastigotes according to their ultrastructural, biological, biochemical, and immunological properties (20). Moreover, the characterized amastigotes, like intracellular ones, differed from promastigotes in a variety of biochemical characteristics, including proteinase and dehydrogenase activities and nitric oxide susceptibility (19, 20, 34).
The development of in vitro models of chemoresistance has greatly facilitated studies on the molecular basis of drug resistance or drug susceptibility. Unfortunately, most of the mechanisms responsible for Leishmania drug resistance were characterized with the promastigote forms, which are mainly encountered in the insect vector (24, 37, 38). In a previous study we selected pentamidine-resistant amastigotes of Leishmania mexicana and showed that the stability of the chemoresistant phenotypes was dependent on the level of resistance induced (33). Since the biochemical mechanisms that underly antimonial agent resistance in Leishmania field isolates are still poorly understood, the molecular and biochemical characterization of the Sb(III)-resistant amastigotes could greatly help in providing an understanding of antimony resistance. Furthermore, any information on the transmission of the chemoresistant phenotypes during the parasite life cycle could help in providing an understanding of the epidemiology of Leishmania chemoresistance in the field.
The mode of action of Sb(V) remains poorly understood (4–6). Several properties of the Sb(V) agents have been suggested to contribute to their activities. Carbohydrates form water-soluble complexes with antimony and may serve to deliver antimonial drugs to host macrophages (31). Relatively nontoxic Sb(V) may be a prodrug that is converted to more toxic Sb(III) at or near the site of action (9, 10). This in vivo metabolic conversion was suggested more than 50 years ago and was supported by the observation that serum from patients treated with meglumine contains 15 to 25% Sb(III) compounds (7, 14, 15, 26, 30). We and other investigators have shown that Sb(III) compounds are highly toxic to promastigotes of different Leishmania species (23, 30–32). Interestingly, we recently demonstrated that axenically grown amastigotes of three Leishmania species were significantly more susceptible to Sb(III) than to Sb(V) and that L. infantum is the more Sb(III)-susceptible species (32). We report here that amastigotes of L. infantum grown axenically were poorly susceptible to meglumine [Sb(V)], unlike those growing inside THP-1 cells. Altogether, these results strongly suggest the hypothesis that fairly toxic Sb(V) is converted in vivo into highly active Sb(III) compounds.
In order to clarify more precisely the mechanism of Sb(V) toxicity, in vitro Sb(III) pressure was directly used on axenically cultured L. infantum amastigotes. By using an appropriate drug pressure protocol (33), we could demonstrate the feasibility of inducing Sb(III) resistance in the Leishmania stage detected in mammals. The three mutants selected (LdiR2, LdiR10, and LdiR20) were 14, 26, and 32 times more resistant to Sb(III), respectively, than the parental wild-type clone (LdiWT). A low level of resistance (LdiR2) was obtained in a relatively short period of selection (less than 2 months of drug pressure), whereas induction of a higher level of resistance (LdiR20) required about 6 months. These resistant amastigotes were able to grow axenically in the presence of Sb(III) at levels as high as those potentially generated inside macrophages during human chemotherapy. The antimony concentration achieved in the serum of humans or dogs treated with sodium stibogluconate or meglumine was about 20 μg/ml (27, 39). Since 15 to 25% of the serum antimony content consisted of Sb(III) (7, 26, 30), the calculated peak concentration of Sb(III) in serum should be between 3 and 5 μg/ml. These concentrations were not toxic to THP-1 cells but remained highly toxic to wild-type extracellular L. infantum amastigotes (IC50, 1.32 ± 0.06 μg/ml). The intramacrophagic Sb(III) concentration should be higher because macrophages are able to concentrate Sb(III) compounds by factors of 43 for potassium antimony tartrate and 24 for Sb(III)-mannan (31), leading to intramacrophagic concentrations ranging from 72 to 215 μg/ml.
In accordance with the hypothesis described above, extracellular as well as intramacrophagic Sb(III)-resistant amastigotes were highly cross-resistant to meglumine [Sb(V)], with the degree of Sb(V) resistance depending on the level of Sb(III) resistance induced. Previous work with J774 macrophages (31) has shown that macrophages could fairly concentrate Sb(V) species [factors of 3.8 for Sb(V)-mannan species and 0.33 for sodium stibogluconate]. Under our experimental conditions, the calculated intracellular concentration of Sb(V) should be between 39.6 and 456 μg/ml, depending on the Sb(V) species considered. As we have demonstrated in our study, these concentrations of Sb(V) are not toxic for the extracellular wild-type and Sb(III)-resistant amastigotes (the IC50 for LdiWT was about 1.28 mg/ml). These observations point out the fact that macrophages should play an important role in the toxicity of meglumine, probably in metabolizing the drug. Altogether, these data indicate that in vivo Sb(V) should be reduced into trivalent forms, which are concentrated by macrophages in order to eradicate intracellular Leishmania parasites.
The hypothesis that a stage-specific parasite reductase which is expressed only in intracellular amastigotes and not in axenically grown amastigotes is responsible for the Sb(V)-to-Sb(III) conversion should be also considered. However, several experiments suggest that the axenically cultured amastigotes of different Leishmania species are very similar to amastigotes isolated from tissues or infected macrophages and differ from promastigotes in a variety of biochemical characteristics, especially various enzymatic activities (1, 20, 27). Moreover, amastigotes of Leishmania have been cultured in a wide range of mammalian cells, some of which have been used in in vitro screens. The activities of drugs measured in mammalian cell lines showed variations not only between Leishmania species but also between in vitro systems of screening. L. donovani was found to be less sensitive to sodium stibogluconate in THP-1 cells (IC50, 5.5 μg/ml) (13) than in U 937 cells (IC50, 6 ng/ml) (21). Moreover, sodium stibogluconate, which is highly active against L. donovani and L. mexicana in macrohage models, showed disappointing activity in a fibroblast cell line (22), suggesting that different host cells have different abilities to reduce the drug. This would indicate that drug reduction occurred in host cells.
Surprisingly, sodium stibogluconate (Pentostam), the second antimonial drug in clinical use, was toxic to axenically grown amastigotes (IC50, about 104 μg/ml) (8, 32) as well as to intramacrophagic ones (IC50, <10 μg/ml). Because data suggest that the susceptibilities of promastigotes of L. panamensis or L. donovani to sodium stibogluconate (Pentostam) is due to the m-chlorocresol component and not to the sodium stibogluconate component (11, 29), we measured the susceptibilities of wild-type and Sb(III)-resistant amastigotes to m-chlorocresol. The current study indicates that wild-type axenic amastigotes of L. infantum are highly susceptible to m-chlorocresol (IC50, 0.54 μg/ml), with the IC50 being in a concentration range similar to that of the m-chlorocresol concentration in Pentostam. In the same way, m-chlorocresol was found to be highly toxic to intracellular amastigotes (IC50, about 0.4 μg/ml). Interestingly, both axenically grown and intracellular Sb(III)-resistant amastigotes of L. infantum were not cross-resistant to m-chlorocresol or to sodium stibogluconate (Pentostam). Thus, the higher in vitro toxicity of the m-chlorocresol compared to that of Sb(III) explains the lack of cross-resistance that we observed. Altogether these results demonstrate that m-chlorocresol should be responsible for the leishmanicidal activity of sodium stibogluconate (Pentostam) in our in vitro models. In the same way, previous studies have shown that the induction of spontaneous resistance to sodium stibogluconate (Pentostam) induces a cross-resistance to m-chlorocresol (11).
In conclusion we have shown that Sb(III) species at the range of concentrations potentially generated inside macrophages are highly toxic to both extracellular and intracellular amastigotes. Moreover, the induction of a Sb(III) resistance by direct drug pressure on the relevant parasite stage induced strong cross-resistance to meglumine. Thus, meglumine antimoniate may serve as a passive system for delivering Sb(V) antimony to the reticuloendothelial system, in which active Sb(III) antimony species were generated in situ. m-Chlorocresol, which is toxic to both extracellular and intracellular amastigotes of L. infantum, may contribute to the overall activity of sodium stibogluconate (Pentostam) in vivo. Collectively, these results suggest that the emergence of parasite resistance to antimonial compounds is a potential risk of inadequate meglumine treatment and could explain the relapses that occur in some patients after treatment (12). Studies on the biochemical mechanisms involved in the Sb(III) resistance of axenically grown amastigotes of L. infantum are in progress.
ACKNOWLEDGMENTS
This work was supported by grants from the ORSTOM Institute, CJF INSERM 96-04, and Conseil Regional of Languedoc Roussillon. D.S. received a grant from ORSTOM, and K.Z. is a recipient of a fellowship from INSERM.
REFERENCES
- 1.Bates P A, Robertson C D, Tetley L, Coombs G H. Cultivation and characterization of Leishmania mexicanaamastigote-like forms. Parasitology. 1992;105:193–202. doi: 10.1017/s0031182000074102. [DOI] [PubMed] [Google Scholar]
- 2.Bates P A. Axenic culture of Leishmaniaamastigotes. Parasitol Today. 1993;9:143–146. doi: 10.1016/0169-4758(93)90181-e. [DOI] [PubMed] [Google Scholar]
- 3.Bates P A. Complete developmental cycle of Leishmania mexicanain axenic culture. Parasitology. 1992;108:1–9. doi: 10.1017/s0031182000078458. [DOI] [PubMed] [Google Scholar]
- 4.Berman J D. Chemotherapy for leishmaniasis: biochemical mechanisms, clinical efficacy and future strategies. Rev Infect Dis. 1988;10:560–586. doi: 10.1093/clinids/10.3.560. [DOI] [PubMed] [Google Scholar]
- 5.Berman J D, Waddell D, Hanson B D. Biochemical mechanisms of the antileishmanial activity of sodium stibogluconate. Antimicrob Agents Chemother. 1985;27:916–920. doi: 10.1128/aac.27.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman J D, Gallalee J V, Best J M. Sodium stibogluconate (Pentostam) inhibition of glucose catabolism via the glycolytic pathway, and fatty acid beta oxidation in Leishmania mexicanaamastigotes. Biochem Pharmacol. 1987;36:197–201. doi: 10.1016/0006-2952(87)90689-7. [DOI] [PubMed] [Google Scholar]
- 7.Burguera J L, Burguera M, Petit de Pena Y, Lugo A, Anez N. Selective determination of antimony(III) and antimony(V) in serum and urine and of total antimony in skin biopsies of patients with cutaneous leishmaniasis treated with meglumine antimoniate. Trace Elem Med. 1993;10:66–70. [Google Scholar]
- 8.Callahan H L, Portal A C, Devereaux R, Grögl M. An axenic amastigote system for drug screening. Antimicrob Agents Chemother. 1997;41:818–822. doi: 10.1128/aac.41.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombs G H, Craft J A, Hart D T. A comparative study of Leishmania mexicanaamastigotes and promastigotes. Enzyme activities and subcellular locations. Mol Biochem Parasitol. 1982;5:199–211. doi: 10.1016/0166-6851(82)90021-4. [DOI] [PubMed] [Google Scholar]
- 10.Coombs G H, Tetley L, Moss V A, Vickerman K. Three-dimensional structure of the Leishmaniaamastigote as revealed by computer-aided reconstruction from serial sections. Parasitology. 1986;92:13–23. doi: 10.1017/s0031182000063411. [DOI] [PubMed] [Google Scholar]
- 11.Ephros M, Waldman E, Zilberstein D. Pentostam induces resistance to antimony and the preservative chlorocresol in Leishmania donovanipromastigotes and axenically grown amastigotes. Antimicrob Agents Chemother. 1997;41:1064–1068. doi: 10.1128/aac.41.5.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faraut-Gambarelli F, Piarroux R, Deniau M, Giusano B, Marty P, Michel G, Faugère B, Dumon H. In vitro and in vivo resistance of Leishmania infantumto meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob Agents Chemother. 1997;41:827–830. doi: 10.1128/aac.41.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebre-Hiwot A, Tadesse G, Croft S L, Frommel D. An in vitromodel for screening antileishmanial drugs: the human leukemia monocyte cell line, THP-1. Acta Trop. 1992;51:237–245. doi: 10.1016/0001-706x(92)90042-v. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin L C. Pentostam® (sodium stibogluconate); a 50-year personal reminiscence. Trans R Soc Trop Med Hyg. 1995;89:339–341. doi: 10.1016/0035-9203(95)90572-3. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin L G, Page J E. A study of the excretion of organic antimonials using a polarographic procedure. Biochem J. 1943;22:236–240. doi: 10.1042/bj0370198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grögl M, Thomason T N, Franke E. Drug resistance in leishmaniasis: it’s implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am J Trop Med Hyg. 1992;47:117–126. doi: 10.4269/ajtmh.1992.47.117. [DOI] [PubMed] [Google Scholar]
- 17.Hassan H F, Coombs G H. Leishmania mexicana, purine metabolizing enzymes of amastigotes and promastigotes. Exp Parasitol. 1985;59:15–28. doi: 10.1016/0014-4894(85)90066-9. [DOI] [PubMed] [Google Scholar]
- 18.Huber W, Koella J C. A comparison of three methods of estimating EC50in studies of drug resistance of malaria parasites. Acta Trop. 1993;55:257–261. doi: 10.1016/0001-706x(93)90083-n. [DOI] [PubMed] [Google Scholar]
- 19.Lemesre J L, Sereno D, Daulouède S, Veyret B, Brajon N, Vincendeau P. Leishmania spp.: nitric oxide-mediated metabolic inhibition of promastigote and axenically-grown amastigote forms. Exp Parasitol. 1997;86:58–68. doi: 10.1006/expr.1997.4151. [DOI] [PubMed] [Google Scholar]
- 20.Lemesre J L. Methods for the culture in vitro of different stages of tissue parasites. International publication no. WO 94/26899. 1994. Pending patent PCT/FR94/00577. [Google Scholar]
- 21.Martinez S, Looker D L, Marr J J. A tissue culture system for the growth of several species of Leishmania: growth kinetics and drug sensitivities. Am J Trop Med Hyg. 1988;38:304–307. doi: 10.4269/ajtmh.1988.38.304. [DOI] [PubMed] [Google Scholar]
- 22.Mattock N M, Peters W. The experimental chemotherapy of leishmaniasis. II. The activity in tissue culture of some antiparasitic and antimicrobial compounds in clinical use. Ann Trop Med Parasitol. 1975;69:359–371. [PubMed] [Google Scholar]
- 23.Mottram J C, Coombs G H. Leishmania mexicana: enzyme activities of amastigotes and promastigotes and their inhibition by antimonials and arsenicals. Exp Parasitol. 1985;59:151–160. doi: 10.1016/0014-4894(85)90067-0. [DOI] [PubMed] [Google Scholar]
- 24.Ouellette M, Papadopoulou B. Mechanisms of drug resistance in Leishmania. Parasitol Today. 1993;9:150–153. doi: 10.1016/0169-4758(93)90135-3. [DOI] [PubMed] [Google Scholar]
- 25.Peters B S, Fish D, Golden R, Evans D A, Bryceson A D M, Pinching A J. Visceral leishmaniasis in HIV infection and AIDS: clinical feature and response to therapy. Q J Med. 1990;77:1101–1111. doi: 10.1093/qjmed/77.2.1101. [DOI] [PubMed] [Google Scholar]
- 26.Petit de Pena Y, Gallignani M, Burguera M, Burguera J L, Anez N, Lugo Y. Selective determination of antimony(III) and antimony(V) in blood serum and urine by hydride generation and atomic absorption spectrometry. J Braz Chem Soc. 1990;1:72–75. [Google Scholar]
- 27.Rainey P M, Spithill T W, McMahon-Pratt D, Pan A A. Biochemical and molecular characterization of Leishmania pifanoiamastigotes in continuous axenic culture. Mol Biochem Parasitol. 1991;49:111–118. doi: 10.1016/0166-6851(91)90134-r. [DOI] [PubMed] [Google Scholar]
- 28.Rees P H, Keating M I, Kager P A, Hockmeyer W T. Renal clearance of pentavalent antimony (sodium stibogluconate) Lancet. 1980;ii:226–229. doi: 10.1016/s0140-6736(80)90120-8. [DOI] [PubMed] [Google Scholar]
- 29.Roberts W L, Rainey P M. Antileishmanial activity of sodium stibogluconate fractions. Antimicrob Agents Chemother. 1993;37:1842–1846. doi: 10.1128/aac.37.9.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts W L, Rainey P M. Antimony quantification in Leishmaniaby electrothermal atomic absorption spectroscopy. Anal Biochem. 1993;211:1–6. doi: 10.1006/abio.1993.1223. [DOI] [PubMed] [Google Scholar]
- 31.Roberts W L, Berman J D, Rainey P M. In vitro antileishmanial properties of tri- and pentavalent antimonial preparations. Antimicrob Agents Chemother. 1995;39:1234–1239. doi: 10.1128/aac.39.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sereno D, Lemesre J L. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob Agents Chemother. 1997;41:972–976. doi: 10.1128/aac.41.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sereno D, Lemesre J L. In vitro life cycle of pentamidine-resistant amastigotes: stability of the chemoresistant phenotypes is dependent on the level of resistance induced. Antimicrob Agents Chemother. 1997;41:1898–1903. doi: 10.1128/aac.41.9.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sereno D, Lemesre J L. Use of an enzymatic in vitro micromethod to quantify amastigote stage of axenically grown amastigote forms of Leishmania amazonensis. Parasitol Res. 1997;83:401–403. doi: 10.1007/s004360050272. [DOI] [PubMed] [Google Scholar]
- 35.Sereno D, Michon P, Brajon N, Lemesre J L. Phenotypic characterization of Leishmania mexicanapentamidine-resistant promastigotes: modulation of the resistance during developmental life cycle. C R Acad Sci. 1997;320:981–987. doi: 10.1016/s0764-4469(97)82471-7. [DOI] [PubMed] [Google Scholar]
- 36.Tsuchiya S, Kobayashi Y, Goto Y, Okumara H, Nakal S, Konno T, Tada K. Induction of maturation in cultured human monocytic leukemia cells by phorbol diester. Cancer Res. 1982;42:1530–1536. [PubMed] [Google Scholar]
- 37.Ullman B. Multidrug resistance and P-glycoproteins in parasitic protozoa. J Bioenergetic Biomembranes. 1995;27:77–84. doi: 10.1007/BF02110334. [DOI] [PubMed] [Google Scholar]
- 38.Ullman B, Carrero-Valenzuella E, Coons T. Leishmania donovani: isolation and characterization of sodium stibogluconate (Pentostam)-resistant cell lines. Exp Parasitol. 1989;69:157–163. doi: 10.1016/0014-4894(89)90184-7. [DOI] [PubMed] [Google Scholar]
- 39.Valladares J E, Arebolla J, Esteban M, Arbois M. Disposition of antimony after the administration of N-methylglucamine antimoniate to dogs. Vet Res. 1996;138:181–183. doi: 10.1136/vr.138.8.181. [DOI] [PubMed] [Google Scholar]