Abstract
The aim of this study was to measure the concentrations of enrofloxacin (ERFX) and other fluoroquinolones; orbifloxacin (OBFX), marbofloxacin (MBFX), and ofloxacin (OFLX) in the plasma and bile of rabbits after a single intravenous (IV) injection. Twenty male rabbits were divided into four groups and given each drug by IV injection into the ear vein at a dose of 5.0 mg/kg BW. The concentration of ERFX, ciprofloxacin (CPFX), OBFX, MBFX and OFLX in plasma and bile were determined by HPLC. CPFX, metabolite of ERFX, was also measured by HPLC in plasma and bile of rabbits receiving ERFX. Several pharmacokinetic parameters in plasma were calculated and biliary clearance (CLbile) was calculated from extent of biliary excretion and accumulation of AUC of each drug. After IV injection, elimination half-life (t1/2β) was 4.13, 3.68, 6.60, 5.14 hr; volume of distribution at a steady state (Vdss) was 1.24, 0.503, 0.771, 1.02 L/kg; and total body clearance (CLtot) was 1.05, 0.418, 0.271, 0.453 L/kg/hr, respectively. The values for CLbile for ERFX, OBFX, MBFX, and OFLX were 0.0048, 0.0050, 0.0057, and 0.0094 L/kg/hr, respectively. These values represent 0.48%, 1.2%, 2.1%, and 2.3% of the total body clearance (CLtot) of each drug, respectively. The biliary clearance of CPFX was also measured and found to be 0.0199 L/kg/hr with ERFX administration. The results showed that ERFX, OBFX, MBFX, and OFLX were not excreted into the bile to a significant extent, making them safe drugs to use in rabbits.
Keywords: bile, elimination, fluoroquinolones, pharmacokinetics, rabbit
Raising rabbits is gaining popularity in numerous nations as a companion animal due to their small spatial requirements, and low investment costs. Moreover, producing rabbits can fulfill the growing consumer demand for low-fat meat [34, 45]. Similar to other tiny animals, rabbits are vulnerable to various microbial infections, and the infective agents that cause these infections are typically the most prevalent [32]. At present, there is a restricted selection of antimicrobial drugs authorized for utilization in rabbits. Occasionally, these authorized medications are inadequate in treating serious infections, and hence other antimicrobials are frequently used in an off-label manner, resulting in the possibility of harmful drug residues in rabbit meat and development of antimicrobial resistance in bacteria. The techniques for managing rabbits intended for meat, fur or wool production differ significantly from those used for keeping pet or indoor rabbits. This indicates a need for rethinking strategies for treating infectious diseases in rabbits [23]. Bacterial illnesses are prevalent in rabbits and frequently involve gram-negative infections affecting different systems [43].
Fluoroquinolones belong to a category of potent antibacterial that have a broad spectrum of bactericidal activity against a vast majority of bacteria, mycoplasma, and certain anaerobic bacteria [42]. The mechanism of action of fluoroquinolones involves interfering with the bacterial topoisomerase type II, which hinders the synthesis of DNA in bacteria. This is achieved by inhibiting the catalytic function of both DNA gyrase and topoisomerase IV−two crucial bacterial enzymes responsible for regulating the chromosomal supercoiling necessary for DNA synthesis [31]. Because of their excellent antibacterial effect, they are widely used in the veterinary field [9, 23].
Various fluoroquinolones have been studied for their pharmacokinetics in rabbits in previous reports, including enrofloxacin (ERFX) [5, 6, 12, 13], ofloxacin (OFLX) [22], norfloxacin [23, 30], difloxacin [1, 14], moxifloxacin [8, 16], danofloxacin [15], ibafloxacin [24], ofloxacin [3], orbifloxacin (OBFX) [25], marbofloxacin (MBFX) [2, 26], and levofloxacin [34].
Moreover, the intestinal microbiota can also influence the production characteristics of economically significant animals, including rabbits [11]. The presence of intestinal microorganisms is crucial for the metabolism of bile acids. A prior investigation discovered that the amount of lithocholic acid, a metabolite of bile acid metabolism known to have a protective impact on the intestinal epithelial barrier and associated with the onset of inflammatory bowel disease, was notably reduced in a group experiencing diarrhea. This suggests that the interaction between gut microbes and metabolites plays a role in this disease’s pathogenesis [20].
Rabbits have a unique digestive system that relies on a complex interplay of microorganisms in the cecum and colon to digest and absorb nutrients from their food. Disruption of this delicate balance can lead to overgrowth of harmful bacteria, such as Clostridium spp., and can result in diarrhea, enteritis, and even death [29, 41]. Antibiotics that are excreted through bile can have a prolonged effect on the gastrointestinal microbiota, leading to dysbiosis even after the antibiotic has been discontinued. Therefore, it is important to avoid the use of these antibiotics in rabbits unless absolutely necessary and under the supervision of a veterinarian. Alternative routes of excretion, such as renal excretion, may be preferred in rabbits to minimize the risk of dysbiosis. In general, fluoroquinolones are excreted by the kidneys, primarily through urine [42].
The study aimed to investigate the pharmacokinetics and biliary excretion of ERFX and other fluoroquinolones, OBFX, MBFX, and OFLX following intravenous (IV) injection in rabbits.
MATERIALS AND METHODS
Drugs and chemicals
ERFX was obtained from Bayer Medical Co., Ltd., Tokyo, Japan (Product name: Baytril 2.5%). MBFX was obtained from Meiji Seika Pharma Co., Ltd., Tokyo, Japan (Product name: Marbofloxacin 2%). OBFX was obtained from Dainippon Sumitomo Pharma Co., Ltd, Osaka, Japan (Product name: Victas®S injection 5%). The powder of ciprofloxacin (CPFX) and OFLX was obtained from Daiichi Sankyo Co., Ltd., Tokyo, Japan. For intravenous administration of OFLX, the powder of OFLX was dissolved in sterilized distilled water (DW) with the help of small amount of 2 N acetic acid. The other solutions and chemicals utilized in the current study were of a quality suitable for HPLC or analytical purposes.
Animals
This study utilized twenty male Japanese white rabbits that were clinically healthy, weighed between 2.7–3.2 kg, and were 16 weeks old. Sankyo Labo Service Corp. (Tokyo, Japan) provided the rabbits, and they were divided into four groups, each receiving a different drug. The rabbits were treated in accordance with the ‘Guide for the Care and Use of Laboratory Animals’, which was approved by the Faculty of Agriculture at Tokyo University of Agriculture and Technology. The rabbits were kept in pens with good ventilation and at an ambient temperature. Prior to the pharmacokinetic study, the rabbits were fasted starting from the day before, but they were allowed to consume water freely.
Pharmacokinetic study
The rabbits were given medetomidine (0.1 mg/kg, IM) and midazolam (0.5 mg/kg, IM) to induce anesthesia, and isoflurane was used to maintain it during the experiments. A polyvinyl chloride tube cannula, 5Fr (1.7 mm in outer diameter, Atomu Medical, Tokyo, Japan), was inserted into the choledochal duct to collect bile samples after a midline incision was made. In addition, a polyethylene tube cannula (Natsume-Seisakusyo, Tokyo, Japan) with an inner diameter of 0.8 and an outer diameter of 1.2 was inserted into a femoral artery to collect blood samples. ERFX was administered to the rabbits intravenously via an injection in the ear vein at a dose of 5.0 mg/kg, which is the clinically recommended dose [17]. The doses of other three drugs were the same as ERFX.
Before administering the fluoroquinolones, 1.0 mL of blood was collected from the femoral artery. Subsequently, blood was collected at 0.5, 1, 2, 3, 4, 6, and 8 hr after dosing. Plasma was separated by centrifuging the blood at 1,600 g for 10 min. Total excretory bile was collected at intervals of 0.5, 1, 2, 3, 4, 6, and 8 hr after injection. Plasma and bile samples were stored at −20°C until analysis.
Measurement of fluoroquinolones concentration in plasma and bile
A high-performance liquid chromatography (HPLC) method with fluorometric detection was used to determine the concentrations of ERFX, CPFX, OBFX, MBFX, and OFLX in both plasma and bile. In addition, the plasma and bile of rabbits that received ERFX were also analyzed by HPLC to measure CPFX, which is a metabolite of ERFX.
As determined previously by Kung et al. and Marin et al. [19, 25] with some modifications, 200 µL of plasma or bile was mixed with 1,000 µL of acetonitrile and vortexes for 30 sec. The mixture was then centrifuged at 20,000 g for two min at 5°C. After centrifugation, the supernatant was collected and filtered using a 0.45-µm HPLC filter (Chromatodisc®, 4P, Kurabo Biomedical Industries, Ltd., Osaka, Japan). Next, 120 µL of the filtrate was combined with 500 µL of mobile phase buffer and 50 µL of the resulting mixture was injected into the HPLC column. The fluoroquinolones peaks in the samples were identified by comparing the detection time with that of the standard substance.
The HPLC system used in this study was obtained from Shimadzu Corporation in Kyoto, Japan, and included an LC-10AD pump, an SPD-6A UV detector, an integrator (Chromatopac C-R7A plus), and a loop injector (Model 7125). The mobile phase buffer consisted of 7 mM tetramethylammonium chloride and 20 mM sodium dihydrogen phosphate dehydrate, which was adjusted to pH 2.0 with 2N phosphoric acid. The mobile phase was a mixture of acetonitrile and the buffer (16.5% and 83.5%, respectively). The mobile phase chemicals and solutions were purchased from Fujifilm Wako Pure Chemical Corporation in Osaka, Japan. The fluoroquinolones were separated using a reversed-phase C18 column (Mightysil RP-18, 4.6 µm × 250 mm, Kanto Chemical Co., Tokyo, Japan) at a flow rate of 1.0 mL/min. The excitation and emission wavelengths used for ERFX and CPFX were 300 nm and 504 nm, respectively, while those for OBFX, MBFX, and OFLX were 338 nm and 425 nm, 295 nm and 500 nm, respectively.
Pharmacokinetic analysis
The way in which the fluoroquinolones were eliminated from plasma was analyzed through pharmacokinetics, using a model with two compartments that were open. The concentration of the drugs in plasma (Cp(t)) was represented by the following equation:
Cp(t)=A exp (-αt) + B exp (-βt) (Eq.1)
A, α, B, and β were determined by the nonlinear least-squares method using the curve fitting program, MULTI [44].
Non-compartmental analysis was used to calculate various pharmacokinetic parameters in plasma. The area under the concentration versus time curve (AUC i.v.) was determined using the trapezoidal method, from time zero to the last sampling time, and by integration, from the last sampling time to infinity. The total body clearance (CLtot=Dose i.v./AUC i.v.), mean residence time after intravenous administration (MRT i.v.), and distribution volume at a steady state (Vdss=Dose i.v. ×MRT i.v../AUC i.v.) were calculated using conventional methods. The biliary clearance (CLbile) was determined based on the extent of biliary excretion and accumulation of AUC for each drug.
RESULTS
After each trial, a clinical examination of all rabbits was conducted, and no abnormalities were detected before or after the administration of the drugs. Additionally, there were no observed local or adverse reactions, including signs of irritation, pain, or lameness, following intravenous injection of the studied drugs in rabbits. Figure 1 illustrates the semi-logarithmic plasma concentration-time profiles of ENFX, OBFX, MBFX, and OFLX after a single intravenous injection of 5 mg/kg body weight of each drug. Table 1 shows the mean values (± SD) of the pharmacokinetic parameters obtained from the study. After intravenous injection, the concentrations of ENFX, OBFX, MBFX, and OFLX in plasma exhibited a biphasic decline and remained detectable for up to 8 hr post-injection. The t1/2α was 0.35, 0.37, 0.39, 0.40 hr; t1/2β was 4.13, 3.68, 6.60, 5.14 hr; Vdss was 1.24, 0.503, 0.771, 1.02 L/kg; CLtot was 1.05, 0.418, 0.271, 0.453 L/kg/hr, and MRT was 3.98, 4.21, 8.28, 5.12 hr for ENFX, OBFX, MBFX and OFLX, respectively.
Fig. 1.

Time course of enrofloxacin (ERFX), orbifloxacin (OBFX), marbofloxacin (MBFX), and ofloxacin (OFLX) in plasma after intravenous injection at a dose of 5.0 mg/kg in rabbits. The points indicate the average of the measured values, and the vertical lines indicates the Standard Deviation (n=5). Ciprofloxacin (CPFX), metabolite of ERFX, simultaneously detected with ERFX in plasma after ERFX injection. Each solid line was calculated by Eq. 1 using pharmacokinetic parameters in Table 1.
Table 1. Pharmacokinetic parameters of enrofloxacin (ERFX), orbifloxacin (OBFX), marbofloxacin (MBFX), and ofloxacin (OFLX) after intravenous (IV) injection (5 mg/kg BW) in rabbits (n=5).
| Parameters | ERFX Mean ± SD | OBFX Mean ± SD | MBFX Mean ± SD | OFLX Mean ± SD |
|---|---|---|---|---|
| A (µg/mL) | 3.18 ± 1.33 | 5.69 ± 1.13 | 4.91 ± 1.10 | 4.44 ± 0.99 |
| α (hr−1) | 2.24 ± 0.90 | 1.94 ± 0.47 | 1.84 ± 0.32 | 1.84 ± 0.47 |
| B (µg/mL) | 0.570 ± 0.15 | 1.70 ± 0.35 | 1.78 ± 0.28 | 1.05 ± 0.20 |
| β (hr−1) | 0.180 ± 0.05 | 0.195 ± 0.04 | 0.115 ± 0.03 | 0.140 ± 0.02 |
| t 1/2α (hr) | 0.35 ± 0.15 | 0.37 ± 0.09 | 0.39 ± 0.06 | 0.40 ± 0.11 |
| t 1/2β (hr) | 4.13 ± 1.23 | 3.68 ± 0.74 | 6.60 ± 2.39 | 5.14 ± 1.16 |
| AUC0-8hr (μg·hr/mL) | 4.11 ± 0.846 | 9.90 ± 0.626 | 12.3 ± 1.66 | 7.75 ± 1.23 |
| AUC0-inf (μg·hr/mL) | 4.98 ± 1.17 | 12.1 ± 1.23 | 19.7 ± 6.44 | 12.7 ± 6.18 |
| MRTiv (hr) | 3.98 ± 1.35 | 4.21 ± 1.05 | 8.28 ± 3.18 | 5.12 ± 0.61 |
| CLtot (L/hr/Kg) | 1.05 ± 0.26 | 0.418 ± 0.038 | 0.271 ± 0.064 | 0.453 ± 0.153 |
| Vdss (L/Kg) | 1.24 ± 0.48 | 0.503 ± 0.139 | 0.771 ± 0.207 | 1.02 ± 0.26 |
A & B; Zero time plasma drug concentration intercepts of biphasic intravenous disposition curve. α: distribution rate constant; β: elimination rate constant; t1/2α; distribution half-life, t1/2β; elimination half-life after IV injection, AUC; area under plasma concentration-time curve, MRTiv; mean residence time after IV injection, Vdss; volume of distribution at steady-state, CLtot; total body clearance.
Figure 2 illustrates how much OBFX, MBFX, and OFLX are excreted through bile during an 8-hr period after being given. Within that time frame, the cumulative amount of each drug that was excreted through bile was 0.99%, 1.38%, and 1.43% of the dose given, respectively. The total amount of ERFX and CPFX found in the bile during the 8-hr period was 1.09% of the given dose, which is represented by ERFX. The biliary clearance (CLbile) of the four fluoroquinolones-ERFX, OBFX, MBFX, and OFLX-were measured and reported in Table 2. The values for CLbile for ERFX, OBFX, MBFX, and OFLX were 0.0048, 0.0050, 0.0057, and 0.0094 L/kg/hr, respectively. These values represent 0.48%, 1.2%, 2.1%, and 2.3% of the total body clearance (CLtot) of each drug, respectively. The biliary clearance of CPFX was also measured and found to be 0.0199 L/kg/hr with ERFX administration. The Cmax in bile for ERFX, CPFX, OBFX, MBFX and OFLX were 6.09, 3.56, 14.4, 10.8, and 17.1 µg/mL, respectively.
Fig. 2.
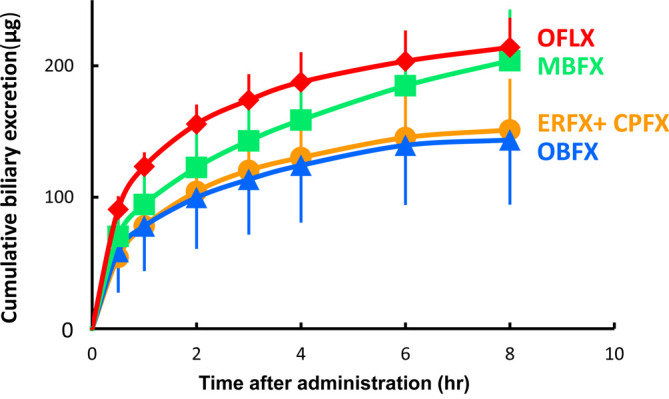
Extent of biliary excretion of enrofloxacin (ERFX), orbifloxacin (OBFX), marbofloxacin (MBFX), and ofloxacin (OFLX) for 8 hr after intravenous injection at a dose of 5.0 mg/kg in rabbits. Ciprofloxacin (CPFX), metabolite of ERFX, simultaneously detected with ERFX in bile after ERFX injection. The points indicate the average of the measured values, and the vertical lines indicates the Standard Deviation (n=5).
Table 2. Biliary excretion profiles of enrofloxacin (ERFX), orbifloxacin (OBFX), marbofloxacin (MBFX), and ofloxacin (OFLX) after intravenous injection (5 mg/kg BW) in rabbits (n=5). Ciprofloxacin (CPFX), metabolite of ERFX, simultaneously detected with ERFX in bile after ERFX injection.
| ERFX Mean ± SD | CPFX Mean ± SD | OBFX Mean ± SD | MBFX Mean ± SD | OFLX Mean ± SD | |
|---|---|---|---|---|---|
| Cmax in bile (µg/mL) | 6.09 ± 1.96 | 3.56 ± 0.78 | 14.4 ± 2.2 | 10.8 ± 1.1 | 17.1 ± 2.7 |
| CLbile (L/hr/kg) | 0.0048 ± 0.0010 | 0.0199 ± 0.0054 | 0.0050 ± 0.0013 | 0.0057 ± 0.0012 | 0.0094 ± 0.0011 |
| CLbile / CLtot (%) | 0.48 ± 0.15 | -* | 1.21 ± 0.37 | 2.14 ± 0.21 | 2.32 ± 1.06 |
| Amount excreted in bile0-8hr (μg/kg BW) | 20.2 ± 6.85 | 31.4 ± 6.8 | 50.0 ± 15.6 | 69.1 ± 11.4 | 71.7 ± 8.49 |
| Cumulative bile excretion of each drugs of dose0-8hr (%) | 1.09 ± 0.27** | 0.99 ± 0.31 | 1.38 ± 0.22 | 1.43 ± 0.17 |
* Total body clearance of CPFX cannot be calculated because CPFX is a metabolite of ERFX. ** Sum of ERFX and CPFX found in the bile for 8 hr of administered dose as ERFX.
DISCUSSION
The authors stated that this study is the first to evaluate the biliary excretion of multiple fluoroquinolones in rabbits after IV injection, to their knowledge. While previous studies have examined the pharmacokinetics of IV administration of fluoroquinolones in rabbits, this study specifically evaluated biliary excretion. The data collected in this study showed that the plasma concentration of the studied fluoroquinolones declined in a biphasic manner, consistent with a two compartment open model, which is consistent with other pharmacokinetic studies involving fluoroquinolones in rabbits as difloxacin [1], moxifloxacin [16], and danofloxacin [15].
The half-life of distribution (t1/2α) in this study for ERFX, OBFX, MBFX, and OFLX was estimated to be 0.35, 0.37, 0.39, and 0.40 hr, respectively. This duration was longer than the half-life of distribution of marbofloxacin in rabbits 0.23 hr [2] and shorter than ofloxacin in rabbits 0.70 hr [3].
The half-life of elimination (t1/2β) after IV dosing was 4.13, 3.65, 6.60, and 5.14 hr for ERFX, OBFX, MBFX and OFLX, respectively which was nearly similar to danofloxacin in rabbits 4.88 hr [15] and difloxacin in rabbits 4.19 hr [14] for enrofloxacin in our study, longer than other fluoroquinolones in rabbits as enrofloxacin 2.5 hr [6], 3 hr [13], ofloxacin 1.59 hr [22], moxifloxacin 1.84 hr [16], ibafloxacin 3.00 hr, [24], ofloxacin 1.77 hr [3], orbifloxacin 2.50 hr, [25], norfloxacin 3.18 hr [23], levofloxacin 2.06 hr [34], and shorter than marbofloxacin in rabbits 8.66 hr [26]. Rabbits’ rapid elimination of drugs could be attributed to their elevated heart rate and cardiac output [27, 34].
The Vdss after IV administration was found to be 1.24, 0.503, 0.771, and 1.02 L/kg for ERFX, OBFX, MBFX and OFLX, respectively. These results indicate that the drugs have moderate ability to penetrate biological membranes. The volume of drug distribution observed in this study is lower than that reported for difloxacin in rabbits, which was found to be 1.5 L/kg [1], moxifloxacin 1.95 L/kg [16], danofloxacin 3.16 L/kg [15], ofloxacin 3.81 L/kg [3], orbifloxacin 1.71 L/kg [25], difloxacin 1.95 L/kg [14], marbofloxacin 1.99 L/kg [26], norfloxacin 1.71 L/kg [23], and levofloxacin 1.37 L/kg [34].
The MRTi.v. refers to the average duration that a drug molecule remains in the body following its intravenous administration. The authors of this study found that the MRT in rabbits for ERFX, OBFX, MBFX and OFLX was 3.98, 4.21, 8.28, and 5.12 hr, respectively. The obtained results were nearly similar to MRT recorded for danofloxacin 4.14 hr [15], difloxacin 4.82 hr [14], longer than MRT for other fluoroquinolones reported in rabbits as ofloxacin 2.18 hr [3], orbifloxacin 1.88 hr [25], norfloxacin 2.14 hr [23], and levofloxacin 2.27 hr [34]. Longer MRT was reported for marbofloxacin in healthy rabbits 8.41 hr [2].
The CLtot was 1.05, 0.418, 0.271, and 0.453 L/kg/hr for ERFX, OBFX, MBFX and OFLX, respectively. The total body clearance for other fluoroquinolones reported in rabbits was 0.76 L/kg/hr for danofloxacin [15], ofloxacin 0.18 L/kg/hr [3], difloxacin 0.41 L/kg/hr [14], orbifloxacin 0.91 L/kg/hr [25], marbofloxacin 0.42 L/kg/hr [26], norfloxacin 0.42 L/kg/hr [23], and levofloxacin 0.60 L/kg/hr [34]. Also, the total clearance of ERFX in rabbits was 1.71 L/kg/hr [13]. The findings of this study suggest that when administering fluoroquinolones to rabbits via injection, frequent dosing will be necessary.
These differences in PK of fluoroquinolones in rabbits might be due to differences in rabbit breed, size of the animals in the studies, the provision of other drugs (e.g., anesthetic administration), and the presence of infection in some studies [34].
ERFX is known to be relatively safe as an antimicrobial for use in rabbits, with recommended therapeutic doses being 5 mg/kg administered orally, subcutaneously, intramuscularly and intravenously [17]. It is assumed that orally administered fluoroquinolones are rapidly absorbed in the upper small intestine and rarely reach the lower small intestine, colon or cecum, possibly due to their high lipophilicity, but no studies have been found that have examined this in detail. Therefore, it would be interesting to investigate the pharmacokinetics of fluoroquinolone antimicrobials after oral administration to determine their absorption and bioavailability after oral administration, which would also contribute to the elucidation of their safety after oral administration. Investigation of pharmacokinetics fluoroquinolones after oral administration, together with confirmation of linearity of kinetics at high doses, is considered to be a future issue.
Drugs excreted through biliary excretion may be difficult to use in rabbits due to the unique physiology of their digestive system. Unlike many other animals, rabbits have a limited ability to break down and eliminate certain drugs that are excreted through the bile. This is because rabbits have a unique digestive system that relies heavily on bacterial fermentation to break down food, which can also affect drug metabolism and elimination [21, 40]. Rabbits have a relatively small bile acid pool, lower biliary flow rate, and limited ability to reabsorb bile acids. These factors contribute to an increased sensitivity of rabbits to the toxic effects of certain drugs that are excreted via the biliary route. Additionally, the biliary system in rabbits is anatomically different from other animals, making them more susceptible to bile stasis and subsequent complications [18]. Bile stasis can lead to the formation of gallstones or sludge, which can cause cholestasis, inflammation, and liver damage. Furthermore, rabbits have a highly efficient enterohepatic circulation, which means that drugs excreted via the bile can be rapidly reabsorbed in the intestine and re-enter the systemic circulation. As a result, drugs that are excreted through biliary excretion can accumulate in the rabbit’s system and cause toxicity. Signs of toxicity may include gastrointestinal upset, loss of appetite, diarrhea, lethargy, and even death. Therefore, it is important to avoid administering drugs that are excreted through biliary excretion to rabbits.
The excretion of marbofloxacin in bile is 10% in preruminant calves and 45% in ruminant cattle [37]. Sitafloxacin is mainly eliminated through both renal and biliary excretion in rats [35]. Variations in digestive functions, such as ruminant versus non-ruminant, herbivore versus carnivore, etc., contribute to differences in drug absorption and can be responsible for inter-species differences in drug metabolism [38].
To determine whether a compound is eliminated through biliary or non-biliary excretion, the molecular weight (MW) of the compound is an important factor. Studies conducted in rats using compounds with MW ranging from 150 to over 700 have shown that % of compounds excreted in the bile compared to urine increases as the MW of the compound increases [7]. To summarize, in most species, substances that have MW <300 are mainly eliminated by renal clearance via glomerular filtration, whereas those with MW >600, including drugs, metabolites, and conjugates, are usually eliminated through the bile by active carrier-mediated transport [4, 10]. The preferred method of elimination for substances with MWs ranging between 300 and 600–800 may vary widely among species, resulting in a greater likelihood of interspecies differences. Animals, including rabbits, guinea pigs, and humans, which have limited biliary excretion abilities, have been categorized as poor biliary execrators. Meanwhile, rats, chickens, and dogs, which possess strong biliary excretion capabilities, have been classified as good biliary execrators. Certain species, such as cats and sheep, are intermediate in their ability to excrete substances through bile, with the threshold molecular weight for significant biliary excretion varying from species to species. It is worth noting that the classification of poor or good biliary excreters is not linked to the rate of bile flow, which is high in rabbits, a species considered a poor biliary excreter, and much lower in dogs, which are categorized as good biliary execrators [38].
Substances eliminated through bile have a certain level of polarity that allows them to be transported by a carrier-mediated mechanism from hepatic parenchymal cells into bile [4]. Drugs and their metabolites that are excreted through bile enter the duodenum and some of them may be reabsorbed by passive diffusion, depending on their lipid solubility [36]. Because the molecular weight of the selected fluoroquinolones (ERFX, CPFX, OBFX, MBFX, and OFLX) ranges from approximately 331–395 Da, which is less than 500 Da, it was anticipated that they would not be excreted in bile to a large extent.
Enrofloxacin’s primary elimination route is renal excretion, while ciprofloxacin is eliminated via both hepatic and renal pathways. Both enrofloxacin and ciprofloxacin undergo intestinal recirculation through bile excretion [39]. In Yellow River carp, bile excretion may be the primary pathway for eliminating enrofloxacin [46]. Moxifloxacin and ciprofloxacin were eliminated via the bile route in humans [36]. Dogs predominantly eliminate enrofloxacin through bile excretion; however, the small amounts of ERFX excretion observed in rabbit bile indicate that there could be variations in the extent of biliary excretion of ERFX among different species. Similarly, the other three fluoroquinolones were also inadequately excreted in rabbit bile. These results suggest that these fluoroquinolones may be considered safe for use in rabbits, as they are not extensively excreted in their bile.
The excretion of the glucuronide conjugates of fluoroquinolones depends on both the species and the specific fluoroquinolone administered [28]. They may be excreted in either urine or bile. It has been suggested that fluoroquinolones may undergo enterohepatic circulation, which involves the release of the original drug or biologically active metabolites by β-glucuronidases in the gastrointestinal tract [33].
Horses and rabbits are hindgut fermenters and their caecum and colon play a vital role in the digestion of feed through microbial action. This makes them vulnerable to enterocolitis caused by antimicrobial drugs, which disrupt their normal microflora, leading to an overgrowth of pathogenic microorganisms such as Clostridium species. In rabbits, C. spiriforme has been identified as the primary cause of enterotoxaemia and death [38]. To minimize the risk of drug-induced enterocolitis, antibiotics that are extensively excreted in the bile or eliminated by enterocyte efflux after parenteral administration, such as oxytetracycline and doxycycline, should be administered to horses and rabbits with caution. Lincomycin and clindamycin have also been linked to enterocolitis in horses, while certain penicillins (amoxicillin, ampicillin) and cephalosporins (ceftiofur) are associated with enterocolitis in rabbits, regardless of their method of administration. Therefore, these antibiotics should be avoided in hindgut fermenters [38]. The low biliary excretion of fluoroquinolones in rabbits in this study suggests that fluoroquinolones are antimicrobial agents that can avoid adverse effects on the gut flora of rabbits, which are hindgut fermenters.
In conclusion, lack of local reaction or any other adverse effect and favorable kinetics of the studied fluoroquinolones (ERFX, OBFX, MBFX and OFLX) and the hardly excretion of these drugs into the bile made it used as safe drugs in rabbits. It is important to carefully consider the use of drugs that are excreted through biliary excretion to avoid potential toxicity in rabbits.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Abd El-Aty AM, Goudah A, Ismail M, Shimoda M. 2005. Disposition kinetics of difloxacin in rabbit after intravenous and intramuscular injection of Dicural. Vet Res Commun 29: 297–304. doi: 10.1023/B:VERC.0000048503.05426.68 [DOI] [PubMed] [Google Scholar]
- 2.Abo-el-Sooud K, Goudah A. 2010. Influence of Pasteurella multocida infection on the pharmacokinetic behavior of marbofloxacin after intravenous and intramuscular administrations in rabbits. J Vet Pharmacol Ther 33: 63–68. doi: 10.1111/j.1365-2885.2009.01110.x [DOI] [PubMed] [Google Scholar]
- 3.Ahmad M, Raza H, Murtaza G, Akhtar N. 2008. Pharmacokinetic variations of ofloxacin in normal and febrile rabbits. Pak Vet J 28: 181–185. [Google Scholar]
- 4.Baggot J. 2007. Veterinary dosage forms. pp. 3941–3977. In: Encyclopedia of Pharmaceutical Technology 3rd ed. (Swarbrick J ed.), Taylor & Francis eBooks, London. [Google Scholar]
- 5.Bregante MA, Saez P, Aramayona JJ, Fraile L, Garcia MA, Solans C. 1999. Comparative pharmacokinetics of enrofloxacin in mice, rats, rabbits, sheep, and cows. Am J Vet Res 60: 1111–1116. [PubMed] [Google Scholar]
- 6.Broome RL, Brooks DL, Babish JG, Copeland DD, Conzelman GM. 1991. Pharmacokinetic properties of enrofloxacin in rabbits. Am J Vet Res 52: 1835–1841. [PubMed] [Google Scholar]
- 7.Calabrese E. 1983. Cyanide toxicity. pp. 278–281. In: Principles of Animal Extrapolation, Wiley, New York. [Google Scholar]
- 8.Cárceles CM, Serrano JM, Marín P, Escudero E, Fernández-Varón E. 2006. Pharmacokinetics of moxifloxacin in rabbits after intravenous, subcutaneous and a long-acting poloxamer 407 gel formulation administration. J Vet Med A Physiol Pathol Clin Med 53: 300–304. doi: 10.1111/j.1439-0442.2006.00827.x [DOI] [PubMed] [Google Scholar]
- 9.Chen JC, Kang JJ, Zhang M, Shao HT, Song ZW, Ma KL, Yang F, Yang F. 2023. Pharmacokinetics of danofloxacin after single oral and intravenous administration in non-laying hens. J Vet Pharmacol Ther 46: 119–124. doi: 10.1111/jvp.13098 [DOI] [PubMed] [Google Scholar]
- 10.Dooley JS, Lok AS, Garcia-Tsao G, Pinzani M. 2018. Sherlock’s Diseases of the Liver and Biliary System, John Wiley & Sons, Hoboken. [Google Scholar]
- 11.Drouilhet L, Achard CS, Zemb O, Molette C, Gidenne T, Larzul C, Ruesche J, Tircazes A, Segura M, Bouchez T, Theau-Clément M, Joly T, Balmisse E, Garreau H, Gilbert H. 2016. Direct and correlated responses to selection in two lines of rabbits selected for feed efficiency under ad libitum and restricted feeding: I. Production traits and gut microbiota characteristics. J Anim Sci 94: 38–48. doi: 10.2527/jas.2015-9402 [DOI] [PubMed] [Google Scholar]
- 12.Elmas M, Yazar E, Uney K, Er Karabacak A. 2006. Influence of Escherichia coli endotoxin-induced endotoxaemia on the pharmacokinetics of enrofloxacin after intravenous administration in rabbits. J Vet Med A Physiol Pathol Clin Med 53: 410–414. doi: 10.1111/j.1439-0442.2006.00854.x [DOI] [PubMed] [Google Scholar]
- 13.Elmas M, Üney K, Yazar E, Karabacak A, Traş B. 2007. Pharmacokinetics of enrofloxacin following intravenous and intramuscular administration in Angora rabbits. Res Vet Sci 82: 242–245. doi: 10.1016/j.rvsc.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 14.Fernández-varón E, Cárceles CM, Marín P, Vancraeynest D, Montes A, Sotillo J, García-Martínez JD. 2008. Disposition kinetics and pharmacokinetics--pharmacodynamic integration of difloxacin against Staphylococcus aureus isolates from rabbits. Res Vet Sci 84: 90–94. doi: 10.1016/j.rvsc.2007.04.002 [DOI] [PubMed] [Google Scholar]
- 15.Fernández-Varón E, Marin P, Escudero E, Vancraeynest D, Cárceles CM. 2007. Pharmacokinetic-pharmacodynamic integration of danofloxacin after intravenous, intramuscular and subcutaneous administration to rabbits. J Vet Pharmacol Ther 30: 18–24. doi: 10.1111/j.1365-2885.2007.00807.x [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Varón E, Bovaira MJ, Espuny A, Escudero E, Vancraeynest D, Cárceles CM. 2005. Pharmacokinetic-pharmacodynamic integration of moxifloxacin in rabbits after intravenous, intramuscular and oral administration. J Vet Pharmacol Ther 28: 343–348. doi: 10.1111/j.1365-2885.2005.00665.x [DOI] [PubMed] [Google Scholar]
- 17.Ivey ES, Morrisey JK. 2000. Therapeutics for rabbits. Vet Clin North Am Exot Anim Pract 3: 183–220, vii. doi: 10.1016/S1094-9194(17)30101-9 [DOI] [PubMed] [Google Scholar]
- 18.Kararli TT. 1995. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos 16: 351–380. doi: 10.1002/bdd.2510160502 [DOI] [PubMed] [Google Scholar]
- 19.Küng K, Riond JL, Wanner M. 1993. Pharmacokinetics of enrofloxacin and its metabolite ciprofloxacin after intravenous and oral administration of enrofloxacin in dogs. J Vet Pharmacol Ther 16: 462–468. doi: 10.1111/j.1365-2885.1993.tb00212.x [DOI] [PubMed] [Google Scholar]
- 20.Lajczak-McGinley NK, Porru E, Fallon CM, Smyth J, Curley C, McCarron PA, Tambuwala MM, Roda A, Keely SJ. 2020. The secondary bile acids, ursodeoxycholic acid and lithocholic acid, protect against intestinal inflammation by inhibition of epithelial apoptosis. Physiol Rep 8: e14456. doi: 10.14814/phy2.14456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B, Cui Y, Ali Q, Zhu X, Li D, Ma S, Wang Z, Wang C, Shi Y. 2022. Gut microbiota modulate rabbit meat quality in response to dietary fiber. Front Nutr 9: 849429. doi: 10.3389/fnut.2022.849429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marangos MN, Zhu Z, Nicolau DP, Klepser ME, Nightingale CH. 1997. Disposition of ofloxacin in female New Zealand white rabbits. J Vet Pharmacol Ther 20: 17–20. doi: 10.1046/j.1365-2885.1997.00812.x [DOI] [PubMed] [Google Scholar]
- 23.Marín P, García-Martínez F, Hernándis V, Escudero E. 2018. Pharmacokinetics of norfloxacin after intravenous, intramuscular and subcutaneous administration to rabbits. J Vet Pharmacol Ther 41: 137–141. doi: 10.1111/jvp.12422 [DOI] [PubMed] [Google Scholar]
- 24.Marín P, Cárceles CM, Escudero E, Bermejo R, Fernández-Varón E. 2007. Development of a method for the determination of ibafloxacin in plasma by HPLC with flourescence detection and its application to a pharmacokinetic study. J Chromatogr Sci 45: 242–245. doi: 10.1093/chromsci/45.5.242 [DOI] [PubMed] [Google Scholar]
- 25.Marín P, Fernández-Varón E, Escudero E, Vancraeynest D, Cárceles CM. 2008. Pharmacokinetic-pharmacodynamic integration of orbifloxacin in rabbits after intravenous, subcutaneous and intramuscular administration. J Vet Pharmacol Ther 31: 77–82. doi: 10.1111/j.1365-2885.2007.00927.x [DOI] [PubMed] [Google Scholar]
- 26.Marín P, Álamo LF, Escudero E, Fernández-Varón E, Hernandis V, Cárceles CM. 2013. Pharmacokinetics of marbofloxacin in rabbit after intravenous, intramuscular, and subcutaneous administration. Res Vet Sci 94: 698–700. doi: 10.1016/j.rvsc.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 27.Mitchell M, Tully TN. 2008. Manual of exotic pet practice 1st ed, Elsevier, Amsterdam. [Google Scholar]
- 28.Nix DE, Schentag JJ. 1988. The quinolones: an overview and comparative appraisal of their pharmacokinetics and pharmacodynamics. J Clin Pharmacol 28: 169–178. doi: 10.1002/j.1552-4604.1988.tb05740.x [DOI] [PubMed] [Google Scholar]
- 29.Oglesbee BL, Lord B. 2020. Gastrointestinal diseases of rabbits. pp. 174-187. In: Ferrets, Rabbits, and Rodents, 4th ed. (Quesenberry KE, Mans C, Orcutt CJ, Carpenter JW eds.), Elsevier, Amsterdam. [Google Scholar]
- 30.Park SC, Yun HI, Oh TK. 1998. Comparative pharmacokinetic profiles of two norfloxacin formulations after oral administration in rabbits. J Vet Med Sci 60: 661–663. doi: 10.1292/jvms.60.661 [DOI] [PubMed] [Google Scholar]
- 31.Pham TDM, Ziora ZM, Blaskovich MAT. 2019. Quinolone antibiotics. MedChemComm 10: 1719–1739. doi: 10.1039/C9MD00120D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rougier S, Galland D, Boucher S, Boussarie D, Vallé M. 2006. Epidemiology and susceptibility of pathogenic bacteria responsible for upper respiratory tract infections in pet rabbits. Vet Microbiol 115: 192–198. doi: 10.1016/j.vetmic.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 33.Sarkozy G. 2001. Quinolones: a class of antimicrobial agents. Vet. Med. −. Czech 46: 257–274. doi: 10.17221/7883-VETMED [DOI] [Google Scholar]
- 34.Sitovs A, Voiko L, Kustovs D, Kovalcuka L, Bandere D, Purvina S, Giorgi M. 2020. Pharmacokinetic profiles of levofloxacin after intravenous, intramuscular and subcutaneous administration to rabbits (Oryctolagus cuniculus). J Vet Sci 21: e32. doi: 10.4142/jvs.2020.21.e32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tachibana M, Tanaka M, Mitsugi K, Jin Y, Takaichi M, Okazaki O. 2004. Pharmacokinetics, tissue distribution, and excretion of sitafloxacin, a new fluoroquinolone antibiotic, in rats, dogs, and monkeys. Arzneimittelforschung 54: 898–905. [DOI] [PubMed] [Google Scholar]
- 36.Thabit AK. 2020. Antibiotics in the biliary tract: a review of the pharmacokinetics and clinical outcomes of antibiotics penetrating the bile and gallbladder wall. Pharmacotherapy 40: 672–691. doi: 10.1002/phar.2431 [DOI] [PubMed] [Google Scholar]
- 37.Thomas V, Deleforge J, Boisrame B. 1994. Pharmacokinetics of marbofloxacin in preruminant and ruminant cattle. pp. 60–61. In: Proceedings of the Sixth Congress of the European Association for Veterinary Pharmacology and Toxicology, Edinburgh. [Google Scholar]
- 38.Toutain PL, Ferran A, Bousquet-Mélou A. 2010. Species differences in pharmacokinetics and pharmacodynamics. Handb Exp Pharmacol 199: 19–48. doi: 10.1007/978-3-642-10324-7_2 [DOI] [PubMed] [Google Scholar]
- 39.Trouchon T, Lefebvre S. 2016. A review of enrofloxacin for veterinary use. Open J Vet Med 6: 40–58. doi: 10.4236/ojvm.2016.62006 [DOI] [Google Scholar]
- 40.Varga M.2014. Rabbit basic science. pp. 3–108. In: Textbook of Rabbit Medicine, Elsevier, Amsterdam. [Google Scholar]
- 41.Wang J, Xia S, Fan H, Shao J, Tang T, Yang L, Sun W, Jia X, Chen S, Lai S. 2022. Microbiomics revealed the disturbance of intestinal balance in rabbits with diarrhea caused by stopping the use of an antibiotic diet. Microorganisms 10: 841. doi: 10.3390/microorganisms10050841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfson JS, Hooper DC. 1989. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev 2: 378–424. doi: 10.1128/CMR.2.4.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie S, Trott DJ, Saputra S, Ebrahimie E, Dehcheshmeh MM, Page C, Woodward N, Griffiths N, Kimble B, Govendir M. 2022. Pharmacokinetic profile and effect on the faecal microbiome of a single dose of pradofloxacin oral suspension in the rabbit (Oryctolagus cuniculus). J Vet Pharmacol Ther 45: 203–212. doi: 10.1111/jvp.13038 [DOI] [PubMed] [Google Scholar]
- 44.Yamaoka K, Tanigawara Y, Nakagawa T, Uno T. 1981. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn 4: 879–885. doi: 10.1248/bpb1978.4.879 [DOI] [PubMed] [Google Scholar]
- 45.Yang F, Yang F, Shi W, Si H, Kong T, Wang G, Zhang J. 2018. Development of a multiroute physiologically based pharmacokinetic model for orbifloxacin in rabbits. J Vet Pharmacol Ther 41: 622–631. doi: 10.1111/jvp.12496 [DOI] [PubMed] [Google Scholar]
- 46.Yang F, Zhang CS, Duan MH, Wang H, Song ZW, Shao HT, Ma KL, Yang F. 2022. Pharmacokinetics and tissue distribution of enrofloxacin following single oral administration in Yellow River carp (Cyprinus carpio haematoperus). Front Vet Sci 9: 822032. doi: 10.3389/fvets.2022.822032 [DOI] [PMC free article] [PubMed] [Google Scholar]


