Abstract
Numerous disabling motor and non-motor symptoms occur during Parkinson’s disease (PD), including speech disorders, often referred to as hypokinetic dysarthria. PD is the most common cause of this type of dysarthria. About 90% of PD patients experience hypokinetic dysarthria, which is exacerbated as the disease progresses and makes it very difficult for other people to understand the person with PD. This disorder is characterized by a monotonous speech pattern, reduced and monotonous loudness, decreased stress, a breathy or hoarse voice quality, an increase in speech rate, rapid repetition of phonemes, and impreciseness in consonant production. However, patients may also have sensory symptoms including inaccurate perceptions of their own loudness and decreased awareness of speech problems. Hypokinetic dysarthria in PD may not only result from dopamine degeneration in the nigrostriatal pathway but also from disturbances in the motor and somatosensory systems. All speech components, such as phonation, articulation, respiration, resonance, and prosody should be assessed carefully in PD patients with hypokinetic dysarthria. Taking medical history, an oral motor assessment, a perceptual evaluation of speech characteristics, intelligibility, efficiency, and participation in communication all need to be a part of the assessment. The tasks of maximum phonation time, diadochokinetic rate, reading sentences, words, and passages, describing pictures, and spontaneous speech are used to assess the features of speech components and intelligibility. The evaluation should include physiological, acoustic, or imaging modalities as well. Speech therapy is typically the main treatment of speech problems in PD. The management of PD-related hypokinetic dysarthria basically focuses on speaker-oriented and communication-oriented strategies. In addition to these strategies, Augmentative Alternative Communication (AAC) should be considered in patients with severe dysarthria. Loudness, intelligibility, and sound perception may all significantly improve with the Lee Silverman Voice Therapy LOUD (LSVT LOUD) program which is an evidence-based program. The beneficial effect of pharmacological and surgical treatment approaches has not been proven in improving speech. Deep brain stimulation may carry the risk of the deterioration of speech as the illness progresses.
Keywords: Assessment, hypokinetic dysarthria, Parkinson’s disease, speech disorders, treatment
The most prevalent form of Parkinsonism, Parkinson’s disease (PD), is caused by the degeneration of dopamine-producing neurons in the substantia nigra.[1] There are numerous motor symptoms in PD.[2] Hypokinetic dysarthria is a commonly used term for speech disorder that is one of these motor symptoms. Although this disorder is seen in most of the patients in the later stages, it can also occur in the early period.[3] It can have a detrimental impact on an individual’s quality of life by leading to social isolation, communication, and participation challenges.[4-6] Despite all these, it has been reported that few of patients sought to speech and language therapy.[7] The individuals’ lack of awareness about speech difficulties or the lack of speech and language therapists (SLT) in the region is two contributing factors to this situation. This study aims to give speech-language therapists and candidates a broad perspective by synthesizing information on the pathophysiology, assessment, and intervention of hypokinetic dysarthria associated with PD, to guide clinical applications, and to raise awareness of other health professionals working collaboratively.
Hypokinetic Dysarthria in Parkinson’s Disease
Parkinson’s disease is the most common cause of hypokinetic dysarthria.[1] 89% of patients with PD experience some form of speech disorder, which becomes more severe as the illness progresses and causes significant difficulties in communicating with others.[6,8,9] Dysarthria is a motor speech disorder characterized by “abnormalities in the range, speed, strength, steadiness, accuracy or tone of movements required for speech production.” The phonatory, articulatory, respiratory, and prosodic components of speech are commonly observed to be impaired in hypokinetic dysarthria.[10] Thus, speech disorders that are typically observed in PD include reduced loudness and stress, monoloudness, monopitch, breathy and hoarse voice, rapidly repeated phonemes, tendency for rapid or accelerated rate, imprecise articulation, respiratory deficiencies, and short utterances.[10-12] In individuals, rigidity, a limited range of motion, but occasionally rapid, repetitive movements, can be observed.[10]
Reduced vocal volume, monoloudness, monopitch, breathiness, and harsh voice quality are symptoms of phonatory impairments in individuals who have PD.[10] Individuals may also have voice tremor.[13] Hypophony is the most prominent characteristic of hypokinetic dysarthria in PD.[8] Hypophonia’s perceptual characteristics include reduced loudness and breathy voice quality.[14] Hypophonia is frequently accompanied by pharyngeal, laryngeal, and thoracic muscle rigidity, which reduces respiratory support, weakens vocal fold adduction, and affects vocal resonance.[13]. Impaired self-monitoring and awareness related to reduced loudness may limit an individual with PD ability to increase loudness.[13]
Symptoms of articulation difficulties in individuals with PD are reduced range of motion and reduced articulatory contact, which results in imprecise consonant sounds and repeated phonemes.[10] In addition, speaking rapidly distorts how consonants are produced.[15]
Speech i.e., nonfluent, monoloud, monopitch, and rapid is evidence of prosodic disturbances. Blockages or delayed, repetitive vowel phonemes are signs of speech initiation problems, and unnaturally prolonged pauses throughout the process affect the speech fluency, similar to the freezing of walking, freezing of voice-speech can also be seen in this situation.[13,16] Some individuals have short rashes of speech.[10] These are related to attempting to produce longer utterances while providing less support for the breath, having vague articulatory contact, and employing a reduced range of motion.[13]
Individuals with PD may experience some respiratory problems as a result of a decrease in vital capacity.[15] A reduced respiratory cycle or reduced respiratory range of motion may result in shallow breath support for speech production.[15]
Dysarthria in PD has a detrimental impact on individuals’ lives and their ability to communicate.[5,17] According to Miller et al. (2006)[5], communication problems in individuals with PD have a direct impact on socialization and cause anxiety and isolation in social situations. These undesirable conditions are not brought about by dysarthria alone. Decreased levels of communicative involvement have been linked to negative judgments of speech disorders, reduced speech, exhaustion, linguistic, cognitive, and emotional issues, and dysphagia. Despite the fact that many individuals had favorable experiences with speech therapy, some individuals felt that the social aspects of communication were not adequately addressed by speech interventions. It was stressed as a result that it is crucial to emphasize on not only speech components but also linguistic-cognitive demands and psychosocial aspects of communication.[18]
Pathophysiology of Hypokinetic Dysarthria in PD
Dopaminergic and non-dopaminergic pathways have been suggested as potential contributors to the pathophysiology of hypokinetic dysarthria in PD. PD’s motor symptoms, including speech abnormalities, emerge when the nigrostriatal route from the substantia nigra to the striatum is denervated.[19] The Cortico-Basal Ganglia-Cerebellar circuit has been associated with this neural pathway, according to various studies.[19] The three main cerebrocortical dysfunctions during a speech in PD are over-activation of the bilateral dorsolateral prefrontal cortex and the rostral part of the pre-supplementary motor area inhibition of the primary motor cortex and cerebellum.[19] These variances have led to hypokinetic dysarthria being linked to motor symptoms such as bradykinesia, rigidity, hypokinesia, and tremor.[20]
Non-dopaminergic mechanisms are another underlying cause of hypokinetic dysarthria in PD. The impairment of error detection or repair systems in the processing of sensory feedback may potentially be related to the impairment in voice control in individuals.[3,21] Speech perception difficulties are a sign of somatosensory input processing issues in the basal ganglia.[8,22] Poor control of speech production, particularly reduced vocal loudness, may be explained by the inadequate perception of speech volume and impaired sensory processing.[3,22,23] Individuals can increase their speech volume when direct auditory cues for loudness are presented.[20]
Assessment of Hypokinetic Dysarthria in PD
Assessment is the process of gathering reliable and accurate data regarding a situation, reaching a decision about the subject, and using the data to make conclusions.[24] A good dysarthria assessment should include formal and informal assessment methods. A case history analysis, observation, symptom identification, and data evaluation are used to perform an informal assessment.[25] Formal tests, often known as standardized tests, are assessments that include standardized administration and scoring methods.[24] Based on these methods, a protocol for PH-associated hypokinetic dysarthria assessment can be developed. The assessment protocol should focus on sensory-motor problems that impair speech components such phonation, articulation, respiration, resonance and prosody. Case history, standardized tests, orofacial examination, the maximal phonation time, the diadochokinetic rate (DDK), and other necessary elements such as recording of speech samples (spontaneous speech, reading, picture description, and repetition), intelligibility and consistency analysis, instrumental evaluation (phonatory aerodynamic system, videostroboscopy, nasometer, and acoustic measurements), having information related to individual-caregiver needs, demands and priorities, etc., (for evaluating psychosocial influence, participation in communication, communication effectiveness) and also evaluation of accompanying swallowing, cognitive-linguistic problems should all be included in a comprehensive assessment.[15,26]
The perceptual analysis method is the approach most frequently used in the assessment of motor speech disorders. However, the assessment should also take other approaches into account. A comprehensive assessment of the acoustic, laryngeal, and aerodynamic systems should be conducted as part of the assessment of dysarthria.[10,27,28] To ensure the reliability of the acoustic assessment, the guideline prepared by Rusz et al. (2021) states that the recording setting, recording procedure, and acoustic data should be appraised according to certain standards. The assessment should just not limit its emphasis to speech-only components. The assessment should also take into consideration the individual’s perception of how their speech impairment affects their ability to participate in communication during daily activities and their speech intelligibility.[10,28,29]
A case history may be the first step in assessing hypokinetic dysarthria in individuals with PD. The presence of any concurrent conditions and the drugs prescribed should be questioned, in addition to the case history’s demographic profiles, and details regarding any visual, hearing, swallowing, saliva control, language, and cognitive problems. Consideration should also be given to the individual’s expectations for therapy and their needs for communication.[10,29]
The structural and functional integrity of the orofacial mechanism can be assessed after gathering the case history. Orofacial symmetry and oral motor abilities are assessed via a cranial nerve examination.[29] The control of involuntary movements is part of the oral motor examination, along with the symmetry of the face, lips, tongue, jaw, and velum at rest. Accuracy, range, strength, continuity, and speed are the main considerations while performing some tasks. Examination of reflexes can also be performed at this stage.[10]
The individual’s maximum phonation time is used to assess the individual’s voice quality, velar movement, and respiratory support.[1,10] Individuals are told to take a deep breath and maintain the/a/phoneme for as long as they can, without straining, at a comfortable loudness and pitch on one breath.[28]
DDK is the measurement of an individual’s ability to perform rapid alternating muscle movements in a repetitive motion.[1] This test also assesses the rate and regularity of consonant-vowel repetitions (/pʌ/, /tʌ/, /kʌ/ or /pʌtʌkʌ/).[28]. Additional characteristics that can be investigated include a range of motion, imprecise phoneme production, loudness, pitch, nasal emission, hypernasality, rhythm, and pause.[1] Sequential and changing movement rates are assessed as part of the DDK. The individual should be instructed to take a deep breath in and repeat the/pʌtʌkʌ/syllables as rapidly and precisely as they can until instructed to stop. There are 12 repetitions required, and the task must be performed in one breath. The individual should be told to repeat the/pʌpʌpʌ/ syllables as quickly as they can until instructed to stop for alternate rates of movement. The task must be performed in one breath and for five seconds. Alternatively, syllable repetitions /tʌ/ or /kʌ/ can be used.[28]
The patient is instructed to count, read aloud, and speak spontaneously while their speech production is being perceptually evaluated. These tasks are used to assess the patients’ nasal emission or hypernasality, any changes in loudness or pitch, accentuation, pauses, accuracy, and speed of speech output.[29] For these tasks, reading recommendations range from 80 to 120 words printed in 24-point size. Individuals should be asked to talk about a topic of their choice, such as their hobbies, hometown, family, or early years. A minimum of 60–90 seconds should be assigned for the monologue. Picture description is a common alternative. Riddles or singing should be avoided.[28] These tasks are meant to ensure accuracy/consistency in acoustic analysis.
Acoustic assessments are performed in addition to perceptual assessment. Praat and Computerized Speech Lab (CSL, model 4500; KayPENTAX, Lincoln Park, NJ) are two of the most used software tools for clinically assessing both speech and voice performance.[30] For the acoustic assessment, a quiet room and a steady chair should be chosen. In addition, the audio signal’s quality should be examined (avoiding artifacts at 50 Hz), and ventilation sources such as open windows, doors, or air conditioners should be eliminated. A predetermined distance of 4–10 cm should separate the microphone from the lips and it should be positioned at a 45–90° angle from the front of the mouth. For individuals with hypokinetic dysarthria, a minimum phonation time of 6 seconds is optimal in acoustic analysis. 14 variables should be considered in the analysis, such as the fundamental frequency, shimmer, jitter, harmonic-to-noise ratio, DDK, and voice onset time.[27,28]
A variety of tests are used to examine speech difficulties in PD; The Movement Disorder Society Unified Parkinson’s Disease Rating Scale is one of these tests (MDS-UPDRS). Parts II and III of this test, which is administered by neurologists, include questions about speech difficulties. Part II of the test questions the individual’s awareness of their speech, and Part III of the test includes a question that the doctor will score relying on the individual’s speech.[31] Based on the results, a speech and language therapist may be consulted to conduct a comprehensive assessment to consider any speech impairment. SLT commonly use the Frenchay Dysarthria Assessment to assess speech problems in individuals with dysarthria. The Robertson Dysarthria Profile is also used.[32] A specific assessment of intelligibility can be obtained by the listener transcribing the speaker’s speech and dividing the number of correctly understood words by the number of sentences.[33,34] One of the tests that make use of this mathematical method is the Assessment of Intelligibility of Dysarthric Speech.[33] Tests are required to determine how dysarthria affects individuals’ psychological and social well-being. For instance, the Voice Handicap Index evaluates the amount of functional, emotional, and physical problems individuals have with their voices.[35] In addition, the Dysarthria Impact Profile was developed to assess how dysarthria affected the individual’s social and psychological well-being.[36]
Individuals’ communication participation and communication effectiveness are both impacted by speech problems. The Communicative Effectiveness Survey-Revised, which is specifically developed for PD, and the Communicative Participation Item Bank, can be used to assess the above.[37,38] Individuals can then identify the communication situations in which they struggle the most.
The International Classification of Functioning, Disability, and Health (ICF) standards must be followed during evaluation. Evaluation carried out within the scope of ICF will serve as a guide in terms of determining the nature and severity of hypokinetic dysarthria, defining the disorder, figuring out how it affects one’s quality of life, health, and socialization, providing prognostic information and planning the intervention.[25]
Intervention of Hypokinetic Dysarthria in PD
Hypokinetic dysarthria can be managed with intervention for restorative or compensatory purposes. Speech therapy, pharmacotherapy, and surgical procedures such as deep brain stimulation (DBS), thalamotomy, pallidotomy, and vocal cord augmentation are all potential treatments available for hypokinetic dysarthria caused by PD.
Speech Language Therapy
There is currently a lack of access to speech therapy for the treatment of hypokinetic dysarthria, which is present in the majority of individuals with PD.[39] Speech therapy should be delivered more frequently and earlier.[3] The goal of the intervention is to improve individuals’ communication in all PD processes so that the underlying problems can be addressed, and individuals can engage in family and social life.[13] The management of PD-related hypokinetic dysarthria mainly focuses on speaker-oriented and communication-oriented strategies. In addition to these strategies, Augmentative Alternative Communication (AAC) should be considered in patients with severe dysarthria.
Speaker-oriented strategies basically include traditional dysarthria therapies and standardized interventions. Speaker-oriented strategies use the methods for improving intelligibility. Exercises used in traditional dysarthria therapy for individuals who have PD include those that improve phonation, articulation, breathing, and prosody abilities.[40] Depending on the extent of the effect on the person, each component can be handled separately. Exercises such as “a” phonation as long and loudly as possible, prolonged “a” phonation by changing the pitch, and instrumental biofeedback may be advised for the phonation component.[1] Over-articulation and intelligibility drills may be suggested for the articulation component.[1] Pacing boards, intonation profiles, contrastive stress drills, and chunking utterances into syntactic units can be considered for the prosody component.[1,10] Speaking shortly after exhaling, ceasing phonation early, and the optimal breath group are all advised for the respiratory component.[1]
As standardized speaker-oriented strategies, it is possible to execute an intervention that uses exercise-focused approaches such as Lee Silverman Voice Therapy LOUD (LSVT LOUD), SPEAKOUT! ® or Expiratory Muscle Strength Training (EMST). Interventions that emphasize re-amplification, self-monitoring, feedback, and attention to effort are the main approaches.[13] An effective, certified, and standardized intervention approach for speech therapy in PD is called LSVT LOUD.[41] 1-h intensive sessions take place 4 times a week for 4 weeks.[41] Although LSVT LOUD is a widely used therapy method, the homework assignments and generalization exercises are customized to each individual in order to improve motivation, engagement, and neuroplasticity.[42] The most effective treatments for hypophonia have been demonstrated to be intensive ones, such LSVT, and it has been noted that the loudness is maintained for at least 2 years following the treatment.[43] Following LSVT and respiratory therapy, individuals with PD exhibit less monopitch in terms of pitch. Consequently, LSVT can result in the generalization of positive effects across the entire speech production system (including voice, intonation, articulation, and rate).[43] The Parkinson’s Voice Project SPEAKOUT! ® method of loudness is another highly effective and widely used method. It is a brand-new form of therapy that may be used with either one-on-one or group support and involves raising the cognitive load.[44] However, it was discovered that EMST was successful in enhancing speech breathing.[12] Contrarily, traditional speech treatment tends to focus on more speech components, is low-intensity, and does not systematically address the sensory processing issues linked to the self-perception of loudness of individuals with PD.[42] Rate control is one of them, and it can improve listener perceptions, especially when used in combination with a loud voice.[13] There is a need for further randomized controlled trials despite the fact that speech therapy is effective for hypokinetic dysarthria in PD.
Communication-oriented strategies are often used along with speaker-oriented strategies. Communication-oriented strategies aim to improve understanding between speaker and listener including the elimination of background noise, establishing eye contact and gesturing active listening, identification of the topic before beginning conversation and reducing communication distance.[45]
AAC treatment approaches including speech-generating devices or communication applications for portable devices may help in patients with the severe dysarthria.[46]
Pharmacological Treatments
Pharmacological treatments for hypokinetic dysarthria in PD are controversial in terms of their effectiveness.[3] Although it was stated that voice and speech parameters (such as speed, intensity of movement, and pitch ranges) may be favorably improved, it is concluded that the advantages did not include communication or intelligibility.[13] Furthermore, according to some researchers, long-term, high-dose levodopa treatment may impair speech fluency.[47]. Levodopa load may result in deteriorating speech, articulation, rhythm, intelligibility and rate.[3] The disease, length of treatment, and stage of the disease are the primary factors that affect how speech components react to L-dopa.[3]
Surgical Procedures
Thalamotomy, one of the surgical procedures, is no longer used since it has a negative impact on speech.[19] The positive and negative effects of posteroventral pallidotomy on hypokinetic dysarthria in PD are debatable.[8,48,49] Furthermore, motor limb impairment is more responsive to DBS than dysarthria. A number of speech problems can be brought on by stimulation of the target and nearby structures.[19] However, low frequency in traditional subthalamic nucleus DBS (STN-DBS) or adaptive DBS can be used to alter neurostimulation parameters to improve speech.[50] In conclusion, it seems that the potentially harmful synergistic effects of levodopa and STN-DBS happen over time and subsequently contribute to the deterioration of speech as the illness progresses.[3]
Conclusion
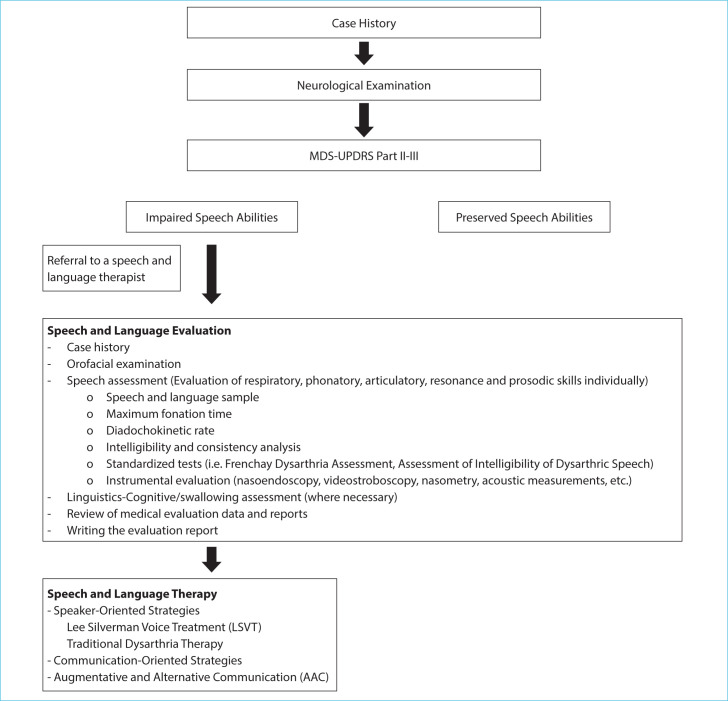
The speech components that are affected by hypokinetic dysarthria caused by PD must be determined in order to give an effective service. Assessments on the individual’s awareness of their experience of dysarthria are considered to be beneficial in addition to focusing on speech components. Individuals with PD with hypokinetic dysarthria should also be evaluated for their ability to engage in social and familial interactions through communication, too. Assessing language, cognition, emotional, and other factors that may affect communication in individuals with PD may be crucial for intervention planning, even though dysarthria is not the only condition that affects communication involvement abilities. The effect of surgical and pharmacological interventions on speech is a subject of debate. Behavioral approaches are still effective treatments for hypokinetic dysarthria, particularly the LSVT.[8] It is critical to not overlook the speech impairments that individuals with PD experience. A multidisciplinary approach should be used, and the therapy process should include caregivers or communication partners. In addition, environmental regulations are recommended. Since PD is a neurodegenerative illness, assessment and treatments might need to be administered and provided on a frequent basis. Fig. 1 shows the algorithmic approach for managing PD-related hypokinetic dysarthria.
Figure 1.
Algorithmic approach for managing PD-related hypokinetic dysarthria.
Footnotes
Please cite this article as ”Sapmaz Atalar M, Oguz O, Genc G. Hypokinetic Dysarthria in Parkinson’s Disease: A Narrative Review. Med Bull Sisli Etfal Hosp 2023;57(2):163–170”.
Disclosures
Peer-review
Externally peer-reviewed.
Conflict of Interest
None declared.
Authorship Contributions
Concept – M.S.A., O.O.; Design – G.G.; Supervision – G.G., O.O; Data collection &/or processing – M.S.A.; Analysis and/ or interpretation – M.S.A., O.O.; Literature search – M.S.A.; Writing – M.S.A., O.O., G.G.; Critical review – G.G.
References
- 1.Freed DB. 3rd ed. San Diego, CA: Plural Publishing; 2020. Motor Speech Disorders: Diagnosis and Treatment. [Google Scholar]
- 2.Schrag A, Horsfall L, Walters K, Noyce A, Petersen I. Prediagnostic presentations of Parkinson's disease in primary care: a case-control study. Lancet Neurol. 2015;14:57–64. doi: 10.1016/S1474-4422(14)70287-X. [DOI] [PubMed] [Google Scholar]
- 3.Moreau C, Pinto S. Misconceptions about speech impairment in Parkinson's disease. Mov Disord. 2019;34:1471–5. doi: 10.1002/mds.27791. [DOI] [PubMed] [Google Scholar]
- 4.McAuliffe MJ, Baylor CR, Yorkston KM. Variables associated with communicative participation in Parkinson's disease and its relationship to measures of health-related quality-of-life. Int J Speech Lang Pathol. 2017;19:407–7. doi: 10.1080/17549507.2016.1193900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller N, Noble E, Jones D, Burn D. Life with communication changes in Parkinson's disease. Age Ageing. 2006;35:235–9. doi: 10.1093/ageing/afj053. [DOI] [PubMed] [Google Scholar]
- 6.Trail M, Fox C, Ramig LO, Sapir S, Howard J, Lai EC. Speech treatment for Parkinson's disease. Neuro Rehabilitation. 2005;20:205–21. [PubMed] [Google Scholar]
- 7.Miller N, Noble E, Jones D, Deane KH, Gibb C. Survey of speech and language therapy provision for people with Parkinson's disease in the United Kingdom: individuals' and carers' perspectives. Int J Lang Commun Disord. 2011;46:179–88. doi: 10.3109/13682822.2010.484850. [DOI] [PubMed] [Google Scholar]
- 8.Dashtipour K, Tafreshi A, Lee J, Crawley B. Speech disorders in Parkinson's disease: pathophysiology, medical management and surgical approaches. Neurodegener Dis Manag. 2018;8:337–8. doi: 10.2217/nmt-2018-0021. [DOI] [PubMed] [Google Scholar]
- 9.Hartelius L, Svensson P. Speech and swallowing symptoms associated with Parkinson's disease and multiple sclerosis: a survey. Folia Phoniatr Logop. 1994;46:9–17. doi: 10.1159/000266286. [DOI] [PubMed] [Google Scholar]
- 10.Duffy JR. 3rd ed. St. Louis, MO: Mosby; 2013. Motor Speech Disorders: Substrates, Differential Diagnosis and Management. [Google Scholar]
- 11.American Speech-Language-Hearing Association (ASHA) Distinguishing Perceptual Characteristics and Physiologic Findings by Dysarthria Type. Available at: https://www.asha.org/practice-portal/clinical-topics/dysarthria-inadults/distinguishing-perceptual-characteristics/ Accessed Nov 20, 2022.
- 12.Darling-White M, Huber JE. The impact of expiratory muscle strength training on speech breathing in individuals with Parkinson's disease: a preliminary study. Am J Speech Lang Pathol. 2017;26:1159–66. doi: 10.1044/2017_AJSLP-16-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller N. Communication changes in Parkinson's disease. Pract Neurol. 2017;17:266–74. doi: 10.1136/practneurol-2017-001635. [DOI] [PubMed] [Google Scholar]
- 14.Lombard LE, Steinhauer KM. A novel treatment for hypophonic voice: twang therapy. J Voice. 2007;21:294–9. doi: 10.1016/j.jvoice.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Hegde MN, Freed D. 2nd ed. San Diego, CA: Plural Publishing; 2017. Assessment of Communication Disorders in Adults: Resources and Protocols. [Google Scholar]
- 16.Ricciardi L, Ebreo M, Graziosi A, Barbuto M, Sorbera C, Morgante L, et al. Speech and gait in Parkinson's disease: when rhythm matters. Parkinsonism Relat Disord. 2016;32:42–7. doi: 10.1016/j.parkreldis.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Walshe M, Miller N. Living with acquired dysarthria: the speaker's perspective. Disabil Rehabil. 2011;33:195–203. doi: 10.3109/09638288.2010.511685. [DOI] [PubMed] [Google Scholar]
- 18.Yorkston K, Baylor C, Britton D. Speech Versus Speaking: The Experiences of People with Parkinson's Disease and Implications for Intervention. Am J Speech Lang Pathol. 2017;26:561–8. doi: 10.1044/2017_AJSLP-16-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinto S, Ozsancak C, Tripoliti E, Thobois S, Limousin-Dowsey P, Auzou P. Treatments for dysarthria in Parkinson's disease. Lancet Neurol. 2004;3:547–56. doi: 10.1016/S1474-4422(04)00854-3. [DOI] [PubMed] [Google Scholar]
- 20.Ramig LO, Fox C, Sapir S. Speech treatment for Parkinson's disease. Expert Rev Neurother. 2008;8:297–309. doi: 10.1586/14737175.8.2.297. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Wang EQ, Metman LV, Larson CR. Vocal responses to perturbations in voice auditory feedback in individuals with Parkinson's disease. PloS One. 2012;7:e33629. doi: 10.1371/journal.pone.0033629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho AK, Bradshaw JL, Iansek T. Volume perception in parkinsonian speech. Mov Disord. 2000;15:1125–31. doi: 10.1002/1531-8257(200011)15:6<1125::aid-mds1010>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Huang X, Chen X, Yan N, Jones JA, Wang EQ, Chen L, et al. The impact of Parkinson's disease on the cortical mechanisms that support auditory-motor integration for voice control. Hum Brain Mapp. 2016;37:4248–61. doi: 10.1002/hbm.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shipley KG, McAfee JG. 6th ed. San Diego, CA: Plural Publishing; 2021. Assessment in Speech-Language Pathology: A Resource Manual. [Google Scholar]
- 25.Pindzola RH, Plexico LW, Haynes WO. 9th ed. New York: Pearson; 2015. Diagnosis and Evaluation in Speech Pathology. [Google Scholar]
- 26.Oğuz Ö. İstanbul: Nobel Tıp Kitabevleri; 2020. Motor Konuşma Bozuklukları Tanrıdağ O. Nörobilim ve Dil-Konuşma Bozuklukları. [Google Scholar]
- 27.Patel RR, Awan SN, Barkmeier-Kraemer J, Courey M, Deliyski D, Eadie T, et al. Recommended protocols for instrumental assessment of voice: American Speech-Language-Hearing Association expert panel to develop a protocol for instrumental assessment of vocal function. Am J Speech Lang Pathol. 2018;27:887–905. doi: 10.1044/2018_AJSLP-17-0009. [DOI] [PubMed] [Google Scholar]
- 28.Rusz J, Tykalova T, Ramig LO, Tripoliti E. Guidelines for speech recording and acoustic analyses in dysarthrias of movement disorders. Mov Disord. 2021;36:803–14. doi: 10.1002/mds.28465. [DOI] [PubMed] [Google Scholar]
- 29.Altaher AM, Chu SY, Kam RBM, Razak RA. A report of assessment tools for individuals with dysarthria. Open Public Health J. 2019;12:384–6. [Google Scholar]
- 30.Boersma P, Weenink D. Praat: doing phonetics by computer Version 6.1.08. 2019. Available at: http://www.praat.org/.AccessedNov20,2022.
- 31.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 32.Robertson SJ. Buckinghamshire: Winslow; 1982. Robertson Dysarthria Profile. [Google Scholar]
- 33.Yorkston KM, Beukelman DR. Tigard, OR: C.C. Publications; 1981. Assessment of Intelligibility of Dysarthric Speech. [Google Scholar]
- 34.Yorkston KM, Beukelman DR, Strand EA, Bell KR. 2nd ed. Austin, TX: Pro Ed; 1999. Management of Motor Speech Disorders in Children and Adults. [Google Scholar]
- 35.Jacobson BH, Johnson A, Grywalski C, Silbergleit A, Jacobson G, Benninger MS, et al. The voice handicap index (VHI) development and validation. Am J Speech Lang Pathol. 1997;6:66–70. [Google Scholar]
- 36.Walshe M, Peach RK, Miller N. Dysarthria impact profile: development of a scale to measure psychosocial effects. Int J Lang Commun Disord. 2009;44:693–715. doi: 10.1080/13682820802317536. [DOI] [PubMed] [Google Scholar]
- 37.Baylor C, Yorkston K, Eadie T, Kim J, Chung H, Amtmann D. The Communicative Participation Item Bank (CPIB): item bank calibration and development of a disorder-generic short form. J Speech Lang Hear Res. 2013;56:1190–208. doi: 10.1044/1092-4388(2012/12-0140). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donovan NJ. Examining the item-level psychometric properties of the communicative effectiveness survey-revised for people with Parkinson's disease and dysarthria. Clin Arch Commun Disord. 2018;3:42–51. [Google Scholar]
- 39.Schalling E, Johansson K, Hartelius L. Speech and communication changes reported by people with Parkinson's disease. Folia Phoniatr Logop. 2017;69:131–41. doi: 10.1159/000479927. [DOI] [PubMed] [Google Scholar]
- 40.Sackley CM, Smith CH, Rick C, Brady MC, Ives N, Patel R, et al. Lee Silverman voice treatment versus standard NHS speech and language therapy versus control in Parkinson's disease (PD COMM pilot): study protocol for a randomized controlled trial. Trials. 2014;15:213. doi: 10.1186/1745-6215-15-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramig L, Halpern A, Spielman J, Fox C, Freeman K. Speech treatment in Parkinson's disease: randomized controlled trial (RCT) Mov Disord. 2018;33:1777–91. doi: 10.1002/mds.27460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox C, Ebersbach G, Ramig L, Sapir S. LSVT LOUD and LSVT BIG: behavioral treatment programs for speech and body movement in Parkinson disease. Parkinsons Dis. 2012:391946. doi: 10.1155/2012/391946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atkinson-Clement C, Sadat J, Pinto S. Behavioral treatments for speech in Parkinson's disease: meta-analyses and review of the literature. Neurodegener Dis Manag. 2015;5:233–48. doi: 10.2217/nmt.15.16. [DOI] [PubMed] [Google Scholar]
- 44.Levitt JS, Walker-Batson D. The effects of the “Speak with Intent” instruction for individuals with Parkinson's disease. J Commun Disorder Assist Technol. 2018;1:1–15. [Google Scholar]
- 45.Tjaden K. Speech and swallowing in Parkinson's disease. Top Geriatr Rehabil. 2008;24:115–26. doi: 10.1097/01.TGR.0000318899.87690.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez HH, Walter BL, Rush TE, Ahmed A. 3rd ed. New York: Springer Publishing Company; 2021. A Practical Approach to Movement Disorders: Diagnosis and Management. [Google Scholar]
- 47.Tykalová T, Rusz J, Čmejla R, Klempíř J, Růžičková H, Roth J, et al. Effect of dopaminergic medication on speech dysfluency in Parkinson's disease: a longitudinal study. J Neural Transm. 2015;122:1135–42. doi: 10.1007/s00702-015-1363-y. [DOI] [PubMed] [Google Scholar]
- 48.Schulz GM, Peterson T, Sapienza CM, Greer M, Friedman W. Voice and speech characteristics of persons with Parkinson's disease pre-and post-pallidotomy surgery: preliminary findings. J Speech Lang Hear Res. 1999;42:1176–94. doi: 10.1044/jslhr.4205.1176. [DOI] [PubMed] [Google Scholar]
- 49.Tröster AI, Woods SP, Fields JA, Hanisch C, Beatty WW. Declines in switching underlie verbal fluency changes after unilateral pallidal surgery in Parkinson's disease. Brain Cogn. 2002;50:207–17. doi: 10.1016/s0278-2626(02)00504-3. [DOI] [PubMed] [Google Scholar]
- 50.Moreau C, Pennel-Ployart O, Pinto S, Plachez A, Annic A, Viallet F, et al. Modulation of dysarthropneumophonia by low-frequency STN DBS in advanced Parkinson's disease. Mov Disord. 2011;26:659–63. doi: 10.1002/mds.23538. [DOI] [PubMed] [Google Scholar]