Key Points
Question
Are there racial and ethnic disparities in survival among participants enrolled in clinical trials receiving standardized initial care for early-stage breast cancer?
Findings
In this cohort study with 9479 participants, pooled survival data suggest that survival differences exist even within clinical trial participants receiving similar initial care. Subgroups, defined by tumor subtype, age, and/or body mass index, that may drive racial and ethnic disparities in survival were identified.
Meaning
These findings suggest potential factors contributing to racial and ethnic disparities in survival of patients with breast cancer; it is critical to evaluate interventions for improvement.
This cohort study assesses the association of race and ethnicity with survival among clinical trial participants with early-stage breast cancer according to tumor subtype, age, and body mass index (BMI).
Abstract
Importance
Black women in the United States have higher breast cancer (BC) mortality rates than White women. The combined role of multiple factors, including body mass index (BMI), age, and tumor subtype, remains unclear.
Objective
To assess the association of race and ethnicity with survival among clinical trial participants with early-stage BC (eBC) according to tumor subtype, age, and BMI.
Design, Setting, and Participants
This cohort study analyzed survival data, as of November 12, 2021, from participants enrolled between 1997 and 2010 in 4 randomized adjuvant chemotherapy trials: Cancer and Leukemia Group B (CALGB) 9741, 49907, and 40101 as well as North Central Cancer Treatment Group (NCCTG) N9831, legacy groups of the Alliance of Clinical Trials in Oncology. Median follow-up was 9.8 years.
Exposures
Non-Hispanic Black and Hispanic participants were compared with non-Hispanic White participants within subgroups of subtype (hormone receptor positive [HR+]/ERBB2 [formerly HER2] negative [ERBB2−], ERBB2+, and HR−/ERBB2−), age (<50, 50 to <65, and ≥65 years), and BMI (<18.5, 18.5 to <25.0, 25.0 to <30.0, and ≥30.0).
Main Outcomes and Measures
Recurrence-free survival (RFS) and overall survival (OS).
Results
Of 9479 participants, 436 (4.4%) were Hispanic, 871 (8.8%) non-Hispanic Black, and 7889 (79.5%) non-Hispanic White. The median (range) age was 52 (19.0-89.7) years. Among participants with HR+/ERBB2− tumors, non-Hispanic Black individuals had worse RFS (hazard ratio [HR], 1.49; 95% CI, 1.04-2.12; 5-year RFS, 88.5% vs 93.2%) than non-Hispanic White individuals, although the global test for association of race and ethnicity with RFS was not significant within any tumor subtype. There were no OS differences by race and ethnicity in any subtype. Race and ethnicity were associated with OS in young participants (age <50 years; global P = .008); young non-Hispanic Black participants (HR, 1.34; 95% CI, 1.04-1.71; 5-year OS, 86.6% vs 92.0%) and Hispanic participants (HR, 1.62; 95% CI, 1.16-2.29; 5-year OS, 86.2% vs 92.0%) had worse OS than young non-Hispanic White participants. Race and ethnicity were associated with RFS in participants with BMIs of 25 to less than 30, with non-Hispanic Black participants having worse RFS (HR, 1.81; 95% CI, 1.23-2.68; 5-year RFS, 83.2% vs 87.3%) than non-Hispanic White participants.
Conclusions and Relevance
In this cohort study, racial and ethnic survival disparities were identified in patients with eBC receiving standardized initial care, and potentially at-risk subgroups, for whom focused interventions may improve outcomes, were found.
Introduction
For several decades, non-Hispanic Black women have had substantially higher breast cancer (BC) mortality rates than non-Hispanic White women.1,2,3,4,5,6,7 Recent data from the Surveillance, Epidemiology, and End Results program show that between 2015 and 2019, Black women had 41% higher BC mortality compared with White women despite a 4% lower incidence of BC.8 The emergence and subsequent widening of this disparity over the past 40 years suggests that potentially modifiable and time-varying factors contribute.1 Such disparities are also known to exist among Hispanic women, although there is a relative dearth of literature studying this important population.9,10,11 While lack of quality and timely initial adjuvant treatment is an important contributor, prospective study of BC survival in patients receiving standardized adjuvant therapy, as in the clinical trial setting, can facilitate identification of other factors.10,11,12
A previous pooled analysis of SWOG clinical trials showed that, while no racial disparities were observed for most cancer types, non-Hispanic Black women with BC were more likely to die than non-Hispanic White women.13 While this study did not investigate potential contributing factors, subsequent studies have suggested that racial and ethnic differences in BC survival vary by tumor subtype.14,15,16,17 Age has also been associated with survival in patients with BC. Both younger and older age have been associated with differences in treatments and adherence as well as worse mortality.18,19,20,21,22 Body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) has also been shown to be associated with BC survival, although the role of BMI in racial and ethnic survival disparities, particularly among groups that are underreported and underrepresented in clinical trials, such as Hispanic patients, merits additional study.20,21,23,24,25,26 In general, the extent of disparities within subgroups defined by of age, BMI, and tumor subtype are unclear yet likely are important in addressing BC. In this pooled analysis of 4 prospective adjuvant BC clinical trials, we assessed whether race and ethnicity were associated with recurrence-free survival (RFS) and overall survival (OS) among women enrolled in clinical trials for early-stage BC (eBC) according to tumor subtype, age, and BMI.14,15,16,17,20,21,24,25
Methods
Data and Patients
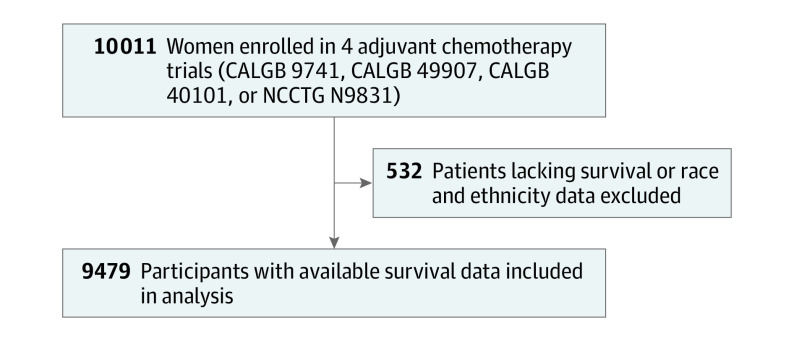
We included participants enrolled in 4 adjuvant chemotherapy trials: Cancer and Leukemia Group B (CALGB) C9741 (NCT00003088), C49907 (NCT00005970), C40101 (NCT00024102) and North Central Cancer Treatment Group (NCCTG) N9831 (NCT00041119) (eMethods in Supplement 1).27,28,29,30 CALGB and NCCTG are now part of the Alliance for Clinical Trials in Oncology (Alliance). All participants with available survival and race and ethnicity data were included (Figure 1). Three of the trials (CALGB 9741, CALGB 49907, and CALGB 40101) evaluated adjuvant chemotherapy regimens for all BC subtypes, while NCCTG N9831 included only patients with human epidermal growth factor receptor 2–positive (ERBB2+) BC. CALGB 49907 studied older women with BC and included only patients ages 65 years and older. Relevant ethical review committees approved all trials. All participants in these trials provided informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Figure 1. Flow Diagram of Participants.
CALGB indicates Cancer and Leukemia Group B; NCCTG, North Central Cancer Treatment Group.
Measures and Outcomes
Tumor hormone receptor (HR) and ERBB2 status were assessed in each trial as previously published.27,28,29,30 In this study, we analyzed the following subtypes: hormone receptor–positive/ERBB2-negative (HR+/ERBB2−), ERBB2+, and HR-negative/ERBB2-negative (HR−/ERBB2−; also known as triple negative). Race and ethnicity were analyzed in 4 groups: Hispanic, non-Hispanic Black, non-Hispanic White, and other race and ethnicity (including American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander). The other race and ethnicity group was created because the sample sizes of the individual groups were too small to analyze separately. Race and ethnicity were patient-reported based on investigator options in 2 of the 4 trials included. For the other 2 trials, documentation of how race and ethnicity were collected is not available. Age at diagnosis was divided into 3 groups: younger than 50 years, 50 to younger than 65 years, and 65 years or older. Participant BMI at time of trial randomization was separated into 4 groups: less than 18.5, 18.5 to less than 25.0, 25.0 to less than 30.0, and 30.0 or greater, corresponding to the US Center for Disease Control and Prevention definitions of underweight, healthy weight, overweight, and obesity, respectively.31
RFS events included local, regional, or distant BC recurrence or death due to any cause.32 Participants alive without an RFS event were censored at the time of last follow-up. OS was defined using deaths from any cause as an event. Participants without known death were censored at the time of last contact, including any participants lost to follow-up. All time-to-event measures started at the time of trial randomization.
Statistical Analysis
Median follow-up was estimated using the reverse Kaplan-Meier method.33,34,35 Baseline characteristics between race and ethnicity groups were compared using a χ2 test.36 All results presented are multivariable analyses. Cox proportional hazards models were used to estimate multivariable-adjusted hazard ratios (HRs) and 95% CIs for the association between race and ethnicity and RFS or OS.34,35 Multivariable models included race and ethnicity, tumor subtype, BMI, age, stage, and treatment group if the variable was not the subgroup being evaluated. Participants with missing data for subtype or stage were not included in the multivariable analyses controlling for subtype or stage, respectively. We also conducted separate analyses within strata of age and strata of BMI. Participants with missing data for BMI were not included in analyses within strata of BMI. No participants had missing age data. P values are provided only for global tests and not for comparisons of different levels of the variable with the reference group. We present point estimates and 95% CIs comparing each level of the variable with the reference group. Results of analyses were also depicted with forest plots.37 Kaplan-Meier estimators were used to estimate survival at specific time points and to generate survival curves.34,35 The prespecified level of significance was .05. The Alliance Statistics and Data Management Center conducted statistical analyses on the study database frozen on November 12, 2021, using SAS version 9.3 (SAS Institute).
Results
Patient Characteristics
Of 10 011 women enrolled in the included trials, 9479 (94.7%) had available survival and race and ethnicity data and were included in this pooled analysis. Their median (IQR) follow-up time was 9.8 (6.7-13.2) years, and 435 participants (4.6%) were designated as lost to follow-up. All participants were female. There were 436 (4.6%) Hispanic, 871 (9.2%) non-Hispanic Black, 7889 (83.2%) non-Hispanic White participants, and 283 (3.0%) patients with another race and ethnicity (Table). At enrollment, median (range) age was 52 (19.0-89.7) years, and median (range) BMI was 28.3 (12.3-77.3). The distribution of tumor subtypes differed significantly among race and ethnicity groups, with a larger proportion of non-Hispanic Black participants with HR−/ERBB2− BC compared with non-Hispanic White participants (197 [25.6%] vs 1049 [14.7%]; P < .001). Additionally, BMI differed significantly across race and ethnicity groups; obesity was more frequent in non-Hispanic Black participants than non-Hispanic White participants (511 [59.5%] vs 3008 [38.7%]; P < .001). The baseline characteristics between Hispanic and non-Hispanic White participants were similar.
Table. Participant Baseline Characteristics.
| Characteristic | Participants, No. (%) | ||||
|---|---|---|---|---|---|
| Hispanic (n = 436) | Non-Hispanic Black (n = 871) | Non-Hispanic White (n = 7889) | Non-Hispanic other (n = 283)a | Total (N = 9479) | |
| Trial | |||||
| CALGB 40101 | 206 (47.2) | 357 (41.0) | 2865 (36.3) | 95 (33.6) | 3523 (37.2) |
| CALGB 49907 | 30 (6.9) | 59 (6.8) | 460 (5.8) | 10 (3.5) | 559 (5.9) |
| CALGB 9741 | 80 (18.3) | 216 (24.8) | 1625 (20.6) | 39 (13.8) | 1960 (20.7) |
| NCCTG N9831 | 120 (27.5) | 239 (27.4) | 2939 (37.3) | 139 (49.1) | 3437 (36.3) |
| Age category, y | |||||
| <50 | 212 (48.6) | 424 (48.7) | 3285 (41.6) | 138 (48.8) | 4059 (42.8) |
| 50 to <65 | 162 (37.2) | 335 (38.5) | 3385 (42.9) | 109 (38.5) | 3991 (42.1) |
| ≥65 | 62 (14.2) | 112 (12.9) | 1219 (15.5) | 36 (12.7) | 1429 (15.1) |
| Stage | |||||
| I | 113 (26.2) | 177 (20.5) | 1798 (22.9) | 52 (18.7) | 2140 (22.7) |
| II | 268 (62.2) | 533 (61.8) | 4823 (61.5) | 186 (66.9) | 5810 (61.7) |
| III | 50 (11.6) | 152 (17.6) | 1225 (15.6) | 40 (14.4) | 1467 (15.6) |
| Missing | 5 | 9 | 43 | 5 | 62 |
| Breast cancer subtype | |||||
| ERBB2+ | 172 (43.4) | 320 (41.6) | 3376 (47.3) | 152 (59.1) | 4020 (47.0) |
| HR+/ERBB2− | 141 (35.6) | 252 (32.8) | 2711 (38.0) | 72 (28.0) | 3176 (37.1) |
| HR−/ERBB2− | 83 (21.0) | 197 (25.6) | 1049 (14.7) | 33 (12.8) | 1362 (15.9) |
| Missing | 40 | 102 | 753 | 26 | 921 |
| BMI categoryb | |||||
| Underweight | 4 (0.9) | 8 (0.9) | 129 (1.7) | 5 (1.8) | 146 (1.6) |
| Healthy weight | 92 (21.3) | 112 (13.0) | 2315 (29.8) | 115 (40.8) | 2634 (28.2) |
| Overweight | 146 (33.9) | 228 (26.5) | 2328 (29.9) | 96 (34.0) | 2798 (29.9) |
| Obesity | 189 (43.9) | 511 (59.5) | 3008 (38.7) | 66 (23.4) | 3774 (40.4) |
| Missing | 5 | 12 | 109 | 1 | 127 |
Abbreviations: −, negative; +, positive; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CALGB, Cancer and Leukemia Group B; HR, hormone receptor; NCCTG, North Central Cancer Treatment Group.
Other race and ethnicity includes American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander.
BMI groups were determined based on Center for Disease Control and Prevention definitions of underweight, healthy weight, overweight, and obesity as follows: less than 18.5, 18.5 to less than 25.0, 25.0 to less than 30.0, and 30.0 or greater respectively.
Survival and Race and Ethnicity
Survival Within Tumor Subtypes by Race and Ethnicity
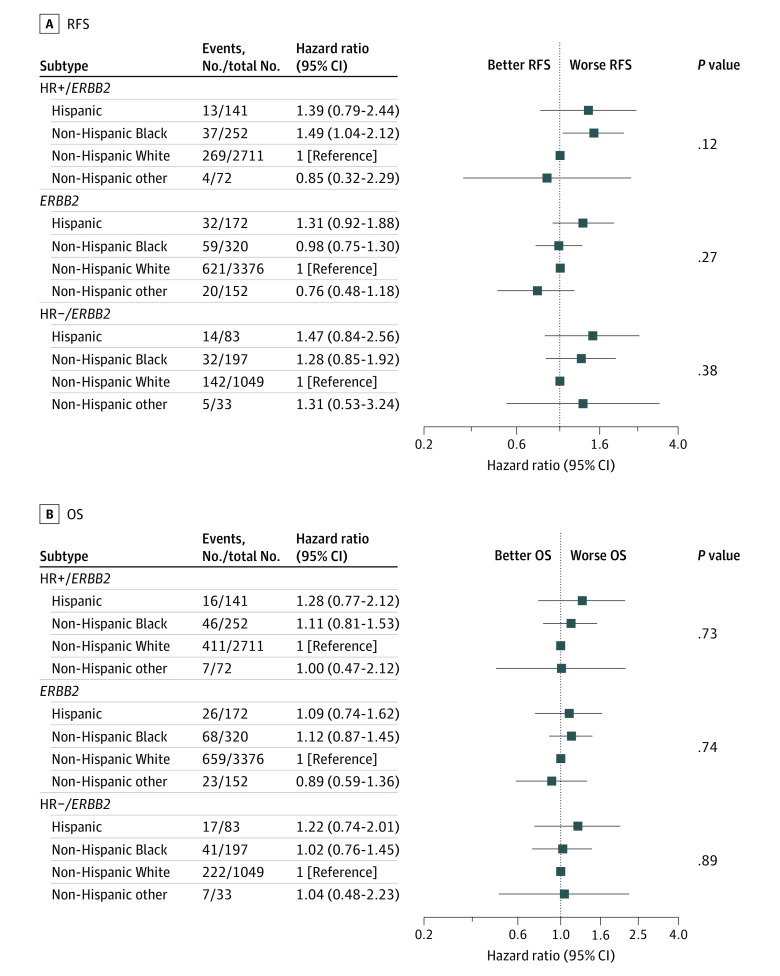
We evaluated differences in RFS and OS among racial and ethnic groups for participants with the same BC subtype. Of all 9479 participants, 8588 (90.6%) had available tumor subtype data and were included in these analyses. Global tests for the association of the race and ethnicity variable with tumor subtype were not statistically significant within any subtype. However, we did observe an association among participants with HR+/ERBB2− tumors, with non-Hispanic Black individuals having worse RFS than non-Hispanic White individuals (HR, 1.49; 95% CI, 1.04-2.12; 5-year RFS, 88.5% vs 93.2%) (Figure 2). There were no differences in RFS observed between non-Hispanic Black and non-Hispanic White participants among those with ERBB2+ tumors (HR, 0.98; 95% CI, 0.75-1.30; 5-year RFS, 84.0% vs 85.1%) or HR−/ERBB2− tumors (HR, 1.28; 95% CI, 0.85-1.92; 5-year RFS, 84.1% vs 86.6%). There were no differences in RFS between Hispanic and non-Hispanic White participants, and no OS differences by race and ethnicity in any tumor subtype. Kaplan-Meier estimates of RFS and OS in BC subtype by race and ethnicity are depicted in eFigure 1 and eFigure 2 in Supplement 1.
Figure 2. Forest Plots of Hazard Ratios Comparing Survival in Tumor Subtype by Race/Ethnicity.
− Indicates negative; +, positive; HR, hormone receptor; OS, overall survival; and RFS, recurrence-free survival.
Survival Within Age Categories by Race and Ethnicity
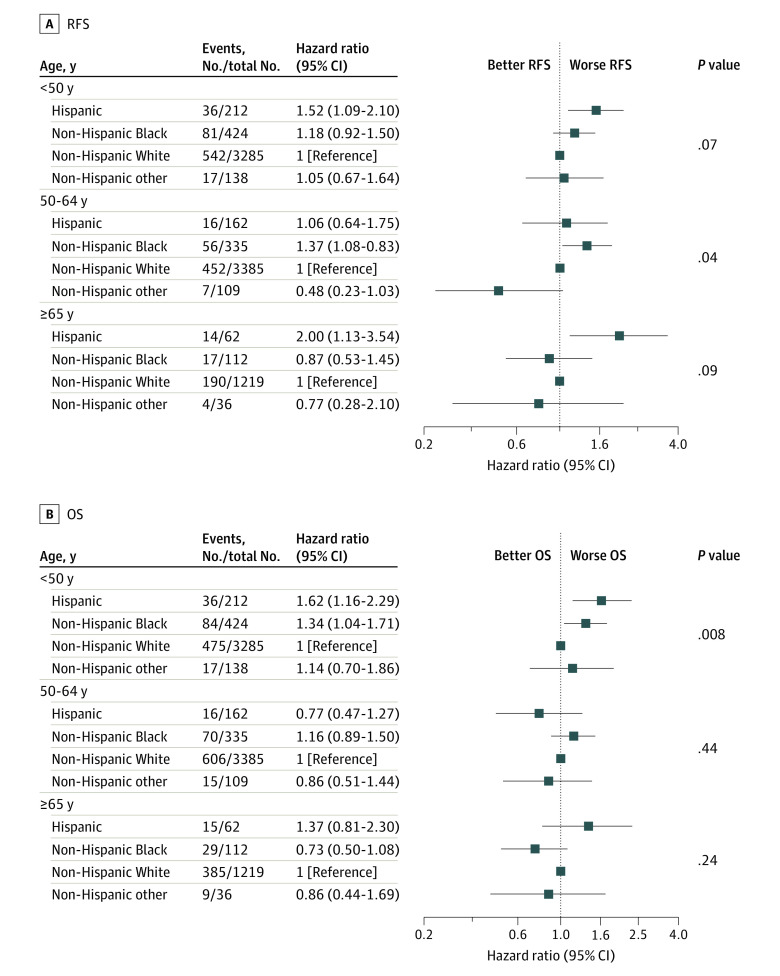
We next evaluated survival differences within age groups. All 9479 participants had available age data and were included in these analyses. Within the middle age group, ages 50 to younger than 65 years, race and ethnicity were associated with RFS (global P = .04); non-Hispanic Black participants had worse RFS than non-Hispanic White participants (HR, 1.37; 95% CI, 1.03-1.83; 5-year RFS, 84.6% vs 89.1%) (Figure 3). Among younger (ages <50 years) and older (ages ≥65 years) patients, race and ethnicity were not significantly associated with RFS (global P = .07 and global P = .09, respectively). Both younger and older Hispanic participants had worse RFS than non-Hispanic White participants (younger: HR, 1.52; 95% CI, 1.09-2.10; 5-year RFS, 82.6% vs 85.9%; older: HR, 2.00; 95% CI, 1.13-3.54; 5-year RFS, 78.0% vs 87.9%). Race and ethnicity were significantly associated with OS in young participants (global P = .008). Specifically, young non-Hispanic Black (HR, 1.34; 95% CI, 1.04-1.71; 5-year OS, 86.6% vs 92.0%) and Hispanic (HR, 1.62; 95% CI, 1.16-2.29; 5-year OS, 86.2% vs 92.0%) participants had worse OS than young non-Hispanic White participants. There was no notable association observed between race and ethnicity and OS among participants ages 50 and older. Kaplan-Meier estimates of RFS and OS in age category by race and ethnicity are depicted in eFigure 3 and eFigure 4 in Supplement 1.
Figure 3. Forest Plots of Hazard Ratios Comparing Survival in Age Category by Race/Ethnicity.
OS indicates overall survival; RFS, recurrence-free survival.
When further analyzed within subgroups jointly defined by subtype and age, race and ethnicity were associated with RFS in older participants with HR+/ERBB2− BCs (global P = .002). Among participants ages 65 years or older with HR+/ERBB2− tumors (n = 696), Hispanic participants (n = 23) had more than 6 times the risk of having an RFS event than non-Hispanic White participants (n = 537) (HR, 6.30; 95% CI, 2.41-16.50). Although the global test was not statistically significant for participants younger than 50 years with HR+/ERBB2− for RFS (P = .06) (n = 1288), non-Hispanic Black participants (n = 103) had considerably worse RFS (HR, 1.98; 95% CI, 1.15-3.42) than non-Hispanic White participants. This was also observed for OS with a non–statistically significant global test (P = .15) but with a considerably worse OS for non-Hispanic Black participants compared with non-Hispanic White participants (HR, 1.86; 95% CI, 1.08-3.18).
Survival Within BMI Categories by Race and Ethnicity
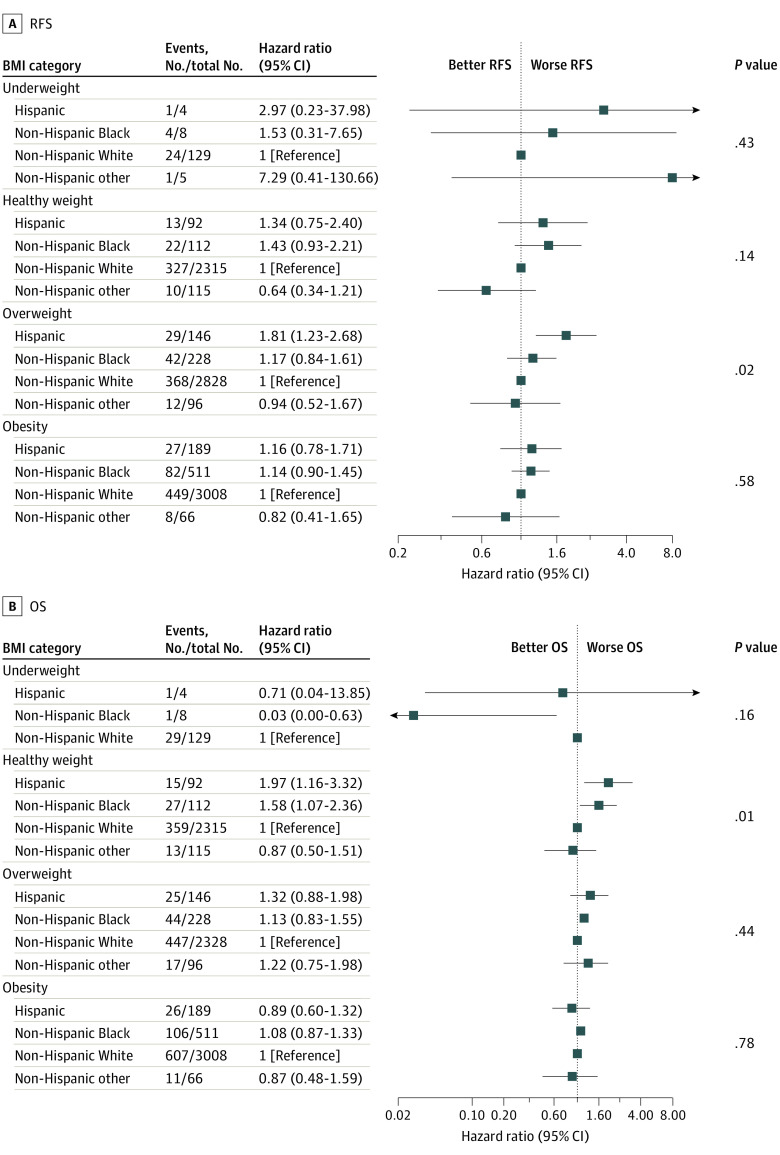
We also studied survival differences between race and ethnicity groups within BMI categories. Of all 9479 participants, 9352 (98.7%) had available BMI data and were included in these analyses. For participants with underweight and obesity, race and ethnicity were not significantly associated with RFS or OS on global testing. However, among participants with overweight, race and ethnicity were significantly associated with RFS (global P = .02), with Hispanic participants having worse RFS than non-Hispanic White participants (HR, 1.81; 95% CI, 1.23-2.68; 5-year RFS, 83.7% vs 87.3%) (Figure 4). For those with healthy weight, the association between race and ethnicity and RFS was not significant, although there was a significant association between race and ethnicity and OS (global P = .01): both non-Hispanic Black and Hispanic participants had worse OS than non-Hispanic White participants (non-Hispanic Black: HR, 1.58; 95% CI, 1.07-2.36; 5-year OS, 85.7% vs 91.5%; Hispanic: HR, 1.97; 95% CI, 1.16-3.32; 5-year OS, 85.0% vs 91.5%). Kaplan-Meier estimates of RFS and OS in BMI category by race and ethnicity are depicted in eFigure 5 and eFigure 6 in Supplement 1.
Figure 4. Forest Plots of Hazard Ratios Comparing Survival in Body Mass Index Category by Race/Ethnicity.
Body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) groups were determined based on Center for Disease Control and Prevention definitions of underweight, healthy weight, overweight, and obesity as follows less than 18.5, 18.5 to less than 25.0, 25.0 to less than 30.0, and 30.0 or greater, respectively. Point estimates and 95% CIs for overall survival (OS) of participants in the non-Hispanic other race and ethnicity group with underweight could not be estimated. RFS indicates recurrence-free survival.
Analysis of subgroups jointly defined within BMI and subtype showed that, among patients with HR+/ERBB2− BC and overweight (n = 1054), non-Hispanic Black individuals (n = 69) had considerably worse RFS than non-Hispanic White individuals (n = 858) (HR, 2.12; 95% CI, 1.09-4.12), although the global test for race and ethnicity was not significant (global P = .08). This association was not statistically significant for OS. Within other subgroups defined by both BMI and subtype, there were no significant associations of race and ethnicity with RFS or OS.
Discussion
We identified disease and patient subgroups with similarities and differences in survival among racial and ethnic groups for participants enrolled in 4 Alliance adjuvant chemotherapy clinical trials for eBC. While race and ethnicity, in general, were not associated with survival when stratified by subtype, non-Hispanic Black patients with HR+/ERBB2− tumors had worse RFS than non-Hispanic White patients. There were no OS differences by race and ethnicity in any subtype. We also observed survival differences within strata of some age groups: in middle-aged participants, non-Hispanic Black individuals had worse RFS than non-Hispanic White individuals. Among young participants, both non-Hispanic Black and Hispanic participants had worse OS than non-Hispanic White participants. Importantly, these survival differences existed even within clinical trial populations that ostensibly received standardized initial cancer care.
Prior work within the National Comprehensive Cancer Network Breast Cancer Outcomes Database and in the Carolina Breast Cancer Study demonstrated that racial disparities vary by tumor subtype, with differences in survival between non-Hispanic Black and non-Hispanic White patients with HR+ BC and no differences for patients with triple negative or ERBB2+ BC.17,38 However, these patients were not exclusively clinical trial participants. In contrast, there have been other analyses of survival differences in HR+ BCs within adjuvant clinical trials; however, unlike the present study, Hispanic patients and other race and ethnicity groups besides non-Hispanic Black and non-Hispanic White have not been specifically examined in these studies.39,40 We would advocate for the dedicated study of these patients in gaining understanding of disparities facing patients with HR+ BC and have included these populations in our analyses.
There are multiple plausible explanations for the differences observed between non-Hispanic Black and non-Hispanic White participants with HR+/ERBB2− BC in other studies and suggested in our study.41 Black patients are less likely to receive endocrine therapy, and some observational data suggests lower rates of adherence to endocrine therapy.40,42,43 Similarly, Black patients with HR+ BC are reported to have lower estrogen receptor staining levels.44 To better understand and address these factors, some investigators call for further biological delineation of HR+ subtype, evaluation of social determinants of health, and investigation into ancestry and country of origin in future studies.41,45
We found that, overall, young non-Hispanic Black and Hispanic patients have worse OS than young non-Hispanic White patients. It is reported that non-Hispanic Black and Hispanic women are more likely to be diagnosed with BC at a young age compared with non-Hispanic White women.7,46 While we identified differences in OS for young non-Hispanic Black and Hispanic women compared with non-Hispanic White women, there was no association between race and ethnicity and RFS in these groups. One possible explanation for this may be related to comorbidities: young BC survivors, who typically have longer life expectancies than older survivors if cured from BC, are at increased risk of developing cardiac toxic effects, depression, and secondary cancers.47 Disparities in treatment of these conditions, as well as other non–cancer-related comorbidities, may be driving the racial and ethnic disparities in OS but not RFS in younger patients. Additionally, while sample size was small, we observed that among older participants with HR+/ERBB2− BCs, Hispanic participants had more than 6 times worse RFS than non-Hispanic White participants. Both extremes of age and Hispanic ethnicity have been associated with reduced adherence to endocrine therapy, which may be a contributor.18
It is unclear why Hispanic participants in this study had significantly worse RFS than non-Hispanic White participants if they had overweight, but there was no such association in patients with obesity. Obesity has been associated with increased BC mortality in several studies, although more recent studies suggest that this association holds in White women but not in Black women.24,25,48,49,50,51 This is consistent with our data in the clinical trial setting and suggests that differences in obesity rates by race likely do not account for racial and ethnic disparities in BC survival.52 Of note, Hispanic ethnicity is underreported and underrepresented in clinical trials, and future studies with improved inclusion of Hispanic patients are necessary to confirm these findings.26,53
The strengths of this pooled analysis of clinical trials, including prospective collection of survival data with long follow-up, are particularly important given the outcomes of RFS and OS. Within each clinical trial, participants received similar initial treatment, and outcomes have been well ascertained. In addition to adjustment for baseline clinicopathologic risk factors, our analysis was adjusted for chemotherapy treatment received, thereby facilitating study of other factors associated with survival. By studying racial and ethnic disparities in survival in the clinical trial setting, which standardized initial care and treatment, our results emphasize that other factors, such as inequities in subsequent treatment, survivorship care, or biological differences, may contribute to these disparities. As such, this study extends the understanding of potential contributors to disparities in BC survival. We hope this will inform ongoing efforts to mitigate disparities in BC survival, such as patient navigation programs and the GETSET study54 (NCT04379570), as well as motivate the development of future interventions aimed at improving racial and ethnic inequities in long-term care after BC.55,56
Limitations
This study has limitations. Despite the pooled nature of this analysis, there are small sample sizes within certain strata. For example, small numbers of very young patients precluded our ability to specifically study women under the age of 40 years, a group that has been shown to exhibit differences in tumor genomics and worse BC outcomes. It is possible that such biologic heterogeneity affected outcomes in the group of women younger than 50 years.17,57,58,59 The small sample sizes within certain subgroups, such as Hispanic participants and those with underweight BMI, likely impacts the precision of our findings. To mitigate this risk and limit the number of statistical comparisons made, we provide P values only for global tests comparing the main variables (race and ethnicity, age, tumor subtype, and BMI) rather than comparison of the many different levels of a variable. In the present study, it is possible that the global race and ethnicity variable was not significantly associated with RFS within any subtype due to sample size or the relatively low proportion of non-Hispanic Black and Hispanic participants, populations that are underrepresented in oncology clinical trials.26,60,61 Importantly, in this and many other ways, the clinical trial population does not represent typical clinical settings, and this may affect the generalizability of these data. Additionally, there was no adjustment made for multiple comparisons other than using a global test for the main variables of interest. However, throughout the manuscript, we report the testing that was done and provide estimates of observed effects.
Information on cause of death could not be reliably ascertained from all 4 of the included studies and therefore we were unable to examine differences in BC-specific survival. Additionally, as specified previously, there is not documentation of how race and ethnicity were collected for participants in every trial, which may have led to misclassification of race and ethnicity in some participants.
Conclusions
In this cohort study of clinical trial participants treated for eBC, we observed worse survival among Black or Hispanic participants within subgroups defined by age, BMI, or tumor subtype. These data suggest that, in addition to addressing the social and structural factors that contribute to racial and ethnic disparities overall, it may be necessary to identify and address subgroup-specific mechanisms underlying the observed associations. It is critical to evaluate specific contributors to racial and ethnic disparities in survival as these may inform future interventions to improve these disparities.
eMethods. Supplemental Methods
eFigure 1. Kaplan-Meier Estimates of Recurrence-Free Survival in Breast Cancer Subtype by Race and Ethnicity
eFigure 2. Kaplan-Meier Estimates of Overall Survival in Breast Cancer Subtype by Race and Ethnicity
eFigure 3. Kaplan-Meier Estimates of Recurrence-Free Survival in Age Category by Race and Ethnicity
eFigure 4. Kaplan-Meier Estimates of Overall Survival in Age Category by Race and Ethnicity
eFigure 5. Kaplan-Meier Estimates of Recurrence-Free Survival in Body Mass Index (BMI) Category by Race and Ethnicity
eFigure 6. Kaplan-Meier Estimates of Overall Survival in Body Mass Index (BMI) Category by Race and Ethnicity
eReferences.
Data Sharing Statement
References
- 1.Jatoi I, Sung H, Jemal A. The emergence of the racial disparity in U.S. breast-cancer mortality. N Engl J Med. 2022;386(25):2349-2352. doi: 10.1056/NEJMp2200244 [DOI] [PubMed] [Google Scholar]
- 2.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and White American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24(9):1342-1349. doi: 10.1200/JCO.2005.03.3472 [DOI] [PubMed] [Google Scholar]
- 3.Hines RB, Johnson AM, Lee E, Erickson S, Rahman SMM. Trends in breast cancer survival by race-ethnicity in Florida, 1990-2015. Cancer Epidemiol Biomarkers Prev. 2021;30(7):1408-1415. doi: 10.1158/1055-9965.EPI-20-1746 [DOI] [PubMed] [Google Scholar]
- 4.Nnorom SO, Akinyemi O, Tran J, et al. Color or money?: the impact of socioeconomic status and race/ethnicity on breast cancer mortality. Am J Surg. 2022;224(6):1403-1408. doi: 10.1016/j.amjsurg.2022.07.013 [DOI] [PubMed] [Google Scholar]
- 5.Prakash O, Hossain F, Danos D, Lassak A, Scribner R, Miele L. Racial disparities in triple negative breast cancer: a review of the role of biologic and non-biologic factors. Front Public Health. 2020;8:576964. doi: 10.3389/fpubh.2020.576964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azin A, Tahmasebi H, Brar A, et al. Racial, ethnic and socioeconomic disparities in diagnosis, treatment, and survival of patients with breast cancer. Am J Surg. 2023;225(1):154-161. doi: 10.1016/j.amjsurg.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 7.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313(2):165-173. doi: 10.1001/jama.2014.17322 [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 9.Goel N, Yadegarynia S, Lubarsky M, et al. Racial and ethnic disparities in breast cancer survival: emergence of a clinically distinct Hispanic Black population. Ann Surg. 2021;274(3):e269-e275. doi: 10.1097/SLA.0000000000005004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004-2006. J Clin Oncol. 2010;28(27):4135-4141. doi: 10.1200/JCO.2009.27.2427 [DOI] [PubMed] [Google Scholar]
- 11.Nurgalieva ZZ, Franzini L, Morgan RO, Vernon SW, Liu CC, Du XL. Impact of timing of adjuvant chemotherapy initiation and completion after surgery on racial disparities in survival among women with breast cancer. Med Oncol. 2013;30(1):419. doi: 10.1007/s12032-012-0419-1 [DOI] [PubMed] [Google Scholar]
- 12.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2016;2(3):322-329. doi: 10.1001/jamaoncol.2015.3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albain KS, Unger JM, Crowley JJ, Coltman CA Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101(14):984-992. doi: 10.1093/jnci/djp175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warner ET, Tamimi RM, Hughes ME, et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33(20):2254-2261. doi: 10.1200/JCO.2014.57.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163(1):49-56. doi: 10.1001/archinte.163.1.49 [DOI] [PubMed] [Google Scholar]
- 16.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat. 2011;127(3):729-738. doi: 10.1007/s10549-010-1191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Partridge AH, Hughes ME, Warner ET, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34(27):3308-3314. doi: 10.1200/JCO.2015.65.8013 [DOI] [PubMed] [Google Scholar]
- 18.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602-606. doi: 10.1200/JCO.2003.07.071 [DOI] [PubMed] [Google Scholar]
- 19.Sedrak MS, Sun C-L, Ji J, et al. Low-intensity adjuvant chemotherapy for breast cancer in older women: results from the prospective multicenter HOPE Trial. J Clin Oncol. 2023;41(2):316-326. doi: 10.1200/JCO.22.01440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208(3):341-347. doi: 10.1016/j.jamcollsurg.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One. 2009;4(11):e7695. doi: 10.1371/journal.pone.0007695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman RA, Keating NL, Lin NU, et al. Breast cancer-specific survival by age: worse outcomes for the oldest patients. Cancer. 2018;124(10):2184-2191. doi: 10.1002/cncr.31308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marret H, Perrotin F, Bougnoux P, et al. Low body mass index is an independent predictive factor of local recurrence after conservative treatment for breast cancer. Breast Cancer Res Treat. 2001;66(1):17-23. doi: 10.1023/A:1010699912768 [DOI] [PubMed] [Google Scholar]
- 24.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378-397. doi: 10.3322/caac.21405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol. 2016;34(35):4203-4216. doi: 10.1200/JCO.2016.68.4480 [DOI] [PubMed] [Google Scholar]
- 26.Keegan G, Crown A, DiMaggio C, Joseph KA. Insufficient reporting of race and ethnicity in breast cancer clinical trials. Ann Surg Oncol. Published online September 1, 2023. doi: 10.1245/s10434-023-14201-z [DOI] [PubMed] [Google Scholar]
- 27.Muss HB, Berry DA, Cirrincione CT, et al. ; CALGB Investigators . Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055-2065. doi: 10.1056/NEJMoa0810266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez EA, Suman VJ, Davidson NE, et al. Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol. 2011;29(34):4491-4497. doi: 10.1200/JCO.2011.36.7045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shulman LN, Berry DA, Cirrincione CT, et al. Comparison of doxorubicin and cyclophosphamide versus single-agent paclitaxel as adjuvant therapy for breast cancer in women with 0 to 3 positive axillary nodes: CALGB 40101 (Alliance). J Clin Oncol. 2014;32(22):2311-2317. doi: 10.1200/JCO.2013.53.7142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21(8):1431-1439. doi: 10.1200/JCO.2003.09.081 [DOI] [PubMed] [Google Scholar]
- 31.Zierle-Ghosh A, Jan A. Physiology, Body Mass Index. StatPearls Publishing LLC; 2023. [PubMed] [Google Scholar]
- 32.Tolaney SM, Garrett-Mayer E, White J, et al. Updated standardized definitions for efficacy end points (STEEP) in adjuvant breast cancer clinical trials: STEEP version 2.0. J Clin Oncol. 2021;39(24):2720-2731. doi: 10.1200/JCO.20.03613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shuster JJ. Median follow-up in clinical trials. J Clin Oncol. 1991;9(1):191-192. doi: 10.1200/JCO.1991.9.1.191 [DOI] [PubMed] [Google Scholar]
- 34.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 35.Cox DR. Regression models and life-tables. J Royal Stat Soc. Series B (Methodological). 1972;34(2):187-220. [Google Scholar]
- 36.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 2nd ed. John Wiley & Sons; 1981. [Google Scholar]
- 37.Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ. 2001;322(7300):1479-1480. doi: 10.1136/bmj.322.7300.1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16(24):6100-6110. doi: 10.1158/1078-0432.CCR-10-1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim G, Pastoriza JM, Qin J, et al. Racial disparity in distant recurrence-free survival in patients with localized breast cancer: a pooled analysis of National Surgical Adjuvant Breast and Bowel Project trials. Cancer. 2022;128(14):2728-2735. doi: 10.1002/cncr.34241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadigh G, Gray RJ, Sparano JA, et al. Assessment of racial disparity in survival outcomes for early hormone receptor-positive breast cancer after adjusting for insurance status and neighborhood deprivation: a post hoc analysis of a randomized clinical trial. JAMA Oncol. 2022;8(4):579-586. doi: 10.1001/jamaoncol.2021.7656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones VC, Kruper L, Mortimer J, Ashing KT, Seewaldt VL. Understanding drivers of the Black:White breast cancer mortality gap: a call for more robust definitions. Cancer. 2022;128(14):2695-2697. doi: 10.1002/cncr.34243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lund MJ, Brawley OP, Ward KC, Young JL, Gabram SS, Eley JW. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109(3):545-557. doi: 10.1007/s10549-007-9675-8 [DOI] [PubMed] [Google Scholar]
- 43.Reeder-Hayes KE, Jackson BE, Baggett CD, et al. Race, geography, and risk of breast cancer treatment delays: a population-based study 2004-2015. Cancer. 2023;129(6):925-933. doi: 10.1002/cncr.34573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purrington KS, Gorski D, Simon MS, et al. Racial differences in estrogen receptor staining levels and implications for treatment and survival among estrogen receptor positive, HER2-negative invasive breast cancers. Breast Cancer Res Treat. 2020;181(1):145-154. doi: 10.1007/s10549-020-05607-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine—a time for reckoning with racism. N Engl J Med. 2021;384(5):474-480. doi: 10.1056/NEJMms2029562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero-Cordoba SL, Salido-Guadarrama I, Rebollar-Vega R, et al. Comprehensive omic characterization of breast cancer in Mexican-Hispanic women. Nat Commun. 2021;12(1):2245. doi: 10.1038/s41467-021-22478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tesch ME, Partridge AH. Treatment of breast cancer in young adults. Am Soc Clin Oncol Educ Book. 2022;42(42):1-12. doi: 10.1200/EDBK_360970 [DOI] [PubMed] [Google Scholar]
- 48.Lu Y, Ma H, Malone KE, et al. Obesity and survival among Black women and White women 35 to 64 years of age at diagnosis with invasive breast cancer. J Clin Oncol. 2011;29(25):3358-3365. doi: 10.1200/JCO.2010.34.2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Lu W, Zheng W, et al. Obesity and weight change in relation to breast cancer survival. Breast Cancer Res Treat. 2010;122(3):823-833. doi: 10.1007/s10549-009-0708-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caan BJ, Kwan ML, Hartzell G, et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19(10):1319-1328. doi: 10.1007/s10552-008-9203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901-1914. doi: 10.1093/annonc/mdu042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549-1555. doi: 10.1001/jama.295.13.1549 [DOI] [PubMed] [Google Scholar]
- 53.Bazan JG, Obeng-Gyasi S, Gamez ME. Reporting of race and Hispanic ethnicity in breast cancer studies from the National Cancer Database. JAMA Oncol. 2022;8(10):1507-1509. doi: 10.1001/jamaoncol.2022.3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Get Set. Accessed September 25, 2023. https://getsetstudy.org/
- 55.Sheppard VB, Isaacs C, Luta G, et al. Narrowing racial gaps in breast cancer chemotherapy initiation: the role of the patient-provider relationship. Breast Cancer Res Treat. 2013;139(1):207-216. doi: 10.1007/s10549-013-2520-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simon MA, Trosman JR, Rapkin B, et al. Systematic patient navigation strategies to scale breast cancer disparity reduction by improved cancer prevention and care delivery processes. JCO Oncol Pract. 2020;16(12):e1462-e1470. doi: 10.1200/JOP.19.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waks AG, Kim D, Jain E, et al. Somatic and germline genomic alterations in very young women with breast cancer. Clin Cancer Res. 2022;28(11):2339-2348. doi: 10.1158/1078-0432.CCR-21-2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luen SJ, Viale G, Nik-Zainal S, et al. Genomic characterisation of hormone receptor-positive breast cancer arising in very young women. Ann Oncol. 2023;34(4):397-409. doi: 10.1016/j.annonc.2023.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guzmán-Arocho YD, Rosenberg SM, Garber JE, et al. Clinicopathological features and BRCA1 and BRCA2 mutation status in a prospective cohort of young women with breast cancer. Br J Cancer. 2022;126(2):302-309. doi: 10.1038/s41416-021-01597-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loree JM, Anand S, Dasari A, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5(10):e191870-e191870. doi: 10.1001/jamaoncol.2019.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aldrighetti CM, Niemierko A, Van Allen E, Willers H, Kamran SC. Racial and ethnic disparities among participants in precision oncology clinical studies. JAMA Netw Open. 2021;4(11):e2133205. doi: 10.1001/jamanetworkopen.2021.33205 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eFigure 1. Kaplan-Meier Estimates of Recurrence-Free Survival in Breast Cancer Subtype by Race and Ethnicity
eFigure 2. Kaplan-Meier Estimates of Overall Survival in Breast Cancer Subtype by Race and Ethnicity
eFigure 3. Kaplan-Meier Estimates of Recurrence-Free Survival in Age Category by Race and Ethnicity
eFigure 4. Kaplan-Meier Estimates of Overall Survival in Age Category by Race and Ethnicity
eFigure 5. Kaplan-Meier Estimates of Recurrence-Free Survival in Body Mass Index (BMI) Category by Race and Ethnicity
eFigure 6. Kaplan-Meier Estimates of Overall Survival in Body Mass Index (BMI) Category by Race and Ethnicity
eReferences.
Data Sharing Statement